Abstract
Drug repurposing is using an existing drug for a new treatment that was not indicated before. It has received immense attention during the COVID-19 pandemic emergency. Drug repurposing has become the need of time to fasten the drug discovery process and find quicker solutions to the over-exerted healthcare scenario and drug needs. Drug repurposing involves identifying the drug, evaluating its efficiency using preclinical models, and proceeding to phase II clinical trials. Identification of the drug candidate can be made through computational and experimental approaches. This approach usually utilizes public databases for drugs. Data from primary and translational research, clinical trials, anecdotal reports regarding off-label uses, and other published human data information available are included. Using artificial intelligence algorithms and other bioinformatics tools, investigators systematically try to identify the interaction between drugs and protein targets. It can be combined with genetic data, clinical analysis, structure (molecular docking), pathways, signatures, targets, phenotypes, binding assays, and artificial intelligence to get an optimum outcome in repurposing. This article describes the strategies involved in drug repurposing and enlists a series of repurposed drugs and their indications.
Keywords: drug repurposing, clinical trials, molecular docking, drug discovery, post-market safety
INTRODUCTION
STAGES OF DRUG REPURPOSING
IMPORTANCE OF DRUG REPURPOSING
AN IDEAL CANDIDATE FOR REPURPOSING
STRATEGIES FOR DRUG REPURPOSING
Phenotypic Screening
Target-Based Methods
Knowledge-Based Methods
Signature-Based Methods
Pathway- or Network-Based Methods
Targeted Mechanism-Based Methods
Molecular Docking
Application of Drug repurposing in Drug Discovery
Challenges
CONCLUSIONS
REFERENCES
INTRODUCTION
Drug repurposing is the technique of using an existing drug or drug candidate for a new treatment or medical condition for which it was not indicated before [1]. It was initially developed to treat a different medical condition. It has been described as a serendipitous process that happens unexpectedly. In this process, the undesired side effects of drug molecules can also be a pointer to exploring the possibility of its effectiveness in an entirely different medical condition [2]. Usually, drugs with established safety in humans and tested and developed for efficacy in a particular disease other than the one for which they were developed [3]. This process brings the drugs directly to preclinical and clinical trials, skipping the drug development process, and thus reducing risk and costs [4].
STAGES OF DRUG REPURPOSING
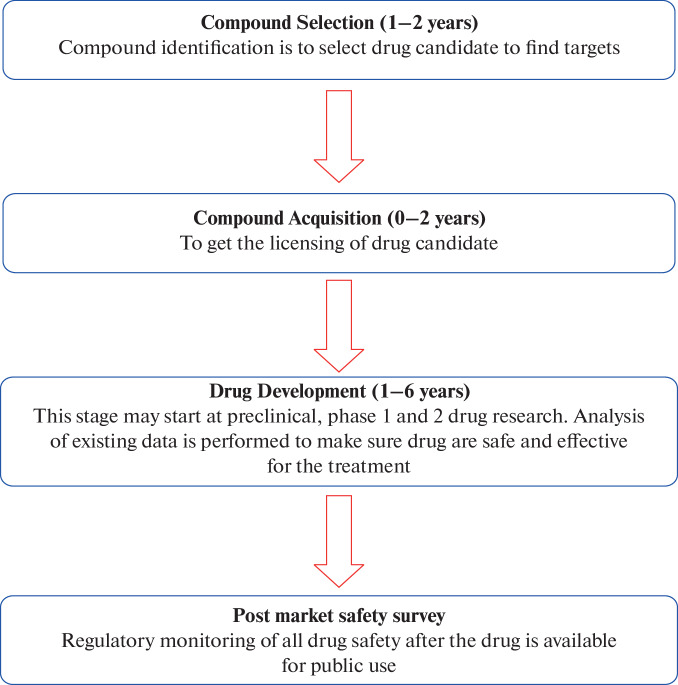
The stages of repurposing are elucidated in Fig. 1 [17].
Fig. 1.
Stages of Repurposing.
IMPORTANCE OF DRUG REPURPOSING
Repurposing can identify new compounds based on phenotypic benefits without explicitly defining the mechanism of action. This can be directly tested in preclinical animal models, and these results are more applicable to clinical applications and research. It may progress directly straight to Phase II clinical trials [5]. There is a minimum risk of failure with repurposed drugs [6]. The difference between traditional drug discovery and drug repurposing is described in Table 1.
Table 1. .
| TRADITIONAL DRUG DISCOVERY | DRUG REPURPOSING |
|---|---|
|
Include 5 stages: • Discovery and preclinical • Safety review • Clinical research • FDA review • FDA post-market safety monitoring |
Include 4 stages: • Compound identification • Compound acquisition • Development • FDA post-market safety monitoring |
| Generally, more time consuming | Less time consuming |
| High investment or cost | Lesser investment compared to traditional drug discovery |
| More risk of failure | Less risk of failure |
| Clinical efficacy and safety profile should be evaluated | Clinical efficacy and safety profiles already exist |
AN IDEAL CANDIDATE FOR REPURPOSING
A drug has undergone clinical drug development and has been marketed as an ideal candidate for repurposing. A drug with well-established safety and toxicity studies in previous clinical trials, approved by the regulatory authorities, can skip clinical trials with sufficient data support and justification. The mechanism of action of the selected drug shall be established [3].
Those drugs that have gone through several stages of clinical development and have been unsuccessful for reasons other than safety are ideal candidates for repurposing. Some drug repurposing has occurred during clinical trials, like the well-known Viagra (sildenafil) indicated initially for hypertension and angina. The new role of treating erectile dysfunction was unravelled during the clinical trials.
There are examples of abandoned drugs with their toxicity resurfaced with different indications. Thalidomide, indicated for vomiting, was used to treat nausea in pregnant women and resulted in several congenital disabilities. Due to this tragic effect on fetal development, its use was banned or restricted in several countries. Later, the drug was repurposed for leprosy and multiple myeloma [18]. However, this type of repurposing has met objections from the scientific community [19]. The benefit-to–to-risk ratio can be considered during repurposing cases. A rational decision from the regulatory authorities is essential to address the concern “Is it worth the risk? Or can an existing therapy perform better than the repurposed drug?”.
STRATEGIES FOR DRUG REPURPOSING
This approach usually utilizes public databases for drugs. Data from primary and translational research, clinical trials, anecdotal reports regarding off-label uses, and other published human data information available are included. Using artificial intelligence algorithms and other bioinformatics tools, investigators systematically try to identify the interaction between drugs and protein targets. In-silico drug repositioning is a powerful technology with significant advantages, including speed and reduced costs [20]. There are usually three kinds of approaches; computational approach, biological experimental approach, and mixed approach, which are described in Table 2 [17].
Table 2. .
Drug repurposing can be drug-oriented, disease-oriented, and treatment-oriented
| Drug-oriented Information of drugs | Disease-oriented | Treatment oriented |
|---|---|---|
| Off-label use of drugs | Information on disease pathway | Disease omics data |
| Phenotypic screening | Disease omics data | All information related to treatment strategies |
| Target 3D structure of the drug | Genetics data of disease | Genetics genomics |
| Chemical structure of drugs and ligands | Protein interaction network | Proteomics metabolics |
| An adverse effect of drugs |
Traditional phenotype-based screening methods do not need prior knowledge, and the repositioned drugs are just serendipitously tested. The integrated knowledge and elucidated drug action mechanisms increase with the complexity of modelling methods [4]. These methods are listed in Table 3.
Table 3. .
Methods of Repurposing [4]
| Method | Required knowledge# |
|---|---|
| Blinded search or screening method |
Off-label use Phenotypic screening |
| Target-based methods | Phenotypic screening; Target 3D structure, chemical structure information of drugs and ligands |
| Knowledge-based methods |
Drug–target information, chemical structure information of targets and adverse effects (clinical trial information) Regulatory approval labels and adverse effects Available pathway information of disease |
| Signature-based methods |
Disease omics data; Genetics data; Drug omics data Disease omics and drug omics data |
| Pathway- or network-based methods | Disease omics data, available pathway information, and protein interaction network; Drug omics data |
| Targeted mechanism-based methods | Drug omics data, disease pathway and protein interaction network |
# Repurposing methods require various skills and knowledge of various factors, that is, it may be drug-oriented or disease-oriented, or treatment-oriented [4]
Phenotypic Screening
The phenotypic screening method was used to discover molecules and biologics approved by the regulatory bodies. These methods do not include pharmaceutical or biological information and therefore are less likely to help elucidate any mechanisms of action of drugs. Most depend on serendipitous identification from tests aimed at specific diseases and medicines. The advantage of these methods (off-label use and phenotypic screening) is that they have a high chance of application to many drugs or conditions [4, 21].
Target-Based Methods
This method requires specific knowledge about the targets, such as 3D protein structures. Knowledge-based methods require knowledge about drugs or diseases, such as adverse effects, regulatory approval labels, records of clinical trials, and published disease biomarkers (potential targets) or disease pathways). These methods enable researchers to quickly screen any number of drug molecules with a known chemical structure (e.g., Simplified Molecular Input Line-Entry System SMILES). Target-based drug repositioning methods include.
• In-vitro and in vivo high-throughput and/or high-content screening (HTS/HCS) of drugs for a protein or biomarker of interest and
• In-silico screening of drugs or compounds from drug libraries, such as ligand-based screening or docking.
Compared to blinded methods, targeted-based methods improve the chances of drug discovery as targets are directly linked to the disease mechanism. Integrating target information and drug repurposing increases the possibility of finding therapeutically beneficial compounds [4, 21, 22].
Knowledge-Based Methods
These methods apply bioinformatics or cheminformatics approaches to include the available information of drugs, drug-target networks, chemical structures of targets and drugs, clinical trial information, FDA approval labels, signalling or metabolic pathways, and so on, into drug-repositioning studies. The information content of blinded and target-based methods may not be sufficient to identify new mechanisms beyond the known targets. Knowledge-based methods incorporate known information into predicting unknown mechanisms, such as novel drug targets, obscure drug-drug similarities, and new disease biomarkers. Knowledge-based methods include much-known information into the drug repositioning process to improve prediction accuracy [4, 21].
Signature-Based Methods
Signature-based drug-repurposing methods use gene signatures derived from disease omics data with or without treatment to discover unknown off-target or disease mechanisms. Such genomics data can be assessed through publicly available databases, such as NCBI-GEO (http://www.ncbi.nlm.nih.gov/geo/), SRA Sequence Read Archive (http://www.ncbi. nlm.nih.gov/Traces/sra/), CMAP Connectivity Map and CCLE Cancer Cell Line Encyclopedia. Signature-based methods help uncover unknown mechanisms of action of molecules and drugs. Using computational approaches includes molecular-level mechanisms, such as significantly changed genes [4, 21].
Pathway- or Network-Based Methods
This method utilizes genetic disease data, available signalling or metabolic pathways, and protein interaction networks to reconstruct disease-specific pathways, thereby identifying the key target for repurposing drugs. They help narrow general signalling networks from a large number of proteins down to a specific network with a few proteins (or targets). These methods use pathway analysis or network biology methods to discover essential pathways from diseases' genetic, genomic, proteomic, and metabolic data to find new targets for repositioned drugs. Example: Signalling mechanisms of metastatic subtypes of breast cancer because the subtype signalling mechanisms are hard to elucidate from existing breast cancer pathways or the gene signatures [4, 21].
Targeted Mechanism-Based Methods
These methods integrate treatment omics (genetic) data, available signalling pathway information, and protein interaction networks to delineate the unknown mechanisms of the action of drugs. It aims to discover drug action mechanisms by identifying off-target or targeted pathways of treated drugs using drug omics data (before and after drug treatment). For example, drug resistance is an issue in cancer therapy. Although patients initially respond well to a drug, they often acquire resistance to that drug after a few months of treatment. Hence, successful drug treatment needs additional information about the mechanisms of action of drugs to find better drug targets. However, there are fewer studies on targeted mechanism-based methods that developed elegant computational models to predict the drug effects and related targeted pathways. This is because of the difficulties in deriving effective computational models [4, 21].
Molecular Docking
Molecular docking is a versatile tool used to predict the geometry and to score the interaction of a protein in a complex with a small-molecule ligand. Docking can be performed by docking a known drug into a large set of different target structures or a database of approved medications into one intended target. Molecular docking is a convenient and fast method to screen large libraries of ligands and targets, with a full range of sampling options [23]. 3D structures of the target shall be available through crystallography, nuclear magnetic resonance (NMR), or comparative models to carry out docking. Drawbacks and limitations include approximate scoring function and imperfect binding mode placement algorithms. However, these problems can be overcome by postprocessing docking results with more accurate scoring functions and other criteria [24].
Application of Drug Repurposing in Drug Discovery
Besides developing new treatment options (such as immunotherapy and host-directed therapies), scientists worldwide are working to repurpose existing drugs against SARS-CoV-2 [27]. Table 2 gives a detailed list of repurposed drugs, and a few are repurposed for the treatment of COVID-19. Another example is metformin, an antidiabetic drug that shows anticancer effects by decreasing the incidence of different cancers and inhibiting the proliferation and migration of cancer cells, activating apoptosis, and reducing EMT (epithelial-mesenchymal transition) and metastasis. Repurposing helps overcome antibiotic resistance. For example, TB strains resistant to currently used drug combinations are found in all parts of the world. The product antibiotic, pyridomycin, discovered in the 1950s, is repurposed to treat TB, which takes the place of isoniazid [25, 26]. Table 4 contains a detailed list of repurposed drugs with their initial indication and repurposed use.
Table 4. .
Examples of repurposed drugs
| S. No. | Drug | Discovered | Repurposed | Ref. |
|---|---|---|---|---|
| 1 | Amiloride | Acid-sensing ion channel antagonist | Secondary progressive multiple sclerosis (SPMS) | [26] |
| 2 | Anastrazole | Ovulation induction | Breast cancer | [9] |
| 3 | Angiotensin-converting enzyme 2 (ACE2) inhibitor, angiotensin receptor blocker (ARB) and statins | Antihypertensives | Effective against SARS-CoV-2 (COVID-19) [Few controversies are seen apart from promising results] | [27] |
| 4 | Aripiprazole | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 5 | Artesunate | Anti-infective | Active against fungal biofilms | [28] |
| 6 | Aspirin and ibuprofen | Inflammation | Antibacterial and antifungal | [25] |
| 7 | Atorvastatin (generic Lipitor) | Hyper-cholesterolaemia | Cavernous angioma | [26] |
| 8 | Auranofin | Rheumatoid arthritis | Antibacterial and antifungal | [25] |
| 9 | Avermectin B1a | Anti-infective | Active against fungal biofilms | [28] |
| 10 | Azathioprine | Crohn’s disease | Antibacterial and antifungal | [25] |
| 11 | Bacitracin | Anti-infective | Active against fungal biofilms | [28] |
| 12 | Benzbromarone | Vasodilator | Active against fungal biofilms | [28] |
| 13 | Bithionate disodium | Anti-infective | Active against fungal biofilms | [28] |
| 14 | Bleomycin | Antitumor | Active against fungal biofilms | [28] |
| 15 | Bromperidol | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 16 | Broxyquinoline | Anti-infective | Active against fungal biofilms | [28] |
| 17 | Capecitabine | Colon cancer | Breast cancer | [9] |
| 18 | Carboplatin | Antitumor | Active against fungal biofilms | [28] |
| 19 | Celecoxib | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 20 | Chloroquine | Anti-malarial | Active against fungal biofilms | [28] |
| 21 | Cisplatin | Antitumor | Active against fungal biofilms | [28] |
| 22 | Clarithromycin | Anti-infective | Active against fungal biofilms | [28] |
| 23 | Clarithromycin, pioglitazone, and treosulfan |
Antibiotic Antidiabetic |
Non-small cell lung cancer | [29] |
| 24 | Clomiphene | Fertility | Antibacterial and antifungal | [25] |
| 25 | Cyclophosphamide | As immuno-modulator in autoimmune diseases | Breast cancer | [9] |
| 26 | Cyclosporine | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 27 | Dacarbazine | Antitumor | Active against fungal biofilms | [28] |
| 28 | Daunorubicin | Acute myeloid leukemia, acute lymphocytic leukemia, chronic myelogenous leukemia, and Kaposi’s sarcoma | Antibacterial and antifungal | [25] |
| 29 | Dexpramipexole | ALS and other neurological diseases: phase 3 trials did not meet the endpoint | Hypereosinophilic syndromes | [26] |
| 30 | Diazepam | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 31 | Digoxin | Treatment for cardiac diseases | Anticancer | [30] |
| 32 | Dihydroartemisinin | Anti-infective | Active against fungal biofilms | [28] |
| 33 | Disulfiram (Antabuse) | Reduces ethanol tolerance in alcoholism | Metastatic breast cancer & Alzheimer’s disease | [26] |
| 34 | Docetaxel | Hormone-refractory prostate cancer | Breast cancer and active against fungal biofilms | [9, 28] |
| 35 | Doxepin | Antipsychotic/Antidepressant | Active against fungal biofilms | 28] |
| 36 | Doxorubicin | Antibiotic from Streptomyces peucetiusbacterium, | Bladder, breast, stomach, lung, ovarian, and thyroid cancers | [9, 25] |
| 37 | Ebastine | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 38 | Ebselen | Bipolar disorder and ischemic stroke | Antibacterial and antifungal | [25] |
| 39 | Edaravone | Neuroprotective agent in acute ischemic stroke and ALS | Multiple sclerosis | [26] |
| 40 | Eltrombopag | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 41 | Esketamine (S enantiomer of ketamine) | Intravenous anesthetic | Treatment-resistant major depressive disorder (TRD) | [31] |
| 42 | Etodolac | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 43 | Everolimus (Votubia, Evertor) | Immunosuppressants during organ transplants, wound healing | Breast cancer | [9] |
| 44 | Exemestane | Ovulation induction | Breast cancer | [9] |
| 45 | Favipiravir | Inhibitors of RNA-dependent RNA polymerase of virus (Antiviral drug) | Effective against SARS-CoV-2 (COVID-19) [More studies ae required] | [27, 34] |
| 46 | Fenofibrate | Reduces, triglyceride-rich particles (LDL) in plasma | Reduces macrophage recruitment in abdominal aortic aneurysm | [26] |
| 47 | Finasteride | Benign prostatic hyperplasia | Antibacterial and antifungal | [25] |
| 48 | Floxuridine | Colorectal cancer | Antibacterial and antifungal | [25] |
| 49 | Fluorouracil | Keratoacanthomas, actinic keratosis, and skin warts | Breast cancer | [9] |
| 50 | Fluorouracil | Solid tumors | Antibacterial and antifungal | [25] |
| 51 | Fluoxetine | Antipsychotic/Antidepressant, Serotonin selective reuptake inhibitor (SSRI) | Active against fungal biofilms, secondary progressive multiple sclerosis (SPMS) |
] |
| 52 | Fluvastatin | Lipid-lowering | Active against fungal biofilms | [28] |
| 53 | Fulvestrant | Antiestrogen | Breast cancer | [9] |
| 54 | Gallium nitrate | Lymphoma and bladder cancer | Antibacterial and antifungal | [25] |
| 55 | Gemcitabine | Anti-viral drug | Breast cancer | [9] |
| 56 | Goserelin | Prostate cancer, uterine fibroids, assisted reproduction | Breast cancer | [9] |
| 57 | γ-Secretase inhibitors (GSI) | Alzheimer disease: prevent amyloid precursor cleavage | Several inhibitors are being tested against a variety of cancers | [26] |
| 58 | Human Albumin | Blood additive | Immuno-restoration | [26] |
| 59 | Hydroxychloroquine | Antimalarial | Antiviral drug (HIV, Chicken guinea, dengue, SARS-CoV-2) | [34] |
| 60 | Imipramine | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 61 | Iodoquinol | Anti-infective | Active against fungal biofilms | [28] |
| 62 | Itraconazole | Antifungal | Anticancer | [30] |
| 63 | Ivermectin | Anti-parasitic | Effective against SARS-CoV-2 (COVID-19) [safe in conventional doses] | [27] |
| 64 | Ketoprofen | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 65 | Ketorolac | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 66 | Letrozole | Ovulation induction | Breast cancer | [9] |
| 67 | Lopinavir/ritonavir | Antiviral drug | Effective against SARS-CoV-2 (COVID-19) Drug needs to be further investigated] | [27, 34] |
| 68 | Lorazepam | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 69 | Losartan | Blood pressure reduction | Alzheimer disease | [26] |
| 70 | Lovastatin | Lipid-lowering | Active against fungal biofilms | [28] |
| 71 | Loxapine | Antipsychotic and antischizophrenia | Irritability associated with autism | [26] |
| 72 | Mebendazole | Antiparasitic/Helminthiasis/Anti-infective | Brain cancer (i.e., medulloblastoma and glioblastoma)/Antibacterial and antifungal/Active against fungal biofilms | [25, 28, 29] |
| 73 | Meloxicam | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 74 | Metformin | Diabetes | Anti-nonsmall cell lung cancer, and augmented resistance in aging, Colo rectal cancer | [26, 32] |
| 75 | Methotrexate | Leukemia | Breast cancer | [9] |
| 76 | Mibefradil (Posicor) | Antihypertensive, calcium channel blocker | Short term use as an adjuvant in cancer therapy | [26] |
| 77 | Midazolam | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 78 | Mifepristone | Emergency contraceptive | Cushing’s syndrome | [29] |
| 79 | Mycophenolic acid | Immunosuppressant | Anticancer | [30] |
| 80 | Nelfinavir | HIV protease inhibitor | Solid tumors | [26] |
| 81 | Niclosamide | Helminthiasis/Anti-infective |
Antibacterial and antifungal and active against fungal biofilms Treats multidrug-resistant leukemia |
[25, 28, 33] |
| 82 | Nitazoxanide | Antiprotozoal agent | Influenza | [26] |
| 83 | Nitroxoline | Anti-infective | Active against fungal biofilms | [28] |
| 84 | Nortriptyline | Antipsychotic/Antidepressant | Active against fungal biofilms | [28] |
| 85 | Oxyclozanide | Helminthiasis | Antibacterial and antifungal | [25] |
| 86 | Paclitaxel | Ovarian cancer, atrial restenosis | Breast cancer | [9] |
| 87 | Pentetic acid | Hypocalcaemia | Antibacterial and antifungal | [25] |
| 88 | Perhexiline maleate | Anti-anginal | Active against fungal biofilms | [28] |
| 89 | Phenobarbitone | Anticonvulsant | Active against fungal biofilms | [28] |
| 90 | Pimozide | Severe Tourette’s syndrome and schizophrenia | Antibacterial and antifungal | [25] |
| 91 | Promethazine | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 92 | Propanolol | Antiarrhythmic | Active against fungal biofilms | [28] |
| 93 | Pyrvinium pamoate | Anti-infective | Active against fungal biofilms | [28] |
| 94 | Quinacrine | Helminthiasis | Antibacterial and antifungal | [25] |
| 95 | Raloxifene | Osteoporosis in postmenopausal women | Breast cancer | [9] |
| 96 | Rapamune | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 97 | Remdesvir | Inhibitors of RNA-dependent RNA polymerase in virus (Antiviral drug) | Effective against SARS-CoV-2 |
[27, 34] |
| 98 | Ribavirin | Antiviral drug | Effective against SARS-CoV-2(COVID-19) | [27, 34] |
| 99 | Rifampicin | Anti-infective | Active against fungal biofilms | [28] |
| 100 | Riluzol | Glutamate antagonist | Secondary progressive multiple sclerosis (SPMS) | [26] |
| 101 | Saracatinib | Cancer therapy | Mild to moderate Alzheimer disease | [26] |
| 102 | Sildenafil (Viagra) | Angina | Erectile dysfunction, | [29] |
| 103 | Silymarin | Anti-hepatotoxic | Active against fungal biofilms | [28] |
| 104 | Simvastatin | Hyper-cholesterolemia (Lipid-lowering) | Antibacterial and antifungal | [25, 28] |
| 105 | Sirolimus and Zoledronic acid | Prophylaxis of organ rejection, Osteoporosis respectively | Osteosarcoma (combined with Metzolimos, metronomic cyclophosphamide, methotrexate) | [29] |
| 106 | Statins | Hyper-cholesterolaemia | Oncology | [26] |
| 107 | Streptozotocin | Pancreatic islet cell cancer | Antibacterial and antifungal | [25] |
| 108 | Sulfadiazine | Anti-infective | Active against fungal biofilms | [28] |
| 109 | Sulfadimethoxine | Anti-infective | Active against fungal biofilms | [28] |
| 110 | Sulfamethoxazole | Anti-infective | Active against fungal biofilms | [28] |
| 111 | Sulfamethoxy-pyridazine | Anti-infective | Active against fungal biofilms | [28] |
| 112 | Tacrolimus | Anti-inflammatory/Immunomodulatory | Active against fungal biofilms | [28] |
| 113 | Tamoxifen | Breast cancer, Albright syndrome, ovulation induction | Antibacterial and antifungal | [9, 25] |
| 114 | Teicoplanin | Antibacterial | Antiviral, potentially repurposable for COVID-19 treatment | [27] |
| 115 | Telmisartan | Blood pressure reduction | Abdominal aortic aneurysm | [26] |
| 116 | Thalidomide | Antiemetic | Hanson’s disease, Leprosy | [26] |
| 117 | Thiotepa | Immunosuppressant | Breast cancer | [9] |
| 118 | Tigecycline | Anti-infective | Active against fungal biofilms | [28] |
| 119 | Tocilizumab | Immunosuppressive drug for cytokine release syndrome | Severe COVID-19 infection | [27, 34] |
| 120 | Toremifene | Infertility with an ovulatory disorder | Breast cancer | [9] |
| 121 | Valproic acid | Anticonvulsant | Active against fungal biofilms | [28] |
| 122. | Verapamil | Antiarrhythmic | Active against fungal biofilms | [28] |
| 123. | Vinblastine | Hodgkin lymphoma, non-Hodgkin’s lymphoma, histiocytosis | Breast cancer | [9] |
| 124. | Yohimbine hydrochloride | Vasodilator | Active against fungal Biofilms | [28] |
Challenges
Although screening efforts are relatively inexpensive, approved drug clinical trials are expensive. The advantage of drug repurposing is that the early stages of clinical development are complete; hence, the drugs can proceed to clinical studies. However, doing clinical studies directly without preclinical studies is a risk. Inaccurate identification of drugs may prove to have no significant impact on therapy or mortality rates, and it may result in a loss in terms of treatment and expense.
Identifying a new therapeutic indication for an existing drug is a significant challenge. Choosing the right therapeutic area for the drug under investigation, evaluating the clinical trials with respect to the new therapeutic use, and deciding which stage of the clinical study or preclinical study shall be restarted are a few challenges repurposing. New preclinical and clinical trials may be required to be carried out if the available data are not satisfactory and do not comply with the requirements of regulatory agencies.
Another critical issue is patent application and intellectual property rights (IPR). Patents or IPR can prevent some repurposed drugs from entering the market, and IP protection for drug repurposing is minimal. Hence, the regulatory constraints and the risk of abandoning repurposing projects due to unsatisfactory results can discourage the investment of money or resources towards drug repurposing. A spike in market demand can be an excellent motivator to shift researchers and investors into action.
CONCLUSIONS
There are numerous diseases for which good therapeutic options have not been developed. The concept of repurposing a drug enables exploring the hidden potential of many molecules and better utilization of therapeutic agents. For better drug repositioning, more in-depth understanding along with integrated approaches between computational and experimental methods may be required to ensure high success rates of repositioned drugs.
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the authors.
REFERENCES
- 1.Oprea T.I. AAPS J. 2012;14:759–763. doi: 10.1208/s12248-012-9390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strittmatter S.M. Nat Med. 2014;20:590–591. doi: 10.1038/nm.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinić J., Efferth T., García-Sosa A.T., Grahovac J., Padrón J.M., Pajeva I., Rizzolio F., Saponara S., Spengler G., Tsakovska I. Drug Resist. Updates. 2020;52:100713. doi: 10.1016/j.drup.2020.100713. [DOI] [PubMed] [Google Scholar]
- 4.Jin G., Wong S.T.C. Drug Discovery Today. 2014;19:637–644. doi: 10.1016/j.drudis.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner N., Zeng X.Y., Osborne B., Rogers S., Ye J.M. Trends Pharmacol. Sci. 2016;37:379–389. doi: 10.1016/j.tips.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Rudrapal, M., Khairnar, S.J., and Jadhav, A.G., Drug Repurposing (DR): An Emerging Approach in Drug Discovery, 2020. 10.5772/intechopen.93193
- 7.Hughes J.P., Rees S., Kalindjian S.B., Philpott K.L. Br. J. Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalita J., Chetia D., Rudrapal M. Med. Chem. 2020;16:928–937. doi: 10.2174/1573406415666190806154722. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S., Verma S.S., Aggarwal S., Gupta S.C. Semin. Cancer Biol. 2020;68:8–20. doi: 10.1016/j.semcancer.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha Y., Erez T., Reynolds I.J., Kumar D., Ross J., Koytiger G., Kusko R., Zeskind B., Risso S., Kagan E., Papapetropoulos S., Grossman I., Laifenfeld D. Br. J. Pharmacol. 2018;175:168–180. doi: 10.1111/bph.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal P. J. Pharmacovigil. 2018;6:1–2. doi: 10.1016/j.drudis.2020.10.010. [DOI] [Google Scholar]
- 12.Allarakhia M. Drug Des. Dev. Ther. 2013;7:753–766. doi: 10.2147/DDDT.S46289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parvathaneni V., Kulkarni N.S., Muth A., Gupta V. Drug Discovery Today. 2019;24:2076–2085. doi: 10.1016/j.drudis.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padhy B.M., Gupta Y.K. J. Postgrad. Med. 2011;57:153. doi: 10.4103/0022-3859.81870. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal P. J. Pharmacovigil. 2015;2:1–2. doi: 10.4172/2329-6887.S2-e002. [DOI] [Google Scholar]
- 16.Pantziarka P., Bouche G., Meheus L., Sukhatme V., Sukhatme V.P., Vikas P. Ecancermedicalscience. 2014;8:442. doi: 10.3332/ecancer.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue H., Li J., Xie H., Wang Y. Int. J. Biol. Sci. 2018;14:1232–1244. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J.H., Scialli A.R. Toxicol. Sci. 2011;122:1–6. doi: 10.1093/toxsci/kfr088. [DOI] [PubMed] [Google Scholar]
- 19.Pannikar V. Lepr. Rev. 2003;74:286–288. doi: 10.47276/lr.74.3.286. [DOI] [PubMed] [Google Scholar]
- 20.Abbruzzese C., Matteoni S., Signore M., Cardone L, Nath K., Glickson J.D., Paggi M.G. J. Exp. Clin. Cancer Res. 2017;36:169. doi: 10.1186/s13046-017-0642-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.March-Vila E., Pinzi L., Sturm N., Tinivella A., Engkvist O., Chen H., Rastelli G. Front. Pharmacol. 2017;8:298. doi: 10.3389/fphar.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napolitano F., Zhao Y., Moreira V.M., Tagliaferri R., Kere J., D’mato M., Greco D. J. Cheminform. 2013;5:30. doi: 10.1186/1758-2946-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitchen D.B., Decornez H., Furr J.R., Bajorath J. Nat. Rev. Drug Discovery. 2004;3:935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 24.Sgobba M., Caporuscio F., Anighoro A., Portioli C., Rastelli G. Eur. J. Med. Chem. 2012;58:431–440. doi: 10.1016/j.ejmech.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Miró-Canturri A., Ayerbe-Algaba R., Smani Y. Front. Microbiol. 2019;10:41. doi: 10.3389/fmicb.2019.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schein C.H. Med. Res. Rev. 2020;40:586–605. doi: 10.1002/med.21627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jean S.S., Hsueh P.R. Expert Rev. Anti Infect. Ther. 2020;18:843–847. doi: 10.1080/14787210.2020.1771181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Mello T.P., Silva L.N., Ramos L.S., Frota H.F., Branquinha M.H., dos Santos A.L.S. Curr. Top. Med. Chem. 2020;20:509–516. doi: 10.2174/156802662007200316142626. [DOI] [Google Scholar]
- 29.Hernandez J.J., Pryszlak M., Smith L., Yanchus C., Kurji N., Shahani V.M., Molinski S.V. Front. Oncol. 2017;7:273. doi: 10.3389/fonc.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shim J.S., Liu J.O. Int. J. Biol. Sci. 2014;10:654–663. doi: 10.7150/ijbs.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Das J. J. Med. Chem. 2020;63:13514–13525. doi: 10.1021/acs.jmedchem.0c01193. [DOI] [PubMed] [Google Scholar]
- 32.Sang, J., Tang, R., Yang, M., and Sun, Q., Biomed. Res. Int., 2020, pp. 1–9. 10.1155/2020/9312149 [DOI] [PMC free article] [PubMed]
- 33.Hamdoun S., Jung P., Efferth T. Front. Pharmacol. 2017;8:1–11. doi: 10.3389/fphar.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng Y.L., Salim C.K., Chu J.J.H. Pharmacol. Ther. 2021;228:1–14. doi: 10.1016/j.pharmthera.2021.107930. [DOI] [PMC free article] [PubMed] [Google Scholar]