Abstract
Worldwide, breast cancer is still a leading cause of cancer death in women. Indeed, over the years, several anti-breast cancer drugs have been developed; however, the complex heterogeneous nature of breast cancer disease reduces the applicability of conventional targeted therapies with the upsurge in side effects and multi-drug resistance. Molecular hybrids generated by a combination of two or more active pharmacophores emerged as a promising approach in recent years for the design and synthesis of anti-breast cancer drugs. The hybrid anti-breast cancer molecules are well known for their several advantages compared to the parent moiety. These hybrid forms of anti-breast cancer molecules demonstrated remarkable effects in blocking different pathways contributing to the pathogenies of breast cancer and improved specificity. In addition, these hybrids are patient compliant with reduced side effects and multi-drug resistance. The literature revealed that molecular hybrids are applied to discover and develop novel hybrids for various complex diseases. This review article highlights the recent progress (∼2018–2022) in developing molecular hybrids, including linked, merged, and fused hybrids, as promising anti-breast cancer agents. Furthermore, their design principles, biological potential, and future perspective are discussed. The provided information will lead to the development of novel anti-breast cancer hybrids with excellent pharmacological profiles in the future.
This review highlights the potential of a molecular hybridization approach in breast cancer treatment. It provides insights into the current progress in developing linked, merged, and fused hybrids as potential anti-breast cancer agents.
1. Introduction
Breast cancer is one of the significant endocrine cancers diagnosed in women, and in 2020, globally, 684 996 deaths were reported due to breast cancer (WHO, 2021).1 Breast cancer is the second leading cause of cancer death for women in the United States after lung cancer. It is estimated that in 2022, around 43 250 women and 530 men will die of breast cancer (ACS, 2022).2 Broadly, breast cancer can be classified into three main subtypes: hormone receptor-positive (HR+), human epidermal growth factor receptor-2 (HER-2)-positive, and triple-negative breast cancer. Of all the breast cancers diagnosed, approximately 65% are HR+ with high estrogen and progesterone receptor expression. For many years, endocrine therapy, including selective estrogen receptor modulators (tamoxifen), aromatase inhibitors (letrozole, anastrozole, and exemestane), and the selective estrogen receptor degrader (fulvestrant), has been in use for the treatment of HR+ breast cancer.3–5 As observed, nearly one out of five breast cancers are HER-2 positive with overexpression of human epidermal growth factor receptor-2 and treated with anti-HER-2 therapy, particularly with the drug trastuzumab.6 Triple-negative breast cancer (TNBC) is a heterogeneous type that accounts for approximately 10–20% of breast cancers.7–9 TNBC is negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2, therefore unresponsive to hormonal or targeted molecular therapy and responsible for 25% of breast cancer-related deaths. TNBC treatment is still a substantial clinical challenge compared to that of other subtypes due to its high metastatic potential and limited therapeutic options. The most commonly explored and actively studied strategies for TNBC treatment include targeted therapies and immunotherapy medicines.10,11 The increasing number of breast cancer cases and associated mortalities makes it a considerable challenge. The available breast cancer treatments mainly target various strategies and pathways. They are effectively helpful in increasing the overall survival rate of the patients but are associated with adverse side effects and the development of drug resistance due to prolonged use. As a result, there is a need to identify and improve new therapeutic strategies for treating breast cancer.
Hybrid molecules, generated by combining two or more pharmacophores, have gained remarkable attention in academia and industry to control more than one target type and fight against different diseases. Fink et al. developed the concept of hybrid molecules in 1996 by combining acetylcholinesterase (AChE) and monoamine oxidase (MAO) inhibitory activity in one molecular framework that exhibited promising pharmacological activity against Alzheimer's disease.12 Since then, the concept of molecular hybridization, involving a combination of two or more pharmacophoric moieties in one molecule, appeared as a promising approach for rationally designing multi-targeting drugs with improved pharmacological potency.13–19 The hybrid molecules are operative due to predictable pharmacokinetics and pharmacodynamic profiles, improved drug bioavailability, better transportation across membranes, and protection against enzymatic degradation. In addition, hybrid molecules effectively minimize drug–drug interactions and potential drug resistance. Various strategies were adopted to design multifunctional hybrids with the required pharmacological properties, including framework combination,20 categorized based on linkages such as non-cleavable linker hybrids, cleavable linker hybrids, merged hybrids, and fused hybrids (Fig. 1a).
Fig. 1. (a) Types of hybrids based on linkages. (b) Types of hybrids depending on their approach to target interaction.
The cleavable linker hybrids are specially designed to release two drugs during metabolism that interact independently with the respective targets. The non-cleavable linker hybrid molecules are composed of pharmacophores that are separated by a distinct metabolically stable linker group. The merged hybrids are formed when scaffolds are combined by taking advantage of the common structural features of the starting compounds to generate smaller and simpler molecules. In fused hybrids/mutual prodrugs, two moieties are fused without using any linker. In addition, the hybrid molecules, depending on their approach to target interaction, are grouped as (i) acting on a single independent target, (ii) acting on two independent targets, and (iii) acting on two related targets (Fig. 1b).
The advancement in understanding related to the molecular complexity of cancer, mechanism of anticancer drug resistance, and availability of extensive clinical data suggests that the fight against cancer requires a multidimensional approach comprising the designing of hybrid molecules, drug combination, and co-delivery of medicines.21,22 Hybrid drugs have gained extensive attention in the anticancer drug development process because of their ability to control multiple cell proliferation targets, reduce the possibility of drug interaction and resistance, and have an unpretentious pharmacokinetic profile.23–26 Furthermore, compared to a combination of drugs, a single medication of hybrid drug reduces the treatment regimen complications, side effects, and toxicity and are therefore compatible with patients. Several anticancer hybrids have reached clinical trials; a few examples are estramustine,27,28 curaxin,29,30 lucitanib,31,32 fimepinostat,33,34 CUDC-101,35,36etc. (Fig. 2). Several natural products including geldanamycin, daunorubicin, porphyrins, anthraquinone, taxol, piperine, curcumin, etc. have been effectively utilized for designing partial or full natural hybrids for cancer treatment.20,37,38 Moreover, organometallic hybrids with platinum, iridium, gold, and iron have been studied extensively as anticancer agents.39–42 FDA has approved only a few hybrid drugs. Among them, lapatinib (Tykerb) and sunitinib (Sutent) are successful hybrid drugs available in the market for breast cancer and renal cell carcinoma treatment, respectively (Fig. 2).43–45 In the field of anticancer hybrid molecules, enormous data and a few recently published reviews are available in the literature.21–26,46–58 Considering our experience in breast cancer,3–5,59–63 we decided to review the recent literature on molecular hybrids and discuss by categorizing them into linked, merged, and fused hybrids, highlighting their significance in breast cancer research and treatment. This review discusses the chemical structure and biological evaluation data of the series' most promising hybrid and highlights the strategies and approaches to design the selected hybrid.
Fig. 2. Anticancer hybrids in clinical trials or approved by FDA and on the market.
2. Molecular hybrids with anti-breast cancer activity
2.1. Linker-based hybrids
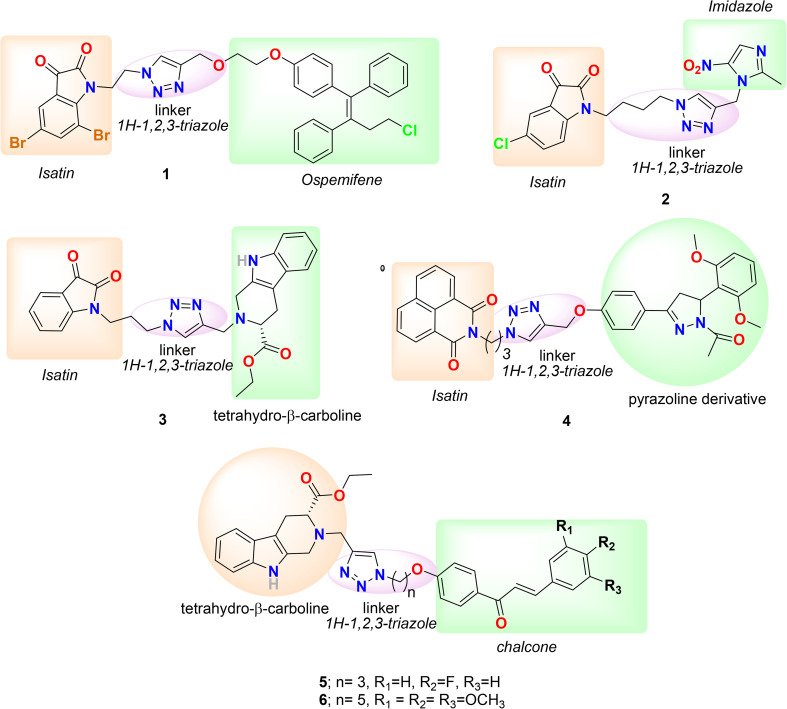
The first hybrid we selected to discuss in the category of linker hybrid is 1 (Fig. 3), designed and synthesized by Vipan Kumar and coworkers in 2018 by conjugating ospemifene and isatin via a 1H-1,2,3-triazole linkage.64 The triarylethylene pharmacophore-bearing raloxifene, tamoxifen, and toremifene are effective selective estrogen receptor modulators (SERMs) with anti-breast cancer activity in pre-and post-menopausal women. Recently, ospemifene (Osphena), a triphenylethylene derivative and a SERM, was approved by the FDA for the treatment of moderate to severe dyspareunia (vaginal and vulvar atrophy associated with menopause). Ospemifene displayed estrogen antagonistic activity in the breast and uterus and estrogen agonistic activity in the brain, bones, and vagina and has a safe pharmacological profile in clinical use.65,66 Considering the significance of ospemifine, the group linked it with isatin through a 1H-1,2,3-triazole linkage and evaluated its anti-proliferative activity against MCF-7 (ER+) and triple-negative MDA-MB-231 (ER−) cell lines using MTT assay. Conjugate 1 (Fig. 3) is the most promising hybrid of the series, bearing a bromo-substituent at the C-5 and C-7 positions of the isatin ring and with IC50 = 1.56 and 48.46 μM in MCF-7 and MDA-MB-231 cells, respectively, and in docking studies displayed a high binding affinity with the ERα active site. Imidazole and its derivatives are of great interest in medicinal chemistry, and in particular, imidazolium salt derivatives are effective in inducing G1 phase cell cycle arrest and apoptosis in tumor cells.67 Keeping in mind the significance of imidazole derivatives, the group designed and synthesized a series of conjugates with nitroimidazole by linking them to isatin via 1H-1,2,3-triazole and evaluated them against MCF-7 and MDA-MB-231 cell lines.68 The conjugates were not as effective as the standard compound (plumbagin); however, a few of the hybrid's activities were comparable to that of the anti-breast cancer drug tamoxifen. Hybrid 2, bearing a chloro-substituent at the C-5 position of the isatin ring and a butyl chain length, was found to be most effective against MCF-7 and MDA-MB-231 cell lines with IC50s of 54.25 and 26.12 μM, respectively. Additionally, conjugate 2 (Fig. 3) efficiently inhibited the growth of HEK-293 cells (control cells) with an IC50 value of 12.11 μM.
Fig. 3. Chemical structure of linker hybrids 1–6.
Naturally occurring and synthesized tetrahydro-β-carboline (THβC) derivatives are known for their diverse biological profile, including anti-proliferative activity against MCF-7 and MDA-MB-231 cell lines, low cytotoxicity, and ability to inhibit mitosis and block the G0/G1 phase of the cell cycle.69–72 Following this, the group designed and synthesized a series of 1H-1,2,3-triazole-linked THβC–isatin conjugates by using different substituents and stereochemistry at the C-1 and C-5 positions of THβC and isatin rings.73 The series was selectively active against ER+ (MCF-7) cell lines and inactive against ER− (MDA-MB-231) cell lines. The most promising conjugate of the series was 3 (Fig. 3), which exhibited an IC50 = 37.42 μM against MCF-7 cells and >100 μM against MDA-MB-231 cells. The activity of 3 against MCF-7 cells is similar to that of a THβC derivative, peganumine A (IC50 = 38.5 μM), and better than that of the anti-breast cancer drug tamoxifen (IC50 = 50 μM). Molecular docking studies of 3 with ERα suggest that the hybrid has a good binding affinity for receptors through numerous hydrophobic, H-bonding, and cation–π interactions.
Chalcones are known as inhibitors of various anticancer targets, including thioredoxin reductase and tubulin polymerization.74,75 Likewise, the naphthalimides are known as DNA-intercalating agents and exhibit promising activities against cancer cells.76–79 Considering the significance of chalcone and naphthalimides, the group synthesized hybrids of substituted chalcones/pyrazolines and 1,8-naphthalimides by linking them using 1H-1,2,3-triazole.80 The in vitro antiproliferative activity of the synthesized hybrids was evaluated on human breast cancer cell lines, including MCF-7 (ER+) and MDA-MB-231 (ER−). The naphthalimide–chalcone/pyrazoline hybrids were not very effective in both cell lines. Only two conjugates bearing a spacer length of 3 carbons, a pyrazoline ring and a mono-methoxy or dimethoxy phenyl ring, were moderately active in MCF-7 cell lines, and compound 4 (Fig. 3) exhibited the best activity with IC50 = 62.23 μM. The antiproliferative activity of these compounds against MCF-7 cells was significantly less compared to that of the reference molecule, plumbagin (IC50 = 3.5 μM), but similar to that of the standard drug, tamoxifen (IC50 = 50 μM). In addition, both compounds were non-cytotoxic against normal/non-tumor cell lines, i.e., human embryonic kidney (HEK-293) cell line with IC50 >100 μM. The SAR studies of the series suggest that the presence of an ethyl spacer, chalcone, and unsubstituted/nitro-substituted/ferrocene in the hybrid deteriorated the activity of hybrids. In contrast, a propyl spacer, a pyrazoline ring, and electron-donating substituents favor the activity. In addition, the group designed and synthesized a novel hybrid series by conjugating tetrahydro-β-carboline and chalcones/ferrocenylchalcones using a triazole linkage and examined the in vitro antiproliferative activity against the ER+ MCF-7 and triple-negative MDA-MB-231 cell lines.81 The series was moderately effective against both cell lines. However, the chalcone-bearing hybrids were more active, and ferrocenyl chalcone-containing hybrids were more selective for MCF-7. The most active compound against MCF-7 was found to be 5 (Fig. 3), having a 4-fluoro group on the phenyl ring of chalcone and a propyl chain as linker, with IC50 = 10.33 μM, indicating the significance of an electron-withdrawing group on the aryl ring with a shorter alkyl chain as the spacer for activity against ER+ cells. In comparison, the most promising compound against MDA-MB-231 cells was 6 (Fig. 3), bearing a pentyl chain spacer and a trimethoxy group on the phenyl ring of chalcone, with IC50 = 21.99 μM, suggesting the significance of electron-donating substituents with longer alkyl spacer length for activity against triple-negative breast cancer cells. Furthermore, recently Kumar et al. published a review focused on the significance of molecular hybrids in cancer chemotherapy. Molecular hybrids currently approved or in clinical development are discussed with a focus on their mechanism of action and identified drug targets.22
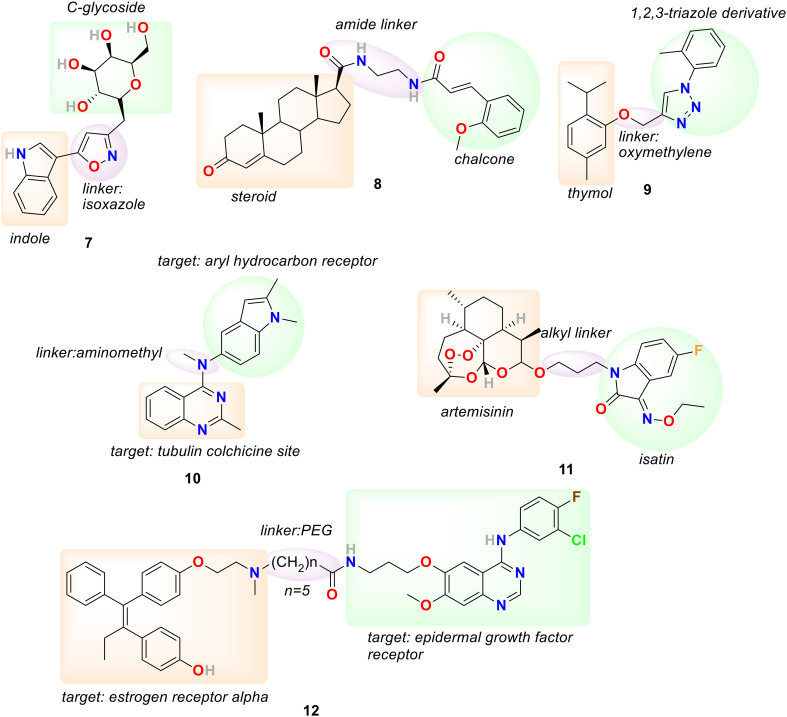
C-Glycosides have attracted much attention recently as an effective therapeutic agent because their conformational differences are minimal compared to O- or N-glycosides, and they are resistant to enzymatic or acidic hydrolysis.82,83 In addition, heterocyclic pharmacophores such as pyrazoline, isoxazole, and indole are well known for their medicinal relevance due to their widespread potential biological activities, including anticancer and antioxidant activities and as COX-2 inhibitors.84–88 In 2020, Ram Sagar and his coworkers designed and synthesized a series of C-glycosides and indole hybrids using pyrazole and isoxazole linkers and evaluated their anticancer activity against various cell lines, including MCF-7, MDA-MB-453, MDA-MB-231 and MCF-10A.89 The hybrids displayed promising anticancer activity at micromolar levels (22.3–35.5 μM) in MDA-MB-231 and limited activity for MDA-MB-453. The hybrid 7 (Fig. 4) bearing an isoxazole linkage exhibited promising activity against MCF-7 cells with IC50 = 0.67 μM and COX-2 inhibition activity with % inhibition of 61% and 95% at 25 and 50 μM, respectively.
Fig. 4. Chemical structure of linker hybrids 7–12.
Considering the significance of steroid-based hybrids in drug discovery, Q. Hou et al. in 2020 synthesized a series of novel steroid and chalcone hybrids linked together with hydrophilic amide linkages.90 MTT assay results of the series suggested their sensitivity towards triple-negative breast cancer (TNBC) cells. Compound 8 (Fig. 4) exhibited promising cytotoxicity against MDA-MB-231 with an IC50 value of 0.42 μM, efficiently suppressing colony formation of MDA-MB-231 cells and, in a concentration-dependent manner, arrested the cell cycle of MDA-MB-231 cells at S phase. Furthermore, compound 8 accelerated MDA-MB-231 cell apoptosis by upregulating the cellular reactive oxygen species levels, regulating Bcl-2/Bax proteins, and activating the caspase-3 signaling pathway. In 2021, M. M. Alam et al. synthesized a series of hybrids by conjugating thymol and 1,2,3-triazole through an oxymethylene linker and evaluated their cytotoxicity against breast cancer cell lines, including MCF-7 and MDA-MB-231 cells.91 The most active hybrid of the series was 9 (Fig. 4) with IC50 = 6.17 and 10.52 μM against MCF-7 cells and MDA-MB-231 cells, respectively, working as a thymidylate synthase inhibitor, and exhibited cell cycle arrest at the G2/M phase and initiation of apoptosis.
Colchicine binding site inhibitors (CBSIs) are responsible for inhibiting tubulin assembly and suppressing microtubule formation, resulting in cell division and cell proliferation arrest.92,93 Furthermore, aryl hydrocarbon receptors (AhRs) are associated with regulating breast cancer metastasis and proliferation.94,95 In 2021, Li Chen, Jianbo Sun, and coworkers adapted the fragment-based drug design strategy to design and synthesize a hybrid scaffold targeting the tubulin colchicine site and the AhR binding site.96 In this respect, quinazoline/quinoline scaffolds selected to target the tubulin colchicine site were connected via an aminomethyl linker to the indole/aromatic nucleus chosen to target the AhR binding site. The hybrids were developed as a dual receptor inhibitors targeting both tubulin colchicine and AhR binding sites, inhibiting the malignant proliferation and metastasis of breast cancer cells. The in vitro activity of all the hybrid compounds was tested against MCF-7 cells with overexpressed microtubules by MTT assay. The compound bearing indole rings on the linker exhibited better activity than those with a benzene ring. The most promising compound of the series was 10 (Fig. 4), having a quinoline ring with an additional N atom introduced as a substituent to C-3 and an indole ring on the linker with IC50 = 0.9 nM. Hybrid 10 effectively induced inner microtubule destruction and supposedly caused apoptosis. In addition, hybrid 10 exhibited potency to antagonize β-tubulin adduct band generation and microtubule depolymerization by binding to colchicine sites. The docking study of hybrid 10 with microtubule suggested that the indole ring procured the hydrophobic cavity with residues ASN-258, MET-259, LYS-352, and THR-314, and the 1-N of quinazoline established a hydrogen bond with an essential residue Cys 241. Additionally, 10 regulated the levels of G2/M-related and apoptosis-related proteins and encouraged cell-cycle arrest in the G2/M phase and cell apoptosis. Hybrid 10 could disrupt mitochondrial function to promote breast-cancer-cell apoptosis and stimulate the generation of ROS in MCF-7 cells. Furthermore, hybrid 10 was effective in synergistically antagonizing microtubule polymerization and AhR expression and subsequently promoting the apoptosis of MCF-7 cells. The experiment results also suggested that 10 could inhibit the cell migration of MCF-7 cells by inhibiting AhR. The in vivo anticancer activity of 10 was determined using MCF-7 breast cancer xenograft mouse models. Hybrid 10 at a low dose of 1 mg kg−1 reduced the tumor weight by 81.1% and induced the apoptosis of cells in solid tumors.
Artemisinin derivatives are known to be effective against drug-resistant and drug-sensitive breast cancer and have minimal cytotoxicity to normal cells.97 In addition, isatin-based molecules are also well known as effective in vivo anti-breast cancer agents, and many isatin derivatives are under clinical trials.98 In particular, the isatin derivatives with substitution at the C-2 and C-3 positions are more influential for biological activity. In 2022, Xiangyang Sun and coworkers designed and synthesized a series of artemisinin and isatin hybrids by linking them with 1,2,3-triazole and alkyl linkers and evaluated their toxicity against MCF-7, MDA-MB-231 and doxorubicin-resistant MCF-7 (MCF-7/DOX) and against normal breast cancer cell lines (MCF-10A).99 Most of the synthesized hybrids exhibited moderate activity against MCF-7, MDA-MB-231, and MCF-7/DOX cell lines, and a few were more effective than artemisinin. The hybrids bearing alkyl linkers were more effective than hybrids with 1,2,3-triazole linkage, and hybrid 11 (Fig. 4) was the most promising compound with IC50 = 20.7, 23.9, and 21.7 μM against MCF-7, MDA-MB-231 and MCF-7/DOX, respectively. Hybrid 11 was non-toxic towards MCF-10A with IC50 >100 μM and highly selective, with selectivity index (SI) = >1.3 and resistance index (RI) = 0.96, suggesting no cross-resistance with doxorubicin.
Tamoxifen, an estrogen receptor antagonist, is the most commonly used drug in endocrine therapies against ER+ breast cancer, but its prolonged usage is associated with several risks and tamoxifen resistance.3 It is evident that ER+ breast cancer cells resist endocrine therapy because of receptor tyrosine kinase overexpression and increased activity. The most common tyrosine kinase targets emerging in breast cancer treatment include insulin-like growth factor receptor (IGFR), epidermal growth factor receptor (EGFR/HER1), and human epidermal growth factor receptor-2 (HER-2).100,101 An EGFR inhibitor, gefitinib, can upregulate ER mRNA levels and induce re-expression of ERα and therefore proficiently reverse tamoxifen resistance in tamoxifen-resistant MCF-7 cells.102 In addition, in combination with 4-hydroxy tamoxifen, gefitinib augmented apoptosis in EGFR-positive and ER+ breast cancer in vitro and in vivo.103 In 2022, Darius P. Zlotos, Stefan A. Laufer, and coworkers prepared a series of compounds by conjugating tamoxifen and its active metabolite endoxifen with gefitinib by a covalent linkage including (CH2)nCONH-amide groups (n = 4–6, 9, and 15) and PEG linkers.104 All the synthesized hybrids exhibited substantial EGFR inhibition, ER alpha binding, and antagonistic activity. The most promising hybrid 12 (Fig. 4) bearing an amide linkage between endoxifen and gefitinib exhibited promising activity in the nanomolar range on both targets, including ER alpha (IC50 = 4.6 nM) and EGFR (IC50 = 2.5 nM). Compound 12 displayed a significant effect in cellular viability assays on MCF-7 (IC50 = 1.35 μM), MDA-MB-231 (IC50 = 0.89 μM), MDA-MB-468 (IC50 = 0.55 μM), and BT-549 (IC50 = 0.46 μM) breast cancer cells. In addition, compound 12 exhibited IC50 = 460–889 nM against TNBC cells, signifying an ER-independent mechanism of action.
2.2. Merged hybrids
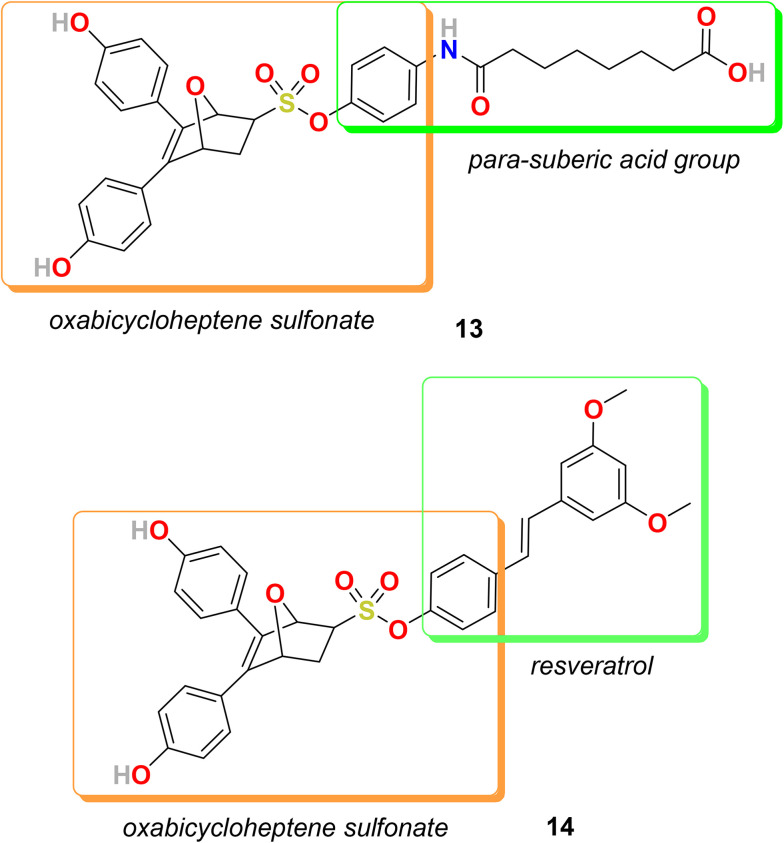
In the category of merged hybrids, two representative hybrids, 13 and 14 (Fig. 5), were designed and synthesized by Hai-Bing Zhou, and coworkers. Exo-5,6-bis(4-hydroxyphenyl)-7-oxabicyclo[2.2.1]hept-5-ene-2-sulfonic acid phenyl ester (OBHS) is a compound based on an inherently three-dimensional 7-oxabicyclo[2.2.1]hept-5-ene core and bears no resemblance to other estrogen antagonists but performs as a partial antagonist to both ERα and ERβ. The relative binding affinity (RBA) of OBHC for ERα is 9.3% and for ERβ is 1.7% [estradiol RBA = 100%] with α/β ratio = 5.5.105 Also, estrogen receptor interaction with multi-protein complexes, including histone deacetylases (HDACs), regulates the transcription of specific genes. Therefore, HDAC inhibitors control ERα gene expression and transcriptional activity.106,107 It is also evident that combined HDAC and ER inhibitors can reduce the proliferation and viability of tamoxifen-resistant cells.108 Therefore, the group decided to design and synthesize a new series of bifunctional hybrid agents by conjugating an ER antagonist (OBHS, oxabicycloheptene sulfonate) with the HDAC inhibitor suberoylanilide hydroxamic acid (SAHA) to obtain compounds with enhanced efficacy and selectivity but with high affinity for ER.109 The most promising compound of the OBHS–HDACi conjugate series was 13 bearing a para-suberic acid group on the phenyl sulfonate unit. The RBAs of 13 for ERα and ERβ are 12.4% and 0.44%, respectively, with ERα/ERβ selectivity of 28. Compound 13 exhibited an excellent ERα antagonistic activity with IC50 = 0.05 μM and reasonable ERβ antagonistic activity with IC50 = 0.16 μM. Compound 13 also displayed strong inhibition of HDACs and strong selectivity for HDAC1 (IC50 = 0.107 μM) over HDAC6 (IC50 = 8.34 μM). Compound 13 showed higher potency against MCF-7 cells with IC50 = 19.1 μM in comparison to DU 145 cells (prostate cancer) with IC50 = 34.8 μM and with no toxicity towards normal cells (VERO cells IC50 = >100 μM).
Fig. 5. Chemical structure of merged hybrids 13 and 14.
Further, in 2018, the group decided to develop an antiproliferative agent to inhibit NF-κB activation by exerting an anti-inflammatory effect. Hence, resveratrol, an anti-inflammatory drug and known inhibitor of NF-κB, was conjugated with an OBHS scaffold to develop a novel series of dual inhibitors targeting both ER and NF-κB.110 Several of these novel hybrids displayed excellent ER binding affinity, antagonistic activity, and substantial anti-inflammatory activity. The series displayed promising results in macrophage RAW 264.7 cells for NO inhibition. In vivo analysis in murine MCF-7 breast cancer tumor models in Balb/c nude mice suggested that hybrid 14 is more productive than tamoxifen in inhibiting the growth of tumor cells. The relative binding affinity (RBA) values of 14 on ERα and ERβ are 10.01% and 5.61%, with an α/β ratio of 1.78. Compound 14 bearing a resveratrol moiety on the sulfonate part exhibited interesting results in a transcriptional activation assay. Compound 14 efficiently antagonized ERα with IC50 = 0.007 μM and agonized ERβ with IC50 = 0.004 μM and showed moderate effect in antagonizing ERβ with IC50 = 0.934 μM. Compound 14 displayed excellent results for the inhibition of nitric oxide production in an anti-inflammatory assay with NO inhibition IC50 = 0.44 μM and NO production % inhibition = 95.58%. The anti-inflammatory activity of 14 against breast cancer cells (MCF-7) was also promising with IC50 = 3.7 μM. Moreover, the enantiomers of compound 14 were separated successfully and their biological activities were compared.
2.3. Fused hybrids
In 2018, S. B. Tsogoeva and coworkers prepared a series of novel hybrids by fusing artemisinin and estrogen and evaluated their in vitro antimalarial, antiviral and anticancer activity.111 2-Methoxyestradiol is an endogenous metabolite of 17β-estradiol and regarded as a promising anticancer agent because it can inhibit cancer cell proliferation effectively in both in vitro and in vivo analysis, showed no severe toxicity in clinical trials, possesses minimal estrogen receptor agonist activity, and is subsequently free of the usual hormone-related side effects.112,113 In contrast, artemisinin, an antimalarial drug, also possesses anticancer efficiency. In particular, a semisynthetic derivative of artesunic acid is safe and well tolerated in patients with metastatic breast cancer in a phase I clinical trial.114 All the synthesized hybrids displayed reasonable antimalarial activity with EC50 in the range from 3.8 to 128.8 nM, whereas estrogen precursors of the hybrids displayed no antimalarial activity. The hybrids also showed better anti-human cytomegalovirus (HCMV) activity, EC50 between 0.22 and 0.38 μM, compared to the parent compound artesunic acid. The anticancer potential of all the hybrids was evaluated against various human breast cancer and cervical cancer cell lines by MTT assay. All the hybrids displayed more noticeable anti-proliferative activity than their estrogen precursor. Hybrid 15 (Fig. 6) displayed remarkable efficacy compared to its precursor building blocks on various breast cancer cell lines, including MCF-7 (EC50 = 0.45 μM), MDA-MB-231 (EC50 = 0.86 μM), MDA-MB-361 (EC50 = 0.18 μM) and T47D (EC50 = 0.93 μM) and cervical cancer cell line C33A (EC50 = 0.15 μM).
Fig. 6. Chemical structure of fused hybrids 15 and 20.
Triple-negative breast cancer (TNBC) is a subtype of breast cancer that is very aggressive, resistant to endocrine therapy, challenging to treat, and has a high mortality rate. Evidence suggests that the intratumoral heterogeneity of TNBC is promoted by the cancer stem cell (CSC) subpopulation and plays a significant role in all types of therapy resistance and metastasis of tumors.115,116 Furthermore, the abnormal activation of epidermal growth factor (EGFR) in tumor cells because of gene mutation and protein overexpression can result in TNBC.117,118 Therapies directed individually against stem cells using stem cell inhibitors or EGFR with monoclonal antibodies or small-molecule inhibitors were ineffective against TNBC.119 Therefore, in 2021, Shaohua Gou and coworkers decided to develop novel hybrids having both EGFR and cancer cell stemness inhibitory activity.120 In this respect, the group introduced CSC inhibitors, including perphenazine, fluphenazine, and the BBI608 derivatives, into the secondary amine of lapatinib, an FDA-approved EGFR TKI inhibitor for the treatment of metastatic breast cancer, and evaluated them against lapatinib-induced MDA-MB-231 drug-resistant cells. The prepared hybrids showed good activity against all the treated breast cancer cell lines, specifically against lapatinib-resistant cells, i.e., MDA-MB-231/lapatinib cells. The compounds 16 and 17 (Fig. 6), hybrids of lapatinib derivative with CSC inhibitors including perphenazine and BB1608, respectively, exhibited respectable cytotoxicity against MDA-MB-231/lapatinib cells with IC50 = 1.03 ± 0.52 and 1.69 ± 0.48 μM, respectively, which is 28.53 and 17.69 times less than that of lapatinib. The resistant factors (RFs) calculated using the formula IC50 (MDA-MB-231/lapatinib)/IC50 (MDA-MB-231) for compounds 16 and 17 were 0.67 and 0.82, respectively. Compounds 16 and 17 exhibited satisfactory ligand lipophilicity efficiency (3.50 and 3.52, respectively) in MDA-MB-231/lapatinib cells. Both compounds (16 and 17) effectively maintained the lapatinib activity on EGFR and reversed the lapatinib-mediated MDA-MB-231 cell resistance by inhibiting the AKT/ERK signaling pathway. Compounds 16 and 17 showed a strong ability to inhibit cancer cell stemness by inhibiting the overexpression of surface antigens and markers, including CD44+, ALDH, SOX2, CD24−, Kif4, and ALDH1A1, etc. In addition, compounds 16 and 17 effectively suppressed the invasion and metastasis of breast cancer cells via inhibiting the Wnt/β-catenin signaling pathway and MMP2/9 protein expression. Further, compound 16, based on its promising activity to reverse lapatinib-induced drug resistance and inhibit MDA-MB-231/lapatinib cell invasion and migration by suppressing the cancer cell stemness, was selected for in vivo testing. The tumor-initiating potential of 16-treated MDA-MB-231/lapatinib cells was studied via a tumorigenesis experiment in vivo. The results revealed that treatment with 16 reduces the tumor-initiating potential by inhibiting breast cancer cell stemness.
In recent years, selenium (Se)-containing compounds emerged as a promising scaffold against breast cancer and other diseases because they modulate cellular redox status.121,122 The introduction of aryl selenium, particularly the phenyl selenide (PhSe) group, into small molecules is known to have an advantageous effect on their redox activity, uptake, efficacy, and antagonizing drug resistance.123,124 Therefore, in 2022, the group developed new hybrids by incorporating the PhSe group into the OBSH pharmacophore and evaluated their anti-breast cancer activity.125 The hybrid compounds displayed reasonable ERα binding affinities and significant anti-proliferative activities in both BC MCF-7 cells and tamoxifen-resistant BC LCC2 cells. In a cell viability assay, compound 18 (Fig. 6) showed promising anti-proliferative activity against ERα-positive breast cancer cell lines, including tamoxifen-sensitive MCF-7 (IC50 = 0.05 μM) and tamoxifen-resistant LCC2 (IC50 = 2.67 μM) cell lines. In comparison, 18 exhibited poor inhibitory activity against ERα-negative MDA-MB-231 breast cancer cell line (IC50 = 16.01 μM) and breast normal cell line MCF-10A (IC50 = >60 μM) and a high selectivity index between MCF-7 and MCF-10A with SI = >1200. Compound 18 displayed a low relative binding affinity for ERα and ERβ of 0.36% and 0.10%, with an α/β ratio of 3.60. Moreover, using dual luciferase reporter assay, compound 18 displayed respectable ERα antagonistic activity (IC50 = 0.31 μM) in HEK-293T cells. Mechanistic studies with 18 suggested that the compound targets ER and HK1 and causes mitochondrial dysfunction by downregulating mitochondrial-bound HK1 protein and promoting ROS production, consequently accelerating redox imbalance in cancer cells. The pharmacokinetic profile of compound 18 is adequate, with oral bioavailability of 23.20%. In addition, compound 18 demonstrated significant tumor growth suppression in vivo in tamoxifen-sensitive and -resistant breast cancer tumor xenograft models.
The diaminopyrimidine and aryl thiazole scaffolds are privileged structures for developing anticancer agents.126,127 Keeping in mind the therapeutic significance of diaminopyrimidine and aryl thiazole, Yihua Chen and coworkers synthesized a series of hybrids of 2,4-diaminopyrimidine and aryl thiazole derivatives and determined their anti-proliferative activities against two cell lines including MCF-7 and MDA-MB-231.128 The biological activity of compounds bearing para-substitutions with benzyl alkoxy groups on benzene rings showed relatively better activity. The most promising compound of the series was 19 (Fig. 6) with a methoxy benzyl group at the para position of benzene and exhibited IC50 of 3.18 and 2.00 μM against MCF-7 and MDA-MB-231 cell lines, respectively. Compound 19 displayed the ability to inhibit MDA-MB-231 and MCF-7 cell colony formation at 2.0 mM and also worked in a dose-dependent manner, including the number and size of the colony, suggesting its ability to inhibit breast cancer proliferation in vivo partly. The study of compound 19 on cell cycle distribution indicates that it possibly encourages cell-cycle arrest at the G0/G1 phase and successively inhibits breast cancer cell proliferation. The anti-migration effect of compound 19 on MDA-MB-231 cells was determined, and the results suggest that it successfully reduces MDA-MB-231 cell migration in a dose-dependent manner in a wound healing migration model with IC50 = 6.20 μM.
Quinacrine (QC) is a substantial scaffold applicable in developing therapeutic agents for various diseases, including cancer, and with the capability to efficiently kill cancer cells by inhibiting topoisomerases and the NF-κB and Wnt-TCF signaling pathways as well as inducing p53 and p21 tumor suppressors.129,130 Furthermore, the [1,3]thiazinan-4-ones group is a noteworthy scaffold associated with a substantial affinity toward various anticancer targets, including non-membrane protein tyrosine phosphatase (SHP-2), JNK-stimulating phosphatase-1 (JSP-1), tumor necrosis factor TNF-a, anti-apoptotic bio complex Bcl-XL-BH3 and integrin.131–133 In 2018, V. Raja Solomon and his coworkers selected the primary scaffold of quinacrine, 9-aminoacridin, to link it with the [1,3]thiazinan-4-ones pharmacophore. The respective hybrid's cytotoxic effect was evaluated against cancer and non-cancer cell lines.134 Most of the hybrids exhibited good anticancer activity, and the hybrid 20 (Fig. 6) bearing thiophen-2-yl group at the C-2 position of the [1,3]thiazinan-4-ones scaffold emerged as the most effective hybrid against various tested cancer cell lines. The IC50 values of compound 20 against MDA-MB-468, MDA-MB-231, and MCF-7 cells were 1.73, 2.80, and 0.69 μM, respectively. The data signifies that compound 20, compared to the standard cisplatin, was more effective in MDA-MB-231 (8.4-fold) and MCF-7 (37.3-fold) cell lines. Additionally, hybrid 20 on MCF-7 cells was sixfold more effective than the parent QC with an IC50 of 0.69 μM vs. 4.19 μM. The effect of compound 20 on cell cycle expression was examined using a flow cytometry assay, and the result revealed that at the 2 μM dose, it arrested the MCF-7 and MDA-MB-231 cell populations in the S phase. In contrast, at the same dose, compound 20 exhibited no effect on the cell cycle progression of 184B5 non-cancer cells. The S phase arrest leads to cell death because of inhibition of DNA replication caused by the down-regulation of cyclin A coupled with the continued up-regulation of cyclin E during the S phase and not by DNA damage.
3. Conclusion and future perspective
In recent years, molecular hybridization has emerged as a promising approach for lead discovery by efficiently enabling structural modifications to generate ligands with new molecular structures and activity. This modern approach delivered novel potential hybrids with dual activity, better affinity, efficacy, controlled secondary effect, and limited drug–drug interaction compared to the parent moiety. For complex diseases, single-target drugs have limited pharmacological actions; therefore, hybrid molecules with different pharmacological properties are one of the possible approaches to counter pathological conditions with multiple pathways. In particular, for diseases with limited commercial drug options or the available drug possessing high toxicity, molecular hybridization is an interesting strategy to counter these challenges. Furthermore, over the last few years, we have witnessed the emergence of many new diseases with no particular cure, a drop in the number of new drug approvals, and a continuous increase in multi-drug resistance. One of the promising strategies to resolve these issues is rationally designed multi-targeting hybrids.
Regardless of massive progress in breast cancer treatment, cancer treatment failure is widespread in patients due to drug resistance, low potency and efficacy, and high toxicity. With the increase in breast cancer-related clinical data and improved understanding of the molecular complexity of cancer cells and drug resistance mechanism, it is evident that to win the war against breast cancer a multidimensional approach using drug combination, co-delivery of medicines, or designing hybrid drugs is required. To overcome the drawbacks of cancer treatment failure, molecular hybrids targeting many points of signaling networks and various structures within a cancer cell emerged as a promising approach. Hybrid drugs can efficiently control multiple cell proliferation-related targets and bear an encouraging pharmacological profile with reduced drug–drug interaction and are utilized to overcome cancer drug resistance. In the last two decades, a continuous increase has been evident in the number of anticancer hybrid-related publications. The synthesis of anticancer hybrids by combining pharmacologically relevant moieties, including FDA-approved drugs, small molecules, natural products, and organometallics, has been explored extensively with reassuring outcomes.
This review discussed the recently reported organic hybrids with anti-breast cancer profiles, focusing on linked, fused, and merged hybrids (Table 1). The detailed analysis of hybrids and their activity suggests a substantial increase in biological potential and reduced toxicity compared to their parent pharmacophores. Some hybrids display encouraging specificity and are efficiently active against triple-negative and multi-drug-resistant breast cancer. However, it is also evident that some of these hybrids possess a high molecular mass, indicating that utilization of these hybrids in clinical trials could be challenging. To design potent anticancer hybrids, an attachment point of the joining hybrids needs special attention so that the optimized parent molecule structure is not altered and significant affinity, selectivity, and metabolism profile can be maintained. In addition, the particular focus must be on the size of the hybrid, flexibility, and physiochemical parameters to improve the overall pharmacokinetics and pharmacodynamics of the designed hybrid. However, with the advancement in the understanding of various aspects of breast cancer drug discovery and development areas and with effective multidisciplinary collaborations, it is apparent that the future holds more effective and safe hybrids for breast cancer treatment. Certainly, the molecular hybridization approach is a remarkable opportunity for the medicinal chemist to design and develop anti-breast cancer drugs; however; the availability of drug libraries and clinical data followed by the understanding of the mechanism of action and special attention during the designing process will minimize the failure of hybrid drugs in clinical trials. We anticipate that the information incorporated in this review will provide knowledge and inspiration to researchers around the globe engaged in developing therapeutic agents for breast cancer research and treatment.
Anti-breast cancer activity of hybrids 1–20 against MCF-7 and MDA-MB-231 cells.
| Entry | Chemical structure of hybrids | Activity against cells | Ref. | |
|---|---|---|---|---|
| MCF-7 cells (μM) | MDA-MB-231 (μM) | |||
| 1 |

|
IC50 = 1.56 | IC50 = 48.46 | 64 |
| 2 |

|
IC50 = 54.25 | IC50 = 26.12 | 68 |
| 3 |

|
IC50 = 37.42 | IC50 > 100 | 73 |
| 4 |

|
IC50 = 62.23 | — | 80 |
| 5 |

|
IC50 = 10.33 | — | 81 |
| 6 |

|
— | IC50 = 21.99 | 81 |
| 7 |

|
IC50 = 0.67 | — | 89 |
| 8 |

|
IC50 = 0.42 | — | 90 |
| 9 |

|
IC50 = 6.17 | IC50 = 10.52 | 91 |
| 10 |

|
IC50 = 0.0009 | — | 96 |
| 11 |

|
IC50 = 20.7 | IC50 = 23.9 | 99 |
| 12 |

|
IC50 = 1.35 | IC50 = 0.89 | 104 |
| 13 |

|
IC50 = 19.1 | — | 109 |
| 14 |

|
IC50 = 3.7 | — | 110 |
| 15 |

|
EC50 = 0.45 | EC50 = 0.86 | 111 |
| 16 |

|
— | IC50 = 0.45 (lapatinib-resistant cells) | 120 |
| 17 |

|
IC50 = 0.45 (lapatinib-resistant cells) | 120 | |
| 18 |

|
IC50 = 0.05 | IC50 = 16.01 | 125 |
| 19 |

|
IC50 = 3.18 | IC50 = 2.00 | 128 |
| 20 |

|
IC50 = 0.69 | IC50 = 2.80 | 134 |
Conflicts of interest
There is no conflict of interest to declare.
Supplementary Material
Acknowledgments
Dr. Shagufta and Dr. Irshad Ahmad are thankful to the School of Graduate Studies and Research, American University of Ras Al Khaimah, for the support.
Biographies
Biography
Shagufta.

Dr. Shagufta is an Associate Professor of Chemistry at the American University of Ras Al Khaimah, UAE. She attended the Department of Chemistry and Biochemistry, Stephenson Life Science Research Center, University of Oklahoma, USA, and Leiden Academic Centre for Drug Research, Leiden University, Leiden, the Netherlands, as a postdoctoral research associate. She received her Ph.D. in Chemistry (2008) from Central Drug Research Institute/Lucknow University, India. Her research focused on organic and medicinal chemistry. She is involved in the development of novel chemical entities as anticancer agents. Her research area includes medicinal chemistry, homogeneous and heterogeneous catalysis for organic transformations, and nanotechnology.
Biography
Irshad Ahmad.

Dr. Irshad Ahmad is a Professor of Chemistry at the American University of Ras Al Khaimah, UAE. He received his Ph.D. in Chemistry (2006) from CSMCRI/Bhavnagar University, India. He attended the Korea Research Institute of Chemical Technology, S. Korea, as an invited scientist; the Van't Hoff Institute for Molecular Sciences, University of Amsterdam, the Netherlands; the Leibniz Institute for Surface Modification, Germany; and the Department of Chemistry and Biochemistry, Stephenson Life Science Research Center, University of Oklahoma, USA. He has received Visiting Professor Positions at the University of Toronto, Canada, and Wayne State University, USA. His research area is asymmetric catalysis, supramolecular chemistry, nanotechnology, and medicinal chemistry.
References
- https://www.who.int/news-room/fact-sheets/detail/breast-cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer
- https://www.stopbreastcancer.org/information-center/facts-figures/ https://www.stopbreastcancer.org/information-center/facts-figures/
- Shagufta Ahmad I. Tamoxifen a pioneering drug: An update on the therapeutic potential of tamoxifen derivatives. Eur. J. Med. Chem. 2018;143:515–531. doi: 10.1016/j.ejmech.2017.11.056. [DOI] [PubMed] [Google Scholar]
- Shagufta Ahmad I. Recent developments in steroidal and nonsteroidal aromatase inhibitors for the chemoprevention of estrogen-dependent breast cancer. Eur. J. Med. Chem. 2015;102:375–386. doi: 10.1016/j.ejmech.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Shagufta Ahmad I. Mathew S. Rehman S. Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer. RSC Med. Chem. 2020;11:438–454. doi: 10.1039/C9MD00570F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. Hurvitz S. Human epidermal growth factor receptor-2-positive breast cancer: Current management of early, advanced, and recurrent disease. Curr. Opin. Obstet. Gynecol. 2011;23(1):37–43. doi: 10.1097/GCO.0b013e3283414e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L. Niu Y. Triple negative breast cancer: special histological types and emerging therapeutic methods. Cancer Biol. Med. 2020;17(2):293–306. doi: 10.20892/j.issn.2095-3941.2019.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]; , PMID: 32587770; PMCID: PMC7309458
- Yao H. He G. Yan S. Chen C. Song L. Rosol T. J. Deng X. Triple-negative breast cancer: is there a treatment on the horizon? Oncotarget. 2017;8(1):1913–1924. doi: 10.18632/oncotarget.12284. doi: 10.18632/oncotarget.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]; , PMID: 27765921; PMCID: PMC5352107
- Yin L. Duan J. J. Bian X. W. et al. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. doi: 10.1186/s13058-020-01296-5. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Zhan Z. Yin X. Fu S. Deng X. Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Front. Oncol. 2021;11:731535. doi: 10.3389/fonc.2021.731535. doi: 10.3389/fonc.2021.731535. [DOI] [PMC free article] [PubMed] [Google Scholar]; , PMID: 34778045; PMCID: PMC8581040
- Tarantino P. Corti C. Schmid P. et al. Immunotherapy for early triple negative breast cancer: research agenda for the next decade. npj Breast Cancer. 2022;8:23. doi: 10.1038/s41523-022-00386-1. doi: 10.1038/s41523-022-00386-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink D. M. Palermo M. G. Bores G. M. Huger F. P. Kurys B. E. Merriman M. C. Olsen G. E. Petko W. O'Malley G. J. Imino 1,2,3,4-tetrahydrocyclopent[b]indole carbamates as Dual Inhibitors of Acetylcholinesterase and Monoamine Oxidase. Bioorg. Med. Chem. Lett. 1996;6:625–630. doi: 10.1016/0960-894X(96)00072-8. doi: 10.1016/0960-894X(96)00072-8. [DOI] [Google Scholar]
- Viegas-Junior C. Danuello A. da Silva B. V. Barreiro E. J. Fraga C. A. Molecular hybridization: a useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007;14(17):1829–1852. doi: 10.2174/092986707781058805. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]; , PMID: 17627520
- Ivasiv V. Albertini C. Gonçalves A. E. Rossi M. Bolognesi M. L. Molecular Hybridization as a Tool for Designing Multitarget Drug Candidates for Complex Diseases. Curr. Top. Med. Chem. 2019;19(19):1694–1711. doi: 10.2174/1568026619666190619115735. doi: 10.2174/1568026619666190619115735. [DOI] [PubMed] [Google Scholar]; , PMID: 31237210
- Bérubé G. An overview of molecular hybrids in drug discovery. Expert Opin. Drug Discovery. 2016;11(3):281–305. doi: 10.1517/17460441.2016.1135125. doi: 10.1517/17460441.2016.1135125. [DOI] [PubMed] [Google Scholar]; , Epub 2016 Jan 12. PMID: 26727036
- Design of Hybrid Molecules for Drug Development, ed. M. Decker, Elsevier Ltd., 2017, https://www.sciencedirect.com/book/9780081010112/design-of-hybrid-molecules-for-drug-development [Google Scholar]
- Meunier B. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 2007;41(1):69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- Keith C. T. Borisy A. A. Stockwell B. R. Multicomponent therapeutics for networked systems. Nat. Rev. Drug Discovery. 2005;4(1):71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- Morphy R. Rankovic Z. Designed multiple ligands. An emerging drug discovery paradigm. J. Med. Chem. 2005;48(21):6523–6654. doi: 10.1021/jm058225d. [DOI] [PubMed] [Google Scholar]
- Choudhary S. Singh P. K. Verma H. Singh H. Silakari O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018;151:62–97. doi: 10.1016/j.ejmech.2018.03.057. doi: 10.1016/j.ejmech.2018.03.057. [DOI] [PubMed] [Google Scholar]; , Epub 2018 Mar 24. PMID: 29605809
- Szumilak M. Wiktorowska-Owczarek A. Stanczak A. Hybrid Drugs—A Strategy for Overcoming Anticancer Drug Resistance? Molecules. 2021;26(9):2601. doi: 10.3390/molecules26092601. doi: 10.3390/molecules26092601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalini Kumar V. Have molecular hybrids delivered effective anti-cancer treatments and what should future drug discovery focus on? Expert Opin. Drug Discovery. 2021;16(4):335–363. doi: 10.1080/17460441.2021.1850686. doi: 10.1080/17460441.2021.1850686. [DOI] [PubMed] [Google Scholar]
- Zheng W. Zhao Y. Luo Q. Zhang Y. Wu K. Wang F. Y. Multi-Targeted Anticancer Agents. Curr. Top. Med. Chem. 2017;17:3084–3098. doi: 10.2174/1568026617666170707124126. [DOI] [PubMed] [Google Scholar]
- Nepali K. Sharma S. Sharma M. Bedi P. M. S. Dhar K. L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014;77:422–487. doi: 10.1016/j.ejmech.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Fortin S. Bérubé G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discovery. 2013;8:1029–1047. doi: 10.1517/17460441.2013.798296. [DOI] [PubMed] [Google Scholar]
- Fu R.-G. Sun Y. Sheng W.-B. Liao D.-F. Designing multi-targeted agents: An emerging anticancer drug discovery paradigm. Eur. J. Med. Chem. 2017;136:195–211. doi: 10.1016/j.ejmech.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Saha P. Debnath C. Berube G. Steroid-Linked Nitrogen Mustards as Potential Anticancer Therapeutics: A Review. J. Steroid Biochem. 2013;137:271–300. doi: 10.1016/j.jsbmb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Perry C. M. McTavish D. Estramustine Phosphate Sodium. A Review of its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy in Prostate Cancer. Drugs Aging. 1995;7(1):49–74. doi: 10.2165/00002512-199507010-00006. [DOI] [PubMed] [Google Scholar]
- Preet R. Mohapatra P. Mohanty S. Sahu S. K. Choudhuri T. Wyatt M. D. et al. Quinacrine has Anticancer Activity in Breast Cancer Cells through Inhibition of Topoisomerase Activity. Int. J. Cancer. 2012;130(7):1660–1670. doi: 10.1002/ijc.26158. [DOI] [PubMed] [Google Scholar]
- Dermawan J. K. T. Gurova K. Pink J. Dowlati A. De S. Narla G. et al. Quinacrine Overcomes Resistance to Erlotinib by Inhibiting FACT, NF-kappa B, and Cell-Cycle Progression in Non-Small Cell Lung Cancer. Mol. Cancer Ther. 2014;13(9):2203–2214. doi: 10.1158/1535-7163.MCT-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello E. Taraboletti G. Colella G. Zucchetti M. Forestieri D. Licandro S. A. et al. The Tyrosine Kinase Inhibitor E-3810 Combined with Paclitaxel Inhibits the Growth of Advanced-Stage Triple-Negative Breast Cancer Xenografts. Mol. Cancer Ther. 2013;12(2):131–140. doi: 10.1158/1535-7163.MCT-12-0275-T. [DOI] [PubMed] [Google Scholar]
- Molina A. M. Hutson T. E. Larkin J. Gold A. M. Wood K. Carter D. et al. A Phase 1b Clinical Trial of the Multi-Targeted Tyrosine Kinase Inhibitor Lenvatinib (E7080) in Combination with Everolimus for Treatment of Metastatic Renal Cell Carcinoma (RCC) Cancer Chemother. Pharmacol. 2014;73(1):181–189. doi: 10.1007/s00280-013-2339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younes A. Berdeja J. G. Patel M. R. Flinn I. Gerecitano J. F. Neelapu S. S. et al. Safety, Tolerability, and Preliminary Activity of CUDC-907, a First-in-class, Oral, Dual Inhibitor of HDAC and PI3K, in Patients with Relapsed or Refractory Lymphoma or Multiple Myeloma: An Open-Label, Dose-Escalation, Phase 1 Trial. Lancet Oncol. 2016;17(5):622–631. doi: 10.1016/S1470-2045(15)00584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Pursell N. Ma A. Atoyan R. Samson M. Borek M. et al. CUDC-907, a Dual HDAC and PI3K Inhibitor, Potentially Targets Cancer Cells and the Microenvironment in Hematological Malignancies. Blood. 2013;122(21):4930. doi: 10.1182/blood.V122.21.4930.4930. [DOI] [Google Scholar]
- Cai X. Zhai H. X. Wang J. Forrester J. Qu H. Yin L. et al. Discovery of 7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptan Amide (CUDC-101) as a Potent Multi-Acting HDAC, EGFR, and HER2 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2010;53(5):2000–2009. doi: 10.1021/jm901453q. [DOI] [PubMed] [Google Scholar]
- Galloway T. J. Wirth L. J. Colevas A. D. Gilbert J. Bauman J. E. Saba N. F. et al. A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in Combination with Chemoradiation in Patients with Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015;21(7):1566–1573. doi: 10.1158/1078-0432.CCR-14-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M. Hybrid molecules incorporating natural products: applications in cancer therapy, neurodegenerative disorders and beyond. Curr. Med. Chem. 2011;18(10):1464–1475. doi: 10.2174/092986711795328355. doi: 10.2174/092986711795328355. [DOI] [PubMed] [Google Scholar]; , PMID: 21428895
- Micale N. Molonia M. S. Citarella A. Cimino F. Saija A. Cristani M. Speciale A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules. 2021;26(15):4665. doi: 10.3390/molecules26154665. doi: 10.3390/molecules26154665. [DOI] [PMC free article] [PubMed] [Google Scholar]; , PMID: 34361819; PMCID: PMC8348089
- Zhang N. Chen H. Liu A. Y. Shen J. J. Shah V. Zhang C. et al. Gold Conjugate-based Liposomes with Hybrid Cluster Bomb Structure for Liver Cancer Therapy. Biomaterials. 2016;74:280–291. doi: 10.1016/j.biomaterials.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Ye R. R. Tan C. P. He L. Chen M. H. Ji L. N. Mao Z. W. Cyclometalated Ir(III) Complexes as Targeted Theranostic Anticancer Therapeutics: Combining HDAC Inhibition with Photodynamic Therapy. Chem. Commun. 2014;50(75):10945–10948. doi: 10.1039/C4CC05215C. [DOI] [PubMed] [Google Scholar]
- Cincinelli R. Musso L. Dallavalle S. Artali R. Tinelli S. Colangelo D. et al. Design, Modeling, Synthesis and Biological Activity Evaluation of Camptothecin-Linked Platinum Anticancer Agents. Eur. J. Med. Chem. 2013;63:387–400. doi: 10.1016/j.ejmech.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Ding S. Qiao X. Kucera G. L. Bierbach U. Using a Build-and-Click Approach for Producing Structural and Functional Diversity in DNA-Targeted Hybrid Anticancer Agents. J. Med. Chem. 2012;55(22):10198–10203. doi: 10.1021/jm301278c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdam F. L. Guchelaar H. J. Beijnen J. H. Schellens J. H. Lapatinib for advanced or metastatic breast cancer. Oncologist. 2012;17(4):536–542. doi: 10.1634/theoncologist.2011-0461. doi: 10.1634/theoncologist.2011-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]; , Epub 2012 Apr 3. PMID: 22477724; PMCID: PMC3336826
- Hurvitz S. A. Kakkar R. Role of lapatinib alone or in combination in the treatment of HER2-positive breast cancer. Breast Cancer. 2012;4:35–51. doi: 10.2147/BCTT.S29996. [DOI] [PMC free article] [PubMed] [Google Scholar]; , PMID: 24367193; PMCID: PMC3846547
- Schmid T. A. Gore M. E. Sunitinib in the treatment of metastatic renal cell carcinoma. Ther. Adv. Urol. 2016;8(6):348–371. doi: 10.1177/1756287216663979. doi: 10.1177/1756287216663979. [DOI] [PMC free article] [PubMed] [Google Scholar]; , Epub 2016 Aug 23. PMID: 27904651; PMCID: PMC5117167
- Tilekar K. Shelke O. Upadhyay N. Lavecchia A. Ramaa C. S. Current status and future prospects of molecular hybrids with thiazolidinedione (TZD) scaffold in anticancer drug discovery. J. Mol. Struct. 2022;1250(2):131767. doi: 10.1016/j.molstruc.2021.131767. doi: 10.1016/j.molstruc.2021.131767. [DOI] [Google Scholar]
- Viegas-Junior C. Danuello A. da Silva Bolzani V. Barreiro E. J. Fraga C. A. M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007;14(17):1829–1852. doi: 10.2174/092986707781058805. doi: 10.2174/092986707781058805. [DOI] [PubMed] [Google Scholar]
- Abbot V. Sharma P. Dhiman S. Noolvi M. N. Patel H. M. Bhardwaj V. Small hybrid heteroaromatics: resourceful biological tools in cancer research. RSC Adv. 2017;7:28313–28349. doi: 10.1039/C6RA24662A. [DOI] [Google Scholar]
- Ebenezer O. Shapi M. Tuszynski J. A. A Review of the Recent Developments of Molecular Hybrids Targeting Tubulin Polymerization. Int. J. Mol. Sci. 2022;23(7):4001. doi: 10.3390/ijms23074001. doi: 10.3390/ijms23074001. [DOI] [PMC free article] [PubMed] [Google Scholar]; , PMID: 35409361; PMCID: PMC8999808
- Gao F. Sun Z. Kong F. Xiao J. Artemisinin-derived hybrids and their anticancer activity. Eur. J. Med. Chem. 2020;188:112044. doi: 10.1016/j.ejmech.2020.112044. doi: 10.1016/j.ejmech.2020.112044. [DOI] [PubMed] [Google Scholar]; , Epub 2020 Jan 8. PMID: 31945642
- Xu Z. Zhao S. J. Liu Y. 1,2,3-Triazole-containing hybrids as potential anticancer agents: Current developments, action mechanisms and structure-activity relationships. Eur. J. Med. Chem. 2019;183:111700. doi: 10.1016/j.ejmech.2019.111700. doi: 10.1016/j.ejmech.2019.111700. [DOI] [PubMed] [Google Scholar]; , Epub 2019 Sep 16. PMID: 31546197
- Wang G. Sun S. Guo H. Current status of carbazole hybrids as anticancer agents. Eur. J. Med. Chem. 2022;229:113999. doi: 10.1016/j.ejmech.2021.113999. doi: 10.1016/j.ejmech.2021.113999. [DOI] [PubMed] [Google Scholar]; , Epub 2021 Nov 20. PMID: 34838335
- Shaveta M. S. Singh P. Hybrid molecules: The privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem. 2016;124:500–536. doi: 10.1016/j.ejmech.2016.08.039. doi: 10.1016/j.ejmech.2016.08.039. [DOI] [PubMed] [Google Scholar]; , Epub 2016 Aug 21. PMID: 27598238
- Jia Y. Wen X. Gong Y. Wang X. Current scenario of indole derivatives with potential anti-drug-resistant cancer activity. Eur. J. Med. Chem. 2020;200:112359. doi: 10.1016/j.ejmech.2020.112359. doi: 10.1016/j.ejmech.2020.112359. [DOI] [PubMed] [Google Scholar]; , Epub 2020 Apr 26. PMID: 32531682
- Soltan O. M. Shoman M. E. Abdel-Aziz S. A. Narumi A. Konno H. Abdel-Aziz M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021;225:113768. doi: 10.1016/j.ejmech.2021.113768. doi: 10.1016/j.ejmech.2021.113768. [DOI] [PubMed] [Google Scholar]; , Epub 2021 Aug 14. PMID: 34450497
- Gao F. Zhang X. Wang T. Xiao J. Quinolone hybrids and their anti-cancer activities: An overview. Eur. J. Med. Chem. 2019;165:59–79. doi: 10.1016/j.ejmech.2019.01.017. doi: 10.1016/j.ejmech.2019.01.017. [DOI] [PubMed] [Google Scholar]; , Epub 2019 Jan 11. PMID: 30660827
- Bollu R. Palem J. D. Bantu R. Guguloth V. Nagarapu L. Polepalli S. Jain N. Rational design, synthesis and anti-proliferative evaluation of novel 1,4-benzoxazine-[1,2,3]triazole hybrids. Eur. J. Med. Chem. 2015;89:138–146. doi: 10.1016/j.ejmech.2014.10.051. doi: 10.1016/j.ejmech.2014.10.051. [DOI] [PubMed] [Google Scholar]; , Epub 2014 Oct 18. PMID: 25462234
- Kerru N. Singh P. Koorbanally N. Raj R. Kumar V. Recent advances (2015-2016) in anticancer hybrids. Eur. J. Med. Chem. 2017;142:179–212. doi: 10.1016/j.ejmech.2017.07.033. doi: 10.1016/j.ejmech.2017.07.033. [DOI] [PubMed] [Google Scholar]; , Epub 2017 Jul 20. PMID: 28760313
- Shagufta Ahmad I. Transition metal complexes as proteasome inhibitors for cancer treatment. Inorg. Chim. Acta. 2020;506:119521. doi: 10.1016/j.ica.2020.119521. [DOI] [Google Scholar]
- Shagufta Ahmad I. An insight into the therapeutic potential of quinazoline derivatives as anticancer agents. MedChemComm. 2017;8:871–885. doi: 10.1039/C7MD00097A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagufta Ahmad I. Panda G. Quest for steroidomimetics: Amino acids derived steroidal and nonsteroidal architectures. Eur. J. Med. Chem. 2017;133:139–151. doi: 10.1016/j.ejmech.2017.03.054. [DOI] [PubMed] [Google Scholar]
- Shagufta Ahmad I. Recent insight into the biological activities of synthetic xanthone derivatives. Eur. J. Med. Chem. 2016;116:267–280. doi: 10.1016/j.ejmech.2016.03.058. [DOI] [PubMed] [Google Scholar]
- Shagufta Srivastava A. K. Sharma R. Mishra R. Balapure A. K. Murthy P. S. R. Substituted phenanthrenes with basic amino side chains: a new series of anti-breast cancer agents. Bioorg. Med. Chem. 2006;14(5):1497–1505. doi: 10.1016/j.bmc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kumar S. Gu L. Plama G. Kaur M. Pillay A. S. Singh P. Kumar V. Design, synthesis, anti-proliferative evaluation and docking studies of 1H-1,2,3-triazole tethered ospemifene-isatin conjugates as selective estrogen receptor modulators. New J. Chem. 2018;42:3703–3713. doi: 10.1039/C7NJ04964A. [DOI] [Google Scholar]
- Descoteaux C. Leblanc V. Belanger G. Parent S. Asselin E. Berube G. Steroids. 2008;73:1077. doi: 10.1016/j.steroids.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Adsule S. Banerjee S. Ahmed F. Padhye S. Sarkar F. H. Bioorg. Med. Chem. Lett. 2010;20:1247. doi: 10.1016/j.bmcl.2009.11.128. [DOI] [PubMed] [Google Scholar]
- Zeng X. Yang X. Zhang Y. Qing C. Zhang H. Synthesis and antitumor activity of 1-mesityl-3-(2-naphthoylmethano)-1H-imidazolium bromide. Bioorg. Med. Chem. Lett. 2010;20:1844–1847. doi: 10.1016/j.bmcl.2010.01.163. [DOI] [PubMed] [Google Scholar]
- Kumar S. Saha S. T. Gu L. Palma G. Perumal S. Pillay A. S. Singh P. Anand A. Kaur M. Kumar V. 1H-1,2,3-triazole tethered nitroimidazole-isatin conjugates: synthesis, docking, and anti-proliferative evaluation against breast cancer. ACS Omega. 2018;3:12106–12113. doi: 10.1021/acsomega.8b01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. B. Di Y. T. Bao Y. Yuan C. M. Chen G. Li D. H. Bai J. He H. P. Hao X. J. Pei Y. H. Jing Y. K. Li Z. L. Hua H. M. Org. Lett. 2014;16:4028–4031. doi: 10.1021/ol501856v. [DOI] [PubMed] [Google Scholar]
- Mahale S. Bharate S. B. Manda S. Joshi P. Jenkins P. R. Vishwakarma R. A. Chaudhuri B. Cell Death Dis. 2015;6:1743. doi: 10.1038/cddis.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Singh A. Kumar K. Kumar V. Recent insights into synthetic β-carbolines with anti-cancer activities. Eur. J. Med. Chem. 2017;142:48–73. doi: 10.1016/j.ejmech.2017.05.059. [DOI] [PubMed] [Google Scholar]
- Mohamed H. A. Girgis N. M. R. Wilcken R. Bauer M. R. Tinsley H. N. Gary B. D. Piazza G. A. Boeckler F. M. Abadi A. H. J. Med. Chem. 2011;54:495–509. doi: 10.1021/jm100842v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B. Singh A. Gu L. Saha S. T. Pillay A. S. Cele N. Singh P. Kaur M. Kumar V. Diastereoselective approach to rationally design tetrahydra-β-carboline-isatin conjugates as potential SERMs against breast cancer. RSC Adv. 2019;9:9809–9819. doi: 10.1039/C9RA00744J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmaoglu S. Algul O. Aktas D. Gobek A. Gulbol G. Synthesis and anti-proliferative activity of fluoro-substituted chalcones. Bioorg. Med. Chem. Lett. 2016;26:3172. doi: 10.1016/j.bmcl.2016.04.096. [DOI] [PubMed] [Google Scholar]
- Ducki S. Antimitotic Chalcones and Related Compounds as Inhibitors of Tubulin Assembly. Anti-Cancer Agents Med. Chem. 2009;9(3):336–347. doi: 10.2174/1871520610909030336. [DOI] [PubMed] [Google Scholar]
- Noro J. Maciel J. Duarte D. Olival A. C. D. Baptista C. Silva A. C. D. Alves M. J. Lin P. K. T. Org. Chem.: Curr. Res. 2015;4:144. [Google Scholar]
- Brãna M. F. Cacho M. Garcia M. A. de Pascual-Teresa B. Ramos A. Dominguez M. T. Pozuelo J. M. Abradelo C. Rey-Stolle M. F. Yuste M. Banez-Coronel M. J. Med. Chem. 2004;47:1391. doi: 10.1021/jm0308850. [DOI] [PubMed] [Google Scholar]
- Liang X. Xu K. Xu Y. Liu J. Qian X. B1-induced caspase-independent apoptosis in MCF-7 cells is mediated by down-regulation of Bcl-2 via p53 binding to P2 promoter TATA box. Toxicol. Appl. Pharmacol. 2011;256:52. doi: 10.1016/j.taap.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Liang X. Xu Y. Xu K. Liu J. Qian X. B1, a Novel Amonafide Analogue, Overcomes the Resistance Conferred by Bcl-2 in Human Promyelocytic Leukemia HL60 Cells. Mol. Cancer Res. 2010;8:1619. doi: 10.1158/1541-7786.MCR-10-0341. [DOI] [PubMed] [Google Scholar]
- Shalini Pankaj Saha S. T. Kaur M. Oluwakemi E. Awolade P. Singh P. Kumar V. Synthesis and in vitro anti-proliferative evaluation of naphthalimide-chalcone/pyrazoline conjugates as potential SERMs with computational validation. RSC Adv. 2020;10:15836–15845. doi: 10.1039/D0RA01822H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B. Gu L. Pillay R. P. Cele N. Awolade P. Singh P. Kaur M. Kumar V. Design, synthesis, and anti-proliferative evaluation of 1H-1,2,3-triazole grafted tetrahydro-βcarboline-chalcone/ferrocenylchalcone conjugates in estrogen responsive and triple negative breast cancer cells. New J. Chem. 2020;44:11137. doi: 10.1039/D0NJ00879F. [DOI] [Google Scholar]
- Yang Y. Yu B. Recent Advances in the Chemical Synthesis of C-Glycosides. Chem. Rev. 2017;117(19):12281–12356. doi: 10.1021/acs.chemrev.7b00234. doi: 10.1021/acs.chemrev.7b00234. [DOI] [PubMed] [Google Scholar]
- Kaliappan K. P. Subrahmanyam A. V. A New Versatile Strategy for C-Aryl Glycosides. Org. Lett. 2007;9:1121–1124. doi: 10.1021/ol0701159. [DOI] [PubMed] [Google Scholar]
- George R. F. Fouad M. A. Gomma I. E. O. Synthesis and cytotoxic activities of some pyrazoline derivatives bearing phenyl pyridazine core as new apoptosis inducers. Eur. J. Med. Chem. 2016;112:48–59. doi: 10.1016/j.ejmech.2016.01.048. [DOI] [PubMed] [Google Scholar]
- Fioravanti R. et al. Synthesis and biological evaluation of N-substituted-3,5-diphenyl-2-pyrazoline derivatives as cyclooxygenase (COX-2) inhibitors. Eur. J. Med. Chem. 2010;45:6135–6138. doi: 10.1016/j.ejmech.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Padamaja A. Rajasekhar C. Muralikrishna A. Padmavathi V. Synthesis and antioxidant activity of oxazolyl, thiazolyl, sulphonylmethyl pyrazoles and isoxazoles. Eur. J. Med. Chem. 2011;46:5034–5038. doi: 10.1016/j.ejmech.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Puel J. L. Pujol R. Ladrech R. Eybalin M. α-Amino-3-heteroaryl-5-methyl-4-isoxazole propionic acid electrophysiological and neurotoxic effect in the guinea-Pig Cochlea. Neuroscience. 1991;45:63–72. doi: 10.1016/0306-4522(91)90103-U. [DOI] [PubMed] [Google Scholar]
- Kamei C. Masuda Y. Oka M. Shimizu M. Effects of antiepileptics on both behavioral and electrographic seizure patterns induced by maximal electroshock in rats. Epilepsia. 1978;19:625–636. doi: 10.1111/j.1528-1157.1978.tb05042.x. [DOI] [PubMed] [Google Scholar]
- Kumari P. Mishra V. S. Narayana C. Khanna A. Chakrabarty A. Sagar R. Design and efficient synthesis of pyrazoline and isoxazole bridged indole C-glycoside hygrids as potential anticancer agents. Sci. Rep. 2020;10:6660. doi: 10.1038/s41598-020-63377-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q. Lin X. Lu X. Bai C. Wei H. Luo G. Xiang H. Discovery of novel steroidal-chalcone hybrids with potent and selective activity against triple-negative breast cancer. Bioorg. Med. Chem. 2020;28:115763. doi: 10.1016/j.bmc.2020.115763. [DOI] [PubMed] [Google Scholar]
- Alam M. M. Malebari A. M. Nazreena S. Neamatallah T. Almalki A. S. A. Elhenawy A. A. Obaid R. J. Alshariff M. A. Design, synthesis and molecular docking studies of thymol based 1,2,3-triazole hybrids as thymidylate synthase inhibitors and apoptosis inducers against breast cancer cells. Bioorg. Med. Chem. 2021;38:116136. doi: 10.1016/j.bmc.2021.116136. [DOI] [PubMed] [Google Scholar]
- Lu Y. Chen J. Xiao M. Li W. Miller D. D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 2012;29(11):2943–2971. doi: 10.1007/s11095-012-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin E. C. O'Boyle N. M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals. 2020;13(1):8. doi: 10.3390/ph13010008. doi: 10.3390/ph13010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. Kaina B. The aryl hydrocarbon receptor (AhR) in the regulation of cell−cell contact and tumor growth. Carcinogenesis. 2010;31:1319–1328. doi: 10.1093/carcin/bgq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan A. Q. Leng L. Li J. Luo X. M. Fan Y. J. Yang Q. Y. Xie Q. H. Chen Y. S. Ni C. S. Guo L. M. Tang H. Chen X. Tang N. J. TCDD-induced antagonism of MEHP-mediated migration and invasion partly involves aryl hydrocarbon receptor in MCF-7 breast cancer cells. J. Hazard. Mater. 2020;398:122869. doi: 10.1016/j.jhazmat.2020.122869. [DOI] [PubMed] [Google Scholar]
- Wang K. Zhong H. Li N. Yu N. Wang Y. Chen L. Sun J. Discovery of Novel Anti-Breast-Cancer Inhibitors by Synergistically Antagonizing Microtubule Polymerization and Aryl Hydrocarbon Receptor Expression. J. Med. Chem. 2021;64:12964–12977. doi: 10.1021/acs.jmedchem.1c01099. [DOI] [PubMed] [Google Scholar]
- Zhu S. Yu Q. Huo C. Li Y. He L. Ran B. Chen J. Li Y. Liu W. Ferroptosis: A Novel Mechanism of Artemisinin and its Derivatives in Cancer Therapy. Curr. Med. Chem. 2021;28(2):329–345. doi: 10.2174/1875533XMTAzlNzkj1. [DOI] [PubMed] [Google Scholar]
- Nath P. Mukherjee A. Mukherjee S. Banerjee S. Das S. Banerjee S. Isatin: A Scaffold with Immense Biodiversity. Mini-Rev. Med. Chem. 2021;21(9):1096–1112. doi: 10.2174/2211536609666201125115559. doi: 10.2174/2211536609666201125115559. [DOI] [PubMed] [Google Scholar]; , PMID: 33238872
- Wang Y. Ding R. Tai Z. Hou H. Gao F. Sun X. Artemisinin-isatin hybrids with potential antiproliferative activity against breast cancer. Arabian J. Chem. 2022;15:103639. doi: 10.1016/j.arabjc.2021.103639. [DOI] [Google Scholar]
- Knowlden J. M. Hutcheson I. R. Barrow D. Gee J. M. Nicholson R. I. Insulin-like growth factor-i receptor signaling in tamoxifen-resistant breast cancer: A supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- Jin K. Kong X. Shah T. Penet M. F. Wildes F. Sgroi D. C. Ma X. J. Huang Y. Kallioniemi A. Landberg G. Bieche I. Wu X. Lobie P. E. Davidson N. E. Bhujwalla Z. M. Zhu T. Sukumar S. The hoxb7 protein renders breast cancer cells resistant to tamoxifen through activation of the EGFR pathway. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2736–2741. doi: 10.1073/pnas.1018859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Zhang B. Liu J. Li C. Dong W. Fang S. Li M. Song B. Tang B. Wang Z. Zhang Y. Mechanism of gefitinibmediated reversal of tamoxifen resistance in MCF-7 breast cancer cells by inducing ERα re-expression. Sci. Rep. 2015;5:7835. doi: 10.1038/srep07835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y. Bae S. Y. You D. Jung S. P. Choi H. J. Kim I. Lee S. K. Yu J. Kim S. W. Lee J. E. Kim S. Nam S. J. EGFR is a therapeutic target in hormone receptor-positive breast cancer. Cell. Physiol. Biochem. 2019;53:805–819. doi: 10.33594/000000174. [DOI] [PubMed] [Google Scholar]
- Abdelmalek C. M. Hu Z. Kronenberger T. Kublbeck J. Kinnen F. J. M. Hesse S. S. Malik A. Kudolo M. Niess R. Gehringer M. Zender L. Witt-Enderby P. A. Zlotos D. P. Laufer S. A. Gefitinib-tamoxifen hybrid ligands as potent agents against triple-negative breast cancer. J. Med. Chem. 2022;65:4616–4632. doi: 10.1021/acs.jmedchem.1c01646. [DOI] [PubMed] [Google Scholar]
- Zhou H. B. Comninos J. S. Stossi F. Katzenellenbogen B. S. Katzenellenbogen J. A. Synthesis and evaluation of estrogen receptor ligands with bridged oxabicyclic cores containing a diarylethylene motif: estrogen antagonists of unusual structure. J. Med. Chem. 2005;48:7261–7274. doi: 10.1021/jm0506773. [DOI] [PubMed] [Google Scholar]
- Munster P. N. Marchion D. Thomas S. Egorin M. Minton S. Springett G. Lee J. H. Simon G. Chiappori A. Sullivan D. Daud A. Phase I trial of vorinostat and doxorubicin in solid tumours: histone deacetylase 2 expression as a predictive marker. Br. J. Cancer. 2009;101:1044–1050. doi: 10.1038/sj.bjc.6605293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H. Li H. Avraham S. Jiang S. Avraham H. K. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int. J. Cancer. 2003;107:353–358. doi: 10.1002/ijc.11403. [DOI] [PubMed] [Google Scholar]
- Raha P. Thomas S. Thurn K. T. Park J. Munster P. N. Combined histone deacetylase inhibition and tamoxifen induces apoptosis in tamoxifen-resistant breast cancer models, by reversing Bcl-2 overexpression. Breast Cancer Res. 2015;17:26. doi: 10.1186/s13058-015-0533-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. Li C. Zhang S. Hu Z. Wu J. Dong C. Huang J. Zhou H.-B. Novel bioactive hybrid compound dual targeting estrogen receptor and histone deacetylase for the treatment of breast cancer. J. Med. Chem. 2015;58:4550–4572. doi: 10.1021/acs.jmedchem.5b00099. [DOI] [PubMed] [Google Scholar]
- Ning W. Hu Z. Tang C. Yang L. Zhang S. Dong C. Huang J. Zhou H.-B. Novel Hybrid Conjugates with Dual Suppression of Estrogenic and Inflammatory Activities Display Significantly Improved Potency against Breast Cancer. J. Med. Chem. 2018;61(18):8155–8173. doi: 10.1021/acs.jmedchem.8b00224. [DOI] [PubMed] [Google Scholar]
- Frohlich T. Kiss A. Wolfling J. Memyak E. Kulmany A. E. Minorics R. Zupko I. Leidenberger M. Friedrich O. Kappes B. Hahn F. Marschall M. Schneider G. Tsogoeva S. B. Synthesis of artemisinin-estrogen hybrids highly active against HCMV, P. falciparum, and cervical and breast cancer. ACS Med. Chem. Lett. 2018;9:1128–1133. doi: 10.1021/acsmedchemlett.8b00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani N. J. Sarkar M. A. Venitz J. Figg W. D. 2-Methoxyestradiol, a promising anticancer agent. Pharmacotherapy. 2003;23:165. doi: 10.1592/phco.23.2.165.32088. [DOI] [PubMed] [Google Scholar]
- Luc J. G. Y. Paulin R. Zhao J. Y. Freed D. H. Michelakis E. D. Nagendran J. 2-Methoxyestradiol: A Hormonal Metabolite Modulates Stimulated T-Cells Function and proliferation. Transplant. Proc. 2015;47:2057. doi: 10.1016/j.transproceed.2015.05.021. [DOI] [PubMed] [Google Scholar]
- von Hagens C. Walter-Sack I. Goeckenjan M. Osburg J. Storch-Hagenlocher B. Sertel S. Elsässer M. Remppis B. A. Edler L. Munzinger J. Efferth T. Schneeweiss A. Strowitzki T. Prospective open uncontrolled phase I study to define a well-tolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2) Breast Cancer Res. Treat. 2017;164:359. doi: 10.1007/s10549-017-4261-1. [DOI] [PubMed] [Google Scholar]
- Podgorski I. I. Pinterić M. Marčinko D. PopovićHadžija M. P. Filić V. Ciganek I. Pleše D. Balog T. Sobočanec S. Combination of sirtuin 3 and hyperoxia diminishes tumorigenic properties of MDA-MB-231 cells. Life Sci. 2020;254:117812. doi: 10.1016/j.lfs.2020.117812. [DOI] [PubMed] [Google Scholar]
- Ginsburg O. Bray F. Coleman M. P. Vanderpuye V. Eniu A. Kotha S. R. Sarker M. Huong T. T. Allemani C. Dvaladze A. Gralow J. Yeates K. Taylor C. Oomman N. Krishnan S. Sullivan R. Kombe D. Blas M. M. Parham G. Kassami N. Conteh L. The global burden of women's cancers: a grand challenge in global health. Lancet. 2017;389:847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan N. Luo X. Shen Y. Olson Z. Agrawal A. Endo Y. Rotstein D. S. Pelosof L. C. Wu W. J. A novel bispecific antibody targeting EGFR and VEGFR2 is effective against triple negative breast cancer via multiple mechanisms of action. Cancers. 2021;13:1027. doi: 10.3390/cancers13051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai K. Hung M. C. Yamaguchi H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer Res. 2016;6:1609–1623. [PMC free article] [PubMed] [Google Scholar]
- Gordeeva O. TGF β family signaling pathways in pluripotent and teratocarcinoma stem cells' fate decisions: balancing between selfrenewal, differentiation, and cancer. Cells. 2019;8:1500. doi: 10.3390/cells8121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Lv Z. Chen F. Wang X. Gou S. Conjugates Derived from Lapatinib Derivatives with Cancer Cell Stemness Inhibitors Effectively Reversed Drug Resistance in Triple-Negative Breast Cancer. J. Med. Chem. 2021;64:12877–12892. doi: 10.1021/acs.jmedchem.1c01013. [DOI] [PubMed] [Google Scholar]
- Chuai H. Zhang S. Q. Bai H. Li J. Wang Y. Sun J. Wen E. Zhang J. Xin M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021;223:113621. doi: 10.1016/j.ejmech.2021.113621. [DOI] [PubMed] [Google Scholar]
- Hou W. Xu H. Incorporating selenium into heterocycles and natural products−from chemical properties to pharmacological activities. J. Med. Chem. 2022;65:4436–4456. doi: 10.1021/acs.jmedchem.1c01859. [DOI] [PubMed] [Google Scholar]
- Ali W. Spengler G. Kincses A. Nove M. Battistelli C. Latacz G. Starek M. Dabrowska M. Honkisz-Orzechowska E. Romanelli A. Rasile M. M. Szymańska E. Jacob C. Zwergel C. Handzlik J. Discovery of phenylselenoether-hydantoin hybrids as ABCB1 efflux pump modulating agents with cytotoxic and antiproliferative actions in resistant T-lymphoma. Eur. J. Med. Chem. 2020;200:112435. doi: 10.1016/j.ejmech.2020.112435. [DOI] [PubMed] [Google Scholar]
- Jardim G. A. M. Lima D. J. B. Valenca W. O. Lima D. J. B. Cavalcanti B. C. Pessoa C. Rafique J. Braga A. L. Jacob C. da Silva E. N. da Cruz E. H. G. Synthesis of selenium-quinone hybrid compounds with potential antitumor activity via Rh-catalyzed C-H bond activation and click reactions. Molecules. 2018;23(1):83. doi: 10.3390/molecules23010083. doi: 10.3390/molecules23010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. Xie B. Li Q. Xiao Y. Hu Z. Deng X. Fang P. Dong C. Zhou H.-B. Huang J. Discovery of Novel Bicyclic Phenylselenyl-Containing Hybrids: An Orally Bioavailable, Potential, and Multiacting Class of Estrogen Receptor Modulators against Endocrine-Resistant Breast Cancer. J. Med. Chem. 2022;65:7993–8010. doi: 10.1021/acs.jmedchem.2c00525. [DOI] [PubMed] [Google Scholar]
- Wang S. Griffiths G. Midgley C. A. Barnett A. L. Cooper M. Grabarek J. Ingram L. Jackson W. Kontopidis G. McClue S. J. McInnes C. McLachlan J. Meades C. Mezna M. Stuart I. Thomas M. P. Zheleva D. I. Lane D. P. Jackson R. C. Glover D. M. Blake D. G. Fischer P. M. Discovery and characterizationof 2-anilino-4-(thiazol-5-yl)pyrimidine transcriptional CDK inhibitors as anticancer agents. Chem. Biol. 2010;17:1111–1121. doi: 10.1016/j.chembiol.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Kaur R. Kaur G. Gill R. K. Soni R. Bariwal J. Recent developments in tubulin polymerization inhibitors: an overview. Eur. J. Med. Chem. 2014;87C:89–124. doi: 10.1016/j.ejmech.2014.09.051. [DOI] [PubMed] [Google Scholar]
- Zhou W. Huang A. Zhang Y. Lin Q. Guo W. You Z. Yi Z. Liu M. Chen Y. Design and optimization of hybrid of 2,4-diaminopyrimidine and arylthiazole scaffold as anticancer cell proliferation and migration agents. Eur. J. Med. Chem. 2015;96:269–280. doi: 10.1016/j.ejmech.2015.04.027. [DOI] [PubMed] [Google Scholar]
- Mohapatra P. Preet R. Das D. Satapathy S. R. Choudhuri T. Wyatt M. D. Kundu C. N. Quinacrine-mediated autophagy and apoptosis in colon cancer cells is through a p53- and p21-dependent mechanism. Oncol. Res. 2012;20:81–91. doi: 10.3727/096504012X13473664562628. [DOI] [PubMed] [Google Scholar]
- Satapathy S. R. Siddharth S. Das D. Nayak A. Kundu C. N. Enhancement of cytotoxicity and inhibition of angiogenesis in oral cancer stem cells by a hybrid nanoparticle of bioactive quinacrine and silver: implication of base excision repair cascade. Mol. Pharmaceutics. 2015;12:4011–4025. doi: 10.1021/acs.molpharmaceut.5b00461. [DOI] [PubMed] [Google Scholar]
- Geronikaki A. Eleftheriou P. Vicini P. Alam I. Dixit A. Saxena A. K. 2-Thiazolylimino/heteroarylimino-5-arylidene-4-thiazolidinones as new agents with SHP-2 inhibitory action. J. Med. Chem. 2008;51:5221–5228. doi: 10.1021/jm8004306. [DOI] [PubMed] [Google Scholar]
- Cutshall N. S. O'Day C. Prezhdo M. Rhodanine derivatives as inhibitors of JSP-1. Bioorg. Med. Chem. Lett. 2005;15:3374–3379. doi: 10.1016/j.bmcl.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Degterev A. Lugovskoy A. Cardone M. Mulley B. Wagner G. Mitchison T. Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat. Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- Solomon V. R. Pundir S. Le H.-T. Lee H. Design and synthesis of novel quinacrine-[1,3]-thiazinan-4-one hybrids for their anti-breast cancer activity. Eur. J. Med. Chem. 2018;143:1028–1038. doi: 10.1016/j.ejmech.2017.11.097. [DOI] [PubMed] [Google Scholar]