Abstract
Childhood interstitial lung diseases (chILDs) are rare and heterogeneous diseases with significant morbidity and mortality. An accurate and quick aetiological diagnosis may contribute to better management and personalised treatment. On behalf of the European Respiratory Society Clinical Research Collaboration for chILD (ERS CRC chILD-EU), this review summarises the roles of the general paediatrician, paediatric pulmonologists and expert centres in the complex diagnostic workup. Each patient's aetiological chILD diagnosis must be reached without prolonged delays in a stepwise approach from medical history, signs, symptoms, clinical tests and imaging, to advanced genetic analysis and specialised procedures including bronchoalveolar lavage and biopsy, if necessary. Finally, as medical progress is fast, the need to revisit a diagnosis of “undefined chILD” is stressed.
Short abstract
Childhood interstitial lung diseases are rare and severe diseases. A stepwise approach to an aetiological diagnosis includes specific investigations performed in expert centres. The term “undefined chILD” must be regularly reassessed. https://bit.ly/3YIWKvn
Introduction
Rare lung diseases in children comprise a variety of conditions that include cystic fibrosis, primary ciliary dyskinesia, congenital malformations of the lung, pulmonary hypertension, abnormal ventilatory drive and childhood interstitial lung diseases (chILDs). The latter is, by itself, a heterogeneous group of very rare lung diseases with an overall estimated prevalence of 1.6–46 per million depending on the few available reports [1–13]. Thus, they appear to be around 10 times rarer than in adults, covering different aetiologies with some of them being extremely severe [14]. Most general practitioners and paediatricians will face none or one of these patients in their whole career and even paediatric pulmonologists may manage only a few cases of chILD. Unspecific and often inconspicuous, clinical signs could also delay the diagnosis and worsen the prognosis for chILD [1, 5]. When an ILD in a child is suspected, further investigations should be performed by experienced radiologists, geneticists and pathologists. Despite an exhaustive workup, a proportion of 6–12% of chILD remains unexplained or undefined [5, 11, 15].
In the past years, most European countries have identified specialised clinicians in expert centres who developed a chILD network. The Clinical Research Collaboration for chILD-EU, supported by the European Respiratory Society (ERS CRC chILD-EU), focuses on improving the diagnosis and management of chILD. The present review provides an up-to-date overview of the stepwise diagnostic process of chILD, from the involvement of the general paediatrician to the minimal set of required tests performed in expert centres, and discusses the current definition of “undefined chILD”.
chILD definition and classification
The diagnosis of chILD has been defined as the presence of at least three of the following criteria: 1) respiratory symptoms, 2) clinical signs of respiratory insufficiency, 3) hypoxaemia or low pulsed oxygen saturation and 4) diffuse parenchymal lung disease on chest radiography or thoracic computed tomography (CT) scan [16, 17].
Respiratory symptoms and clinical signs of chILD are non-specific. They include tachypnoea, dyspnoea on exertion (such as feeding in infants/neonates) or at rest, persistent dry cough, failure to thrive, retractions, crackles, digital clubbing, cyanosis and, less frequently, chest wall deformity (pectus excavatum and others) [17]. Other clinical signs that can point to a chILD aetiology are haemoptysis, presence of pulmonary hypertension, signs of peripheral hypothyroidism, recurrent fever, skin lesions, joint pain and/or neurological issues such as hypotonia, developmental delay, chorea and sensorial defects [18, 19]. In neonates, an unexplained respiratory distress, especially in a term baby, should also raise suspicion of chILD [20, 21].
Based on this definition, the general paediatrician facing a patient with persistent respiratory symptoms consistent with chILD should actively pursue this diagnosis to identify such rare cases from the bulk of recurrent respiratory tract infections and other more frequently occurring entities. Detailed history, description of findings at examination and a chest radiograph should be performed before referring the patient to a paediatric pulmonologist or a paediatric pulmonary department. These specialists will exclude differential diagnoses, confirm the diagnosis of chILD and push forward the investigations to try to define the chILD aetiology.
The 2004 report of the ERS Task force on chronic ILD in immunocompetent children presented the first classification system for children that was closely linked to the classification system in adults [22]. In 2007, pathologists, together with clinicians, proposed a classification system based on the histology of lung tissue for children <2 years of age [15]. This system was later extended to all paediatric age groups. Altogether, the main identified groups of chILD are 1) ILD related to primary parenchymal disorder (mostly group A of the chILD-EU classification [23]), 2) ILD specific to infancy, 3) ILD related to systemic disease processes and 4) ILD related to exposure/environmental insults [23–28]. Among these categories, the most frequent diagnoses are neuroendocrine cell hyperplasia of infancy (NEHI) (also called persistent tachypnoea of infancy (PTI) in the absence of histology), inherited surfactant disorders, diffuse alveolar haemorrhage, pulmonary alveolar proteinosis, sarcoidosis, autoinflammatory diseases and connective tissue diseases. These disorders are highly heterogeneous, but sometimes present similarly and as a consequence may be difficult to diagnose. More than 50 conditions can be associated with a chILD and deciphering which exact aetiology is affecting the patient can be long and unsuccessful, emphasising the importance of a systematic diagnostic workup.
Diagnostic workup for chILD
Over the past decade, USA and European Union work groups have proposed some diagnostic approaches [16, 17]. The first was by Kurland et al. [16] in 2013, in the framework of the American Thoracic Society, and was based on a careful family screening for ILD, followed by the elimination of other diagnoses before proceeding to more specific chILD investigations such as CT scan, genetic tests and lung biopsy. At that time, the number of involved genes was limited to surfactant-related genes (SFTPB, SFTPC, ABCA3 and NKX2-1), pulmonary alveolar proteinosis genes (CSF2RA and CSF2RB) and FOXF1 for diffuse abnormalities of lung development. This first publication was of major help, and allowed improved diagnosis and classification of ILD in children. Two years later, Bush et al. [17] on behalf of the chILD-EU working group proposed another flowchart for the diagnosis of chILD, primarily based on CT scan and placing blood tests, especially genetic testing, before more invasive tests such as bronchoalveolar lavage and lung biopsy. The genetic evolution reflected the expansion and the wider availability of new molecular techniques allowing the study of a panel of genes (next-generation sequencing (NGS) and whole-exome sequencing (WES)) instead of one by one (Sanger sequencing). This led to the discovery of new genetic entities in chILD, such as MARS mutations, other cytosolic aminoacyl-tRNA synthetase (ARS) mutations or OAS1 in pulmonary alveolar proteinosis [29–33], COPA and STING1 mutations for ILD related to autoinflammatory disorders [34–36], and many other even rarer diseases related to mutations in FLNA, TBX4, NHLRC2 or ZNFX1 [25, 37–41].
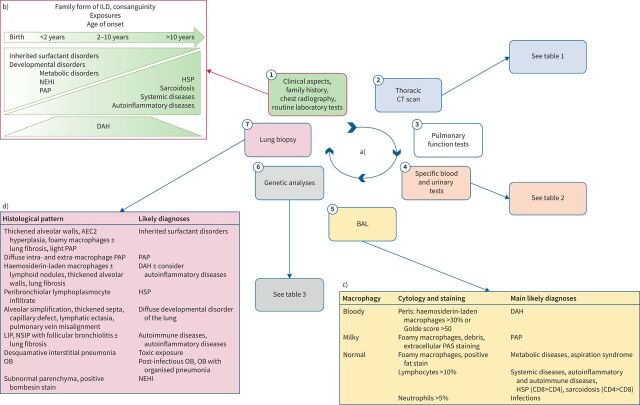
The multiplication of new aetiologies has raised the question of prioritisation of the diagnostic approach, from the clinical approach to the choice and timing of genetic tests, but also the place of lung biopsy (figure 1a) and finally, when are we allowed to label a patient as “undefined chILD”?
FIGURE 1.
Investigations in childhood interstitial lung disease (chILD) diagnostic workup. a) The investigations are performed one after the other to reach a chILD aetiology. The first step is usually done by the general paediatrician (framed in red), and includes family history, clinical evaluation, chest radiography and routine laboratory tests. b) Age at onset is crucial information that helps to consider some aetiologies more than others. c) Bronchoalveolar lavage (BAL) eliminates infectious causes of ILD and provides clues to the most likely diagnoses. d) When thoracic computed tomography (CT) scan, specific blood tests, BAL and genetic analyses could not assess a diagnosis, lung biopsy is the last step of the diagnostic process, allowing to assess the most likely diagnoses in most cases. NEHI: neuroendocrine cell hyperplasia of infancy; PAP: pulmonary alveolar proteinosis; HSP: hypersensitivity pneumonitis; DAH: diffuse alveolar haemorrhage; PAS: Periodic acid–Schiff; AEC2: type 2 alveolar epithelial cells; LIP: lymphoid interstitial pneumonia; NSIP: non-specific interstitial pneumonia; OB: obliterans bronchiolitis.
Medical history, family screening and careful clinical examination
This remains the first and major step of chILD workup as valuable information can be retrieved from the patient and their family history.
Establishing a genealogical tree, also called a pedigree chart, is mandatory in all chILD. It is estimated that up to 20–30% of chILDs are due to monogenic diseases, some of them being associated with extrapulmonary involvement. Thus, collecting information on relatives and siblings can be highly useful: oxygen therapy, lung transplantation, neonatal respiratory distress or unexplained death, neurological issues such as hypotonia, developmental delay, chorea (NKX2-1), cerebral aneurysms (FARSA and FARSB), sensorial defects (ARS), peripheral hypothyroidism (NKX2-1), autoimmune diseases or general symptoms such as fever, skin lesions, joint pains (autoinflammatory disorders, connective tissue diseases), age and cause of death of older generation family members may be of interest. Consanguinity is also very important to be aware of, as it could increase the risk of rare recessive homozygous disease (ABCA3, MARS/other ARS genes and SFTPB).
The age at onset of the ILD is crucial information. Even though it is now well documented that almost all chILD can occur at any age, some diagnoses are much more frequent in newborns, infants or older children, as shown in figure 1b [42–44].
The habits and living conditions of the patient can orientate one to chILD related to lung toxicity (e.g. drugs, medications and radiation) or hypersensitivity pneumonitis and other exposure-related diseases (e.g. birds, hay, mould and air conditioners).
CT scan: the cornerstone of chILD diagnosis
As far as the patient's medical status makes it possible, and when the diagnosis of chILD is suspected, a high-resolution CT (HRCT) scan is the first-line investigation to be performed [45, 46]. The HRCT scan will allow to confirm ILD and to identify the ILD pattern. As the technique and the interpretation of the HRCT scan are crucial, it should be performed in expert centres for paediatric imaging using protocols for optimisation of image quality while keeping individual radiation exposure to the lowest level (especially in neonates and infants) [47–49]. The use of intravenous contrast is indicated if lymphadenopathies, gross structural abnormalities, or associated cardiac or vessel abnormalities need to be differentiated. The lung parenchyma analysis will search for elementary lesions of ILD such as ground-glass anomalies, consolidations, thickening of the bronchovascular interstitium, thickening of the interlobular septa, visualisation of intralobular lines, cystic lesions and micronodules or nodules. Their association, distribution, extent as well as the presence of signs of fibrosis will be sought [50, 51].
In the majority of cases, the picture remains non-specific, i.e. it is helpful for general ILD diagnosis but unable to identify a specific aetiology. The CT pattern observed varies depending on the age of the child. Infants most often present with diffuse ground-glass anomalies associated or not with other abnormalities/findings. Older children may have more cystic, nodular or even fibrosing abnormalities. However, in specific cases, the aetiological diagnosis may be guided by typical lesions, such as images of “crazy paving” favouring alveolar proteinosis or other patterns detailed in table 1.
TABLE 1.
Childhood interstitial lung disease diagnostic assessment based on computed tomography scan pattern
| Elementary lesions | Distribution | Suspected diagnoses |
| GGO | Dense, diffuse | Inherited surfactant disorders |
| GGO, peripheral traction cysts | Diffuse | Inherited surfactant disorders |
| GGO, peripheral and/or parenchymal traction cysts, traction bronchiectasis, reticulations | Inherited surfactant disorders (older age); autoinflammatory disorders | |
| Diffuse (sometimes ill-defined centrilobular) nodules, diffuse GGO ± alveolar consolidation | Patchy | Diffuse alveolar haemorrhage |
| GGO, cysts, honeycombing and reticulations | Peripheral | Connective tissue diseases, systemic and autoimmune diseases |
| GGO | Paramediastinal, paracardial, middle lobe, lingula (usual); others (aberrant) | Persistent tachypnoea of infancy/neuroendocrine cell hyperplasia of infancy |
| GGO and air trapping | Centrilobular | Hypersensitivity pneumonitis |
| Reversed halo sign | Organising pneumonia | |
| Crazy paving | More intense in lower lobes | Pulmonary alveolar proteinosis |
| Micronodules, hilar lymphadenopathies | Lymphatic distribution | Sarcoidosis |
| Centrilobular nodules | Diffuse | Hypersensitivity pneumonitis |
GGO: ground-glass opacities.
Gas exchange and pulmonary function tests
Oxygen saturation at rest, during sleep and with exercise, the absence or presence of clinical signs, and pulmonary hypertension are used in the Fan severity score for chILD (rated 1 (low severity) to 5 (high severity)) [52]. Blood gas may be of interest to determine impairment of gas exchange. The 6-min walk test is particularly interesting in chILD because of its high sensitivity and ease of use from the age of 4–5 years [53].
The first pulmonary function tests (PFTs) should be performed as soon as possible after chILD diagnosis, if the child's condition allows it and depending on their age [54–60]. Although testing seldom provides an aetiology to the ILD, it allows to objectively assess the functional consequences of the pathology.
ILD is often characterised by a restrictive ventilatory disorder, with a decrease in total lung capacity and vital capacity. Measurement of diffusing capacity of the lung for carbon monoxide (DLCO) should be systematically performed according to the age of the child. Additionally, measurement of pulmonary compliance (an invasive examination which requires the placement of an oesophageal catheter) is done exceptionally to complete the evaluation [59]. In infants, PFTs can only be performed during sleep and therefore require the use of chloral pre-medication, the use of which is unauthorised in some countries and subject to signed informed consent in others. Between the ages of 3 and 6 years, PFTs require active cooperation (accepting at least a nose clip and a mouthpiece). After the age of 6–8 years, exploration approaches that of adults. Functional residual capacity is the most common measurement. Resistance, volumes and forced expiratory flows can complete the examination as association with an obstructive pathology is not exceptional.
Specific clinical laboratory tests
A number of blood and urinary tests are performed during the chILD workup. Most of them should be done only based on clinical orientation and age of onset. They are listed in table 2.
TABLE 2.
Biological investigations in childhood interstitial lung disease
| Investigation | Indicates |
| Haematology | |
| Complete blood count | |
| Reticulocytes | Anaemia and/or diffuse alveolar haemorrhage |
| Haemostasis | Anaemia and/or diffuse alveolar haemorrhage |
| Biochemistry | |
| Serum electrolytes, creatinine | |
| Liver enzymes | Hepatomegaly and/or pulmonary alveolar proteinosis |
| Thyroxine, thyroid-stimulating hormone | Surfactant disorder (NKX2-1) |
| Serum protein electrophoresis, sedimentation rate | Autoinflammatory/inflammatory disorder |
| Angiotensin converting enzyme | Sarcoidosis |
| Iron, ferritin | Anaemia and/or diffuse alveolar haemorrhage and inflammatory syndrome |
| Calcium, ionised calcium, phosphorous | Sarcoidosis |
| Lactate dehydrogenase | Alveolar lung injury |
| Proteinuria | Autoinflammatory/inflammatory disorder |
| Calciuria | Sarcoidosis |
| Ammonaemia | |
| Chromatography of blood and urine amino acids | Metabolic disorder, e.g. lysinuric proteinuria |
| Chromatography of urinary organic acids | |
| Serologies | |
| Epstein–Barr virus serology and viral load | |
| Cytomegalovirus serology and viral load | If subacute, neonatal or immune deficiency |
| HIV-1/HIV-2 serology and viral load | Pneumocystis jirovecii, immune deficiency |
| Mycoplasma pneumoniae serology and nasopharyngeal PCR | Subacute |
| Chlamydia pneumoniae serology and nasopharyngeal PCR | Subacute |
| Chlamydia trachomatis serology and nasopharyngeal PCR | Subacute in newborns |
| Ureaplasma urealyticum serology and nasopharyngeal PCR | |
| IgG precipitins | Hypersensitivity pneumonitis, farmer's lung, bird fancier's lung |
| Immunology | |
| Post-vaccinal serologies | Immune deficiency |
| IgG, IgA, IgM and IgG subclasses | Immune deficiency, autoinflammatory/inflammatory disorder |
| C3, C4, CH50 | |
| Lymphocyte count/differential | |
| Circulating immune complexes | |
| Antinuclear antibodies | |
| ANCAc (PR3), ANCAp (MPO) | |
| Rheumatoid factor | |
| Anti-CCP | |
| Anti-cardiolipin | |
| Scleroderma, polymyositis and myositis antibodies (KU, PM-Scl75, TIF1γ, MDA-5, PM-Scl100, Ml2, KJ; anti-synthetase (PL7, PL12, OJ, centromere, SRP, JO1), smooth muscle, glomerular basement membrane) | Muscular, oesophageal and/or cutaneous involvement |
| GM-CSF auto-antibodies | Pulmonary alveolar proteinosis |
| IFN-α signature, IFN-α dosage | Immune deficiency, autoinflammatory/inflammatory disorder |
ANCA: anti-neutrophil cytoplasmic antibody; PR3: proteinase 3; MPO: myeloperoxidase; CCP: cyclic citrullinated peptide; GM-CSF: granulocyte–macrophage colony-stimulating factor; IFN: interferon.
Bronchoalveolar lavage
As long as the medical condition of the patient allows it, flexible bronchoscopy with bronchoalveolar lavage (BAL) should be performed [61–65]. It allows cytological and microbiological analysis (bacteria, viral and fungi) of the alveolar fluid and collects the following information: volume and appearance of the fluid, cell count and staining (May–Grünwald Giemsa and Papanicolaou for cellular morphology, Perls to detect the presence of iron-containing cell samples, Periodic acid–Schiff (PAS) to detect polysaccharides such as glycogen, glycoproteins, glycolipids and mucins, and targeted staining (Ziehl and Grocott to detect mycobacteria and fungi, respectively)). A sample of BAL is kept (supernatant and cell pellets) for additional studies, in particular immunohistochemistry.
The BAL profile can guide the diagnosis, first with its macroscopic aspect and second with its cell count and specific staining (figure 1c). A global increase of the BAL cell count in the presence of a proven case of chILD and after exclusion of an infection may reflect alveolitis; however, the reference values for total BAL cell counts vary widely, so that this variable is not useful in everyday practice [61, 66]. The cytological examination makes it possible to search for pathogenic agents, viral inclusions, unusual macrophages (e.g. haemosiderin-laden macrophages and foamy macrophages), foreign bodies and abnormal cell populations [18]. Surfactant protein analysis by Western blot may be of interest but is not used routinely [67].
These results, together with those of the HRCT scan, allow a definite chILD diagnosis for some conditions, including hypersensitivity pneumonitis, pulmonary alveolar proteinosis, pulmonary haemorrhage and several infectious conditions such as Pneumocystis jirovecii infection [64].
Cardiac ultrasound
Cardiac ultrasound must be carried out early and systematically as part of the severity assessment. It has three main purposes in the evaluation of chILD: 1) the search for pulmonary hypertension, which is an important prognostic factor and part of the Fan severity score items [52], but can also guide toward specific aetiologies such as diffuse developmental disorders of the lung and surfactant disorders in newborns [68], 2) the search for a left-sided heart pathology (to eliminate a differential diagnosis), and 3) the search for cardiac involvement in the context of a general illness (search for pericarditis and associated heart malformation).
Genetic tests
A genetic cause is currently identified in ∼20% of patients with chILD (table 3) [11]. Genetic analysis is recommended for all paediatric patients with chronic ILD, whether sporadic or familial with no identified cause [69–72]. The analysis must be carried out by specialised genetics centres, and the detection of a genetic anomaly must always be explained to the patient and their family during genetic counselling consultation [71, 73].
TABLE 3.
Main genes and proteins currently implicated in childhood interstitial lung disease (chILD) groups, their mode of transmission and the associated phenotypes
| Gene (protein) | Inheritance pattern | Phenotypes |
| Inherited surfactant disorders | ||
| SFTPA1, SFTPA2 | AD | Very rarely chILD, adult ILD and adenocarcinoma of the lung |
| SFTPB | AR | Neonatal respiratory distress ± PH |
| SFTPC | AD | Neonatal respiratory distress; ILD in infants or children, adults |
| ABCA3 | AR | Neonatal respiratory distress ± PH; ILD in infants or children, adults |
| NKX2-1 | AD | Brain–lung–thyroid syndrome |
| PAP | ||
| MARS | AR | PAP; hepatomegaly with cholestasis, anaemia, neurological impairment |
| CSF2RA, CSF2RB | GR and AR | PAP (infants, children, adults) |
| GATA2 | AR | Secondary PAP; immune deficiency with myelodysplasia |
| Autoinflammatory disorders | ||
| TMEM173 | AD | Early chILD with autoimmune and inflammatory disease ± joint and skin involvement |
| COPA | AD | Early chILD or DAH with autoimmune and inflammatory disease ± joint and kidney involvement |
| ZNFX1 | chILD with severe viral infections, neurological symptoms, thrombotic microangiopathy | |
| OAS1 | AD | PAP with immunodeficiency and autoinflammation |
| Other chILD | ||
| FLNA | GA and GD | chILD with emphysema; cardiac abnormalities, neurological impairment; girls>boys |
| NHLRC2 | AR | FINCA |
| Diffuse abnormalities of lung development | ||
| FOXF1 | AD | chILD with PH; alveolar capillary dysplasia ± misalignment of pulmonary veins |
| TBX4, FGFR2 | AD and AR | chILD with PH; acinar dysplasia |
| EIF2AK4 | AR | chILD with PH; pulmonary haemangiomatosis; veno-occlusive disease |
AR: autosomal recessive; AD: autosomal dominant; GR: gonosomal recessive; GD: gonosomal dominant; PH: pulmonary hypertension; PAP: pulmonary alveolar proteinosis; DAH: diffuse alveolar haemorrhage; FINCA: fibrosis, neurodegeneration and cerebral angiomatosis.
The majority of patients in whom a genetic abnormality related to chILD is identified have a mutation in the genes encoding proteins of surfactant metabolism [25, 74–80]. Mutations in the SFTPB and SFTPC genes, encoding surfactant protein (SP)-B and SP-C, the surfactant transporter ABCA3 (ATP binding cassette subfamily A member 3), and the transcription factor NKX2-1 (or TTF1 (thyroid transcription factor 1)) are most often implicated [81–85]. SFTPA1 and SFTPA2 (SP-A1 and SP-A2) and FLNA (filamin A) mutations have also very rarely been involved in chILD (but more often in adult ILD) [86–89]. If alveolar proteinosis is suspected, the genes MARS (methionyl-tRNA synthetase), particularly when elevated liver values are noted, and CSF2RA and CSF2RB (subunits α and β of the receptor) are studied [29, 90–92]. Other ARS (FARSA, FARSB, YARS, IARS and LARS) mutations have also been associated with rare cases of syndromic chILD [30, 31, 93–95].
Genetic abnormalities responsible for autoinflammatory diseases with autoimmunity have also been described in early chILD such as SAVI syndrome (STING-associated vasculitis of infancy) related to mutations in TMEM173 (transmembrane protein 173) and COPA syndrome due to mutations in COPA (coatomer protein complex subunit α) [34–36, 96–98].
Finally, in the event of chILD secondary to a metabolic disease such as Niemann–Pick disease (NPC1 and NPC2), mucopolysaccharidosis, alveolar microlithiasis (SLC34A2) or dibasic protein intolerance (SLC7A7), a targeted analysis of the involved genes is carried out.
Lung biopsy
The indications for lung biopsy are currently declining with the progress of genetic diagnostics. Previously considered as the gold standard for chILD diagnosis, it is now discussed as a last line of investigation [16, 17]. The lung biopsy is usually done as a surgical thoracoscopic or an open procedure depending on the centre's expertise and the child's age [99–101]. Transbronchial and transthoracic biopsies are not recommended for the diagnosis of chILD, as the samples obtained may be too small. Transbronchial biopsies are also limited by the size of the fibrescope, the biopsy forceps being larger than the operator channel of the smallest fibrescope. Histology analysis of the lung sample requires an expert pathologist as lung architecture has to be analysed in relation to the age of the child and the low number of lung biopsies reduces the experience of pathologists in centres without chILD expertise. If possible, a sample is dedicated to histology, another is fixed in glutaraldehyde buffer for electron microscopy and a frozen sample could be used for further somatic genetic analyses. Microscopic examination is carried out on standard stains (haematoxylin/eosin), special stains (Perls, PAS, Grocott, reticulin and Masson's Trichrome) and immunostaining (TTF-1, bombesin, surfactant proteins and vascular markers). The topography of the lesions is evaluated at low magnification and the elementary lesions are systematically analysed. The lesion profile must be correlated with the imaging data. This morphological analysis can identify specific histological profiles (figure 1d).
In the case of chILD with extrapulmonary involvement, the diagnosis may be obtained by biopsy of an organ that is easier to access than the lung. This is the case, for example, for sarcoidosis (salivary glands, adenopathy, liver, etc.) or dermatomyositis (skin, muscle, etc.) [102].
A stepwise approach to chILD diagnosis
As described in the previous section, the number of investigations required to approach a chILD diagnosis may be substantial and time consuming. Meanwhile, supporting treatment including oxygen therapy and nutritional supplementation are started [16, 17, 25]. The final diagnosis is often awaited to start more specific medications such as corticosteroids, azithromycin, hydroxychloroquine or anti-fibrosing therapies [103–112]. In chILD related to connective tissue diseases or autoinflammatory syndromes, immunosuppressive drugs such as mycophenolate mofetil, azathioprine, rituximab or Janus kinase inhibitors may be discussed [96, 97, 113–116].
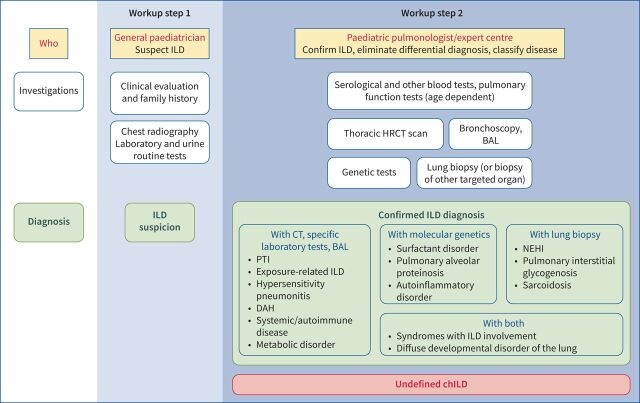
Figure 2 attempts to schematise the step-by-step workup of chILD. Workup step 1 includes clinical evaluation, family history, routine biological tests, chest radiography and echocardiography. A normal chest radiograph does not rule out the presence of chILD. It may often allow suspecting a chILD diagnosis by the paediatrician. However, in all cases, the confirmation of the ILD diagnosis and its classification requires specialised expertise and sometimes non-routine testing. Workup step 2 is thus realised in expert centres and includes HRCT and further explorations (BAL and specific laboratory tests) that could allow assessing, in typical cases, the diagnoses of exposure-related ILD, hypersensitivity pneumonitis, systemic and autoimmune diseases, metabolic diseases, PTI/NEHI or diffuse alveolar haemorrhage. However, in most cases, genetic tests and/or lung biopsy are still required at that stage. Genetic tests can confirm the diagnosis of surfactant disorders, monogenic forms of pulmonary alveolar proteinosis, SAVI, COPA syndrome and others (table 3). Lung biopsy can confirm the diagnosis of NEHI, sarcoidosis and pulmonary interstitial glycogenosis. Diffuse developmental disorders usually require both investigations to precisely determine the stage of developmental arrest (acinar dysplasia, congenital alveolar dysplasia and alveolar capillary dysplasia with or without misalignment of pulmonary veins) and the extent of the lesions.
FIGURE 2.
Stepwise approach to childhood interstitial lung disease (chILD) diagnosis. Workup step 1 represents the general paediatrician's role in chILD diagnostic workup. If the chILD suspicion is confirmed, the patient is referred to paediatric pulmonologists in expert centres for workup step 2: chILD confirmation and aetiological assessment. After specific investigations, usually including molecular analyses and/or lung biopsy, a chILD aetiology can be assessed in most cases. Other patients meet the definition of “undefined chILD”. HRCT: high-resolution computed tomography; BAL: bronchoalveolar lavage; PTI: persistent tachypnoea of infancy; DAH: diffuse alveolar haemorrhage; NEHI: neuroendocrine cell hyperplasia of infancy.
Multidisciplinary team approach and periodic re-investigation of “undefined chILD”
At each step of the diagnostic process, multidisciplinary team meetings are of major help to ascertain the diagnostic suspicion or its confirmation and discuss the management of the patient. In chILD, an assessment of the diagnosis reached by the referring team by a central peer review team did not confirm the diagnosis in 13% [11]. However, expert clinicians also need training in subcategorisation of the final diagnosis, as the overall inter-rater agreement was only 64% [11].
Undefined chILD?
While these multiple investigations are still pending, chILD with no identified cause may be labelled as a “working diagnosis of undefined ILD”. However, when performed, a significant proportion of chILDs (up to 12%) still remain unclassified. Based on the current (and evolving) knowledge, only those should be labelled as “undefined chILD”.
It is indeed important to regularly reconsider undefined diagnoses regarding the evolution of the disease, the repetition of biological tests (especially autoimmune tests that can become positive with time) and the advances in chILD classifications in terms of new reported entities or new available molecular diagnoses. Some centres offer NGS panels, others propose WES systematically or after a negative NGS panel, and whole-genome sequencing is now available in some countries, blurring the line between diagnosis and research.
Points for clinical practice
chILDs are rare and heterogeneous diseases with significant morbidity and mortality.
The number of different chILD aetiologies is high and the diagnostic process requires a stepwise approach.
The role of the general paediatrician facing a chILD suspicion is to question about the family and medical history and to initiate investigations (routine laboratory tests and chest radiography) before referring the patient rapidly to a specialised centre.
Specific investigations, including CT scan, laboratory tests, BAL, PFTs, genetic testing and eventually lung biopsy, are run in expert centres and their results are discussed during multidisciplinary team meetings.
This diagnostic workup, when complete, allows identification of a chILD aetiology in most cases.
The remaining patients meet the definition of “undefined chILD”. As medical progress is rapid, this diagnosis must be regularly reassessed.
Conclusions
The chILD diagnostic process can be simple and relatively short if a systematic two-step approach is followed. The role of the general paediatrician is crucial in untangling the personal and family medical history and the clinical signs, and in referring the patient to specialised centres when chILD is suspected. Even if easily accessible, the HRCT scan should be performed in a specialised centre to optimise its profitability. Lung biopsy is being dethroned by the fantastic progress in molecular diagnostics. However, a low number of expert geneticists may induce a prolonged delay in getting the results. Thus, for each patient, a multidisciplinary case-by-case discussion based on coherent algorithms (figure 2) could minimise chILD diagnostic delay and reduce the proportion of undefined chILD, allowing a maximum of these young patients to receive personalised treatments and to benefit from an improved prognosis.
Acknowledgements
The authors thank the European Respiratory Society Clinical Research Collaboration chILD-EU – The European Research Collaboration for Children's Interstitial Lung Disease. They also thank the COST Innovator Grant (IG16125): Open ILD: An Open Access Repository of Pluripotent Stem Cells from Children and Adults with Interstitial Lung Disease.
ERS CRC chILD-EU group members: Killian Hurley, Ireland; Adam Jaffe, Australia; Ernst Eber, Austria; Petr Pohunek, Czech Republic; Chiara Sileo, France; Aurore Coulomb, France; Elias Seidl, Germany; Florian Gothe, Germany; Nicolaus Schwerk, Germany; Effrosyne Manali, Greece; Deborah Snijders, Italy; Nicola Ullmann, Italy; Suzanne Terheggen-Lagro, The Netherlands; Katarzyna Krenke, Poland; Stanislaw Bogusławski, Poland; Antonio Moreno, Spain; Dilber Ademhan Tural, Turkey; Tugba Sismanlar, Turkey; Andrew Bush, UK; Jeanette Boyd, UK; Steve Cunningham, UK; Robin Deterding, USA.
Provenance: Commissioned article, peer reviewed.
Previous articles in this series: No. 1: Simonneau G, Fadel E, Vonk Noordegraaf A, et al. Highlights from the International Chronic Thromboembolic Pulmonary Hypertension Congress 2021. Eur Respir Rev 2023; 32: 220132. No. 2: Buschulte K, Cottin V, Wijsenbeek M, et al. The world of rare interstitial lung diseases. Eur Respir Rev 2023; 32: 220161. No. 3: Dumoulin DW, Bironzo P, Passiglia F, et al. Rare thoracic cancers: a comprehensive overview of diagnosis and management of small cell lung cancer, malignant pleural mesothelioma and thymic epithelial tumours. Eur Respir Rev 2023; 32: 220174. No. 4: Cullivan S, Gaine S, Sitbon O. New trends in pulmonary hypertension. Eur Respir Rev 2023; 32: 220211.
This article has an editorial commentary: https://doi.org/10.1183/16000617.0006-2023
Number 5 in the Series “The world of rare lung diseases” Edited by Michael Kreuter, Marc Humbert, Thomas Wagner and Marlies Wijsenbeek
Author contributions: N. Nathan, M. Griese and R. Epaud wrote the manuscript. K. Michel, B. Willemse, A. Torrent-Vernetta, N. Emiralioglu, J. Carlens, H. Marczak, C. Gilbert and C. Delestrain critically reviewed the manuscript. The ERS CRC chILD-EU group participated in manuscript conception. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors.
Conflict of interest: None declared.
References
- 1.Dinwiddie R, Sharief N, Crawford O. Idiopathic interstitial pneumonitis in children: a national survey in the United Kingdom and Ireland. Pediatr Pulmonol 2002; 34: 23–29. doi: 10.1002/ppul.10125 [DOI] [PubMed] [Google Scholar]
- 2.Laverty A, Jaffe A, Cunningham S. Establishment of a web-based registry for rare (orphan) pediatric lung diseases in the United Kingdom: the BPOLD registry. Pediatr Pulmonol 2008; 43: 451–456. doi: 10.1002/ppul.20783 [DOI] [PubMed] [Google Scholar]
- 3.Kornum JB, Christensen S, Grijota M, et al. The incidence of interstitial lung disease 1995–2005: a Danish nationwide population-based study. BMC Pulm Med 2008; 8: 24. doi: 10.1186/1471-2466-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griese M, Haug M, Brasch F, et al. Incidence and classification of pediatric diffuse parenchymal lung diseases in Germany. Orphanet J Rare Dis 2009; 4: 26. doi: 10.1186/1750-1172-4-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan N, Taam RA, Epaud R, et al. A national internet-linked based database for pediatric interstitial lung diseases: the French network. Orphanet J Rare Dis 2012; 7: 40. doi: 10.1186/1750-1172-7-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casamento K, Laverty A, Wilsher M, et al. Assessing the feasibility of a web-based registry for multiple orphan lung diseases: the Australasian Registry Network for Orphan Lung Disease (ARNOLD) experience. Orphanet J Rare Dis 2016; 11: 42. doi: 10.1186/s13023-016-0389-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saddi V, Beggs S, Bennetts B, et al. Childhood interstitial lung diseases in immunocompetent children in Australia and New Zealand: a decade's experience. Orphanet J Rare Dis 2017; 12: 133. doi: 10.1186/s13023-017-0637-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares JJ, Deutsch GH, Moore PE, et al. Childhood interstitial lung diseases: an 18-year retrospective analysis. Pediatrics 2013; 132: 684–691. doi: 10.1542/peds.2013-1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young L, Nevel R, Casey A, et al. A national registry for childhood interstitial and diffuse lung diseases in the United States. Eur Respir J 2018; 52: Suppl. 62, OA3786. doi: 10.1183/13993003.congress-2018.OA3786 [DOI] [Google Scholar]
- 10.Tang X, Li H, Liu H, et al. Etiologic spectrum of interstitial lung diseases in Chinese children older than 2 years of age. Orphanet J Rare Dis 2020; 15: 25. doi: 10.1186/s13023-019-1270-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griese M, Seidl E, Hengst M, et al. International management platform for children's interstitial lung disease (chILD-EU). Thorax 2018; 73: 231–239. doi: 10.1136/thoraxjnl-2017-210519 [DOI] [PubMed] [Google Scholar]
- 12.Torrent-Vernetta A, Gaboli M, Castillo-Corullón S, et al. Incidence and prevalence of children's diffuse lung disease in Spain. Arch Bronconeumol 2022; 58: 22–29. doi: 10.1016/j.arbres.2021.06.001 [DOI] [PubMed] [Google Scholar]
- 13.Nathan N. Childhood interstitial lung diseases (chILD) recognition: when epidemiology increases a rare disease incidence. Arch Bronconeumol 2022; 58: 217–218. doi: 10.1016/j.arbres.2022.01.009 [DOI] [PubMed] [Google Scholar]
- 14.Griese M. Chronic interstitial lung disease in children. Eur Respir Rev 2018; 27: 170100. doi: 10.1183/16000617.0100-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deutsch GH, Young LR, Deterding RR, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med 2007; 176: 1120–1128. doi: 10.1164/rccm.200703-393OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurland G, Deterding RR, Hagood JS, et al. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med 2013; 188: 376–394. doi: 10.1164/rccm.201305-0923ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bush A, Cunningham S, de Blic J, et al. European protocols for the diagnosis and initial treatment of interstitial lung disease in children. Thorax 2015; 70: 1078–1084. doi: 10.1136/thoraxjnl-2015-207349 [DOI] [PubMed] [Google Scholar]
- 18.Nathan N, Berdah L, Borensztajn K, et al. Chronic interstitial lung diseases in children: diagnosis approaches. Expert Rev Respir Med 2018; 12: 1051–1060. doi: 10.1080/17476348.2018.1538795 [DOI] [PubMed] [Google Scholar]
- 19.Nathan N, Berdah L, Delestrain C, et al. Interstitial lung diseases in children. Presse Med 2020; 49: 103909. doi: 10.1016/j.lpm.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 20.Nogee LM. Interstitial lung disease in newborns. Semin Fetal Neonatal Med 2017; 22: 227–233. doi: 10.1016/j.siny.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush A, Griese M, Seidl E, et al. Early onset children's interstitial lung diseases: discrete entities or manifestations of pulmonary dysmaturity? Paediatr Respir Rev 2019; 30: 65–71. doi: 10.1016/j.prrv.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 22.Clement A. Task force on chronic interstitial lung disease in immunocompetent children. Eur Respir J 2004; 24: 686–697. doi: 10.1183/09031936.04.00089803 [DOI] [PubMed] [Google Scholar]
- 23.Griese M, Irnstetter A, Hengst M, et al. Categorizing diffuse parenchymal lung disease in children. Orphanet J Rare Dis 2015; 10: 122. doi: 10.1186/s13023-015-0339-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement A, Nathan N, Epaud R, et al. Interstitial lung diseases in children. Orphanet J Rare Dis 2010; 5: 22. doi: 10.1186/1750-1172-5-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathan N, Borensztajn K, Clement A. Genetic causes and clinical management of pediatric interstitial lung diseases. Curr Opin Pulm Med 2018; 24: 253–259. doi: 10.1097/MCP.0000000000000471 [DOI] [PubMed] [Google Scholar]
- 26.Rice A, Tran-Dang M-A, Bush A, et al. Diffuse lung disease in infancy and childhood: expanding the chILD classification. Histopathology 2013; 63: 743–755. doi: 10.1111/his.12185 [DOI] [PubMed] [Google Scholar]
- 27.Fan LL, Dishop MK, Galambos C, et al. Diffuse lung disease in biopsied children 2 to 18 years of age. Application of the chILD classification scheme. Ann Am Thorac Soc 2015; 12: 1498–1505. doi: 10.1513/AnnalsATS.201501-064OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griese M. Etiologic classification of diffuse parenchymal (interstitial) lung diseases. J Clin Med 2022; 11: 1747. doi: 10.3390/jcm11061747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadchouel A, Wieland T, Griese M, et al. Biallelic mutations of methionyl-tRNA synthetase cause a specific type of pulmonary alveolar proteinosis prevalent on Réunion Island. Am J Hum Genet 2015; 96: 826–831. doi: 10.1016/j.ajhg.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krenke K, Szczałuba K, Bielecka T, et al. FARSA mutations mimic phenylalanyl-tRNA synthetase deficiency caused by FARSB defects. Clin Genet 2019; 96: 468–472. doi: 10.1111/cge.13614 [DOI] [PubMed] [Google Scholar]
- 31.Antonellis A, Oprescu SN, Griffin LB, et al. Compound heterozygosity for loss-of-function FARSB variants in a patient with classic features of recessive aminoacyl-tRNA synthetase-related disease. Hum Mutat 2018; 39: 834–840. doi: 10.1002/humu.23424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magg T, Okano T, Koenig LM, et al. Heterozygous OAS1 gain-of-function variants cause an autoinflammatory immunodeficiency. Sci Immunol 2021; 6: eabf9564. doi: 10.1126/sciimmunol.abf9564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seidl E, Schramm D, Schön C, et al. Pulmonary alveolar proteinosis due to heterozygous mutation in OAS1: whole lung lavages for long-term bridging to hematopoietic stem cell transplantation. Pediatr Pulmonol 2022; 57: 273–277. doi: 10.1002/ppul.25728 [DOI] [PubMed] [Google Scholar]
- 34.Watkin LB, Jessen B, Wiszniewski W, et al. COPA mutations impair ER-Golgi transport and cause hereditary autoimmune-mediated lung disease and arthritis. Nat Genet 2015; 47: 654–660. doi: 10.1038/ng.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Jesus AA, Marrero B, et al. Activated STING in a vascular and pulmonary syndrome. N Engl J Med 2014; 371: 507–518. doi: 10.1056/NEJMoa1312625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeremiah N, Neven B, Gentili M, et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest 2014; 124: 5516–5520. doi: 10.1172/JCI79100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galambos C, Mullen MP, Shieh JT, et al. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur Respir J 2019; 54: 1801965. doi: 10.1183/13993003.01965-2018 [DOI] [PubMed] [Google Scholar]
- 38.Haarman MG, Kerstjens-Frederikse WS, Berger RMF. The ever-expanding phenotypical spectrum of human TBX4 mutations: from toe to lung. Eur Respir J 2019; 54: 1901504. doi: 10.1183/13993003.01504-2019 [DOI] [PubMed] [Google Scholar]
- 39.Szafranski P, Coban-Akdemir ZH, Rupps R, et al. Phenotypic expansion of TBX4 mutations to include acinar dysplasia of the lungs. Am J Med Genet A 2016; 170: 2440–2444. doi: 10.1002/ajmg.a.37822 [DOI] [PubMed] [Google Scholar]
- 40.Rapp CK, Van Dijck I, Laugwitz L, et al. Expanding the phenotypic spectrum of FINCA (fibrosis, neurodegeneration, and cerebral angiomatosis) syndrome beyond infancy. Clin Genet 2021; 100: 453–461. doi: 10.1111/cge.14016 [DOI] [PubMed] [Google Scholar]
- 41.Vavassori S, Chou J, Faletti LE, et al. Multisystem inflammation and susceptibility to viral infections in human ZNFX1 deficiency. J Allergy Clin Immunol 2021; 148: 381–393. doi: 10.1016/j.jaci.2021.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathan N, Pautrat J, L'Hermine AC, et al. Pulmonary sarcoid-like granulomatous disease in an 11-month-old girl. BMJ Case Rep 2013; 2013: bcr2012008024. doi: 10.1136/bcr-2012-008024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cottin V, Nunes H, Brillet P-Y, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J 2005; 26: 586–593. doi: 10.1183/09031936.05.00021005 [DOI] [PubMed] [Google Scholar]
- 44.Cottin V, Reix P, Khouatra C, et al. Combined pulmonary fibrosis and emphysema syndrome associated with familial SFTPC mutation. Thorax 2011; 66: 918–919. doi: 10.1136/thx.2010.151407 [DOI] [PubMed] [Google Scholar]
- 45.Guillerman RP. Imaging of childhood interstitial lung disease. Pediatr Allergy Immunol Pulmonol 2010; 23: 43–68. doi: 10.1089/ped.2010.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semple TR, Ashworth MT, Owens CM. Interstitial lung disease in children made easier … well, almost. Radiographics 2017; 37: 1679–1703. doi: 10.1148/rg.2017170006 [DOI] [PubMed] [Google Scholar]
- 47.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380: 499–505. doi: 10.1016/S0140-6736(12)60815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 2013; 346: f2360. doi: 10.1136/bmj.f2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brody AS, Guillerman RP. Ten rules for ordering chest CTs. Pediatr Pulmonol 2021; 56: 1868–1871. doi: 10.1002/ppul.25399 [DOI] [PubMed] [Google Scholar]
- 50.Brody AS. Imaging considerations: interstitial lung disease in children. Radiol Clin North Am 2005; 43: 391–403. doi: 10.1016/j.rcl.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 51.Brody AS. New perspectives in imaging interstitial lung disease in children. Pediatr Radiol 2008; 38: Suppl. 2, S205–S207. doi: 10.1007/s00247-008-0766-4 [DOI] [PubMed] [Google Scholar]
- 52.Fan LL, Kozinetz CA. Factors influencing survival in children with chronic interstitial lung disease. Am J Respir Crit Care Med 1997; 156: 939–942. doi: 10.1164/ajrccm.156.3.9703051 [DOI] [PubMed] [Google Scholar]
- 53.Klepper SE, Muir N. Reference values on the 6-minute walk test for children living in the United States. Pediatr Phys Ther 2011; 23: 32–40. doi: 10.1097/PEP.0b013e3182095e44 [DOI] [PubMed] [Google Scholar]
- 54.Amsallem F, Gauthier R, Ramonatxo M, et al. Respiratory function testing in infants: recommendations on normal values. Rev Mal Respir 2008; 25: 405–432. doi: 10.1016/S0761-8425(08)71583-3 [DOI] [PubMed] [Google Scholar]
- 55.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beydon N. Épreuves fonctionnelles respiratoires de l'enfant de 3 à 5 ans: quel matériel, quelles mesures? [PFTing in children aged 3–5 years: which material, which measurements?] Arch Pediatr 2010; 17: 442–445. doi: 10.1016/j.arcped.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 57.Hughes JMB, Pride NB. Examination of the carbon monoxide diffusing capacity (DlCO) in relation to its Kco and Va components. Am J Respir Crit Care Med 2012; 186: 132–139. doi: 10.1164/rccm.201112-2160CI [DOI] [PubMed] [Google Scholar]
- 58.Kim Y-J, Christoph K, Yu Z, et al. Pulmonary diffusing capacity in healthy African-American and Caucasian children. Pediatr Pulmonol 2016; 51: 84–88. doi: 10.1002/ppul.23205 [DOI] [PubMed] [Google Scholar]
- 59.Khirani S, Nathan N, Ramirez A, et al. Work of breathing in children with diffuse parenchymal lung disease. Respir Physiol Neurobiol 2015; 206: 45–52. doi: 10.1016/j.resp.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 60.Ring AM, Carlens J, Bush A, et al. Pulmonary function testing in children's interstitial lung disease. Eur Respir Rev 2020; 29: 200019. doi: 10.1183/16000617.0019-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Blic J, Midulla F, Barbato A, et al. Bronchoalveolar lavage in children. ERS Task Force on bronchoalveolar lavage in children. Eur Respir J 2000; 15: 217–231. doi: 10.1183/09031936.00.15121700 [DOI] [PubMed] [Google Scholar]
- 62.Midulla F, Villani A, Merolla R, et al. Bronchoalveolar lavage studies in children without parenchymal lung disease: cellular constituents and protein levels. Pediatr Pulmonol 1995; 20: 112–118. doi: 10.1002/ppul.1950200211 [DOI] [PubMed] [Google Scholar]
- 63.Popler J, Wagner BD, Tarro HL, et al. Bronchoalveolar lavage fluid cytokine profiles in neuroendocrine cell hyperplasia of infancy and follicular bronchiolitis. Orphanet J Rare Dis 2013; 8: 175. doi: 10.1186/1750-1172-8-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ratjen F, Costabel U, Griese M, et al. Bronchoalveolar lavage fluid findings in children with hypersensitivity pneumonitis. Eur Respir J 2003; 21: 144–148. doi: 10.1183/09031936.03.00035703a [DOI] [PubMed] [Google Scholar]
- 65.Wuyts WA, Dooms C, Verleden GM. The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2013; 187: 777. doi: 10.1164/ajrccm.187.7.777 [DOI] [PubMed] [Google Scholar]
- 66.Griese M, Felber J, Reiter K, et al. Airway inflammation in children with tracheostomy. Pediatr Pulmonol 2004; 37: 356–361. doi: 10.1002/ppul.10432 [DOI] [PubMed] [Google Scholar]
- 67.Griese M, Lorenz E, Hengst M, et al. Surfactant proteins in pediatric interstitial lung disease. Pediatr Res 2016; 79: 34–41. doi: 10.1038/pr.2015.173 [DOI] [PubMed] [Google Scholar]
- 68.Soreze Y, Sileo C, Coulomb l'Hermine A, et al. Interstitial lung diseases in the neonatal period. In: Sinha IP, Bhatt JM, Cleator A, et al., eds. Respiratory Diseases of the Newborn Infant (ERS Monograph). Sheffield, European Respiratory Society, 2021; pp. 213–230. [Google Scholar]
- 69.Borie R, Kannengiesser C, Nathan N, et al. Familial pulmonary fibrosis. Rev Mal Respir 2015; 32: 413–434. doi: 10.1016/j.rmr.2014.07.017 [DOI] [PubMed] [Google Scholar]
- 70.Borie R, Kannengiesser C, Crestani B. Familial forms of nonspecific interstitial pneumonia/idiopathic pulmonary fibrosis: clinical course and genetic background. Curr Opin Pulm Med 2012; 18: 455–461. doi: 10.1097/MCP.0b013e328356b15c [DOI] [PubMed] [Google Scholar]
- 71.Borie R, Kannengiesser C, Amselem S, et al. Multidisciplinary team dedicated to suspected heritable pulmonary fibrosis. Eur Respir J 2018; 52: Suppl. 62, PA2233. doi: 10.1183/13993003.congress-2018.PA2233 [DOI] [Google Scholar]
- 72.Borie R, Kannengiesser C, de Fontbrune FS, et al. Management of suspected monogenic lung fibrosis in a specialised centre. Eur Respir Rev 2017; 26: 160122. doi: 10.1183/16000617.0122-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelada L, Wakefield C, Vidic N, et al. Genomic testing for children with interstitial and diffuse lung disease (chILD): parent satisfaction, understanding and health-related quality of life. BMJ Open Respir Res 2022; 9: e001139. doi: 10.1136/bmjresp-2021-001139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nogee LM. Genetics of pediatric interstitial lung disease. Curr Opin Pediatr 2006; 18: 287–292. doi: 10.1097/01.mop.0000193310.22462.1f [DOI] [PubMed] [Google Scholar]
- 75.Epaud R, Jonard L, Ducou-le-Pointe H, et al. Pathologies génétiques du surfactant. [Genetic disorders of surfactant.] Arch Pediatr 2012; 19: 212–219. doi: 10.1016/j.arcped.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 76.Gupta A, Zheng SL. Genetic disorders of surfactant protein dysfunction: when to consider and how to investigate. Arch Dis Child 2017; 102: 84–90. doi: 10.1136/archdischild-2012-303143 [DOI] [PubMed] [Google Scholar]
- 77.Hartl D, Griese M. Interstitial lung disease in children – genetic background and associated phenotypes. Respir Res 2005; 6: 32. doi: 10.1186/1465-9921-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayasaka I, Cho K, Akimoto T, et al. Genetic basis for childhood interstitial lung disease among Japanese infants and children. Pediatr Res 2018; 83: 477–483. doi: 10.1038/pr.2017.217 [DOI] [PubMed] [Google Scholar]
- 79.Kröner C, Wittmann T, Reu S, et al. Lung disease caused by ABCA3 mutations. Thorax 2017; 72: 213–220. doi: 10.1136/thoraxjnl-2016-208649 [DOI] [PubMed] [Google Scholar]
- 80.Kröner C, Reu S, Teusch V, et al. Genotype alone does not predict the clinical course of SFTPC deficiency in paediatric patients. Eur Respir J 2015; 46: 197–206. doi: 10.1183/09031936.00129414 [DOI] [PubMed] [Google Scholar]
- 81.Nogee LM, Garnier G, Dietz HC, et al. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest 1994; 93: 1860–1863. doi: 10.1172/JCI117173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nogee LM, de Mello DE, Dehner LP, et al. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med 1993; 328: 406–410. doi: 10.1056/NEJM199302113280606 [DOI] [PubMed] [Google Scholar]
- 83.Shulenin S, Nogee LM, Annilo T, et al. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med 2004; 350: 1296–1303. doi: 10.1056/NEJMoa032178 [DOI] [PubMed] [Google Scholar]
- 84.Devriendt K, Vanhole C, Matthijs G, et al. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure. N Engl J Med 1998; 338: 1317–1318. doi: 10.1056/NEJM199804303381817 [DOI] [PubMed] [Google Scholar]
- 85.Nogee LM, Dunbar AE 3rd, Wert SE, et al. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med 2001; 344: 573–579. doi: 10.1056/NEJM200102223440805 [DOI] [PubMed] [Google Scholar]
- 86.Nathan N, Giraud V, Picard C, et al. Germline SFTPA1 mutation in familial idiopathic interstitial pneumonia and lung cancer. Hum Mol Genet 2016; 25: 1457–1467. doi: 10.1093/hmg/ddw014 [DOI] [PubMed] [Google Scholar]
- 87.Legendre M, Butt A, Borie R, et al. Functional assessment and phenotypic heterogeneity of SFTPA1 and SFTPA2 mutations in interstitial lung diseases and lung cancer. Eur Respir J 2020; 56: 2002806. doi: 10.1183/13993003.02806-2020 [DOI] [PubMed] [Google Scholar]
- 88.Lange M, Kasper B, Bohring A, et al. 47 patients with FLNA associated periventricular nodular heterotopia. Orphanet J Rare Dis 2015; 10: 134. doi: 10.1186/s13023-015-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shelmerdine SC, Semple T, Wallis C, et al. Filamin A (FLNA) mutation – a newcomer to the childhood interstitial lung disease (ChILD) classification. Pediatr Pulmonol 2017; 52: 1306–1315. doi: 10.1002/ppul.23695 [DOI] [PubMed] [Google Scholar]
- 90.Hildebrandt J, Yalcin E, Bresser H-G, et al. Characterization of CSF2RA mutation related juvenile pulmonary alveolar proteinosis. Orphanet J Rare Dis 2014; 9: 171. doi: 10.1186/s13023-014-0171-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki T, Maranda B, Sakagami T, et al. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur Respir J 2011; 37: 201–204. doi: 10.1183/09031936.00090610 [DOI] [PubMed] [Google Scholar]
- 92.Lenz D, Stahl M, Seidl E, et al. Rescue of respiratory failure in pulmonary alveolar proteinosis due to pathogenic MARS1 variants. Pediatr Pulmonol 2020; 55: 3057–3066. doi: 10.1002/ppul.25031 [DOI] [PubMed] [Google Scholar]
- 93.Schuch LA, Forstner M, Rapp CK, et al. FARS1-related disorders caused by bi-allelic mutations in cytosolic phenylalanyl-tRNA synthetase genes: look beyond the lungs! Clin Genet 2021; 99: 789–801. doi: 10.1111/cge.13943 [DOI] [PubMed] [Google Scholar]
- 94.Nowaczyk MJM, Huang L, Tarnopolsky M, et al. A novel multisystem disease associated with recessive mutations in the tyrosyl-tRNA synthetase (YARS) gene. Am J Med Genet A 2017; 173: 126–134. doi: 10.1002/ajmg.a.37973 [DOI] [PubMed] [Google Scholar]
- 95.Fuchs SA, Schene IF, Kok G, et al. Aminoacyl-tRNA synthetase deficiencies in search of common themes. Genet Med 2019; 21: 319–330. doi: 10.1038/s41436-018-0048-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frémond M-L, Legendre M, Fayon M, et al. Use of ruxolitinib in COPA syndrome manifesting as life-threatening alveolar haemorrhage. Thorax 2020; 75: 92–95. doi: 10.1136/thoraxjnl-2019-213892 [DOI] [PubMed] [Google Scholar]
- 97.Frémond M-L, Nathan N. COPA syndrome, 5 years after: where are we? Joint Bone Spine 2020; 88: 105070. doi: 10.1016/j.jbspin.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 98.Lepelley A, Martin-Niclós MJ, Le Bihan M, et al. Mutations in COPA lead to abnormal trafficking of STING to the Golgi and interferon signaling. J Exp Med 2020; 217: e20200600. doi: 10.1084/jem.20200600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Langston C, Patterson K, Dishop MK, et al. A protocol for the handling of tissue obtained by operative lung biopsy: recommendations of the chILD pathology co-operative group. Pediatr Dev Pathol 2006; 9: 173–180. doi: 10.2350/06-03-0065.1 [DOI] [PubMed] [Google Scholar]
- 100.Chan CD, Niyogi A, Jaffray B, et al. Lung biopsy in children: when is it useful? Arch Dis Child 2021; 106: 291–293. doi: 10.1136/archdischild-2019-318443 [DOI] [PubMed] [Google Scholar]
- 101.Lamoshi AY, Nakayama DK. Usefulness of lung biopsy in pediatric pulmonary conditions. Am Surg 2015; 81: 31–33. doi: 10.1177/000313481508100121 [DOI] [PubMed] [Google Scholar]
- 102.Nathan N, Marcelo P, Houdouin V, et al. Lung sarcoidosis in children: update on disease expression and management. Thorax 2015; 70: 537–542. doi: 10.1136/thoraxjnl-2015-206825 [DOI] [PubMed] [Google Scholar]
- 103.Perisson C, Epaud R, Fayon M, et al. Beneficial effect of azithromycin in children with diffuse parenchymal lung disease. Eur Respir J 2014; 44: Suppl. 58, P3495. [Google Scholar]
- 104.Epaud R, Kron C, Flamei F, et al. Azithromycin in interstitial lung disease associated with surfactant metabolism disorders. Am J Respir Crit Care Med 2010; 181: A3993. doi: 10.1164/ajrccm-conference.2010.181.1_MeetingAbstracts.A3993 [DOI] [Google Scholar]
- 105.Thouvenin G, Nathan N, Epaud R, et al. Diffuse parenchymal lung disease caused by surfactant deficiency: dramatic improvement by azithromycin. BMJ Case Rep 2013; 2013: bcr2013009988. doi: 10.1136/bcr-2013-009988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Parnham MJ, Erakovic Haber V, Giamarellos-Bourboulis EJ, et al. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther 2014; 143: 225–245. doi: 10.1016/j.pharmthera.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 107.Braun S, Ferner M, Kronfeld K, et al. Hydroxychloroquine in children with interstitial (diffuse parenchymal) lung diseases. Pediatr Pulmonol 2015; 50: 410–419. doi: 10.1002/ppul.23133 [DOI] [PubMed] [Google Scholar]
- 108.Griese M, Köhler M, Witt S, et al. Prospective evaluation of hydroxychloroquine in pediatric interstitial lung diseases: study protocol for an investigator-initiated, randomized controlled, parallel-group clinical trial. Trials 2020; 21: 307. doi: 10.1186/s13063-020-4188-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Williamson M, Wallis C. Ten-year follow up of hydroxychloroquine treatment for ABCA3 deficiency. Pediatr Pulmonol 2014; 49: 299–301. doi: 10.1002/ppul.22811 [DOI] [PubMed] [Google Scholar]
- 110.Misra HP, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem 2000; 204: 119–126. doi: 10.1023/A:1007023532508 [DOI] [PubMed] [Google Scholar]
- 111.Deterding R, Griese M, Deutsch G, et al. Study design of a randomised, placebo-controlled trial of nintedanib in children and adolescents with fibrosing interstitial lung disease. ERJ Open Res 2021; 7: 00805-2020. doi: 10.1183/23120541.00805-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Griese M, Kappler M, Stehling F, et al. Randomized controlled phase 2 trial of hydroxychloroquine in childhood interstitial lung disease. Orphanet J Rare Dis 2022; 17: 289. doi: 10.1186/s13023-022-02399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sanchez GAM, Reinhardt A, Ramsey S, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 2018; 128: 3041–3052. doi: 10.1172/JCI98814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Crow YJ, Neven B, Frémond M-L. JAK inhibition in the type I interferonopathies. J Allergy Clin Immunol 2021; 148: 991–993. doi: 10.1016/j.jaci.2021.07.028 [DOI] [PubMed] [Google Scholar]
- 115.Jamois C, Gibiansky L, Chavanne C, et al. Rituximab pediatric drug development: pharmacokinetic and pharmacodynamic modeling to inform regulatory approval for rituximab treatment in patients with granulomatosis with polyangiitis or microscopic polyangiitis. Clin Transl Sci 2022; 15: 2172–2183. doi: 10.1111/cts.13351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishi K, Ogura M, Tamai N, et al. Successful rituximab treatment for severe rapidly progressive interstitial lung disease with anti-MDA5 antibody-positive juvenile dermatomyositis: a case report and literature review. Pediatr Rheumatol Online J 2022; 20: 60. doi: 10.1186/s12969-022-00723-5 [DOI] [PMC free article] [PubMed] [Google Scholar]