Abstract
BACKGROUND
Early treatment to prevent severe coronavirus disease 2019 (Covid-19) is an important component of the comprehensive response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic.
METHODS
In this phase 3, double-blind, randomized, placebo-controlled trial, we used a 2-by-3 factorial design to test the effectiveness of three repurposed drugs — metformin, ivermectin, and fluvoxamine — in preventing serious SARS-CoV-2 infection in nonhospitalized adults who had been enrolled within 3 days after a confirmed diagnosis of infection and less than 7 days after the onset of symptoms. The patients were between the ages of 30 and 85 years, and all had either overweight or obesity. The primary composite end point was hypoxemia (≤93% oxygen saturation on home oximetry), emergency department visit, hospitalization, or death. All analyses used controls who had undergone concurrent randomization and were adjusted for SARS-CoV-2 vaccination and receipt of other trial medications. CoV-2 vaccination and receipt of other trial medications.
RESULTS
A total of 1431 patients underwent randomization; of these patients, 1323 were included in the primary analysis. The median age of the patients was 46 years; 56% were female (6% of whom were pregnant), and 52% had been vaccinated. The adjusted odds ratio for a primary event was 0.84 (95% confidence interval [CI], 0.66 to 1.09; P = 0.19) with metformin, 1.05 (95% CI, 0.76 to 1.45; P = 0.78) with ivermectin, and 0.94 (95% CI, 0.66 to 1.36; P = 0.75) with fluvoxamine. In prespecified secondary analyses, the adjusted odds ratio for emergency department visit, hospitalization, or death was 0.58 (95% CI, 0.35 to 0.94) with metformin, 1.39 (95% CI, 0.72 to 2.69) with ivermectin, and 1.17 (95% CI, 0.57 to 2.40) with fluvoxamine. The adjusted odds ratio for hospitalization or death was 0.47 (95% CI, 0.20 to 1.11) with metformin, 0.73 (95% CI, 0.19 to 2.77) with ivermectin, and 1.11 (95% CI, 0.33 to 3.76) with fluvoxamine.
CONCLUSIONS
None of the three medications that were evaluated prevented the occurrence of hypoxemia, an emergency department visit, hospitalization, or death associated with Covid-19. (Funded by the Parsemus Foundation and others; COVID-OUT ClinicalTrials.gov number, NCT04510194.)
Widely available early outpatient treatments for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are still needed to prevent severe coronavirus disease 2019 (Covid-19) owing to waning vaccine efficacy and the emergence of new variants.1–3 Access to current outpatient therapeutics remains limited, serious drug–drug interactions may prevent use, and the effectiveness of monoclonal antibodies is challenged by viral evolution.4–7
Medications filling this need may include metformin, ivermectin, and fluvoxamine on the basis of biophysical modeling, which predicted that protein translation may be an antiviral therapy target. Previous studies have shown that metformin has actions against proteins involved in translation.8,9 In addition, metformin is under investigation as an antiviral agent10,11 and has shown in vitro activity against SARS-CoV-2 and other RNA viruses.12–15 Metformin has also shown antiinflammatory actions, including reducing levels of interleukin-1β and interleukin-6 and decreasing the risk of thrombosis and inflammasome activation;16,17 the drug has also shown protection against lipopolysaccharide-induced lung injury in mice inoculated with SARS-CoV-2.18 Observational studies have shown associations between the use of metformin and less severe Covid-19 in patients who were already receiving metformin.19–24
Ivermectin has shown in vitro activity against SARS-CoV-2 but at levels that were 50 to 100 times as high as those that are achievable in humans.25,26 In a small, randomized trial involving 398 volunteers, investigators assessed the use of ivermectin at a dose of 300 μg per kilogram of body weight per day for 5 days and found no effect on symptom resolution, although the study population was young and had few coexisting illnesses.27 The ongoing use of ivermectin, possibly because of concern that the evaluated dose was too low, has suggested the need for more data.
Fluvoxamine has antiinflammatory actions that are mediated by the sigma-1 receptor,28 and the same biophysical model predicted that fluvoxamine would perturb sigma-1 receptor–mediated virion assembly as viral proteins transfer from the cytoplasm to the endoplasmic reticulum.8 Two randomized trials of fluvoxamine at a dose of 100 mg two or three times per day showed a reduction of approximately 25% in hospitalizations or prolonged acute care visits29–31; however, starting fluvoxamine at 100 mg can cause side effects. In a nonrandomized prospective cohort study, investigators found that fluvoxamine at a dose of 50 mg twice daily may be effective and had a better side-effect profile than the 100-mg dose, which suggested a need to study the lower dose.32
We conducted COVID-OUT, a phase 3, randomized, double-blind, placebo-controlled trial, using a 2-by-3 factorial design to test these three oral, generic medications for early outpatient treatment of SARS-CoV-2 infection. We hypothesized that each medication would prevent progression to severe Covid-19.
METHODS
TRIAL DESIGN AND PATIENTS
This was an investigator-initiated clinical trial conducted at six institutions in the United States. On December 30, 2020, we began the enrollment of patients to evaluate metformin as compared with placebo. In the portion of the trial involving the 2-by-3 factorial design, enrollment began on May 21, 2021. The original randomization of patients to receive either metformin or placebo continued for all pregnant women. All the trial patients were recruited remotely, and trial drugs were delivered to the patients at home. Enrollment ended on January 28, 2022.
Follow-up for the primary end point in the last patients who were enrolled ended on February 14, 2022, during which time all the investigators except the statistician remained unaware of group-level results. The investigators continue to remain unaware of individual-level treatment assignments because follow-up assessments of long Covid-19 are ongoing. Details regarding the trial design are provided in the protocol, available with the full text of this article at NEJM.org.
Eligibility criteria included an age of 30 to 85 years; a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) associated with overweight or obesity; proof of SARS-CoV-2 infection within the past 3 days; and an onset of symptoms within 7 days before randomization. Details regarding the inclusion and exclusion criteria and BMI values are provided in the Supplementary Appendix, available at NEJM.org. All the patients provided written informed consent.
RANDOMIZATION
The patients underwent randomization with equal probability of assignment to each trial group that was recruiting at the time of enrollment. The six trial groups were assigned to receive the following drugs or combinations of drugs: group 1, metformin plus fluvoxamine; group 2, metformin plus ivermectin; group 3, metformin plus placebo; group 4, placebo plus fluvoxamine; group 5, placebo plus ivermectin; and group 6, placebo plus placebo. The main effect of each medication in the trial was assessed while controlling for the effects of other medications in the trial. Randomization was stratified according to trial site, and schedules were pregenerated by means of a mass-weighted urn design that limits deviations from the targeted equal allocation similar to permuted blocks. Details regarding the randomization procedures are provided in the Supplementary Appendix.
TREATMENT
The groups received the trial drugs according to the following doses: immediate-release metformin administered with an increase in dose over 6 days to 1500 mg per day for 14 days, ivermectin at a dose of 390 to 470 μg per kilogram per day for 3 days, and fluvoxamine at a dose of 50 mg twice daily for 14 days. For the analysis, the metformin group included the patients who had received metformin alone or metformin in combination with either fluvoxamine or ivermectin. The metformin control group included the patients who had received placebo, fluvoxamine alone, or ivermectin alone. The ivermectin group included the patients who had received ivermectin alone or ivermectin in combination with metformin. Patients in the ivermectin control group were randomly assigned to receive either placebo or metformin alone. The fluvoxamine and fluvoxamine control groups were constructed similarly. The control groups that were used in the comparisons with the active-drug groups included only concurrently randomly assigned patients.
All the patients who were not pregnant and who had been enrolled after the trial had been expanded to include ivermectin and fluvoxamine received two blinded trial drugs that were prepacked into a pillbox. Although groups 1 and 2 (metformin in combination with either fluvoxamine or ivermectin) were the only groups with two active medications, all the patients received two types of pills to maintain the blinding and have a similar pill burden in each group.
End Points
The primary end point was severe Covid-19 through 14 days, defined as a composite of hypoxemia (≤93% oxygen saturation on home oximetry), emergency department visit, hospitalization, or death. At the time that the investigational new drug application was obtained for all the trial drugs, primary end points were typically assessed at 14 days in Covid-19 treatment trials.31,33 Hypoxemia was defined as a home oximeter reading of 93% or less or the need for supplemental oxygen to maintain an oxygen saturation of at least 94%. As so defined, hypoxemia was used as a marker of severe Covid-19, as previously defined by the Food and Drug Administration (FDA) and used in other Covid-19 trials.34 The FDA later issued a safety communication on the accuracy of pulse oximeters after the trial began.34 In addition, numerous patients recorded apparently spurious readings. (Details regarding sources of bias in the ascertainment of hypoxemia are provided in the Supplementary Appendix.) The statistical analysis plan prespecified the performance of secondary analyses, including an analysis that was limited to only the health care components of the primary end point. (FDA guidance recommends analyzing the components of a composite primary end point that are less common but indicate severe disease [in this case, emergency department visit, hospitalization, or death],35 a method that has been commonly used in Covid-19 trials.)
Key secondary end points were daily symptom severity,36 a modified total symptom score based on current standards for Covid-specific symptoms,37 and drug discontinuations. The confidence intervals for secondary analyses were not adjusted for multiplicity, and each medication had its own hypothesized mechanism of action. Participants could opt into three optional substudies of biospecimens that were obtained on days 1, 5, and 10.38
OVERSIGHT
The trial was approved by the central institutional review board of consultant Advarra and was conducted in compliance with the provisions of the Declaration of Helsinki, Good Clinical Practice Guidelines, and local regulatory requirements.39 The trial was funded by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The funders had no role in the design or conduct of the trial and were not involved in the collection or analysis of the data, in the writing of the manuscript, or in making the decision to submit the manuscript for publication. The authors assume responsibility for the fidelity of the trial to the protocol and for the accuracy and completeness of the data and analyses.
STATISTICAL ANALYSIS
We selected an enrollment target of 1350 patients to ensure the participation of 728 patients in the analysis cohorts for fluvoxamine and ivermectin, assuming a frequency of withdrawal of 10%. We determined that this sample size would provide 80% power to detect a 35% relative risk reduction in the active-drug group, assuming a 28% event rate in the control group and additive effects of each trial drug on the log-odds scale. We also determined that this enrollment target would provide at least 90% power to detect a 35% relative risk reduction with metformin under the same assumptions. The statistical power would be higher if the other trial drugs were not efficacious. A prespecified recalculation of the final overall enrollment target was conducted by the statistician in an unblinded manner on November 23, 2021, after a prespecified blinded review of interim event rates in the placebo group, according to baseline vaccination status and a lower-than-anticipated frequency of withdrawal.
Safety, efficacy, and futility were monitored by the members of an independent data and safety monitoring board who reviewed three full interim reports. Efficacy monitoring boundaries for each trial drug were calculated with the use of a Kim–DeMets alpha-spending function after control of the overall one-sided type I error rate at 0.025; futility monitoring guidelines were based on conditional power under the hypothesized effect. At the third interim review, after enrollment of approximately 1100 patients, the data and safety monitoring board recommended stopping fluvoxamine because of futility, with a conditional power of less than 3%.
The prespecified analysis in the modified intention-to-treat population excluded patients who had confirmed that they had not received any trial drug. This approach was informed by prior remote trials showing that 5 to 10% of participants who had provided consent had withdrawn before receiving trial materials.33,40,41 The results in the intention-to-treat population are provided in Tables S6 to S8 in the Supplementary Appendix. The main effect of each trial drug on the primary end point was estimated with the use of logistic regression after adjustment for other trial-drug assignments and SARS-CoV-2 vaccination status. All analyses were performed with the use of concurrently randomized controls only.
The control group for each drug consisted of patients who had been enrolled when the trial drug had been available. Missing data for primary end points and vaccination status were multiply imputed with the use of chained equations and predictive mean matching. Testing for statistical significance was based on a likelihood-ratio test; a two-sided P value of less than 0.05 was considered to indicate statistical significance.42 There was no prespecified plan to adjust for multiple testing, so the results of secondary analyses are reported with point estimates and 95% confidence intervals without P values. The widths of the confidence intervals have not been adjusted for multiple testing and should not be used to infer definitive treatment effects.
We assessed symptom severity using generalized estimating equations after adjustment for baseline symptom severity, receipt of other trial medications, and SARS-CoV-2 vaccination status. Missingness of data in daily symptom logs was approximately 25% across symptoms and was not imputed. All analyses were performed with the use of R software, version 4.1.
RESULTS
PATIENTS
A total of 1431 patients were enrolled in the trial from December 30, 2020, through January 28, 2022. Of these patients, 108 (7.5%) were excluded from the modified intention-to-treat population, which left 1323 patients for inclusion in the primary analysis (Fig. 1). The median age of the patients was 46 years (interquartile range [IQR], 37 to 55); 741 (56.0%) were female, of whom 45 (6.1%) were pregnant. The median BMI was 30 (IQR, 27 to 34). The mean (±SD) number of days from symptom onset to the initiation of a trial drug was 4.8±1.9 days. Overall, 690 of the patients (52.2%) had received Covid-19 vaccination (Table 1).
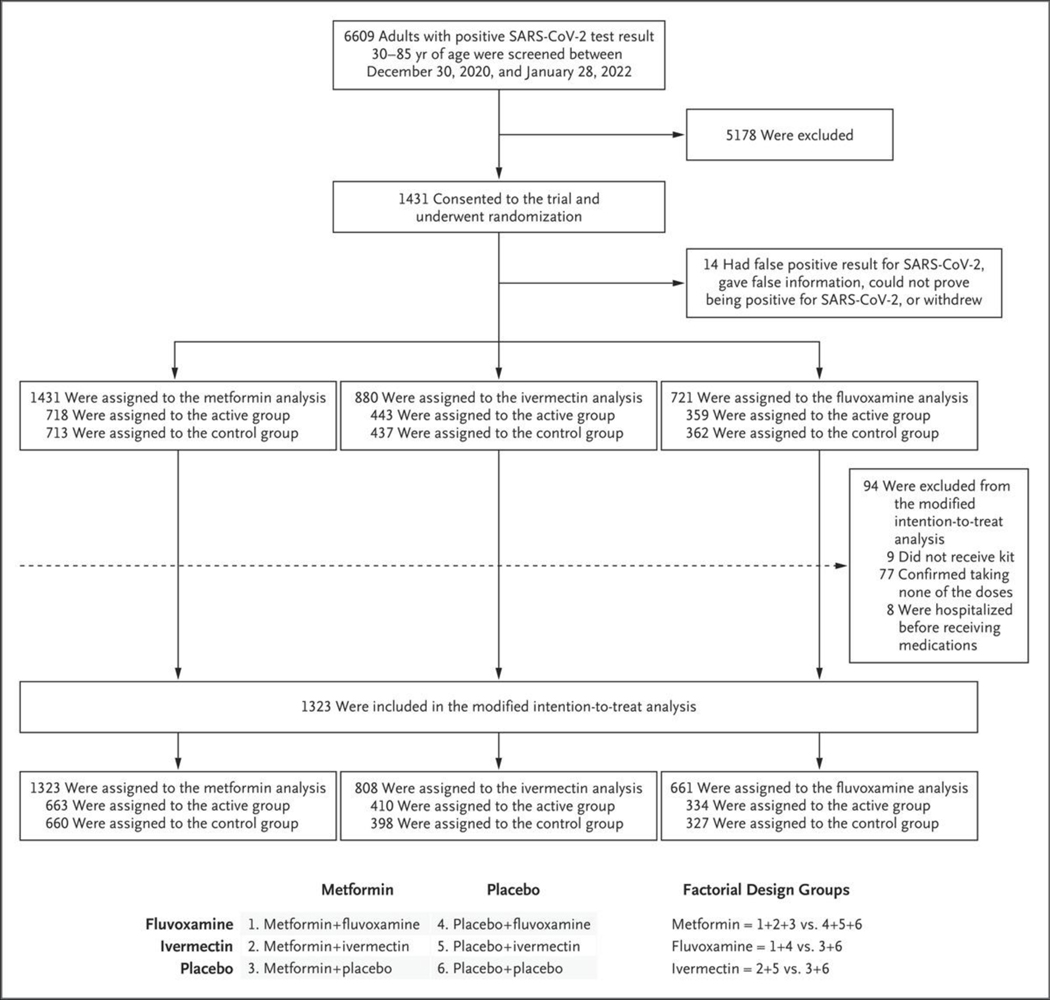
Figure 1. Enrollment and Factorial Design.
Shown is the 2-by-3 factorial design that was developed to test the effectiveness of three repurposed drugs — metformin, ivermectin, and fluvoxamine — in preventing serious coronavirus disease 2019 (Covid-19) in nonhospitalized adults. Although groups 1 and 2 were the only groups with two active drugs, all the patients received two types of pills to maintain the blind and have a similar pill burden in each group. The primary analysis was performed in the modified intention-to-treat population, which excluded patients who had confirmed that they had not received any trial drug. Details regarding the number of patients who were excluded from the trial are provided in the Supplementary Appendix.
Table 1.
Characteristics of the Patients at Baseline (Modified Intention-to-Treat Population).*
| Characteristic | Metformin | Ivermectin | Fluvoxamine† | |||
|---|---|---|---|---|---|---|
| Active (N = 663) | Control (N = 660) | Active (N = 410) | Control (N = 398) | Active (N = 334) | Control (N = 327) | |
| Demographic | ||||||
| Median age (IQR) —yr | 46 (38–55) | 45 (37–55) | 46 (39–55) | 45 (37–56) | 46 (38–53) | 43 (37–53) |
| Female sex — no. (%)† | 359 (54.1) | 382 (57.9) | 216 (52.7) | 226 (56.8) | 170 (50.9) | 188 (57.5) |
| Race or ethnic group‡ | ||||||
| Native American | 10 (1.5) | 17(2.6) | 7 (1.7) | 9 (2.3) | 8 (2.4) | 9 (2.8) |
| Asian | 25 (3.8) | 26 (3.9) | 19 (4.6) | 18 (4.5) | 9 (2.7) | 12 (3.7) |
| Hawaiian or Pacific Islander | 5 (0.8) | 4 (0.6) | 2 (0.5) | 3 (0.8) | 2 (0.6) | 3 (0.9) |
| Black | 55 (8.3) | 45 (6.8) | 30 (7.3) | 29 (7.3) | 28 (8.4) | 23 (7.0) |
| White | 545 (82.2) | 546 (82.7) | 340 (82.9) | 322 (80.9) | 272 (81.4) | 267 (81.7) |
| Other | 43 (6.5) | 37 (5.6) | 24 (5.9) | 29 (7.3) | 21 (6.3) | 23 (7.0) |
| Latinx | 76 (11.5) | 84 (12.7) | 41 (10.0) | 57(14.3) | 42 (12.6) | 46 (14.1) |
| Medical history | ||||||
| Body-mass index | ||||||
| Median (IQR) | 30 (27–34) | 30 (27–34) | 30 (27–34) | 30 (27–34) | 29 (27–34) | 30 (27–34) |
| ≥30 — no. (%) | 316 (47.7) | 330 (50.0) | 194 (47.3) | 189 (47.5) | 155 (46.4) | 157 (48.0) |
| Cardiovascular disease§ | 178 (26.8) | 175 (26.5) | 94 (22.9) | 90 (22.6) | 104 (31.1) | 74 (22.6) |
| Diabetes | 10(1.5) | 16 (2.4) | 8 (2.0) | 5 (1.3) | 4 (1.2) | 3 (0.9) |
| Primary series of vaccines | 359 (54.1) | 331 (50.2) | 222 (54.1) | 227 (57.0) | 186 (55.7) | 187 (57.2) |
| Symptom duration | ||||||
| No. of days | 4.8±1.9 | 4.8±1.9 | 4.6±l.9 | 4.8±1.8 | 5.0±2.2 | 4.7±1.8 |
| ≤4 days — no./total no. (%) | 298/653 (45.6) | 305/642 (47.5) | 199/406 (49.0) | 174/391 (44.5) | 147/330 (44.5) | 146/322 (45.3) |
| Predominant variant — no. (%) | ||||||
| Alpha before 6/19/21 | 79 (11.9) | 80(12.1) | 11 (2.7) | 11 (2.8) | 12 (3.6) | 11 (3.4) |
| Delta from 6/19/21 to 12/12/21 | 440 (66.4) | 431 (65.3) | 278 (67.8) | 275 (69.1) | 278 (83.2) | 275 (84.1) |
| Omicron after 12/12/21 | 144 (21.7) | 149 (22.6) | 121 (29.5) | 112 (28.1) | 46 (14.0) ¶ | 41 (12.5)¶ |
| Insurance status — no. (%) | ||||||
| Medicaid | 92 (13.9) | 108 (16.4) | 70 (17.1) | 60 (15.1) | 43 (12.9) | 42 (12.8) |
| Medicare | 52 (7.8) | 48 (7.3) | 27 (6.6) | 31 (7.8) | 27 (8.1) | 21 (6.4) |
| Private | 410 (61.8) | 413 (62.6) | 257 (62.7) | 230 (57.8) | 206 (61.7) | 197 (60.2) |
| None | 97 (14.6) | 81 (12.3) | 52 (12.7) | 67 (16.8) | 55 (16.5) | 58 (17.7) |
Plus-minus values are means ±SD. IQR denotes interquartile range.
A total of 6% of the women were pregnant during the trial.
Race or ethnic group was reported by the patients. The category of “other” included patients for whom data were not provided.
Cardiovascular disease defined as hypertension, hyperlipidemia, coronary artery disease, past myocardial infarction, heart failure, pacemaker placement, arrhythmias or pulmonary hypertension.
Enrollment in the fluvoxamine group was stopped on January 7, 2022, by the data and safety monitoring board for a lack of conditional power.
END POINTS
A primary event (hypoxemia, emergency department visit, hospitalization, or death) occurred in 333 of 1305 patients with complete data (25.5%); of these patients, 134 of 686 (19.5%) were vaccinated, as compared with 199 of 615 (32.4%) who were were unvaccinated. The adjusted odds ratio for a primary event was 0.84 (95% confidence interval [CI], 0.66 to 1.09; P = 0.19) with metformin, 1.05 (95% CI, 0.76 to 1.45; P = 0.78) with ivermectin, and 0.94 (95% CI, 0.66 to 1.36; P = 0.75) with fluvoxamine.
The results of a prespecified secondary analysis that included the components of the primary end point are detailed in Table 2. The adjusted odds ratio for emergency department visit, hospitalization, or death was 0.58 (95% CI, 0.35 to 0.94) with metformin, 1.39 (95% CI, 0.72 to 2.69) with ivermectin, and 1.17 (95% CI, 0.57 to 2.40) with fluvoxamine. For hospitalization or death, the adjusted odds ratio was 0.47 (95% CI, 0.20 to 1.11) with metformin, 0.73 (95% CI, 0.19 to 2.77) with ivermectin, and 1.11 (95% CI, 0.33 to 3.76) with fluvoxamine.
Table 2.
Primary Composite End Point and Its Components.*
| End Point | Metformin | Ivermectin | Fluvoxamine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Active (N = 663) | Control (N = 660) | Adjusted Odds Ratio (95% CI)† | Active (N = 410) | Control (N = 398) | Adjusted Odds Ratio (95% CI)† | Active (N = 334) | Control (N = 327) | Adjusted Odds Ratio (95% CI)† | |
| no./total no. (%) | no. /total no. (%) | no./total no. (%) | |||||||
| Primary composite | 154/652 (23.6) | 179/653 (27.4) | 0.84 (0.66–1.09) | 105/407 (25.8) | 96/391 (24.6) | 1.05 (0.76–1.45) | 79/329 (24.0) | 80/321 (24.9) | 0.94 (0.66–1.36) |
| Hypoxemia only | 147/650 (22.6) | 158/651 (24.3) | 0.94 (0.72–1.22) | 96/406 (23.6) | 88/390 (22.6) | 1.04 (0.75–1.46) | 71/328 (21.6) | 73/320 (22.8) | 0.93 (0.64–1.35) |
| Emergency department visit, hospitalization, or death | 27/652 (4.1) | 48/655 (7.3) | 0.58 (0.35–0.94) | 23/406 (5.7) | 16/394 (4.1) | 1.39 (0.72–2.69) | 18/329 (5.5) | 15/324 (4.6) | 1.17 (0.57–2.40) |
| Hospitalization or death | 8/652 (1.2) | 18/655 (2.7) | 0.47 (0.20–1.11) | 4/406 (1.0) | 5/394 (1.3) | 0.73 (0.19–2.77) | 6/329 (1.8) | 5/324 (1.5) | 1.11 (0.33–3.76) |
| Death | 1/657 (0.2) | 0/655 (0) | NA | 1/408 (0.2) | 0/396 (0) | NA | 0/330 (0) | 0/325 (0) | NA |
The primary end point was a composite of hypoxemia (≤93% on home oximetry), emergency department visit, hospitalization, or death by 14 days. Analyses used concurrently randomized controls and were adjusted for SARS-CoV-2 vaccination and other trial medications. The primary analysis was performed in the modified intention-to-treat cohort. Comparison of absolute event rates across groups is not valid because of differences in timing of enrollment, which resulted in differences in vaccination rates and the prevalence of SARS-CoV-2 variants.
Adjusted odds ratios and 95% confidence intervals are based on a logistic-regression model that was adjusted for baseline vaccination status and the receipt of other medications during the trial; multiple imputation was used with chained equations and predictive mean matching. The complete case-analysis results without imputation are provided in Table S4; the analysis in the intention-to-treat population is presented in Tables S6 to S8.
We assessed the effect of the three drugs by a priori subgroups for the overall and more severe components of the primary composite end point (Figs. S1 and S2). Effects were consistent across subgroups, including according to vaccination history, variant period, and the presence or absence of pregnancy. Because hospitalization and death were rare, subgroups were not assessed again after sequential removal of the variable for the emergency department visit. Through day 28, hospitalization or death occurred in 8 of 596 patients (1.3%) receiving metformin and in 19 of 601 controls (3.2%) (Figs. S7 and S8).
SYMPTOMS AND ADVERSE EVENTS
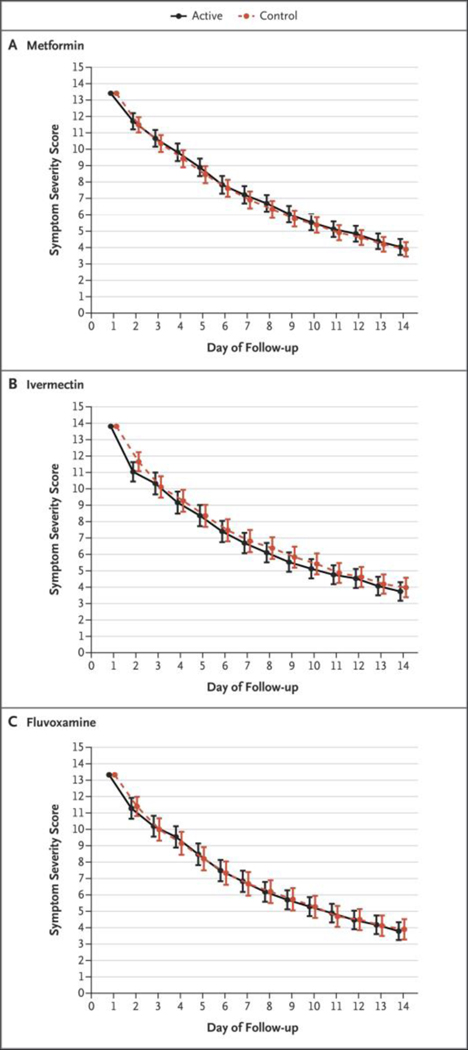
Patients were asked to circle the severity of daily symptoms in paper diaries during a period of 14 days. Neither overall symptoms nor Covid-19–specific symptoms were reduced faster with placebo than with any of the trial drugs (Fig. 2). Individual symptoms are provided in Figure S5. No medication-related serious adverse events occurred.
Figure 2. Total Scores on a Symptom Severity Scale during a 14-Day Period.
The three panels present the composite symptom scores in the active treatment groups and the control groups among the patients who received metformin, ivermectin, or fluvoxamine. Scores were calculated with the use of a generalized estimating equation after adjustment for the baseline score, vaccination status, and receipt of other medications during the trial. Shown on the y axis is the composite score of 14 symptoms, which were graded as none (0), mild (1), moderate (2), or severe (3). Overall, 80% of the patients contributed data on the symptom logs; the frequency of missing data was approximately 25% on each of the 14 days. Additional details regarding specific symptoms are provided in Figures S4 and S5 in the Supplementary Appendix.
DISCUSSION
In this placebo-controlled clinical trial of metformin, ivermectin, and fluvoxamine for early outpatient treatment of SARS-CoV-2 infection, none of the three drugs had a significant effect on the composite primary end point of hypoxemia, emergency department visit, hospitalization, or death. A possible benefit for the prevention of the more severe components of the primary end point (emergency department visit, hospitalization, or death) was shown for metformin. However, this finding was a prespecified secondary end point and thus cannot be considered to be definitive pending the results of other trials.
In a previous clinical trial of metformin, investigators enrolled 421 adults during different waves of SARS-CoV-2 variants to evaluate whether extended-release metformin at a dose of 750 twice daily would convey benefit over placebo, including in patients who were already taking up to 1000 mg of immediate-release metformin for such conditions as diabetes, prediabetes, weight loss, polycystic ovarian syndrome, or nonalcoholic fatty liver disease.43 The relative risk of hospitalization or a prolonged emergency department visit with metformin was 1.03 (95% Bayesian credible interval, 0.64 to 1.66). In this trial, patients started metformin at a dose of 1500 mg per day without dose adjustment, which may have caused side effects and discontinuations; in the per-protocol analysis, hospitalization occurred in 8 of 168 patients (4.8%) in the metformin group and in 14 of 179 patients (7.8%) in the control group.43 A higher dose of metformin may not improve antiinflammatory actions, as suggested in a recent study of macular degeneration.44 Also, immediaterelease metformin may have higher peak systemic exposure than the extended-release formulation, which may be relevant in SARS-CoV-2 infection.45
We did not find evidence that fluvoxamine at a low dose of 50 mg twice daily prevented a primary event in this population. In two randomized, double-blind, placebo-controlled trials, investigators found that higher-dose fluvoxamine (100 mg two to three times daily) resulted in a 25 to 30% reduction in hospitalization or a prolonged emergency department stay.29 Because agonism of the sigma-1 receptor may be an important mechanism of fluvoxamine against Covid-19, the dose may need to be higher to produce an effect, especially in patients who have overweight or obesity.46,47
Likewise, we did not find evidence that ivermectin prevented a primary event in this population of U.S. adults who were 30 years of age or older and who had overweight or obesity. Because a previous randomized trial of ivermectin at a dose of 300 μg per kilogram per day did not show any significant effect, we chose a higher dose, a median of 430 μg per kilogram (range, 390 to 470 μg per kilogram) per day.27 Ivermectin has been studied around the world, and the effect of ivermectin would be expected to be greater in patients with chronic Strongyloides stercoralis parasitic infection who had Covid-19 progression and received dexamethasone, thereby preventing life-threatening hyperinfection with strongyloides species. However, strongyloidiasis is rare in the United States outside of Appalachia.48,49
As in other fields of medicine, it appears that there may be a role for both repurposed generic medications as well as newly developed, targeted therapies. For example, in the field of obesity medicine, new agents (e.g., semaglutide) are used alongside repurposed generic medications, such as topiramate and bupropion, and even concurrently in the same patients. Previous studies have suggested that metformin improved the sustained virologic response of antiviral drugs against hepatitis C.50 The proposed mechanisms of action against Covid-19 for metformin include antiinflammatory and antiviral activity and the prevention of hyperglycemia during acute illness. Further investigation is needed to determine whether any of these proposed mechanisms has any clinically meaningful activity in the treatment of Covid-19.
Our trial has several limitations. Since enrollment was limited to patients who were between 30 and 85 years of age and who had overweight or obesity in the United States (where the prevalence of overweight or obesity was >72% in 2018), the findings may not be generalizable beyond this population.51 The percentage of patients who identified as Black or Latinx was smaller than that in the U.S. population, a discrepancy that is exacerbated among patients with Covid-19. The composite end point that included hypoxemia was defined before statements from the FDA pointed to accuracy problems with home oxygen monitors, especially nonprescription brands.34 The hypoxemia component of the primary end point is the most susceptible to bias owing to inherent limits of oximetry to accurately detect and report oxygen saturation,34 measurement error caused by cold hands or improper fit, misclassification caused by transient atelectasis-induced hypoxemia, and recall bias for hypoxemia reported by the patients (19%) without written documentation. A visit to an emergency department or hospitalization is more memorable and less susceptible to recall bias than a reduced oxygen level. Although we could objectively verify emergency department visits by medical record review, emergency department visits are influenced by the patient’s willingness and ability to seek health care. Thus, hospitalization is perhaps the most accurate and well-documented end point, because objective criteria are necessary for admission.
Our loss to follow-up for the primary analysis was 1.4%, and the frequency of missing data regarding daily symptoms (21 to 24%) was similar to that in other remotely managed trials of Covid-19 treatments.52 Due to the rarity of the hospitalization (2.0% overall) or mortality (0.2%), small numbers of missing unknown events could have affected the precision of estimates. Given the number of comparisons that were made by assessing a secondary outcome within subgroups, any suggestion of metformin activity in a subgroup should be interpreted with caution because there was no control for multiplicity. Finally, the factorial design that included trial drug groups that were open to enrollment during different waves of viral variants does not permit comparisons of marginal event rates between drugs, because such rates may be confounded by temporal trends. Thus, all comparisons were performed against concurrent randomized controls.
In this randomized trial involving adults with overweight and obesity, none of the three trial drugs prevented a primary event of hypoxemia, emergency department visit, hospitalization, or death. The analysis of a prespecified secondary outcome suggested a possible reduction in a composite end point of emergency department visit, hospitalization, or death with metformin. None of the trial drugs resulted in a lower severity of symptoms than identically matched placebo.
Supplementary Material
Acknowledgments
Supported by the Parsemus Foundation, Rainwater Charitable Foundation, Fast Grants, and UnitedHealth Group Foundation. The fluvoxamine placebo tablets were donated by Apotex Pharmaceuticals. The ivermectin placebo and active tablets were donated by Edenbridge Pharmaceuticals. Dr. Bramante was supported by grants (KL2TR002492 and UL1TR002494) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) and by a grant (K23 DK124654-01-A1) from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. Dr. Buse was supported by a grant (UL1TR002489) from NCATS. Dr. Nicklas was supported by a grant (K23HL133604) from the National Heart, Lung, and Blood Institute of the NIH. Dr. Odde was supported by the Institute for Engineering in Medicine, the Medtronic Professorship for Engineering in Medicine, and by grants (U54 CA210190 and P01 CA254849) from the National Cancer Institute of the NIH. Dr. Murray was supported in part by the Medtronic Faculty Fellowship.
Footnotes
References
- 1.Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system work-force. N Engl J Med 2021;385:1330–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med 2021;385:1184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022;386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022;386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzolini C, Kuritzkes DR, Marra F, et al. Prescribing nirmatrelvir-ritonavir: how to recognize and manage drug-drug interactions. Ann Intern Med 2022;175:744–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castle BT, Dock C, Hemmat M, et al. Biophysical modeling of the SARS-CoV-2 viral cycle reveals ideal antiviral targets. June 16, 2020 ( 10.1101/2020.05.22.111237v2). preprint. [DOI]
- 9.Karam BS, Morris RS, Bramante CT, et al. mTOR inhibition in COVID-19: a commentary and review of efficacy in RNA viruses. J Med Virol 2021;93:1843–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farfan-Morales CN, Cordero-Rivera CD, Reyes-Ruiz JM, et al. Anti-flavivirus properties of lipid-lowering drugs. Front Physiol 2021; 12: 749770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng F, He M, Jung JU, Lu C, Gao SJ. Suppression of Kaposi’s sarcoma-associated herpesvirus infection and replication by 5′-AMP-activated protein kinase. J Virol 2016;90:6515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020;583:459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Campo JA, García-Valdecasas M, Gil-Gómez A, et al. Simvastatin and metformin inhibit cell growth in hepatitis C virus infected cells via mTOR increasing PTEN and autophagy. PLoS One 2018; 13(1):e0191805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakashima K, Takeuchi K, Chihara K, Hotta H, Sada K. Inhibition of hepatitis C virus replication through adenosine monophosphate-activated protein kinase-dependent and -independent pathways. Microbiol Immunol 2011; 55:7 74–82. [DOI] [PubMed] [Google Scholar]
- 15.Schaller MA, Sharma Y, Dupee Z, et al. Ex vivo SARS-CoV-2 infection of human lung reveals heterogeneous host defense and therapeutic responses. JCI Insight 2021; 6(18):e148003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postler TS, Peng V, Bhatt DM, Ghosh S. Metformin selectively dampens the acute inflammatory response through an AMPK-dependent mechanism. Sci Rep 2021;11:18721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin G, Wei Z, Ji C, et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci Rep 2016;6:36222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xian H, Liu Y, Rundberg Nilsson A, et al. Metformin inhibition of mitochondrial ATP and DNA synthesis abrogates NLRP3 inflammasome activation and pulmonary inflammation. Immunity 2021; 54(7):1463.e11–1477.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bramante CT, Buse J, Tamaritz L, et al. Outpatient metformin use is associated with reduced severity of COVID-19 disease in adults with overweight or obesity. J Med Virol 2021;93: 4273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo P, Qiu L, Liu Y, et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg 2020;103:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Yang X, Yan P, Sun T, Zeng Z, Li S. Metformin in patients with COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:704666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol (Lausanne) 2021;11:600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bramante CT, Ingraham NE, Murray TA, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev 2021; 2(1):e34–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Portmann-Baracco A, Bryce-Alberti M, Accinelli RA. Antiviral and anti-inflammatory properties of ivermectin and its potential use in COVID-19. Arch Bronconeumol (Engl Ed) 2020; 56:8 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popp M, Stegemann M, Metzendorf M-I, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev 2021;7(7):CD015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Medina E, López P, Hurtado IC, et al. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA 2021; 325: 1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Facente SN, Reiersen AM, Lenze EJ, Boulware DR, Klausner JD. Fluvoxamine for the early treatment of SARS-CoV-2 infection: a review of current evidence. Drugs 2021; 81:2 081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10(1):e 42–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee TC, Vigod S, Bortolussi-Courval É, et al. Fluvoxamine for outpatient management of COVID-19 to prevent hospitalization: a systematic review and meta-analysis. JAMA Netw Open 2022;5 (4): e226269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 2020; 324:2 292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis 2021; 8(2):o fab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med 2020; 383: 517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. Pulse oximeter accuracy and limitations: FDA safety communication. February 19, 2021 (https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication).
- 35.Food and Drug Administration. Multiple endpoints in clinical trials: guidance for industry. January 2017. (https://www.fda.gov/media/102657/download).
- 36.Food and Drug Administration. Assessing COVID-19-related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID-19 prevention or treatment. Guidance for industry. September 2020. (https://www.fda.gov/media/142143/download). [Google Scholar]
- 37.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes new monoclonal antibody for treatment of COVID-19 that retains activity against omicron variant. FDA news release. February 11, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-monoclonal-antibody-treatment-covid-19-retains). [Google Scholar]
- 38.Bramante CT, Proper JL, Boulware DR, et al. Vaccination against SARS-CoV-2 is associated with a lower viral load and likelihood of systemic symptoms. Open Forum Infect Dis 2022;9(5): ofac066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191–4. [DOI] [PubMed] [Google Scholar]
- 40.Skipper CP, Pastick KA, Engen NW, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med 2020;173:623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connors JM, Brooks MM, Sciurba FC, et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19: the ACTIV-4B randomized clinical trial. JAMA 2021;326:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng X-L, Rubin DB. Performing likelihood ratio tests with multiply-imputed data sets. Biometrika 1992;79:103–11. [Google Scholar]
- 43.Reis G, Dos Santos Moreira Silva EA, Medeiros Silva DC, et al. Effect of early treatment with metformin on risk of emergency care and hospitalization among patients with COVID-19: the TOGETHER randomized platform clinical trial. Lancet Reg Health Am 2022;6:100142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Blitzer AL, Ham SA, Colby KA, Skondra D. Association of metformin use with age-related macular degeneration: a casecontrol study. JAMA Ophthalmol 2021;139:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmins P, Donahue S, Meeker J, Marathe P. Steady-state pharmacokinetics of a novel extended-release metformin formulation. Clin Pharmacokinet 2005;44:721–9. [DOI] [PubMed] [Google Scholar]
- 46.Bolo NR, Hodé Y, Nédélec JF, Lainé E, Wagner G, Macher JP. Brain pharmacokinetics and tissue distribution in vivo of fluvoxamine and fluoxetine by fluorine magnetic resonance spectroscopy. Neuropsychopharmacology 2000; 23: 428–38. [DOI] [PubMed] [Google Scholar]
- 47.Dodds MG, Doyle EB, Reiersen AM, Brown F, Rayner CR. Fluvoxamine for the treatment of COVID-19. Lancet Glob Health 2022;10(3):e332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newberry AM, Williams DN, Stauffer WM, Boulware DR, Hendel-Paterson BR, Walker PF. Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis. Chest 2005;128:3681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stauffer WM, Alpern JD, Walker PF. COVID-19 and dexamethasone: a potential strategy to avoid steroid-related strongyloides hyperinfection. JAMA 2020;324:623–4. [DOI] [PubMed] [Google Scholar]
- 50.Yu J-W, Sun L-J, Zhao Y-H, Kang P, Yan B-Z. The effect of metformin on the efficacy of antiviral therapy in patients with genotype 1 chronic hepatitis C and insulin resistance. Int J Infect Dis 2012;16(6):e436–e441. [DOI] [PubMed] [Google Scholar]
- 51.Hales CM, Carroll MD, Fryar CD, Og-den CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief 2020;360:1–8. [PubMed] [Google Scholar]
- 52.Oldenburg CE, Pinsky BA, Brogdon J, et al. Effect of oral azithromycin vs placebo on COVID-19 symptoms in outpatients with SARS-CoV-2 infection: a randomized clinical trial. JAMA 2021;326:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.