Abstract
Background
Few prospective studies of Long COVID risk factors have been conducted. The purpose of this study was to determine whether sociodemographic factors, lifestyle, or medical history preceding COVID-19 or characteristics of acute severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are associated with Long COVID.
Methods
In March 26, 2020, the COVID-19 Citizen Science study, an online cohort study, began enrolling participants with longitudinal assessment of symptoms before, during, and after SARS-CoV-2 infection. Adult participants who reported a positive SARS-CoV-2 test result before April 4, 2022 were surveyed for Long COVID symptoms. The primary outcome was at least 1 prevalent Long COVID symptom greater than 1 month after acute infection. Exposures of interest included age, sex, race/ethnicity, education, employment, socioeconomic status/financial insecurity, self-reported medical history, vaccination status, variant wave, number of acute symptoms, pre-COVID depression, anxiety, alcohol and drug use, sleep, and exercise.
Results
Of 13 305 participants who reported a SARS-CoV-2 positive test, 1480 (11.1%) responded. Respondents’ mean age was 53 and 1017 (69%) were female. Four hundred seventy-six (32.2%) participants reported Long COVID symptoms at a median 360 days after infection. In multivariable models, number of acute symptoms (odds ratio [OR], 1.30 per symptom; 95% confidence interval [CI], 1.20–1.40), lower socioeconomic status/financial insecurity (OR, 1.62; 95% CI, 1.02–2.63), preinfection depression (OR, 1.08; 95% CI, 1.01–1.16), and earlier variants (OR = 0.37 for Omicron compared with ancestral strain; 95% CI, 0.15–0.90) were associated with Long COVID symptoms.
Conclusions
Variant wave, severity of acute infection, lower socioeconomic status, and pre-existing depression are associated with Long COVID symptoms.
Keywords: long COVID, SARS-CoV-2, patient-reported outcomes, COVID-19, Post-Acute Sequelae of SARS-CoV-2 (PASC)
Persistent symptoms were highly prevalent and commonly persisted beyond 1 year. Number of symptoms during acute SARS-CoV-2 infection, financial insecurity, pre-existing depression, and earlier variants are associated with Long COVID symptoms independent of vaccination, medical history, and other factors.
Symptoms attributable to coronavirus disease 2019 (COVID-19), including fatigue, memory/concentration problems (“brain fog”), and shortness of breath may persist after acute infection. Symptoms may be due to Long COVID, a type of postacute sequelae of COVID-19 not explainable by other known medical conditions [1]. Although prevalence estimates vary, Long COVID may occur in up to 30% of individuals after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [2–7]. Most individuals with Long COVID were not hospitalized for acute infection, and Long COVID can occur regardless of illness severity, vaccination status, and SARS-CoV-2-targeted treatment [8, 9], although the risk may be lower among vaccinated individuals and those with asymptomatic infection [9, 10]. Symptoms have been reported for up to 24 months [11], and there are currently no proven treatments for Long COVID.
The existing literature on risk factors for Long COVID largely relies on assessments performed after SARS-CoV-2 infection, captured in electronic health record diagnostic codes, or focused on individuals recruited from Long COVID clinics or after hospitalization for acute COVID-19 [12–14]. Furthermore, cohort studies that require in-person participation may exclude individuals unable to attend study visits or far from research sites, and thus survey-based approaches, particularly those that leverage preinfection data, may contribute to our understanding of Long COVID and its antecedent factors [10, 15, 16]. The objectives of this study were to estimate Long COVID symptom prevalence and determine whether sociodemographic factors, lifestyle, or medical history preceding COVID-19, or characteristics of acute SARS-CoV-2 infection were associated with development of Long COVID symptoms.
METHODS
Design, Setting, and Participants
The COVID-19 Citizen Science (CCS) study is an online cohort study that began enrolling participants on March 26, 2020 [17]. The CCS is hosted on the Eureka Research Platform (University of California, San Francisco [UCSF], San Francisco, CA), a digital platform for clinical research studies including a mobile application (app) and web-based software. Participants are recruited through email invitations to participants in other Eureka Research Platform studies, press releases, word-of-mouth, and by partner organizations. Participants must be 18 years of age or older, register for a Eureka Research Platform account, have an iOS or Android smartphone with a cell phone number (or enroll in the web-based study launched January 21, 2021), agree to participate in English, and provide consent. There are no geographic restrictions, but 98% are US residents. After providing electronic consent, participants complete baseline, daily, weekly, and monthly surveys. The CCS methods have been previously described [17]. For this analysis, we included data collected from March 26, 2020 to May 18, 2022. The primary analyses included all individuals who reported a positive COVID-19 test result (polymerase chain reaction [PCR], antigen, or antibody) more than 30 days before May 4, 2022 and responded to a survey about Long COVID symptoms, but for longitudinal symptom comparison we also included those without a positive COVID-19 test and compared them to those without SARS-CoV-2 infection. Sample size was not determined a priori. Results are reported in accordance with STROBE guidelines [18].
Patient Consent Statement
All participants provided digitally signed informed consent. The study was reviewed and approved by the UCSF Institutional Review Board (Number 17-21879).
Long COVID Symptoms
Participants who reported a COVID-19 positive test more than 30 days prior were offered a cross-sectional survey about Long COVID symptoms in January 2022 and May 2022 (surveys went out on different days depending on participant's last answered survey). The survey asked about the presence, duration, and severity of Long COVID symptoms using a nonvalidated instrument. Severity was assessed using a Likert-scale asked for each reported symptom: “How bad do you think these symptoms were?” (1–5, very mild to very severe). In addition, the survey asked about healthcare use and missed days of work or school due to Long COVID symptoms.
Longitudinal Symptoms
In addition to cross-sectional surveys, participants were surveyed on a daily (before August 2021) or weekly (after August 2021) basis regarding symptoms including the following: scratchy throat, painful sore throat, cough, runny nose, fever or chills, temperature >100.4°F or 38.0°C, muscle aches, nausea, vomiting, diarrhea, shortness of breath, unable to taste or smell, and red or painful eyes. We did not use these surveys to classify people as having Long COVID. We categorized the surveys for each individual by time period relative to date of SARS-CoV-2 positive test into 30–60 days preinfection, 0–30 days preinfection, 0–30 days postinfection, 30–90 days postinfection, 90–180 days postinfection, 180–365 days postinfection, and >365 days postinfection. For each period, we summed the proportion of respondents averaging ≥1 symptom and the average number of symptoms reported by Long COVID status among respondents to the cross-sectional survey and among nonrespondents infected with SARS-CoV-2, and individuals without infection (averaged over all time points since March 2020).
Other Variables
Variant wave was classified by the date of first positive test: Initial (before March 11, 2021), Alpha (March 11, 2021–July 3, 2021), Delta (July 4, 2021–December 25, 2021), and Omicron (December 26, 2021–April 4, 2022) [19]. Participants self-reported demographics, medical history, and vaccine history via surveys. Most participants (969 of 1480, 65.5%) completed baseline surveys before SARS-CoV-2 infection, whereas the remainder enrolled after infection. Participants were queried about lifestyle factors before and after COVID including number of days per week they exercised, typical amount of sleep, and number of alcoholic drinks they consumed. Standardized instruments including the Patient Health Questionnaire (PHQ)-8 for depression [20] and Generalized Anxiety Disorder (GAD)-7 for anxiety [21] were used in a self-administered format and were assessed before and after COVID for those who completed surveys before infection. Socioeconomic status was assessed using the MacArthur Scale of Subjective Social Status [22].
Statistical Analysis
The presence of Long COVID symptoms was defined based on ≥1 symptom reported more than 30 days after SARS-CoV-2 positive test on the cross-sectional survey. First, we compared demographics, pre-COVID medical history, socioeconomic variables, and lifestyle patterns among those with Long COVID symptoms compared to those without Long COVID symptoms using χ2 tests for categorical variables and t tests for continuous variables. Then we described the prevalence of each reported symptom and patterns of symptom persistence. To assess risk factors associated with Long COVID and adjust for potential confounders, we constructed multivariable logistic regression models in a prespecified staged approach using a complete case approach to missing data. In an initial (baseline) model (Stage 1), we included age, sex, variant wave, number of symptoms during acute infection, and past medical history. For the next model (Stage 2), we added vaccine status and timing, Hispanic ethnicity, and sociodemographic variables including socioeconomic status, education level, and employment in healthcare. In the final model (Stage 3), in which we prespecified including pre-COVID variables with P < .10 in univariate analysis, we added pre-COVID anxiety, depression, and financial insecurity. We additionally conducted sensitivity analyses considering only those with pre-COVID baseline assessments, only those with persistent symptoms, only those with severe/very severe symptoms, and only those with PCR or antigen testing. Statistical significance was considered to be P < .05 for all analyses other than the prespecified potential predictor selection process in Stage 3. All analyses were conducted with SAS version 9.4 (Cary, NC).
RESULTS
As of May 18, 2022, 13 305 participants reported a diagnosis of COVID-19 more than 30 days before the survey, 1480 (11.1%) responded to a survey about Long COVID symptoms, and 476 of these (32.2% of respondents) reported Long COVID symptoms. Compared to non-respondents, survey respondents were more likely to be infected during the Omicron wave and less likely during the Initial wave, more likely to have been vaccinated before infection, and had a higher number of acute symptoms (P < .001 for each). Among those with Long COVID symptoms, mean age was 53.1 (standard deviation = 13.3) and 356 (75.1%) were female (Table 1). Among those with Long COVID symptoms, the median time from first SARS-CoV-2 positive test to survey response was 360 days (interquartile range [IQR], 129–506), and among those without it was 129 days (IQR, 108–343).
Table 1.
Participant Characteristics
| Characteristics | Long COVID Symptoms, N = 476 | No Long COVID Symptoms, N = 1004 | Did Not Answer Survey, N = 11 825 | P Value Comparing Symptoms Versus No Symptoms |
|---|---|---|---|---|
| Age, mean (SD) | 53.13 (13.27) | 52.50 (14.13) | … | .42 |
| Female sex at birth | 356 (75.11%) | 661 (65.90%) | 8153 (69.34%) | .0004 |
| Race/Ethnicitya | ||||
| White | 433 (92.52%) | 924 (92.77%) | 10 374 (89.12%) | .86 |
| Black or African American | 15 (3.21%) | 33 (3.31%) | 493 (4.24%) | .91 |
| Asian | 12 (2.56%) | 24 (2.41%) | 452 (3.88%) | .80 |
| Native Hawaiian or Pacific Islander | 3 (0.64%) | 1 (0.10%) | 42 (0.36%) | .07 |
| American Indian or Alaska Native | 14 (2.99%) | 11 (1.10%) | 207 (1.78%) | .009 |
| Other/Does Not Know | 25 (5.34%) | 32 (3.21%) | 642 (5.51%) | .05 |
| Hispanic Ethnicity | 55 (11.60%) | 75 (7.48%) | 1378 (11.72%) | .009 |
| MacArthur SES, mean (SD) | 6.64 (1.54) | 7.08 (1.46) | 6.54 (1.68) | .0000 |
| Highest Educational Level | ||||
| No high school degree | 0 | 3 (0.30%) | 79 (0.67%) | .0000 |
| High school graduate (or equivalent) | 16 (3.38%) | 15 (1.50%) | 622 (5.29%) | |
| College degree (including associate's) | 279 (58.86%) | 486 (48.60%) | 6697 (56.97%) | |
| Graduate degree | 171 (36.08%) | 488 (48.80%) | 4188 (35.62%) | |
| Other | 8 (1.69%) | 8 (0.80%) | 168 (1.43%) | |
| US resident | 461 (96.85%) | 984 (98.01%) | 11 552 (97.72%) | .17 |
| Primary Employment | ||||
| Healthcare | 95 (19.96%) | 187 (18.63%) | 2743 (23.20%) | .06 |
| Education | 71 (14.92%) | 154 (15.34%) | 1410 (11.93%) | |
| Retail | 11 (2.31%) | 14 (1.39%) | 333 (2.82%) | |
| Transportation | 12 (2.52%) | 12 (1.20%) | 213 (1.80%) | |
| Arts, entertainment, and recreation | 13 (2.73%) | 33 (3.29%) | 298 (2.52%) | |
| Hospitality and food services | 4 (0.84%) | 23 (2.29%) | 288 (2.44%) | |
| Finance and insurance | 26 (5.46%) | 46 (4.58%) | 700 (5.92%) | |
| Scientific and technical services | 30 (6.30%) | 100 (9.96%) | 792 (6.70%) | |
| Utilities | 3 (0.63%) | 6 (0.60%) | 85 (0.72%) | |
| Construction | 9 (1.89%) | 8 (0.80%) | 231 (1.95%) | |
| Manufacturing | 14 (2.94%) | 23 (2.29%) | 324 (2.74%) | |
| Other | 188 (39.50%) | 398 (39.64%) | 4405 (37.26%) | |
| COVID-19 Variant Wave | ||||
| Initial | 236 (49.58%) | 244 (24.30%) | 5861 (49.56%) | <.0001 |
| Alpha | 21 (4.41%) | 48 (4.78%) | 710 (6.00%) | |
| Delta | 103 (21.64%) | 207 (20.62%) | 2202 (18.62%) | |
| Omicron | 116 (24.37%) | 505 (50.30%) | 3052 (25.81%) | |
| Days since COVID-19, median (IQR) | 360.0 (129–506) | 129.0 (108–343) | … | <.0001 |
| Hospitalized due to COVID | 7 (1.47%) | … | 29 (0.25%) | .0001 |
| Max number of acute COVID-19 symptoms, mean (SD) | 5.44 ± 2.82 | 3.99 ± 2.39 | 4.08 ± 2.73 | <.0001 |
| 1st vaccine dose before COVID-19 | 213 (44.75%) | 710 (70.72%) | 5111 (43.22%) | <.0001 |
| 1st vaccine dose after COVID-19 | 216 (45.38%) | 211 (21.02%) | 3276 (27.70%) | <.0001 |
| No reported vaccine | 47 (9.87%) | 83 (8.27%) | 3438 (29.07%) | .31 |
| Average days/week physical activity pre-COVID, mean (SD) | 2.49 ± 1.75 | 2.70 ± 1.90 | 2.52 ± 1.87 | .18 |
| Average days/week physical activity post-COVID (all), mean (SD) | 2.20 ± 1.73 | 2.62 ± 1.84 | 2.19 ± 1.84 | .0000 |
| Average days/week physical activity post-COVID (only those with pre-COVID data), mean (SD) | 2.14 ± 1.73 | 2.55 ± 1.83 | 2.17 ± 1.82 | .009 |
| Average hours of sleep/night pre-COVID, mean (SD) | 6.60 ± 0.93 | 6.85 ± 0.81 | 6.79 ± 0.92 | .0001 |
| Average hours of sleep/night post-COVID, mean (SD) | 6.62 ± 0.99 | 6.88 ± 0.86 | 6.83 ± 1.05 | .0000 |
| Average hours of sleep/night post-COVID (only those with pre-COVID data), mean (SD) | 6.68 ± 1.01 | 6.89 ± 0.85 | 6.89 ± 1.03 | .002 |
| BMI, mean (SD) | 30.58 (7.81) | 28.12 (6.91) | 29.47 (7.74) | <.0001 |
| Tobacco use | 29 (6.09%) | 52 (5.18%) | 974 (8.75%) | .47 |
| Marijuana use | 30 (6.30%) | 70 (6.97%) | 953 (8.56%) | .63 |
| Alcoholic drinks/week pre-COVID, mean (SD) | 4.19 ± 5.71 | 4.81 ± 5.59 | 4.35 ± 5.65 | .15 |
| Alcoholic drinks/week post-COVID (only those with pre-COVID data), mean (SD) | 3.77 ± 5.28 | 4.41 ± 5.36 | 3.88 ± 5.35 | .12 |
| Alcoholic drinks/week post-COVID, mean (SD) | 2.99 ± 4.65 | 4.24 ± 5.26 | 3.28 ± 5.03 | <.0001 |
| Financial insecurity pre-COVID | 66 (30.00%) | 92 (13.31%) | 806 (18.58%) | <.0001 |
| Financial insecurity post-COVID (only those with pre-COVID data) | 60 (28.44%) | 72 (11.06%) | 577 (16.09%) | <.0001 |
| Financial insecurity post-COVID | 149 (32.68%) | 131 (13.72%) | 1382 (20.64%) | <.0001 |
| Anxiety (GAD-7) pre-COVID, mean (SD) | 5.83 ± 4.69 | 3.51 ± 3.50 | 4.40 ± 4.41 | <.0001 |
| Anxiety (GAD-7) post-COVID (only those with pre-COVID data), mean (SD) | 5.41 ± 4.82 | 3.19 ± 3.57 | 3.94 ± 4.34 | <.0001 |
| Anxiety (GAD-7) post-COVID, mean (SD) | 5.04 ± 4.66 | 3.08 ± 3.60 | 4.18 ± 4.53 | <.0001 |
| Depression (PHQ-9) pre-COVID, mean (SD) | 6.27 ± 4.72 | 3.61 ± 3.56 | 4.56 ± 4.47 | <.0001 |
| Depression (PHQ-9) post-COVID (only those with pre-COVID data), mean (SD) | 6.36 ± 5.13 | 3.50 ± 3.79 | 4.43 ± 4.58 | <.0001 |
| Depression (PHQ-9) post-COVID, mean (SD) | 5.85 ± 4.91 | 3.31 ± 3.74 | 4.71 ± 4.85 | <.0001 |
| Hypertension | 146 (30.67%) | 270 (26.89%) | 2954 (26.01%) | .11 |
| Diabetes | 40 (8.40%) | 68 (6.77%) | 850 (7.48%) | .23 |
| Coronary artery disease | 28 (5.88%) | 32 (3.19%) | 452 (3.98%) | .05 |
| Heart failure | 6 (1.26%) | 10 (1.00%) | 126 (1.11%) | .75 |
| Stroke or TIA | 14 (2.94%) | 20 (1.99%) | 236 (2.08%) | .002 |
| Atrial fibrillation | 29 (6.09%) | 41 (4.08%) | 424 (3.73%) | .06 |
| Obstructive sleep apnea | 85 (17.86%) | 124 (12.35%) | 1474 (12.98%) | .005 |
| Chronic obstructive pulmonary disease | 27 (5.67%) | 15 (1.49%) | 275 (2.42%) | <.0001 |
| Asthma | 68 (14.29%) | 85 (8.47%) | 1237 (10.89%) | .0009 |
| Cancer | 28 (5.88%) | 74 (7.37%) | 544 (4.79%) | .48 |
| Immunodeficiency | 29 (6.09%) | 20 (1.99%) | 396 (3.49%) | .0002 |
| HIV | 4 (0.84%) | 5 (0.50%) | 71 (0.63%) | .13 |
| Pregnant | 3 (0.63%) | 8 (0.80%) | 152 (1.34%) | .90 |
| COVID before baseline survey | 237 (49.79%) | 274 (27.29%) | 6432 (54.39%) | <.0001 |
Abbreviations: BMI, body mass index; COVID, coronavirus disease 2019; GAD, Generalized Anxiety Disorder; HIV, human immunodeficiency virus; IQR, interquartile range; PHQ, Patient Health Questionnaire; SD, standard deviation; SES, socioeconomic status; TIA, transient ischemic attack.
NOTES: Baseline and post-COVID characteristics among those with Long COVID symptoms, without Long COVID symptoms, and nonresponders. P values are for univariate unadjusted comparison between those with and without Long COVID symptoms reported on the cross-sectional survey using χ2 tests for categorical variables and t tests for continuous variables.
Participants could check all of the above so totals do not add to 100%, and each race/ethnicity was considered separately rather than as a single categorical variable.
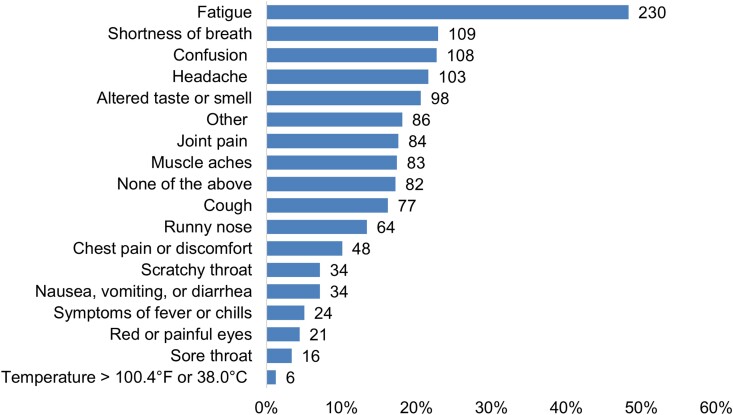
The most common Long COVID symptom was fatigue, reported by 230 of 476 (48.3%) (Figure 1). Other common symptoms included shortness of breath (109, 22.9%), confusion (108, 22.7%) headache (103, 21.6%), and altered taste or smell (98, 20.6%). A minority of participants (62 of 476, 13.0%) reported at least 1 severe or very severe symptom. Healthcare contact about Long COVID was reported by 219 of 476 (46.0%) of participants. Missing work or school due to Long COVID was reported by 124 (26.1%) participants, with 57 (12.0%) missing 1–5 days, 24 (5.0%) missing 6–10 days, and 43 (9.0%) missing 11 days or more.
Figure 1.
Patient-reported symptoms of Long COVID among people reporting symptoms at least 1 month after COVID-19 (N = 476). Numbers to the right of bars represent number of participants reporting that symptom. Participants could report more than 1 symptom. Number and proportion with each symptom.
Long COVID symptoms lasted for varying durations, with approximately half of participants reporting ongoing symptoms (227 of 476, 47.7%) (Table 2). Of participants with COVID-19 at least 1 year before survey completion who reported experiencing Long COVID symptoms, 133 of 237 (56.1%) reported still experiencing symptoms at the time of survey completion.
Table 2.
Duration of Long COVID Symptoms Among People Reporting Symptoms at Least 1 Month After COVID-19
| Duration of Symptoms | All (N = 476) | COVID-19 at Least 3 Months Ago (N = 452) | COVID-19 at Least 1 Years Ago (N = 237) |
|---|---|---|---|
| Less than 3 months | 141 (29.6%) | 132 (29.2%) | 66 (27.8%) |
| 3–4 months | 50 (10.5%) | 46 (10.2%) | 10 (4.2%) |
| 5–6 months | 20 (4.2%) | 20 (4.4%) | 3 (1.3%) |
| More than 6 months | 33 (6.9%) | 33 (7.3%) | 21 (8.9%) |
| I am still having Long COVID symptoms | 227 (47.7%) | 216 (47.8%) | 133 (56.1%) |
| Prefer not to answer | 5 (1.1%) | 5 (1.1%) | 4 (1.7%) |
Abbreviations: COVID, coronavirus disease 2019.
NOTES: Number and proportion reporting each duration of symptoms and those reporting ongoing symptoms by time of initial infection (any time, more than 3 months before survey, and more than 1 year before survey). Having symptoms longer than 3 months is most consistent with the World Health Organization definition [14].
In all 3 multivariable models, the number of acute COVID-19 symptoms during initial infection was associated with prevalent Long COVID symptoms with 1.3 times higher odds per additional acute symptom (95% confidence interval [CI], 1.20–1.40 for Model 3) (Table 3). Variant wave was also associated with prevalent Long COVID symptoms, with later wave participants less likely to have Long COVID symptoms despite shorter follow-up time (median follow up 360 days [IQR, 129–506] among symptomatic, 129 days [IQR, 108–343] among asymptomatic). When models were further adjusted for vaccination before or after COVID, ethnicity, and social determinants of health (subjective socioeconomic status, highest level of education, employment in healthcare), Hispanic ethnicity and lower subjective socioeconomic status were associated with Long COVID, with odds ratios (ORs) of 1.73 (95% CI, 1.02–2.83) and 0.81 per unit higher (95% CI, .73–.91), respectively (Model 2) (Table 3). Vaccination status was not statistically significantly associated with Long COVID symptoms (OR = .81 for preinfection, 95% CI = .44–1.49; OR = 1.57 for postinfection vaccination, 95% CI = .60–4.13). After additional adjustment for anxiety, depression, and financial insecurity, pre-existing depression (OR = 1.08 per point on PHQ-8; 95% CI, 1.01–2.16) and financial insecurity (OR = 1.64; 95% CI, 1.02–2.63) were associated with Long COVID.
Table 3.
Multivariable Models of Factors Potentially Associated With Long COVID
| Model 1 N = 1024 | Model 2 N = 1021 | Model 3 N = 905 | ||||
|---|---|---|---|---|---|---|
| Variable | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Age | 1.00 (.99– 1.01) | .90 | 1.00 (.99–1.02) | .66 | 1.01 (.99–1.03) | .14 |
| Wave | ||||||
| Initial | Reference | Reference | Reference | Reference | Reference | Reference |
| Alpha | 0.44 (.17–1.10) | .08 | .64 (.23–1.77) | .39 | 1.20 (.37–3.90) | .76 |
| Delta | 0.33 (.21–.53) | <.001 | 0.48 (0.22–1.06) | .07 | 0.57 (0.21–1.50) | .25 |
| Omicron | 0.17 (.11–.26) | <.001 | 0.25 (.12–0.54) | <.001 | 0.37 (.15–.90) | .04 |
| Number of initial symptoms (per symptom) | 1.27 (1.20–1.35) | <.001 | 1.27 (1.20–1.35) | <.001 | 1.30 (1.20–1.40) | <.001 |
| Female sex | 1.24 (.88–1.77) | .22 | 1.16 (.80–1.66) | .44 | .86 (.57–1.29) | .47 |
| Myocardial infarction | 0.58 (.27–1.25) | .16 | 0.63 (.29–1.37) | .24 | 0.57 (.23–1.39) | .21 |
| Stroke | 0.82 (.30–2.19) | .69 | 0.96 (.34–2.68) | .94 | 1.46 (.47–4.52) | .51 |
| Atrial fibrillation | 0.51 (.26–1.01) | .05 | 0.47 (0.23–0.96) | .04 | 0.61 (.28–1.31) | .20 |
| Sleep apnea | 1.00 (.63–1.58) | .99 | 1.07 (0.67–1.70) | .79 | 1.14 (.68–1.89) | .63 |
| COPD or asthma | 0.79 (.17–3.68) | .76 | 0.87 (.18–4.14) | .86 | 0.89 (.17–4.80) | .89 |
| Immunodeficiency | 0.54 (.27–1.07) | .08 | 0.51 (.25–1.03) | .06 | 0.57 (.26–1.28) | .18 |
| Vaccination before COVID-19 | N/A | N/A | 0.85 (.49–1.49) | .57 | 0.81 (.44–1.49) | .50 |
| Vaccination after COVID-19 | N/A | N/A | 1.41 (.65–3.06) | .39 | 1.57 (.60–4.13) | .36 |
| Hispanic ethnicity | N/A | N/A | 1.70 (1.02–2.83) | .04 | 1.73 (.95–3.14) | .07 |
| Subjective socioeconomic status (per unit higher) | N/A | N/A | 0.81 (.73–.91) | <.001 | 0.90 (.79–1.03) | .12 |
| Highest education | N/A | N/A | ||||
| No high school | N/A | N/A | N/A | N/A | N/A | N/A |
| High school graduate | N/A | N/A | 0.72 (.12–4.40) | .72 | 0.69 (0.08–6.03) | .74 |
| College degree | N/A | N/A | 0.84 (.22–3.25) | .80 | 1.17 (.23–5.97) | .85 |
| Graduate degree | N/A | N/A | 0.73 (.19–2.86) | .65 | .99 (.19–5.12) | .99 |
| Other | N/A | N/A | Reference | Reference | Reference | Reference |
| Healthcare worker | N/A | N/A | 1.02 (.98–1.06) | .28 | 1.02 (.98–1.07) | .25 |
| Pre-COVID-19 Depressive symptoms | N/A | N/A | N/A | N/A | 1.08 (1.01–1.16) | .03 |
| Pre-COVID-19 anxiety symptoms | N/A | N/A | N/A | N/A | 1.04 (.97–1.12) | .25 |
| Pre-COVID-19 financial insecurity | N/A | N/A | N/A | N/A | 1.64 (1.02–2.63) | .04 |
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID, coronavirus disease 2019; N/A, not applicable; OR, odds ratio.
Model 1 includes age, COVID-19 wave, number of initial symptoms, sex, myocardial infarction, stroke, atrial fibrillation, sleep apnea, COPD or asthma, and immunodeficiency.
Model 2 includes Model 1 factors, plus receipt of COVID-19 vaccination before or after COVID-19 diagnosis, ethnicity, subjective socioeconomic status, highest level of education, and primary employment.
Model 3 includes Model 2 factors, plus pre-COVID-19 depressive symptoms, anxiety symptoms, and financial insecurity.
Among those with pre-COVID baseline data, there was a greater decrease in frequency of physical activity after COVID-19 among those with Long COVID (difference in change between symptomatic and asymptomatic: 0.19 days/week, 95% CI = .04–.35, P = .02), but no differences in change in sleep duration, alcohol intake, anxiety, or depression by Long COVID status (Supplementary Table 1). For both groups (with and without Long COVID), physical activity, alcohol intake, and anxiety scores were lower and average sleep was longer post-COVID compared with pre-COVID (Supplementary Table 1).
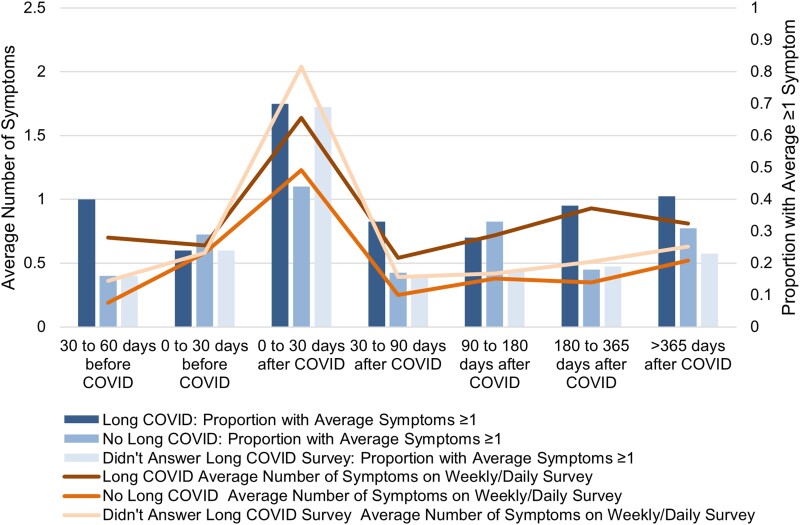
Among those who responded to daily or weekly survey requests including nonrespondents to the cross-sectional Long COVID survey, we plotted the average number of symptoms reported (line) and the proportion who reported an average of 1 or more symptoms over all surveys answered (bars) during time periods before and after SARS-CoV-2 infection for those infected (Figure 2). The estimated proportion reporting an average of 1 or more symptoms on all surveys was highest in the 0–30 days after acute infection in all groups. The estimated proportion with an average of 1 or more symptom across completed surveys for each time period was generally higher among those reporting Long COVID symptoms (at 180–365 days: 38% vs 18% without Long COVID symptoms) and substantially higher than among individuals without SARS-CoV-2 infection (n = 57 415, 11%). Of note, in the period 30–60 days before SARS-CoV-2 infection, those who later reported Long COVID symptoms reported a higher number of symptoms compared to those who did not ultimately report Long COVID symptoms (0.70 ± 1.06 vs 0.19 ± 0.47), with a similar pattern in the proportion reporting an average of 1 or more symptoms (40% vs 16%). Similarly, the average number of symptoms was higher among those reporting Long COVID (at 180–365 days: 0.81 ± 1.81) compared to those not reporting Long COVID symptoms (0.35 ± 0.98) and those without SARS-CoV-2 infection (n = 57 415, 0.32 ± 0.60).
Figure 2.
Average number of symptoms and proportion with symptoms on the weekly/daily surveys during each time period. We found higher proportions with symptoms and a higher number of symptoms reported among those with Long COVID. Of note, symptoms queried on the daily/weekly surveys included whether participants had 1 or more of the following: a scratchy throat, a painful sore throat, a cough, a runny nose, symptoms of fever or chills, a temperature >100.4°F or 38.0°C, muscle aches, nausea, vomiting, or diarrhea, shortness of breath, unable to taste or smell, and red or painful eyes. These symptoms are more typical during acute infection so some individuals with Long COVID did not have any of these symptoms but still reported Long COVID symptoms.
Sensitivity Analyses
First, we considered whether selection bias from those who joined the study and completed baseline surveys after SARS-CoV-2 infection impacted the findings. Among 969 individuals with preinfection baseline surveys, 239 (25%) reported Long COVID symptoms compared with 237 of 511 who completed baseline surveys after SARS-CoV-2 infection (46%; P < .0001), which suggests that individuals with Long COVID symptoms after SARS-CoV-2 infection were more likely to participate than those without Long COVID symptoms, consistent with selection bias. More importantly, mean levels of reported physical activity, sleep duration, alcohol intake, financial insecurity, depression, and anxiety were similar between those with baseline surveys obtained before SARS-CoV-2 infection and those who joined the cohort after infection, which suggests that differential recall is unlikely to bias these variables (Supplementary Table 2). The general pattern of results for the multivariable models were unchanged when only individuals with baseline surveys before infection (n = 239 with and n = 730 without symptoms) were included (Supplementary Table 3).
Second, we considered only persistent symptoms (n = 227) compared to those with no symptoms (n = 1004), excluding those whose symptoms resolved (Supplementary Table 3). Overall results were similar with 1 exception: female sex was associated with persistent symptoms (OR = 1.34; 95% CI, 1.21–1.49) (Supplementary Table 3). We also compared those with severe or very severe symptoms (n = 62) to those without symptoms (n = 1004), with no substantive differences in our findings (Supplementary Table 3).
Third, we excluded 1 individual with Long COVID with only a positive antibody test and 63 individuals (11 with Long COVID and 52 without) with unknown test type; including those with only PCR or antigen testing did not result in any substantive changes in our findings (Supplementary Table 3).
DISCUSSION
In this cross-sectional assessment of mostly nonhospitalized individuals who reported prior SARS-CoV-2 infection in the COVID Citizen Science online cohort, persistent symptoms including fatigue, shortness of breath, headache, brain fog/confusion, and altered taste/smell were highly prevalent. A minority of participants reported severe or very severe symptoms, but half of participants infected more than 1 year earlier reported ongoing symptoms. We found that preinfection socioeconomic status, financial insecurity, and depression assessed before SARS-CoV-2 infection were associated with Long COVID symptoms. We also found that the number of symptoms during acute infection was associated with reporting Long COVID symptoms independent of vaccination and variant wave, and that more recent variant waves are associated with lower odds of Long COVID even after adjusting for vaccine status.
Comparison of Symptom Patterns and Persistence
Our findings of common symptoms (fatigue, shortness of breath, confusion, and headache) are consistent with prior reports [1–3]. Similarly, we found that symptoms were persistent for more than 12 months among approximately half of those infected with SARS-CoV-2 who reported symptoms lasting at least 1 month. This is consistent with the prior literature that symptoms present for more than 3 months tend not to self-resolve [1], but it is higher than estimates that only 15% of individuals with symptoms at 3 months continue to have symptoms beyond 1 year [2].
Risk of Long COVID by Symptoms and Variant Wave
The number of symptoms during acute infection was associated with Long COVID, consistent with prior reports that acute illness severity is associated with Long COVID [8]. This raises the question of whether reducing acute symptoms through acute treatment might modify the risk of developing Long COVID. A second interesting finding is that the variant wave is associated with Long COVID symptoms even with adjustment for timing of vaccination (preinfection, postinfection, or not vaccinated) and number of symptoms during acute infection; more recent variants were associated with lower odds of Long COVID. One prior study suggested that there may be some subtle differences in Long COVID symptoms by variant wave (more dyspnea with ancestral strain, more neuropsychiatric and myalgic symptoms with Alpha, and hair loss with Delta for example) [23, 24]. Our findings are consistent with 3 prior studies that suggested that there may be a lower prevalence of Long COVID with the more recent variant waves (Epsilon, Omicron) [15, 25, 26].
Demographics, Social Determinants of Health, and Long COVID
Even within a relatively homogenous cohort, we found that Hispanic ethnicity, lower socioeconomic status, and financial insecurity were associated with Long COVID symptoms. In contrast to prior reports, we did not identify female sex as associated with Long COVID symptoms after adjustment, except in sensitivity analysis of persistent symptoms [10, 28]. Despite extensive research documenting the role of adverse social determinants of health increasing risk of acute SARS-CoV-2 infection, there are limited prior data regarding associations between social determinants and Long COVID. Consistent with our study, one prior study found that financial concerns were associated with worse health utility and quality of life among those recovering from COVID-19 [27]. Similarly, an online survey-based study found that graduate education and urban residence were associated with lower odds of Long COVID [15]. The implications are that clinical trials of potential therapeutics should make intentional efforts to include those at highest risk, including those of lower socioeconomic status, and social determinants of health should be considered in public health approaches to address Long COVID.
Depression, Anxiety and Long COVID
In our adjusted analysis, pre-COVID depression was associated with Long COVID symptoms. More importantly, we found that anxiety and depression scores did not increase after COVID among those with preinfection baseline surveys, suggesting that individuals with depression may be at elevated risk of Long COVID. One prior study from 3 large cohorts identified that depression, anxiety, perceived stress, and loneliness measured before the pandemic were associated with post-COVID conditions, although we found that anxiety decreased after SARS-CoV-2 infection among those with and without Long COVID [29]. Our findings are consistent with studies which lack preinfection assessments that have found concurrent depression or anxiety to be associated with Long COVID symptoms [10, 11, 28, 30, 31]. Further research into mechanisms of how depression may be an antecedent factor to Long COVID are needed. Fluvoxamine, a selective serotonin reuptake inhibitor, may have protective effects in acute SARS-CoV-2 infection [32, 33], but understanding whether treatment with antidepressants or naltrexone [34] may prevent or treat Long COVID requires clinical trials [35].
Strengths and Limitations
Strengths of this online cohort are a large sample size, data collection before infection in many participants, and data from participants infected during different variant waves; most prior studies predominantly included individuals infected with earlier variants. The primary limitation arises from responder bias: those with Long COVID are more likely to respond to surveys, although over 1000 individuals without symptoms also responded. This may induce bias in our estimate of Long COVID symptom prevalence but is less likely to bias ascertainment of factors associated with Long COVID. Those who reported Long COVID symptoms had a higher number of symptoms and a higher proportion reporting 1 or more symptoms in the 30–60 days pre-COVID, which may represent differences in survey response or true differences in prevalence of symptoms. The second limitation is that a subset of individuals joined the study after SARS-CoV-2 infection, limiting the ability for prospective comparisons to those who had already joined the study. Symptoms may be misclassified as attributable to Long COVID: specifically, some may be attributable to specific medical conditions rather than Long COVID, and some may have been present before SARS-CoV-2 infection (as suggested by the higher proportion with symptoms on the longitudinal surveys 30–60 days pre-COVID among those who would go on to report Long COVID symptoms). In our primary analysis, we included a small number of individuals with unknown test type and one individual with only antibody testing, but results were similar when we limited our sample to those with PCR or antigen testing. We did not assess the effect of repeat SARS-CoV-2 infection. Finally, external generalizability may be limited because the study sample overrepresented those who identify as White, female, and of higher socioeconomic status.
CONCLUSIONS
In conclusion, in this cross-sectional assessment of Long COVID symptoms within an online cohort, we found that Long COVID symptoms were highly prevalent and commonly persisted, consistent with prior reports. We found that having more symptoms during acute infection, lower socioeconomic status, financial stress, and pre-COVID depression were associated with Long COVID. Finally, Long COVID symptoms were less prevalent among those infected with recent variants even accounting for vaccination status and acute symptoms.
Supplementary Material
Acknowledgments
Financial support. Eureka Research Platform is supported by National Institutes of Health (NIH)/National Institute of Biomedical Imaging and Bioengineering (NIBIB ) Grant 3U2CEB021881-05S1. The COVID-19 Citizen Science Study is supported by Patient-Centered Outcomes Research Institute Contract COVID-2020C2-10761 and Bill & Melinda Gates Foundation Contract INV-017206. MSD is supported by NIH/National Heart, Lung, and Blood Institute Grant K12HL143961. MJPe is supported by NIH/National Institute of Allergy and Infectious Diseases Grant K23AI157875.
Contributor Information
Matthew S Durstenfeld, Division of Cardiology at ZSFG, and Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Michael J Peluso, Division of HIV, Infectious Disease, Global Medicine, University of California, San Francisco, San Francisco, California, USA.
Noah D Peyser, Division of Cardiology, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Feng Lin, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, California, USA.
Sara J Knight, Division of Epidemiology, Department of Internal Medicine, University of Utah, Salt Lake City, Utah, USA.
Audrey Djibo, CVS Health Clinical Trial Services, Blue Bell, Pennsylvania, USA.
Rasha Khatib, Advocate Aurora Research Institute, Milwaukee, Wisconsin, USA.
Heather Kitzman, Baylor Scott and White Health and Wellness Center, Dallas, Texas, USA.
Emily O’Brien, Department of Population Health Sciences, Duke University School of Medicine, Durham, North Carolina, USA.
Natasha Williams, Institute for Excellence in Health Equity, Center for Healthful Behavior Change, NYU Grossman School of Medicine, New York, New York, USA.
Carmen Isasi, Department of Epidemiology & Population Health, Albert Einstein College of Medicine, Bronx, New York, USA.
John Kornak, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, California, USA.
Thomas W Carton, Louisiana Public Health Institute, New Orleans, Louisiana, USA.
Jeffrey E Olgin, Division of Cardiology, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Mark J Pletcher, Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, California, USA.
Gregory M Marcus, Division of Cardiology, Department of Medicine, University of California, San Francisco, San Francisco, California, USA.
Alexis L Beatty, Division of Cardiology, Department of Medicine, University of California, San Francisco, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California, San Francisco, San Francisco, California, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Peluso MJ, Kelly JD, Lu S, et al. Persistence, magnitude, and patterns of postacute symptoms and quality of life following onset of SARS-CoV-2 infection: cohort description and approaches for measurement. Open Forum Infect Dis 2022; 9:ofab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Burden of Disease Long COVID Collaborators; Hanson WS, Abbafati C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022; 328:1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Groff D, Sun A, Ssentongo AE, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 2021; 4:e2128568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hirschtick JL, Titus AR, Slocum E, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis 2021; 73:2055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021; 18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayoubkhani D, Pawelek P, Gaughan C. Technical article: Updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK: 26 April 2020 to 1 August 2021. UK Office for National Statistics, Technical Article. London 2021. Released 16 September 2021. Accessed 29 April 2022. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021
- 7. Yomogida K, Zhu S, Rubino F, Figueroa W, Balanji N, Holman E. Post-acute sequelae of SARS-CoV-2 infection among adults aged ≥18 years - Long Beach, California, April 1-December 10, 2020. MMWR Morb Mortal Wkly Rep 2021; 70:1274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie Y, Bowe B, Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun 2021; 12:6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022; 28:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hastie CE, Lowe DJ, McAuley A, et al. Outcomes among confirmed cases and a matched comparison group in the long-COVID in Scotland study. Nat Commun 2022; 13:5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li D, Liao X, Liu Z, et al. Healthy outcomes of patients with COVID-19 two years after the infection: a prospective cohort study. Emerg Microbes Infect 2022; 11:2680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang Y, Pinto MD, Borelli JL, et al. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler looking for clarity in the haze of the pandemic. Clin Nurs Res 2022; 31:1390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hill E, Mehta H, Sharma S, et al. Risk Factors Associated with Post-Acute Sequelae of SARS-CoV-2 in an EHR Cohort: A National COVID Cohort Collaborative (N3C) Analysis as part of the NIH RECOVER program [preprint]. medRxiv. 2022. doi: 10.1101/2022.08.15.22278603. [DOI]
- 14. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; 594:259–64. [DOI] [PubMed] [Google Scholar]
- 15. Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open 2022; 5:e2238804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Q, Ailshire JA, Crimmins EM. Long COVID and symptom trajectory in a representative sample of Americans in the first year of the pandemic. Sci Rep 2022; 12:11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beatty AL, Peyser ND, Butcher XE, et al. The COVID-19 citizen science study: protocol for a longitudinal digital health cohort study. JMIR Res Protoc 2021; 10:e28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147:573–7. [DOI] [PubMed] [Google Scholar]
- 19. Lauring AS, Tenforde MW, Chappell JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, COVID-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ 2022; 376:e069761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009; 114:163–73. [DOI] [PubMed] [Google Scholar]
- 21. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder. Arch Intern Med 2006; 166:1092. [DOI] [PubMed] [Google Scholar]
- 22. Adler N, Singh-Manoux A, Schwartz J, Stewart J, Matthews K, Marmot MG. Social status and health: a comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Soc Sci Med 2008; 66:1034–45. [DOI] [PubMed] [Google Scholar]
- 23. Fernández-de-Las-Peñas C, Cancela-Cilleruelo I, Rodríguez-Jiménez J, et al. Associated-onset symptoms and post-COVID-19 symptoms in hospitalized COVID-19 survivors infected with Wuhan, alpha or Delta SARS-CoV-2 variant. Pathogens 2022; 11(7). doi: 10.3390/pathogens11070725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spinicci M, Graziani L, Tilli M, et al. Infection with SARS-CoV-2 variants is associated with different long COVID phenotypes. Viruses 2022; 14:2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022; 399:2263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morioka S, Tsuzuki S, Suzuki M, et al. Post COVID-19 condition of the omicron variant of SARS-CoV-2. J Infect Chemother 2022; 28:1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Case KR, Wang CP, Hosek MG, et al. Health-related quality of life and social determinants of health following COVID-19 infection in a predominantly Latino population. J Patient Rep Outcomes. 2022; 6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sneller MC, Liang CJ, Marques AR, et al. A longitudinal study of COVID-19 sequelae and immunity: baseline findings. Ann Intern Med. 2022; 175:969–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S, Quan L, Chavarro JE, et al. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post-COVID-19 conditions. JAMA Psychiatry. 2022; 79:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mazza MG, Palladini M, Villa G, De Lorenzo R, Rovere Querini P, Benedetti F. Prevalence, trajectory over time, and risk factor of post-COVID-19 fatigue. J Psychiatr Res 2022; 155:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margalit I, Yelin D, Sagi M, et al. Risk factors and multidimensional assessment of long COVID fatigue: a nested case-control study. Clin Infect Dis 2022; 75:1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA 2020; 324:2292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022;10:e42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Kelly B, Vidal L, McHugh T, Woo J, Avramovic G, Lambert JS. Safety and efficacy of low dose naltrexone in a long COVID cohort; an interventional pre-post study. Brain Behav Immun Health 2022; 24:100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonnet U, Juckel G. COVID-19 outcomes: does the use of psychotropic drugs make a difference? Accumulating evidence of a beneficial effect of antidepressants-A scoping review. J Clin Psychopharmacol 2022; 42:284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.