Abstract
Objective:
This study sought to determine whether gastric symptoms are associated with later eating disorder (ED) symptoms during early adolescence, and whether this relationship is moderated by parental warmth/acceptance and/or the child's sex.
Method:
Longitudinal data from the Adolescent Brain Cognitive DevelopmentSM Study were utilized. Participants ages 9–10 years old (N = 4,950; 2,370 female) completed measures at baseline and 1 year later (Y1). At baseline, gastric symptoms were measured by parent-reported items from the Child Behavior Checklist (CBCL), and perceived parental acceptance was measured by youth report on the Children's Report of Parent Behavior Inventory (CRPBI) Acceptance subscale separately for mothers and fathers. ED symptoms at Y1 were assessed by parent report on a computerized version of the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS). Linear mixed-effects models were conducted separately for maternal and paternal acceptance to test relationships among variables.
Results:
A three-way interaction between baseline gastric symptoms, sex, and maternal acceptance predicted Y1 ED symptoms (β = 0.08; p < .01). Post-hoc analyses revealed that the interaction between gastric symptoms and maternal acceptance was significant for girls only (β = −0.06, p < .01), such that low maternal acceptance was associated with a stronger relationship between baseline gastric symptoms and Y1 ED symptoms. No statistically significant main effects or interactions were found in the model for paternal acceptance.
Discussion:
Gastric symptoms and low perceived maternal acceptance may interact to result in heightened risk for EDs in young adolescent girls.
Keywords: adolescents, eating disorders, females, gastrointestinal symptoms, maternal acceptance, parenting, paternal acceptance, risk factors, sex
1 ∣. INTRODUCTION
Eating disorders (EDs) typically arise in adolescence (Hudson, Hiripi, Pope Jr, & Kessler, 2007; Volpe et al., 2016) and are associated with significant physical and mental health risks. Gastrointestinal (GI) complaints are common among individuals diagnosed with EDs (Boyd, Abraham, & Kellow, 2005). Adults diagnosed with EDs retrospectively report having experienced childhood GI complaints, and these childhood GI symptoms are associated with earlier onset and severity of the ED (Gendall, Joyce, Carter, McIntosh, & Bulik, 2005). Parenting styles may also influence the development of EDs during adolescence, perhaps differently in females and males (Tata, Fox, & Cooper, 2001). However, due to the reliance on retrospective reports and the effects of abnormal eating behaviors on GI functioning, it is unclear whether pre-adolescent GI symptoms may be associated with a higher risk for EDs in adolescence and how parenting styles may influence this association.
1.1 ∣. GI complaints and EDs
Many people diagnosed with EDs have frequent GI complaints, which include bloating, abdominal pain, feeling overly “full,” vomiting, diarrhea, and constipation (Dalton, 2017). GI symptoms are associated with a number of other psychiatric conditions (Lee et al., 2017; North, Alpers, Thompson, & Spitznagel, 1996) and are exacerbated by stress, anxiety, and other mental health problems (Bhatia & Tandon, 2005; Van Oudenhove, Törnblom, Störsrud, Tack, & Simrén, 2016). The directionality of the relationship between GI complaints and EDs is unclear. Although there is evidence to suggest that childhood GI complaints may increase an individual's risk for subsequently developing an ED, this can be challenging to assess given the documented GI sequelae of EDs.
GI complaints are often reported by patients with anorexia nervosa (AN) as a major reason for restricting food, which may also contribute to prolonged disease course (Lee, Lee, Ngai, Lee, & Wing, 2001). One study reported that in their sample of women with bulimia nervosa (BN), over 30% retrospectively reported GI complaints in childhood, and these complaints were associated with earlier onset and higher severity of the illness in adulthood (Gendall et al., 2005). Similarly, a recent review also found that GI disorders treated through dietary means (e.g., by removing certain foods from the diet deemed problematic to the GI system) in childhood increased the risk for later disordered eating (Conviser, Fisher, & McColley, 2018). Conversely, GI complications may be induced or exacerbated by malnutrition, purging, or the re-introduction of food to the GI tract. In a sample of 101 inpatient females receiving treatment for EDs, 98% of them reported GI symptoms, with symptoms related to irritable bowel syndrome (IBS) being most common (Boyd et al., 2005). In another sample of 234 participants with current or past ED, 64% reported IBS symptoms, with the average onset of GI issues being a decade later than the onset of the ED (Perkins, Keville, Schmidt, & Chalder, 2005). Reports of the persistence of GI-related complications after recovery from an ED are mixed, with some evidence that they decrease simultaneously with ED symptoms (Norris et al., 2016), and other research pointing toward sustained GI problems even as ED symptoms remit (Boyd, Abraham, & Kellow, 2010).
Taken together, while GI problems may be a physical consequence of disordered eating behaviors (e.g., caloric restriction, binge eating, purging), there is also evidence to suggest that childhood GI complaints, regardless of cause, are associated with risk for developing an ED. GI discomfort in childhood may lead to aversive visceral conditioning, increasing vulnerability to AN in some individuals (Zucker & Bulik, 2020). Recent studies have demonstrated brain-related abnormalities in interoceptive processing in individuals with EDs (Berner et al., 2018; Kerr et al., 2016; Kerr, Moseman, Avery, Bodurka, & Simmons, 2017; Strigo et al., 2013). Prospective research is necessary to clarify the nature of the relationship between GI complaints and EDs. Large, population-based studies of development may provide an opportunity to examine whether pre-adolescent GI symptoms are associated with a higher risk for ED development in adolescence.
1.2 ∣. Parenting, EDs, and sex differences
While it is well-established that parents do not directly cause EDs (Academy for Eating Disorders, 2015; Schaumberg et al., 2017), it may be that parenting and other familial and social factors may be protective or increase risk of EDs in children who are already susceptible due to other factors (e.g., GI symptoms, anxiety, etc.), and may thus provide a modifiable avenue to inform prevention efforts (Le Grange, Lock, Loeb, & Nicholls, 2010). Parenting styles are a parent's consistent pattern of responding within various contexts of the parent–child relationship. They are often described as a balance between demand-ingness and responsiveness with variable intensities of behaviors such as warmth, autonomy-granting, or behavioral/psychological control (Gorostiaga, Aliri, Balluerka, & Lameirinhas, 2019). The nature of these parent–child interactions can heavily influence a child's life-long socioemotional outcomes. The application of adaptive parenting styles (e.g., warm, accepting, and responsive) are critical for a child's healthy socioemotional development, whereas maladaptive styles (e.g., demanding, harsh, or neglectful) are often associated with various child psychopathologies. For instance, a recent systematic review reported that negative parenting styles were generally associated with higher reports of adolescent anxiety, depression, and suicidal ideation, whereas positive parenting styles were associated with lower reports of these same internalizing symptoms (Gorostiaga et al., 2019). Although research is sparse, there is also evidence that parenting styles may be associated with the development of EDs. For instance, one study reported that less adaptive parenting styles were related to ED symptoms in non-clinical individuals (Haycraft & Blissett, 2010). Of note, differential results have been reported regarding the association between parenting and child psychopathology based on the sex of both the parent and child (Franz & McKinney, 2018). For example, a longitudinal study found that maternal acceptance predicted reduced weight concerns (a risk factor of developing an ED) in adolescents, whereas conflicts between the adolescent and father predicted increased weight concerns (Lam & McHale, 2012). Regarding the sex of the child, one study reported that maternal and paternal overprotection were associated with low body satisfaction for both male and female children, but was related to disordered eating for females only (Tata et al., 2001). Thus, given the influence of parenting behaviors on child psychopathology, parenting is an important element to consider as a potential risk factor for ED. However, as noted by Le Grange et al. (2010), the existing longitudinal research on the effects of parenting and family functioning in the subsequent development of EDs is inconsistent and methodologically flawed. Thus, prospective research is necessary to understand the potential role of parenting factors in the development of EDs.
1.3 ∣. Current study
Given findings from past research, we sought to elucidate potential risk factors for ED onset using the large, population-based, longitudinal Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®). Our primary aim was to address whether gastric symptoms in pre-adolescent children are associated with ED symptoms 1 year later. Our secondary, exploratory aim was to examine potential moderating influences of the child's sex and parental acceptance.
2 ∣. METHOD
2.1 ∣. Participants
The ABCD Study (N = 11,875; Jernigan, Brown, & Coordinators, 2018) is a 10-year, population-based study that tracks developmental changes through adolescence across 21 sites in the United States. Each year, children and their parents/guardians undergo a comprehensive range of assessments of mental and physical health (https://abcdstudy.org). The current report examines participants from the curated dataset from the National Data Archive ABCD Data Release 2.0.1 which includes the complete baseline sample (age: 9–10 years; data collected between 2016 and 2018) and a subsample of the 1-year follow-up (Y1; age: 10–11 years; data collected between 2017 and 2019) assessments (Yang & Jernigan, 2019). In order to address longitudinal changes, we included the subset of participants (n = 4,950) with both baseline and Y1 assessment results available at the time of analysis.
ABCD Study procedures are approved by a central institutional review board and conducted in accordance with the Declaration of Helsinki. Parents/caregivers provided written informed consent and children provided assent for study participation.
2.2 ∣. Measures
2.2.1 ∣. Demographics (parent report)
Height and weight were measured in order to calculate body mass index (BMI).
Using the PhenX survey toolkit (Hamilton et al., 2011), parents reported their child's sex, race, and ethnicity. In the curated dataset, the race and ethnicity information were collapsed into a “race/ethnicity” variable with five categories: Asian, Black, Hispanic, White, and Other. Parents also reported their household income, highest level of education, and marital status (see Table 1).
TABLE 1.
Sample demographics
| n | |
|---|---|
| Sex | |
| Female | 2,370 |
| Male | 2,580 |
| Race/ethnicity | |
| Asian | 114 |
| Black | 464 |
| Hispanic | 941 |
| White | 2,942 |
| Other | 489 |
| Parent education | |
| <H.S diploma | 182 |
| H.S. diploma/GED | 347 |
| Some college | 1,208 |
| Bachelor's degree | 1,356 |
| Post-graduate degree | 1,851 |
| Household income | |
| $50k or below | 1,133 |
| $50k–$100k | 1,392 |
| $100k or above | 2059 |
| Parent marital status | |
| Married or living together | 3,800 |
| Not married or living together | 1,133 |
2.2.2 ∣. ED symptoms (parent report)
As part of the baseline and Y1 assessments, parents completed an adapted, computerized version of the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS; Kaufman, Townsend, & Kobak, 2017). The K-SADS contains seven items assessing current ED symptoms, to which the parent responds “yes” or “no” with regard to their child. In the computerized K-SADS, some items were used as a screener, with subsequent items automatically coded “no” and not administered if no screening items were endorsed. To create an ED symptom composite score, the number of ED items answered in the affirmative were summed. Items include both psychological (e.g., “Self-worth tied to weight”) and behavioral (e.g., “Inappropriate compensatory behaviors to prevent weight gain”) symptoms. Youth did not report their own ED symptoms at the baseline or Y1 assessments. Please see the Supporting Information for the list of items included in this scale.
2.2.3 ∣. Gastric symptoms (parent report)
Parents completed the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001) at the baseline assessment. The CBCL contains a subscale addressing any physical problems with no known cause that occurred any time in the 6 months prior to assessment. Three items on this subscale address nausea, stomachaches, and vomiting. Parents' responses to these three items were on a 3-point Likert scale (“Not true,” “Somewhat/Sometimes true,” “Very true/Often true”) and were summed to create a composite score reflecting youth gastric symptoms at baseline.
2.2.4 ∣. Parental acceptance (youth report)
At the baseline assessment, youth completed the Acceptance subscale of the Children's Report of Parent Behavior Inventory (CRPBI; Schludermann & Schludermann, 1988) which consists of five items reflecting parental warmth and acceptance. Youth rated each item on a Likert scale ranging from 1 (“Not like him/her”) to 3 (“A lot like him/her”). The subscale is scored by calculating the mean of the five items. Youth completed the measure regarding the parent/caregiver who participated in the study assessment with them. They were then asked if there is a second caregiver they spend a significant amount of time with. If they responded yes, they were asked the relation of that caregiver to themselves (e.g., mother, father, grandparent) and then completed the CRPBI again in reference to the second caregiver. Therefore, all participants completed the CRPBI at least once, and most participants completed it a second time for a second caregiver.
For our measures of paternal (CRPBI-P) and maternal (CRPBI-M) acceptance, data were included if the CRPBI was completed for the focal parent. In order for each participant's data to only be included once for each parent, preference was given to the primary/study caregiver. For example, if the parent completing the study with the child was reported as their biological mother, but the child also indicated the secondary caregiver was their mother, only the score of the primary/study caregiver (i.e., first CRPBI completed) was included. This procedure resulted in sample sizes of 3,040 for CRPBI-P and 4,146 for CRPBI-M at baseline.
2.2.5 ∣. Anxiety (parent report)
The CBCL includes a DSM-oriented scale (items consistent with the related DSM-5 categories) assessing anxiety symptoms. Parents responded to each CBCL item on a Likert scale ranging from 1 (“Not true”) to 3 (“Very true/Often true”). We used T-scores (normed for age and sex) from the Anxiety Problems Subscale as a measure of anxiety symptoms at baseline.
2.3 ∣. Statistical analyses
Linear mixed-effects models were conducted using the lme4 package (Bates, Maechler, Bolker, & Walker, 2015) in R (R Core Team, 2019). Models included random effects for family and ABCD site. In order to determine which covariates to include in our models, we first selected demographic variables typically used as covariates in analyses of the ABCD Study data (Y1 age in months, race/ethnicity, parent education, household income, parent marital status) as well as variables that are known to be related to GI and ED symptoms (baseline CBCL anxiety T-scores, BMI). We then conducted linear mixed-effects models for each of these variables to determine if they were related to our predictor (gastric symptoms, maternal acceptance, paternal acceptance) and/or outcome (ED symptoms) variables. We thus analyzed four models for each potential covariate, including random effects for family and ABCD site. We retained covariates associated with both the outcome and at least one predictor. Using this procedure, parent education and Y1 age were eliminated as covariates, as they were not associated with any of our predictor or outcome variables. BMI was also excluded, as it was associated with ED symptoms but not with any predictor variables.
We then examined the relationships among baseline gastric symptoms, parental acceptance, and sex in the prediction of ED symptoms at Y1. Each model included sex, gastric symptoms, maternal or paternal acceptance scores, and the interactions among these variables as predictors. Models were conducted separately for maternal and paternal acceptance due to multicollinearity between these measures. Models included random effects for family and ABCD site. Baseline ED symptoms, baseline CBCL anxiety T-scores, race/ethnicity, household income, and parent marital status were included as covariates in each model. Only participants with complete data for the variables in each model were included. The R package MuMIn (Bartoń, 2020) was used to estimate R2 values for each model, and the package effectsize (Ben-Shachar, Makowski, & Lüdecke, 2020) was used to obtain standardized regressors. R code for variable creation and analyses is available at https://github.com/klkerr/abcd_ed_gi.
3 ∣. RESULTS
3.1 ∣. Descriptive statistics
Descriptive statistics for continuous predictors and covariates are shown in Table 2. Changes for most variables from baseline to Y1 were statistically significant, likely due to the large sample size. All effect sizes were small or negligible. Change in BMI reflects typical physical growth. Correlations among the continuous predictor variables, covariates, and relevant demographic variables (all baseline measures) are displayed in Table 3. Maternal and paternal acceptance scores were moderately correlated, as were gastric symptoms and anxiety. All other correlations were negligible.
TABLE 2.
Descriptive statistics and changes from baseline to Year 1
| Baseline M (SD) | Year 1 M (SD) | t | p | Cohen's d | |
|---|---|---|---|---|---|
| Age (months) | 119.87 (7.39) | 132.01 (7.62) | |||
| BMI | 18.51 (3.79) | 19.42 (4.17) | −37.13 | <.001 | −0.23 |
| Gastric symptoms | 3.42 (0.81) | 3.38 (0.79) | −2.99 | .003 | −0.04 |
| CBCL anxiety T-score | 53.41 (5.99) | 53.38 (5.94) | 0.31 | .76 | 0.00 |
| CRPBI-M | 2.81 (0.28) | 2.83 (0.27) | 4.83 | <.001 | 0.07 |
| CRPBI-P | 2.69 (0.36) | 2.77 (0.31) | 13.10 | <.001 | 0.23 |
| ED symptoms | 0.19 (0.51) | 0.22 (0.58) | 2.43 | .02 | 0.04 |
Abbreviations: BMI, body mass index; CBCL, Child Behavior Checklist; CRPBI, Children's Report of Parent Behavior Inventory, Acceptance subscale, mother (CRPBI-M) or father (CRPBI-P) as reference parent; ED, eating disorder.
TABLE 3.
Pearson's correlations between baseline predictor and demographic variables
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. BMI | 1 | |||||
| 2. Gastric symptoms | .01 | 1 | ||||
| 3. CBCL anxiety T-score | −.01 | .31** | 1 | |||
| 4. CRPBI-M | −.02 | .02 | −.02 | 1 | ||
| 5. CRPBI-P | −.01 | .01 | −.05* | .45** | 1 | |
| 6. Age | .08** | .00 | −.02 | .02 | .04* | 1 |
Note: All measures were assessed at baseline.
Abbreviations: BMI, body mass index; CBCL, Child Behavior Checklist; CRPBI, Children's Report of Parent Behavior Inventory, Acceptance subscale, mother (CRPBI-M) or father (CRPBI-P) as reference parent.
p < .05
p < .01.
3.2 ∣. Linear mixed-effects models
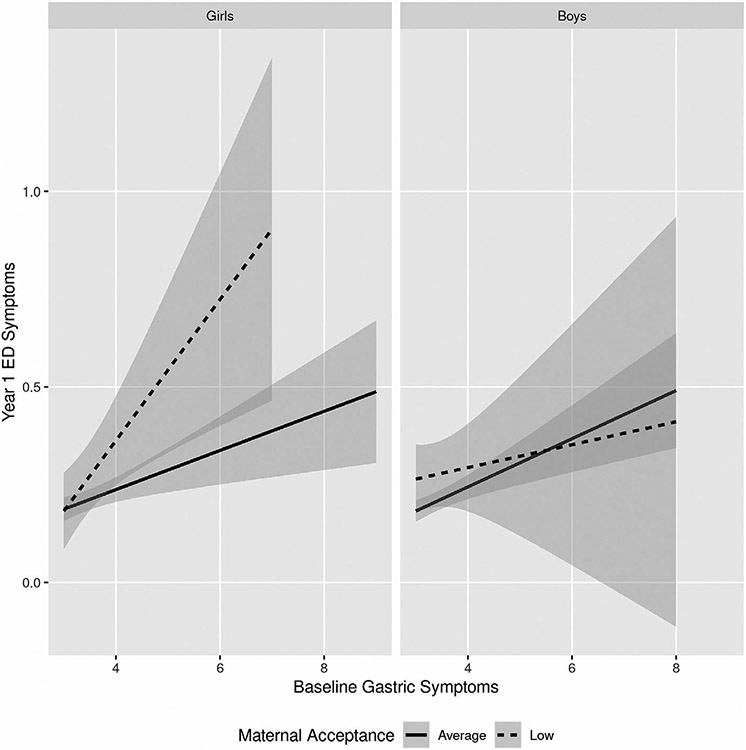
Results of the linear mixed-effects models are shown in Table 4 (paternal acceptance) and Table 5 (maternal acceptance). Tables containing the effects of all variables in each model, including covariates, can be found in the Supporting Information. No statistically significant main effects or interactions were found in the model for paternal acceptance. In the maternal acceptance model, results revealed that baseline gastric symptoms positively predicted Y1 ED symptoms. However, this main effect was qualified by a three-way interaction between Gastric Symptoms, Sex, and Maternal Acceptance. As seen in Figure 1, posthoc analyses revealed that for girls, there was a statistically significant interaction between Gastric Symptoms and Maternal Acceptance (β = −0.06, t = −2.72, p < .01), while for boys, the interaction was not significant (β = 0.01, t = 0.56, p = .58). For girls, low perceived maternal acceptance was associated with a stronger relationship between baseline gastric symptoms and Y1 ED symptoms. Please see Supporting Information for tables containing all data for the sex-specific models.
TABLE 4.
Linear mixed-effects model predicting Year 1 ED symptoms from baseline gastric symptoms, sex, and paternal acceptance
| b | SEb | β | t | p | |
|---|---|---|---|---|---|
| Sex | −0.02 | 0.02 | −0.03 | −0.87 | .38 |
| CRPBI-P | 0.03 | 0.04 | 0.02 | 0.78 | .43 |
| Gastric symptoms | 0.01 | 0.02 | 0.01 | 0.53 | .60 |
| CRPBI-P × Sex | −0.05 | 0.05 | −0.04 | −1.00 | .32 |
| Gastric symptoms × Sex | 0.02 | 0.02 | 0.03 | 0.74 | .46 |
| Gastric symptoms × CRPBI-P | 0.00 | 0.05 | 0.00 | −0.04 | .97 |
| Gastric symptoms × Sex × CRPBI-P | 0.00 | 0.07 | 0.00 | 0.01 | .99 |
Note: n = 3,054; R2 = .21. ED symptoms were summed from parent responses on the computerized Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) interview. Covariates were included for baseline ED symptoms, race/ethnicity, parent marital status, household income, and anxiety. See the Supporting Information for the full model, including effects of covariates.
Abbreviations: CRPBI-P, Children's Report of Parent Behavior Inventory, Acceptance subscale, father as reference parent; ED, eating disorder.
TABLE 5.
Linear mixed-effects model predicting Year 1 ED symptoms from baseline gastric symptoms, sex, and maternal acceptance
| b | SEb | β | t | p | |
|---|---|---|---|---|---|
| Sex | −0.01 | 0.02 | −0.01 | −0.46 | .65 |
| CRPBI-M | −0.04 | 0.05 | −0.02 | −0.78 | .44 |
| Gastric symptoms | 0.03 | 0.02 | 0.05 | 2.12 | .03 |
| CRPBI-M × Sex | 0.02 | 0.06 | 0.01 | 0.26 | .79 |
| Gastric symptoms × Sex | −0.01 | 0.02 | −0.02 | −0.58 | .56 |
| Gastric symptoms × CRPBI-M | −0.17 | 0.06 | −0.07 | −2.96 | .003 |
| Gastric symptoms × Sex × CRPBI-M | 0.21 | 0.08 | 0.08 | 2.68 | .007 |
Note: n = 4,168; R2 = .19. ED symptoms were summed from parent responses on the computerized Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) interview. Covariates were included for baseline ED symptoms, race/ethnicity, parent marital status, household income, and anxiety. See the Supporting Information for the full model, including effects of covariates.
Abbreviations: CRPBI-M, Children's Report of Parent Behavior Inventory, Acceptance subscale, mother as reference parent; ED, eating disorder.
FIGURE 1.
Interaction between maternal acceptance and gastric symptoms in the prediction of eating disorder symptoms 1 year later. Post-hoc analyses were performed using linear mixed-effects models separately for boys and girls. Results revealed that for girls, maternal acceptance moderated the effect of baseline gastric symptoms in the prediction of Year 1 eating disorder (ED) symptoms, such that higher gastric symptoms and lower self-reported maternal acceptance at baseline were associated with increased ED symptoms 1 year later. No such interaction effect existed for boys
4 ∣. DISCUSSION
These results elucidate relationships among gastric symptoms, parenting, sex, and the onset of ED symptoms in late childhood and early adolescence. While controlling for various factors including anxiety symptoms and baseline ED symptoms, the presence of gastric symptoms in children ages 9–10 years interacted with the child's perception of maternal acceptance to predict ED symptoms 1 year later, such that gastric symptoms were related to ED symptoms in the presence of low perceived maternal acceptance. This effect was also influenced by the child's sex, such that these relationships were statistically significant in girls but not boys. To our knowledge, this study is the first to examine the presence and severity of ED symptoms longitudinally using the ABCD sample. These findings inform our understanding of the development of EDs and may contribute to improving prevention efforts and the identification of children at risk.
While GI dysfunction was included in the earliest conceptualizations of AN (Lasègue, 1873/1997), recent work has emphasized the importance of early aversive GI experience as a central feature in a conceptualization of AN focused on visceral learning and interoceptive sensations (Zucker & Bulik, 2020). Early aversive visceral sensations are thought to contribute to sensitization to gastric pain and avoidance behaviors such as food restriction. It is thus hypothesized that GI symptoms in childhood increase risk for the later development of AN. Previous research has shown that digestive problems are indeed associated with increased risk for AN and other EDs (Hedman et al., 2019; Marchi & Cohen, 1990; Marild et al., 2017; Zerwas et al., 2017). However, most of these studies are based on examining medical records of disorders such Celiac disease, and the risks associated may be confounded by the effects of dietary treatments (Conviser et al., 2018). Our findings thus uniquely add to the body of evidence for GI symptoms as a risk factor for EDs by prospectively linking these symptoms in a large sample of children.
The association between gastric symptoms and increased risk for ED symptoms in the ABCD sample was qualified by an interaction with adolescent-reported maternal acceptance to predict ED symptoms in adolescent girls. It is important to note that while research has established that parental factors do not cause EDs and are not a primary mechanism in risk for their development, they do constitute one of a myriad of social and cultural factors that may interact with brain and biology to influence risk (Le Grange et al., 2010). This is in line with our findings, which did not show evidence of a main effect for parental acceptance. Thus, low maternal acceptance on its own is not a sufficient risk factor for the subsequent development of ED symptoms. Rather, maternal acceptance interacted with gastric symptoms and the child's sex in its relation to ED symptoms. Previous studies also support a link between parenting behaviors and ED symptoms (Krug et al., 2016; Martinson, Esposito-Smythers, & Blalock, 2016), including studies using longitudinal methods (Zubatsky, Berge, & Neumark-Sztainer, 2015) and research-coded observations of parenting (Rozenblat et al., 2017).
Our aim in examining the interaction between parental acceptance and gastric symptoms was exploratory, as little research has examined these effects, and to our knowledge no past research has explored these associations as they relate to EDs. We sought to examine their interaction for two primary reasons. First, EDs are complex disorders with biopsychosocial underpinnings. Often, these facets have been studied in isolation. This could mask the potential additive or interactive effects of various risk factors. For example, while speculative, it is possible that parents' responses to their child's GI concerns may influence aversive visceral conditioning, such that either ignoring or overreacting to a child's GI complaints may influence the child's attention to these aversive interoceptive sensations. Indeed, a past study found that parenting was associated with risk for irritable bowel syndrome in a school-based sample of adolescents (Xing, Hou, Zhou, Qin, & Pan, 2014). Our other motivation for exploring these interactive effects was that parenting represents a modifiable risk factor, as demonstrated by programs that are effective in improving the parent–child relationship (e.g., Tuning in to Teens, Havighurst, Kehoe, & Harley, 2015). Interventions aimed at parenting behaviors may help to buffer the ED-related effects of these early childhood GI symptoms.
Our results did not support effects of fathers' warmth and acceptance on later ED symptoms, either as a main effect or a moderator. Past studies specifically examining the effects of fathers' parenting have reported mixed results. Similar to our research, Zubatsky et al. (2015) did not find any associations between paternal parenting style and adolescents' disordered eating 5 years later. In contrast, a cross-sectional study (McEwen & Flouri, 2009) reported that fathers' psychological control and overprotection as reported by the adolescent were associated with higher ED symptoms in this non-clinical sample. As in other areas of developmental research, fathers are relatively understudied, and further research is needed to more clearly determine the effects of paternal parenting behaviors. The ABCD Study provides an opportunity to continue examining these effects over time, as youths' report of fathers' parenting are provided longitudinally.
It should be noted that the effects of gastric symptoms, maternal acceptance, and the interaction between them were only statistically significant in adolescent girls. These results are in line with a recent longitudinal study in Australian adolescents that found low parental warmth and monitoring predicted ED symptoms in adolescent girls but not boys (Krug et al., 2016). With regard to gastric symptoms, previous studies of premorbid GI problems have largely focused on females (Hedman et al., 2019; Marild et al., 2017), precluding the examination of interactions with sex. One study reported a link between autoimmune or autoinflammatory disorders in childhood and later ED development that was particularly strong in boys, but after limiting the disorders to those with a GI component, the effects persisted only for girls (Zerwas et al., 2017). It is therefore possible that these are sex-specific risk factors that may contribute to the higher prevalence of EDs in adolescent girls as compared to boys. This may be at least partially due to visceral sensations and bodily changes that occur with female pubertal development (Zucker & Bulik, 2020). Of note, sex differences in functional GI disorders (i.e., those without a known organic cause) have been reported in adults, with a higher prevalence in women than men (Koloski, Talley, & Boyce, 2002), but a recent study reported no sex differences in children and adolescents, with the exception of functional constipation being more common in boys (Lewis, Palsson, Whitehead, & van Tilburg, 2016).
This is the first study to our knowledge to utilize a large, population-based dataset to examine the interactive effects of two disparate risk factors—gastric symptoms and parental acceptance—to predict ED symptoms during early adolescence. The use of the ABCD Study dataset has many advantages, including a large sample that is relatively representative of the US population, a wide variety of measures across multiple domains, prospective rather than retrospective reporting, and the ability to conduct follow-up studies in the future to explore these effects as the study participants progress through adolescence. There are a few limitations, however, in utilizing this dataset in the study of EDs. Our GI symptom measure was limited to gastric symptoms, without assessing symptoms of the lower GI tract (e.g., constipation, diarrhea), and parents were instructed to only endorse symptoms without a known organic cause. The first two waves of data collection also did not include self-reported ED symptoms for the adolescents. It is likely that some parents are unaware of their child's ED-related thoughts and behaviors, and these would thus not have been accurately assessed. Incongruencies in the ABCD dataset between parent and child reports of other mental health symptoms and behaviors have previously been reported (DeVille et al., 2020). Future waves of data collection will assess self-reported ED symptoms, at which time we will be able to conduct a longitudinal analysis with multiple timepoints and also determine how well parent- and self-reported ED symptoms match. Relatedly, as yet, the ABCD Study does not include observational measures of parenting behaviors. It is thus important to emphasize that our findings relate to the child's perception of maternal warmth and acceptance, which is important in its own right but also has different implications for interpretation. Additionally, the age of the current sample is still younger than the typical age of onset for EDs, and indeed, there was a low rate of any reported ED symptoms in our sample. However, studying this younger age range may help identify early risk factors for ED diagnosis. While our effect sizes were relatively small, this is typical of studies with large samples, including the ABCD Study (Owens et al., 2020). Effects of this size may more accurately reflect those of the general population and are likely still meaningful (Funder & Ozer, 2019). Finally, the lack of statistically significant effects for adolescent boys could be due to the inadequacy of current assessment tools for ED symptoms in boys as well as differences in symptom presentation (Gorrell & Murray, 2019). For example, a past study reported links between maternal attachment and muscle preoccupation in boys (Meesters, Muris, Hoefnagels, & van Gemert, 2007). Further research is needed to refine the potential relationship between GI symptoms and parenting behaviors in the risk for ED symptoms in adolescent boys.
This study provides evidence for an interactive effect between gastric symptoms and child-reported maternal warmth and acceptance in their relation to later ED symptoms in young adolescent girls. Applications of these findings may inform prevention. For example, young adolescent girls presenting with GI symptoms should be monitored for the development of aberrant eating behaviors or cognitions, particularly if there are indications of perceived low acceptance and warmth from their mother. Future directions include replication of these results in the entire ABCD sample; following these effects over time as more waves of data collection are completed; and examining other factors, such as neurobiological measures, that may contribute to risk for EDs during adolescence.
Supplementary Material
ACKNOWLEDGMENTS
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/scientists/workgroups/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the NIMH Data Archive (NDA). The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1504041.
REFERENCES
- Academy for Eating Disorders. (2015, May). Nine truths about eating disorders. https://www.aedweb.org/resources/online-library/publications/nine-truths.
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1), 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Ben-Shachar M, Makowski D, & Lüdecke D (2020). Compute and interpret indices of effect size. CRAN. R package https://github.com/easystats/effectsize. [Google Scholar]
- Berner LA, Simmons AN, Wierenga CE, Bischoff-Grethe A, Paulus MP, Bailer UF, … Kaye WH (2018). Altered interoceptive activation before, during, and after aversive breathing load in women remitted from anorexia nervosa. Psychological Medicine, 48(1), 142–154. 10.1017/S0033291717001635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia V, & Tandon RK (2005). Stress and the gastrointestinal tract. Journal of Gastroenterology and Hepatology, 20(3), 332–339. 10.1111/j.1440-1746.2004.03508.x [DOI] [PubMed] [Google Scholar]
- Boyd C, Abraham S, & Kellow J (2005). Psychological features are important predictors of functional gastrointestinal disorders in patients with eating disorders. Scandinavian Journal of Gastroenterology, 40(8), 929–935. 10.1080/00365520510015836 [DOI] [PubMed] [Google Scholar]
- Boyd C, Abraham S, & Kellow J (2010). Appearance and disappearance of functional gastrointestinal disorders in patients with eating disorders. Neurogastroenterology and Motility, 22(12), 1279–1283. 10.1111/j.1365-2982.2010.01576.x [DOI] [PubMed] [Google Scholar]
- Conviser JH, Fisher SD, & McColley SA (2018). Are children with chronic illnesses requiring dietary therapy at risk for disordered eating or eating disorders? A systematic review. International Journal of Eating Disorders, 51(3), 187–213. 10.1002/eat.22831 [DOI] [PubMed] [Google Scholar]
- Dalton C (2017). What is a functional GI disorder? Ask the expert. Chapel Hill, NC: UNC Center for Functional GI & Motility Disorders. https://www.med.unc.edu/ibs/files/2017/10/What-Is-Functional-GI.pdf [Google Scholar]
- DeVille DC, Whalen D, Breslin FJ, Morris AS, Khalsa SS, Paulus MP, & Barch DM (2020). Prevalence and family-related factors associated with suicidal ideation, suicide attempts, and self-injury in children aged 9 to 10 years. JAMA Network Open, 3(2), e1920956. 10.1001/jamanetworkopen.2019.20956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AO, & McKinney C (2018). Parental and child psychopathology: Moderated mediation by gender and parent-child relationship quality. Child Psychiatry and Human Development, 49(6), 843–852. 10.1007/s10578-018-0801-0 [DOI] [PubMed] [Google Scholar]
- Funder D, & Ozer D (2019). Evaluating effect size in psychological research: Sense and nonsense. Advances in Methods and Practices in Psychological Science, 2(2), 156–168. [Google Scholar]
- Gendall KA, Joyce PR, Carter FA, McIntosh VV, & Bulik CM (2005). Childhood gastrointestinal complaints in women with bulimia nervosa. International Journal of Eating Disorders, 37(3), 256–260. 10.1002/eat.20088 [DOI] [PubMed] [Google Scholar]
- Gorrell S, & Murray SB (2019). Eating disorders in males. Child and Adolescent Psychiatric Clinics of North America, 28(4), 641–651. 10.1016/j.chc.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiaga A, Aliri J, Balluerka N, & Lameirinhas J (2019). Parenting styles and internalizing symptoms in adolescence: A systematic literature review. International Journal of Environmental Research and Public Health, 16(17), 3192. 10.3390/ijerph16173192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, … Haines J (2011). The PhenX Toolkit: Get the most from your measures. American Journal of Epidemiology, 174(3), 253–260. 10.1093/aje/kwr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havighurst SS, Kehoe CE, & Harley AE (2015). Tuning in to teens: Improving parental responses to anger and reducing youth externalizing behavior problems. Journal of Adolescence, 42, 148–158. 10.1016/j.adolescence.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Haycraft E, & Blissett J (2010). Eating disorder symptoms and parenting styles. Appetite, 54(1), 221–224. 10.1016/j.appet.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Hedman A, Breithaupt L, Hübel C, Thornton LM, Tillander A, Norring C, … Bulik CM (2019). Bidirectional relationship between eating disorders and autoimmune diseases. Journal of Child Psychology and Psychiatry and Allied Disciplines, 60(7), 803–812. 10.1111/jcpp.12958 [DOI] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG Jr., & Kessler RC (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry, 61(3), 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown SA, & Coordinators AC (2018). Introduction. Developmental Cognitive Neuroscience, 32, 1–3. 10.1016/j.dcn.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoń K (2020). MuMIn: Multi-model inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn. [Google Scholar]
- Kaufman J, Townsend LD, & Kobak K (2017). The computerized Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS): Development and administration guidelines. Journal of the American Academy of Child & Adolescent Psychiatry, 56(10), S357. [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, & Simmons WK (2017). Influence of visceral interoceptive experience on the brain's response to food images in anorexia nervosa. Psychosomatic Medicine, 79(7), 777–784. 10.1097/PSY.0000000000000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, & Simmons WK (2016). Altered insula activity during visceral interoception in weight-restored patients with anorexia nervosa. Neuropsychopharmacology, 41(2), 521–528. 10.1038/npp.2015.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloski NA, Talley NJ, & Boyce PM (2002). Epidemiology and health care seeking in the functional GI disorders: A population-based study. The American Journal of Gastroenterology, 97(9), 2290–2299. 10.1111/j.1572-0241.2002.05783.x [DOI] [PubMed] [Google Scholar]
- Krug I, King RM, Youssef GJ, Sorabji A, Wertheim EH, Le Grange D, … Olsson CA (2016). The effect of low parental warmth and low monitoring on disordered eating in mid-adolescence: Findings from the Australian Temperament Project. Appetite, 105, 232–241. 10.1016/j.appet.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Lam CB, & McHale SM (2012). Developmental patterns and family predictors of adolescent weight concerns: A replication and extension. International Journal of Eating Disorders, 45(4), 524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laségue C (1997). On hysterical anorexia(a). Obesity Research, 5(5), 492–497. 10.1002/j.1550-8528.1997.tb00676.x [DOI] [PubMed] [Google Scholar]
- Le Grange D, Lock J, Loeb K, & Nicholls D (2010). Academy for eating disorders position paper: The role of the family in eating disorders. International Journal of Eating Disorders, 43(1), 1–5. 10.1002/eat.20751 [DOI] [PubMed] [Google Scholar]
- Lee C, Doo E, Choi JM, Jang SH, Ryu HS, Lee JY, … Brain-Gut Axis Research Group of Korean Society of Neurogastroenterology and Motility. (2017). The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: Systematic review and meta-analysis. Journal of Neurogastroenterology and Motility, 23(3), 349–362. 10.5056/jnm16220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee AM, Ngai E, Lee DT, & Wing YK (2001). Rationales for food refusal in Chinese patients with anorexia nervosa. International Journal of Eating Disorders, 29, 224–229. [DOI] [PubMed] [Google Scholar]
- Lewis ML, Palsson OS, Whitehead WE, & van Tilburg M (2016). Prevalence of functional gastrointestinal disorders in children and adolescents. The Journal of Pediatrics, 177, 39–43.e3. 10.1016/j.jpeds.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Marchi M, & Cohen P (1990). Early childhood eating behaviors and adolescent eating disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 29(1), 112–117. 10.1097/00004583-199001000-00017 [DOI] [PubMed] [Google Scholar]
- Marild K, Stordal K, Bulik CM, Rewers M, Ekbom A, Liu E, & Ludvigsson JF (2017). Celiac disease and anorexia nervosa: A nationwide study. Pediatrics, 139(5), e20164367. 10.1542/peds.2016-4367 [DOI] [PubMed] [Google Scholar]
- Martinson LE, Esposito-Smythers C, & Blalock DV (2016). The effect of parental monitoring on trajectories of disordered eating attitudes and behaviors among adolescents: An individual growth curve analysis. Appetite, 107(12), 180–187. 10.1016/j.appet.2016.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C, & Flouri E (2009). Fathers' parenting, adverse life events, and adolescents' emotional and eating disorder symptoms: The role of emotion regulation. European Child and Adolescent Psychiatry, 18(4), 206–216. 10.1007/s00787-008-0719-3 [DOI] [PubMed] [Google Scholar]
- Meesters C, Muris P, Hoefnagels C, & van Gemert M (2007). Social and family correlates of eating problems and muscle preoccupation in young adolescents. Eating Behaviors, 8(1), 83–90. 10.1016/j.eatbeh.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Norris ML, Harrison ME, Isserlin L, Robinson A, Feder S, & Sampson M (2016). Gastrointestinal complications associated with anorexia nervosa: A systematic review. International Journal of Eating Disorders, 49(3), 216–237. [DOI] [PubMed] [Google Scholar]
- North CS, Alpers DH, Thompson SJ, & Spitznagel EL (1996). Gastrointestinal symptoms and psychiatric disorders in the general population. Findings from NIMH epidemiologic catchment area project. Digestive Diseases and Sciences, 41(4), 633–640. 10.1007/BF02213117 [DOI] [PubMed] [Google Scholar]
- Owens MM, Potter A, Hyatt C, Albaugh M, Thompson WK, Jernigan T, Yuan D, Hahn S, Allgaier N, & Garavan H (2020, November 12). Recalibrating expectations about effect size: A multimethod survey of effect sizes in the ABCD study. 10.31234/osf.io/tn9u4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SJ, Keville S, Schmidt U, & Chalder T (2005). Eating disorders and irritable bowel syndrome: Is there a link? Journal of Psychosomatic Research, 59, 57–64. [DOI] [PubMed] [Google Scholar]
- R Core Team. (2019). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Rozenblat V, Ryan J, Wertheim E, King R, Olsson CA, Letcher P, & Krug I (2017). Relationships between self-reported and observed parenting behaviour, adolescent disordered eating attitudes and behaviours, and the 5-HTTLPR polymorphism: Data from the Australian temperament project. European Eating Disorders Review, 25(5), 381–388. 10.1002/erv.2530 [DOI] [PubMed] [Google Scholar]
- Schludermann E, & Schludermann S (1988). Children's report on parent behavior (CRPBI-108, CRPBI-30). Winnipeg, Canada: Department of Psychology, University of Manitoba. [Google Scholar]
- Schaumberg K, Welch E, Breithaupt L, Hübel C, Baker JH, Munn-Chernoff MA, … Bulik CM (2017). The science behind the Academy for Eating Disorders' nine truths about eating disorders. European Eating Disorders Review, 25(6), 432–450. 10.1002/erv.2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN, Oberndorfer T, Klabunde M, Reinhardt LE, & Kaye WH (2013). Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. The International Journal of Eating Disorders, 46(1), 23–33. 10.1002/eat.22045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata P, Fox J, & Cooper J (2001). An investigation into the influence of gender and parenting styles on excessive exercise and disordered eating. European Eating Disorders Review, 9, 194–206. [Google Scholar]
- Van Oudenhove L, Törnblom H, Störsrud S, Tack J, & Simrén M (2016). Depression and somatization are associated with increased postprandial symptoms in patients with irritable bowel syndrome. Gastroenterology, 150(4), 866–874. 10.1053/j.gastro.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Volpe U, Tortorella A, Manchia M, Monteleone AM, Albert U, & Monteleone P (2016). Eating disorders: What age at nset? Psychiatry Research, 238, 225–227. [DOI] [PubMed] [Google Scholar]
- Yang R, & Jernigan TL (2019). Adolescent Brain Cognitive Development Study (ABCD) 2.0.1 release (Data set). NIMH Data Repositories. 10.15154/1504041 [DOI] [Google Scholar]
- Xing Z, Hou X, Zhou K, Qin D, & Pan W (2014). Impact of parental-rearing styles on irritable bowel syndrome in adolescents: A school-based study. Journal of Gastroenterology and Hepatology, 29(3), 463–468. 10.1111/jgh.12388 [DOI] [PubMed] [Google Scholar]
- Zerwas S, Larsen JT, Petersen L, Thornton LM, Quaranta M, Koch SV, … Bulik CM (2017). Eating disorders, autoimmune, and autoinflammatory disease. Pediatrics, 140(6), e20162089. 10.1542/peds.2016-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubatsky M, Berge J, & Neumark-Sztainer D (2015). Longitudinal associations between parenting style and adolescent disordered eating behaviors. Eating and Weight Disorders–Studies on Anorexia, Bulimia and Obesity, 20(2), 187–194. 10.1007/s40519-014-0154-z [DOI] [PubMed] [Google Scholar]
- Zucker NL, & Bulik CM (2020). On bells, saliva, and abdominal pain or discomfort: Early aversive visceral conditioning and vulnerability for anorexia nervosa. International Journal of Eating Disorders, 53(4), 508–512. 10.1002/eat.23255 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in the NIMH Data Archive (NDA). The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1504041.