Significance
Plants mobilize the endomembrane system for restoring homeostasis when stimulated by stresses. Though the function of ufmylation in ER stress and autophagy has been described in mammals, their detailed roles in plant development and stress responses remain elusive. In this study, we identified Ufl1 as an ATG8 interactor in Arabidopsis and demonstrated that Ufl1 maintains ER homeostasis upon salt stress via regulating ER-phagy by mechanistic connection with the autophagy-related (ATG) machinery. Our study thus sheds light on the pleiotropic functions of the ufmylation cascade in plants under abiotic stresses and underpins the cross-talk among ER stress, salt stress, and autophagy.
Abstract
In plants, the endomembrane system is tightly regulated in response to environmental stresses for maintaining cellular homeostasis. Autophagosomes, the double membrane organelles forming upon nutrient deprivation or stress induction, degrade bulky cytosolic materials for nutrient turnover. Though abiotic stresses have been reported to induce plant autophagy, few receptors or regulators for selective autophagy have been characterized for specific stresses. Here, we have applied immunoprecipitation followed by tandem mass spectrometry using the autophagosome marker protein ATG8 as bait and have identified the E3 ligase of the ufmylation system Ufl1 as a bona fide ATG8 interactor under salt stress. Notably, core components in the ufmylation cascade, Ufl1 and Ufm1, interact with the autophagy kinase complexes proteins ATG1 and ATG6. Cellular and genetic analysis showed that Ufl1 is important for endoplasmic reticulum (ER)-phagy under persisting salt stress. Loss-of-function mutants of Ufl1 display a salt stress hypersensitive phenotype and abnormal ER morphology. Prolonged ER stress responses are detected in ufl1 mutants that phenocopy the autophagy dysfunction atg5 mutants. Consistently, expression of ufmylation cascade components is up-regulated by salt stress. Taken together, our study demonstrates the role of ufmylation in regulating ER homeostasis under salt stress through ER-phagy.
Post-translational modifications (PTMs) diversify protein functions and dynamically coordinate signaling networks in a timely, malleable, and energy-efficient way. In well-studied PTMs like ubiquitination or SUMOylation, a small modifier protein is attached to the active sites of the substrates by covalent bonds through multienzyme processing steps. The modification endows substrate proteins new fates of degradation, trafficking, novel interactions, or signaling (1–3). The ubiquitination process is achieved by three types of enzymes: the activating enzyme E1, the conjugating enzyme E2, and the ligase E3. Ubiquitination, though best known in targeting substrate proteins for degradation, also maintains cellular homeostasis by orchestrating versatile signaling or trafficking pathways (3). Dysfunction of this system leads to serious human diseases or disorders, for example neurodegeneration, immunity disorders, and cancer (4).
Ufmylation, a novel PTM mediated by the ubiquitin-like protein, ubiquitin-fold modifier 1 (Ufm1), is characterized by its multiple functions in diverse cellular processes (5–7). Ufm1 shares a similar tertiary structure with ubiquitin and is processed via comparable actions. The protease enzyme Ufm1-specific peptidase (UFSP) cleaves its C terminus to expose the glycine residue. The E1 enzyme Ufm1-activating Enzyme 5 (Uba5) then activates the Ufm1 at the exposed glycine via thioester bonds. Activated Ufm1 is transferred to the E2 enzyme Ufm1-conjugating Enzyme 1 (Ufc1) then to the E3 enzyme Ufm1-protein Ligase 1 (Ufl1) for subsequent substrate recognition and ufmylation (6–8). The ufmylation system is highly conserved in mammals and plants but not in fungi (5). Unlike the extensively studied E1s, E2s, and especially numerous E3s for ubiquitination, only a single enzyme for each reaction step has been identified for the ufmylation system, except for UFSP with two homologs discovered in mammalian cells yet only one is widely accepted to be active. Besides the core E1-E2-E3 and protease proteins in the ufmylation cascade, two satellite components, the cyclin-dependent Kinase 5 (CDK5) regulatory subunit associated Protein 3 (CDK5RAP3/C53) and the DDRGK domain-containing Protein 1/ Ufm1-binding Protein 1 (DDRGK1/UFBP1/), are also functionally conserved in their functions in ufmylation (9–13).
In mammals, mutations of the ufmylation cascade cause genetic disorders and diseases (11, 14–20). Uba5 and UFBP1 are essential for erythroid development, where loss-of-function mutations of Uba5 and UFBP1 are embryonically lethal in mice (8, 11). Genetic studies identified human families with uba5 mutations that suffer from early-onset intractable epilepsy, autosomal recessive cerebellar ataxia, infantile-onset encephalopathy, and fatal congenital neuropathy (14, 16, 17, 19, 20). Mutations or knock out (KO) of core ufmylation enzymes causes cancer, heart failure, and liver health problems, proving the important functions of the ufmylation cascade in disease progression (21–24). At the cellular level, ufmylation is found to be essential for maintaining endoplasmic reticulum (ER) homeostasis, regulating autophagic flux and modulating ribosome integrity (10, 13, 24–26). Likewise, the ufmylation component and putative substrate protein DDRGK1/UFBP1 promotes ER expansion and plasma cell development by regulating unfolded protein responses (UPRs) (13). Recently, the roles of ufmylation in autophagy have been investigated. Turnover of the autophagic receptor p62 is affected in ufmylation mutants in mammalian cells, and the ER-localized Ufl1 interactor C53 has also been characterized as a novel cross-kingdom ER-phagy receptor (10, 26, 27). Although increasing knowledge on the functions of ufmylation and the UFMylome in mammals, the pleiotropic roles of ufmylation remain enigmatic in plant development and stress responses.
Plants encounter adverse environmental stresses during their sessile life cycles. Stress-triggered regulations by hormones and subsequent endomembrane responses enable plants to self-regulate for cellular homeostasis and survival (28–31). ER as the central hub of lipid and protein biosynthesis undergoes rapid adjustments in stress responses via ER stress pathways (32). Under stress conditions, when protein folding requirements exceed the ER capacity, UPRs trigger ER stress responses that simultaneously constrain protein synthesis and increase chaperone-mediated protein folding (32, 33). Under adverse environments such as salt stress, misfolded proteins accumulate in the ER, trigger UPR, and induce ER-PM contact sites (EPCSs) expansion (32). Nevertheless, how the expanded ER structures recover when stress is relieved remains largely elusive.
In this study, we began by screening for potential autophagy regulators under salt stress, in order to investigate mechanisms of salt stress-induced autophagy in plants. In the mass spectrometry (MS) screening using the autophagy marker protein ATG8e as bait, we identified Ufl1 as a potential ATG8e interactor upon salt treatment. Biochemical and cellular analysis further confirmed that the cytoplasmic/ER-associated Ufl1 colocalized and interacted with ATG8s upon salt stress induction in Arabidopsis. Genetic analysis using T-DNA insertional mutants of Ufl1 showed that ufl1 mutants exhibited lower survival rates and shorter roots under salt stress. Subcellular analyses by confocal laser scanning microscopy (CLSM) and transmission electron microscopy (TEM) revealed abnormal ER expansion with accumulated ER sheets in ufl1 mutants under salt stress. The expression levels of the ufmylation components were evaluated under salt stress and showed significant upregulation by salt stress. We also analyzed the ER stress indicator proteins in atg5 or ufl1 mutants under prolonged salt stress treatments, showing that both mutants exhibited higher expression levels of ER stress factors. To conclude, this study reveals the novel function of the ufmylation system in ER remodeling via ER-phagy under salt stress. Ufmylation is therefore a crucial modulator at the intersection of environmental stresses, autophagy, and ER stress/homeostasis in plants.
Results
Identification of Ufl1 as an ATG8e Novel Interactor under Salt Stress.
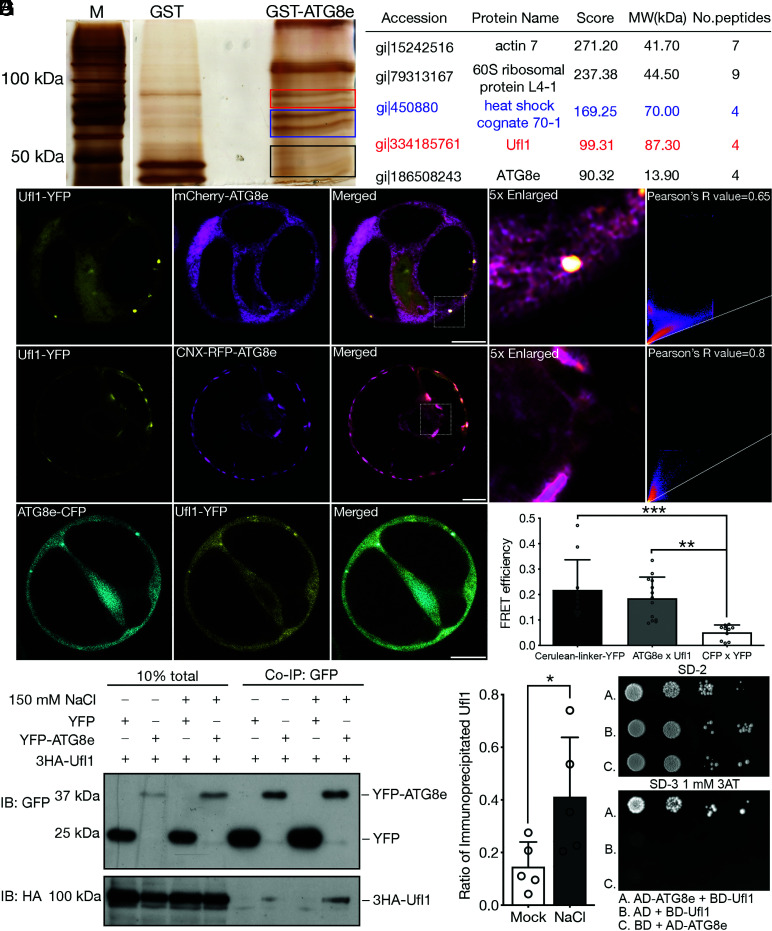
In plants, autophagy is actively triggered by stresses for efficient nutrient recycling to maintain plant growth and survival in adverse environment (34, 35). Although abiotic stresses including drought, heat, and salt stresses are reported to be closely associated with the autophagy pathway, few proteins or mechanisms have been identified in stress-triggered autophagy (29, 36–38). The autophagy receptor NBR1 has been reported to mediate salt stress-induced autophagy in Arabidopsis, yet other receptors or ancillary proteins in the pathway remain enigmatic (39). ATG8, the ubiquitin-like protein that decorates the autophagosomal double membranes and recruits cargoes or receptors during autophagosome biogenesis, is commonly used as a bait for identifying novel autophagy regulators and receptors in mammals (40). We therefore aimed at screening for potential participants in plant autophagy under salt stress by using ATG8 as a bait for the immunoprecipitation (IP) and tandem MS analysis in Arabidopsis. Purified GST-tagged ATG8e was incubated with protoplast lysates isolated from Arabidopsis suspension cultured cells PSB-D treated with salt stress (SI Appendix, Fig. S1A). Protein bands in ATG8e-pull down group distinct from the negative control (GST-only) group were cut for gel purification, peptide extraction, followed by MS/MS analysis (Fig. 1A). Interestingly, Ufl1, the E3 ligase for the ufmylation cascade, was identified as a novel ATG8e interactor upon salt stress (Fig. 1A), which was not detected in MS analysis to screen for ATG8e interactors under mock condition (Dataset S1). To investigate the subcellular localization of Ufl1, fluorescent protein-tagged Ufl1-YFP/RFP was transiently single expressed or co-expressed with distinct organelle markers in PSB-D protoplasts for CLSM observation. The fluorescent tag was fused at the C terminus because that the N terminus of Ufl1 is reported to be functionally important for DDRGK1 and Ufc1 binding (41). Recent study has shown that Ufl1 localizes both in cytosol and as an ER-associated protein in Arabidopsis, in which ER stress induction triggers its increased colocalization with its adaptor proteins and appears as puncta-like structures (10). Consistently, both Ufl1-YFP and Ufl1-RFP showed mainly cytosolic pattern with a few punctae partially colocalizing with mCherry-ATG8e or the ER marker protein Calnexin (CNX)-RFP in protoplasts under salt stress treatment (Fig. 1B and SI Appendix, Fig. S1 B and C ). To further confirm the interaction of ATG8e and Ufl1, Ufl1-YFP was co-expressed with the ER-anchored CNX-RFP-ATG8e. Indeed, Ufl1-YFP was recruited by CNX-RFP-ATG8e to exhibit significantly increased ER pattern (Fig. 1C). The physical interaction between ATG8e and Ufl1 was further confirmed by fluorescence resonance energy transfer (FRET), co-IP, and yeast two-hybrid (Y2H) assays (Fig. 1 D–G). In the co-IP analysis, protoplasts were treated with or without salt stress prior to protein extraction, showing that overexpressed Ufl1 barely interacted with ATG8e under mock condition where increased Ufl1-ATG8e interaction was detected with salt stress treatment (Fig. 1 E and F). Interestingly, among the Arabidopsis ATG8 homologs, Ufl1 also specifically interacted with ATG8a and ATG8i besides ATG8e (SI Appendix, Fig. S2), suggesting the evolutionally conserved functions of ATG8 homologs under salt stress. To conclude, Ufl1 is a novel interactor of ATG8s under salt stress in Arabidopsis.
Fig. 1.
Identification of Ufl1 as an ATG8e interactor under salt stress condition. (A) Purified recombinant GST-ATG8e was used as bait for IP from Arabidopsis PSB-D cell lysate upon salt stress treatment. Proteins pulled-out by GST-ATG8e were separated by SDS-PAGE, and silver-stained protein bands were subjected to tandem MS analysis for subsequent protein identification. The Right panel lists promising hits in the GST-ATG8 pull down group. The label of boxes in red, blue, and black on the gel represents the portion of gel cut where candidates in the lists labeled by the same color were identified. (B) Ufl1-YFP colocalized with mCherry-ATG8e in punctate upon their co-expression in Arabidopsis protoplasts. (Scale bar, 10 μm.) (C) CNX-RFP-ATG8e recruited Ufl1-YFP to the ER upon their co-expression in Arabidopsis protoplasts. (Scale bar, 10 μm.) (D) FRET analysis of ATG8e-CFP with Ufl1-YFP in Arabidopsis protoplasts. (Scale bar, 10 μm.) **P < 0.01, ***P < 0.001. (E) Co-immunoprecipitation (Co-IP) analysis of 3HA-Ufl1 with YFP-ATG8e in Arabidopsis protoplasts with or without salt stress treatment. YFP-ATG8e or free YFP was transiently co-expressed with 3HA-Ufl1, followed by cell lysis and IP using GFP-trap magnetic beads for subsequent western blot analysis. (F) Quantification analysis of the ratio of co-immunoprecipitated Ufl1 by ATG8e shown in (E). Means ± SD; n = 5 individual experiments, two-tailed unpaired t test, *P < 0.05. (G) Y2H analysis between ATG8e and Ufl1 in serial dilution. Transformants were screened on synthetic drop-out medium lacking Trp and Leu (SD-2). Positive colonies were then grown on SD medium lacking His, Trp, and Leu (SD-3) with 1 mM 3AT to inhibit self-activation.
Ufl1 and the Modifier Ufm1 Interacts with Early Autophagy Machinery ATG1 and ATG6.
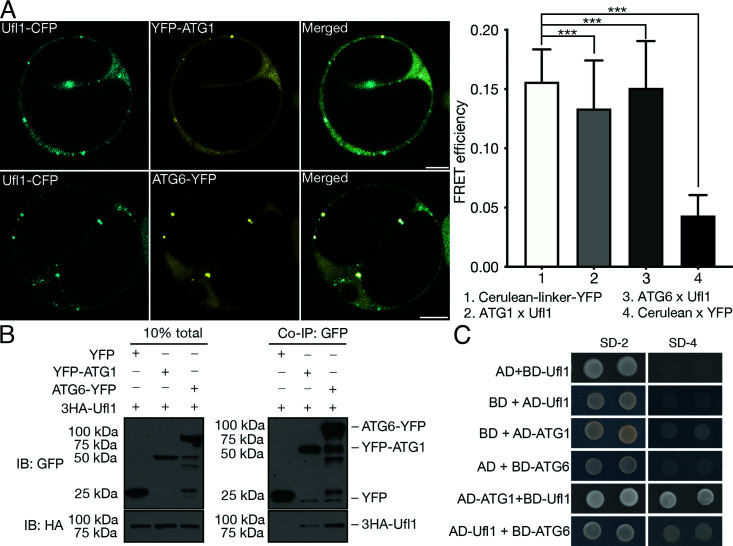
Current knowledge of the interplay between the ufmylation system and the autophagy pathway in plants is limited to C53, and the ancillary protein that forms a complex with Ufl1 and DDRGK1 on ER membrane, to act as an ER-phagy receptor induced by ER-stressors (10). Since we identified the core ufmylation E3 ligase Ufl1 as an ATG8 interactor under salt stress, we further investigated whether other core ATG machinery proteins might directly associate with Ufl1 or other ufmylation components. Two autophagy proteins ATG1 (ULK1 in mammals) and ATG6 (Beclin 1 in mammals) were found to robustly interact with Ufl1 (Fig. 2 A–C). ATG1 and ATG6 both form kinase complexes essential for autophagy initiation and progression (42), where ATG6 is reported to localize along the early secretory pathway and in majority the initiated phagophores (43).
Fig. 2.
Ufl1 interacts with the early autophagy machinery. (A) FRET analysis of Ufl1-CFP with YFP-ATG1 or ATG6-YFP in Arabidopsis protoplasts. (Scale bar, 10 μm.) ***P < 0.001. (B) Co-IP analysis of 3HA-Ufl1 with YFP-ATG1 or ATG6-YFP in Arabidopsis protoplasts. YFP-tagged ATG1/ATG6 or free YFP was transiently co-expressed with 3HA-Ufl1, followed by cell lysis and IP using GFP-trap magnetic beads and subsequent western blot analysis. (C) Y2H analysis between Ufl1 and ATG1 or ATG6.
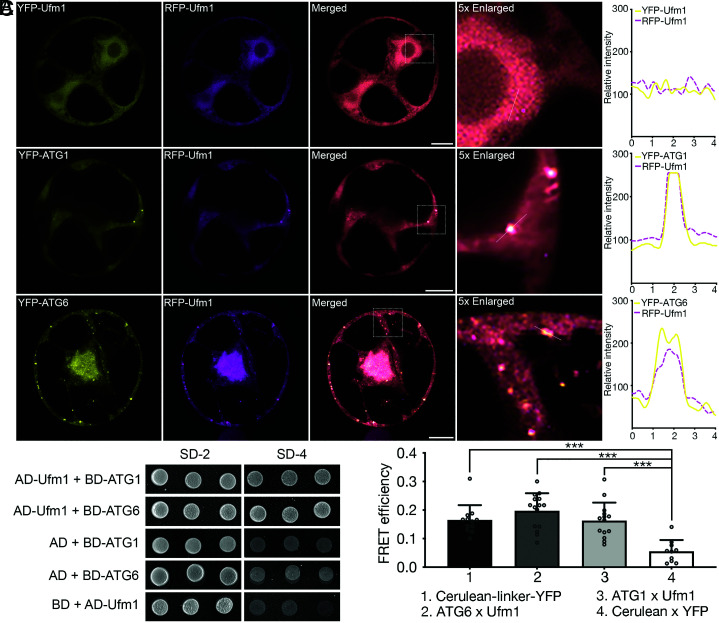
We then tested the in vivo and in vitro interaction of the small ubiquitin-like modifier protein in the ufmylation complex, Ufm1, and its conjugation enzyme Ufc1 with each other or with ATG1 and ATG6. Indeed, Ufl1, Ufc1, and Ufm1 substantially colocalized under confocal analysis and interacted steadily with each other in co-IP and Y2H assays (SI Appendix, Fig. S3 A–C). Intriguingly, RFP/YFP-Ufm1 displayed a cytosolic expression pattern when singly expressed, but when co-expressed with ATG1 or ATG6, Ufm1 is relocated to punctate-like structures that colocalize with both ATG proteins (Fig. 3A). FRET and Y2H analyses further confirmed the interaction between Ufm1 and ATG1/6 (Fig. 3 B and C). In short, the ufmylation core proteins Ufl1 and Ufm1 interacted with core autophagy machinery proteins ATG1 and ATG6, where Ufm1 is relocated to membrane associated structures upon co-expressing with ATG1/6.
Fig. 3.
Ufm1 closely associates with the core autophagy machinery. (A) Transient co-expression of RFP-Ufm1 with YFP-UFM1, YFP-ATG1, or ATG6-YFP, respectively, in Arabidopsis protoplasts, followed by confocal imaging analysis. Puncta colocalization efficiency was evaluated by ImageJ. (Scale bars, 10 μm.) (B) Y2H analysis between Ufm1 and ATG1 or ATG6. (C) FRET analysis of CFP-Ufm1 with YFP-ATG1 or ATG6-YFP in Arabidopsis protoplasts. ***P < 0.001
Ufl1 Localizes at the ER and Autophagosomes upon Salt Stress.
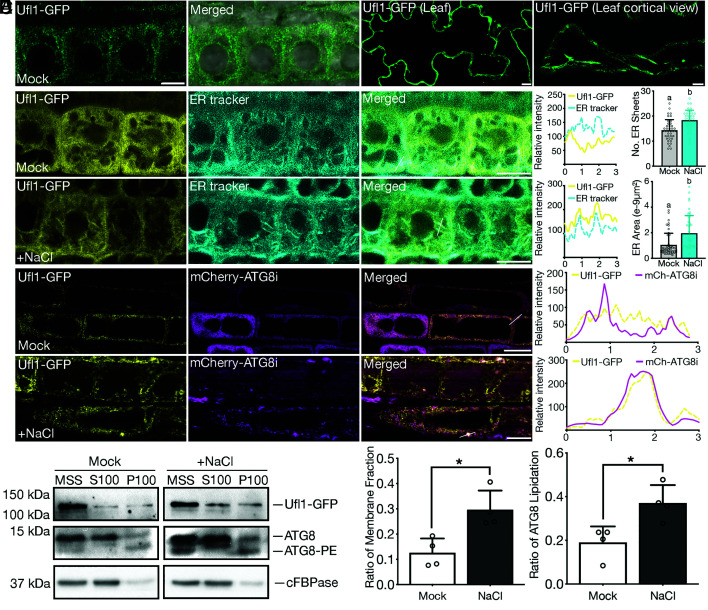
To characterize the in situ localization and function of Ufl1 in plants, we generated transgenic plants stably expressing Ufl1-GFP. Consistent with the case in Arabidopsis protoplasts, Ufl1-GFP also showed mainly cytosolic distribution and exhibited a partial ER-associated pattern under mock conditions in both root tips and leaves (Fig. 4A). In double transgenic plants expressing Ufl1-GFP along with distinct endomembrane organelle markers, Ufl1-GFP colocalized with the ER marker mCherry-HDEL but no other organelles along the secretory pathway (SI Appendix, Fig. S4).
Fig. 4.
Ufl1 localizes with the ER and shows increased membrane association under salt stress condition. (A) Confocal analysis of Ufl1-GFP in root or leave cells of 5-d-old transgenic Arabidopsis seedlings. (B) Confocal imaging analysis of Ufl1-GFP in root tip meristem cells of 5-d-old Arabidopsis seedlings under mock or salt stress conditions after ER-Tracker™ dye staining. (C) Quantification of the number of ER sheets and the ER surface area, respectively, as shown in (B) from ≥ three biological replicates. n ≥ 40 individual cells from ≥20 individual seedlings were counted. (D) Confocal imaging analysis of Ufl1-GFP and mCherry-ATG8i in root tip elongation zone cells of double transgenic seedlings under mock or salt stress conditions. (E) Western blot analysis of the subcellular distribution of Ufl1-GFP and ATG8 in transgenic 5-d-old Arabidopsis seedlings expressing Ufl1-GFP under mock or salt stress conditions. Cellular cytosolic and membrane fractions were separated by medium speed (MS, 10,000 × g) centrifugation and ultracentrifugation (100,000 × g). MSS, medium speed supernatant; S100, supernatant collected after 100,000 × g centrifugation; P100, pellet fraction after 100,000 × g centrifugation. (F) Quantification of the ratio of the membrane fraction (P100 over MSS) and the ratio of ATG8 lipidation, respectively, as shown in (D) with at least three replicates. *P < 0.05.
We next investigated whether salt stress may cause the redistribution of Ufl1-GFP in plants. Interestingly, increasing amounts of ER sheets stained by the ER-tracker accumulated in root tip cells under salt stress and colocalized with Ufl1-GFP (Fig. 4 B and C). Of note, Ufl1-GFP also partially colocalized with mCherry-ATG8i punctae upon salt stress treatment (Fig. 4D). Consistent with the confocal analysis, biochemical analysis showed that the membrane association of Ufl1-GFP increased significantly under salt stress when compared with the control groups (Fig. 4 E and F). The amount of membrane associated ATG8 upon salt stress treatment was also higher than that in the mock group (Fig. 4 E and F).
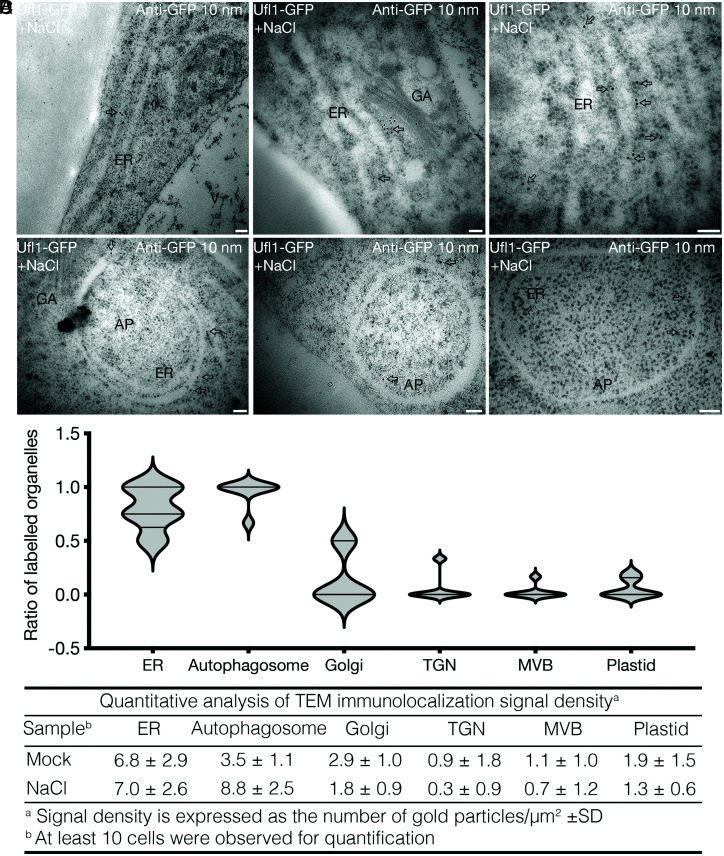
To confirm the subcellular localization of Ufl1 on the ER and autophagosomes under salt stress, we performed immunogold labeling using GFP antibodies in the root tips of Ufl1-GFP transgenic seedlings followed by TEM observation. Concordantly, gold particles for anti-GFP located alongside the ER in the high-pressure-frozen root tip cells of Ufl1-GFP transgenic plants with or without NaCl treatment (Fig. 5 A, C, and D and SI Appendix, Fig. S5A). Notably, gold particles can label the autophagosomal structures engulfing the ER sheets under salt stress (Fig. 5 B–D). Both inner and outer membranes of the forming autophagosomes can be labeled by gold particles in the presence or absence of the engulfed ER (Fig. 5B), probably because of the Ufl1-ATGs association (Figs. 1 and 2). In mammalian cells, immunogold-TEM labeling of proteins in the ULK1 complex or associated with the PI3K complex have been shown locating on both sides of autophagosome membranes during its formation, though they subsequently redistribute to the outer surface and dissociate from the completed autophagosome (44–46), yet ATG8 decorates both sides of the autophagosome membranes (47, 48). Consistently, the Arabidopsis autophagy regulator protein SH3P2, which interacts with multiple ATG proteins including ATG6 and ATG8 as Ufl1, displays similar immunogold labeling on both sides of the autophagosomes (49). Meanwhile, other organelles including Golgi, trans-Golgi network (TGN), multivesicular body (MVB), and plastids were less labeled by gold particles, proving the solid association of Ufl1 on ER and autophagosome membranes under salt stress (SI Appendix, Fig. S5 B and C and Fig. 5 C and D). Previous evidence has shown partial Golgi localization of the ufmylation component DDRGK1 in mammal (7). Here we also observed a few gold labeling of Ufl1 on Golgi, which may indicate conserved function and localization of ufmylation proteins in plants (Fig. 5 A, C, and D). In summary, Ufl1 localizes mainly to the cytosol and ER under normal conditions, whereas salt stress induced a significant increment of Ufl1 relocation to the ER sheets and the surrounding autophagosomes.
Fig. 5.
Ufl1 localizes in both the ER and autophagosome in Arabidopsis under salt stress condition. (A and B) Ultra-thin sections were prepared from root tip cells of 5-d-old transgenic seedlings expressing Ufl1-GFP under salt stress treatment by HPF and freeze substitution. Immunogold labeling with anti-GFP antibodies showed (A) ER and (B) autophagosome distribution. Arrows indicate examples of gold particles labeling on the corresponding organelles. AP, autophagosome; ER, endoplasmic reticulum; GA, Golgi apparatus. (Scale bars, 100 nm.) (C and D) Quantification of organelles with gold particle labeling from sections represented by (A) and (B), with at least three replicates in over five cells.
ufl1 Mutants are Hypersensitive to Salt Stress.
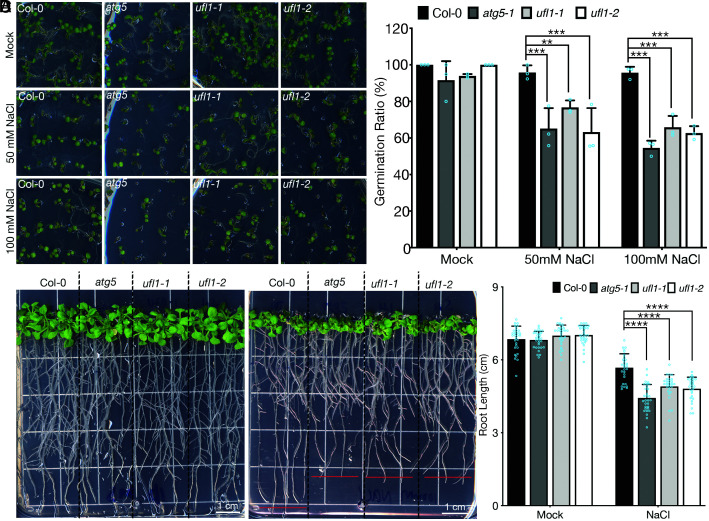
To evaluate the functional roles of Ufl1 upon salt stress in plant, we obtained T-DNA insertional mutants and verified their single insertion by next-generation sequencing (NGS). The mutants were then subjected to phenotypic analysis including seedling germination and plant development under salt stress (SI Appendix, Fig. S6 A and B). To evaluate the autophagy relevant phenotypes of ufl1 mutants under salt stress, mutants of core ATG machinery, atg5 or atg7, were used as positive controls in the phenotypic assays. Both atg mutants and ufl1 mutants grew normally and were comparable to the wild-type Col-0 plants under normal long day growth conditions (SI Appendix, Fig. S6C). However, when germinated on NaCl-containing MS plates, the germination ratio of atg5 and ufl1 mutants significantly decreased compared to the wild-type seedlings (Fig. 6 A and B). We next examined mutant seedlings growth and development upon salt stress. Seedlings germinated on normal MS plates were transferred to NaCl-containing plates at day 6 after germination to grow for 2 to 4 wk. Consistently, atg and ufl1 mutants were hypersensitive to persisting salt stress, displaying shorter roots compared to the Col-0 seedlings (Fig. 6 C and D). In accordance with the recent study, ufl1 mutants exhibited an ER stress-related phenotype, where ufl1 and atg5 mutants all had lower germination rates than Col-0 on DTT plates (Fig. S7 A and B) (10).
Fig. 6.
ufl1 mutants are hypersensitive to salt stress conditions. (A) Phenotypic analysis of Col-0, atg5, and ufl1 mutants (ufl1-1 and ufl1-2) directly germinated on salt stress plates with 50 mM or 100 mM NaCl. (B) Quantification of seedling germination ratio shown in (A) with at least three replicates. (C) Phenotypic analysis of seedlings of Col-0, atg5, and ufl1 mutants (ufl1-1 and ufl1-2) germinated on normal plates for 6 d, followed by another 10 d on salt stress plates with 100 mM NaCl before photography. (D) Quantification of seedling root length shown in (C) with at least three replicates. n ≥ 28 seedlings from biologically independent Col-0, atg5, ufl1-1, and ufl1-2 plants, respectively. **P < 0.01, ***P < 0.001, ****P < 0.0001.
To further investigate whether loss of other ufmylation components also leads to salt stress hypersensitive responses as the cases of Ufl1, we evaluated the phenotypes of ufm1 and ufc1 T-DNA insertional mutants under salt stress. Both mutants displayed etiolated leaves and decreased survival rates after prolonged exposure to salt stress (SI Appendix, Fig. S8 A and B). In short, loss of Ufm1 or Ufc1 phenocopied the hypersensitive phenotype of ufl1 mutants under salt stress. To conclude, loss-of-function of ufmylation led to plant growth defects upon salt stress.
Abnormal Expansion and Accumulation of ER Sheets in ufl1 Mutants under Salt Stress.
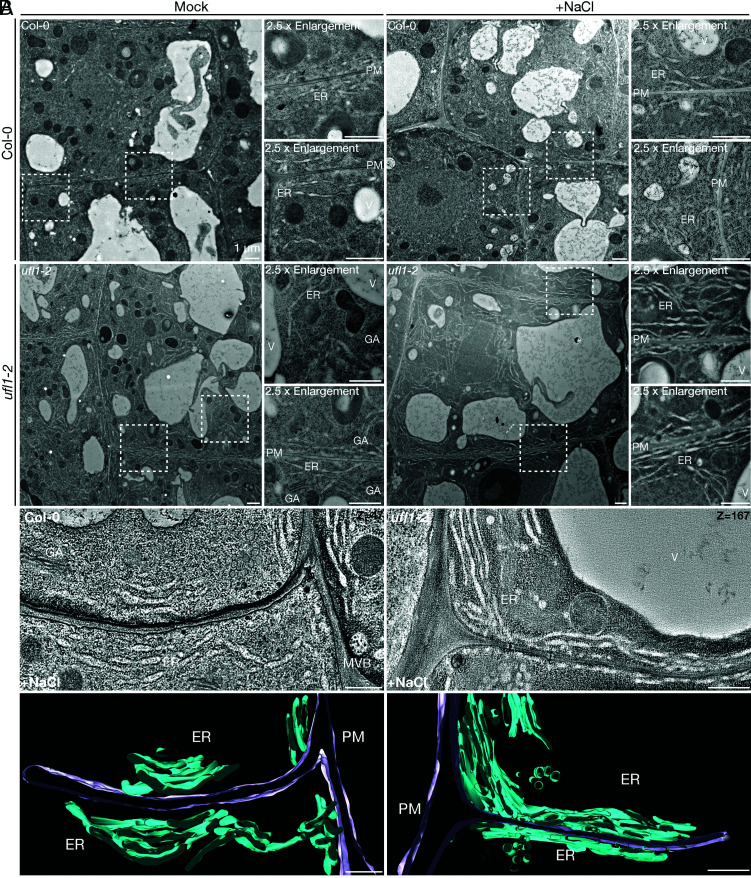
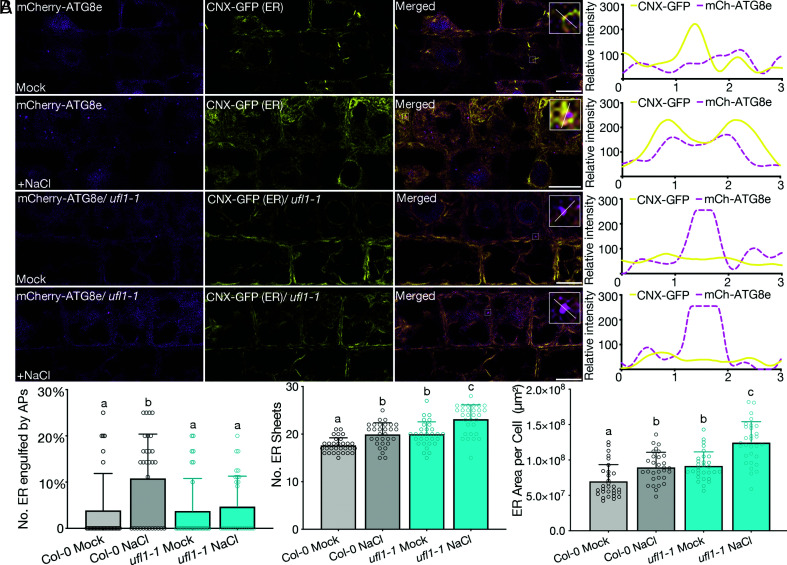
Recent studies have revealed a cross-talk between salt stress and ER stress pathways in plants, suggesting that the defective growth of ufl1 mutants upon salt stress may be due to the failure of ER homeostasis re-establishment under ER stress (33). To assess this hypothesis, we investigated the ER morphology in ufl1 mutants upon salt treatment by CLSM and EM analysis. Seedlings pre-treated with NaCl were stained by ER-tracker before confocal imaging. Serendipitously, extensively elongated ER sheets were observed in ufl1 mutants (SI Appendix, Fig. S9). 2D TEM and 3D-electron tomography (ET) analysis further showed massive ER network accumulation in ufl1 mutants under salt stress (Fig. 7 A and B). Accumulated ER were substantially observed in ufl1 mutants upon salt stress, with a considerable amount in close proximity to the plasma membranes (PMs) (Fig. 7B and Movie S1 and S2). Since Ufl1 localized to ER and was associated with autophagy under salt stress, we thus hypothesized that the massive ER sheets accumulation in ufl1 mutants under salt stress might be due to a failure in degradation and turnover of abrogated ER sheets by ER-phagy. We therefore generated transgenic lines to investigate the ER-phagy status under salt stress. We first stably co-expressed the autophagosome marker mCherry-ATG8e and the ER marker CNX-GFP in Col-0 or ufl1 mutant backgrounds. ER formed punctate structures and colocalized with ATG8e in the Col-0 background; however in ufl1 mutants, ER sheets accumulated and showed less punctate-like structures that colocalized with ATG8e under salt stress (Fig. 8 A and B). We also generated GFP-ATG8e lines in Col-0 or ufl1 mutant backgrounds to observe the ER structure by ER-tracker staining. Consistently, the amount and area of ER sheets increased significantly in ufl1 mutants upon salt stress, with less ATG8e-positive punctate-like structures adjacent ER when compared with Col-0 seedlings (SI Appendix, Fig. S10 A and B). Taken together, loss-of-function of Ufl1 impeded the efficient degradation of abrogated ER after salt stress induction by ER-phagy, which led to the accumulation of massive ER sheets in the mutant cells.
Fig. 7.
TEM and 3D-ET analysis of massive ER network in ufl1 mutants under salt stress condition. (A) Ultra-thin sections were prepared from HPF and freeze substitution root tip cells of 5-d-old Arabidopsis seedlings under mock or salt stress conditions. TEM analysis shows cortical cells in transition zones in Col-0 and ufl1-2 mutant root tips. GA, Golgi Apparatus; ER, endoplasmic reticulum; PM, plasma membrane; V, vacuole. (Scale bars, 500 nm.) (B) 3D-ET analysis of high-pressure-frozen 5-d-old ufl1-1 mutant root tip comparing with Col-0 seedlings under salt stress. Cortical cells in transition zones were selected for analysis. In the 3D model, ER was highlighted in turquoise and PM in lilac. MVB, multivesicular body. (Scale bars, 500 nm.)
Fig. 8.
Loss of Ufl1 impedes normal ER-phagy with ER accumulation in plants under salt stress condition. (A) Confocal imaging analysis showing root tip transition zone cortical cells of 5-d-old double transgenic lines co-expressing mCherry-ATG8e and CNX-GFP in Col-0 and ufl1-1 backgrounds treated with or without 100 mM NaCl. (Scale bars, 10 μm.) (B) Quantification of the total number of ER in close association with or engulfed by autophagosomes, the number of ER sheets, and the ER surface area, respectively, as shown in (A) from ≥ three biological replicates. n ≥ 30 individual cells from ≥15 individual seedlings were counted.
Ufmylation Components Are Closely Regulated by Salt Stress and Associated with Salt Stress-Triggered UPRs.
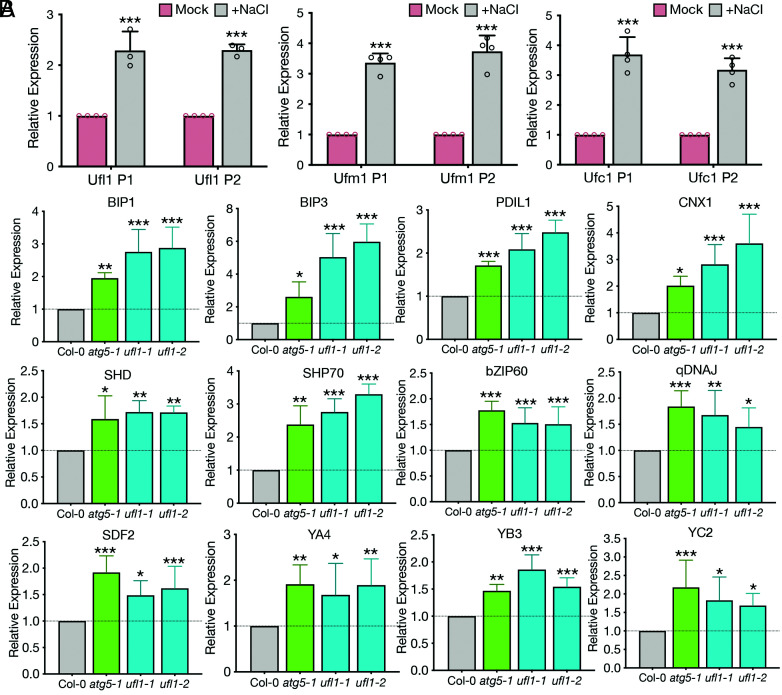
Salt stress induces a signaling cascade involving the processing of AtbZIP17, whose translocation to the nucleus up-regulates the salt stress-responsive genes (33). In response to ER stress, bZIP17 moves into the nucleus (33), where nuclear-localized bZIP17 binds to their target promoters to increase gene expression of ER chaperones, folding catalysts, and components in the ER-associated degradation (ERAD) machinery, who work coincidently to restore the ER homeostasis (50). Based on the previous discoveries that ufl1 mutants accumulated abnormal ER networks that did not colocalize with autophagosomal structures upon salt stress, the core components in the ufmylation cascade interacted with core ATG machinery proteins, and ufmylation mutants displayed hypersensitive salt stress phenotypes, we hypothesized that the ufmylation cascade may be regulated by salt stress and related to ER stress pathways. Ufl1, Ufc1 and Ufm1 were significantly up-regulated upon salt treatment in Arabidopsis seedlings (Fig. 9A). Interestingly, a variety of ER stress indicator genes also displayed significant increase in ufl1 as well as atg5 mutant backgrounds upon salt treatment, indicating that the ER was under severe stress in these mutants with difficulty in returning to homeostasis (Fig. 9B). To conclude, the ufmylation cascade is tightly regulated by salt stress, while its dysfunction led to the failure of regaining ER homeostasis from ER stresses during salt stress.
Fig. 9.
Ufmylation components are regulated by salt stress and contribute to salt stress-triggered UPRs. (A) qRT-PCR analysis comparing Ufl1, Ufc1 or Ufm1 expression levels in 5-d-old Col-0 seedlings under mock or salt stress conditions. Two sets of primers (P1, P2) targeting different sites were designed for each gene. n ≥ three biological replicates. (B) qRT-PCR analysis comparing selective ER stress marker genes in 5-d-old Col-0 seedlings with atg5, ufl1-1 and ufl1-2 mutants under salt stress condition. n ≥ three biological replicates. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Membranes draw the boundary for cellular organisms, where cells respond to stresses in dynamic and flexible ways via rapid remodeling of endomembrane systems (28, 51). Plants as sessile organisms that encounter persisting adverse and changing environmental conditions continuously self-regulate to re-establish cellular homeostasis. Dynamic turnover and recycling of essential macromolecules and organelles are tightly regulated by autophagy under stress (52, 53). Evidence has accumulated indicating the cross-talks of autophagy with salt, drought, and heat stress signaling pathways in plants (36, 37, 54). Although extensive studies have demonstrated the functional importance of the core ATG machinery proteins in plant autophagosome biogenesis, the nature of ancillary regulators and underlying mechanisms of abiotic stress inducing autophagy remain enigmatic. In this study, we identified and characterized the ufmylation E3 ligase Ufl1 as a novel interactor of ATG8 in Arabidopsis upon salt stress. More importantly, the ER over-accumulation in ufl1 mutants underpins future studies to reveal the detailed mechanisms of ufmylation in re-establishing ER homeostasis under stresses in plants.
In mammals, Ufl1 and the ufmylation cascade have been reported to participate in pleiotropic pathways. The core ufmylation components are identified as p62 regulators, where Ufl1 deficiency affected the p62 accumulation yet not LC3 flux (27). Furthermore, the interaction of Uba5 with GABARAP proteins has been structurally resolved, where GABARAPs regulate the localization and function of Uba5 on the ER membrane in a lipidation-independent manner (55, 56). In plants, a recent study defined C53 as an ER-phagy receptor binding to ATG8, and this interaction is negatively regulated by the competitive binding of Ufm1 to C53 (10). Interestingly, though independent of the IRE1-XBP1-mediated UPR pathway in plants, C53 is up-regulated by ER stress and C53 KO cells displayed activate UPRs in both PERK and IRE1α pathways in mammals (9, 26). Nonetheless, the functional roles of the ufmylation system in plants especially in stress-induced autophagy remain largely elusive (10). By screening for ATG8 interaction partners upon salt stress using IP-MS, we revealed that Ufl1 is a potential interactor of ATG8e in Arabidopsis, which has been further confirmed by Y2H, Co-IP, and FRET analysis (Fig. 1). Interestingly, the ufmylation components were shown to interact with ATG1 and ATG6, the core components for the early autophagic ATG1 and PI3K kinase complexes (Figs. 2 and 3), suggesting that the ufmylation system may serve as a scaffold for the docking of the ATG machinery during autophagosome biogenesis in eukaryotes. Our results expand the knowledge of functional linkages between the ufmylation system and autophagy.
Under ER stress, eukaryotes trigger UPRs that lead to ER network expansion, ER stress-responsive genes upregulation and elicit chaperone proteins to assist protein folding to regain homeostasis (32, 57, 58). We revealed that salt stress also causes dramatic ER expansion in Arabidopsis along with the activation of UPRs (Figs. 7 and 9 and SI Appendix, Figs. S9 and S10). Our results support the recent finding of an interplay between salt stress and ER stress pathways in plants, both of which involve the processing of AtbZIP17 to the nucleus for stress genes regulation (33). Further, ER stress has been shown to up-regulate the expression of Ufm1, Ufbp1, and Ufl1 in mammals (12). The abscission of IRE1-XBP1-mediated UPR pathway in xbi1 mutant directly decreases the Uba5, Ufl1, and C53 protein amount (13, 59). Vice versa, the knockdown of Ufm1, Ufbp1, or Ufl1 triggers the ER stress, apoptosis pathways, and ER amplification in mammalian cells (12, 13). Although the major role of ufmylation in RPL26-mediated ribosome stalling on ER has been reported, a scenario of the feedback loop in ER stress communicating with the ufmylation cascade has not been resolved (25). On the other hand, at the end of ER stress responses, autophagosomes as the bulky organelle degradation machine assume the role of recycling extra ER sheets via ER-phagy (57, 58, 60). Interestingly, the ufmylation components Ufl1, Ufc1 and Ufm1 are indeed significantly up-regulated upon salt treatment (Fig. 9A). Loss of Ufl1 causes pronounced ER accumulation (Fig. 7 and SI Appendix, Fig. S9), and the UPR genes are highly up-regulated (Fig. 9B). The defective function of ER-phagy in ufl1 mutants leads to ER over-accumulation under salt stress (Fig. 8 and SI Appendix, Fig. S10). We thus provided novel evidence for salt stress-induced ER expansion and ER-phagy indispensable of Ulf1 to mediate the degradation of bulky ER sheets under prolonged salt stress. Here, our study has not only unveiled a novel interactor of ATG8 under salt stress in Arabidopsis, but also underscored the role of ufmylation cascade in modulating ER-phagy cross-talking with ER stress responses, although more participants and detailed underlying mechanism are remaining to be investigated (Fig. 10 A and B). Future studies on the roles of ufmylation in stress responses and the identification of more ufmylation substrates will provide an integrated understanding of the multi-function ufmylation complex and broaden our current knowledge of how plants maintain ER homeostasis upon environmental stresses.
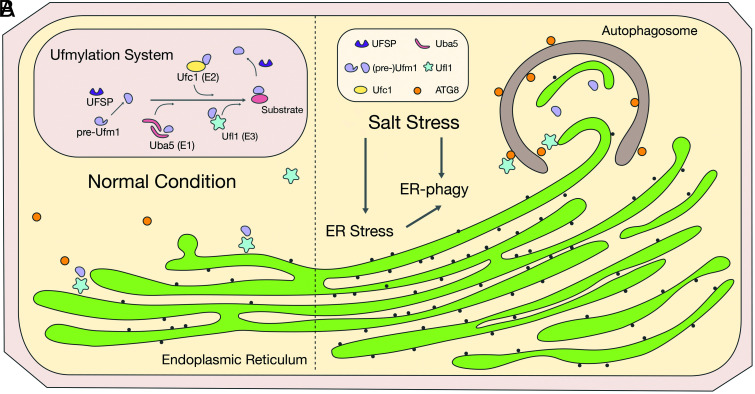
Fig. 10.
Working model of ufmylation functions in mediating ER turnover via ER-phagy under salt stress condition in plants. (A) Under normal condition, ER functions normally as the secretory protein factory. (B) Under salt stress condition, UPR and ER stress responses are triggered, where ER-localized Ufl1 complex interacts with ATG8s for the active degradation and turnover of the expanded or dysfunctional ER under stress condition.
Materials and Methods
Plasmid Construction.
All constructs used in this study were cloned from complementary DNAs from Arabidopsis seedlings into the destination plasmids via restriction digestion or gateway cloning and confirmed by Sanger sequencing as previously described (28).
Plant Materials, Growth, and Phenotypic Analysis.
T-DNA insertional lines were obtained from the Arabidopsis Information Resource (TAIR) and verified by genotyping and NGS. Transgenic plants were generated by floral dipping (61). Arabidopsis wave lines were obtained from the Nottingham Arabidopsis Stock Centre. Arabidopsis plant system biology dark-type culture (PSB-D) suspension cells were incubated at 25 °C and subcultured every 5 d. Arabidopsis seeds were grown at 22 °C under a long day (16 h light/8 h dark) photoperiod. NaCl was applied to seedlings for 6 to 8 h before further analysis. For phenotypic analysis, seeds were directly grown on or transferred to NaCl plates for the indicated times.
Transient Expression in Protoplasts.
Transient expression in Arabidopsis PSB-D protoplasts was performed as described previously (62). The transfected protoplasts were incubated for 12 to 16 h before confocal imaging or western blot analysis.
Confocal Microscopy and FRET Analysis.
Confocal images were captured by a Leica SP8 laser scanning confocal system with a 63 × water lens or Leica STELLARIS 8 confocal microscope with a 93 × glycerol lens following manufacturer’s instructions. FRET analysis was conducted on the same Leica SP8 confocal system according to the manufacturer’s instruction using 405 nm and 514 nm lasers. For surface rendering of ER sheets, Imaris 9.8.0 (Bitplane) was used as previously described (63).
TEM and 3D ET Analysis.
High-pressure freezing (HPF), freeze substitution, ultra-thin sectioning, and imaging were performed as previously described (64). Immunogold labeling for EM was performed with anti-GFP at optimized concentrations, then with goat anti-rabbit 10 nm gold-coupled secondary antibodies at 1:40 dilution. TEM examination was performed with a Hitachi H-7650 transmission electron microscope with a charge-coupled device (CCD) camera operating at 80 kV (Hitachi High-Technologies). ET, 3D reconstruction, and modeling were performed as described previously (63, 64).
Immunoblotting and Co-IP.
Co-IP and immunoblot analysis were performed as previously described (65).
Recombinant Protein Purification.
Recombinant protein expression and purification were performed as previously described (65).
Proteomics and MS.
Proteins were extracted from salt stress-treated PSB-D protoplasts with the collected supernatant co-incubated with purified proteins at 4 °C. Proteins pulled down were separated by SDS-PAGE gel then silver stained and cut out for in-gel trypsin digestion as previously described (66).
Y2H Assay.
Y2H analysis was performed as previously described (65).
Quantitative Reverse Transcription-PCR and RNA-seq Analysis.
Quantitative reverse transcription-PCR and RNA-seq analysis were performed following manufacturer’s instructions.
Statistical Analysis.
Sample numbers and the number of biological replicates for each experiment are indicated in figure legends or method sections. Data are presented as mean values ± SD. Statistical analysis was performed using paired t test or ANOVA using GraphPad Prism version 9.1.2 for Mac (GraphPad Software). Differences in means were considered statistically significant at *P < 0.05. Significance levels are *P < 0.05, **P < 0.01, and ***P < 0.001.
Additional details for Materials and Methods are described in the SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
3D electron tomography (ET) analysis of Col-0 Arabidopsis root tip cells upon salt stress. 3D electron tomography movie with the ER (turquoise) and the PM (lilac) highlighted in 3D modelling in the 5-day-old Col-0 root tip cells upon salt stress treatment.
3D electron tomography (ET) analysis of ufl1-2 mutant Arabidopsis root tip cells upon salt stress. 3D electron tomography movie with the ER (turquoise) and the PM (lilac) highlighted in 3D modelling in the 5-day-old ufl1-2 mutant root tip cells upon salt stress treatment.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (91854201), the Research Grants Council of Hong Kong (AoE/M-05/12, CUHK 14100818, 14101219, C4033-19E, C4002-17G, C4002-20W, C4002-21EF, C2009-19G, C4041-18E, and R4005-18), the Chinese University of Hong Kong (CUHK) Research Committee, and CAS-Croucher Funding Scheme for Joint Laboratories to L.J.
Author contributions
B.L. and Y.Z. designed research; B.L., F.N., Y.Z., M.K.T., C.D., L.H., S.G., and S.W.L. performed research; B.L., F.N., Y.Z., W.C., S.H., Y.D., and L.J. analyzed data; L.J. edited the paper; and B.L. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Baiying Li, Email: zorralee@gmail.com.
Liwen Jiang, Email: ljiang@cuhk.edu.hk.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Millar A. H., et al. , The scope, functions, and dynamics of posttranslational protein modifications. Annu. Rev. Plant Biol. 70, 119–151 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Komander D., Rape M., The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Pickart C. M., Eddins M. J., Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta 1695, 55–72 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Popovic D., Vucic D., Dikic I., Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Komatsu M., et al. , A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 23, 1977–1986 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang S. H., et al. , Two novel ubiquitin-fold modifier 1 (Ufm1)-specific proteases, UfSP1 and UfSP2. J. Biol. Chem. 282, 5256–5262 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Tatsumi K., et al. , A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 285, 5417–5427 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatsumi K., et al. , The Ufm1-activating enzyme Uba5 is indispensable for erythroid differentiation in mice. Nat. Commun. 2, 181 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J., Lei G., Mei M., Tang Y., Li H., A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing protein 1 (DDRGK1) and modulates NF-kappaB signaling. J. Biol. Chem. 285, 15126–15136 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephani M., et al. , A cross-kingdom conserved ER-phagy receptor maintains endoplasmic reticulum homeostasis during stress. Elife 9, e58396 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Y., et al. , UFBP1, a key component of the Ufm1 conjugation system, is essential for ufmylation-mediated regulation of erythroid development. PLoS Genet. 11, e1005643 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemaire K., et al. , Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS One 6, e18517 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu H., et al. , Ufbp1 promotes plasma cell development and ER expansion by modulating distinct branches of UPR. Nat. Commun. 10, 1084 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera-Serrano M., et al. , A homozygous UBA5 pathogenic variant causes a fatal congenital neuropathy. J. Med. Genet. 57, 835–842 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Cai Y., et al. , Indispensable role of the Ubiquitin-fold modifier 1-specific E3 ligase in maintaining intestinal homeostasis and controlling gut inflammation. Cell Discov. 5, 7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colin E., et al. , Biallelic variants in UBA5 reveal that disruption of the UFM1 cascade can result in early-onset encephalopathy. Am. J. Hum. Genet. 99, 695–703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duan R., et al. , UBA5 mutations cause a new form of autosomal recessive cerebellar ataxia. PLoS One 11, e0149039 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J., et al. , UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat. Cell Biol. 22, 1056–1063 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Muona M., et al. , Biallelic variants in UBA5 link dysfunctional UFM1 ubiquitin-like modifier pathway to severe infantile-onset encephalopathy. Am. J. Hum. Genet. 99, 683–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nahorski M. S., et al. , Biallelic UFM1 and UFC1 mutations expand the essential role of ufmylation in brain development. Brain 141, 1934–1945 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo H. M., et al. , Modification of ASC1 by UFM1 is crucial for ERalpha transactivation and breast cancer development. Mol. Cell 56, 261–274 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Kulsuptrakul J., Wang R., Meyers N. L., Ott M., Puschnik A. S., A genome-wide CRISPR screen identifies UFMylation and TRAMP-like complexes as host factors required for hepatitis A virus infection. Cell Rep. 34, 108859 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang R., et al. , CDK5RAP3, a UFL1 substrate adaptor, is crucial for liver development. Development 146, dev169235 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Li J., et al. , Ufm1-specific ligase Ufl1 regulates endoplasmic reticulum homeostasis and protects against heart failure. Circ. Heart Fail. 11, e004917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., et al. , UFMylation of RPL26 links translocation-associated quality control to endoplasmic reticulum protein homeostasis. Cell Res. 30, 5–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Zhang M., Wu J., Lei G., Li H., Transcriptional regulation of the Ufm1 conjugation system in response to disturbance of the endoplasmic reticulum homeostasis and inhibition of vesicle trafficking. PLoS One 7, e48587 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeJesus R., et al. , Functional CRISPR screening identifies the ufmylation pathway as a regulator of SQSTM1/p62. Elife 5, e17290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B., et al. , A distinct giant coat protein complex II vesicle population in Arabidopsis thaliana. Nat. Plants 7, 1335–1346 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Signorelli S., Tarkowski L. P., Van den Ende W., Bassham D. C., Linking autophagy to abiotic and biotic stress responses. Trends Plant Sci. 24, 413–430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Z., et al. , Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 63, 635–674 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Verma V., Ravindran P., Kumar P. P., Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 16, 86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howell S. H., Endoplasmic reticulum stress responses in plants. Annu. Rev. Plant Biol. 64, 477–499 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Liu J. X., Srivastava R., Che P., Howell S. H., Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 51, 897–909 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janse van Rensburg H. C., Van den Ende W., Signorelli S., Autophagy in plants: Both a puppet and a puppet master of sugars. Front. Plant Sci. 10, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Signorelli S., Masclaux-Daubresse C., Moriyasu Y., Van den Ende W., Bassham D. C., Editorial: Sugars and autophagy in plants. Front. Plant Sci. 10, 1190 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao Y., et al. , COST1 regulates autophagy to control plant drought tolerance. Proc. Natl. Acad. Sci. U.S.A. 117, 7482–7493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Xiong Y., Bassham D. C., Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 5, 954–963 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Zhou J., et al. , NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet. 9, e1003196 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su W., et al. , Poplar autophagy receptor NBR1 enhances salt stress tolerance by regulating selective autophagy and antioxidant system. Front. Plant Sci. 11, 568411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak I., et al. , Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M., et al. , RCAD/Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ. 22, 1922–1934 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizushima N., Yoshimori T., Ohsumi Y., The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Cao Y., Klionsky D. J., Physiological functions of Atg6/Beclin 1: A unique autophagy-related protein. Cell Res. 17, 839–849 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Eisenberg-Lerner A., Kimchi A., PKD is a kinase of Vps34 that mediates ROS-induced autophagy downstream of DAPk. Cell Death Differ. 19, 788–797 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kishi-Itakura C., Koyama-Honda I., Itakura E., Mizushima N., Ultrastructural analysis of autophagosome organization using mammalian autophagy-deficient cells. J. Cell Sci. 127, 4089–4102 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Noda N. N., Ohsumi Y., Inagaki F., ATG systems from the protein structural point of view. Chem. Rev. 109, 1587–1598 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Kabeya Y., et al. , LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hess M. W., et al. , Combining high-pressure freezing with pre-embedding immunogold electron microscopy and tomography. Traffic 19, 639–649 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Zhuang X., et al. , A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell 25, 4596–4615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J. X., Howell S. H., Endoplasmic reticulum protein quality control and its relationship to environmental stress responses in plants. Plant Cell 22, 2930–2942 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng Y., Li B., Lin Y., Jiang L., The interplay between endomembranes and autophagy in plants. Curr. Opin. Plant Biol. 52, 14–22 (2019). [DOI] [PubMed] [Google Scholar]
- 52.Liao C. Y., Bassham D. C., Combating stress: The interplay between hormone signaling and autophagy in plants. J. Exp. Bot. 71, 1723–1733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuang X., Chung K. P., Luo M., Jiang L., Autophagosome biogenesis and the endoplasmic reticulum: A plant perspective. Trends Plant Sci. 23, 677–692 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Luo L., et al. , Autophagy is rapidly induced by salt stress and is required for salt tolerance in Arabidopsis. Front. Plant Sci. 8, 1459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Habisov S., et al. , Structural and functional analysis of a novel interaction motif within UFM1-activating enzyme 5 (UBA5) required for binding to ubiquitin-like proteins and ufmylation. J. Biol. Chem. 291, 9025–9041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huber J., et al. , An atypical LIR motif within UBA5 (ubiquitin like modifier activating enzyme 5) interacts with GABARAP proteins and mediates membrane localization of UBA5. Autophagy 16, 256–270 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuck S., Gallagher C. M., Walter P., ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J. Cell Sci. 127, 4078–4088 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng Y., Li B., Zhang W., Jiang L., ER-Phagy and ER stress response (ERSR) in plants. Front. Plant Sci. 10, 1192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J., et al. , A critical role of DDRGK1 in endoplasmic reticulum homoeostasis via regulation of IRE1alpha stability. Nat. Commun. 8, 14186 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu S., Ye H., Cui Y., Jiang L., AtSec62 is critical for plant development and is involved in ER-phagy in Arabidopsis thaliana. J. Integr. Plant Biol. 62, 181–200 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clough S. J., Bent A. F., Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- 62.Miao Y., Jiang L., Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc. 2, 2348–2353 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Cao W., et al. , Correlation of vacuole morphology with stomatal lineage development by whole-cell electron tomography. Plant Physiol. 188, 2085–2100 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui Y., et al. , A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nat. Plants 5, 95–105 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Shen J. B., et al. , A plant Bro1 domain protein BRAF regulates multivesicular body biogenesis and membrane protein homeostasis. Nat. Commun. 9, 3784 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu T., et al. , Mass spectrometry analysis of the variants of histone H3 and H4 of soybean and their post-translational modifications. BMC Plant Biol. 9, 98 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
3D electron tomography (ET) analysis of Col-0 Arabidopsis root tip cells upon salt stress. 3D electron tomography movie with the ER (turquoise) and the PM (lilac) highlighted in 3D modelling in the 5-day-old Col-0 root tip cells upon salt stress treatment.
3D electron tomography (ET) analysis of ufl1-2 mutant Arabidopsis root tip cells upon salt stress. 3D electron tomography movie with the ER (turquoise) and the PM (lilac) highlighted in 3D modelling in the 5-day-old ufl1-2 mutant root tip cells upon salt stress treatment.
Data Availability Statement
All study data are included in the article and/or SI Appendix.