Significance
Understanding the biophysical properties of mycobacterial membranes is of crucial interest, especially in the context of drug discovery, but there is no realistic model of mycobacterial membranes. In this work, we have developed models of the inner membrane of mycobacteria mainly constituted of mannosylated phosphatidylinositol lipids. Our results provide significant and unexpected insights into the molecular details of mycobacterial membranes. Especially, they highlight how recently determined proteins can be accommodated into this specific membrane and how its lipid composition can affect antibiotics diffusion.
Keywords: mycobacteria inner membrane, phosphatidyl-myoinositol mannosides, antibiotics diffusion, multi-scale molecular dynamics
Abstract
Mycobacterium tuberculosis (Mtb) is the causative agent of tuberculosis (TB), a disease that claims ~1.6 million lives annually. The current treatment regime is long and expensive, and missed doses contribute to drug resistance. Therefore, development of new anti-TB drugs remains one of the highest public health priorities. Mtb has evolved a complex cell envelope that represents a formidable barrier to antibiotics. The Mtb cell envelop consists of four distinct layers enriched for Mtb specific lipids and glycans. Although the outer membrane, comprised of mycolic acid esters, has been extensively studied, less is known about the plasma membrane, which also plays a critical role in impacting antibiotic efficacy. The Mtb plasma membrane has a unique lipid composition, with mannosylated phosphatidylinositol lipids (phosphatidyl-myoinositol mannosides, PIMs) comprising more than 50% of the lipids. However, the role of PIMs in the structure and function of the membrane remains elusive. Here, we used multiscale molecular dynamics (MD) simulations to understand the structure-function relationship of the PIM lipid family and decipher how they self-organize to shape the biophysical properties of mycobacterial plasma membranes. We assess both symmetric and asymmetric assemblies of the Mtb plasma membrane and compare this with residue distributions of Mtb integral membrane protein structures. To further validate the model, we tested known anti-TB drugs and demonstrated that our models agree with experimental results. Thus, our work sheds new light on the organization of the mycobacterial plasma membrane. This paves the way for future studies on antibiotic development and understanding Mtb membrane protein function.
Tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb). In 2021 alone, there were an estimated 10.6 million new Mtb infections, leading to 1.6 million deaths (1). Thus, Mtb is one of the world’s leading infectious killers, despite the availability of both a treatment regime and vaccine. The current course of antibiotics for drug susceptible TB cases can last as long as 6 mo and consists of four drugs given in combination (2). This is not only expensive and demanding for the patient, but also encourages non-compliance that contributes toward the rise in multi-drug-resistant and extremely drug-resistant TB (3). It is obvious that new treatments and a better vaccine are needed to meet the World Health Organization’s “End TB Strategy.” Their plan aims to reduce TB-related deaths by 90% by 2030 and thereby curtail the enormous public health cost caused by TB (4). The COVID-19 pandemic has undone some of the progress that had been made in the treatment of TB as fewer people were able to be diagnosed or access medication (5), which might result in a worldwide surge of untreatable cases. Thus, there is a pressing need for innovative research into the mechanisms of Mtb virulence and its ability to survive within the host for extended periods to help develop alternative intervention strategies.
One issue in anti-TB drug discovery is the complexity of the mycobacterial cell envelope (6, 7). This cell envelope consists of an array of lipids contributing to both hydrophobic and polar regions of various thicknesses and densities, making it extremely challenging to predict how molecules will cross this barrier and enter the cell. The Mtb cell envelope has four distinct layers: the outer layer (or the mycomembrane) comprised mycolic acid esters and other complex lipids (such as phthiocerol dimycoserosates), an arabinogalactan-peptidoglycan layer, the periplasmic space and the inner membrane, or the plasma membrane (8). The mycobacterial plasma membrane plays a key role in controlling nutrient/antibiotic uptake and contains important membrane proteins that are targets for antitubercular drugs (9), such as SQ109 that inhibits the transporter MmpL3 (10). The membrane composition is also known to change during different growth stages of the bacteria (7) and may modulate drug and membrane protein diffusion. Additionally, recent studies showed that Mtb plasma membrane organization, such as the formation of functional membrane microdomains (11, 12), can affect the survival ability of mycobacteria. Therefore, understanding the molecular organization and dynamics of the Mtb plasma membrane is essential for developing effective drug candidates.
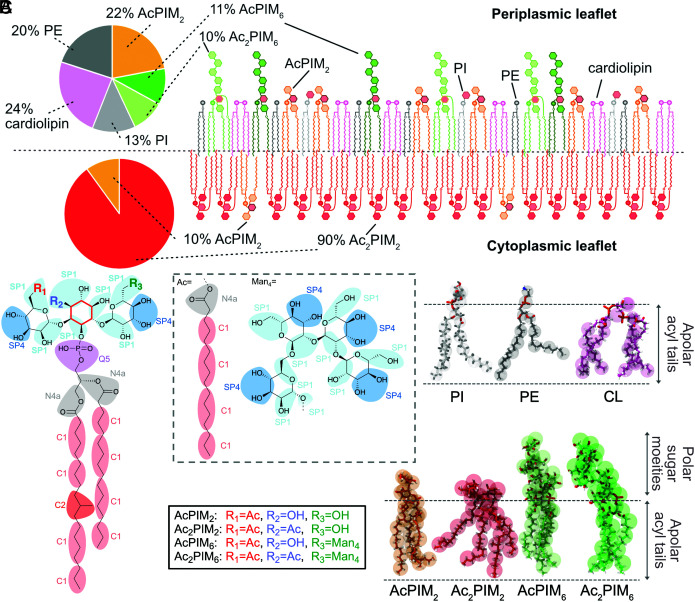
The mycobacterial plasma membrane is composed of a variety of lipids and glycolipids, the most abundant being (Fig. 1A): cardiolipin (CL), phosphatidylethanolamine (PE), phosphatidyl-myoinositol (PI), trehalose monomycolate (TMM), and phosphatidyl-myoinositol mannosides (PIMs) (13), with the PIMs accounting for over half the dry weight of the plasma membrane lipids (13). PIM2 lipids are comprised of a modified PI core decorated with two mannose residues and one acyl chain (Fig. 1B). Furthermore, additional modifications of an acyl group and up to four mannose sugars can be added to the core headgroup, further diversifying the PIM structure (14, 15) (Fig. 1B and SI Appendix, Fig. S1). It is still unclear if the plasma membrane is symmetric or asymmetric (16). In one widely cited and accepted study, it is proposed to be asymmetric (13), with Ac2PIM2 being the dominant species in the cytoplasmic leaflet accompanied by AcPIM2, while the periplasmic leaflet is more varied, containing AcPIM2, AcPIM6, Ac2PIM6, CL, PI, PE, and TMM (6, 13, 17, 18). PIM6 lipids can be further modified to lipomannan (LM) and lipoarabinomannan (LAM) that make up the bulk of the periplasmic space (19). Additionally, lipids have been shown to modulate membrane protein function (20, 21). However, due to the complexity of the mycobacterial cell envelope, the dynamics and properties of the plasma membrane are extremely difficult to probe experimentally.
Fig. 1.
Structure of the mycobacterial lipids. (A) Schematic of an asymmetrical model of the mycobacterial plasma membrane and composition as previously defined (13). (B) Schematic of the core of the PIM lipids found in mycobacteria with the groupings for CG beads. The inositol core is highlighted in red. The bead types for MARTINI 3 are shown. (C) Overlay of the AT (sticks) and CG (spheres) models for each lipid, with chemical characteristics shown to the Right.
Over the last decade, molecular dynamics (MD) simulations have emerged as a powerful strategy for studying structural and functional aspects of biomembranes (22, 23). However, at the time of writing, there are no complete models of the mycobacterial plasma membrane that capture a possible asymmetry and the diverse range of lipids (24). This limits our ability to understand Mtb plasma membrane biology, model drug–lipid interactions—important for anti-TB drug discovery efforts—and simulate mycobacterial membrane proteins in a native environment. Therefore, it is essential to develop models of the major phospholipids of the mycobacterial plasma membrane for application in MD simulations.
Herein, multiscale simulations were used to analyze the structure–function relationship of the four main PIM lipids (AcPIM2, Ac2PIM2, AcPIM6, and Ac2PIM6) found in the mycobacterial membrane. An asymmetric bilayer containing these lipids was assembled and simulated, showing the stability of this composition. To highlight the robustness of our approach, additional simulations representing (in total) two different asymmetric growth stages as well as symmetric membrane configurations were performed. Systematic analysis of the distribution of Mtb protein residues in contact with lipids reveals further indications that this membrane could possibly be asymmetric. We have also modeled how antibiotics, used to treat TB infections, diffuse through the membrane, and interact with membrane proteins and demonstrated that our models agree with experimental results (25). Overall, we have developed robust possible representations of the mycobacterial plasma membrane that will enable studies of membrane dynamics, lipid interactions with integral membrane proteins, and diffusion of antibiotics across this barrier.
Results
PIM Properties.
As an initial step toward developing an in-silico model of the mycobacterial plasma membrane, we first examined lipid properties in our CG and AT models. As shown in SI Appendix, Figs. S3–S8, the probability distributions of bonds and angles from the CG and AT simulations align well, which illustrates that the behavior of the acyl chains is similar to other phospholipids (30). The AcxPIMx aggregation in the AT and CG simulations is comparable and shows no permanent clustering. When lipids did come into contact, all areas of the molecule appear to play an equal role in the interactions (SI Appendix, Figs. S15 and S17). The interactions were not dominated by the sugars, as was seen with the previous iteration of the MARTINI 2 force field (62), and the phospholipids present in the mycobacterial membrane did not have strong interactions with the PIM lipids (SI Appendix, Figs. S18 and S19). The PIM lipids show a higher affinity for ions than PC in CG simulations, but the effect is less apparent in AT simulations (SI Appendix, Fig. S14). While the ion concentrations were the same, the number of ions in the simulation box was different (an order of magnitude higher for CG), which could explain these results. Overall, CG simulations of these lipids behave similarly to AT models (interactions with ions as a minor exception), therefore opening the door to significantly longer simulations by decreasing the degrees of freedom in the system.
The area per lipid in CG for each species in a PC bilayer was found to be 0.93 nm2 (AcPIM2), 1.15 nm2 (Ac2PIM2), 1.01 nm2 (AcPIM6), 1.14 nm2 (Ac2PIM6), 0.6 nm2 (PI), 0.55 nm2 (PE), and 1.25 nm2 (CL). The diffusion coefficients for each PIM species in a PC bilayer at 310 K are as follows: 6.7 × 10−7 cm2/s (AcPIM2), 1.1 × 10−7 cm2/s (Ac2PIM2), 7.6 × 10−7 cm2/s (AcPIM6), and 6.5 × 10−7 cm2/s (Ac2PIM6) (SI Appendix, Fig. S9). The difference between AcPIMx and Ac2PIMx shows the effect of the extra acyl tail in terms of how freely these lipids diffuse through the membrane.
The lipid shapes are approximately the same from AT to CG (SI Appendix, Figs. S10–S13), which agrees with the comparison of the surface areas (SI Appendix, Fig. S8C) and the sugar-phosphate z-distances in AcxPIM6 (SI Appendix, Figs. S5C and S7C). The additional mannose moieties project upward away from the membrane in both sets of simulations. Interestingly, the lipid tail region is measured to occupy approximately the same amount of space with three or four acyl chains. This is likely due to the placement of the fourth tail, projecting downward from the inositol sugar (as highlighted in red in Fig. 1B) and hence inhabiting space close to the other acyl chains.
Taken together, the lipid properties in our CG model broadly replicate those seen with AT resolution. This provided the confidence to assemble these lipids into a complex bilayer.
Mycobacterial Membrane Biophysical Properties.
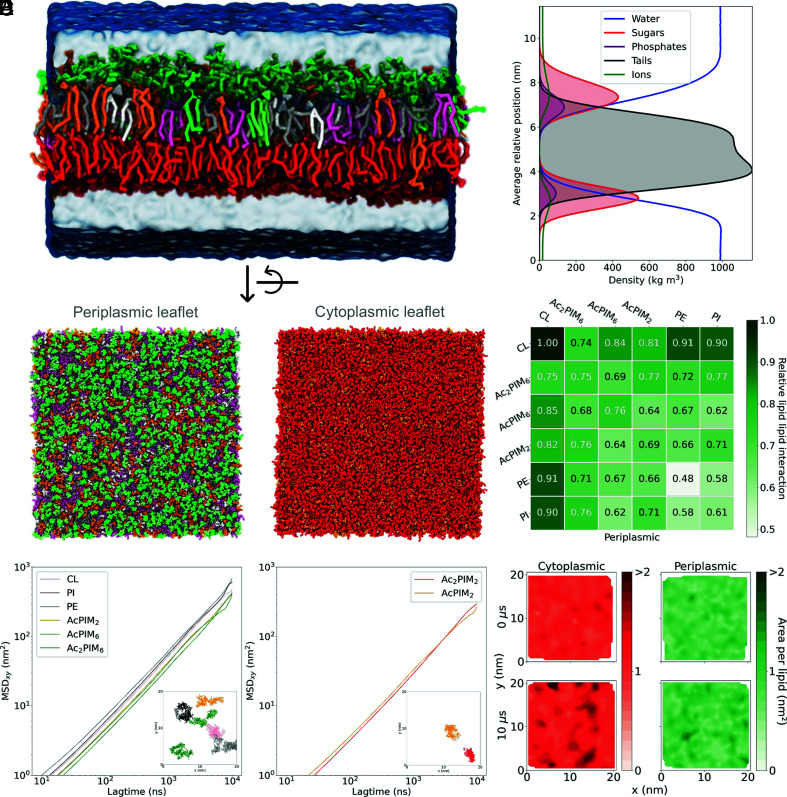
Traditional MD simulations of bacterial membranes use symmetric bilayer representation. However, a study has shown that the mycobacterial plasma membrane could be an asymmetric bilayer, and we used the published composition of the mycobacterial plasma membrane (13) (Fig. 1A) (excluding apolar lipids and TMM) to assemble an asymmetric bilayer (Fig. 2 A and C). This bilayer was found to be stable during 10 µs simulation (Movie S1) and in good agreement with the literature (8, 13). The thickness of the plasma membrane in our simulations was at 5.3 ± 0.1 nm (Fig. 2B), which is slightly narrower than values obtained from imaging studies (6.3 nm for Mycobacterium bovis and 7 nm for Mycobacterium smegmatis) (63–65). However, we did observe thicknesses of up to 7 nm in the density plot (Fig. 2B). In vitro culture conditions or growth stages of the bacteria (65) impact the thickness on the membrane and could explain any minor differences with our model.
Fig. 2.
Mycobacterial membrane model. (A) Side view of the membrane with each lipid type depicted in a different color as shown in Fig. 1A. (B) A density plot showing the density of water, sugar groups, phosphate groups, tail groups, and ions over the simulation box. (C) Snapshots of the periplasmic and cytoplasmic leaflets whose compositions are AcPIM2 22%, AcPIM6 11%, Ac2PIM6 10%, CL 24%, PE 20% and PI 13% (periplasmic leaflet), and AcPIM2 10% and Ac2PIM2 90% (cytoplasmic leaflet). The system size is 50 × 50 × 15 nm. (D) Relative number of neighbors of each lipid type for the periplasmic membrane. (E) Mean squared displacement (MSDxy) (nm2) as a function of lagtime (ns) for each lipid type in the periplasmic and cytoplasmic leaflets. The inserts show the position of the phosphate group of each lipid type over the last 500 ns of the simulation. (F) Contour plots of the area per lipid (nm2) in each leaflet at the starting frame (Upper) and final frame (Lower) of the simulation. A darker color indicates a larger area per lipid.
To test the phase behavior of this membrane, we performed simulations at various temperatures ranging from 290 to 350 K. For the whole range of temperatures, lipids were in the liquid phase and allowed to diffuse freely (Fig. 2E and SI Appendix, Fig. S24). The calculated diffusion coefficients for each species in the periplasmic leaflet at 310 K were as follows: 9.9 × 10−8 cm2/s (AcPIM2), 6.9 × 10−8 cm2/s (AcPIM6), 1 × 10−7 cm2/s (Ac2PIM6), 1.3 × 10−7 cm2/s (PI), 1.5 × 10−7 cm2/s (PE), and 1.2 × 10−7 cm2/s (CL). In the cytoplasmic leaflet, the values were 6.1 × 10−8 cm2/s (AcPIM2) and 8.1 × 10−8 cm2/s (Ac2PIM2). Compared to isolated PIMs in a PC membrane, the diffusion in this plasma membrane was roughly one order of magnitude slower. AcPIM2, which is the only lipid in both leaflets, diffused ~40% slower in the cytoplasmic membrane compared with the periplasmic leaflet. Using a previously reported model of mammalian plasma membrane (22), the diffusion coefficients of PE and PI were calculated to be 3.3 × 10−7 cm2/s and 2.8 × 10−7 cm2/s, showing that the diffusion in the mammalian membrane is equivalent to that in the mycobacterial plasma membrane. The membrane stiffness of our mycobacterial membrane was significantly lower at = 8.2 kBT compared to 13.9 kBT and 19.1 kBT for the mammalian and PC membrane models, respectively (SI Appendix, Fig. S23), highlighting a unique dynamic behavior for the mycobacterial plasma membrane.
Lipid clustering was moderate and membrane composition remained heterogenous over the course of each 10 μs simulation (Fig. 2C). This can also be seen in Fig. 2D where the number of surrounding lipids of the same type for each species in the periplasmic membrane was roughly equivalent to that of any other lipid species. The distribution of the area per lipid in the membrane also suggests high heterogeneity (Fig. 2F). The average area per lipid in the membrane over the course of the simulation was 0.89 nm2 and 1.18 nm2 for the periplasmic and cytoplasmic leaflets, respectively. Thus, the cytoplasmic leaflet appears to be a little denser than the periplasmic leaflet. This can be related to the packing of the four acyl chains of Ac2PIM2 lipids present in high concentration in this leaflet (66). A movie for the change in area per lipid for each leaflet over the course of the simulation can be found in Movies S2 and S3.
As was seen for the individual lipids in CG, the overall bilayer attracted ions; both Cl− and Na+ concentrations were much higher close to the bilayer, especially around the sugar head groups compared to bulk solution (Fig. 2B). It has been shown that lipid–ion interactions can affect the biophysical properties of the membrane, such as fluidity and stiffness, as well as the structure, which could modify the interaction with proteins (67).
The mycobacterial plasma membrane has been reported to change at different growth stages (7, 68), but the study that originally proposed the composition was based solely on late-exponential-phase cells. To explore a wider range of possible membrane compositions, we designed an “PIM-enriched” bilayer, where the amount of PIM lipids in the periplasmic leaflet was increased to reflect cells in rapid growth (7) (see compositions in SI Appendix, Fig. S22). In this case, the overall biophysical properties of that leaflet were very similar to those seen in Fig. 2, but interestingly a change in the diffusion and density of the cytoplasmic leaflet was observed (SI Appendix, Figs. S26 and S27). Increasing the proportion of lipids in the periplasmic leaflet that have three or four acyl chains could mean that the lipid tails make more inter-leaflet interactions and hence slow down diffusion and make the leaflet denser. This decrease in fluidity in the membrane could impact antibiotic diffusion through the membrane (13, 69) which would make the bacteria less susceptible to treatment at a time when it is most vulnerable.
We have also tested alternative plasma membrane compositions with a symmetrical configuration. First, we performed a model of a membrane recapitulating the composition being experimentally derived using a combination of mass spectrometry and NMR (see composition in SI Appendix, Figs. S22 and S38). This symmetrical configuration is stable, and the dynamics of PIM lipids in CG-MD simulations agreed with solid-state NMR results (SI Appendix, Figs. S38 and S39). This data shows that the acyl chains extremities are highly mobile, while the core of the acyl chains displayed a more rigid structure. Interestingly, the sugar moieties adopted an intermediate dynamic regime, as seen from experimental NMR results (SI Appendix, Fig. S38E) and MD simulations (SI Appendix, Fig. S39B). We then designed a model of symmetrical membrane system with a lipid composition that mimics Mtb membranes (see composition in SI Appendix, Fig. S22A). This membrane showed similar interactions of lipids compared to the asymmetric composition and also similar rates of diffusion (SI Appendix, Figs. S25 and S27). Clustering of ions around the sugar headgroups was still observed.
Taken together our observations suggest that PIM lipids play a critical role in the dynamic and structuration of mycobacterial plasma membrane. Both the asymmetric and symmetric configurations of the membrane were equally stable, and our analyses show that both share similar dynamic properties. This work demonstrates the plausible arrangements of the plasma membrane and provides a mechanism to easily study new membrane compositions based on future experimental results.
Asymmetrical Distribution of Residues at the Surface of Mtb Membrane Proteins.
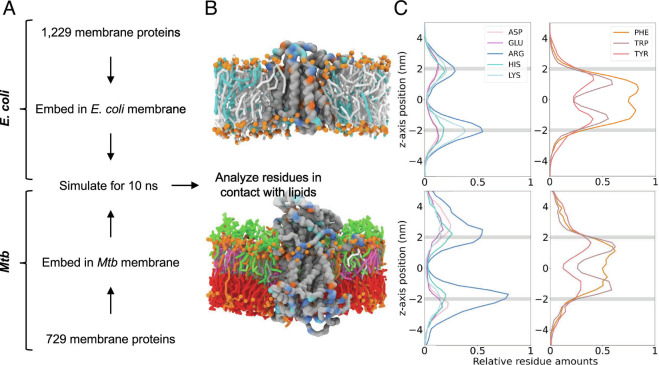
The lipids of the plasma membrane do not exist in isolation, but rather in cooperation with membrane proteins. These proteins could provide insights into how this membrane exists natively (70). To explore this, we used the accurate, fast, and reliable workflows of AlphaFold (49) and memembed (50), to insert all transmembrane proteins from Mtb and E. coli into their native-like membranes (Fig. 3 A and B). We then assessed the distribution of residues in proximity to the lipid bilayer (Fig. 3C).
Fig. 3.
Asymmetry of the mycobacterial membrane. (A) A simplified workflow for the lipid contacts analysis from all membrane proteins. (B) An example of a protein (E. coli protein MraY UniProt id: P0A6W3 and Mtb transporter MmpL3 UniProt id: P9WJV5) embedded in either an E. coli or mycobacterial membrane. The orange spheres represent phosphates. For the E. coli membrane, the cyan sticks show PG and the gray sticks PE. For mycobacterial membrane, the lipid sticks are shown in the colors illustrated in Fig. 1A. Selected residues are colored according to the key in (C). (C) Graphs for E. coli and Mtb (Top and Bottom respectively) showing the relative abundance of selected residues within 8 Å of lipids. The gray lines show the position of the phosphates. The cytoplasmic region is shown by negative z values, the periplasmic region by positive values.
For E. coli, the distribution is as expected and similar to results previously shown (21). The asymmetry of Arg and Lys reflects the “positive inside rule” (71–73) present in bacteria which aids in the correct insertion of the transmembrane helices. The symmetric nature of the lipid bilayer is otherwise reflected in the distribution of the residues (Fig. 3C and SI Appendix, Fig. S28). The Mtb distribution of Arg also shows a strong “positive inside rule.” This is at a higher intensity than in E. coli, likely due to the low levels of Lys observed (Fig. 3C). Furthermore, the decrease in the bulky Phe and Tyr residues (Fig. 3C) in the cytoplasmic leaflet compared to the periplasmic leaflet agrees with the increased density seen in an asymmetric membrane (Fig. 2B and SI Appendix, Fig. S26B).
These results add evidence to an asymmetric model previously proposed (13). In addition to validating the model proposed in this work, it could aid understanding of how mycobacterial membrane proteins differ from those found in other organisms and hence aid targeted therapies.
Asymmetric Interaction of the Membrane with Antibiotics.
We were interested in testing how the organization of this membrane affects the behavior of other molecules, such as antibiotics. We tested two widely used anti-TB antibiotics: ISZ and BDQ (Fig. 4A). To validate the behavior of these small molecules as well as the bond lengths/angles being account for through PyCGTOOL (52) (SI Appendix, Fig. S30), the LogP and solvent accessible surface area were calculated for each and compared to predicted values and AT values, respectively. The values for LogP for neutral BDQ (5.92 for CG, 6.37 predicted) and ISZ (−1.10 for CG, −0.71 predicted) agree very well, as do the solvent accessible surface area values (BDQ values 13.01 ± 0.49 nm2 for CG and 13.69 ± 1.11 for AT; ISZ values 7.48 ± 0.14 nm2 for CG and 7.35 ± 0.04 for AT).
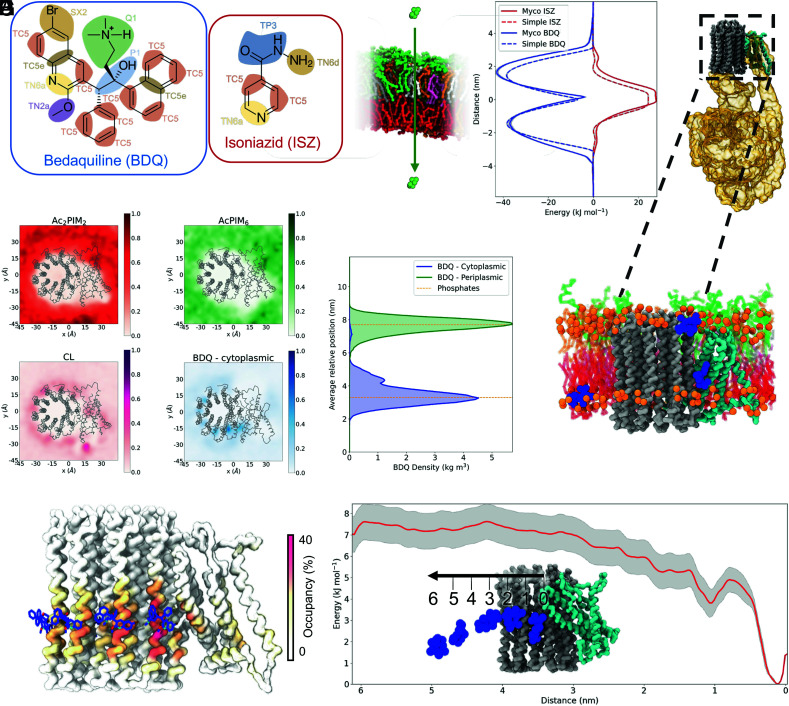
Fig. 4.
Behavior of the antibiotics with mycobacterial membrane and proteins. (A) Chemical structures of BDQ and ISZ with the CG groupings overlaid and bead types shown. (B) PMFs of the two antibiotics being pulled through either a mycobacterial or simple membrane in the z-direction. BDQ is shown in blue and ISZ in red, with the mycobacterial membrane results having a solid line and simple membrane having a dashed line. The error is shown in gray. A schematic of the PMF is shown to the Left. (C) Structure of Mycobacterium smegmatis ATP synthase (PDB: 7JG5) with the c-subunits shown in gray, the a-subunit shown in cyan, and the other components shown as a gold surface. (D) Density in the x and y dimensions of selected lipids and BDQ when starting in the cytoplasmic leaflet relative to the protein shown in gray. (E) Density of the phosphates (orange) and BDQ over the course of the simulations where the antibiotic started in either the periplasmic leaflet (green) or the cytoplasmic leaflet (blue). (F) Snapshot of a single simulation containing a Mtb ATPase model and 8 × BDQ models showing the main positions BDQ occupied. Phosphates are shown in orange, BDQ shown in blue, c-subunits are shown in gray, and the a-subunit is shown in cyan. The lipid sticks are shown in the colors illustrated in Fig. 1A. (G) Comparison of the highest occupancy sites identified with PyLipID (surface) and BDQ from the cryo-EM structure (PDB: 7JGC) (sticks). (H) PMF of BDQ moving through a mycobacterial plasma membrane with the error shown in gray. A schematic of the PMF is shown as an Insert.
ISZ is a first-line anti-TB treatment that targets InhA, a cytosolic enzyme that is essential for mycolic acid synthesis (74). BDQ on the other hand is a last line antibiotic that targets the membrane protein complex of the adenosine triphosphate (ATP) synthase (75, 76). The PMF results for ISZ and BDQ show that the mycobacterial membrane behaves as expected regarding the passage of a small molecule through a lipid-rich membrane, showing favorable interactions with the largely hydrophobic BDQ and unfavorable interactions with hydrophilic ISZ (Fig. 4B). The positive charge present on BDQ could account for the sharp free energy barrier at the membrane mid-plane. To assess the effect of the charge on the passage of BDQ since the protonation state is unknown, we modeled a neutral BDQ where the charged Q1 bead (Fig. 4A) was replaced by SC3. The PMF results with the neutral BDQ show a reduction in the energy barrier at this point (SI Appendix, Fig. S29D). We note that the passage of these drugs through an E. coli inner membrane is symmetric from the mid-plane of the membrane as expected. The interactions of BDQ with the periplasmic leaflet of the mycobacterial membrane were ~6 kJ/mol stronger than for the simple membrane and the mycobacterial cytoplasmic leaflet. This is possibly due to the presence of tetraacylated PIMs in the mycobacterial periplasmic leaflet. Roughly the same difference between the mycobacterial and simple membranes was observed for ISZ at the mid-membrane region, showing less favorable passage through the membrane. These results suggest that ISZ, a first-line TB drug, is unlikely to freely diffuse through the membrane, which is in contrast to the previously proposed uptake mechanism based on passive diffusion (77–79). Small differences were seen for ISZ interactions at the interfaces of the mycobacterial and simple membrane periplasmic membrane. This could be due to the presence of certain PIM lipids in this leaflet. In our simulations, when the BDQ starting position was in the bulk solvent, the drug quickly associated with the membrane, as shown in SI Appendix, Fig. S31, and displayed no strong preference for either leaflet in these simulations.
BDQ has been shown to target the mycobacterial ATP synthase (Rv1304-Rv1311), a membrane protein complex. A cryo-EM structure from M. smegmatis (PDB: 7JGC) shows multiple binding sites for BDQ at the interface of the c-subunits and the interface between the a- and c-subunits (25), where a highly negatively charged area is located (SI Appendix, Fig. S32 A and B). Using the available cryo-EM data, we performed ten simulations of 10 μs each to examine how Mtb ATP synthase complex behaves in our asymmetric mycobacterial plasma membrane model. ATP synthase was stable in the membrane and that there were no significant perturbations of the bilayer by the protein. The lipids of the mycobacterial membrane did not show any strong interactions with the protein (Fig. 4D and SI Appendix, Fig. S33), apart from CL that localizes in the a-subunit and around the c-ring (Fig. 4D) in positions similar to those observed in a previous study (80). The exact values for CL occupancy of each residue are shown in SI Appendix, Fig. S35A. Minimal interactions between BDQ and the ATPase occurred when the drug started in the periplasmic leaflet; however, when BDQ was introduced in the cytoplasmic leaflet, significant occupation of the binding sites on ATPase was observed (Fig. 4 D–G and SI Appendix, Fig. S32 C and D). The interactions were reduced with the neutral BDQ model (SI Appendix, Fig. S29 A–C). Of note, no such leaflet differences were observed when we tested the E. coli symmetric membrane model (SI Appendix, Figs. S36 C and D and S37), but with a symmetric mycobacterial membrane (SI Appendix, Fig. S32). This suggests that the lipid composition plays a crucial role in the regulation of antibiotic interactions.
Furthermore, the interactions of the charged BDQ we observed matched well with what was previously seen in the cryo-EM structure (25), with an occupancy of ~30% of the simulation time on some subunits (Fig. 4G). The occupancy value averaged over all subunits is shown in SI Appendix, Fig. S35B. The simulations were able to identify all three types of sites reported previously (25). Most interactions were seen through the leading site (46% of the time, Koff = 5.2 μs−1) followed by the lagging site (22% of the time, Koff = 7.6 μs−1) and finally further interactions around the rotor (36% of the time, Koff = 1.2 μs−1, SI Appendix, Fig. S34). For the leading site, the PMF calculations confirm this as a binding site, giving a moderate energy well of approximately 7 kJ/mol (Fig. 4H) and making it equivalent to a typical cholesterol–protein binding interaction (81). Tracking the z-position of the central bead from the antibiotic over the course of the unbiased simulations with the protein shows that the binding sites are occupied to some extent by each of the drug molecules and some are flipped to the outer leaflet from the plasma leaflet, as shown in SI Appendix, Fig. S32C.
These studies illustrate that our asymmetrical mycobacterial plasma membrane model can be combined with studies of antibiotics, as well as with studies of membrane proteins and their interactions with lipids and/or antibiotics. Taken together, our simulations suggest that BDQ must enter the target protein from the cytoplasmic side, which agrees with resistance mechanisms against BDQ that involve upregulation of MmpL5, a drug efflux pump (82, 83). Importantly, an E. coli membrane could not capture this feature, highlighting that the mycobacterial membrane model is a more accurate and functionally relevant representation of the Mtb plasma membrane.
Discussion
Here, we provide models for lipids constituting the mycobacterial plasma membrane focused on the PIM lipids, as they represent more than 50% of the total lipid content in Mtb. Thus, understanding how these lipids behave on an individual level and as a constituent of a membrane could provide key insight into the intrinsic resistance of Mtb to antibiotics.
In this CG model, clustering of ions around these lipids was observed at both the single lipid and bilayer levels, probing how this affects the biophysical properties of the bilayer and whether this could be exploited for treatment of TB is an interesting area for future research. The lipids did not cluster together excessively over the timescales studied and all diffused well through the membrane. This confirmed that the mycobacterial cell envelope is dynamic, which could potentially be an important insight into how this cell wall functions. A low membrane bending rigidity compared to a PC and eukaryotic plasma membrane is interesting and could suggest the importance of other cell envelope components in maintaining the shape of the cell.
The simulations confirmed that our asymmetric plasma membrane model is stable with a composition of over 50% PIM lipids. However, considering that this arrangement has not been experimentally validated, a symmetric and “enriched PIM” asymmetric membrane was also shown to share similar biophysical properties. The high proportion of PIM lipids resulted in some unique properties, most notably lower membrane stiffness, which may have important implications for membrane microdomain formation, drug penetration, and integral membrane protein behavior. In this context, the integral membrane proteins from Mtb are of interest for the development of new antibiotics for TB (9), and we show evidence that our membrane model can be used to simulate proteins in a native lipid environment to determine any key lipid interactions, as illustrated by our study of antibiotic BDQ and its target, ATPase. In addition, an overall comparison of plasma membrane protein residues found in contact with the lipid bilayer revealed differences between Mtb and E. coli, supporting the asymmetric nature of the mycobacterial plasma membrane model. This potentially hints at topological differences between proteins in mycobacteria and those within other bacteria. If this is the case, currently unknown proteins which maintain the asymmetry might be present [such as those identified in animal cells (84)] and would constitute interesting drug targets to explore.
Potential limitations of our model stem from the reduced type of lipids we included in this initial development. Thus, future studies will focus on expanding the model to include apolar lipids [such as triglycerides (17)], TMM, and LM/LAM. Moreover, future simulations with proteins and probing protein/lipid interactions with AT resolution could help further elucidate the role of these complex lipids.
In conclusion, our study is a starting point for building an entire mycobacterial cell envelope, as done for gram-negative bacteria (85). Other mycobacterial lipids have already been parameterized (86), and combining them with the model described here has the potential to significantly advance the field that has been lacking experimental strategies for plasma membrane investigations. Additionally, the mycobacterial cell envelope is known to change at different growth stages during its life cycle (7, 68, 87, 88) and in response to different environments (69). With our model and setup methods, as shown with the multiple compositions simulated, it will be possible to understand drug permeability or protein behavior at different stages of infection. Taken together, integrating our mycobacterial plasma membrane model into broader studies of this pathogen, its pathogen/host interactions, as well as into anti-TB drug discovery and development has a potential to reveal new functional insights and yield improved therapies.
Methods
Building the Coarse-grained Lipid Parameters.
The coarse-grained (CG) models of the lipids were parametrized for the newly released MARTINI 3 force field (26) and generated using the protocol described for small molecules (27) on the MARTINI website (http://cgmartini.nl/index.php/martini-3-tutorials/parameterizing-a-new-small-molecule). The bead types and mapping to the PIM molecules were performed manually, comparing with the recently published CG model of PI (28). Atoms were grouped according to functional groups, in sets of three to five non-hydrogen atoms. The CG mapping of AcxPIMx is shown in Fig. 1 B and C. The parameter files that describe the bonds, constraints, and angles were assembled based on the previously described data (26, 28, 29) as an initial estimate.
Simulations of each lipid were set up using a modified version of insane.py python script (30), embedding one copy of a PIM lipid in a 10 × 10 nm2 phosphatidylcholine (PC) bilayer. The system was solvated with MARTINI water (26) and neutralized with 150 mM NaCl, followed by minimization using the steepest descents algorithm. The system was then simulated for 3 μs using a timestep of 20 fs at 310 K. The lipids, solvent, and ions were temperature coupled separately. The velocity rescale (31) and Parrinello-Rahman (32) coupling methods were used with the time constants τT = 1.0 ps and τp= 12.0 ps for temperature and pressure, respectively. Simulations were run using GROMACS version 2021.3 (33). The reaction-field algorithm (34) was used for electrostatics interactions with a cutoff of 1.1 nm. A single cutoff of 1.1 nm was used for the van der Waals interaction. Five repeats were performed for each lipid (SI Appendix, Fig. S2). AcxPIMx lipids parameters are available on P Stansfeld Github webpage (see Data availability section).
Generating Atomistic (AT) Data.
Parameters for the AT AcxPIMx lipids were generated using the CHARMM force field (35). The CHARMM-GUI (36) server was used to set up the lipid systems for GROMACS with the CHARMM36m force field (37, 38). One PIM lipid was embedded in an 8 × 8 nm2 PC bilayer. The system was solvated with 150 mM NaCl and minimized and equilibrated as per the CHARMM-GUI Membrane Builder workflow (39). The system was further minimized using the steepest descents. Simulations were run for 2 μs using a timestep of 2 fs at 310 K. The lipids, solvent, and ions were temperature coupled separately. The velocity rescale (31) and C-rescale coupling methods were used with the time constants τT = 0.1 ps and τp= 1.0 ps for temperature and pressure, respectively. Simulations were run using GROMACS version 2021.3 (33). The particle mesh Ewald (PME) (40) method was used for electrostatic interactions with a cutoff of 1.2 nm. A single cutoff of 1.2 nm was used for the van der Waals interaction. Three repeats were performed for each lipid (SI Appendix, Fig. S2). AcxPIMx models are available through the Membrane Builder workflow (39) under the LPS modeler section (see Data Availability section).
Refining Coarse-grained Parameters.
The AT and CG representations of the four main PIM lipids are shown in Fig. 1C. The distributions of the distances and angles were measured using the gmx tools (distance, gangle and analyze). For the AT simulations, the atoms were grouped according to their mapping, and its center of geometry was used for calculations. The values for each bond/constraint and angle were iteratively refined based on the comparison of probability distribution, and the results are summarized in SI Appendix, Figs. S3–S8. When there was agreement between the AT and CG data, the solvent accessible surface area was measured using the gmx sasa tool to verify that the models behaved the same (SI Appendix, Fig. S8C). Diffusion, shape of the lipids, clustering of ions, aggregation, and interaction with other lipids were also measured on a single lipid level (SI Appendix, SI Methods and Figs. S9–S19). As typical for MARTINI (41), dihedral terms are not defined in the parameters.
Measuring Area per Lipid.
Before setting up the complete bilayer system, the area per lipid was measured. The simulations were assembled as described above for CG, using a homogenous AcxPIMx membrane (SI Appendix, Fig. S2). The area of XY-dimension was measured using gmx energy and then dividing the area by the number of lipids in one leaflet over the course of the trajectory, and the final values were extracted using gmx analyze, as per the protocol described on the MARTINI website (http://cgmartini.nl/index.php/tutorials-general-introduction-gmx5/bilayers-gmx5#Area-per-lipid).
Bilayer Composition and Setup.
The ratio of lipids in each bilayer was obtained by using their molecular weight and the previously reported dry masses (13) of the individual lipids, and the exact calculations can be seen in SI Appendix, Fig. S20. Since specific apolar lipids were not named, they were not included in this study. TMM was not included as there is no available refined AT model, the behavior of the mycolic acid is predicted to be complicated (42), and there is a relatively small proportion of TMM predicted to be present in the plasma membrane. The final composition of the plasma membrane is shown in Fig. 1A.
The PI and PE lipids in mycobacteria are slightly different in structure to the corresponding E. coli lipids described in the MARTINI force field (26, 28): A methyl group replaces the alkene found in one of the acyl chains (SI Appendix, Fig. S1). Before assembling the membrane, the parameters for these lipids were modified by adapting the refined Ac2PIM2 acyl tail parameters and changing the existing tail. The CL parameters were transferred over from the MARTINI 3 beta force field (21).
Simulations of the bilayer were set up using a modified version of insane.py Python script (30), available on the P Stansfeld lab Github page (see Data Availability section), using the composition shown in Fig. 1A, where the area per lipid for the periplasmic membrane was set to 0.92 nm2 and the cytoplasmic leaflet set to 1.13 nm2. The systems were then treated the same as described for the initial CG systems with added equilibration steps as per the Membrane Builder workflow (39) before the production simulation. There were two types of system assembled to represent the asymmetric membrane previously mentioned (13). The first one had an initial simulation box size of 20 × 20 × 15 nm3, and one repeat was performed for 10 μs at 290 K, 300 K, 310 K, 320 K, and 350 K (Fig. 2A). The second one had an initial box size of 50 × 50 × 15 nm3, and a single repeat was performed for 10 μs at 310 K (Fig. 2C and SI Appendix, Fig. S2). The number of lipids in each system is summarized in SI Appendix, Fig. S21. Mycobacterial membrane model can be generated using CHARMM-GUI MARTINI bilayer Maker (89) (see Data Availability section).
To test how the membrane properties change with differing compositions, the same assembly procedure was followed to set up a symmetric membrane and an “PIM-enriched” membrane (SI Appendix, Fig. S22), with one repeat for each in a simulation box of 20 × 20 × 15 nm3, and another in a box of 50 × 50 × 15 nm3, both at 310 K (SI Appendix, Fig. S2). Another membrane to capture an experimentally extracted membrane was simulated for 10 μs in a 20 × 20 × 20 nm3 simulation box at 310 K. The number of lipids in each system is summarized in SI Appendix, Fig. S21 and their compositions in SI Appendix, Fig. S22.
Behavior of the Membrane.
To calculate the bending rigidities, the strategy proposed by Fowler et al. (43) to extract the bending modulus from CG simulations was followed. The membrane mid-plane position in Monge representation was determined by extracting the coordinates of all CG beads at the extremities of lipid fatty acid chains. Using a built-in function of the Mathematica software package, the positions were interpolated to get a smooth function before Fourier-transforming with a fast Fourier transform algorithm. From the so-obtained Fourier modes, the spectral density (or power spectrum) was estimated for a tensionless membrane:
where is the projected area in the plane, is the thermal energy, and J/m2 is the tension associated with lipid protrusions at the nanometer scale. was plotted as a function of and fit it with a second-order polynomial , from which estimates of (and if needed) were obtained (SI Appendix, Fig. S23). The error bars are standard deviations provided by the fitting function in Mathematica.
The density of each constituent was measured using gmx density (Fig. 2B). Diffusion, bilayer thickness, and number of neighbors were measured using LiPyphilic (44) (Fig. 2 D and E and SI Appendix, Figs. S24–S27 and SI Methods). The XY-positions of single lipids were tracked with PLUMED (45) (Fig. 2E). Area per lipid for the membrane was calculated using FATSLiM (46) (Fig. 2F). Plots were created using Matplotlib (47).
Analysis of the Residue Distribution in Membrane Proteins.
All predicted protein structures from Mtb (taxonomy id:83332) and E. coli (taxonomy id:83333) labeled as transmembrane on UniProt (48) were downloaded from the AlphaFold database (49), totaling 729 for Mtb and 1,229 for E.coli. Alphafold was used to obtain a workable dataset of structures, expanding upon the number of experimentally determined Mtb and E. coli membrane protein structures available from the PDB. Each of 1,958 membrane protein structures was orientated in a membrane using memembed (50) with the -n in flag, then converted to CG using martinize2 (51), and inserted into either the asymmetrical mycobacterial (Fig. 1A) or E. coli membrane [75% PE, 15% Phosphatidylglycerol (PG), 10% CL] using a modified version of the insane.py python script (30). Each system was simulated for 10 ns. The residues within 8 Å of the lipids were selected and the density over the z-coordinate plotted (Fig. 3 and SI Appendix, Fig. S28).
Antibiotic Simulations.
Bedaquiline (BDQ) and isoniazid (ISZ) were mapped to CG using PyCGTOOL (52) following 200 ns AT simulations with parameters from CHARMM-GUI (53) (Fig. 4A). The pKa of the amine in BDQ is 8.91 (54), suggesting it will be protonated at physiological pH. This was considered when assigning the MARTINI 3 bead type (Fig. 4A). To validate the use of the protonated state, the same simulations with a neutral BDQ molecule were performed and analyzed (SI Appendix, Fig. S29 A–C). This was done by replacing the Q1 particle with a SC3 bead type (Fig. 4A). In addition, the LogP for these antibiotics was calculated by perturbing the antibiotic in a box containing 878 MARTINI waters or 363 octanol and 32 waters. The Lennard-Jones parameters for each molecule were decoupled over 11 evenly spaced windows, for 50 ns per window. Simulations were run with 20 fs timesteps in the NPT ensemble with the V-rescale thermostat at 323 K and an isotropic Parrinello-Rahman pressure coupling. Free energies were then computed using gmx bar and converted to water/octanol partitioning constants. The values were then compared to data from the ALOGPS online server (55). Alongside this, gmx sasa was used to calculate the solvent accessible surface area for the antibiotics in both CG and AT. The calculations were performed with 4,800 dots per sphere and a probe size of 0.4 nm. Modified van der Waals radii for CG beads of 0.264, 0.230, and 0.191 nm for R, S, and T beads were used, respectively. For the Connolly surface generation, the calculations used 240 dots and a probe of 0.4 nm (SI Appendix, Fig. S30). Parameters for BDQ and ISZ are available on P Stansfeld lab Github webpage (see Data availability section).
BDQ was first simulated with the mycobacterial membrane alone to confirm association with the membrane (SI Appendix, Figs. S2 and S31). The Mtb a- and c-subunits of F-ATPase, BDQ’s target in the mycobacterial membrane, was modeled using SwissModel (56) based on a structure from Mycobacterium smegmatis (25) (PDB: 7JGC, Fig. 4C and SI Appendix, Fig. S32 A and B). The sequence identity between Mtb and M. smegmatis for the F-ATPase was calculated using Clustal Omega (57) at 80% and 72% for c- and a-subunits, respectively. The system with the protein in the asymmetric mycobacterial membrane (Fig. 1A) was assembled using martinize2 (51), memembed (50), and the modified insane.py Python script (30), with eight molecules of BDQ placed either in the periplasmic or cytoplasmic leaflet with five repeats in each membrane (SI Appendix, Fig. S2). Simulations were run for 10 μs using the same setting as described above. The XYZ-positions of BDQ, the lipids, and the backbone beads were tracked over the course of the simulations with PLUMED (45) and plotted with Matplotlib (47), and the results are shown in Fig. 4D and SI Appendix, Figs. S32 C and D and S33. The density of each constituent was measured using gmx density and plotted using Matplotlib (47), and the results are shown in Fig. 4E (with representative positions shown in Fig. 4F). The interaction of BDQ with the protein was calculated using PyLipID (58) (Fig. 4G and SI Appendix, Figs. S34 and S35). The same simulation setup was performed with a model E. coli membrane (75% PE, 15% PG, 10% CL) and with the symmetric mycobacterial membrane (SI Appendix, Fig. S22A). The z-position of BDQ is shown in SI Appendix, Figs. S32, S36 and S37.
PMF Calculations.
The potential of mean force (PMF) calculations for the F-ATPase-BDQ interaction was performed (59). First, a representative pose of BDQ bound to the protein was produced using PyLipID from equilibrium simulations and built into a 12 × 20 × 11 nm3 asymmetric mycobacterial membrane (Fig. 1A) membrane and minimized and equilibrated, as described above. Light (50 kJ/mol/nm2) XY positional restraints were added to Ala 66 on three c-subunits to prevent the protein from rotating in the membrane. Following 50 ns of equilibration, the BDQ was steered away from the protein along the y axis at a rate of 1 nm/ns with a 1,000 kJ/mol/nm2 restraint potential. Frames were extracted at 0.1 nm spacing along this coordinate to seed a total of 58 × 1.5 µs production simulations with a static 1,000 kJ/mol/nm2 umbrella potential imposed to keep the system in the same position along the reaction coordinate. The PMF mdp input files can be downloaded at https://github.com/chelsea-brown/PIM-lipids/tree/main/PMF-files. The PMF profiles were then constructed using the weighted histogram analysis method in GROMACS [gmx wham (60, 61)] and employing 200 rounds of Bayesian bootstrapping to report statistical accuracy (Fig. 4H).
For the membrane crossing PMFs, BDQ or ISZ was placed free in the solvent phase, 7 nm away from the membrane periphery in either an asymmetric mycobacterial membrane (Fig. 1A) or E. coli membrane. The drug was then steered toward and through the membrane and into the solvent phase on the other side. Windows were extracted, simulated (1 µs for ISZ per window and 2 µs per window for BDQ), and analyzed as described above (Fig. 4B and SI Appendix, Fig. S29D for BDQ neutral). The -cycl option was imposed when running gmx wham.
Supplementary Material
Appendix 01 (PDF)
A video showing 50 × 50 nm2 asymmetric bilayer over a 10 μ03BC;s simulation. The cytoplasmic leaflet is on the left and the periplasmic leaflet on the right. The lipids are colored as shown in Figure 1A.
A contour plot showing the area per lipid of the cytoplasmic leaflet of a 20 × 20 nm2 asymmetric bilayer. The darker color indicated a larger area per lipid.
A contour plot showing the area per lipid of the periplasmic leaflet of a 20 × 20 nm2 asymmetric bilayer. The darker color indicated a larger area per lipid.
Acknowledgments
C.M.B. is supported by an MRC studentship (MR/N014294/1). R.A.C. is funded by Wellcome (208361/Z/17/Z). Research in P.J.S.’s lab is funded by Wellcome (208361/Z/17/Z), the MRC (MR/S009213/1), and BBSRC (BB/P01948X/1, BB/R002517/1 and BB/S003339/1). M.C. is supported by the CNRS-MITI grant “Modélisation du vivant” 2020. W.I. is funded by NSF (MCB-2111728). E.F is a Sir Henry Dale Fellow jointly funded by the Wellcome Trust and Royal Society (104193/Z/14/Z and 104193/Z/14/B). M.G. would like to acknowledge the European Union’s Horizon 2020 research and innovation program under grant agreement H2020-PHC-08-2014-643381, TBVAC2020. This work was granted access to the HPC resources of CALMIP supercomputing center (under the allocation 2021-17036) and TGCC Joliot-Curie supercomputer (under the GENCI allocation A0110712941). This project made use of time on ARCHER2 and JADE2 granted via the UK High-End Computing Consortium for Biomolecular Simulation, HECBioSim (http://hecbiosim.ac.uk), supported by EPSRC (grant no. EP/R029407/1). This project also used Athena and Sulis at HPC Midlands+, which were funded by the EPSRC on grants EP/P020232/1 and EP/T022108/1. This work has benefited from the facilities and expertise of the Biophysical and Structural Chemistry platform (BPCS) at IECB, CNRS UAR3033, INSERM US001, and Bordeaux University. We thank the University of Warwick Scientific Computing Research Technology Platform for computational access. We acknowledge Life Science Editors for proofreading the manuscript. We thank C. Cooper for fruitful discussions and M. Costic for comments on the manuscript.
Author contributions
C.M.B., R.A.C., E.F., W.I., P.J.S., and M.C. designed research; C.M.B., R.A.C., A.G., Y.G., Y.K.C., E.L. M.G., and J.N. performed research; C.M.B., R.A.C., A.G., Y.K.C., N.D., M.G. and A.L. analyzed data; and C.M.B., R.A.C., M.G., A.L., W.I., P.J.S., and M.C. wrote the paper.
Competing interest
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Wonpil Im, Email: wonpil@lehigh.edu.
Phillip J. Stansfeld, Email: phillip.stansfeld@warwick.ac.uk.
Matthieu Chavent, Email: matthieu.chavent@ipbs.fr.
Data, Materials, and Software Availability
Data availability: https://github.com/pstansfeld/PIM-lipids. AT systems and CG-membrane setup can be performed using, respectively, CHARMM-GUI bilayer builder (https://charmm-gui.org/input/membrane.bilayer) and CHARMM-GUI MARTINI bilayer Maker (https://charmm-gui.org/?doc=input/martini.bilayer).
Supporting Information
References
- 1.WHO, “Global tuberculosis report 2022” Report Number: 27 (World Health Organization, Geneva, 2022). [Google Scholar]
- 2.Horsburgh C. R., Barry C. E., Lange C., Treatment of tuberculosis. New Engl. J. Med. 373, 2149–2160 (2015). [DOI] [PubMed] [Google Scholar]
- 3.WHO, “Global tuberculosis report 2021” Report Number: 26 (World Health Organization, Geneva, 2021). [Google Scholar]
- 4.WHO, “The end TB strategy” Report Number: 1 WHO Press, World Health Organization; (2022). [Google Scholar]
- 5.WHO, “1.4 million with tuberculosis, lost out on treatment during first year of COVID-19” (2021). https://news.un.org/en/story/2021/03/1087962UNNews
- 6.Chiaradia L., et al. , Dissecting the mycobacterial cell envelope and defining the composition of the native mycomembrane. Sci. Rep. 7, 12807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dulberger C. L., Rubin E. J., Boutte C. C., The mycobacterial cell envelope — a moving target. Nat. Rev. Microbiol. 18, 47–59 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Batt S. M., Minnikin D. E., Besra G. S., The thick waxy coat of mycobacteria, a protective layer against antibiotics and the host’s immune system. Biochem. J. 477, 1983–2006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fullam E., Young R. J., Physicochemical properties and Mycobacterium tuberculosis transporters: Keys to efficacious antitubercular drugs? RSC Med. Chem. 12, 43–56 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacksteder K. A., Protopopova M., Barry C. E. III., Andries K., Nacy C. A., Discovery and development of SQ109: A new antitubercular drug with a novel mechanism of action. Future Microbiol. 7, 823–837 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudehen Y.-M., et al. , Mycobacterial resistance to zinc poisoning requires assembly of P-ATPase-containing membrane metal efflux platforms. Nat. Commun. 13, 4731 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi J. M., et al. , Spatially distinct and metabolically active membrane domain in mycobacteria. Proc. Natl. Acad. Sci. U.S.A. 113, 5400–5405 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bansal-Mutalik R., Nikaido H., Mycobacterial outer membrane is a lipid bilayer and the inner membrane is unusually rich in diacyl phosphatidylinositol dimannosides. Proc. Natl. Acad. Sci. U.S.A. 111, 4958–4963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilleron M., Quesniaux V. F. J., Puzo G., Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis bacillus calmette guérin and Mycobacterium tuberculosis H37Rv and Its implication in toll-like receptor response. J. Biol. Chem. 278, 29880–29889 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Gilleron M., et al. , Acylation state of the phosphatidylinositol mannosides from mycobacterium bovis Bacillus Calmette guérin and ability to induce granuloma and recruit natural killer T cells. J. Biol. Chem. 276, 34896–34904 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Daffé M., Marrakchi H., Unraveling the structure of the mycobacterial envelope. Microbiol. Spectr. 7 (2019), 10.1128/microbiolspec.GPP3-0027-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson M., The mycobacterial cell envelope-lipids. Cold Spring Harb. Perspect Med. 4, a021105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalscheuer R., et al. , The Mycobacterium tuberculosis capsule: A cell structure with key implications in pathogenesis. Biochem. J. 476, 1995–2016 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerin M. E., Korduláková J., Alzari P. M., Brennan P. J., Jackson M., Molecular basis of phosphatidyl-myo-inositol Mannoside biosynthesis and regulation in mycobacteria. J. Biol. Chem. 285, 33577–33583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corradi V., et al. , Emerging diversity in lipid-protein interactions. Chem. Rev. 119, 5775–5848 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corey R. A., et al. , Identification and assessment of cardiolipin interactions with E. coli inner membrane proteins. Sci Adv. 7, 2217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingólfsson H. I., et al. , Lipid organization of the plasma membrane. J. Am. Chem. Soc. 136, 14554–14559 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Pogozheva I. D., et al. , Comparative molecular dynamics simulation studies of realistic eukaryotic, prokaryotic, and archaeal membranes. J. Chem. Inf. Model. 62, 1036–1051 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Adhyapak P., et al. , Lipid clustering in mycobacterial cell envelope layers governs spatially resolved solvation dynamics. Chemistry 17, e202200146 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Guo H., et al. , Structure of mycobacterial ATP synthase bound to the tuberculosis drug bedaquiline. Nature 589, 143–147 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Souza P. C. T., et al. , Martini 3: A general purpose force field for coarse-grained molecular dynamics. Nat. Methods 18, 382–388 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alessandri R., et al. , Martini 3 coarse-grained force field: Small molecules. Adv. Theory Simul. 5, 2100391 (2022). [Google Scholar]
- 28.Borges-Araújo L., Souza P. C. T., Fernandes F., Melo M. N., Improved parameterization of phosphatidylinositide lipid headgroups for the martini 3 coarse-grain force field. J. Chem. Theory Comput. 18, 357–373 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Banerjee P., Lipowsky R., Santer M., Coarse-grained molecular model for the glycosylphosphatidylinositol anchor with and without protein. J. Chem. Theory Comput. 16, 3889–3903 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wassenaar T. A., Ingólfsson H. I., Böckmann R. A., Tieleman D. P., Marrink S. J., Computational lipidomics with insane: A versatile tool for generating custom membranes for molecular simulations. J. Chem. Theory Comput. 11, 2144–2155 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Bussi G., Donadio D., Parrinello M., Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Parrinello M., Rahman A., Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 52, 12 (1981). [Google Scholar]
- 33.Abraham M. J., et al. , GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015). [Google Scholar]
- 34.Barker J. A., Watts R. O., Monte Carlo studies of the dielectric properties of water-like models. Mol. Phys. 26, 789–792 (1973). [Google Scholar]
- 35.Wu E. L., Qi Y., Song K. C., Klauda J. B., Im W., Preferred orientations of phosphoinositides in bilayers and their implications in protein recognition mechanisms. J. Phys. Chem. B 118, 4315–4325 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Lee J., et al. , CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 15, 775–786 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Vanommeslaeghe K., et al. , CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J., et al. , CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 14, 71–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu E. L., et al. , CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Essmann U., et al. , A smooth particle mesh Ewald method. J. Chem. Phys. 103, 8577–8593 (1995). [Google Scholar]
- 41.Marrink S.-J., Downloads: Lipids (2022). (http://cgmartini.nl/index.php/force-fieldparameters/lipids) Accessed 31 October 2022.
- 42.Groenewald B. M., Croft A., Marrink S.-J., Molecular dynamics of mycolic acid monolayers. ChemRxiv [Preprint] (2019). 10.26434/chemrxiv.7881215.v1 (Accessed 2 February 2022). [DOI]
- 43.Fowler P. W., et al. , Membrane stiffness is modified by integral membrane proteins. Soft Matter 12, 7792–7803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith P., Lorenz C. D., LiPyphilic: A python toolkit for the analysis of lipid membrane simulations. J. Chem. Theory Comput. 17, 5907–5919 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Bonomi M., et al. , Promoting transparency and reproducibility in enhanced molecular simulations. Nature Methods 16, 670–673 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Buchoux S., FATSLiM: A fast and robust software to analyze MD simulations of membranes. Bioinformatics 33, 133–134 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Hunter J. D., Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007). [Google Scholar]
- 48.UniProt C., UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 49, D480–D489 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jumper J., et al. , Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nugent T., Jones D. T., Membrane protein orientation and refinement using a knowledge-based statistical potential. BMC Bioinform. 14, 276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroon P. C., Aggregate, Automate, Assemble (University of Groningen, 2020), pp. 16–53. [Google Scholar]
- 52.Graham J. A., Essex J. W., Khalid S., PyCGTOOL: Automated generation of coarse-grained molecular dynamics models from atomistic trajectories. J. Chem. Inf. Model. 57, 650–656 (2017). [DOI] [PubMed] [Google Scholar]
- 53.Kim S., et al. , CHARMM-GUI ligand reader and modeler for CHARMM force field generation of small molecules. J. Comput. Chem. 38, 1879–1886 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.N. C. f. B. Information, PubChem Compound Summary for CID 5388906, Bedaquiline (National Center for Biotechnology Information, 2022). [Google Scholar]
- 55.Tetko I. V., et al. , Virtual computational chemistry laboratory – design and description. J. Comput. Aided Mol. Design 19, 453–463 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Waterhouse A., et al. , SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sievers F., et al. , Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song W., et al. , PyLipID: A python package for analysis of protein-lipid interactions from molecular dynamics simulations. J. Chem. Theory Comput. 18, 1188–1201 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corey R. A., Vickery O. N., Sansom M. S. P., Stansfeld P. J., Insights into membrane protein-lipid interactions from free energy calculations. J. Chem. Theory Comput. 15, 5727–5736 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Souaille M., Roux B. T., Extension to the weighted histogram analysis method: Combining umbrella sampling with free energy calculations. Comput. Phys. Commun. 135, 40–57 (2001). [Google Scholar]
- 61.Hub J. S., de Groot B. L., van der Spoel D., g_wham—A free weighted histogram analysis implementation including robust error and autocorrelation estimates. J. Chem. Theory Comput. 6, 3713–3720 (2010). [Google Scholar]
- 62.Schmalhorst P. S., Deluweit F., Scherrers R., Heisenberg C.-P., Sikora M., Overcoming the limitations of the MARTINI force field in simulations of polysaccharides. J. Chem. Theory Comput. 13, 5039–5053 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann C., Leis A., Niederweis M., Plitzko J. M., Engelhardt H., Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. U.S.A. 105, 3963–3967 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuber B., et al. , Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol 190, 5672–5680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adhyapak P., et al. , Dynamical organization of compositionally distinct inner and outer membrane lipids of mycobacteria. Biophys. J. 118, 1279–1291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes A. V., et al. , Physical properties of bacterial outer membrane models: Neutron reflectometry & molecular simulation. Biophys. J. 116, 1095–1104 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman R., Membrane-ion interactions. J. Membrane Biol. 251, 453–460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Modak B., Girkar S., Narayan R., Kapoor S., Mycobacterial membranes as actionable targets for lipid-centric therapy in tuberculosis. J. Med. Chem. 65, 3046–3065 (2022). [DOI] [PubMed] [Google Scholar]
- 69.Nguyen P. P., Kado T., Prithviraj M., Siegrist M. S., Morita Y. S., Inositol acylation of phosphatidylinositol mannosides: A rapid mass response to membrane fluidization in mycobacteria. J. Lipid Res. 63, 100262 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorent J. H., et al. , Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 16, 644–652 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.von Heijne G., Net N-C charge imbalance may be important for signal sequence function in bacteria. J. Mol. Biol. 192, 287–290 (1986). [DOI] [PubMed] [Google Scholar]
- 72.Baker J. A., Wong W.-C., Eisenhaber B., Warwicker J., Eisenhaber F., Charged residues next to transmembrane regions revisited: “Positive-inside rule” is complemented by the “negative inside depletion/outside enrichment rule”. BMC Biol. 15, 66 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pogozheva I. D., Tristram-Nagle S., Mosberg H. I., Lomize A. L., Structural adaptations of proteins to different biological membranes. Biochim. Biophys. Acta 1828, 2592–2608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson R. J., Groundwater P. W., Todd A., Worsley A. J., Antibacterial Agents: Chemistry, Mode of Action, Mechanisms of Resistance and Clinical Applications (Wiley-Blackwell, Chichester, 2012), p. 378. [Google Scholar]
- 75.Dartois V. A., Rubin E. J., Anti-tuberculosis treatment strategies and drug development: Challenges and priorities. Nat. Rev. Microbiol. 20, 685–701 (2022), 10.1038/s41579-022-00731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krah A., Grüber G., Bond P. J., Binding properties of the anti-TB drugs bedaquiline and TBAJ-876 to a mycobacterial F-ATP synthase. Curr. Res. Struct. Biol. 4, 278–284 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bardou F., Raynaud C., Ramos C., Lanéelle M. A., Lanéelle G., Mechanism of isoniazid uptake in Mycobacterium tuberculosis. Microbiology 144, 2539–2544 (1998). [DOI] [PubMed] [Google Scholar]
- 78.Unissa A. N., Subbian S., Hanna L. E., Selvakumar N., Overview on mechanisms of isoniazid action and resistance in Mycobacterium tuberculosis. Infect. Genet. Evol. 45, 474–492 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Jackson M., et al. , Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol. Microbiol. 31, 1573–1587 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Duncan A. L., Robinson A. J., Walker J. E., Cardiolipin binds selectively but transiently to conserved lysine residues in the rotor of metazoan ATP synthases. Proc. Natl. Acad. Sci. U.S.A. 113, 8687–8692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corey R. A., Stansfeld P. J., Sansom M. S. P., The energetics of protein-lipid interactions as viewed by molecular simulations. Biochem. Soc. Trans. 48, 25–37 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Briffotaux J., Huang W., Wang X., Gicquel B., MmpS5/MmpL5 as an efflux pump in Mycobacterium species. Tuberculosis 107, 13–19 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Degiacomi G., et al. , In vitro study of bedaquiline resistance in Mycobacterium tuberculosis multi-drug resistant clinical isolates. Front. Microbiol. 11, 559469 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarke R. J., Hossain K. R., Cao K., Physiological roles of transverse lipid asymmetry of animal membranes. Biochim. Biophys. Acta 1862, 183382 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Im W., Khalid S., Molecular simulations of gram-negative bacterial membranes come of age. Annu. Rev. Phys. Chem. 71, 171–188 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Augenstreich J., et al. , The conical shape of DIM lipids promotes Mycobacterium tuberculosis infection of macrophages. Proc. Natl. Acad. Sci. U.S.A. 116, 25649–25658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Queiroz A., Riley L. W., Bacterial immunostat: Mycobacterium tuberculosis lipids and their role in the host immune response. Rev. Soc Bras Med. Trop. 50, 9–18 (2017). [DOI] [PubMed] [Google Scholar]
- 88.Abrahams K. A., Besra G. S., Synthesis and recycling of the mycobacterial cell envelope. Curr. Opin. Microbiol. 60, 58–65 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qi Y., et al. , CHARMM-GUI Martini Maker for Coarse-Grained Simulations with the Martini Force Field. Journal of Chemical Theory and Computation 11, 4486–4494 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
A video showing 50 × 50 nm2 asymmetric bilayer over a 10 μ03BC;s simulation. The cytoplasmic leaflet is on the left and the periplasmic leaflet on the right. The lipids are colored as shown in Figure 1A.
A contour plot showing the area per lipid of the cytoplasmic leaflet of a 20 × 20 nm2 asymmetric bilayer. The darker color indicated a larger area per lipid.
A contour plot showing the area per lipid of the periplasmic leaflet of a 20 × 20 nm2 asymmetric bilayer. The darker color indicated a larger area per lipid.
Data Availability Statement
Data availability: https://github.com/pstansfeld/PIM-lipids. AT systems and CG-membrane setup can be performed using, respectively, CHARMM-GUI bilayer builder (https://charmm-gui.org/input/membrane.bilayer) and CHARMM-GUI MARTINI bilayer Maker (https://charmm-gui.org/?doc=input/martini.bilayer).