Abstract
Introduction
A structured set of eight basic dermoscopic parameters (lines, clods, dots, circles, pseudopods, structureless, else, and vessels) including a total of 77 variables with corresponding descriptive and metaphoric vocabulary has been released for evaluation of skin tumors by the International Dermoscopy Society (IDS).
Objectives
To validate the aforementioned criteria for the use in darker phototypes (phototypes IV-VI) via an expert consensus.
Methods
The two-round “Delphi method” was adopted, with an iterative process including two rounds of email questionnaires. Potential panelists were asked to take part in the procedure via email on the basis of their expertise in the dermoscopy of skin tumors in dark phototypes.
Results
A total of 17 participants were involved. All the original variables of the eight basic parameters reached agreement during the first round, except for “pink small clods” (“milky red globules”) and “structureless pink zone” (“milky red areas”). Moreover, during the first round, panelists proposed a change of three existing items and the introduction of four new items, i.e., “black, small clods” (“black globules”), “follicular plugs”, “erosions/ulcerations”, and “white color around vessels” (“perivascular white halo”). All such proposals achieved agreement, thus being included in the final list, for a total of 79 items. There was consistency between the descriptive and metaphoric approaches in terms of scoring.
Conclusions
Albeit most of the original items were considered applicable even for skin of color, there are some points of differences that physicians need to know. No significant preference was found between descriptive and metaphoric terminology among panelists.
Keywords: dermoscopy, neoplasias, neoplastic dermatoses, skin of color, tumors
Introduction
Dermoscopy has nowadays become an invaluable tool for the dermatologist’s daily practice as it allows to highlight relevant findings corresponding to key histological changes that are not visible to the naked eye, thus increasing diagnostic accuracy in the field of both neoplastic and non-neoplastic skin conditions [1,2]. Importantly, to make dermoscopic examination as reproducible as possible it is of utmost importance to follow a systematic analytical approach, with a standardized set of parameters to evaluate and a uniform terminology to use [3–5]. However, over time, many authors employed an arbitrary approach with the use of different terms, even to refer to the same dermoscopic finding, with a consequent heterogeneous semeiology generating confusion among users [4]. In order to face such an issue, the International Dermoscopy Society (IDS) has released two consensus documents encompassing basic dermoscopic variables to assess with the corresponding vocabulary to adopt, one for skin neoplasms and one for non-neoplastic dermatoses (inflammatory, infectious, and infiltrative diseases) [4,5]. Notably, these guidelines were issued considering the literature evidence on light phototypes, with consequent possible limitations if used in dark skin [4,5]. Indeed, it has been shown that dermoscopic patterns of skin disorders may remarkably vary (especially for phototypes V/VI) because of the different color backgrounds as well as specific reaction patterns typical of darker phototypes (e.g., lability of pigment and greater tendency to follicular or sclerotic reactions) [6,7]. For these reasons, the IDS supported a validation process of its consensus document on non-neoplastic dermatoses for use in dark skin, yet such a procedure has not been performed with regard to neoplastic disorders [8].
This document was promoted by the “Imaging in Skin of Color” IDS Task Force with the aim of validating the dermoscopic criteria/terminology provided by the IDS for skin tumors for the use in skin of color by a consensus process involving a panel of experts routinely dealing with dark-skinned patients (phototypes IV, V, and VI).
Materials and Methods
The consensus was performed according to the two-round “Delphi method”, with an iterative process including two rounds of email questionnaires starting from a list of pre-selected items (i.e., dermoscopic criteria provided by the IDS) [5]. Notably, differently from the “modified Delphi method”, the Delphi process makes it possible to gain expert consensus on variable issues by using at least two rounds of questionnaires and involving at least 5–10 participants, without the need for an in-person discussion [9–11]. So, similarly to the validation process for skin of color carried out for non-neoplastic dermatoses [8], we chose to avoid a face-to-face meeting in order to reduce decisional biases because of group interaction [9–11].
Panel Selection
Panel selection was performed by sending an e-mail invitation from the coordinators of the process (E.E. and B.S.A.) to experts in the field of dermoscopy in skin of color (phototypes IV, V, and VI) across the world. In detail, all the members of the “Imaging in Skin of Color” IDS Task Force were invited to join the panel, along with researchers who had published at least five peer-reviewed articles or book chapters on such a topic as either the first or last author. In total, 22 international experts were invited as panel members; participants’ assessments were blinded and anonymity was maintained during the entire process of consensus.
Round 1
The dermoscopic criteria provided by the IDS [5] were tabulated (Table 1) and shared with all the panelists via emails, including eight basic dermoscopic parameters with a total of 77 items. As per the original consensus, descriptive terminology and corresponding metaphoric vocabulary for each dermoscopic parameter were included in the validation process. Instructions and aims of the consensus process were also circulated.
Table 1.
Results of the validation process for the use of the IDS dermoscopic criteria (including both descriptive and metaphoric terminology) for neoplastic dermatoses in skin of color with corresponding agreement rates (percentage of experts giving a score of 4 or 5) and mean scores for each round
| Dermoscopic parameter (Descriptive terminology) | I Round* | II Round* | Dermoscopic parameter (Metaphoric terminology) | I Round* | II Round* |
|---|---|---|---|---|---|
| 1 Lines | |||||
| Lines, reticular | 100 (4.83) | - | Pigment network | 100 (4.91) | - |
| Lines, reticular and thick | 100 (4.75) | - | Broadened network | 100 (4.91) | - |
| Lines, reticular and thin | 100 (4.58) | - | Delicate network | 100 (4.75) | - |
| Lines, reticular and thick or reticular lines that vary in color | 100 (4.66) | - | Atypical pigment network | 100 (4.91) | - |
| Lines, reticular, white | 85 (4.25) | - | - | - | - |
| Lines, reticular, hypopigmented, around brown clods | 92 (4.41) | - | Negative pigment network | 100 (4.33) | - |
| Lines, white, perpendicularly*** | 100 (4.66) | - | Shiny white streaks*** | 84.6 (4.66) | - |
| Lines, branched | 100 (4.75) | - | Branched streaks | 100 (4.66) | - |
| Lines, radial (always at periphery) | 100 (4.83) | - | Streaks | 100 (4.83) | - |
| Lines, radial and segmental | 100 (4.83) | - | Radial streaming | 100 (4.66) | - |
| Lines, radial, connected to a common base | 100 (4.75) | - | Leaflike areas | 100 (4.83) | - |
| Lines, radial, converging to a central dot or clod | 100 (4.91) | - | Spoke wheel area | 100 (4.75) | - |
| Lines, curved and thick | 100 (4.66) | - | Cerebriform pattern | 100 (4.83) | - |
| Lines, brown, curved, parallel, thin | 100 (4.66) | - | Fingerprinting | 100 (4.83) | - |
| Lines, curved and thick, in combination with clods | 100 (4.75) | - | Crypts | 100 (4.83) | - |
| Lines, parallel, short, crossing ridges (volar skin) | 100 (4.83) | - | Fibrillar pattern | 100 (4.83) | - |
| Lines, parallel, thick, on the ridges (volar skin) | 100 (4.83) | - | Parallel ridge pattern | 100 (4.75) | - |
| Lines, parallel, thin, in the furrows and crossing the ridges (volar skin) | 100 (4.83) | - | Lattice-like pattern | 100 (4.75) | - |
| Lines, parallel, thin, in the furrows (volar skin) | 100 (4.83) | - | Parallel furrows pattern | 100 (4.75) | - |
| Lines, angulated or polygonal (facial skin) | 92 (4.58) | - | Rhomboids/zig-zag pattern | 92 (4.50) | - |
| Lines, angulated or polygonal (non-facial skin) | 92 (4.50) | - | Angulated lines/polygons | 92 (4.52) | - |
| 2 Clods | |||||
| Clods, small, round or oval | 100 (4.75) | - | Globules | 100 (4.50) | - |
| Clods, brown, circumferential | 92 (4.58) | - | Rim of brown globules | 92 (4.51) | - |
| Clods, brown, yellow, or orange (rarely black) | 92 (4.52) | - | Comedo-like openings | 92 (4.41) | - |
| Clods, brown or blue, concentric (clod within a clod) | 85 (4.16) | - | Concentric globules | 92 (4.42) | - |
| Clods, brown, blue or black, concentric (clod within a clod)** | - | 100 (4.53) | Concentric globules | 100 (4.42) | - |
| Clods, brown or skin colored, large and polygonal | 100 (4.58) | - | Cobblestone pattern | 100 (4.50) | - |
| Clods, blue, large, clustered | 100 (4.52) | - | Blue-gray ovoid nests | 100 (4.75) | - |
| Clods, blue, small | 100 (4.41) | - | Blue globules | 100 (4.41) | - |
| Clods, black, small | - | 100 (4.53) | Black globules | - | 100 (4.53) |
| Clod within a clod (concentric clods) | 85 (4.25) | - | Variant of spoke wheel area | 85 (4.30) | - |
| Clods, white, shiny*** | 100 (4.66) | - | Shiny white blotches and strands*** | 100 (4.56) | - |
| Clods, pink and small | 72 (3.94) | - | Milky-red globules | 72 (3.98) | - |
| Clods, red or purple | 92 (4.41) | - | Red lacunae | 92 (4.23) | - |
| 3 Dots **** | |||||
| Dots, any color | 100 (4.83) | - | Granularity or granules | 92 (4.54) | - |
| Dots, gray | 100 (4.83) | - | Peppering | 92 (4.52) | - |
| Dots, gray, blue or black** | - | 100 (5.0) | Peppering | 100 (4.52) | - |
| Dots, gray and circles, gray | 100 (4.66) | - | Annular-granular pattern | 100 (4.58) | - |
| Dots, gray, blue or black and circles, gray, blue or black** | - | 100 (4.53) | Annular-granular pattern | 100 (4.58) | - |
| Dots or clods, white, clustered or disseminated | 92 (4.58) | - | Milia-like cyst, cloudy or starry | 100 (4.75) | - |
| Dots, white, four arranged in a square*** | 100 (4.51) | - | Rosettes*** | 92 (4.66) | - |
| Dots, peripheral, arranged in lines | 100 (4.53) | - | Linear dots | 85 (4.52) | - |
| Dots, brown, central (in the center of hypopigmented spaces between reticular lines) | 92 (4.48) | - | Targetoid dots | 92 (4.32) | - |
| 4 Circles | |||||
| Circles, white | 92 (4.58) | - | - | - | - |
| Circles, concentric | 92 (4.16) | - | Circle within a circle | 92 (4.16) | - |
| Circles, incomplete | 92 (4.33) | - | Asymmetric pigmented follicular openings | 100 (4.12) | - |
| 5 Pseudopods | |||||
| Pseudopods, circumferential or lines, radial, circumferential | 100 (4.66) | - | Starburst pattern | 100 (4.66) | - |
| 6 Structureless | |||||
| Structureless zone, brown or black | 100 (4.75) | - | Blotch | 100 (4.75) | - |
| Structureless zone, blue | 100 (4.58) | - | Blue-whitish veil | 100 (4.66) | - |
| Structureless zone, pink | 75 (3.95) | - | Milky-red areas | 72 (3.86) | - |
| Structureless zone, white | 100 (4.83) | - | Scar-like depigmentation | 100 (4.75) | - |
| Structureless zone, white, central | 100 (4.83) | - | Central white patch | 100 (4.66) | - |
| Structureless zone, polychromatic | 85 (4.33) | - | Rainbow pattern | 92 (4.41) | - |
| Structureless, red, interrupted by follicular openings | 82 (4.16) | - | Strawberry pattern | 85 (4.23) | - |
| Structureless, brown (tan), eccentric | 100 (4.58) | - | - | - | - |
| Structureless, any color | 100 (4.75) | - | Homogeneous pattern | 100 (4.83) | - |
| Structureless, brown, interrupted by follicular openings (facial skin) | 100 (4.66) | - | Pseudonetwork | 100 (4.66) | - |
| 7 Else | |||||
| Sharply demarcated, scalloped border | 100 (4.66) | - | Moth-eaten border | 100 (4.75) | - |
| Follicular plugs | - | 92 (4.69) | - | - | - |
| Erosions/Ulcerations | - | 100 (4.84) | - | - | - |
| 8 Vessels | |||||
| 8.1 Morphology | |||||
| Dots | 100 (4.75) | - | - | - | - |
| Clods | 100(4.38) | - | Red-purple lacunes | 100 (4.46) | - |
| Linear | 100 (4.75) | - | - | - | - |
| Coiled | 100 (4.58) | - | Glomerular | 100 (4.50) | - |
| Looped | 100 (4.66) | - | Hairpin | 100 (4.75) | - |
| Serpentine | 100 (4.50) | - | Linear irregular | 100 (4.58) | - |
| Helical | 100 (4.50) | - | Corkscrew | 100 (4.58) | - |
| Curved | 100 (4.44) | - | Comma | 100 (4.41) | - |
| Monomorphous | 92 (4.41) | - | - | - | - |
| Polymorphous | 100 (4.75) | - | - | - | - |
| 8.2 Arrangement | |||||
| Radial | 100 (4.66) | - | Crown vessels | 92 (4.50) | - |
| Serpiginous | 100 (4.66) | - | String of pearls | 100 (4.66) | - |
| Branched | 100 (4.78) | - | Arborizing vessels | 100 (4.83) | - |
| Clustered | 100 (4.83) | - | - | - | - |
| Centered dots | 100 (4.61) | - | Targetoid vessels | 100 (4.63) | - |
| 8.3 White color around vessels | - | 100 (4.53) | Perivascular white halo | - | 100 (4.61) |
Agreement rate (mean score) – Agreement rate is measured from 0% to 100%, mean score is measured from 0 to 5;
This parameter replaces the previous one.
Only visible by polarized dermoscopy.
Dots and clods can be best differentiated if they appear as a pattern. Multiple dots have the same size and shape (they are all small and round), multiple clods vary in size and shape. In general dots are not larger than the diameter of a terminal hair.
Panelists were asked to judge on a 5-point scale the level of agreement on the relevance of each variable (descriptive and metaphoric) for the use in dark-skinned patients (1, no agreement; 2, low agreement; 3, moderate agreement; 4, agreement; and 5, strong agreement). In case of disagreement/poor agreement (score 1–3) on any of the items, participants were invited to justify their choice and provide (optional) suggestions to improve them. Experts were also given the chance to propose additional variables not included in the original list. Each item was considered appropriate for the use in skin of color in case of achievement of a score of 4 or 5 out of 5 by more than 80% of the experts. The agreement threshold of 80% was selected based on the literature guidance on Delphi consensus [10]. Parameters which had not attained 80% agreement would be modified in accordance with suggestions (if any) given by the participants and redistributed, along with new possible proposed items, to the panel of experts for round 2.
Round 2
In round 2, panelists were asked to assess the modified and new parameters (if any) resulting from round 1, following the same methodology as the previous round. At the end of round 2, a comparison between the rating of descriptive and metaphoric terminology for each of the eight basic dermoscopic parameters was carried out. Data were expressed as means ± SD and analysis was performed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA) by the unpaired, two-tailed student’s t-test, with p-value of <0.05 deemed statistically significant.
Results
A total of 17 participants were involved in both rounds of the consensus. With regard to descriptive terminology, all the items received agreement in round 1 except for “pink small clods” and “structureless pink zone”, which reached a mean score of 3.94 and 3.95, respectively. Similarly, corresponding metaphoric terms for such variables (i.e., “milky red globules” and “milky red areas”) did not achieve agreement too, with a mean score of 3.98 and 3.86, respectively. Four new items were proposed during the first round, i.e., (I) “black, small clods” (black globules) for parameter 2 (“CLODS”); (II) follicular plugs and (III) erosions/ulcerations for parameter 7 (“ELSE”); and (IV) white color around vessels (perivascular white halo) for parameter 8 (“VESSELS”). Moreover, the group of experts suggested changing three items when it comes to descriptive terminology, including (I) “clods, brown or blue, concentric (clod within a clod)” to “clods, brown, blue or black, concentric (clod within a clod)”; (II) “dots, gray” to “dots, gray, blue or black”; and (III) “dots, gray and circles, gray” to “dots, gray, blue or black and circles, gray, blue or black”.
All such proposals were rated during the second round and achieved agreement, thus being included in the final list. Therefore, at the end of the validation process, a total of 79 items were identified (72 out of the 77 proposed by the IDS plus seven added in the course of the consensus procedure). Table 1 displays details on agreement rates and mean scores for rounds 1 and 2. Figures 1–4 depict schematic illustrations of the new/changed items and examples of skin tumors typified by such structures.
Figure 1.
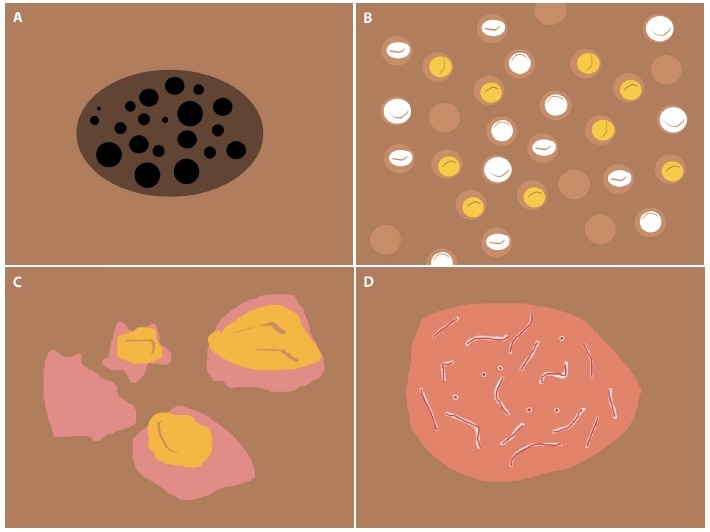
Schematic representation of newly-introduced dermoscopic parameters to use in skin of color: black, small clods (black globules) (a); follicular plugs (b); erosions/ulcerations (c); and white color around vessels (perivascular white halo) (d).
Figure 2.
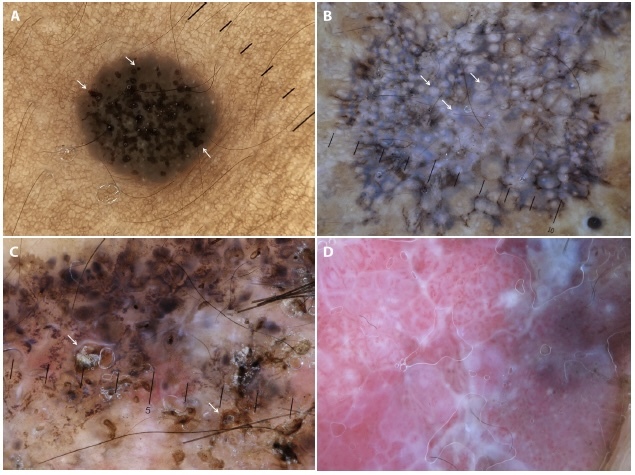
Examples of skin tumors in dark-skinned patients (phototypes V/VI) typified by the newly-introduced dermoscopic structures: black, small clods (black globules) in a seborrheic keratosis (arrows) (a); follicular plugs in an actinic keratosis (arrows) (b); erosions in a basal cell carcinoma (arrows) (c); and white color around vessels (perivascular white halo) in a squamous cell carcinoma (d).
Figure 3.

Schematic representation of modified dermoscopic parameters to use in skin of color: “clods, brown, blue or black, concentric” (clod within a clod) (a); “dots, gray, blue or black” (peppering) (b); and “dots, gray, blue or black and circles, gray, blue or black” (annular-granular pattern) (c).
Figure 4.
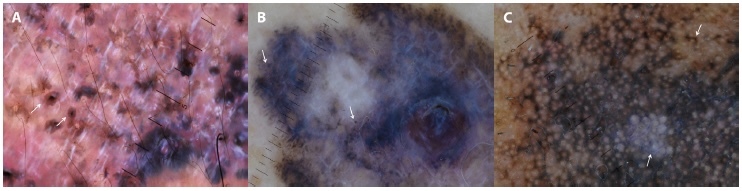
Examples of skin tumors in dark-skinned patients (phototypes V/VI) typified by the modified dermoscopic parameters: black/brown concentric clods (black clod within a brown clod) in a basal cell carcinoma (arrows) (a); “blue/black dots” (blue/black peppering) in a melanoma (arrows) (b); and “blue/black dots and circles” (blue/black annular-granular pattern) in a lentigo maligna (arrows) (c).
Moving to the comparative analysis between descriptive and metaphoric terms of the eight basic parameters, although for the majority of them the mean score was higher for the descriptive counterpart, no statistically significant differences were observed (p-values >0.05).
Discussion
This expert consensus underlines that the whole set of dermoscopic criteria proposed by the IDS for the evaluation of skin tumors may also be used when assessing dark phototypes, apart from “clods, pink and small” and “structureless zone, pink” (and corresponding metaphoric terms, i.e., “milky-red globules” and “milky-red areas”) as “pink”/“milky-red” hue is more difficult to detect in skin of color because of the pigmented background [6, 12].
In general, most of the variables included from the original IDS list (considering both descriptive and metaphoric terminology) received a high mean rate (between 4.5 and 5), with only a few of them reaching agreement with a lower score (< 4.5). In detail, the latter group included the following descriptive items: “reticular white lines” and “lines, reticular, hypopigmented, around brown clods” in the “LINES” category; “clods, brown or blue, concentric (clod within a clod)”, “clods, blue, small”, “clod within a clod (concentric clods)” and “clods, red or purple” in the “CLODS” parameter; “dots, brown, central (in the center of hypopigmented spaces between reticular lines)” in the “DOTS” category; “circles, concentric” and “circles, incomplete” when it comes to the “CIRCLES” parameter; “structureless zone, polychromatic” and “structureless, red, interrupted by follicular openings” considering the “STRUCTURELESS” category; and “clods”, “curved” and “monomorphous” morphology in the “VESSELS” parameter. The reasons underlying a lower scoring for such variables mainly include the higher melanin content and the greater tendency to pigmentary incontinence typical of darker phototypes [6] that may result in lower optical contrast (needed to optimally see concentric, polychromatic or pigmented structures) or the partial obscuration of some findings (e.g., red/purple structures, smaller/thinner vessels, or hypopigmented lines) as well as change in the morphology of some structures (e.g., “incomplete” may become “complete” pigmented circles). This is in line with evidence from the literature. For example, blurred vascular structures and “reticular white lines”/“lines, reticular, hypopigmented, around brown clods” (negative pigment network), commonly found respectively in dermal nevi and dermatofibromas in light phototypes, have been reported less frequently in skin of color [13–15].
On the other hand, homogeneous pigmentary findings (excluding concentric and polychromatic items) and white structures were generally rated high (> 4.5). This is easily explained as diagnosis of skin tumors in dark-skinned patients mainly relies on the prevalence and combination of such features [16]. Additionally, some vessel shapes/arrangements also reached a high score, especially dotted/linear morphologies and clustered/branched distribution patterns, likely resulting from the significant prevalence of these findings in Bowen’s disease and basal cell carcinoma also in skin of color [17, 18].
Besides dermoscopic items included in the original list of the IDS, panelists also proposed and agreed on the introduction of four new variables for the assessment of skin tumors in dark phototypes, including “clods, black, small” (black globules), follicular plugs, erosions/ulcerations, and white color around vessels (perivascular white halo), histologically related to melanin deposits/melanocytes in the epidermis, follicular hyperkeratosis, loss of epidermis/dermis, and acanthosis, respectively. This was due to their significant diagnostic relevance (e.g., follicular plugs are a key clue in actinic keratosis/SCC as they often show a pigmentary pattern similar to lentigo maligna/melanoma – see Figures 2b,4c) but also to the higher prevalence of such structures in skin of color (as the result of a greater tendency to darker pigmentation and follicular/ulcerative reactions as well as a greater contrast between the perivascular white halo and surrounding pigmented skin) [6, 19]. Moreover, during the consensus process a change of three existing parameters (i.e., “dots”, “clod within a clod”, and “dots and circles”) was also included, with darker colors (blue/black) being listed as a possible additional hue for the aforementioned structures, still due to the higher tendency to have more prominent pigmentation in dark phototypes [6, 19].
Finally, the comparative analysis between descriptive and metaphoric terminology highlighted no relevant differences in terms of mean score for each of the eight basic parameters, thereby underlying that both of them are useful and might be complementary. In fact, the metaphoric approach is more related to “blink” (quick) diagnoses (e.g., “arborizing” vessels are a quick hint for a basal cell carcinoma), while descriptive assessment is extremely helpful when “blink” fails in describing a lesion and a more analytical process is needed for a correct dermoscopic diagnosis [20, 21]. The lack of a clear predominance between the two approaches is also emphasized by the consistency observed in the present consensus process when considering the rating of each descriptive item and corresponding metaphoric counterpart (<4.0; 4÷4.5; >4.5), with the only exception of “comedo-like openings”. Indeed, this item was rated lower than the corresponding descriptive terminology, likely because it has a weaker correspondence from a morphological point of view in skin of color as the lower optical contrast typical of dark phototypes often makes epidermal invaginations filled with keratin look like darkly pigmented globules rather than “comedo-like openings” [12].
Conclusions
To conclude, the present validation process provides structured dermoscopic criteria for the assessment of skin tumors in dark phototypes based on parameters proposed by the IDS. Albeit most of the original items were considered applicable even for skin of color, there are some points of differences that physicians need to know. Notably, no significant preference was found between descriptive and metaphoric terminology. The set of criteria validated in this consensus is intended to be the starting point to fill the existing knowledge gap in the field of dermoscopy of skin tumors in skin of color as it might help facilitate the interpretation of reported findings and increase the reproducibility of the studies.
Limitations
The present validation process was based on the Delphi technique, which relies on the opinion of a group of experts, so the results represent the point of view of a limited number of evaluators. Additionally, albeit all the included panelists routinely deal with dark-skinned patients, an interobserver variability does exist in terms of the proportions of each phototype.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Errichetti E, Stinco G. Dermoscopy in General Dermatology: A Practical Overview. Dermatol Ther (Heidelb) 2016;6:471–507. doi: 10.1007/s13555-016-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fee JA, McGrady FP, Rosendahl C, Hart ND. Training Primary Care Physicians in Dermoscopy for Skin Cancer Detection: a Scoping Review. J Cancer Educ. 2020;35:643–50. doi: 10.1007/s13187-019-01647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Errichetti E. Dermoscopy of Inflammatory Dermatoses (Inflammoscopy): An Up-to-Date Overview. Dermatol Pract Concept. 2019;9:169–80. doi: 10.5826/dpc.0903a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Errichetti E, Zalaudek I, Kittler H, et al. Standardization of dermoscopic terminology and basic dermoscopic parameters to evaluate in general dermatology (non-neoplastic dermatoses): an expert consensus on behalf of the International Dermoscopy Society. Br J Dermatol. 2020;182:454–67. doi: 10.1111/bjd.18125. [DOI] [PubMed] [Google Scholar]
- 5.Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: Results of the third consensus conference of the International Society of Dermoscopy. J Am Acad Dermatol. 2016;74:1093–106. doi: 10.1016/j.jaad.2015.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Errichetti E, Ankad BS, Sonthalia S, et al. Dermoscopy in general dermatology (non-neoplastic dermatoses) of skin of colour: a comparative retrospective study by the International Dermoscopy Society. Eur J Dermatol. 2020;30:688–98. doi: 10.1684/ejd.2020.3928. [DOI] [PubMed] [Google Scholar]
- 7.Errichetti E. Dermoscopy of common papulosquamous dermatoses varies between dark (III and IV) and very dark (V and VI) skin phototypes. Dermatol Ther. 2021;34:e14757. doi: 10.1111/dth.14757. [DOI] [PubMed] [Google Scholar]
- 8.Errichetti E, Ankad BS, Jha AK, et al. International Dermoscopy Society criteria for non-neoplastic dermatoses (general dermatology): validation for skin of color through a Delphi expert consensus. Int J Dermatol. 2022;61:461–71. doi: 10.1111/ijd.15729. [DOI] [PubMed] [Google Scholar]
- 9.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- 10.Lynn MR. Determination and quantification of content validity. Nurs Res. 1986;35:382–5. [PubMed] [Google Scholar]
- 11.Graefe A, Armstrong JS. Comparing face-to-face meetings, nominal groups, Delphi and prediction markets on an estimation task. Int J Forecasting. 2016;27:183–95. [Google Scholar]
- 12.Ankad BS, Sakhare PS, Prabhu MH. Dermoscopy of non-melanocytic and pink tumors in brown skin: A descriptive study. Indian J Dermatopathol Diagn Dermatol. 2017;4:41–51. [Google Scholar]
- 13.Lallas A, Reggiani C, Argenziano G, et al. Dermoscopic nevus patterns in skin of colour: a prospective, cross-sectional, morphological study in individuals with skin type V and VI. J Eur Acad Dermatol Venereol. 2014;28:1469–74. doi: 10.1111/jdv.12316. [DOI] [PubMed] [Google Scholar]
- 14.Sarma N, Das A, Gupta A. Melanocytic nevus and Nevoid disorders. In: Ankad BS, Mukherjee SS, Nikam BP, editors. Dermoscopy histopathology correlation. 1st edn. Singapore: Springer Publications; 2021. pp. 15–46. [Google Scholar]
- 15.Kelati A, Aqil N, Baybay H, et al. Beyond classic dermoscopic patterns of dermatofibromas: a prospective research study. J Med Case Reports. 2017;11:266.s. doi: 10.1186/s13256-017-1429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezenwa E, Stein JA, Krueger L. Dermoscopic features of neoplasms in skin of color: A review. Int J Womens Dermatol. 2021;7:145–51. doi: 10.1016/j.ijwd.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinay K, Ankad BS, Narayan VR, et al. A multicentric study on dermoscopic patterns and clinico-dermoscopic-histological correlates of basal cell carcinoma in Indian skin. Clin Exp Dermatol. 2022 Jul 22; doi: 10.1111/ced.15337. [DOI] [PubMed] [Google Scholar]
- 18.Behera B, Kumari R, Thappa DM, Gochhait D, Srinivas BH, Ayyanar P. Dermoscopy of Bowen’s disease: A case series of five patients. Indian J Dermatol Venereol Leprol. 2021;87:576–80. doi: 10.25259/IJDVL_987_20. [DOI] [PubMed] [Google Scholar]
- 19.Neema S, Patil S, Pol D. Basic Principles of Dermoscopy. In: Ankad BS, Bhat YJ, Rambhia KD, editors. IADVL Atlas of Dermoscopy. 1 edn. New Delhi: Jaypee Brothers Medical Publishers; 2022. pp. 6–18. [Google Scholar]
- 20.Giacomel J, Zalaudek I, Marghoob AA. Metaphoric and descriptive terminology in dermoscopy: lessons from the cognitive sciences. Dermatol Pract Concept. 2015;5:69–74. doi: 10.5826/dpc.0502a11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blum A, Argenziano G. Metaphoric and descriptive terminology in dermoscopy: combine “blink” with “think”. Dermatol Pract Concept. 2015;5:23. doi: 10.5826/dpc.0503a05. [DOI] [PMC free article] [PubMed] [Google Scholar]


