Abstract
Introduction
Malignant melanoma is one of the rarest forms of skin cancer but it is the most deadly.
Objective
The objective of this paper was to analyze the epidemiological characteristics and trends of mortality from malignant melanoma in the population of Central Serbia in the period 1999–2015.
Methods
The study was designed as a retrospective descriptive epidemiological study. Standardized mortality rates were used in statistical data processing. A linear trend model and regression analysis were used to examine trends in malignant melanoma mortality.
Results
In Serbia, malignant melanoma mortality shows an increasing trend. The overall age-adjusted melanoma death rate was 2.6 per 100,000 with a higher death rate among men (3.03 per 100,000) than among women (2.1 per 100,000). Malignant melanoma mortality rates increase with age in both sexes and are highest in the age group of 75 and older. The highest increase in mortality in men is recorded in the 65–69 age group, with an average percentage increase of 21.33 (95% CI, 8.40 – 51.05), while in women the largest increase in mortality was recorded in the 35–39 age group, with an average percentage increase of 31.4 and in the 70–74 age group, 12.9.
Conclusions
The trend of increasing mortality from malignant melanoma in Serbia is similar to those in most developed countries. Education and improvement of awareness in the general population and among health professionals are vital to reducing melanoma mortality in the future.
Keywords: malignant melanoma, mortality, risk factors, Serbia
Introduction
Malignant melanoma accounts for 4% of all skin cancers and 1,7% of all cancers [1]. Although it still accounts for less than 5% of all skin malignancies, melanoma causes about 80% of skin cancer deaths [2]. The incidence of cutaneous melanoma has increased sharply in recent years in all parts of the world, with a steady increase in incidence among the white population, while the mortality associated with it remains stable [3–5]. It should also be noted that the incidence of melanoma has increased due to better education and improvement of awareness among patients and due to the development of dermatoscopy [6].
According to Globocan data from 2018, the total number of deaths from malignant melanoma in the world was 60,712, which ranks it 22nd in the structure of mortality from malignant tumors of all localizations [7]. Mortality rates in different populations of the world multiple vary depending on the level of development, so mortality is almost 5 times higher in developed countries than in developing countries [8,9]. Worldwide, the highest mortality rates are registered in regions such as North America, Northern Europe, Australia and New Zealand, while lower rates are commonly found in South American and African countries. For 2018, global data on skin melanoma revealed age-standardized mortality rates of approximately 0.63 deaths per 100,000 inhabitants (0.78 deaths per 100,000 inhabitants for men and 0.50 for women) [7].
Epidemiological studies show that melanoma is more common in older adults than in younger people and is more common in men than in women, but the ratio of these rates between the sexes varies with age [10, 11]. For example, the incidence rate is three times higher in men aged 80 and older than in women of the same age [12]. Although the incidence of melanoma is lower in people under the age of 40, it is one of the most common cancers diagnosed among adolescents and young adults [13].
Although the etiology of melanoma has not been fully elucidated, the findings of studies indicate the importance of the interaction between genetic, biological, and environmental factors. Several meta-analyses have identified key risk factors such as: family history of melanoma, age, history of sunburn and exposure to ultraviolet (UV) radiation, fair-skinned people, increased number of nevi, dysplastic nevi, genetic factors (CDKN2A, CDK4, MC1R genes, TYRP1) [14,15]. The most important and potentially modifying environmental risk factor for the development of malignant melanoma is exposure to ultraviolet (UV) rays due to their genotoxic effects [10,16,17]. According to the latest IARC estimations (2018), the estimated mortality rate in Serbia is 2.5 / 100,000 inhabitants [18]. Compared to other countries, although Serbia has lower mortality rates than the highest estimated in the world, it is in the group of countries with a higher risk of disease. Unless additional efforts in prevention are made, the number of melanoma cases is projected to increase in Serbia over the next 15 years, with a concomitant increase in healthcare costs.
The aim of the research is to analyze the epidemiological characteristics and trends of mortality from malignant melanoma in the population of Central Serbia in the period 1999–2015.
Materials and Methods
The study was designed as a retrospective descriptive epidemiological study. The research used data from the Cancer Registry of Central Serbia, formed on the basis of reports of malignant diseases for the period 1999–2015. The Population Register for Cancer was established in Serbia in 1970 and since 1998, the Cancer Registry of Central Serbia has been admitted to the International (IACR) and European Association of Cancer Registries (ENCR). In the registers, due to numerous data sources and the need for their verification and analysis, the usual time period for data collection is two years, after which the report is published, which is the reason why the last published data from 2015 will be used.
Standardized mortality rates were used in statistical data processing. Mortality rates were calculated based on data on deaths from malignant melanoma in the Cancer Registry of Central Serbia. All reported melanoma deaths were coded according to the International Classification of Disease, 10th Revision (code S43.0). The cases were grouped by gender into 5-year age groups. The size and composition of the population by age and sex were obtained by the 1991, 2002, and 2011 Censuses. The population of Central Serbia by age and sex in the years between the Censuses were estimated based on natural increase and migration.
Age-adjusted mortality rates were calculated by direct standardization, using the world’s population and presented per 100,000 inhabitants. A linear trend model and regression analysis were used to examine trends in malignant melanoma mortality. The percentage of the change in the mortality rate was calculated as the percentage of the difference between the adjusted rates for two consecutive years, and then as the average value of these changes over the entire observation period. Confidence intervals (CI) for average age-adjusted and age-specific mortality rates were estimated with 95% probability. Bilateral P values have been reported and are considered to show statistical significance if they are lesser than 0.05. Data were processed using the statistical package for social sciences, version 19.0 (SPSS Inc, Chicago, IL, USA).
Results
In 2015, a total of 195 melanoma deaths occurred in Central Serbia, which constitutes 1.3% of the total number of cancer deaths and ranks 17th in the structure of cancer mortality of all localizations. During the 17-year observation period, there was a significant decrease in the total mortality of the population (y = −8,7001x + 582.67; p = 0.001; % change = −8.83) (Figure 1), with a significant increase in mortality from all malignant tumors in total (y = 0.6343x + 115.13; p = 0.004; % change = + 0.27) and in both sexes (Figure 2).
Figure 1.
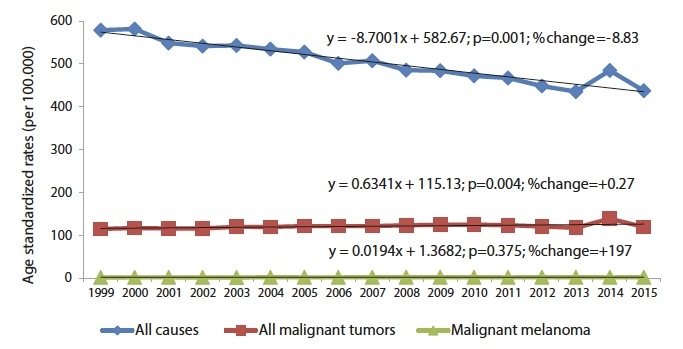
Trends of age-adjusted mortality rates for all causes of death, all malignant tumors and malignant melanoma in Central Serbia in the period 1999–2015.
Figure 2.
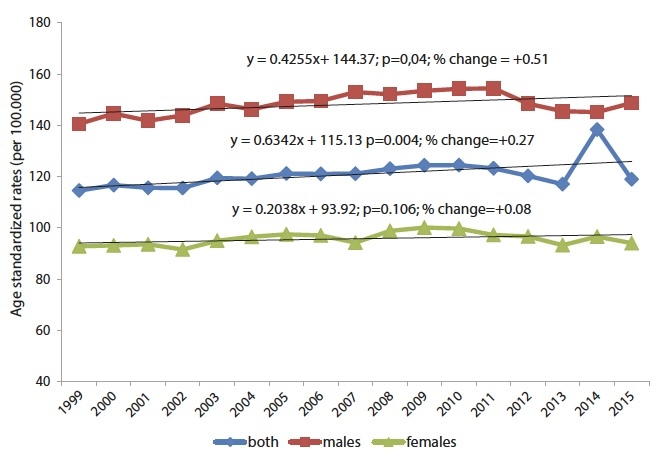
Trends of age-adjusted mortality rates all malignant tumors in Central Serbia by sex, in the period 1999–2015.
In the same period, mortality from malignant melanoma recorded an increasing trend (y = 0.0194x + 1.3682; p = 0.009; % change = + 1.94). Observed by gender, there is a significant trend of increasing mortality in men (y = 0.0486x + 1.5494; p = 0.036; % change = + 6.26), while in women there is a trend of declining mortality from malignant melanoma (y = −0, 0124x + 1.3282; p = 0.088; % change = + 0.43. (Figure 3).
Figure 3.
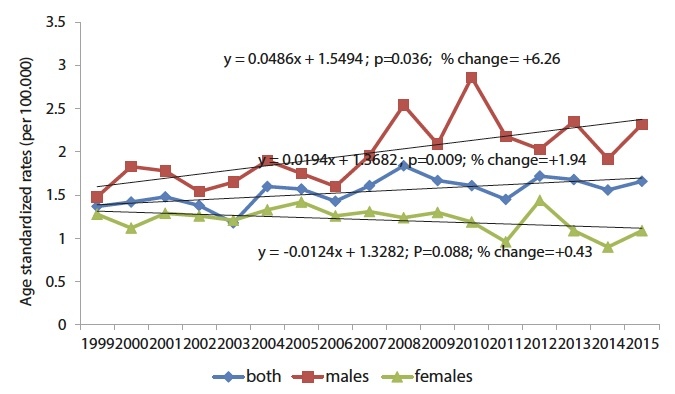
Trends of age-adjusted malignant melanoma mortality rates in Central Serbia, by sex, in the period 1999–2015.
The overall age-adjusted melanoma death rate was 2.6 per 100,000 with a higher death rate among men (3.03 per 100,000) than among women (2.1 per 100,000).
Malignant melanoma mortality rates increase with age in both sexes and are highest in the age group of 75 and older (10.15 per 100,000 for men; 8.32 per 100,000 for women) (Table 1). Low mortality rates have been reported in men and women under the age of 30. The largest increase in mortality in men was recorded in the 65–69 age group, with an average percentage increase of 21.33 (95% CI, 8.40 – 51.05), while in women the largest increase in mortality was recorded in the 35–39 age group, an average percentage increase of 31.4 (95% CI, 23.10 – 85.91) and in the 70–74 age group, 12.9 (95% CI, 14.41 – 40.14).
Table 1.
The average age-specific mortality rates and linear trend of Melanoma malignum in Central Serbia, in 1999–2015.
| Age (years) | Age-specific rates* (per 100,000) | Linear trend | R2 | p | Average annual percentage change (95% CI) |
|---|---|---|---|---|---|
| Male | † | ||||
| 0–4 | 0.00 | † | |||
| 5–9 | 0.00 | † | |||
| 10–14 | 0.00 | † | |||
| 15–19 | 0.00 | † | |||
| 20–24 | 0.27 | † | |||
| 25–29 | 0.42 | † | |||
| 30–34 | 1.07 | † | |||
| 35–39 | 1.69 | † | |||
| 40–44 | 2.03 | † | |||
| 45–49 | 3.33 | † | |||
| 50–54 | 4.95 | † | |||
| 55–59 | 7.99 | † | |||
| 60–64 | 4.15 | y = 5,26 + 0.21·x | 0.274 | 0.031 | 7,93 (−9.16 – 24.97) |
| 65–69 | 4.85 | y = 5.87 + 0.46·x | 0.321 | 0.018 | 21.33 (−8,40 – 51,05) |
| 70–74 | 7.68 | y = −6.23 +0.56·x | 0.620 | < 0.0005 | 10.84 (−8.73 – 30.41) |
| 75+ | 10.15 | y = 7.16 + 0.83·x | 0.669 | < 0.0005 | 10,96 (−9.59 – 31.51) |
| Female | |||||
| 0–4 | 0.00 | † | |||
| 5–9 | 0.00 | † | |||
| 10–14 | 0.00 | † | |||
| 15–19 | 0.00 | † | |||
| 20–24 | 0.16 | † | |||
| 25–29 | 0.42 | † | |||
| 30–34 | 0.88 | † | |||
| 35–39 | 1.48 | y = 2.28 − 0.10·x | 0.273 | 0.032 | 31.41 (−23,10 – 85.91) |
| 40–44 | 1.80 | † | |||
| 45–49 | 2.34 | † | |||
| 50–54 | 2.82 | † | |||
| 55–59 | 3.56 | † | |||
| 60–64 | 3.45 | † | |||
| 65–69 | 3.42 | ||||
| 70–74 | 5.42 | y = 2.90 + 0.32·x | 0.430 | 0.004 | 12.86 (−14.41 – 40.14) |
| 75+ | 8.32 | † | |||
Discussion
Mortality from malignant melanoma in Serbia records an upward trend and mortality rates for malignant melanoma in Serbia remain among the highest in the world. Similar trends are being observed around the world despite numerous efforts to improve primary prevention and early detection, and these increasing rates are affecting public health and the economic burden of the disease [11, 19]. Melanoma mortality rates have increased marginally among fair-skinned populations. Mortality rates are highest in Australia and New Zealand (3.4 per 100,000) and Northern Europe (2.0 per 100,000), while the lowest rates are recorded in South Central Asia and Eastern Asia (0.19) [7].
Trends in melanoma mortality are variable and are affected by latitude, ethnicity, age, and gender [20–22]. In high-risk regions, the mortality rate increased historically until the 1980s, peaking between 1988 and 1990, and then gradually maintained a slow increase. Over the last decade, the death rate has been growing steadily by 1.5% in the most of observed countries, such as New Zealand and Australia [10].
Mortality rates in northern European countries (Norway, Sweden, Netherlands) are among the highest in the world. Thus, according to the latest data from Globocan, in Norway in 2018, the total number of deaths from malignant melanoma was 13.2% of cancer of all localizations, which ranks it 7th in the structure of cancer mortality of all localizations. For the same period, in Sweden, the number of deaths from malignant melanoma accounted for 2.4% of cancer deaths of all localizations, which ranks it 12th, the standardized mortality rate is 2.5 per 100,000 inhabitants. The total number of deaths from malignant melanoma in the Netherlands in 2018 presents 2.0%, of deaths from cancer of all localizations, which ranks it 17th in the structure of cancer mortality of all localizations [7].
Differences in skin type, length, and sun exposure patterns may partly explain the lowest mortality rates recorded in some Middle Eastern countries (Qatar 0.04 per 100,000 inhabitants, Saudi Arabia 0.10 per 100,000 inhabitants, and Yemen 0.11 per 100,000 inhabitants), Africa (Egypt 0.13 per 100,000 inhabitants; Libya 0.14 per 100,000 inhabitants); Asian countries (India - 0.16 per 100,000 inhabitants, China - 0.18 per 100,000 inhabitants and Vietnam 0.08 per 100,000 inhabitants), some Central American countries (Barbados 0.0 per 100,000 inhabitants and Haiti 0.16 per 100,000 inhabitants) and Europe (Albania 0.53 per 100,000 inhabitants; Montenegro 0.89 per 100,000 inhabitants) [18].
The results of our study showed that mortality from malignant melanoma is higher in men than in women, which is in line with most published studies [11, 23]. The average standardized mortality rate for men in the world was 0.78 per 100,000 in 2018, and 0.50 per 100,000 for women in 2018. In terms of the global distribution of malignant melanoma, the standardized malignant melanoma mortality rate expressed on 100,000 inhabitants is higher for men than for women in all regions of the world: Australia and New Zealand (men 5.9; women 2.4); Northern Europe (men 2.5; women 1.6), North America (men 2.6; women 1.2), Western Europe (men 2; women 1.3). Less significant differences in mortality rates are present in less developed regions of the Caribbean (men 0.3, women 0.2), West Africa (men 0.5; women 0.3); East Asia (men 0.4; women 0.3); Southeast Asia (men 0.3; women 0.2); North Africa (men 0.2; women 0.2), Central Asia (men 0.2; women 0.1) [7].
In Europe, the standardized mortality rate of malignant melanoma for men is 3.2 per 100,000, and for women 1.9 per 100,000, with differences varying between regions of Europe: Western Europe (3.3 men and 1.9 women) in Central and Eastern Europe (3.0 and 2.0) in Northern Europe (3.8 and 2.2) in Southern Europe (2.7 and 1.6). The estimated standardized mortality rate for malignant melanoma for men and women in 2018 in Europe is the lowest in Albania (0.8 and 0.6 per 100,000 inhabitants), Montenegro (1.2 and 1.3 per 100,000 inhabitants), Romania (1.9 and 1.4 per 100,000 inhabitants), Spain (1.9 and 1.3 per 100,000 inhabitants). On the other hand, the highest values for men and women are in Croatia (4.6 and 2.5 per 100,000 inhabitants), North Macedonia (4.0 and 2.2 per 100,000 inhabitants), Slovenia (4.8 and 3, 2 per 100,000 inhabitants), Poland (4.0 and 2.4 per 100,000 inhabitants), Slovakia (4.8 and 3.2 per 100,000 inhabitants), Finland (4.4 and 1.7 per 100,000 inhabitants), Norway (6.3 and 4.1 per 100,000 inhabitants) and the Netherlands (4.6 and 3.1 per 100,000 inhabitants) [24].
Men are approximately 1.5 times more likely to develop melanoma than women. The incidence of melanoma is higher in women than in men until they reach the age of 40, however, from the age of 75, the incidence is almost three times higher in men than in women [25]. Mortality from malignant melanoma begins to rise sharply in men aged 40–44 and is especially high in those over 60 years of age. In women, a sharp increase was also observed at the age of 40–44, while the rate is especially high between the ages of 55 and 59. In some countries, the increase in the standardized mortality rate for those over the age of 85 is twenty times higher than for those at the age of 40–44 (Australia 42.65 per 100,000 and 2.62 per 100,000) [18].
When age is taken into account, adolescents and young adult women are more susceptible to melanoma than men [20]. This could be partly due to the widespread use of tanning beds among women, which is associated with an increased risk of melanoma [11]. However, after the age of 40, the incidence rate of melanoma among men is higher than among women [25]. Some believe that this increased sensitivity in men may be partly influenced by androgens [26]. Higher survival rates in women are also attributed to biological differences such as oxidative stress response, sex hormones or vitamin D metabolism and other influencing factors that have yet to be explored [27]. Speculation about the link between steroid hormones and melanoma arose when population studies found that women had a higher survival rate than men, which was evident between 1973 and 1997 when men had a death rate from melanoma that was twice as high as in women [28]. Furthermore, the history of malignant melanoma in women is rare before puberty and then increases sharply during the reproductive period and decreases during the menopausal years, which implies the involvement of estrogen. This phenomenon has led to the suggestion that hormones play an important role in melanoma. However, the results of epidemiological studies that assess the risk of melanoma in relation to hormonal and reproductive factors, such as the use of oral contraceptives, pregnancy and menopause, are contradictory; some studies did not show a cause-and-effect relationship, while others found an increased risk of melanoma [26,29]. Thus, the existence of a link between hormones and melanoma remains uncertain.
Other authors believe that these gender differences may be partly due to low rates of sun protection and more time spent outdoors during life compared to women. In addition, men are less likely to use sunscreen compared to women and are less aware of the importance of preventive measures, so melanoma is usually detected when the disease is present at an advanced stage, while women are more willing to seek medical help earlier and thus to detect changes in the earlier stage of the disease [5].
Many authors state that melanoma is more common in the elderly [30,31]. The results of our study also confirmed that age is a significant risk factor for malignant melanoma. Mortality rates are highest in people aged 75 and older in both sexes, with the highest percentage increase in mortality recorded in the 35–39 age group in women, and in the oldest age groups in men (65–69, 70–74, and 75 and older).
Similar to our results, a study conducted by Karimkhani et al found that malignant melanoma mortality rates are highest in the 75–79, 70–74, and 80 and older age groups [30]. In America, the percentage share of individual age groups in the total number of deaths shows the highest percentage (23.9%) in the 75–84 age group [32].
Older adults have more cumulative sun exposure than younger ones, and each additional decade of intense sun exposure increases the risk of melanoma [33,34]. The results of epidemiological research show that high sun exposure in the first 10 years of life more than doubles the risk of melanoma, while intense, occasional sun exposure during each decade until the age of 29 increases the risk of melanoma by more than 1.5 times [35]. More than five sunburns double the risk of melanoma for both those under 15 and those over 15 years of age. However, other studies have found that the number of sunburns before the age of 30 significantly increased the risk of melanoma, and the positive connection with the risk of melanoma is weaker in burns that occurred in those older than 30 years [36].
Conclusion
The trend of increasing mortality from malignant melanoma in Serbia is similar to those in most developed countries. Due to the high incidence of malignancies and their high mortality rates, the prevention of malignant diseases has a huge public health potential and represents the most effective approach to the control of malignant diseases. Given the strong and continuous trend of demographic aging and the growing burden of the disease, it is estimated that a significant number of new cases of melanoma could be prevented by implementing effective prevention measures ranging from primary, targeted to reducing outdoor and indoor sunbathing exposure in order to reduce the exposure to ultraviolet (UV) radiation, to secondary methods of prevention such as whole-body visual examinations of the skin. Education and improvement of awareness in the general population and among health professionals are vital to reducing melanoma mortality in the future.
The results of this research can be used as a starting point in creating strategies at the community level, as well as for the development of prevention programs aimed at vulnerable and high-risk categories of the population such as children, adolescents and their parents, which would significantly reduce health care costs and total disease burden.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication
References
- 1.GLOBOCAN 2020. Cancer Incidence and Mortality Worldwide. [Accessed March 19, 2020]. Available from: http://https://gco.iarc.fr/
- 2.Gutiérrez-González E, López-Abente G, Aragonés N, et al. Trends in mortality from cutaneous malignant melanoma in Spain (1982–2016): sex-specific age-cohort-period effects. J Eur Acad Dermatol Venereol. 2019;33(8):1522–1528. doi: 10.1111/jdv.15565. [DOI] [PubMed] [Google Scholar]
- 3.McKenna MR, Stobaugh DJ, Deepak P. Melanoma and non-melanoma skin cancer in inflammatory bowel disease patients following tumor necrosis factor-α inhibitor monotherapy and in combination with thiopurines: analysis of the Food and Drug Administration Adverse Event Reporting System. J Gastrointestin Liver Dis. 2014;23:267–271. doi: 10.15403/jgld.2014.1121.233.mrmk. [DOI] [PubMed] [Google Scholar]
- 4.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170(1):11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 5.Guy GP, Jr, Thomas CC, Thompson T, Watson M, Massetti GM, Richardson LC. Vital signs: Melanoma incidence and mortality trends and projections – United States, 1982–2030. MMWR Morb Mortal Wkly Rep. 2015;64(21):591–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Kaminska-Winciorek G, Wydmanski J, Gajda M, Tukiendorf A. Melanoma awareness and prevalence of dermoscopic examination among internet users: a cross-sectional survey. Postepy Dermatol Alergol. 2016;33(6):421–428. doi: 10.5114/pdia.2016.63297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GLOBOCAN 2020. Cancer Incidence and Mortality Worldwide. [Accessed March 19, 2020]. Available from: http://https://gco.iarc.fr/
- 8.Boyers LN, Karimkhani C, Naghavi M, et al. Global mortality from conditions with skin manifestations. J Am Acad Dermatol. 2014;2 doi: 10.1016/j.jaad.2014.08.022. pii: S0190-9622(14)01869-6. [DOI] [PubMed] [Google Scholar]
- 9.Lin L, Yan L, Liu Y, Yuan F, Li H, Ni J. Incidence and death in 29 cancer groups in 2017 and trend analysis from 1990 to 2017 from the Global Burden of Disease Study. J Hematol Oncol. 2019;12(1):96. doi: 10.1186/s13045-019-0783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sneyd MJ, Cox B. A comparison of trends in melanoma mortality in New Zealand and Australia: the two countries with the highest melanoma incidence and mortality in the world. BMC Cancer. 2013;13:372. doi: 10.1186/1471-2407-13-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghazawi FM, Le M, Lagacé F, et al. Incidence, Mortality, and Spatiotemporal Distribution of Cutaneous Malignant Melanoma Cases Across Canada. J Cutan Med Surg. 2019;23(4):394–412. doi: 10.1177/1203475419852048. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65:e17. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 13.Iannacone MR, Youlden DR, Baade PD, Aitken JF, Green AC. Melanoma incidence trends and survival in adolescents and young adults in Queensland, Australia. Int J Cancer. 2015;136(3):603–609. doi: 10.1002/ijc.28956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puig-Butille JA, Escámez MJ, Garcia-Garcia F, et al. Capturing the biological impact of CDKN2A and MC1R genes as an early predisposing event in melanoma and non melanoma skin cancer. Oncotarget. 2014;5(6):1439–1451. doi: 10.18632/oncotarget.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41(14):2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 17.Leiter U, Eigentler T, Garbe C. Epidemiology of skin cancer. Adv Exp Med Biol. 2014;810:120–140. doi: 10.1007/978-1-4939-0437-2_7. [DOI] [PubMed] [Google Scholar]
- 18.International Agency for Research on Cancer. Cancer Mortality Database. [Accessed March 9, 220]. Available from: https://www-dep.iarc.fr/WHOdb/WHOdb.htm.
- 19.Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: Projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Investig Dermatol. 2016;136(6):1161–71. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Watson M, Geller AC, Tucker MA, Guy GP, Jr, Weinstock MA. Melanoma burden and recent trends among non-Hispanic whites aged 15–49 years, United States. Prev Med. 2016;91:294–8. doi: 10.1016/j.ypmed.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27(1):3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Aitken JF, Youlden DR, Baade PD, Soyer HP, Green AC, Smithers BM. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int J Cancer. 2018;142(8):1528–1535. doi: 10.1002/ijc.31141. [DOI] [PubMed] [Google Scholar]
- 23.Khosrotehrani K, Dasgupta P, Byrom L, Youlden DR, Baade PD, Green AC. Melanoma survival is superior in females across all tumour stages but is influenced by age. Arch Dermatol Res. 2015;307(8):731–40. doi: 10.1007/s40487-020-00109-1. [DOI] [PubMed] [Google Scholar]
- 24.Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Weir HK, Marrett LD, Cokkinides V, Barnholtz-Sloan J, Patel P, Tai E, et al. Melanoma in adolescents and young adults (ages 15–39 years): United States, 1999–2006. J Am Acad Dermatol. 2011;65(5 Suppl 1):S38–49. doi: 10.1016/j.jaad.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li WQ, Cho E, Weinstock MA, Mashfiq H, Qureshi AA. Epidemiological assessments of skin outcomes in the nurses’ health studies. Am J Public Health. 2016;106(9):1677–83. doi: 10.2105/AJPH.2016.303315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev Anticancer Ther. 2010;10(11):1811–1823. doi: 10.1586/era.10.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt A, Nanney LB, Boyd AS, King LE, Ellis DL. Oestrogen receptor-β expression in melanocytic lesions. Exp Dermatol. 2006;15:971–980. doi: 10.1111/j.1600-0625.2006.00502.x. [DOI] [PubMed] [Google Scholar]
- 29.Koomen ER, Joosse A, Herings, et al. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: a population-based case–control study. Ann Oncol. 2009;20(2):358–364. doi: 10.1093/annonc/mdn589. [DOI] [PubMed] [Google Scholar]
- 30.Karimkhani C, Green AC, Nijsten T, et al. The global burden of melanoma: results from the Global Burden of Disease Study 2015. Br J Dermatol. 2017;177(1):134–140. doi: 10.1111/bjd.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lasithiotakis KG, Petrakis IE, Garbe C. Cutaneous melanoma in the elderly: epidemiology, prognosis and treatment. Melanoma Res. 2010;20(3):163–70. doi: 10.1097/CMR.0b013e328335a8dd. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Melanoma of the Skin. Available from: https://seer.cancer.gov/statfacts/html/melan.html.
- 33.Veierod MB, Adami HO, Lund E, Armstrong BK, Weiderpass E. Sun and solarium exposure and melanoma risk: effects of age, pigmentary characteristics, and nevi. Cancer Epidemiol Biomarkers Prev. 2010;19(1):111–120. doi: 10.1158/1055-9965.EPI-09-0567. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong BK. How sun exposure causes skin cancer: An epidemiological perspective. In: Hill D, Elwood JM, English D, editors. Prevention of Skin Cancer. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2004. pp. 89–116. [Google Scholar]
- 35.Kricker A, Armstrong BK, Goumas C, et al. Ambient UV, personal sun exposure and risk of multiple primary melanomas. Cancer Causes Control. 2007;18(3):295–304. doi: 10.1007/s10552-006-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfahlberg A, Kölmel KF, Gefeller O Febim Study Group. Timing of excessive ultraviolet radiation and melanoma: epidemiology does not support the existence of a critical period of high susceptibility to solar ultraviolet radiation-induced melanoma. Br J Dermatol. 2001;144(3):471–475. doi: 10.1046/j.1365-2133.2001.04070.x. [DOI] [PubMed] [Google Scholar]


