Introduction
Allogeneic hematopoietic stem cell transplantation ( alloHSCT) is a potentially curative treatment in patients with mycosis fungoides (MF) and may be used in selected patients with advanced disease [1]. The differentiation of MF relapse from other skin diseases that occur after transplantation is challenging. In this case series we present the dermoscopic observation of MF lesions after alloHSCT.
Case Presentation
We have performed a dermoscopic follow-up of MF skin lesions in 3 patients before and after alloHSCT. Dermoscopic images were captured using the DermLite Cam with polarized light and independently analyzed by two certified dermoscopists (G.K-W, A.SZ-S), blinded to the clinical status of the disease. The progression was determined as new MF lesions were confirmed in the histopathologic examination.
The details of the disease, alloHSCT procedure and dermoscopic assessment are presented in Table 1. Treatment was tolerated well, and there was no case of transplant-related mortality.
Table 1.
Dermoscopic assessment of skin lesions in the course of allogeneic hematopoietic stem cell transplantation for mycosis fungoides.
| Patient case number; Gender | |||||||||||
| 1; M | 2; F | 3; F | |||||||||
| Conditioning regimen; Donor; Immunosuppression | |||||||||||
| Fludarabine+Melphalan; MUD; CsA+Mtx | TSI+TLI+ATG; MUD; CsA+MMF | Fludarabine+TBI; MUD; CsA | |||||||||
| Overall response (time after alloHSCT) | |||||||||||
| PR, then PD (treated with DLI), then PR (5,5 years) | CR (3 years) | PD (100 days) | |||||||||
| Day (alloHSCT is day 0) | |||||||||||
| −1 | +14 | +41 | +696 | −1 | +14 | +27 | +87 | −1 | +14 | +56 | +100 |
| Scale (color and distribution) | |||||||||||
| White and yellow patchy | - | - | - | - | - | - | - | - | - | - | - |
| Other structures (color and morphology) | |||||||||||
| - | - | brown parallel lines | brown parallel lines | grey dots and globules; white angulated lines | grey dots and globules; white angulated lines | white diffuse structureless areas; grey dots and globules | white diffuse structureless areas; brown dots and globules | white focal structureless areas; brown globules | whitefocal structureless areas; brown lines | white focal structureless areas; brown focal globules | white focal structureless areas |
| Vessels distribution and morphology | |||||||||||
| unspecific dotted | clustered dotted | - | - | uniform dotted | uniform dotted | peripheral dotted | - | unspecific linear | unspecific linear | unspecific linear | unspecific dotted and linear |
| Color of the background | |||||||||||
| red, orange (salmon) | orange (salmon) | skin-colored | skin-colored | red and purple | orange (salmon) | orange (salmon) | light brown | skin-colored | skin-colored | skin-colored | skin-colored |
Abbreviations: alloHSCT- allogeneic hematopoietic stem cell transplantation; ATG- antithymoglobulin; CsA- cyclosporine A; CR- complete remission; DLI- donor lymphocyte infusion; F- female; M- male; MMF- mycophenolate mofetil; Mtx- methotrexate; no- number; MUD- matched unrelated donor; PD- progressive disease; PR- partial remission TBI- total body irradiation; TLI- total lymphatic irradiation; TSI-total skin irradiation.
In active MF, red and orange color of the background was found with the presence of numerous dotted and/or linear vessels, distributed unspecifically, uniformly, or in clusters. In one case increased white and yellow scaling was noted in patchy distribution. In cases of disease regression, a visible change in background color was noted (from red to skin-colored or light brown) (2/3), with the disappearance of the previously described vessels.
Dermoscopy in the setting of alloHSCT may help to differentiate between a variety of skin conditions after the procedure, including relapse of the primary disease, cutaneous graft versus host disease, drug-induced rashes, infectious skin disorders, and secondary cutaneous neoplasms. Dermoscopy is an auxiliary tool in the differentiation of inflammatory disorders and MF based on the vessels’ morphology and their distribution, color of the background, color and distribution of the scales [2].
Figure 1.
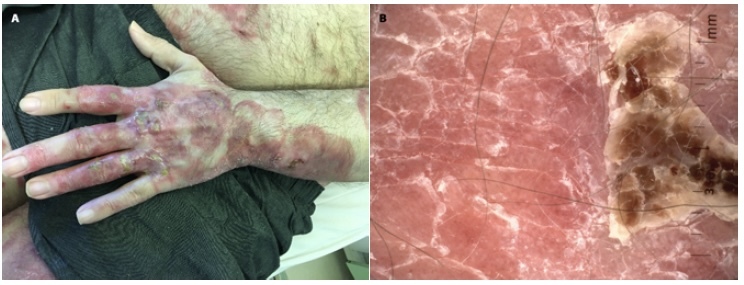
Patient 1, the skin of the left hand in the course of mycosis fungoides before allogeneic stem cell transplantation (alloHSCT). (A) Clinical image of skin lesions: numerous juicy red indurated areas covering >80% of the body surface with a tendency to create crusts can be seen. (B) Dermoscopy: yellow and white scale on juicy red and salmon background is evident. What is more, numerous dotted vessels in unspecific distribution can be seen.
Figure 2.
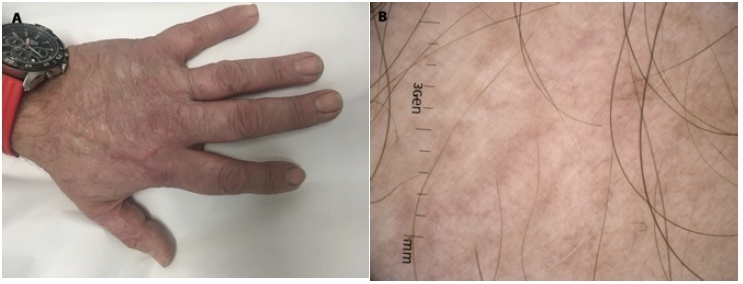
Day +696 after alloHSCT. (A) Clinical image: Full healing of lesions in the course of MF. (B) Dermoscopy: on skin-colored background multiple light brown lines are distributed in unspecific arrangement, creating light brown discolorations, no scale.
Dermoscopic features of MF described in the literature include short linear and dotted vessels [3,4,5,6] and spermatozoa-like vascular structures [3,4,5].
In our cohort, both dotted and linear vessels were noted, and they were indicative of an active disease process.
Background color in MF is red, yellow-orange [5] or a mix of the two colors [2]. The primary red or orange background color, changing to skin-colored or light brown in the case of healing of the lesions was also noted in our patients.
The presence of scale seems to be not specific in the dermoscopy of mycosis fungoides. The large variation in descriptions of its presence and distribution [2,4,6] may be due to the use of a dermoscope with non-polarized light requiring immersion vs. polarized light, where scale is more easily visualized [2]. In our group, scaling was reported in active lesions.
Other noted structures such as white diffuse structureless areas, brown lines and globules were present irrespective of disease status.
Conclusion
Our report adds to the information about the dermoscopic characteristics of MF lesions and their evolution. Observations in the case of disease remission included a change of the background color from initial red-orange to skin-colored or light brown and the disappearance of numerous dotted and linear vessels, while the persistence of vessels was indicative of progressive disease.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome – Update 2017. Eur J Cancer. 2017;77:57–74. doi: 10.1016/j.ejca.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 2.Bilgic SA, Cicek D, Demir B. Dermoscopy in differential diagnosis of inflammatory dermatoses and mycosis fungoides. Int J Dermatol. 2020;59(7):843–850. doi: 10.1111/ijd.14925.3. [DOI] [PubMed] [Google Scholar]
- 3.Bosseila M, Sayed Sayed K, El-Din Sayed SS, Abd El Monaem NA. Evaluation of Angiogenesis in Early Mycosis Fungoides Patients: Dermoscopic and Immunohistochemical Study. Dermatology. 2015;231(1):82–86. doi: 10.1159/000382124. [DOI] [PubMed] [Google Scholar]
- 4.Lallas A, Apalla Z, Lefaki I, et al. Dermoscopy of early stage mycosis fungoides. J Eur Acad Dermatology Venereol. 2013;27(5):617–621. doi: 10.1111/j.1468-3083.2012.04499.x. [DOI] [PubMed] [Google Scholar]
- 5.Bombonato C, Pampena R, Lallas A, Giovanni P, Longo C. Dermoscopy of Lymphomas and Pseudolymphomas. Dermatol Clin. 2018;36(4):377–388. doi: 10.1016/j.det.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Errichetti E, Apalla Z, Lallas A, et al. Dermoscopic spectrum of mycosis fungoides: a retrospective observational study by the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2022;36(7):1045–1053. doi: 10.111/jdv.18078. [DOI] [PMC free article] [PubMed] [Google Scholar]


