Abstract
Introduction
Spitz nevi (SN) are benign melanocytic proliferations frequently occurring in children. Some pigmented SN with a starburst pattern evolve into the “stardust” one, which is characterized by a central, black to gray, hyperpigmented area and remnants of a brown network at the periphery. These dermoscopy changes are often the first alert to induce excision.
Objectives
The aim of this study is to enlarge the case series of stardust SN in children, in order to increase confidence with this new dermoscopic pattern and reduce unnecessary excisions.
Methods
This retrospective observational study was conducted with SN cases received from IDS members. The inclusion criteria were: clinical and/or histopathologic diagnosis of Spitz naevus with starburst appearance in children <12 years old, availability of a dermoscopic image at baseline and after follow-up of at least 1 year, availability of patient data. The dermoscopic images and their changes over time were assessed by three evaluators in consensus.
Results
38 SN were enrolled, with a median age of 7 years and a median FUP duration of 15,5 months. Comparing the evolution with time of FUP, no significant differences were found between growing and involuting lesions in terms of patient age and sex, location and palpability of lesions.
Conclusions
The long follow-up reported in our study could really support the concept of benignity of changing SN. A conservative approach is acceptable for nevi showing the stardust pattern, because it may be considered a physiological evolution of pigmented Spitz nevus, and urgent surgeries could be avoided.
Keywords: spitz nevus, starburst, stardust
Introduction
Spitz nevi (SN) are benign melanocytic proliferations that frequently occur in children [1]. 50% to 75% of patients with a diagnosis of Spitz nevi are found to be younger than 20 years of age and the overall incidence has been estimated between 1.4 and 7 new cases per 100,000 persons per year [2].
Before 1948, this entity was defined as Juvenile melanoma, as the clinical and histological differentiation between Spitz Nevus and melanoma of the adult could not be made with certainty in several cases.
Sophie Spitz [3] first noted a difference in the biological behavior of Spitz nevus compared to adult melanoma. In a series of 13 lesions diagnosed as malignant melanoma in children, only one patient died from melanoma metastases. The other 12 participants had lesions that were locally excised without recurrence or metastases. Sophie Spitz concluded that, since metastases from juvenile melanomas occur only rarely, conservative surgery, rather than the radical surgery usually indicated for adult melanomas, seems justified. The study challenged the standard of practice at that time relating to the diagnosis of melanomas and benign nevi in children [4].
However, in the late 1990s, a new era of controversies on the management of spitzoid lesions started, because cases of spitzoid lesions without malignant histopathological criteria but with nodal metastasis were described. New histological entities were introduced attempting to classify spitzoid tumours with intermediate histopathological features between Spitz naevus and spitzoid melanoma (Spitz naevus with atypia and metastasis, metastasizing Spitz tumour, atypical Spitz tumour, atypical Spitz naevus, melanocytic tumour of unknown malignant potential, melanocytoma). The worrying scenario of possible loco-regional dissemination in connection with the medico-legal liability could lead to the risk of overcalling [5].
The introduction of dermoscopy significantly improved the diagnosis of Spitz naevi, as they were shown to exhibit a peculiar and characteristic pattern of dermoscopic structures.
Pigmented variants were first investigated and shown to display the so-called ‘starburst’ pattern, consisting of a central area of homogeneous black-blue pigmentation and symmetrically distributed peripheral streaks or pseudopods [6].
Several additional patterns were later found to be associated with pigmented Spitz naevus, including globular, homogeneous, reticular and multicomponent patterns.
Histologically, Spitz nevi are composed of spindled and/or epithelioid melanocytes with large nuclei and abundant cytoplasm [7]. The melanocytes seen in Spitz nevi are often larger than those in other types of nevi, arranged perpendicular and parallel to the skin surface, highly cohesive, and they do not destroy the nearby keratinocytes. These spindle cells contain melanin pigment. Additionally, Kamino bodies, which are rounded eosinophilic globules, are often seen. Frequently, solitary melanocytes and nests of melanocytes occurring above the dermo-epidermal junction are detected. Dermal nests and cords of cells could be seen if a dermal component is present. The lack of ulceration, dermal sheets of cells, or significant mitotic activity could exclude the diagnosis of malignant tumors [8]. Guidelines for the management of spitzoid-looking tumors have been proposed by the International Dermoscopy Society (IDS) in 2017 [6]. The first management criterium is symmetry. Lesions displaying asymmetrically distributed spitzoid features (peripheral streaks/pseudopods, dotted vessels, reticular depigmentation) should be excised to rule out melanoma. In symmetrical lesions, the second management-driven criterium is age. Dermoscopically symmetric spitzoid-looking lesions developing after the age of 12 years should be managed with caution because there is a considerable probability that these lesions actually constitute melanoma. Below the age of 12 years, the recommended management of dermoscopically symmetric spitzoid-looking lesions depends if the lesion is nodular or flat, which is the third criterium. For nodular lesions, the recommended management is excision, mainly because the possibility of an atypical Spitz tumor cannot be excluded on the basis of dermoscopic morphology. For flat/raised lesions, follow-up until stabilization is suggested. Monitoring is highly recommended in the subset of lesions displaying a starburst pattern (Reed naevi). Lesions displaying a starburst pattern are expected to grow (Fig 1.), reach stabilization and, then, involute. Ideally, the typical Reed naevus grows symmetrically and gradually acquires a blue-black homogeneous aspect with the disappearance of peripheral projections. After years, the dark pigmented area is gradually restricted to the center of the lesion, while the peripheral part of the naevus exhibits remnants of a delicate brown network. (Fig 2-3-4). This pattern, which may anticipate the involution of the lesion, was first described by Argenziano et al. [1] and later named “stardust” pattern.
Figure 1.
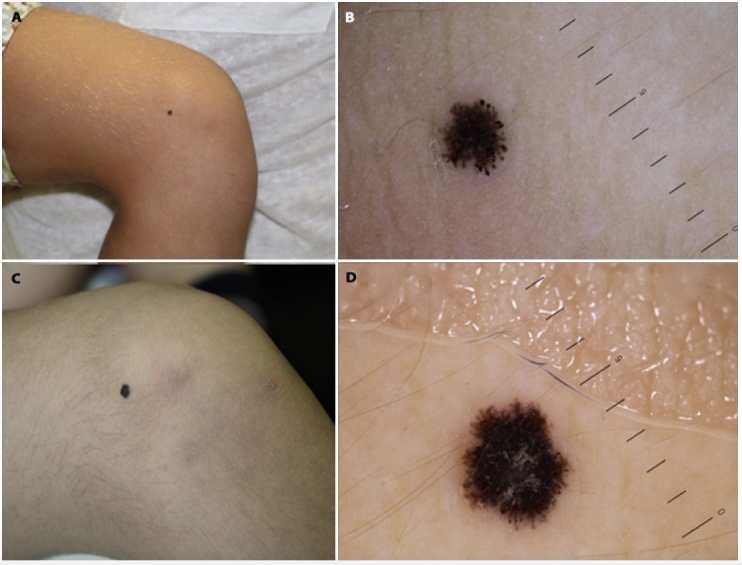
A–B) Male patient aged 5, at his first visit for a SN on the knee. Dermoscopy shows typical SN with hyperpigmented central area and pseudopods at the periphery. C–D) After 1 year, the lesion starts losing pseudopods and grows symmetrically.
Figure 2.

First and last image of SN in a female patient aged 11 and 20 respectively. The classic stardust pattern is evident in the second dermoscopy.
Figure 3.
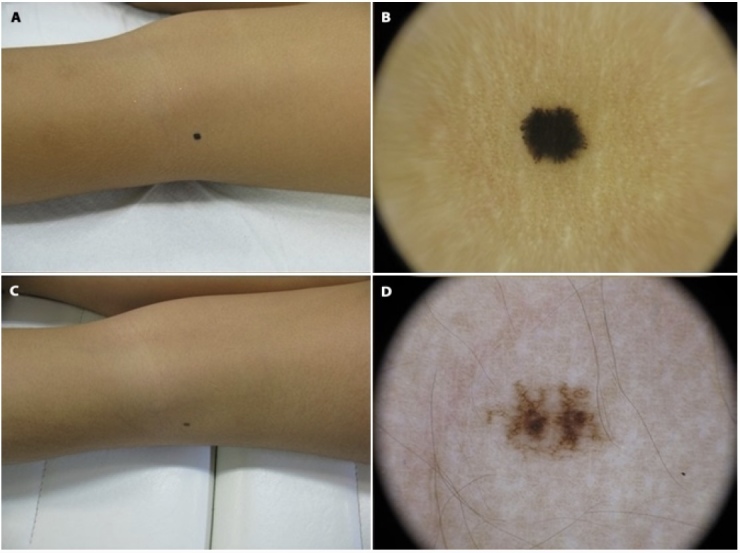
A–B) Female patient, 3 years old at first visit. C–D) FUP visit at 6 years old, where the involution is almost completed, with only brown not organized network.
Figure 4.

A–B) SN in a patient aged 6 at first visit. At dermoscopy, the lesion shows blue-white center with brown streaks at periphery. C–D) Same lesion at 17 years old. Dermoscopy highlights stardust pattern with blue globules in the center on a brown background and brown network fading out at the periphery.
In the IDS guidelines6 “Reed” and “Spitz” names are used interchangeably since a pathological distinction between the two entities cannot be made [9,10].
Objectives
The aim of this study is to enlarge the case series of stardust spitz nevi in children, in order to increase confidence with this new dermoscopic pattern, limit physicians’ concerns, and avoid unnecessary excisions.
Methods
This retrospective observational study was conducted with cases received from members of the International Dermoscopy Society. Inclusion criteria were clinical and/or histopathologic diagnosis of Spitz naevus with starburst appearance in children <12 years old, availability of a dermoscopic image at baseline and after follow-up of at least 1 year, availability of patient’ demographics, lesion location, follow-up duration. Cases lacking adequate clinical information were excluded.
The centers were asked to provide, with images, a database containing the following information: location of the primary lesion, sex, date of birth, age at first and at last visit, number of follow-up visits and number of total months of follow-up. The dermoscopic images were assessed by three evaluators in consensus (GB, CS and GA), with the stardust feature scored as present when at least two of them were in agreement. Cases not assessed as “starburst pattern” were excluded. Moreover, change in dimension (if present stated as growth or involution), palpability at first visit, and eventually change over time were also scored as present when at least two evaluators were in agreement.
Results
We reviewed clinical and dermoscopic images of 38 SN in patients aged from 1 to 12 years (median age, 7 years p<0.01). 21 males and 17 females were included. All patients were Caucasian. The follow-up period varied from a minimum of 3 to a maximum of 120 months, with the median FUP duration of 15,5 months. The median number of follow-up visits was 2.0 with a minimum of 1 and a maximum of 10 (IQR 3.75).
Of 32 SN, 27 (71.1%) were located on the extremities, 3 (7.9 %) on the head and 8 (21 %) on the trunk.
With regards to the clinical presentation at baseline, 36 SN were flat/raised (94.7% – 95% CI: 80.9% to 99.1%, p<0.01) and only 2 were nodular (5.3% – 95% CI: 0.9% to 19%, p<0.01). Almost all the lesions remained flat at the last follow-up visit (97.4% – 95% CI: 84.6% to 99.9%, p<0.01) while 1 of the 2 nodular lesions became flat after regression (2.6% – 95% CI: 0.1). Between the first and the last visit 63.2% of the lesions (N:24 – 95%CI: 46% to 77.6%, p<0.01) had an increase in size, 26.3% had a reduction in size (N:10 – 95%CI: 14% to 43.4%, p=0.05) while 10.5% (N:4 – 95%CI: 3.4% to 25.7%, p=0.14) had only dermoscopic changes without variation in dimension.
Comparing the evolution with time of follow-up, no significant differences were found between growing and involuting lesions in terms of patient age and sex, location and palpability of lesions.
Dermoscopic examination showed findings consistent with the literature.
The starburst pattern evolved into stardust over time. First, a growing phase is observed in which the center of the lesion remains black/blue and the periphery presents a brown network. Successively, an involution phase starts with regression of the network in the periphery and ends with a grey granular pattern in the center of the lesion.
Conclusions
Our study revealed that the Stardust pattern is more frequent in the extremities, with no differences between sex. The median age is consistent with the literature.
Barnhill et al. [11] already described an association between pigmented SN and thighs, whereas the rapidly growing, pink-to-reddish papule variant is more frequent in the head and neck region.
Analyzing the follow-up, we noted that the time needed to pass into a growth phase and then into an involuting one is very variable. The onset of the growth phase ranged between 4 to 24 months from first appearance. Surprisingly, we observed a SN that turned into stardust pattern after being stable for 10 years (Fig 4), while another SN after 10 years was almost completely involved showing greyish remnants of the network (Fig 5). It should be remembered that the IDS guidelines suggest continuing follow-up also for nevi undergoing involution until stabilization (documented evidence of no change for at least 6 months) or complete disappearance. The reason for this SN dynamic behavior is not clear, but can be related to the genetics of melanocytic cells. The most frequently observed genetic alterations in Spitz nevi involve the HRAS gene that drives the symmetrical overgrowth of cells with epithelioid morphology via preferential PI3K/AKT activation [12]. The acquisition and loss of mutations are responsible for the growth and involution of Spitz nevi.
Figure 5.

SN on upper back of male patient. A–B )10 years old. C–D) 20 years old. In D the final involuting phase with greyish remnants of the network.
In our series, despite the change in dimension or in dermoscopy pattern, the symmetry was always maintained as well as the flat/raised appearance as none became nodular.
The long follow-up reported in our study could really support the concept of benignity of changing SN. Since Spitz nevi are not precursors of melanoma6, clinically and dermoscopically typical cases of SN will maintain the same biological behavior, regardless of dermoscopic changes. Moreover, a flat/raised dermoscopically symmetric spitzoid-looking lesion below the age of 12 has an extremely low possibility to be a melanoma as pediatric melanoma is very rare and melanomas arising in children are often nodular and/or amelanotic [13].
A limitation of our study is that our sample is small and descriptive estimates are weighted by large confidence intervals. Other studies are needed to explore the different patterns of evolving SN, and to combine dermoscopy, confocal microscopy and genetic mutations in the optics of the new concept of deep phenotyping.
In conclusion, although the decision to cut off a lesion is demanded to an overall consideration of clinical context (age, site) and guidelines management, a conservative approach is acceptable for nevi showing the stardust pattern, because it resulted to be a physiological evolution of pigmented Spitz nevus, and urgent surgeries could be avoided for these patients.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Argenziano G, et al. Natural evolution of spitz nevi. Dermatology. 2011;222:256–260. doi: 10.1159/000326109. [DOI] [PubMed] [Google Scholar]
- 2.Neri I, Dika ERG, et al. Spitz nevi: defining features and management in children. G Ital Dermatol Venereol. 2014;149:675–682. [PubMed] [Google Scholar]
- 3.SS Melanomas of childhood. Am J Pathol. 1948;24:591–609. [PMC free article] [PubMed] [Google Scholar]
- 4.Spitz K, Piliang M, Mostow E. Sophie Spitz: A woman ahead of her time. Int J Women’s Dermatology. 2019;5:190–191. doi: 10.1016/j.ijwd.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piérard GE, Piérard-Franchimont C, Delvenne P. Simulants of malignant melanoma. Oncol Rev. 2015;9:23–27. doi: 10.4081/oncol.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lallas A, et al. Update on dermoscopy of Spitz/Reed naevi and management guidelines by the International Dermoscopy Society. Br J Dermatol. 2017;177:645–655. doi: 10.1111/bjd.15339. [DOI] [PubMed] [Google Scholar]
- 7.Dika E, Ravaioli GM, Fanti PA, Neri I, Patrizi A. Spitz Nevi and Other Spitzoid Neoplasms in Children: Overview of Incidence Data and Diagnostic Criteria. Pediatr Dermatol. 2017;34:25–32. doi: 10.1111/pde.13025. [DOI] [PubMed] [Google Scholar]
- 8.Ferrara G, et al. Spitz Nevus, Spitz Tumor, and Spitzoid Melanoma. A Comprehensive Clinicopathologic Overview. Dermatol Clin. 2013;31:589–598. doi: 10.1016/j.det.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Bär M, Tschandl P, Kittler H. Differentiation of pigmented Spitz nevi and Reed nevi by integration of dermatopathologic and dermatoscopic findings. Dermatol Pract Concept. 2012;2:13–24. doi: 10.5826/dpc.0201a03.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bär M. Spitz and Reed nevi: acquired or congenital? Dermatol Pract Concept. 2012;2:21–27. doi: 10.5826/dpc.0203a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnhill RL, Barnhill MA, Berwick M, Mihm MC. The histologic spectrum of pigmented spindle cell nevus: A review of 120 cases with emphasis on atypical variants. Hum Pathol. 1991;22:52–58. doi: 10.1016/0046-8177(91)90061-s. [DOI] [PubMed] [Google Scholar]
- 12.Roh MR, Eliades P, SG, et al. Genetics of Melanocytic Nevi. Pigment Cell Melanoma Res. 2015;28:661–672. doi: 10.1111/pcmr.12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haliasos HC, et al. Dermoscopy of Benign and Malignant Neoplasms in the Pediatric Population. Semin Cutan Med Surg. 2010;29:218–231. doi: 10.1016/j.sder.2010.10.003. [DOI] [PubMed] [Google Scholar]


