Abstract
Purpose
The biplane area-length method is commonly used in cardiac magnetic resonance (CMR) to assess left atrial (LA) volume (LAV) and function. Associations between left atrial emptying fraction (LAEF) and clinical outcomes have been reported. However, only limited data are available on the calculation of LAEF using the biplane method compared to 3D assessment. This study aimed to compare volumetric and functional LA parameters obtained from the biplane method with 3D assessment in a large, multiethnic cohort.
Method
158 participants of MESA (Multi-Ethnic Study of Atherosclerosis) underwent CMR that included standard two- and four-chamber steady-state free precession (SSFP) cine imaging for the biplane method. For 3D-based assessment, short-axis SSFP cine series covering the entire LA were obtained, followed by manual delineation of LA contours to create a time-resolved 3D LAV dataset. Paired t-tests and Bland-Altman plots were used to analyze the data.
Results
Standard volumetric assessment showed that LAVmin (bias: −8.35mL, p<0.001), LAVmax (bias: −9.38mL, p<0.001) and LAVpreA (bias: −10.27mL, p<0.001) were significantly smaller using the biplane method compared to 3D assessment. Additionally, the biplane method reported significantly higher LAEFtotal (bias: 7.22%, p<0.001), LAEFactive (bias: 6.08%, p<0.001), and LAEFpassive (bias: 4.51%, p<0.001) with wide limits of agreement.
Conclusions
LA volumes were underestimated using the biplane method compared to 3D assessment, while LAEF parameters were overestimated. These findings demonstrate a lack of precision using the biplane method for LAEF assessment. Our results support the usage of 3D assessment in specific settings when LA volumetric and functional parameters are in focus.
Keywords: Cardiac MRI, biplane area-length method, left atrial volume, left atrial function, left atrial emptying fraction
Graphical Abstract
INTRODUCTION
Left atrial (LA) volume (LAV) is an important predictor of cardiovascular outcomes and function. LA enlargement is associated with various clinical conditions, such as atrial fibrillation (AF), heart failure, myocardial infarction, and hypertension [1–4]. Increased LAV has also been linked to stroke and death [5]. More recently, studies have reported associations between LA emptying fraction (LAEF) as a measure of LA function and clinical endpoints, such as outcome after myocardial infarction, recurrence after ablation for AF, and mitral valve regurgitation [6–8].
Cardiac MRI (CMR) plays an established role in measuring cardiac chamber volumes as well as cardiac function. The biplane area-length method is commonly used in CMR to assess LAV and LA function. Studies have shown that this method allows for good estimation of maximum LA volume (LAVmax) compared to 3D assessment, which is based on short axis (SAX) image acquisition with full volumetric coverage of the LA [9]. However, data on its estimation of minimum LAV (LAVmin) and volume before atrial contraction (LAVpreA) is not available to the same extent [9, 10]. As a result, the effect on the calculation of LAEF using the biplane method compared to 3D assessment is not well investigated in larger, multiethnic cohorts [11, 12].
The Multi-Ethnic Study of Atherosclerosis (MESA) has contributed to understanding the role of LA parameters from CMR assessment in many diseases, such as AF or diabetes mellitus, and in relation to other cardiovascular risk factors [9, 13, 14]. The cohort is well described overall and, therefore, appropriate for investigating the impact of technique on LAEF assessment. In this study, we sought to compare volumetric and functional LA parameters, such as LAEF, obtained from the biplane area-length method with 3D assessment as the reference standard in a cohort of more than 150 MESA participants.
MATERIALS AND METHODS
Study Cohort
This was an ancillary, single center study within MESA and was approved by the Institutional Review Board at our institution. All participants gave written informed consent, in addition to their prior enrollment into the MESA study. MESA protocol has previously been described in detail [15]. 240 MESA participants at our institution were approached to participate in this study. 77 declined participation due to concerns about entering a hospital space during the COVID-19 pandemic. The prospectively recruited cohort of MESA participants (n=163) underwent an additional CMR at our institution between 2018–2020 outside of the original MESA protocol. These additional CMR were analyzed for this study. A total of 5 participants were excluded due to missing imaging series (biplane: n=3, 3D: n=2), resulting in a final study cohort of 158 participants (Figure 1). At inclusion into MESA (between 2000–2002), all participants were free of known cardiovascular disease.
Figure 1.
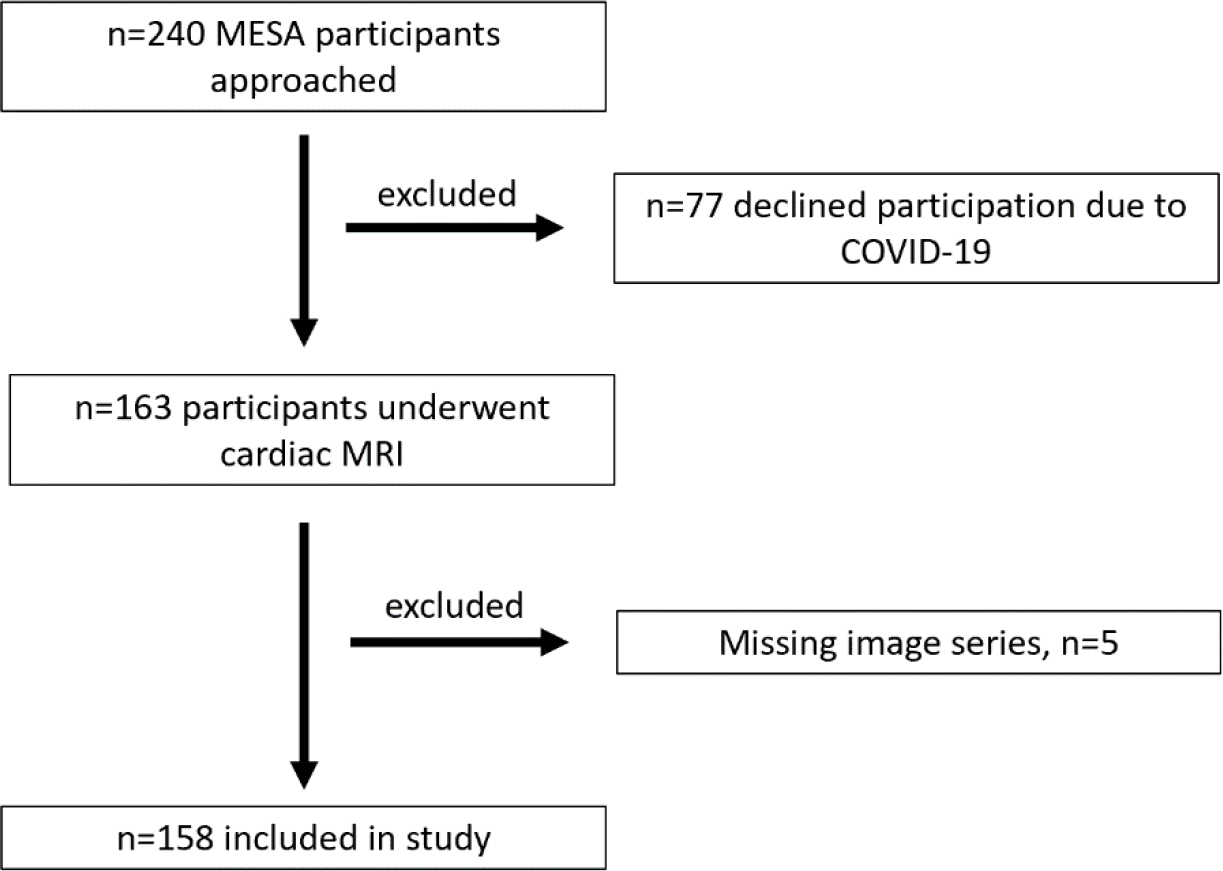
Flow diagram showing selection of the study population.
MRI Protocol
CMR examinations were performed on a 1.5T MRI system (Aera, Siemens Healthineers, Erlangen, Germany). All participants underwent standard two- and four-chamber balanced steady state free precession (SSFP) cine imaging, as well as SAX SSFP cine series covering the left ventricle (LV) and the entire LA (TE: 1.2ms, TR: 35.5ms, flip angle: 59°, spatial resolution: 1.8mm × 1.8mm, slice thickness: 6 mm, slice gap: 3mm, inter-slice distance: 9mm, field of view: 340 mm × 308 mm, matrix: 192 × 180, number of cardiac time points: 25).
Volumetric and Functional Assessment of the Left Atrium
For the biplane area-length method, CMR examinations were evaluated for LA volumes using a commercially available software (cvi42, Version 5.9.0., Circle Cardiovascular Imaging Inc., Calgary, Canada). Two- and four-chamber SSFP cine series were used to contour the LA endocardial boundaries at all time points of the cardiac cycle with the software’s automatic tool for LA assessment. A trainee (S.L.) performed the automated biplane analysis, which was then reviewed in detail by the cardiovascular (CV) radiologist with 3 years of experience in CMR (M.P.). The pulmonary veins and the left atrial appendage were excluded from the LA contours (Figure 2A). For the biplane method, the formula used for estimating the LA volume for each time point is: LA volume = (4-chamber area) × (2-chamber area) × 0.85/atrial length [16].
Figure 2.
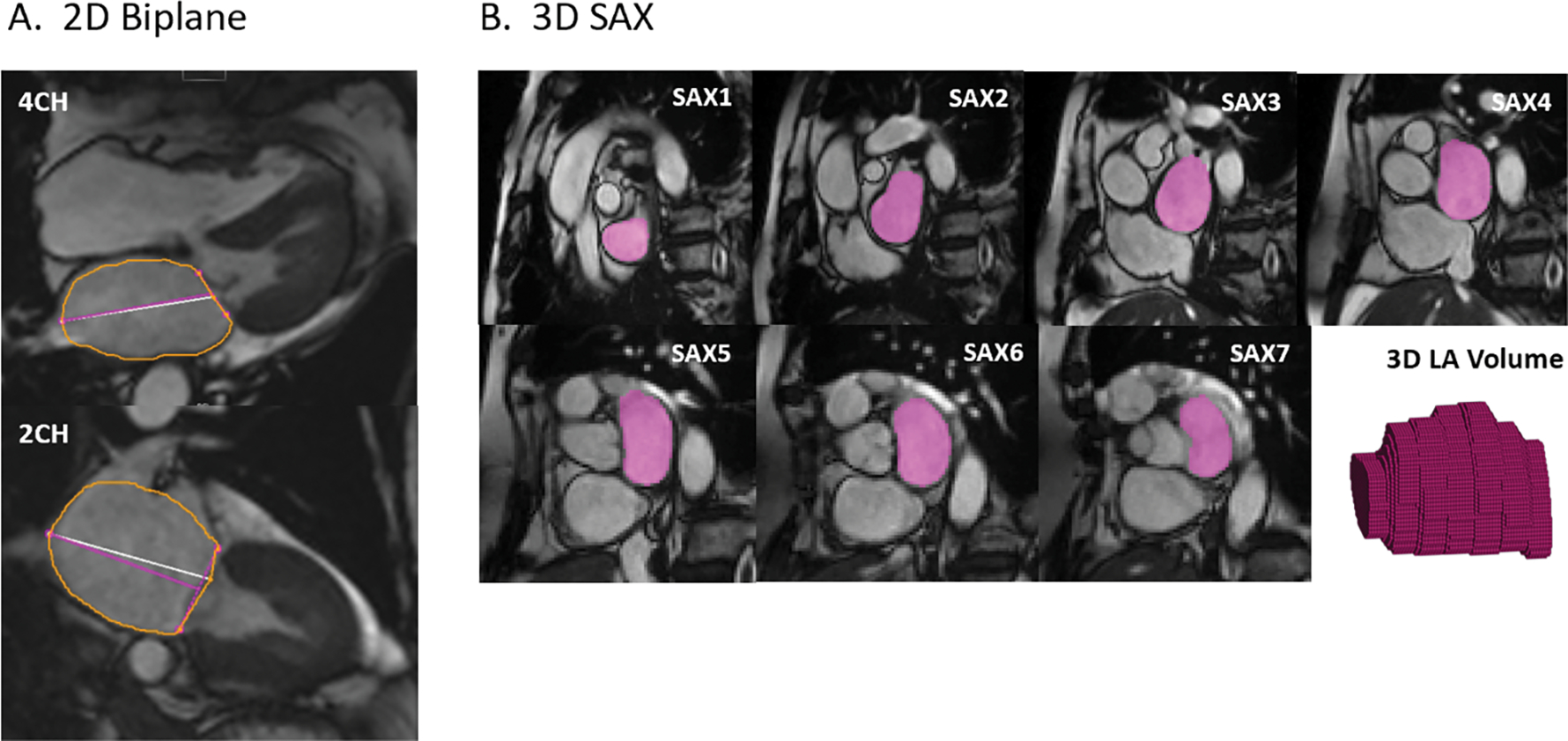
(A) Biplane method: LA endocardial boundaries were contoured on two- and four-chamber images at max LAV. (B) 3D assessment: Contours on SAX images and a 3D volume representation of the LA endocardium for a single time point; the contouring was performed for each time point of the cardiac cycle (25 per patient). At the atrioventricular border, the LA was contoured if less than 50% of left ventricular myocardium was visible.
3D assessment based on 2D SAX images was performed by the CV radiologist (M.P.) using a home-built software programmed in MATLAB (The MathWorks, Natick, MA, USA). The LA endocardial borders were contoured by the CV radiologist on each SAX slice and at all cardiac time points. At the atrioventricular junction, the LA was contoured only if there was less than 50% of LV myocardium visible. This rule was also used to define the mitral valve plane. The contours for each plane were combined into a time-resolved 3D volume dataset for further analysis. The biplane and 3D-based assessments for each participant were performed at least 4 weeks apart. An overview is depicted in Figure 2B.
LAVmax and LAVmin were automatically identified in each of the volume-time curves derived for both methods (biplane and 3D assessment, Figure 3A). The pre-atrial contraction volume (LAVpreA) was manually identified for each participant and each method independently using the volume-time curves as well. If neighboring points were present for LAVpreA, we systematically chose the larger volume. Based on these parameters, total, active, and passive LAEF were calculated using the standard equations in Figure 3B [9].
Figure 3.
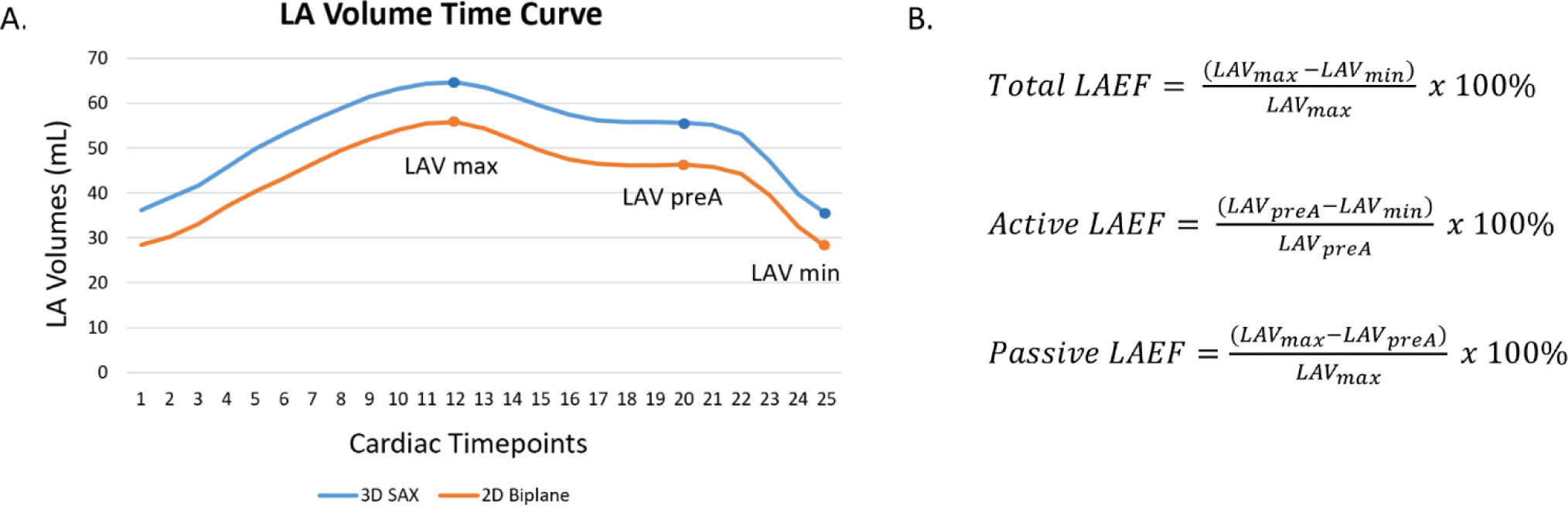
(A) Determination of maximum (LAVmax), minimum (LAVmin), and LAV before atrial contraction (LAVpreA) using the LA volume time curve for each method. (B) The equations used to calculate LAEF parameters.
Interreader assessment was performed on ten random participants by a reader with one year of experience in both biplane and 3D methods (A.M.). For each method, we compared all six parameters: LAVmax, LAVmin, LAVpreA, LAEFtotal, LAEFpassive, and LAEFactive. To evaluate agreement between the two readers, we calculated intraclass correlation coefficients (ICC) for each LAV and LAEF parameter.
Statistical Analysis
Statistical analyses were performed using Excel (Microsoft, Redmond, WA, USA) and Python (Python Software Foundation, Wilmington, DE, USA). All parameters were first tested for normal distribution and if normally distributed, reported as mean and standard deviation (SD). Paired t-tests were then performed. Bland-Altman plots were created to compare the differences between the biplane and 3D methods for all available time points, including the mean difference (bias) and limits of agreement (LoA). The percentage difference was calculated by taking the absolute value of the change in means (bias) divided by the average of the two methods.
RESULTS
Subject Demographics
The study included 158 participants (83 female) with a mean age of 72.7 ± 7.3 years (Table 1). Ethnicity was self-reported as African American (n=36), Chinese American (n=52), or White (n=70). Mean BMI was 26.4 ± 4.7 kg/m2. Although participants were free of a history of cardiovascular events (myocardial infarction, revascularization, stroke, heart failure, or current atrial fibrillation) at their entry into the MESA study in 2000–2002, participants had the following characteristics in 2018–2020 at the time of the cardiac MRI: arterial hypertension (n=90, 57.0%), diabetes (n=20, 12.7%), history of AF (n=12, 7.6%) and former or current smoker (n=74, 46.8%). For each participant, the median number of slices with a visible LA was 6 (IQR: 2, range: 3–9).
Table 1.
Characteristics of the study cohort.
| Characteristics | |
|---|---|
|
| |
| Participants | 158 |
|
| |
| Age (years) ± SD | 72.7 ± 7.3 |
|
| |
| Female | 83 (52.5) |
|
| |
| Race/Ethnicity | |
| White | 70 (44.3) |
| African American | 36 (22.8) |
| Chinese American | 52 (32.9) |
|
| |
| Mean BMI (kg/m2) | 26.4 ± 4.7 |
|
| |
| Conditions at Time of CMR | |
| Hypertension | 90 (57.0) |
| Diabetes | 20 (12.7) |
| History of AF | 12 (7.6) |
| Former or current smoker | 74 (46.8) |
| Median Slice Number ± IQR | 6 ± 2 |
Numbers are mean ± SD (if not stated otherwise) or number of participants (percentage). CMR = cardiac magnetic resonance imaging. AF = atrial fibrillation. IQR = interquartile range. SD = standard deviation.
LA Volumes
Overall, pairwise comparisons for LAVmin, LAVmax and LAVpreA showed significantly smaller values for the biplane method compared to 3D assessment (p<0.001, p<0.001, and p<0.001) (Table 2). Analysis of agreement via Bland-Altman plots resulted in negative biases of −8.35 mL, −9.38 mL and −10.27 mL respectively for these three parameters. When comparing the two methods for each participant, this discrepancy was also seen over all 25 available time points (mean bias ± SD: −9.03 ± 0.80 mL, range: −9.97 mL,−7.18 mL), indicating that LA volumes were generally underestimated using the biplane area-length method versus 3D assessment (Figure 4A). Limits of agreement were wide for LAVmax (−37.49, 18.73 mL), LAVmin (−26.91, 10.21 mL), and LAVpreA (−33.48, 12.95 mL), and the percentage differences were large (LAVmax 14.24%, LAVmin 25.90%, and LAVpreA 20.32%) (Figure 4, Table 2). While the overall trend was underestimation of LAV by the biplane method, a total of 44 participants (27.8%) had larger volumes in at least one LAV parameter (LAVmax: 38 participants, LAVmin: 21 participants, LAVpreA: 24 participants).
Table 2.
Mean values with SD for LAV and LAEF parameters as calculated by the biplane and 3D methods.
| Analysis of | Differences between methods | Agreement between methods | ||||
|---|---|---|---|---|---|---|
| Parameter | Biplane | 3D | p-value | % diff | Bias (95% CI) | LoA |
| LAV max (mL) | 58.18 ± 23.87 | 67.10 ± 21.28 | <0.001 | 14.24 | −9.38 (−11.65, −7.10) | −37.49, 18.73 |
| LAV min (mL) | 26.68 ± 19.66 | 34.62 ± 17.77 | <0.001 | 25.90 | −8.35 (−9.85, −6.84) | −26.91, 10.21 |
| LAV preA (mL) | 45.93 ± 19.15 | 56.32 ± 17.00 | <0.001 | 20.32 | −10.27 (−12.20, −8.33) | −33.48, 12.95 |
| LAEF total (%) | 56.71 ± 12.31 | 49.51 ± 11.15 | <0.001 | 13.56 | 7.22 (6.00, 8.45) | −7.91, 22.35 |
| LAEF active (%) | 47.60 ± 11.48 | 41.23 ± 10.56 | <0.001 | 14.34 | 6.08 (4.59, 7.56) | −11.70, 23.85 |
| LAEF passive (%) | 20.14 ± 7.95 | 15.29 ± 7.73 | <0.001 | 27.38 | 4.51 (2.82, 5.52) | −9.54, 18.56 |
LoA = Limits of Agreement. % diff = percentage difference. CI = confidence interval.
Figure 4.
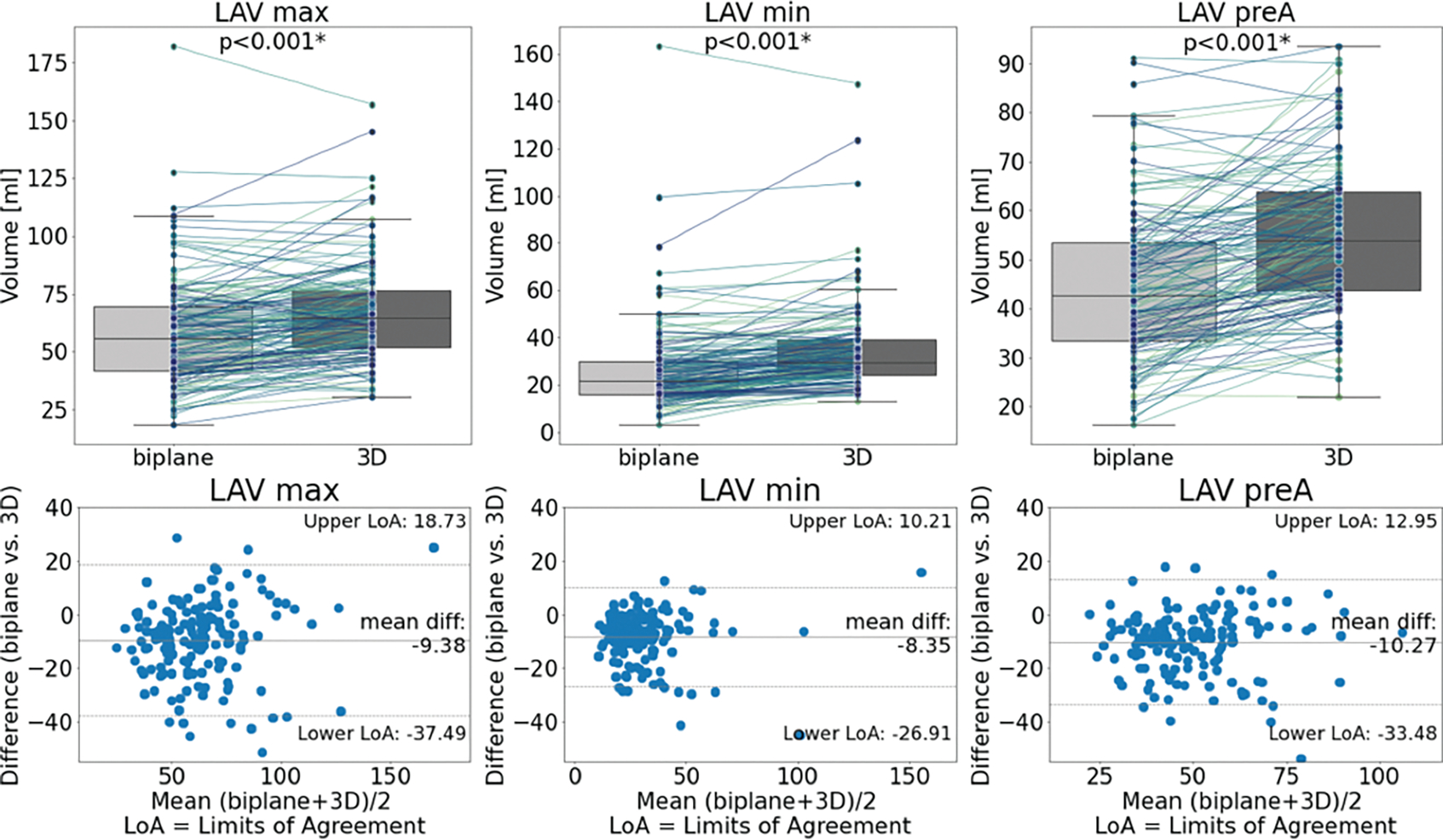
Box plots (top) and Bland-Altman plots (bottom) comparing biplane and 3D methods for each LAV parameter. Overall, the biplane method significantly underestimated the LAVs with wide limits of agreement.
LA Emptying Fractions
The biplane method resulted in significant overestimation of LAEF parameters, including higher LAEFtotal (p<0.001), LAEFactive (p<0.001), and LAEFpassive (p<0.001) compared to 3D assessment (Table 2). Analysis of agreement by Bland-Altman plots showed positive biases with wide limits of agreement for LAEFtotal (bias: 7.22%, LoA: −7.91%, 22.35%), LAEFactive (bias: 6.08%, LoA: −11.7%, 23.85%), and LAEFpassive (bias: 4.51%, LoA: −9.54%, 18.56%) and high percentage differences (Figure 5, Table 2). While the overall trend was overestimation of LAEF by the biplane method, a total of 80 participants (50.6%) had lower values for at least one LAEF parameter (LAEFtotal: 24 participants, LAEFactive: 42 participants, LAEFpassive: 42 participants).
Figure 5.
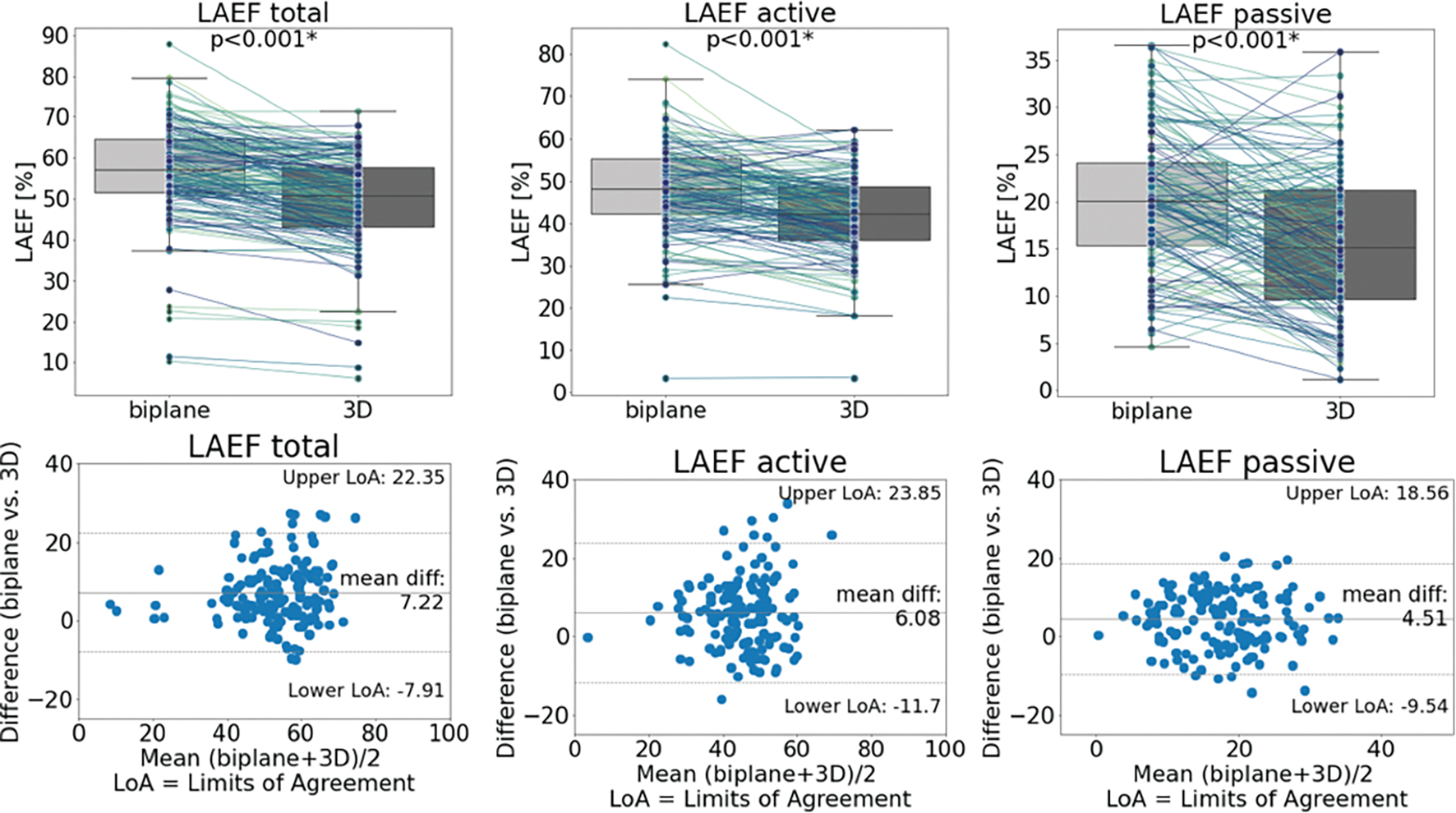
Box plots (top) and Bland-Altman plots (bottom) comparing biplane and 3D methods for each LAEF parameter. Overall, the biplane method overestimated the LAEFs significantly with wide limits of agreement.
Reproducibility and Variability
Interreader assessment revealed excellent agreement for the volumetric parameters derived from the biplane and 3D methods. For the 3D method, the bias was −1.1 mL (LoA: −10.55, 8.4 mL) for LAVmax (ICC: 0.99), −2.4 mL (LoA: −6.2, 1.4 mL) for LAVmin (ICC: 1.00) and −1.0 mL (LoA: −11.3, 9.4 mL) for LAVpreA (ICC: 0.98). Comparison of LAEF parameters for the 3D method also showed excellent agreement for LAEFtotal (bias: 2.3%, LoA: −4.4, 9.0%, ICC: 0.99) and LAEFactive (bias: 2.8%, LoA: −6.7, 12.3%, ICC: 0.92). Bias for LAEFpassive was similarly low (bias: −1.0%, LoA: −13.1, 11.1%), while ICC was less compared to the other parameters (ICC: 0.72).
For the biplane method, there was also excellent agreement for LAV and LAEF parameters. The bias was 3.1 mL (LoA: −3.1, 9.3mL) for LAVmax (ICC: 1.00), 0.2 mL (LoA: −4.8, 5.1mL) for LAVmin (ICC: 1.00), and 5.2 mL (LoA: −0.3, 10.6mL) for LAVpreA (ICC: 0.99). LAEF assessment showed a bias of 1.9% (LoA: −7.8, 11.6%) for LAEFtotal (ICC: 0.98), 4.4% (LoA: −7.2, 16.0%) for LAEFactive (ICC: 0.94) and −3.8% (LoA: −7.7, 0.2%) for LAEFpassive (ICC: 0.98).
DISCUSSION
In this study, we compared the biplane area-length method with the 3D method as the reference standard for the assessment of LA volumetric and functional parameters in a cohort of 158 MESA participants. Our main finding was that LAEF parameters (total, active, and passive) were significantly overestimated using the biplane area-length method when compared to the reference standard of 3D assessment. This result was based on an underestimation of LA volumes for each time point of the cardiac cycle when using the biplane method. Furthermore, limits of agreement between methods were wide and percentage differences were high for LAV and LAEF parameters, indicating large variances on an individual level between biplane and 3D-based assessments.
The importance of LA volume was first described as a marker for AF, stroke, and death in the Framingham study [4, 5]. Over the years, remodeling and enlargement of the LA have been linked to other diseases, such as heart failure, hypertension, diabetes, and demographics such as age and obesity, using different imaging modalities [1, 3, 17–19]. There is general consensus that CMR represents the gold standard for assessment of cardiac chambers and cardiac function [20]. While this primarily refers to 3D-based evaluation of LV function, assessment of the LA is mainly performed using the biplane method [21]. In the reference work for cardiac chamber assessment by Kawel-Boehm et al., only the LAVmax was reported for the 3D-based assessment. This was likely due to the time-consuming nature of the analysis, which is especially relevant if more time points need to be segmented. On the other hand, the biplane area-length method is simpler to use and does not require extra image acquisition, since two- and four-chamber cine SSFP are standard in any cardiac MRI protocol [22]. Regarding LAV assessment in our study, we found that the biplane method, which assumes an ellipsoidal LA, significantly underestimated LAV compared to the reference standard of 3D-based assessment, which depicts the actual LA shape. This underestimation was found at all cardiac time points, which is of interest since previous studies focused primarily on distinct time points in the cardiac cycle [9, 11, 21]. Few studies investigated all time points [23, 24]. Overall, we also found wide limits of agreement and high percentage differences for volumetric assessment, but especially at the fiducial points of maximum, minimum, and pre-atrial contraction, indicating large variance on the level of individual participants. Li et al. reported similar results showing an underestimation of LAV and overestimation of LAEF by the biplane method compared to 3D assessment in a cohort of healthy Chinese participants [12]. Zareian et al. reported a better agreement between the two techniques for LAVmax assessment with narrower limits of agreement compared to our results. However, they investigated a smaller cohort (30 participants) and pre-selected the time points for assessment visually, while we segmented each time point and extracted the volume at the specific time points from the comprehensive volume-time curves. They also used multimodality tissue tracking for contouring LA boundaries, which could explain these differences [21]. For maximum LA volume, Bashir et al. reported a general overestimation of LAV by the biplane area-length method in a cohort of 250 AF patients; LA enlargement as a result of remodeling could explain that finding [10]. They also reported wide limits of agreement of net 40 mL, which indicates large variance on the level of individual patients.
LAEF has been linked to various cardiovascular conditions, such as AF and mitral regurgitation, but most studies reporting LAEF (and LA volumes) have used the biplane method [25, 26]. However, our findings suggest a potential lack of precision using the biplane area-length method for both LAV and LAEF assessment. Our results from 3D-based LAEF assessment are in accordance with previously reported 3D mean values in two studies with small sample sizes of 20 and 15 participants in the same age group [7, 8]. Most studies evaluating the biplane method compared to 3D-based assessment have focused on LAVmax [9, 10, 21, 27]. However, the importance of LAVmin as an imaging marker has grown in recent years, potentially since it has outperformed LAVmax in the assessment of LV diastolic dysfunction, prediction of AF, and prognosis in heart failure in certain studies [24, 28–31]. Since LAVmin is part of the numerator in the formulas used to calculate total and active LAEF, LAEF assessment is, therefore, dependent on precise and accurate LAVmin assessment. In our analysis, we found an underestimation of LAVmin using the biplane method with a higher percentage difference compared to LAVmax assessment. This indicates a greater relative underestimation of LAVmin compared to LAVmax and, moreover, a larger influence of measurement technique on its assessment. This result can be due to the angulation of the two- and four-chamber views, causing them to be uncentered at the time point of LAVmin. This angulation cannot be adjusted retrospectively in the biplane method. On the other hand, if the LA is fully covered using the 3D technique, it is possible to assess the entire LA, and angulation does not play a role. As a result of the greater underestimation of LAVmin than LAVmax by the biplane method, LAEF assessment showed an overestimation compared to the 3D method. Yet, we observed wide limits of agreement with high percentage differences, which again indicate both overestimation and underestimation on an individual level.
We believe that our results have potential implications for future studies, for example, on normal values of LAEF, but also on associations with disease in general. Assessment of LV function is routinely performed using 3D cine imaging; while this method allows for assessment of regional wall motion abnormalities, biplane assessment would not be feasible in this case. If LA function is of interest, such as specific research settings, our results support the usage of a 3D-based approach over the clinical standard of biplane assessment. In addition, interreader analysis on all parameters (LAV and LAEF) showed small but similar differences in bias and limits of agreement between the biplane and 3D methods. This indicates that our findings cannot be explained by measurement variability alone, but that the chosen technique plays an important role.
Although full time-resolved, 3D-based assessment of the LA is likely superior, it is not yet ready for clinical usage. This mainly involves two factors: first, SAX cine series are typically only acquired for the LV in a clinical setting, and for full LA coverage, additional SAX slices would have to be acquired. Even though this would only slightly add to scan protocol duration, any extension needs to be justified. The 3D segmentation of the LA is the second, larger hurdle. There are only limited software options available offering LA assessment beyond biplane assessment using the two- and four-chamber views; therefore, in our study, we used a MATLAB-based solution. The actual contouring of endocardial borders of the LA needs to be performed on each slice in which the LA is visible. The median number of slices with a visible LA was 6 but ranged from 3 to 9 slices, depending on the LA size, and included about 25 individual images, depending on the temporal resolution. Even when segmenting only the two time points of maximum and minimum LA volumes, this technique will add to the CMR post-processing analysis significantly. Fully automatic, artificial intelligence-based solutions could address and overcome this hurdle. However, there are only a few works involving 3D LA assessment based on cine images, and those still have some limitations, such as its development on a cohort of AF patients, or that the segmentation is only done on specific, pre-selected time points [32, 33]. Having results from LA segmentations automatically available could be the next step in imaging and assessment of the LA. This could enable researchers to make full use of the advantages of 3D-based assessment as shown in this study. Furthermore, a better understanding of the impact of 3D-based analysis of LAV and LAEF might affect the classification of patients with diseases such as AF, heart failure, or myocardial infarction.
The study has limitations. It was a single-center study; we included 158 MESA participants who underwent an additional CMR at our institution involving SAX covering the whole LA, which was not part of the original MESA CMR protocol. Participants were of older ages (range: 62–93 years) and do not represent the entire population. However, LAV and LA function from this cohort most likely represent a cross-section of their age group. About a quarter of MESA participants who were approached to participate could not be included due to their concerns of being exposed to COVID-19. In addition, there were a few enlarged LAs in the cohort, which could have impacted the results. While there were about 23% Hispanic participants in the entire MESA cohort, there were none included in this study, since our site did not recruit members of this ethnicity. Our cohort still has a fair distribution of African American, Chinese American, and White participants. MRI exams were acquired on one model from one vendor, so inter-scanner and inter-vendor differences could not be assessed. Although the interreader assessment showed excellent agreement scores, it was performed on a small, random portion of the overall cases which might limit the generalizability.
CONCLUSIONS
In this study of more than 150 multiethnic participants, we demonstrated that the biplane area-length method systemically underestimated LAV for all time points of the cardiac cycle compared to the gold standard of 3D assessment based on LA SAX cine imaging. Furthermore, the underestimation was greater for LAVmin compared to LAVmax. These results led to a significant overestimation of total, active, and passive LAEF as parameters for LA function. While 3D assessment currently appears too time-consuming for clinical use, our results support its usage in specific settings when LA volumetric and functional parameters are in focus.
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. M.P. is currently an AHA postdoctoral research fellow at the Department of Radiology, Northwestern University, and is supported by the Bangerter-Rhyner Foundation and Freiwillige Akademische Gesellschaft Basel.
FUNDING
This work was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 and grant R01HL127659 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). This research was also supported by an American Heart Association Strategically Focused Research Network on atrial fibrillation, Project Number: 18SFRN34110170.
Abbreviations
- CMR
cardiac magnetic resonance imaging
- LA
left atrial
- LAV
left atrial volume
- LAEF
left atrial emptying fraction
- MESA
Multi-Ethnic Study of Atherosclerosis
- SSFP
steady-state free precession
- SAX
short axis
- LAVmin
minimum left atrial volume
- LAVmax
maximum left atrial volume
- LAVpreA
left atrial volume before atrial contraction
- LAEF
left atrial emptying fraction
Footnotes
Conflict of Interest Statement
All authors have no relevant disclosures or conflicts of interest regarding this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA STATEMENT
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- [1].Cuspidi C, Rescaldani M, Sala C, Prevalence of echocardiographic left-atrial enlargement in hypertension: a systematic review of recent clinical studies, Am J Hypertens 26(4) (2013) 456–64. [DOI] [PubMed] [Google Scholar]
- [2].Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Kober L, Bourgoun M, McMurray JJ, Velazquez EJ, Maggioni AP, Ghali J, Arnold JM, Zelenkofske S, Pfeffer MA, Solomon SD, Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study, Eur Heart J 30(1) (2009) 56–65. [DOI] [PubMed] [Google Scholar]
- [3].Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J, Left atrium in heart failure with preserved ejection fraction: structure, function, and significance, Circ Heart Fail 7(6) (2014) 1042–9. [DOI] [PubMed] [Google Scholar]
- [4].Vaziri SM, Larson MG, Benjamin EJ, Levy D, Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study, Circulation 89(2) (1994) 724–30. [DOI] [PubMed] [Google Scholar]
- [5].Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D, Left atrial size and the risk of stroke and death. The Framingham Heart Study, Circulation 92(4) (1995) 835–41. [DOI] [PubMed] [Google Scholar]
- [6].Schuster A, Backhaus SJ, Stiermaier T, Navarra JL, Uhlig J, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Gutberlet M, Bigalke B, Kutty S, Hasenfuss G, Thiele H, Eitel I, Left Atrial Function with MRI Enables Prediction of Cardiovascular Events after Myocardial Infarction: Insights from the AIDA STEMI and TATORT NSTEMI Trials, Radiology 293(2) (2019) 292–302. [DOI] [PubMed] [Google Scholar]
- [7].Butts B, Ahmed MI, Bajaj NS, Cox Powell P, Pat B, Litovsky S, Gupta H, Lloyd SG, Denney TS, Zhang X, Aban I, Sadayappan S, McNamara JW, Watson MJ, Ferrario CM, Collawn JF, Lewis C, Davies JE, Dell’Italia LJ, Reduced Left Atrial Emptying Fraction and Chymase Activation in Pathophysiology of Primary Mitral Regurgitation, JACC Basic Transl Sci 5(2) (2020) 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chou CC, Lee HL, Chang PC, Wo HT, Wen MS, Yeh SJ, Lin FC, Hwang YT, Left atrial emptying fraction predicts recurrence of atrial fibrillation after radiofrequency catheter ablation, PLoS One 13(1) (2018) e0191196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zemrak F, Ambale-Venkatesh B, Captur G, Chrispin J, Chamera E, Habibi M, Nazarian S, Mohiddin SA, Moon JC, Petersen SE, Lima JA, Bluemke DA, Left Atrial Structure in Relationship to Age, Sex, Ethnicity, and Cardiovascular Risk Factors: MESA (Multi-Ethnic Study of Atherosclerosis), Circ. Cardiovasc. Imaging 10(2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bashir A, Rabbat M, Biswas S, Wilber D, Sanagala T, Syed MA, Left atrial volume assessment by area-length method compared to multislice volumetric method using cardiovascular magnetic resonance imaging, J. Cardiovasc. Magn. Reson. 15(1) (2013) E97. [Google Scholar]
- [11].Wandelt LK, Kowallick JT, Schuster A, Wachter R, Stumpfig T, Unterberg-Buchwald C, Steinmetz M, Ritter CO, Lotz J, Staab W, Quantification of left atrial volume and phasic function using cardiovascular magnetic resonance imaging-comparison of biplane area-length method and Simpson’s method, Int. J. Cardiovasc. Imaging 33(11) (2017) 1761–1769. [DOI] [PubMed] [Google Scholar]
- [12].Li W, Wan K, Han Y, Liu H, Cheng W, Sun J, Luo Y, Yang D, Chung YC, Chen Y, Reference value of left and right atrial size and phasic function by SSFP CMR at 3.0 T in healthy Chinese adults, Sci Rep 7(1) (2017) 3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle-Valle TM, Zareian M, Almeida AL, Choi EY, Wu C, Alonso A, Heckbert SR, Bluemke DA, Lima JA, Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis), Circ. Cardiovasc. Imaging 9(8) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Markman TM, Habibi M, Venkatesh BA, Zareian M, Wu C, Heckbert SR, Bluemke DA, Lima JAC, Association of left atrial structure and function and incident cardiovascular disease in patients with diabetes mellitus: results from multi-ethnic study of atherosclerosis (MESA), Eur. Heart J. Cardiovasc. Imaging 18(10) (2017) 1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP, Multi-Ethnic Study of Atherosclerosis: Objectives and Design, Am. J. Epidemiol. 156(9) (2002) 871–881. [DOI] [PubMed] [Google Scholar]
- [16].Russo C, Hahn RT, Jin Z, Homma S, Sacco RL, Di Tullio MR, Comparison of echocardiographic single-plane versus biplane method in the assessment of left atrial volume and validation by real time three-dimensional echocardiography, J Am Soc Echocardiogr 23(9) (2010) 954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB, Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden, Am. J. Cardiol. 90(12) (2002) 1284–9. [DOI] [PubMed] [Google Scholar]
- [18].Peterson LR, Waggoner AD, de las Fuentes L, Schechtman KB, McGill JB, Gropler RJ, Davila-Roman VG, Alterations in left ventricular structure and function in type-1 diabetics: a focus on left atrial contribution to function, J. Am. Soc. Echocardiogr 19(6) (2006) 749–55. [DOI] [PubMed] [Google Scholar]
- [19].Casaclang-Verzosa G, Gersh BJ, Tsang TS, Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation, J. Am. Coll. Cardiol. 51(1) (2008) 1–11. [DOI] [PubMed] [Google Scholar]
- [20].Salerno M, Sharif B, Arheden H, Kumar A, Axel L, Li D, Neubauer S, Recent Advances in Cardiovascular Magnetic Resonance: Techniques and Applications, Circ. Cardiovasc. Imaging 10(6) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, Bluemke DA, Lima JA, Venkatesh BA, Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment, J Cardiovasc Magn Reson 17(1) (2015) 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E, Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update, J. Cardiovasc. Magn. Reson. 22(1) (2020) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ, Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance, J Cardiovasc Magn Reson 12(1) (2010) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pradella M, Anastasopoulos C, Yang S, Moor M, Badertscher P, Gehweiler JE, Spies F, Haaf P, Zellweger M, Sommer G, Stieltjes B, Bremerich J, Osswald S, Kuhne M, Sticherling C, Knecht S, Associations between fully-automated, 3D-based functional analysis of the left atrium and classification schemes in atrial fibrillation, PLoS One 17(8) (2022) e0272011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maceira AM, Cosin-Sales J, Prasad SK, Pennell DJ, Characterization of left and right atrial function in healthy volunteers by cardiovascular magnetic resonance, J Cardiovasc Magn Reson 18(1) (2016) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sievers B, Kirchberg S, Addo M, Bakan A, Brandts B, Trappe HJ, Assessment of left atrial volumes in sinus rhythm and atrial fibrillation using the biplane area-length method and cardiovascular magnetic resonance imaging with TrueFISP, J. Cardiovasc. Magn. Reson. 6(4) (2004) 855–63. [DOI] [PubMed] [Google Scholar]
- [27].Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R, Plein S, Tee M, Eng J, Bluemke DA, Normal values for cardiovascular magnetic resonance in adults and children, J. Cardiovasc. Magn. Reson. 17 (2015) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hoit BD, Left atrial size and function: role in prognosis, J. Am. Coll. Cardiol. 63(6) (2014) 493–505. [DOI] [PubMed] [Google Scholar]
- [29].Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR, Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function, Heart 98(10) (2012) 813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fatema K, Barnes ME, Bailey KR, Abhayaratna WP, Cha S, Seward JB, Tsang TS, Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study, Eur. J. Echocardiogr. 10(2) (2009) 282–6. [DOI] [PubMed] [Google Scholar]
- [31].Shin SH, Claggett B, Inciardi RM, Santos ABS, Shah SJ, Zile MR, Pfeffer MA, Shah AM, Solomon SD, Prognostic Value of Minimal Left Atrial Volume in Heart Failure With Preserved Ejection Fraction, J Am Heart Assoc 10(15) (2021) e019545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Anastasopoulos C, Yang S, Pradella M, Akinci D’Antonoli T, Knecht S, Cyriac J, Reisert M, Kellner E, Achermann R, Haaf P, Stieltjes B, Sauter AW, Bremerich J, Sommer G, Abdulkadir A, Atri-U: assisted image analysis in routine cardiovascular magnetic resonance volumetry of the left atrium, J. Cardiovasc. Magn. Reson. 23(1) (2021) 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lourenco A, Kerfoot E, Dibblin C, Chubb H, Bharath A, Correia T, Varela M, Automatic estimation of left atrial function from short axis CINE-MRI using machine learning, Eur. Heart J. 41(Supplement_2) (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.


