Abstract
Introduction
Digital ulcers represent a current public health issue, due to the relevant difficulties in their management and their tendency to become chronic, non-healing lesions.
Objectives
Our case series represents an opportunity to discuss the main comorbidities of digital ulcers and to present an evidence-based treatment protocol that has proved highly effective in our clinical practice.
Methods
We collected the clinical data about clinical features, associated diseases and diagnostic therapeutical procedures of 28 patients with digital ulcers referred to our Wound Care Service at S. Orsola-Malpighi Hospital.
Results
Digital ulcers were divided into 5 categories, based on the causative agent: peripheral artery disease: 5/16 females and 4/12 males, diabetes-associated wounds: 2/16 females and 1/12 males, mixed wounds: 4/12 males, pressure wounds: 3/16 females and 2/12 males, and immune-mediated diseases associated with wounds: 6/16 females and 1/12 males. Each group received specific management, based on the characteristics of the ulcer and the underlying comorbidities.
Conclusions
The clinical evaluation of digital wounds requires a thorough knowledge of their aetiopathogenesis. A multidisciplinary approach is necessary to achieve a precise diagnosis and correct treatment.
Keywords: digital ulcer, multidisciplinary approach, dermatology, diabetes, ischaemic wound
Introduction
With the increase in life expectancy, more and more patients are suffering from chronic diseases and an increasingly innovative and personal approach, which includes the collaboration between specialists from various branches of medicine and surgery, is now part of a comprehensive care plan [1].
Limb chronic ulcers have an increasing incidence, due to the higher prevalence of chronic vascular and metabolic diseases, such as peripheral artery disease (PAD) and diabetes mellitus, which play the main role in their aetiology.
Within the category of limb chronic ulcers, digital ulcers (DU) represent a small but peculiar group, due to their difficult management and their scarce tendency to heal.
We present an observational study of 28 patients with DU referred to our Wound Care Service, focusing on multidisciplinary and dermatological management.
Methods
We collected the clinical data of patients with DU referred to the Wound Care Service (a tertiary referral center) of the Dermatology Unit of the S.Orsola-Malpighi Hospital over a period of 18 months, from January 2020 to June 2021. Only patients presenting with non-healing digital ulcers of the limbs, present for at least 6 weeks, were enrolled. Demographic and clinical data were collected, including associated comorbidities and their therapy and previous ulcerative episodes in other cutaneous areas. Diagnosis of the disease that caused the DU was based on clinical history, laboratory evaluation and histopathological study, when necessary. Management of these conditions was based on the main International Guidelines for the management of peripheral artery disease in patients with foot ulcers and diabetes [2–4] and on the evidence-based protocol for diabetic foot ulcers [5].
All the digital wounds were assessed for excluding any signs of local or surrounding infection. In particular, the presence of at least 3 or more STONEES criteria (Size is bigger, Temperature elevated, Os, New breakdown, Exudate, Erythema, Smell) lead to a systemic antibiotic treatment. The presence of at least three NERDS criteria (Nonhealing, Exudate Increase, Red friable granulation, Debris) was followed by a topical antimicrobial therapy [3].
The evaluation of pain associated with the DU was performed using the VAS (visual analogue scale). All patients with pain equal or superior to 5 on the VAS scale, despite adequate medication and non-responder to paracetamol, were managed by our Pain Therapy Service.
Despite the cause of the DU, mechanical debridement of the necrotic tissues was performed in all cases. In three cases, the use of local medications was preceded by a total surgical debridement of necrotic tissue. In the other patients, an ambulatorial mechanical debridement was performed. Gauze soaked in iodine was used as the main antiseptic medication.
Results
From January 2020 to June 2021, we treated 28 patients with DU, 12 males and 16 females (Table 1). Median age was 72.6 years (range 38–97 years): average age of males was 68.3 years, that of females 75.9 years. The toes were the most common site of DU (24 patients), with 16 patients having ulcers of several digits (ranging from 2 digits to 4); finger ulcers were present in 4 patients, one of them having DU in 3 fingers, the other 3 in one finger.
Table 1.
Clinical characteristics of the patients with digital ulcers.
| Patient | Age | Sex | Number of wounds | Localization | Aetiology |
|---|---|---|---|---|---|
| 1 | 50 | F | 2 | toe | Diabetes II |
| 2 | 70 | M | 2 | toe | Diabetes II and PAD |
| 3 | 95 | F | 4 | toe | PAD |
| 4 | 80 | M | 3 | finger | SSc |
| 5 | 64 | M | 2 | toe | PAD |
| 6 | 81 | F | 1 | toe | PAD |
| 7 | 50 | M | 1 | finger | Diabetes II and PAD |
| 8 | 82 | F | 1 | toe | Pressure ulcer |
| 9 | 82 | F | 4 | toe | Vasculitis |
| 10 | 83 | M | 2 | toe | Diabetes II |
| 11 | 58 | M | 1 | toe | PAD |
| 12 | 81 | M | 1 | toe | Diabetes II and PAD |
| 13 | 83 | F | 1 | toe | Vasculitis |
| 14 | 46 | M | 3 | toe | Pressure ulcer |
| 15 | 69 | M | 1 | toe | Diabetes II and PAD |
| 16 | 97 | F | 5 | toe | PAD |
| 17 | 89 | F | 2 | toe | Diabetes II |
| 18 | 92 | F | 4 | toe | PAD |
| 19 | 84 | F | 2 | toe | Pressure ulcer |
| 20 | 56 | F | 1 | toe | SSc |
| 21 | 64 | F | 3 | toe | Vasculitis |
| 22 | 80 | F | 2 | toe | PAD |
| 23 | 80 | M | 3 | toe | Pressure ulcer |
| 24 | 83 | F | 2 | toe | Pressure ulcer |
| 25 | 58 | M | 1 | toe | PAD |
| 26 | 58 | F | 1 | finger | SSc |
| 27 | 38 | F | 1 | finger | SSc |
| 28 | 80 | M | 2 | toe | PAD |
M: Male; F: Female; PAD: peripheral artery disease; SSc: systemic sclerosis.
The diseases detected as causes of DU were as follows: peripheral artery disease (PAD): 9 cases, all with DU of the toes; type 2 diabetes mellitus with poor glycaemic control: 3 patients, all with DU of the toes; association of PAD with type 2 diabetes mellitus: 4 patients, 3 with DU of the toes and 1 of a finger; pressure ulcers with toe involvement: 5 patients; vasculitis: 3 patients with DU of the toes; systemic sclerosis (SSc) in 4 patients, 3 with DU of the fingers and 1 of a toe.
PAD-Associated DU
The 9 patients with PAD were evaluated by specific imaging investigation (color duplex ultrasound, computed tomographic angiography, magnetic resonance angiography) and then referred to a vascular surgeon for possible revascularization. Only 4 cases were revascularized, while in the other 5 the procedure was not possible due to the poor general conditions and the high anaesthetic risk. The topical medication of the DU after debridement in all these patients was an Iodine-based antiseptic dressing or a soft silicon foam dressing, in order to diminish the risk of bacterial superinfection of necrotic tissue (Figure 1a). All the cases that underwent revascularization showed a better outcome compared to the other cases, with a significant reduction in healing times. No correlations with any lymphatic drainage impairment were found in this group of patients after specific imaging investigation.
Figure 1.
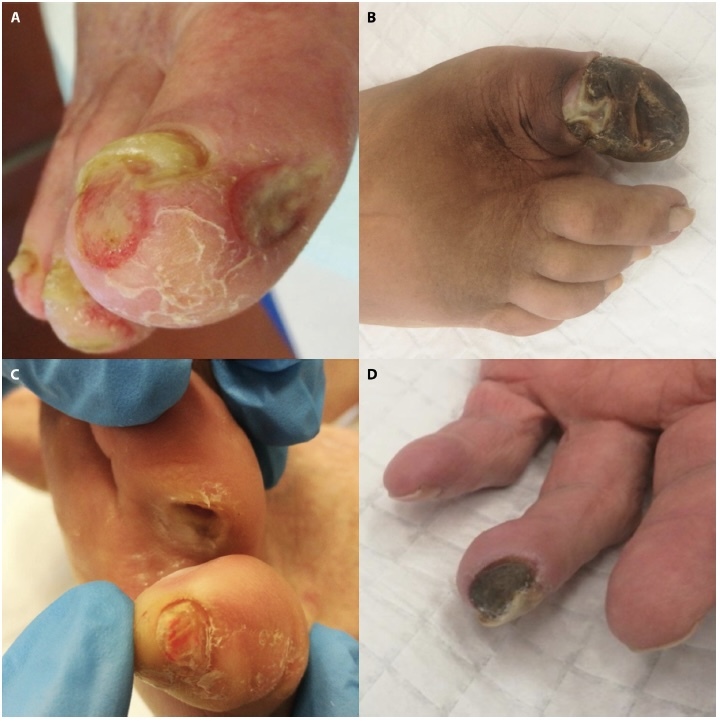
Clinical images of patients with digital ulcers of different aetiology. A 92-year-old female patient with two recently occurring ischemic ulcers at her right first toe, in the context of peripheral artery disease (A); a 50-year-old female patient with extensive necrosis at her distal phalanx of the right first toe, in the context of poorly controlled diabetes mellitus (B); an 82-year-old female patient with a well-demarcated pressure ulcer at the fourth finger of her left foot (C); an 80-year-old male patient with a necrotic ulcer at the distal phalanx of the fourth finger of his left hand, in the context of systemic sclerosis (D).
Diabetes-Associated DU
Seven patients presented type 2 diabetes mellitus in poor glycaemic control, associated with the presence of DU. In 3 cases diabetes was identified as the primary cause of the ulcerative lesions, as laboratory and technical evaluations excluded other comorbidities: these patients were referred to the Diabetology service for a review of their metabolic status and chronic hypoglycaemic therapy and underwent local medications with gauze soaked in iodine to accelerate wound healing. A better clinical outcome was achieved only after the diminishment of these patients’ plasma glucose values.
In 4 patients, type 2 diabetes was associated with arteriopathy of the large vessels of the lower limbs that required prompt revascularization (Figure 1b). Surgery increased the chances of wound healing in these patients.
Obesity and metabolic syndrome are often present in patients with diabetes II and PAD, as they recognize a sedentary lifestyle as a common denominator, associated with unbalanced nutrition. However, no direct correlations were found between these factors and the development of digital ulcerative lesions.
Pressure DU
Five patients with pressure ulcers of the feet developed toe lesions as an effect of continuous forces directed to the distal pulp, causing progressive skin ischemia above bone prominences4. These patients were bedbound due to progressive neurological diseases, such as multiple sclerosis with spinal involvement (4 cases) or a severe form of motor-sensitive polyneuropathy of the lower limbs (1 case). In these cases, mechanical debridement of the eschar followed by topical therapy with iodine gauzes was associated with a physiatrist consultation that lead to the prescription of plantar orthosis and anti-decubitus mattresses. All the procedures allowed wound healing with restoration of the physical integrity of the toe skin (Figure 1c).
Immune-Mediated Disease-Associated DU
Four patients, 3 with DU of the fingers and 1 of a toe suffered from SSc (Figure 1d). A skin biopsy of the DU showed in all cases skin calcinosis. These patients were managed through the close collaboration of rheumatologists.
One patient with DU of the toe was affected by rheumatoid arthritis and 2 by systemic lupus erythematous. A biopsy of the perilesional skin showed leukocytoclastic vasculitis with fibrinoid necrosis of the vessel walls and prominent polymorphonuclear cell infiltration.
The clinical suspicion of autoimmune disease in this group of patients derived from suggestive anamnestic data collection and from the execution of skin biopsies of the ulcerative lesions. A subsequent rheumatological evaluation confirmed the clinical suspicion.
Discussion
Our literature search could not identify any studies that collected such a large number of cases regarding DU of both fingers and toes, making a subdivision based on clinical aspects and on the respective aetiologies. The multidisciplinary management of the different cases represents the standard model to get better the outcome of patients suffering from multiple comorbidities.
PAD-Associated DU
The term peripheral arterial disease defines the lower extremity artery disease, including obstruction at the aortoiliac, femoropopliteal and infrapopliteal arterial segments [5]. Studies have shown an increased risk of cardiovascular mortality, as well as of morbidity from myocardial infarction and stroke in patients with asymptomatic or symptomatic arterial disease [2]. The distal localization of the vascular obstruction leads to the formation of chronic ulcerative lesions of the acral extremities, mainly of the lower limbs, with the appearance of necrotic eschars of the distal phalanges of the toes. These ulcers are at high risk of bacterial superinfection. The association with both uncontrolled type II diabetes mellitus is common, therefore in these patients, the evaluation of the arterial function with imaging techniques should always be associated with laboratory monitoring of the glycaemic state, as type II diabetes comorbidity greatly impairs local wound management [6].
The symptoms and signs of PAD are variable and range from the classic symptom of claudication to other non– joint-related limb symptoms (atypical leg symptoms) or are absent [7–10]. In these cases, the DU may be the first sign that leads to the diagnosis. The vascular examination for PAD includes pulse palpation, auscultation for femoral bruits, and inspection of the legs and feet [8]. To confirm the diagnosis of PAD, abnormal physical examination findings must be confirmed with diagnostic testing [5]. Studies for anatomic imaging assessment (duplex ultrasound, computed tomography angiography [CTA], or magnetic resonance angiography [MRA], invasive angiography) are generally reserved for highly symptomatic patients in whom revascularization is being considered [9].
Patients with PAD associated with skin ulcers fall into a high-risk cardiovascular group, due to the signs of advanced disease10: for this category, a careful multidisciplinary evaluation is important in order to select patients eligible for surgical revascularization and to increase the chance of wound healing, as well as of reducing a progression of disease [11].
Adequate pharmacological therapy associated with a healthy lifestyle is mandatory for the chronic management of this disease and to reduce the global cardiovascular risk [12, 13].
Diabetes-Associated DU
As for diabetic foot ulcers, they occur in between 12 and 25% of patients with type 2 diabetes mellitus [14] and precede 84% of all non-trauma limb amputations in this growing slice of the population [15].
A diabetic foot ulcer is defined as any skin breakdown on the foot of a diabetic person [16]. Early recognition of the skin defect and treatment prevents its progression to a chronic wound that is often recalcitrant to therapy [17–18].
When a patient with a diabetic foot ulcer is first seen, a comprehensive history and treatment plan must be put into place. Then, a laboratory evaluation based on their metabolic status, and the monitoring for any complications (e.g. heart disease, renal failure, retinopathy, neuropathy) must be carried out [19]. It is important to assess the pedal pulses, and unless a pulse is clearly palpable, all patients with foot ulcers should undergo non-invasive vascular testing, to determine if the patient would benefit from revascularization. In an observational study, shorter time to revascularization (<8 weeks) was associated with a higher possibility of healing of ischaemic foot ulcers [20]. In addition, it is mandatory that every patient be evaluated for proper orthotics, and appropriate footwear should be prescribed that adequately protects the foot from trauma induced by shoes and alleviates pressure, considering the fact that pressure and pain sensation are often impaired in these patients [21].
Pressure DU
Prolonged bed rest caused by paraplegia and various central nervous system diseases plays an important role in the formation of pressure acral lesions, which follow prolonged skin ischemia and are especially located above bony prominences. The first signs of ischemia are the formation of calluses at the level of the toepads. If not recognized and treated, they lead to the formation of painless and difficult-to-treat chronic ulcers. The application of physical means such as intermittent pneumatic compression devices and the evaluation from expert physiotherapists is necessary in these patients, in order to prevent the extension of the wound and the arising of other similar lesions in other toes [22].
In people suffering from advanced neurological conditions, spasticity is significantly associated with the development of pressure ulcers in typical and even atypical locations [23]. Severe spastic conditions might facilitate wound onset in various ways: increased tonus of the limbs leads to immobility and to a reduced capacity to reposition the body, with an abnormal pressure redistribution. This prolonged rigidity causes severe soft tissue injury [4, 24]. A comprehensive assessment is warranted with a focus on identifying the source of pressure, contributing factors, underlying comorbidities, and elements affecting wound healing.
Pressure redistribution targeting the duration and/or magnitude of loading is critical [25, 26]. Evidence exists that advanced support surfaces are superior to standard hospital beds in preventing and managing pressure injuries. However, no clear advantage has been identified for one specific advanced support surface over another [27].
There is strong evidence that a moist wound environment accelerates healing in this type of DU: occlusive dressings seem to be superior to more-traditional simple gauze, especially in terms of maintaining a moist wound environment [28]. Ultimately the dressing selection may be guided by the characteristics of the wound, balance of moisture and exudate, bacterial control, debridement balance, ease of use, cost, and patient preference [29].
Immune-Mediated Disease-Associated DU
Inflammatory and autoimmune systemic diseases may first become clinically evident with the appearance of an ischemic digital wound. SSc is the best described among these types of conditions [30]. Cutaneous and/or systemic vasculitis can also present with the formation of necrotic lesions involving the fingers of the limbs, both the upper and lower ones. SSc is an immune-mediated disease that represents a major clinical challenge for physicians and patients. For the patient, SSc is associated with great uncertainty of outcome and development of manifestations that are potentially lethal or can reduce quality of life [31].
The problem of digital ulcers is increasingly recognised [32]. DU occur in around half of SSc cases during their disease history, and about one in five patients might have this complication at any one time [33, 34]. There is now a better appreciation of the effects of digital ulcers, which include impaired function, pain, and loss of employment, as well as the more obvious medical complications of cellulitis, osteomyelitis, digital infarction, and severe pain. Treatment of digital ulcers with drugs or systemic therapies needs to be combined with appropriate expert local care and dressings, and this treatment usually benefits from specialist nurse input. Evidence-based treatments include phosphodiesterase-5 inhibitors and endothelin receptor antagonists, although some studies have not shown a clear treatment benefit [35, 36].
Rheumatoid arthritis (RA) is a chronic, inflammatory autoimmune disorder expressed most commonly as a symmetrical, deforming arthropathy [37]. A well-known cause of ulceration in rheumatoid arthritis is vasculitis. Vessels of different sizes may be affected. These patients will require systemic therapy because mortality can be high [38].
Workup for the patients should include a complete history and thorough physical exam, screening laboratory studies and biopsies. Treatment is a challenge, but stabilizing the autoimmune disease is imperative. Adalimumab with methotrexate (MTX) has shown promise in RA-associated ulcers [39]. Improving wound bed preparation by the application of moisture-retentive dressings has been shown to be beneficial [40].
Systemic lupus erythematosus (SLE) is a systemic autoimmune connective tissue disease that can affect most organ systems. Ulcerations are not infrequent and, like other connective tissue diseases, they are multifactorial [41]. Vasculitis, noninflammatory thrombosis of small or large vessels, venous insufficiency, lupus profundus, lichen planus overlap, and drug-induced lupus syndrome has been associated with leg ulcerations. The ulcers are usually painful, sharply margined, or punched out. Adjacent skin can appear erythematous, purpuric, or rolled and violaceous. Histological examination of vasculitis ulcers in SLE shows a leukocytoclastic vasculitis with fibrinoid necrosis of the vessel walls and prominent polymorphonuclear cell infiltration. Thrombocclusive histologic findings can be associated with the presence of antiphospholipid antibodies (lupus anticoagulant) [42]. SLE-associated leg ulcers are a therapeutic challenge, as local wound care is not always sufficient. The underlying cause of the ulceration needs to be established and treated. If vasculitis is present, systemic corticosteroids with cytotoxic agents should be utilized [37].
Conclusion
The clinical evaluation of DUs requires a thorough knowledge of their possible causes. A careful clinical evaluation is necessary together with laboratory and imaging technique investigations. A multidisciplinary approach in the management of these wounds is the only effective way to achieve a precise diagnosis and a correct treatment, as described in Figure 2.
Figure 2.
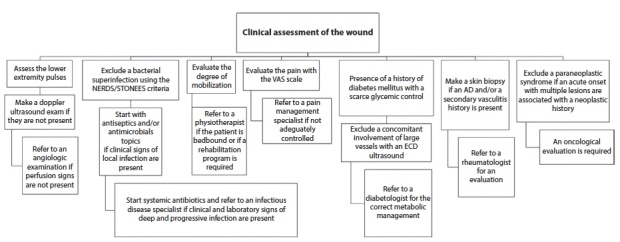
Clinical evaluation in the management of digital wounds. NERDS: Nonhealing, Exudate Increase, Red friable granulation, Debris; STONEES: Size is bigger, Temperature elevated, Os, New breakdown, Exudate, Erythema, Smell; VAS: visual analogue scale; ECD: echo colour doppler; AD: autoimmune disease.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510–515. doi: 10.1161/01.CIR.71.3.510. [DOI] [PubMed] [Google Scholar]
- 2.Ricco JB, Bartelink MLEL. Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: the European Stroke Organization (ESO) :60. [Google Scholar]
- 3.Sibbald RG, Elliott JA, Persaud-Jaimangal R, et al. Wound Bed Preparation 2021. Adv Skin Wound Care. 2021;34(4):183–195. doi: 10.1097/01.ASW.0000733724.87630.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajhosseini B, Longaker MT, Gurtner GC. Pressure Injury. Annals of Surgery. 2020;271(4):671–679. doi: 10.1097/SLA.0000000000003567. [DOI] [PubMed] [Google Scholar]
- 5.Chioncel V, Brezeanu R, Sinescu C. New Directions in the Management of Peripheral Artery Disease. American Journal of Therapeutics. 2019;26(2):e284–e293. doi: 10.1097/MJT.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 6.Hinchliffe RJ, Forsythe RO, Apelqvist J, et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(S1) doi: 10.1002/dmrr.3276. [DOI] [PubMed] [Google Scholar]
- 7.Chan KA, Junia A. Lower extremity peripheral artery disease: a basic approach. Br J Hosp Med. 2020;81(3):1–9. doi: 10.12968/hmed.2019.0263. [DOI] [PubMed] [Google Scholar]
- 8.Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the Clinical Examination Predict Lower Extremity Peripheral Arterial Disease? :11. doi: 10.1001/jama.295.5.536. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12) doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennion DR, Siano KA, Center TAM. Diagnosis and Treatment of Peripheral Arterial Disease. Peripheral Arterial Disease. 2013;88(5):5. [PubMed] [Google Scholar]
- 11.Morley RL, Sharma A, Horsch AD, Hinchliffe RJ. Peripheral artery disease. BMJ. doi: 10.1136/bmj.j5842. Published online February 1, 2018:j5842. [DOI] [PubMed] [Google Scholar]
- 12.Selvin E, Erlinger TP. Prevalence of and Risk Factors for Peripheral Arterial Disease in the United States: Results From the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110(6):738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 13.Khan S, Flather M, Mister R, et al. Characteristics and Treatments of Patients with Peripheral Arterial Disease Referred to UK Vascular Clinics: Results of a Prospective Registry. European Journal of Vascular and Endovascular Surgery. 2007;33(4):442–450. doi: 10.1016/j.ejvs.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. The Lancet. 2005;366(9498):1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 15.Morris AD, McAlpine R, Steinke D, et al. Diabetes and Lower-Limb Amputations in the Community: A retrospective cohort study. Diabetes Care. 1998;21(5):738–743. doi: 10.2337/diacare.21.5.738. [DOI] [PubMed] [Google Scholar]
- 16.Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJM. Evidence-Based Protocol for Diabetic Foot Ulcers. Plastic and Reconstructive Surgery. 2006;117(SUPPLEMENT):193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. [DOI] [PubMed] [Google Scholar]
- 17.Brem H. Healing of Diabetic Foot Ulcers and Pressure Ulcers With Human Skin Equivalent: A New Paradigm in Wound Healing. Arch Surg. 2000;135(6):627. doi: 10.1001/archsurg.135.6.627. [DOI] [PubMed] [Google Scholar]
- 18.Mostow EN. Diagnosis and classification of chronic wounds. Clinics in Dermatology. 1994;12(1):3–9. doi: 10.1016/0738-081X(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues TC, Pecis M, Azevedo MJ, Gross JL. Homeostase pressórica e complicações microvasculares em pacientes diabéticos. Arq Bras Endocrinol Metab. 2005;49(6):882–890. doi: 10.1590/S0004-27302005000600005. [DOI] [PubMed] [Google Scholar]
- 20.Elgzyri T, Larsson J, Nyberg P, Thörne J, Eriksson KF, Apelqvist J. Early Revascularization after Admittance to a Diabetic Foot Center Affects the Healing Probability of Ischemic Foot Ulcer in Patients with Diabetes. European Journal of Vascular and Endovascular Surgery. 2014;48(4):440–446. doi: 10.1016/j.ejvs.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Colagiuri S, Marsden LL, Naidu V, Taylor L. The use of orthotic devices to correct plantar callus in people with diabetes. Diabetes Research and Clinical Practice. 1995;28(1):29–34. doi: 10.1016/0168-8227(95)01050-N. [DOI] [PubMed] [Google Scholar]
- 22.Mervis JS, Phillips TJ. Pressure ulcers: Prevention and management. Journal of the American Academy of Dermatology. 2019;81(4):893–902. doi: 10.1016/j.jaad.2018.12.068. [DOI] [PubMed] [Google Scholar]
- 23.Jaul E. Cohort study of atypical pressure ulcers development: Atypical pressure ulcers development. Int Wound J. 2014;11(6):696–700. doi: 10.1111/iwj.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trompetto C, Marinelli L, Mori L, et al. Pathophysiology of Spasticity: Implications for Neurorehabilitation. BioMed Research International. 2014;2014:1–8. doi: 10.1155/2014/354906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy M, Gill SS, Rochon PA. Preventing Pressure Ulcers: A Systematic Review. JAMA. 2006;296(8):974. doi: 10.1001/jama.296.8.974. [DOI] [PubMed] [Google Scholar]
- 26.Grey JE, Harding KG, Enoch S. Pressure ulcers. BMJ. 2006;332(7539):472–475. doi: 10.1136/bmj.332.7539.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gould L, Stuntz M, Giovannelli M, et al. Wound healing society 2015 update on guidelines for pressure ulcers: Guidelines for the treatment of pressure ulcers. Wound Rep and Reg. 2016;24(1):145–162. doi: 10.1111/wrr.12396. [DOI] [PubMed] [Google Scholar]
- 28.Boyko TV, Longaker MT, Yang GP. Review of the Current Management of Pressure Ulcers. Advances in Wound Care. 2018;7(2):57–67. doi: 10.1089/wound.2016.0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and Management of Lower-Extremity Ulcers. In: Campion EW, editor. N Engl J Med. 16. Vol. 377. 2017. pp. 1559–1567. [DOI] [PubMed] [Google Scholar]
- 30.Costedoat I, Masson M, Barnetche T, et al. Locoregional Treatments for Digital Ulcers in Systemic Sclerosis: A Systematic Review. Acta Derm Venereol. 2021;101(6):adv00478. doi: 10.2340/00015555-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton CP, Khanna D. Systemic sclerosis. The Lancet. 2017;390(10103):1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 32.Nihtyanova SI, Brough GM, Black CM, Denton CP. Clinical burden of digital vasculopathy in limited and diffuse cutaneous systemic sclerosis. Annals of the Rheumatic Diseases. 2008;67(1):120–123. doi: 10.1136/ard.2007.072686. [DOI] [PubMed] [Google Scholar]
- 33.Allanore Y, Denton CP, Krieg T, et al. Clinical characteristics and predictors of gangrene in patients with systemic sclerosis and digital ulcers in the Digital Ulcer Outcome Registry: a prospective, observational cohort. Ann Rheum Dis. 2016;75(9):1736–1740. doi: 10.1136/annrheumdis-2016-209481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mihai C, Landewé R, van der Heijde D, et al. Digital ulcers predict a worse disease course in patients with systemic sclerosis. Ann Rheum Dis. 2016;75(4):681–686. doi: 10.1136/annrheumdis-2014-205897. [DOI] [PubMed] [Google Scholar]
- 35.Khanna D, Denton CP, Merkel PA, et al. Effect of Macitentan on the Development of New Ischemic Digital Ulcers in Patients With Systemic Sclerosis: DUAL-1 and DUAL-2 Randomized Clinical Trials. JAMA. 2016;315(18):1975. doi: 10.1001/jama.2016.5258. [DOI] [PubMed] [Google Scholar]
- 36.Domsic RT, Nihtyanova SI, Wisniewski SR, et al. Derivation and Validation of a Prediction Rule for Two-Year Mortality in Early Diffuse Cutaneous Systemic Sclerosis: A Validated Two-Year Mortality Model for Early Diffuse Scleroderma. Arthritis & Rheumatology. 2014;66(6):1616–1624. doi: 10.1002/art.38381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dabiri G, Falanga V. Connective tissue ulcers. Journal of Tissue Viability. 2013;22(4):92–102. doi: 10.1016/j.jtv.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danning CL, lllei GG, Boumpas DT. Vasculitis associated with primary rheumatologic diseases. Current Opinion in Rheumatology. 1998;10(1):58–65. doi: 10.1097/00002281-199801000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Hirche D, Rubbert A, Lunau L, Krieg T, Eming SA. Successful treatment of refractory rheumatoid arthritis-associated leg ulcerations with adalimumab. Br J Dermatol. 2005;152(5):1062–1064. doi: 10.1111/j.1365-2133.2005.06520.x. [DOI] [PubMed] [Google Scholar]
- 40.Öien RF, Håkansson A, Hansen BU. Leg ulcers in patients with rheumatoid arthritis—a prospective study of aetiology, wound healing and pain reduction after pinch grafting. Rheumatology. 2001;40(7):816–820. doi: 10.1093/rheumatology/40.7.816. [DOI] [PubMed] [Google Scholar]
- 41.Goslen JB. Autoimmune ulceration of the leg. Clinics in Dermatology. 1990;8(3–4):92–117. doi: 10.1016/0738-081X(90)90050-B. [DOI] [PubMed] [Google Scholar]
- 42.Fink AM, Kottas-Heldenberg A, Mayer W, et al. Lupus anticoagulant and venous leg ulceration. Br J Dermatol. 2002;146(2):308–310. doi: 10.1046/j.0007-0963.2001.04546.x. [DOI] [PubMed] [Google Scholar]


