Abstract
Introduction
The Covid-19 pandemic has been shown to have major acute and chronic impacts on the skin. Various studies reported that there has been an increase in the number of patients referred to outpatient dermatology clinics with the complaint of variable hair diseases during the era of Covid-19. Hair seems to be substantially affected by both the infection itself and anxiety/stress provoked by the pandemic. Therefore, understanding the impact of Covid-19 on the clinical course of variable hair diseases has become a major concern in dermatology practice.
Objectives
To examine the frequency and types of various hair diseases, both new-onset and ingravescent, observed in healthcare providers.
Methods
A web-based questionnaire related to the hair diseases seen in healthcare providers both prior to the Covid-19 pandemic and after the start of the pandemic was created. The type of both new-onset and pre-existing hair diseases and ongoing hair diseases observed during Covid-19 were investigated.
Results
A total number of 513 participants were included in the study. One hundred seventy cases were diagnosed with Covid-19. During the Covid-19 pandemic, 228 reported having at least one hair disease; the most common one being telogen effluvium, followed by hair greying and seborrheic dermatitis. There was a statistically significant relationship between the presence of a new-onset hair disease during the pandemic and being diagnosed with Covid-19 (p=0.004).
Conclusion
Our study shows that Covid-19 infection has a significant impact on the emergence of new-onset hair diseases.
Keywords: Covid-19, hair diseases, surveys, questionnaires
Introduction
The Covid-19 pandemic has been shown to have a negative influence on both skin diseases and the dermatological life quality of healthcare providers (HCPs) [1]. Skin serves as one of the first-line-of-defense components of the body against numerous pathogens by forming a physical barrier [2]. Continuous use of personal hygiene equipment impairs the skin barrier, causing skin problems such as drying, lichenification and itching further aggravating a previously-existing dermatological disease or resulting in the development of a new-onset skin disorder [1].
Hair is a skin appendage whose cycle is frequently affected by various external factors including nutritional deficiencies, endocrinopathies, major surgical operations and systemic diseases [3]. In a normal hair cycle, approximately 90% of the hairs are in the anagen phase [4]. The catagen phase begins when the anagen phase ends and approximately 5% of all hair shafts are in the catagen phase at any given time [4]. In the anagen phase, hair follicles continue to grow whereas in the catagen phase, hair follicles start to dwindle and hair growth stops [5]. The telogen phase or ‘resting phase’ lasts about three months and 10–15% of all hairs are in the telogen phase in a normal hair cycle [4,5]. Following the telogen phase, hair shafts fall out. Telogen effluvium (TE) presents itself in the form of excessive hair shedding resulting from the rapid entry of anagen hairs into the telogen phase [3].
The normal cycle of hair growth is influenced and disrupted by various internal and external factors including severe systemic infections such as Covid-19 [6]. Covid-19-induced mild and severe TE cases have continued to be reported in increasing numbers from the start of the pandemic [6,7]. Alopecia areata (AA), an autoimmune hair disorder; seborrheic dermatitis (SD), a chronic inflammatory skin disease; trichotillomania, a psychodermatologic hair disorder are some other entities that are increasingly observed in the era of Covid-19 [8–10]. Both adverse psychosocial effects of the pandemic and cytokine storm induced in the setting of Covid-19 infection are implicated in the development of these hair diseases [6].
In our study, we aimed to investigate the frequencies and types of both new-onset and pre-existing variable hair diseases observed in HCPs during the Covid-19 pandemic.
Methods
Local ethics committee approval was obtained for the present study (the date and decision number: November 19 2021, 2021/028). A web-based survey consisting of 22 questions (Supplementary file 1) was created using Google forms. The survey included four sections: (I) personal information; (II) Covid-19 infection; (III) hair diseases seen prior to the onset of the Covid-19 pandemic; (IV) hair diseases observed during the Covid-19 pandemic. The online questionnaire was carried out among HCPs and a virtual snowball sampling method was used.
IBM SPSS for Windows Version 20.0 was used for the statistical analysis. For descriptive analysis, numerical variables were given as mean ± standard deviation (range: minimum-maximum) and categorical variables were shown as percentages and frequencies. Chi-Square test was used to analyze the relationships between the categorical variables. P values of <0.05 were considered statistically significant.
Results
A total number of 513 HCPs were included in the study. The mean age was 34.84 ± 8.90 years (minimum:19, maximum: 66). Three hundred fifty-eight (69.8%) of the participants were female whereas 155 (30.2%) were male. Two hundred forty-one (47%) respondents reported having Covid-19 related symptoms; whereas 272 (53 %) didn’t exhibit any symptoms. Three hundred twenty-nine (63.2%) participants had a history of close contact with someone with a confirmed diagnosis of Covid-19. Covid-19 real-time polymerase chain reaction test (RT-PCR) was performed on 445 (86.7 %) respondents; 156 (35.1%) cases tested positive, whereas 289 (64.9%) tested negative. Additionally, 14 cases who did not give RT-PCR test or tested negative, were diagnosed with Covid-19 via radiological imaging. So, a total of 170 cases were accepted to have a confirmed diagnosis of Covid-19. Only 12 patients were required to be hospitalized for severe Covid-19 infection.
During the online questionnaire, participants were asked if they had any other contributing factors during the Covid-19 pandemic that might have played a role in the development or worsening of their hair diseases. Thirty-six (7%) cases had a pregnancy, one (0.2%) respondent was receiving chemotherapy, 21 (4.1%) were dieting, 12 (2.3%) had a major surgical operation, 20 (3.9%) had intentional/unintentional loss of more than 5% of their own weight in a period of 6 months during the Covid-19 pandemic. Lastly, 162 (31.6%) participants reported feeling stressed, which had a major impact on their lives during the Covid-19 pandemic. Two hundred fifty-four (49.5%) respondents had at least one hair disease prior to the start of the Covid-19 pandemic. The frequencies and percentages of different types of hair diseases observed in the pre-Covid-19 era are shown in Table 1.
Table 1.
Frequencies and percentages of different types of hair diseases in the pre-Covid-19 and Covid-19 pandemic eras.
| Type of Hair Disease | Pre-Covid-19 (n, %) | Covid-19 (n,%) |
|---|---|---|
| Male pattern hair loss | 36 (13.9) | 29 (12.7) |
| Alopecia areata | 13 (5) | 3 (1.3) |
| Female pattern hair loss | 30 (11.6) | 33 (14.5) |
| Alopecia totalis | 1 (0.4) | 1 (0.4) |
| Telogen effluvium | 108 (41.7) | 111 (48.7) |
| Seborrheic dermatitis | 63 (24.3) | 52 (22.8) |
| Scalp psoriasis | 9 (3.5) | 9 (3.9) |
| Trichodynia | 35 (13.5) | 32 (14) |
| Trichotillomania | 18 (6.9) | 16 (7) |
| Increased hair greying | 72 (27.8) | 71 (31.1) |
| Total | 259 (100) | 228 (100) |
Of 513 patients, only 40 patients had new-onset hair disease that developed during the Covid-19 pandemic. Out of 40 patients, 25 (62.5%) had new-onset TE, 12 (30%) reported increased hair greying, 6 (15%) had new-onset female-pattern hair loss (FPHL), 3 (7.5%) had new-onset male-pattern hair loss (MPHL), 4 (10%) reported trichodynia, 2 (5%) reported new-onset SD, 2 (5%) reported new-onset trichotillomania and 1 (2.5%) reported new-onset scalp psoriasis. Of these 40 patients, only 20 (50%) were diagnosed with Covid-19. The mean duration between the emergence of the new-onset hair disease and Covid-19 diagnosis was 1.24 ± 0.83 months (minimum: 0.25, maximum:3). Of 25 patients with new-onset TE during the Covid-19 pandemic, 22 (88 %) had been tested for SARS-CoV-2 infection. Fourteen (63.6%) out of 22 patients had a positive RT-PCR result. For these patients, the mean time interval between the diagnosis of Covid-19 and the approximate commencement of the acute TE was 1.13 ± 0.66 months (minimum: 0.3, maximum:3). During the era of Covid-19, 228 (44.4 %) reported having any hair disease (including pre-existing, ongoing and new-onset ones); the frequencies and percentages of different types of hair diseases observed in the Covid-19 era are shown in Table 1. Of 228 individuals with a history of any hair disease during Covid-19, 34 (14.9%) had been diagnosed by a physician. Participants who had the same hair disease prior to the Covid-19 pandemic and during the Covid-19 pandemic were asked to express their opinion about the course of the hair disease during the Covid-19 pandemic. Out of 259 respondents, 129 (49.8 %) indicated that there was no change, 121 (46.7%) reported an increase in the sign and symptoms of the specified hair disease whereas 9 (3.5 %) claimed that the severity of the hair disease decreased. Of 121 patients who reported an increase in the severity of the pre-existing hair disease during the Covid-19 pandemic, 51 (42.1%) had TE, 37 (30.6%) had hair greying, 29 (24%) were diagnosed with SD, 16 (13.2%) had symptoms of trichodynia, 16 (13.2%) had FPHL, 12 (9.9 %) were diagnosed with MPHL, 7 (5.8%) had trichotillomania, 5 (4.1%) were suffering from scalp psoriasis, 2 (1.7 %) had AA and only 1 (0.8 %) patient had alopecia totalis. Again, of 121 individuals who reported an increase in the severity of the hair disease and 40 who had new-onset hair disease during the Covid-19 pandemic, 138 (85.7%) thought that stress and anxiety played a role either in the development of the new-onset hair disease or deterioration of the pre-existing disease.
A statistically significant relationship was found between Covid-19 RT-PCR positivity and the presence of any hair disease during the Covid-19 pandemic (p=0.009) (Table 2). In addition, a statistically significant relationship was present between Covid-19 RT-PCR positivity and the presence of any new-onset hair disease during the Covid-19 pandemic (p=0.002). The distribution of Covid-19 RT-PCR results in relation to the presence of any new-onset hair disease during the Covid-19 pandemic is shown in Figure 1. The distribution of Covid-19 infection status in relation to the presence of any hair disease during the Covid-19 pandemic is shown in Figure 2. A statistically significant relationship was found between being diagnosed with Covid-19 and having any specific hair disease during the Covid-19 pandemic (p=0.011). Again, there was a statistically significant relationship between the presence of any new-onset hair disease and the diagnosis of Covid-19 (p=0.004). The distribution of Covid-19 infection status in relation to the presence of any new-onset hair disease is shown in Table 3.
Table 2.
Statistically significant relationship was found between the Covid-19 RT-PCR positivity and having at least one hair disease during the Covid-19 pandemic (p=0.009).
| Covid-19 RT-PCR Results | Presence of any hair disease during Covid-19 pandemic | Total n (%) |
|
|---|---|---|---|
| Not present n (%) | Present | ||
| Negative | 169 (70.4) | 120 (58.5) | 289 (64.9) |
| Positive | 71 (29.6) | 85 (41.5) | 156 (35.1) |
| Total | 240 (100) | 205 (100) | 445 (100) |
Figure 1.
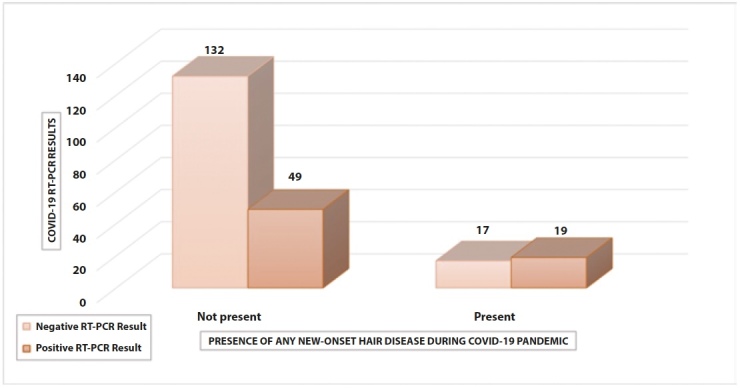
The distribution of Covid-19 RT-PCR results in relation to the presence of any new-onset hair disease during the Covid-19 pandemic.
Figure 2.
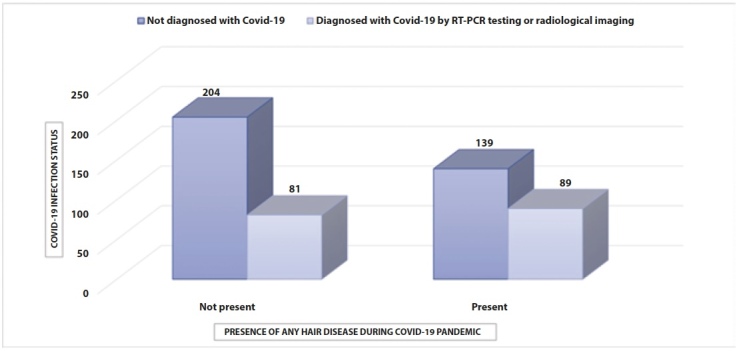
The distribution of Covid-19 infection status in relation to the presence of any hair disease during the Covid-19 pandemic.
Table 3.
The distribution of Covid-19 infection status in relation to the presence of any new-onset hair disease during the Covid-19 pandemic.
| Diagnosed with Covid-19 by RT-PCR testing or radiological imaging | Presence of any new-onset hair disease during Covid-19 pandemic | Total n (%) |
|
|---|---|---|---|
| Not present n (%) | Present | ||
| No | 156 (72.9) | 20 (50) | 176 (69.3) |
| Yes | 58 (27.1) | 20 (50) | 78 (30.7) |
| Total | 214 (100) | 40 (100) | 254(100) |
The distribution of having a new-onset hair disease with regard to the presence of one or more contributing factors for hair disease development (Supplementary File 1-Question 13) is shown in Table 4. There was no statistically significant difference between developing new-onset hair disease and having at least one contributing factor that may be related to the pathogenesis of the new-onset hair disorder (p=0.119).
Table 4.
The distribution of having a new-onset hair disease with regard to the presence of one or more contributing factors for hair disease development.
| Presence of any new-onset hair disease during Covid-19 pandemic | Presence of at least one precipitating factor linked to hair disease | Total n (%) |
|
|---|---|---|---|
| Not present n (%) | Present | ||
| Not present | 145 (86.8) | 69 (79.3) | 214 (84.3) |
| Present | 22 (13.2) | 18 (20.7) | 40 (15.7) |
| Total | 167 (100) | 87 (100) | 254 (100) |
Discussion
From the start of the Covid-19 pandemic, numerous cutaneous manifestations of the infection itself along with the occupational skin problems which result from the continuous use of personal protective equipment have been reported in the literature [11,12]. Urticaria, maculopapular eruption, angioedema, vesicular eruption, pityriasis rosea, erythema multiforme, chilblain-like acral lesions and cutaneous small vessel vasculitis are among the reported cutaneous manifestations of Covid-19 infection [11]. HCPs have worked so hard to provide effective and adequate care for patients with Covid-19 infection. Since effective health care requires optimum protection, continual use of personal protective equipment such as gloves, goggles, masks and hand disinfectants has become an irrevocable rule for all HCPs. Besides the skin diseases observed in the setting of hyperinflammation and immune system activation, occupational skin problems including irritant/allergic contact dermatitis, acne, itching, xerosis, pressure-related skin injuries, skin erythema, retroauricular dermatitis and secondary cutaneous infections continue to be a major concern, especially for HCPs [11,12].
Hair is a part of the integumentary system which has its own growth cycle and this cycle is greatly influenced by intrinsic and extrinsic factors. Diverse drugs, stress, anxiety, chronic illnesses, high fever, extreme loss weight, smoking and iron deficiency may all disrupt the normal hair cycle and cause the hair shafts to enter the telogen phase prematurely which results in excessive hair shedding [13]. Although all humanity is likely to be largely affected by a chaotic emergency situation such as a pandemic, those who work on the front lines to fight against the infection and struggle to provide the utmost care for the patients are more likely to have unfavorable psychosocial impacts [14]. During the era of Covid-19, HCPs have been shown to exhibit signs of post-traumatic stress disorder, depression, anxiety, insomnia, burnout syndrome and somatization [15]. In a study by Ibar et al [16], chronic stress and anxiety among health workers during the Covid-19 pandemic were examined and the relationship with hair cortisol concentration as a biomarker of stress was sought. In a study population of 234 HCPs, 40% showed hair cortisol concentration outside of the normal range [16]. In this study, positive correlation between hair cortisol concentration versus perceived stress and direct correlation between hair cortisol concentration versus emotional exhaustion were also found [16]. This study again underlies the fact that HCPs are exposed to emotional stress and burnout syndrome during the pandemic and hair is largely influenced by the psychosocial impacts of Covid-19. In correlation with this research, in our study of 161 participants who either reported an increase in the severity of the pre-existing hair disease or had new-onset hair disease during the Covid-19 pandemic, 138 (85.7%) thought that stress and anxiety contributed to the development of the new-onset hair disease or progression of the pre-existing disease.
Cases of TE and trichodynia have been observed to increase in number from the start of the pandemic [6,17]. Rizzetto et al [6] reported three cases of TE observed after severe SARS-CoV-2 infection. Furthermore, Mieczkowska et al [18] described a case series consisting of 10 women who were diagnosed with Covid-19 via RT-PCR and antibody test. These patients didn’t have any other triggering factor that would induce TE and TE started 3 to 7 months after Covid-19 infection. In another retrospective study by Cline et al [19], it is found that there was a >400 % increase in the number of patients diagnosed with TE between July and August 2020 when compared to the number of patients diagnosed with TE between November 2019 and February 2020. In another multi-centered study, 214 patients who were diagnosed with acute TE after SARS-CoV-2 infection were evaluated [20]. The mean age was 47.4 years and 78.5 % of all patients were female, 86.4 % developed a fever during the infection. Additionally, 20.8 % of patients required hospitalization and the mean time interval between the Covid-19 diagnosis and significant hair shedding was 57.1 days [20]. In our study cohort, only 40 patients developed new-onset hair disease during the Covid-19 pandemic and the most frequently observed new-onset hair disease was TE. Of these 25 patients with TE, 22 were tested for SARS-CoV-2 infection. Fourteen out of 22 patients had been diagnosed with Covid-19. In our research, the mean time interval between the diagnosis of Covid-19 and the start of hair shedding was found to be 1.13 ± 0.66 months.
Trichodynia is defined as a painful sensation in the scalp often accompanied by TE [21]. Di Landro et al [17] observed 39 patients who presented with TE after Covid-19 infection. Of 39 patients, 7 patients also suffered from severe trichodynia. In this study, the mean age was 64.6 years and both the excessive hair shedding and trichodynia resolved within 2 to 4 months. The authors argued that the hyperinflammatory environment developing in the setting of SARS-Cov-2 infection, was the most probable cause of TE and trichodynia since interleukin-6, interleukin-1ß and tumor necrosis factor-α were shown to induce transition to the catagen phase in hair follicles [17,22]. In our research, 4 new-onset trichodynia cases were detected whereas 16 participants reported exacerbation in trichodynia symptoms during the Covid-19 pandemic.
MPHL is a common hair disease which is characterized by patterned, progressive thinning of hair, seen in genetically susceptible men. The presence of MPHL is thought to be correlated with severe Covid-19 infection [23]. The priming of SARS-CoV-2’s spike protein requires transmembrane protease, serine 2 (TMPRSS2) activity [23]. TMPRSS2 gene transcription is dependent upon androgen receptor activity, so it seems plausible that men are more vulnerable to SARS-CoV-2 infection and fatality rates are rare before puberty [23]. Lee et al [24] evaluated 1605 patients who tested negative for Covid-19 and 336 patients who were diagnosed with Covid-19. The two groups didn’t have any difference in terms of mean age and body mass index [24]. In this study, it was shown that Covid-19 positivity correlated with the increasing baldness score (determined by the Hamilton-Norwood scale) [24]. In another study, 41 Caucasian males who were hospitalized with a diagnosis of SARS-CoV-2 pneumonia were evaluated [25]. Twenty-nine (71%) had clinically evident androgenetic alopecia (Hamilton Norwood scale > 2) and 12 (29%) patients had a Hamilton Norwood scale of 1 or 2 [25]. In another study by Muller Ramos et al [26], it was proven that alopecia and grey hair are correlated with Covid-19 severity. In our study, of 170 patients diagnosed with Covid-19, 21.8 % reported having MPHL and/or increased grey hair prior to the pandemic. Out of 37 patients, only 5 required hospitalization for Covid-19.
AA is an autoimmune, inflammatory, non-cicatricial alopecia which is characterized by relapsing and recurring episodes of patchy or diffuse hair loss. In the hyperinflammatory state of Covid-19, plasma levels of tumor necrosis factor- α, interferon- γ, interleukin-2 and interleukin-1β which are also involved in the pathogenesis of AA, rise substantially [27]. In a study by Rudnicka et al [28], 32 patients with mild-to-moderate AA were evaluated 1–6 weeks before Covid-19 infection and 3 months after the infection [28]. It was found that Covid-19 infection didn’t have any negative impact on the clinical course of AA [28]. In another study with 392 AA patients, 42.5 % had disease relapse about 2 months after Covid-19 infection whereas disease relapse was observed in 12.5 % of all participants without a confirmed diagnosis of SARS-CoV-2 infection [29]. In our study, no new-onset AA was identified, but only 2 (15.4%) out of 13 patients with a pre-existing diagnosis of AA reported an increase in the severity of symptoms.
SD is a chronic inflammatory skin disorder characterized by erythema, greasy scaling and itching which most commonly occurs in the scalp, eyebrows, ears and face [30]. A study by Veraldi et al [30], showed that disease severity increased in 46.5 % of the patients diagnosed with SD. These patients reported wearing anti-Covid-19 masks for 6 to 10 hours a day and 35 % of the patients with worsening SD were health workers [30]. It was asserted that the high temperature and humidity induced by the use of face masks cause abnormalities in microbiota and enhance sweating, thereby provoking an irritant reaction and itching [30]. In line with the results of this study, 29 (90.6%) out of 32 participants with a previous diagnosis of SD, reported an increase in the severity of symptoms of the disease during the pandemic. The higher percentage of participants in our study is not surprising since we have included only HCPs in our study.
Trichotillomania is characterized by repetitive urges to pull out hair from the scalp, eyebrows or other parts of the body [31]. Low self-confidence, social anxiety, major depression and psychosocial dysfunction are associated with trichotillomania [31]. In a study by Pathoulas et al [32], 460 patients presented with a self-reported diagnosis of body-focused repetitive behaviors, 181 patients had a hair-pulling disorder, whereas 141 reported both hair-pulling and skin-picking disorders. A majority of the patients reported an increase in their symptoms during Covid-19 [32]. Our results showed that 38.9% of the individuals with a diagnosis of trichotillomania prior to the pandemic reported having incremental symptoms during Covid-19.
In conclusion, if we were to look at the results of our study altogether, we want to highlight that a statistically significant relationship exists between being diagnosed with Covid-19 and having at least one specific hair disease during the Covid-19 pandemic. Additionally, there seems to be a statistically significant relationship between having a new-onset hair disease during the pandemic and being diagnosed with Covid-19. Our study indicates that hair is vulnerable to the direct effects of SARS-CoV-2 infection and indirect effects of the pandemic (psychosocial impacts, skin problems resulting from the use of personal protective equipment etc.). We believe that the underlying etiopathological mechanisms of the trichological effects of Covid-19 are also possibly related to other pre-existing, ongoing and new-onset (post-Covid-19) systemic as well as dermatological manifestations.
Our research has some limitations since not all the participants were diagnosed by a dermatologist. The severity of the hair disease was not directly evaluated by a physician using a score or scale, so the results might have been subjective. Prospective, randomized-controlled studies with larger sample sizes are needed to confirm our results.
Supplementary Information
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Daye M, Cihan FG, Durduran Y. Evaluation of skin problems and dermatology life quality index in health care workers who use personal protection measures during COVID-19 pandemic. Dermatol Ther. 2020;33(6):e14346. doi: 10.1111/dth.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toncic RJ, Jakasa I, Hadzavdic SL, et al. Altered Levels of Sphingosine, Sphinganine and Their Ceramides in Atopic Dermatitis Are Related to Skin Barrier Function, Disease Severity and Local Cytokine Milieu. Int J Mol Sci. 2020;21(6):1958. doi: 10.3390/ijms21061958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asghar F, Shamim N, Farooque U, Sheikh H, Aqeel R. Telogen Effluvium: A Review of the Literature. Cureus. 2020;12(5):e8320. doi: 10.7759/cureus.8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harland DP. Introduction to Hair Development. Adv Exp Med Biol. 2018;1054:89–96. doi: 10.1007/978-981-10-8195-8_8. [DOI] [PubMed] [Google Scholar]
- 5.Stenn KS, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81(1):449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- 6.Rizzetto G, Diotallevi F, Campanati A, et al. Telogen effluvium related to post severe Sars-Cov-2 infection: Clinical aspects and our management experience. Dermatol Ther. 2021;34(1):e14547. doi: 10.1111/dth.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrantes TF, Artounian KA, Falsey R, et al. Time of onset and duration of post-COVID-19 acute telogen effluvium. J Am Acad Dermatol. 2021;85(4):975–976. doi: 10.1016/j.jaad.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ivenson FD. COVID-19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2021;60(1):127. doi: 10.1111/ijd.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Öner Ü. Children with trichotillomania in COVID-19 outbreak. J Cosmet Dermatol. 2021;20(7):1967–1968. doi: 10.1111/jocd.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alpalhão M, Gaibino N, Filipe P. Seborrheic dermatitis in COVID-19: a case report. Int J Dermatol. 2020;59(12):1543–1544. doi: 10.1111/ijd.15256. [DOI] [PubMed] [Google Scholar]
- 11.Mawhirt SL, Frankel D, Diaz AM. Cutaneous Manifestations in Adult Patients with COVID-19 and Dermatologic Conditions Related to the COVID-19 Pandemic in Health Care Workers. Curr Allergy Asthma Rep. 2020;20(12):75. doi: 10.1007/s11882-020-00974-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcha RJ. Does Wearing a Face Mask During the COVID-19 Pandemic Increase the Incidence of Dermatological Conditions in Health Care Workers? Narrative Literature Review. JMIR Dermatol. 2021;4(1):e22789. doi: 10.2196/22789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi A, Magri F, Sernicola A, et al. Telogen Effluvium after SARS-CoV-2 Infection: A Series of Cases and Possible Pathogenetic Mechanisms. Skin Appendage Disord. 2021;21(5):1–5. doi: 10.1159/000517223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodríguez BO, Sánchez TL. The Psychosocial Impact of COVID-19 on health care workers. Int Braz J Urol. 2020;46(suppl 1):195–200. doi: 10.1590/S1677-5538.IBJU.2020.S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salazar de Pablo G, Vaquerizo-Serrano J, Catalan A, et al. Impact of coronavirus syndromes on physical and mental health of health care workers: Systematic review and meta-analysis. J Affect Disord. 2020;275:48–57. doi: 10.1016/j.jad.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibar C, Fortuna F, Gonzalez D, et al. Evaluation of stress, burnout and hair cortisol levels in health workers at a University Hospital during COVID-19 pandemic. Psychoneuroendocrinology. 2021;128:105213. doi: 10.1016/j.psyneuen.2021.105213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Landro A, Naldi L, Glaser E, Paus R, Tosti A. Pathobiology questions raised by telogen effluvium and trichodynia in COVID-19 patients. Exp Dermatol. 2021;30(7):999–1000. doi: 10.1111/exd.14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mieczkowska K, Deutsch A, Borok J, et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021;60(1):122–124. doi: 10.1111/ijd.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cline A, Kazemi A, Moy J, Safai B, Marmon S. A surge in the incidence of telogen effluvium in minority predominant communities heavily impacted by COVID-19. J Am Acad Dermatol. 2021;84(3):773–775. doi: 10.1016/j.jaad.2020.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Arrones OM, Lobato-Berezo A, Gomez-Zubiaur A, et al. SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35(3):e181–e183. doi: 10.1111/jdv.17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebora A. Trichodynia: a review of the literature. Int J Dermatol. 2016;55(4):382–4. doi: 10.1111/ijd.13204. [DOI] [PubMed] [Google Scholar]
- 22.Hussain N, Agarwala P, Iqbal K, et al. A systematic review of acute telogen effluvium, a harrowing post-COVID-19 manifestation. J Med Virol. 2022;94(4):1391–1401. doi: 10.1002/jmv.27534. [DOI] [PubMed] [Google Scholar]
- 23.Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J Am Acad Dermatol. 2020;83(1):308–309. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Yousaf A, Fang W, Kolodney MS. Male balding is a major risk factor for severe COVID-19. J Am Acad Dermatol. 2020;83(5):e353–e354. doi: 10.1016/j.jaad.2020.07.062. Erratum in: J Am Acad Dermatol. 2021 ;85(3):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goren A, Vaño-Galván S, Wambier CG, et al. A preliminary observation: Male pattern hair loss among hospitalized COVID-19 patients in Spain - A potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020;19(7):1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- 26.Müller Ramos P, Ianhez M, Amante Miot H. Alopecia and grey hair are associated with COVID-19 Severity. Exp Dermatol. 2020;29(12):1250–1252. doi: 10.1111/exd.14220. [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudnicka L, Rakowska A, Waskiel-Burnat A, Kurzeja M, Olszewska M. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: A retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85(3):723–725. doi: 10.1016/j.jaad.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinaldi F, Trink A, Giuliani G, Pinto D. Italian Survey for the Evaluation of the Effects of Coronavirus Disease 2019 (COVID-19) Pandemic on Alopecia Areata Recurrence. Dermatol Ther (Heidelb) 2021;11(2):339–345. doi: 10.1007/s13555-021-00498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veraldi S, Angileri L, Barbareschi M. Seborrheic dermatitis and anti-COVID-19 masks. J Cosmet Dermatol. 2020;19(10):2464–2465. doi: 10.1111/jocd.13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diefenbach GJ, Tolin DF, Hannan S, Crocetto J, Worhunsky P. Trichotillomania: impact on psychosocial functioning and quality of life. Behav Res Ther. 2005;43(7):869–84. doi: 10.1016/j.brat.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Pathoulas JT, Olson SJ, Idnani A, Farah RS, Hordinsky MK, Widge AS. Cross-sectional survey examining skin picking and hair pulling disorders during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84(3):771–773. doi: 10.1016/j.jaad.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


