Introduction
Hidrocystomas (AHC) are benign cystic tumors that originate from apocrine or eccrine sweat glands. While rare, apocrine AHC are typically found as solid, asymptomatic blue to black papules or nodules on the face and neck, especially around the eyelid margin. AHC arises from cystic proliferation of the apocrine gland [1], while the eccrine variant is caused by the retention of eccrine glands [2]. The etiology of apocrine AHC is largely unknown [1]. Pigmented AHC of the nasal epithelium of the eccrine origin have been reported [3], but to our knowledge, our case is one of few [4] AHC on the nasal ala reported. Additionally, we report another case of pigmented AHC and describe the reflectance confocal microscopy (RCM) findings, which are scarce in current literature [5]. Although AHC are benign lesions, based off clinical appearance alone they are often mistaken for basal cell carcinomas, blue nevi, or even melanoma. RCM can noninvasively differentiate between benign and malignant cutaneous lesions, and we review the potential application of this imaging technique in the clinical management of AHC.
Case Presentation
Case 1 is a 69-year-old male with Fitzpatrick Skin type III presented with concerns for a 2-mm homogenous, well demarcated blue-gray papule on a background of sun-damaged hyperpigmented skin (Figure 1, A and B). Initial clinical assessment was determined as a blue nevus potentially superimposed upon a solar lentigo, and a shave biopsy was done to rule out malignant melanoma given the patient reported history of excessive sun exposure and rapid growth of the lesion. The specimen routinely stained with hematoxylin and eosin and histologically diagnosed as a pigmented AHC. Microscopic examination revealed a cystic structure containing focal granular pigmented material whose upper portion was lined by cuboidal epithelial cells. The lesion was histologically diagnosed as a pigmented AHC. The specimen routinely stained with hematoxylin and eosin and histologically diagnosed as a pigmented AHC (Figure 1C).
Figure 1.
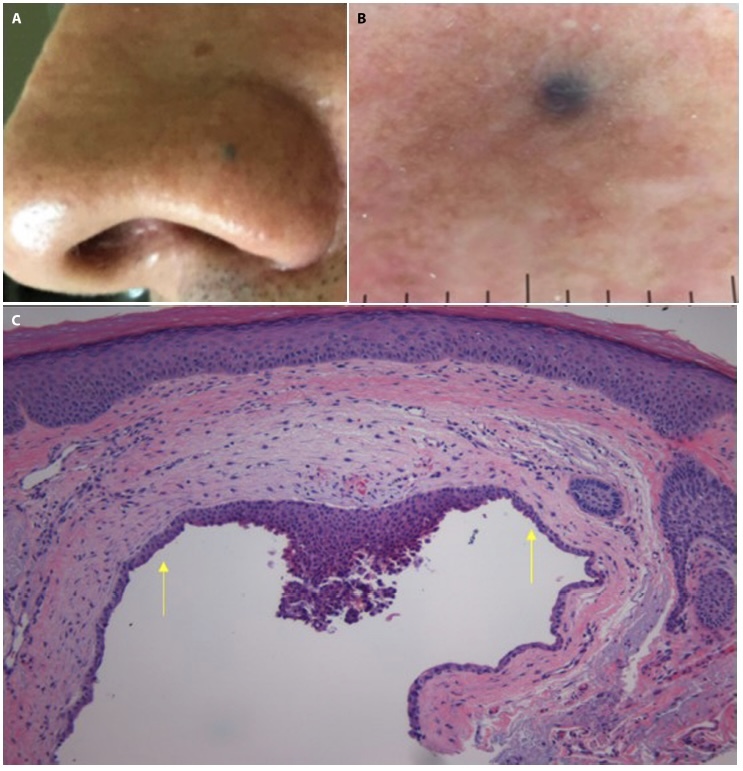
(A) Clinical image of a darkly pigmented papule located on the left nasal ala. (B) Dermoscopic image of the lesion showing a 2-mm homogenous blue-gray papule on a background of brown to red sun-damaged skin. (C) Histopathologic image showing a cystic structure lined by single-to-several layers of cuboidal epithelial cells (arrows) with focal granular pigmented material in the cyst lumen (H&E, x100).
Case 2 is a 63-year-old male presented with a history of non-melanoma skin cancer who presented with concerns for an asymptomatic lesion located on his central forehead. The patient reported that the lesion has been present for an unknown amount of time but has recently grown in size over the last few months. Clinical and dermoscopic examination revealed a well demarcated 4-mm blue homogenous papule (Figure 2, A and B). The lesion was further evaluated with RCM (Vivascope 1500) which revealed a normal honey-combed epidermal architecture surrounding a hypo reflective space. Deeper images reveal dark lacunae near normal adnexal structures (Figure 3A), representative of cystic spaces. These findings along with the absence of concerning features such as pagetoid cells or non-edged papillae favored diagnosis of a benign AHC. However, due to patient concern for the growing lesion, the lesion was removed using a shave biopsy technique and histopathological assessment illustrated a cystic space lined by several layers of cuboidal epithelial cells, confirming the diagnosis of pigmented AHC (Figure 3B).
Figure 2.
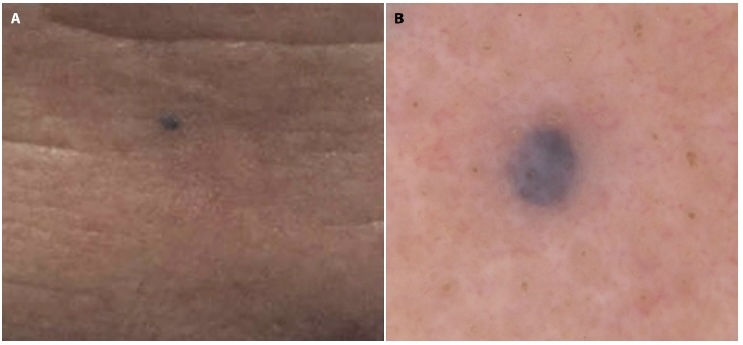
(A) Clinical image of a single darkly pigmented papule located on the central forehead. (B) Dermoscopy image demonstrating a 4-mm blue-violet homogenous well-demarcated papule.
Figure 3.
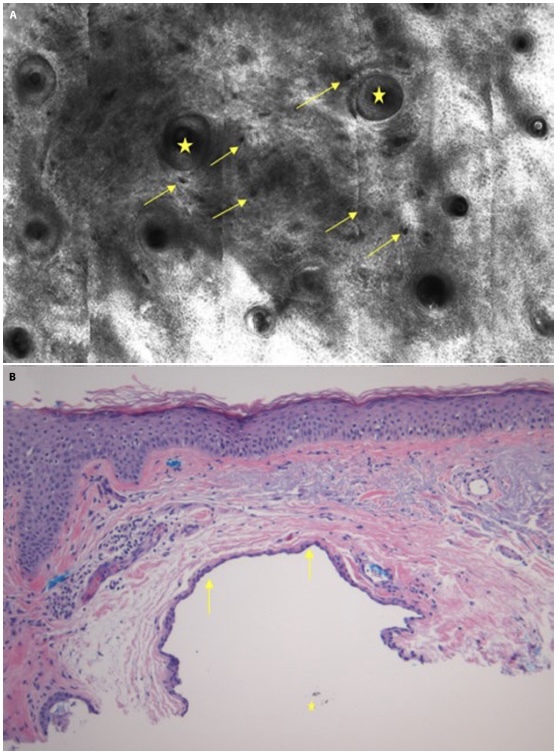
(A) Reflectance confocal microscopic image of the superficial dermis reveals adnexal structures (yellow star) surrounded by several hypoechogenic lacunae (yellow arrows) representative of cystic spaces. (B) Histopathologic image showing a cystic lumen with the upper portion demonstrating an attenuated lining containing 2 layers of flattened to cuboidal epithelial cells (arrows) and pigmented brown granular material (star) in the cystic space (H&E, x100).
Conclusions
This paper reports a unique anatomical presentation of an apocrine variant of pigmented AHC and discusses the differential diagnosis of pigmented papules or nodules on the body, specifically blue nevi and melanoma. AHC can grossly present as a blue papule or nodule that can be mistakenly clinically diagnosed as a blue nevus, pigmented basal cell carcinoma (BCC), or even melanoma. While AHCs and blue nevi are benign, skin cancers like BCC and melanoma have malignant potential, thus histologic evaluation can be of benefit. Blue nevus-like melanomas have two reported pathogenesis: melanoma arising coincidentally with a benign blue nevus or arising de novo and mimicking a blue nevus [6]. While rare, it is important to note an initially benign blue nevus also has the potential to become malignant [7–9]. AHC may be benign, however it is important to be familiar of other malignancies with similar clinical presentations as potential differential diagnosis.
Familiarity with features of AHC may promote accurate clinical diagnosis and avoid the unnecessary financial, cosmetic, and psychological implications associated with physical biopsies. AHC tend to appear as a solitary, homogeneous papule or nodule, typically ranging in size from 3–15mm [10], either skin-colored or with pink, yellow or blue color hue, and can have secondary features such as arborizing vessels seen under dermoscopy [11]. AHC most commonly appears on the eyelid margins [12–15], oral mucosa [16,11,17] and ear [11,17,18]. We would like to report that both our cases of AHC, when compared to more classical presentations, do not have arborizing vessels, and has a less common location on the nasal ala, contributing to the literature of other variants, including eccrine [19] and planar AHC variants [20], reported on the nasal ala.
It is important to utilize advanced imaging technologies to discern differential diagnosis of blue skin lesions with malignant potential, such as blue nevi, BCC, and melanoma from a completely benign entity like hidrocystoma. Recognizing the absence of certain features of BCC such as bright tumor islands with abundant vasculature and pleomorphism of the overlying epidermis [21–24] or features like nucleated round, dendritic, or spindled cells and non-edged papillae which can be found in melanocytic lesions with malignant potential like blue nevi or melanoma [25] can provide clinicians with enough confidence to safely monitor the lesion without sampling. The clinical benefits of utilizing RCM include prevention of unnecessary biopsies, decreased pain, improved cosmetic outcomes, and enhanced surveillance for recurrent malignancy [26]. Data on RCM characteristic findings for hidrocystomas is scarce in current literature [4,22] and we provide further evidence supporting the features of hidrocystoma commonly seen on RCM including homogenous cystic structures adjacent to normal-appearing adnexal structures.
The variations and overlapping features in the clinical presentation of AHC, blue nevus, melanoma and non-melanoma skin cancer may make it challenging to dictate appropriate management therefore, it is important to consider the diagnosis of pigmented AHC clinically when there is a suspicion of blue nevus, BCC, or melanoma. Dermoscopic and reflectance confocal microscopic assessment of the lesion aids in an accurate diagnosis which may to guide appropriate patient care.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Hafsi W, Badri T. StatPearls. StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC; Treasure Island (FL): 2020. Apocrine Hidrocystoma. [Google Scholar]
- 2.Alfadley A, Al Aboud K, Tulba A, Mourad MM. Multiple eccrine hidrocystomas of the face. Int J Dermatol. 2001;40(2):125–129. doi: 10.1046/j.1365-4362.2001.01126.x. [DOI] [PubMed] [Google Scholar]
- 3.Rappazzo KC, Cohen PR. Pigmented hidrocystoma of nasal epithelium (PHONE): report of a man with a pigmented hidrocystoma of his nose and literature review. Dermatol Online J. 2016;22(5):13030/qt9c50d26x. [PubMed] [Google Scholar]
- 4.Anandasabapathy N, Soldano AC. Multiple apocrine hidrocystomas. Dermatol Online J. 2008;14(5):12. [PubMed] [Google Scholar]
- 5.Walker A, Sahni VN, Sahni DR, Curtis J. Use of Reflectance Confocal Microscopy for Hidrocystomas: An Emerging, Cost-Effective, and Powerful Tool. Case Rep Dermatol Med. 2021;2021:5543803. doi: 10.1155/2021/5543803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granter SR, McKee PH, Calonje E, Mihm MC, Jr, Busam K. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus’. Am J Surg Pathol. 2001;25(3):316–323. doi: 10.1097/00000478-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 7.González-Cámpora R, Galera-Davidson H, Vázquez-Ramírez FJ, Díaz-Cano S. Blue nevus: classical types and new related entities. A differential diagnostic review. Pathol Res Pract. 1994;190(6):627–635. doi: 10.1016/S0344-0338(11)80402-4. [DOI] [PubMed] [Google Scholar]
- 8.Connelly J, Smith JL., Jr Malignant blue nevus. Cancer. 1991;67(10):2653–7. doi: 10.1002/1097-0142(19910515)67:10<2653::aid-cncr2820671041>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Lee HY, Na SY, Son YM, et al. A malignant melanoma associated with a blue nevus of the lip. Ann Dermatol. 2010;22(1):119–124. doi: 10.5021/ad.2010.22.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansson M, Brodersen J, Gøtzsche PC, Jørgensen KJ. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6(6):CD012352. doi: 10.1002/14651858.CD012352.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheung WY, Bayliss MS, White MK, et al. Humanistic burden of disease for patients with advanced melanoma in Canada. Support Care Cancer. 2018;26(6):1985–1991. doi: 10.1007/s00520-017-4025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaballos P, Bañuls J, Medina C, Salsench E, Serrano P, Guionnet N. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28(3):378–381. doi: 10.1111/jdv.12044. [DOI] [PubMed] [Google Scholar]
- 13.Sarabi K, Khachemoune A. Hidrocystomas--a brief review. Med-GenMed. 2006;8(3):57. [PMC free article] [PubMed] [Google Scholar]
- 14.Belaldavar BP, Suranagi V, Kalakuntla M, Raj B, Tiwari A. Apocrine Hidrocystoma: A Rare Case Report. Indian J Otolaryngol Head Neck Surg. 2019;71(Suppl 1):59–61. doi: 10.1007/s12070-016-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alagheband M, Maida M. Asymptomatic periorbital, bluish cystic papule. Cortlandt Forum. 2004;18:36–41. [Google Scholar]
- 16.Jakobiec FA, Stacy RC, Colby KA. Pigmented apocrine hidrocystoma of the caruncle. Cornea. 2010;29(11):1320–1322. doi: 10.1097/ICO.0b013e3181d4fd71. [DOI] [PubMed] [Google Scholar]
- 17.Poli PP, Creminelli L, Moramarco V, Del Gobbo A, Ferrante F, Maiorana C. Diagnostic Workup and Treatment of a Rare Apocrine Hidrocystoma Affecting the Oral Mucosa: A Clinical and Histological Case Report. Case Rep Dent. 2017;2017:9382812. doi: 10.1155/2017/9382812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozel HE, Kaynar A. A case of an apocrine hidrocystoma treated by sublabial approach. Kulak Burun Bogaz Ihtis Derg. 2012;22(5):284–287. doi: 10.5606/kbbihtisas.2012.054. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis DG, Drivas EI, Papadakis CE, Feritsian A, Bizakis JG, Skoulakis CE. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2(1):79. doi: 10.1186/1757-1626-2-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rappazoo KC, Cohen PR. Pigmented hidrocystoma of nasal epithelium (PHONE): report of a man with a pigmented hidrocystoma of his nose and literature review. Dermatol Online J. 2016;22(5):13030/qt9c50d26x. [PubMed] [Google Scholar]
- 21.Yanagi T, Sawamura D, Nishie W, Abe M, Shibaki A, Shimizu H. Multiple apocrine hidrocystoma showing plane pigmented macules. J Am Acad Dermatol. 2006;54(2 Suppl):S53–S54. doi: 10.1016/j.jaad.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura S, Yanagi T, Imafuku K, Hata H, Shimizu H. Lipofuscin deposition causes the pigmentation of apocrine hidrocystoma. J Dermatol. 2018;45(1):91–94. doi: 10.1111/1346-8138.14037. [DOI] [PubMed] [Google Scholar]
- 23.Willard K, Warschaw KE, Swanson DL. Use of reflectance confocal microscopy to differentiate hidrocystoma from basal cell carcinoma. Dermatol Surg. 2011;37(3):392–394. doi: 10.1111/j.1524-4725.2011.01893.x. [DOI] [PubMed] [Google Scholar]
- 24.González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47(6):869–784. doi: 10.1067/mjd.2002.124690. [DOI] [PubMed] [Google Scholar]
- 25.Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30(3):413–419. doi: 10.1111/jdv.13408. [DOI] [PubMed] [Google Scholar]
- 26.Agero AL, Busam KJ, Benvenuto-Andrade C, et al. Reflectance confocal microscopy of pigmented basal cell carcinoma. J Am Acad Dermatol. 2006;54(4):638–643. doi: 10.1016/j.jaad.2005.11.1096. [DOI] [PubMed] [Google Scholar]


