Background:
Lamin A/C gene (LMNA)-related dilated cardiomyopathy is a serious and life-threatening condition with a high unmet medical need. This phase 2 study assessed the effects of the oral selective p38 mitogen-activated protein kinase inhibitor ARRY-371797 on functional capacity and cardiac function in patients with LMNA-related dilated cardiomyopathy.
Methods:
Patients with LMNA-related dilated cardiomyopathy in New York Heart Association class II–IIIA, on background heart failure treatment, received ARRY-371797 100 or 400 mg twice daily for 48 weeks. The primary end point was change from baseline in the 6-minute walk test distance at 12 weeks. Secondary end points included changes over time in 6-minute walk test distance, NT-proBNP (N-terminal pro-B-type natriuretic peptide) concentration, left ventricular ejection fraction, and quality-of-life scores on the Kansas City Cardiomyopathy Questionnaire. Data from the 2 dose groups were combined.
Results:
Twelve patients were enrolled; median (minimum, maximum) 6-minute walk test distance at baseline was 314 (246, 412) m. At week 12, the mean (80% CI) increase from baseline in 6-minute walk test distance was 69 (39, 100) m (median, 47 m). Median NT-proBNP concentration declined from 1409 pg/mL at baseline to 848 pg/mL at week 12. Mean left ventricular ejection fraction was stable at week 12. There was a trend toward improvement in Kansas City Cardiomyopathy Questionnaire Overall and Clinical Summary scores at week 12. No clinically significant drug-related safety concerns were identified.
Conclusions:
ARRY-371797 was well tolerated and resulted in potential increases in functional capacity and lower concentrations of cardiac biomarker NT-proBNP in patients with LMNA-related dilated cardiomyopathy.
Registration:
URL: https://clinicaltrials.gov; Unique identifier: NCT02057341.
Keywords: clinical trial, lamin A/C, LMNA-related dilated cardiomyopathy, p38 MAPK
There have been considerable advances in our understanding of the molecular basis of some forms of heart failure (HF), yet there are still very few mechanism-specific therapies for any forms of this morbid syndrome. Family studies and genetic analyses have identified the causal mechanisms underlying multiple different forms of dilated cardiomyopathy (DCM), including one form caused by mutations in the lamin A/C gene (LMNA).1 Estimates of the prevalence of DCM from genetic causes vary depending on the underlying patient population and methodology used in registry studies. Population-based studies indicate that DCM may occur in ≈1:250 to 1:400 patients.2,3 Reports also suggest that ≈6% of DCM cases are potentially attributable to mutations in the LMNA gene,2 which results in a particularly penetrant form of HF with an initial clinical course that manifests as conduction system disease or arrhythmias in early to mid-adulthood, and frequently, early mortality due to sudden cardiac death.4 LMNA-related DCM is characterized by a high burden of ventricular arrhythmia5 and is distinguished from other cardiomyopathies by having an indication for early implantable cardioverter-defibrillator implantation.4,6,7 Over time, most patients develop DCM and progressive HF.1,8,9 While available studies are small, and incompletely controlled for relatedness, they do suggest that LMNA-related DCM may be associated with higher levels of morbidity than other forms of DCM.2,4,9
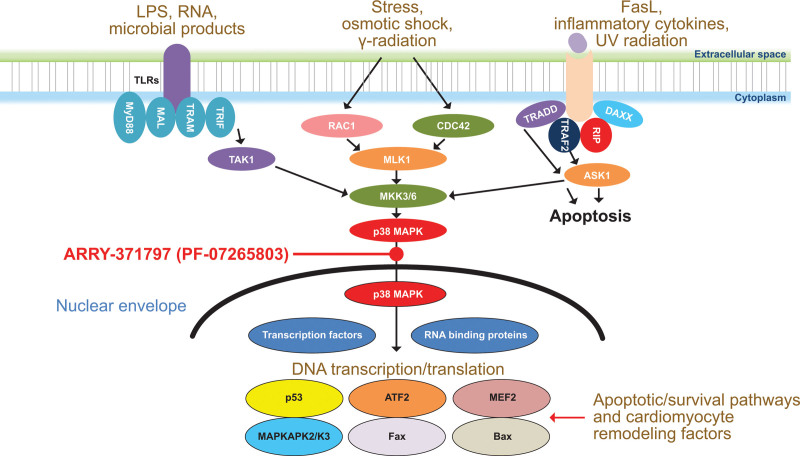
Although the precise mechanism of LMNA-related DCM has yet to be elucidated, identification of causal mutations enables the creation of mechanistically faithful animal models that reproduce components of the pathophysiology along the entire disease course.10 In such animal studies, loss of functional lamin proteins produces strong cellular stress signals that activate the p38 mitogen-activated protein kinase (MAPK) pathway (Figure 1).11–13 Furthermore, p38 MAPK pathway activation has also been demonstrated in biopsies from the hearts of adult patients with LMNA-related DCM.11 Downstream consequences of p38 MAPK activation include enhanced cardiomyocyte apoptosis, cardiomyocyte hypertrophy, decreased contractility, and increased expression of brain natriuretic peptide.14–17 ARRY-371797 (PF-07265803) is a selective, oral, small-molecule inhibitor of the p38 MAPK pathway. In a validated animal model of LMNA-related DCM, ARRY-371797 (PF-07265803) reversed negative left ventricular remodeling.11
Figure 1.
p38 MAPK pathway in LMNA-related dilated cardiomyopathy. ASK1 indicates apoptosis signal-regulating kinase 1; ATF2, activating transcription factor 2; CDC42, cell division control protein 42 homolog; DAXX, death-associated protein 6; FasL, Fas ligand; Fax, failed axon connection; LMNA, lamin A/C gene; LPS, lipopolysaccharide; MAL, myelin and lymphocyte protein; MAPK, mitogen-activated protein kinase; MAPKAPK2/3, mitogen-activated protein kinase-activated protein kinase 2/3; MEF2, myocyte enhancer factor; MKK3/6, mitogen-activated protein kinase kinase 3/6; MLK1, mixed lineage kinase 1; MyD88, myeloid differentiation primary response 88; RAC1, Ras-related C3 botulinum toxin substrate 1; RIP, receptor-interacting protein; TAK1, transforming growth factor β-activated kinase 1; TLR, Toll-like receptor; TRADD, tumor necrosis factor receptor type 1-associated death domain protein; TRAF2, tumor necrosis factor receptor-associated factor 2; TRAM, TRIF-related adaptor molecule; and TRIF, Toll/interleukin-1 receptor domain-containing adapter-inducing interferon-β.11–13.
This phase 2 study assessed the effects of ARRY-371797 (PF-07265803) on cardiac physiology and on overall functional capacity in 12 patients with LMNA-related DCM to test the hypothesis that such molecularly-guided therapy might improve patient symptoms and thus establish a rationale for a future pivotal phase 3 study.
Methods
A full description of the methods of this trial is available in the associated Supplemental Material.18–22
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
This open-label, phase 2, nonrandomized, 2-arm study was conducted at 5 US sites (ClinicalTrials.gov identifier: NCT02057341). The study protocol was approved by each institutional review board or ethics committee and performed in accordance with Good Clinical Practice guidelines established by the International Council for Harmonisation and local regulatory requirements. All patients provided written informed consent.
Results
Patients
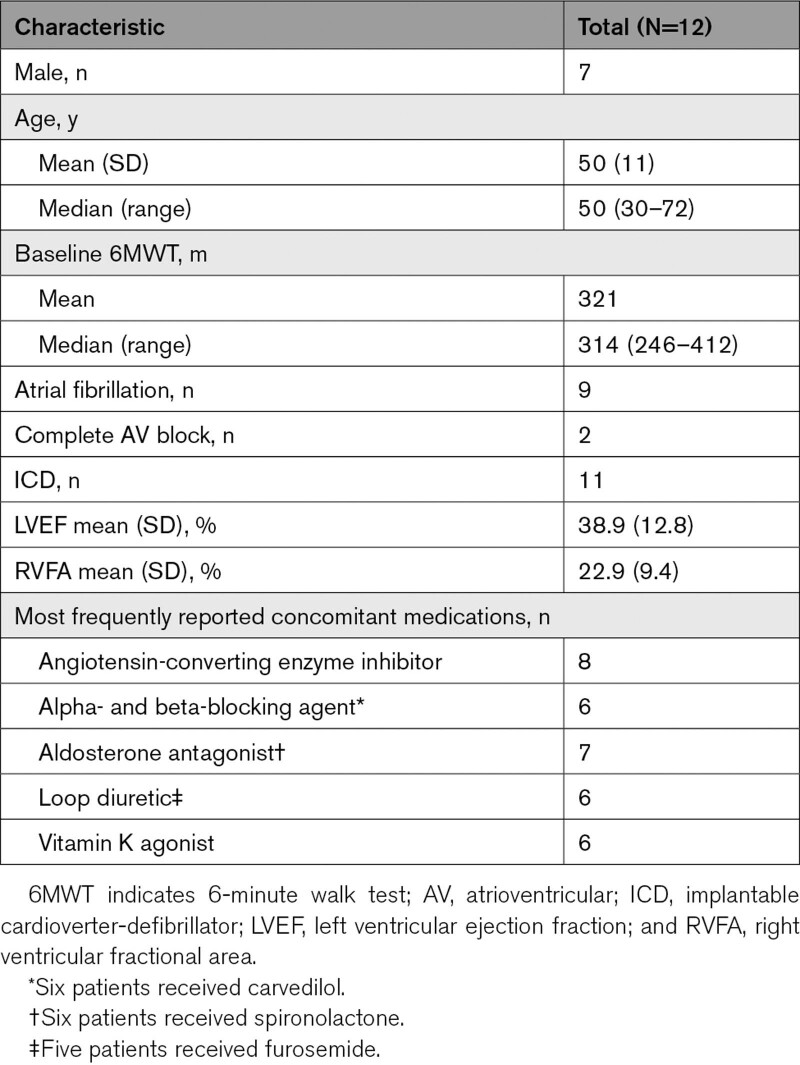
Among 36 screened patients, 12 (7 male; 12 White; mean age, 50 years) were enrolled (Table 1). The 6-minute walk test (6MWT) mean and median (range) baseline values were 321 and 314 m (246–412 m), respectively. Baseline mean left ventricular ejection fraction was 39%, and 9 patients had atrial fibrillation. The most commonly reported concomitant HF medications included angiotensin-converting enzyme inhibitors, beta blockers, aldosterone antagonists, loop diuretics, and vitamin K agonists. Eleven patients had an implantable cardioverter-defibrillator, with the remaining patient having a pacemaker only; no patients had a cardiac resynchronization therapy pacemaker. Six patients received ARRY-371797 (PF-07265803) 100 mg BID, and 6 received ARRY-371797 (PF-07265803) 400 mg BID. Demographic characteristics and 6MWT distance at baseline were generally similar between dose groups.
Table 1.
Demographics and Clinical Characteristics at Baseline
Four patients discontinued treatment early (see Safety, below). Four of the 8 remaining patients continued to receive their assigned dose. One patient receiving ARRY-371797 (PF-07265803) 400 mg BID was downtitrated at week 12 to 200 mg BID in response to serious adverse event (AE) or intolerance, per protocol. Three patients receiving 100 mg BID were dose-escalated to 400 mg BID at week 24 or 28. Eight patients completed 48 weeks of treatment.
Primary Efficacy End Point: 6MWT Distance at Week 12
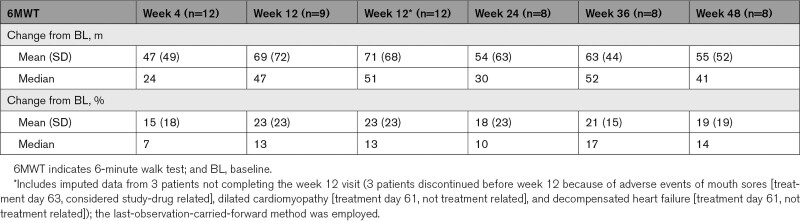
A mean absolute change in the 6MWT distance of 69 m (range, –18 to 203; SD, 72; [80% CI, 39–100]) was observed at week 12, corresponding to a mean percentage change of 23% (range, –5 to 66; SD, 23; [80% CI, 13–32]; Table 2). The increase in absolute 6MWT distance at 12 weeks versus baseline was statistically significant (P=0.02, paired t test). Median absolute and percentage changes were 47 m and 13%. Using imputed data, the mean increase from baseline in 6MWT distance was 71 m (range, –18 to 203; SD, 68; [80% CI, 46–97] versus baseline), corresponding to a mean (SD) percentage change from baseline of 23% (23%). The median absolute and percentage changes using imputed data were 51 m and 13%.
Table 2.
Absolute and Percentage Change From Baseline Data in 6MWT Distance With ARRY-371797 (PF-07265803) Treatment
6MWT Distance at Weeks 4, 24, 36, and 48
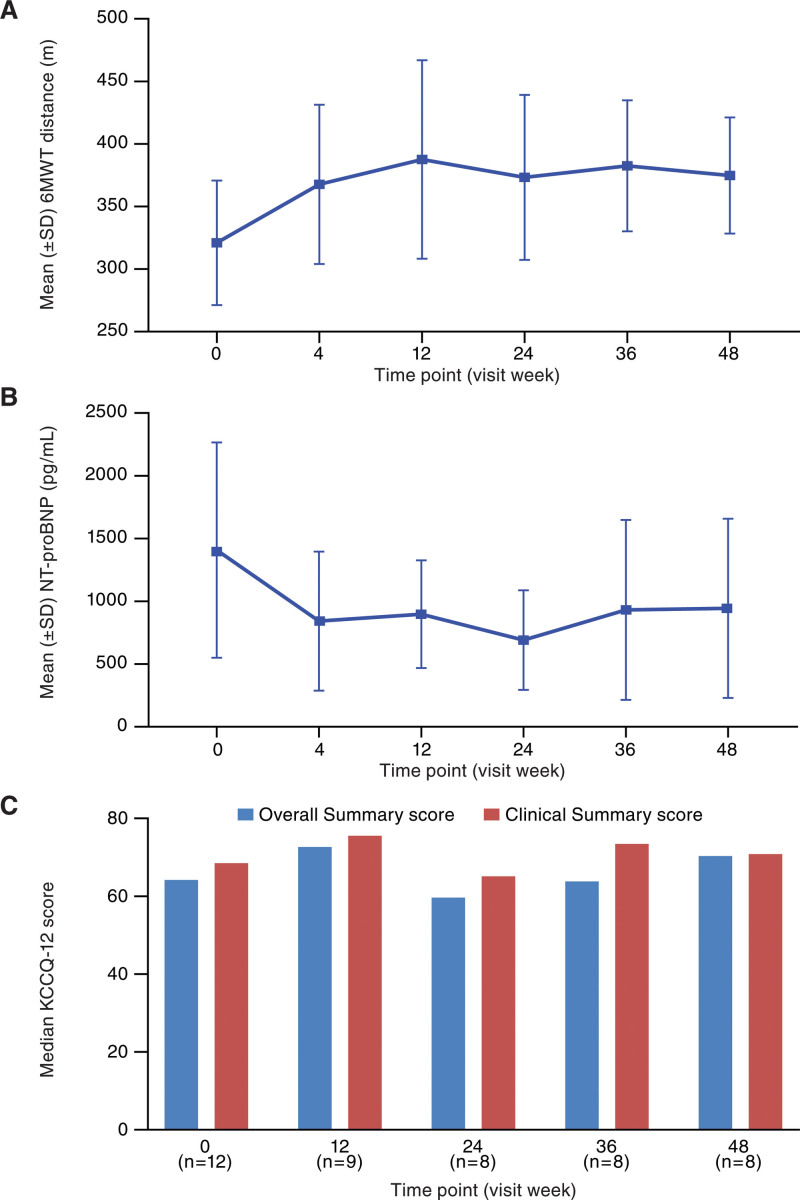
Improvement in mean and median 6MWT distance was observed at the first time point (week 4) and maintained over 48 weeks (Figure 2A; Table 2). Response (≥10% improvement in 6MWT distance) was observed in 5 (41.7%), 5 (41.7%), 4 (33.3%), 6 (50.0%), and 5 (41.7%) patients at weeks 4, 12, 24, 36, and 48, respectively. Increases in absolute 6MWT distance versus baseline were statistically significant at 24, 36, and 48 weeks with P value (paired t test) of 0.04, 0.005, and 0.02, respectively. Response with imputed data for week 12 was observed in 7 (58.3%) patients. 6MWT results up to completion in those who completed 12 versus 48 weeks of treatment were representative of the overall population (Figure S1).
Figure 2.
Clinical endpoints across study duration. Mean 6MWT distance without imputation (A), mean NT-proBNP concentration (B), and median KCCQ-12 OS and CS scores (C) with ARRY-371797 (PF-07265803) treatment. *P of increases in absolute 6MWT distance vs baseline ≤0.05. 6MWT indicates 6-minute walk test; CS, clinical summary; KCCQ-12, Kansas City Cardiomyopathy Questionnaire short form; NT-proBNP, N-terminal pro-B-type natriuretic peptide; and OS, overall summary.
Plasma N-Terminal Pro-B-Type Natriuretic Peptide Concentrations
Decreases in mean NT-proBNP (N-terminal pro-B-type natriuretic peptide) concentration from baseline were observed at all study visits (Figure 2B). Median NT-proBNP concentrations were 1409 pg/mL at baseline, and 758, 848, 633, 638, and 680 pg/mL at weeks 4, 12, 24, 36, and 48, respectively. Mean (median) changes from baseline (decrease) in NT-proBNP concentrations were 560 (437), 445 (384), 341 (250), 297 (211), and 284 (166) pg/mL at weeks 4, 12, 24, 36, and 48, respectively.
Echocardiography
Measurements of left ventricular ejection fraction and right ventricular fractional area remained stable during the treatment with ARRY-371797 (PF-07265803; Figure S2). Mean left ventricular ejection fraction (SD) was 39% (13) at baseline (n=10) and 39% (9) at week 48 (n=7). Mean (SD) right ventricular fractional area was 23% (9) at baseline (n=12) and 30% (10) at week 48 (n=7).
Dose–Response Relationship
The results for 6MWT and left ventricular ejection fraction showed a high degree of variability with no clear dose effect. However, larger reductions in NT-proBNP concentration were observed with the 400-mg dose of ARRY-371797 (PF-07265803 as compared to the 100-mg BID dose in an analysis of aggregated mean change from baseline at all time points, Figure S3).
Kansas City Cardiomyopathy Questionnaire
The Kansas City Cardiomyopathy Questionnaire median Overall Summary and Clinical Summary scores had increased from baseline at all visits except week 24 (Figure 2C). An increase from baseline of >5 points was seen at weeks 12 and 48 for Overall Summary score, and at week 12 for Clinical Summary score.
Safety
ARRY-371797 (PF-07265803) was well tolerated at both dose levels. Sixty-nine AEs were reported in 12 patients. Most AEs were mild in severity (grade 1/2). A total of 9 grade 3/4 AEs were reported in 3 patients, and none were considered related to study drug by the investigator. The most frequently reported AEs, by category, were gastrointestinal disorders (6 patients), laboratory abnormalities (6 patients), cardiac disorders (5 patients), infections and infestations (4 patients), and skin and subcutaneous tissue disorders (4 patients). AEs reported in >1 patient or as grade 3/4 are listed in Table S1.
Six patients reported 18 AEs that were considered related to study drug by the investigator. The majority (n=11) were mild in severity. The most frequently reported study drug-related AEs, by category, were gastrointestinal disorders (4 patients), skin and subcutaneous tissue disorders (3 patients), and laboratory abnormalities (2 patients).
Four patients were withdrawn from the study, one because of an AE considered study drug-related (grade 2 AE of mouth sores). None of the other withdrawals were related to study drug; 2 were the result of physician decisions (availability of a donor-matched heart for transplantation; cardiac resynchronization therapy), and one because of a serious AE (cardiac failure) with the patient subsequently undergoing placement of a left ventricular assist device. There were no deaths during the study.
Discussion
Results from this phase 2 study suggest that treatment with ARRY-371797 (PF-07265803) provides increased functional capacity and lower concentrations of cardiac biomarker NT-proBNP in patients with LMNA-related DCM. While LMNA-related DCM represents ≈ 6% of patients with DCM, it remains underdiagnosed.2 LMNA-related DCM is marked by a high frequency of arrhythmias and accelerated progression of HF.4 Even with optimal conventional therapy, many patients with LMNA-related DCM rapidly progress and experience major cardiac events.1,8,9 As such, there is a large unmet medical need for therapies that can halt disease progression or improve cardiac function in patients with LMNA-related DCM.
In this phase 2 study, improvements in 6MWT distance occurred in patients who had been stable before the study, and consistent favorable changes in NT-proBNP concentrations were also seen. A trend toward improvement in right ventricular fractional area was observed, and median Kansas City Cardiomyopathy Questionnaire Overall Summary and Clinical Summary scores had increased from baseline at all visits except week 24. ARRY-371797 (PF-07265803) was generally well tolerated; most AEs were mild, and only one patient discontinued because of a study drug-related AE. These potential benefits were observed with ARRY-371797 (PF-07265803) despite the late-stage disease and maximal-tolerated baseline therapy in the patients in the study.
This is one of the first therapeutic studies in a molecularly distinct subset of HF with reduced ejection fraction. LMNA-related DCM exhibits discrete features including early-onset and highly penetrant atrioventricular block, high frequency of atrial fibrillation and ventricular arrhythmias, and accelerated progression of HF.1,23–25 Extracardiac features are reported in other autosomal dominant disorders associated with LMNA mutations, and often are present subclinically in families where DCM with atrioventricular block is the predominant phenotype segregating in the kindred.23,26,27 LMNA-related DCM may not only be particularly suited to proximate mechanistic interventions, but may also represent a distinctive pathway within the broader rubric of HF with reduced ejection fraction.
This first-in-patient phase 2 study was designed to understand dosing, safety signals, and the optimal design for a pivotal phase 3 study of ARRY-371797 (PF-07265803) treatment. That phase 3 clinical trial, A Multinational, Randomized, Placebo-controlled Study of ARRY-371797 (PF-07265803) in Patients With Symptomatic Dilated Cardiomyopathy Due to a Lamin A/C Gene Mutation (REALM-DCM, NCT03439514) is a randomized, double-blind, placebo-controlled, multicenter study in adult patients with symptomatic LMNA-related DCM and is currently recruiting patients. REALM-DCM includes a 24-week, double-blind portion in which ≈160 patients with LMNA-related DCM will be randomized to ARRY-371797 (PF-07265803) 400 mg BID or placebo. The primary end point is the change from baseline in 6MWT distance at week 24. Upon completion of the primary outcome measures, the study will then transition into an open-label phase.
Limitations
This was a small, phase 2 trial, conducted in a limited number of patients over a broad age range with a rare disease and without a control group. As such, the data should be interpreted with caution, given the limited sample size, potential impact of age on the end points examined in the trial, such as 6MWT and lack of comparison against standard of care. Nevertheless, the statistically significant increases from baseline in 6MWT distance observed are promising, particularly in a condition for which there are currently no therapies specifically targeting the underlying cause of disease. While there are learning effects associated with the 6MWT,28,29 which are particularly relevant in an uncontrolled study, each patient performed the 6MWT 3 times before the start of treatment to minimize the impact of these effects. Data from the ongoing, Phase 3 REALM-DCM trial will provide more robust data on the impact of treatment with ARRY-371797 (PF-07265803) on functional capacity, cardiac biomarkers, and measures of quality of life, along with safety and tolerability, as compared with placebo in a larger population of patients with LMNA-related DCM than previously studied.
Conclusions
In this phase 2 trial, patients with LMNA-related DCM had increased functional capacity and lower concentrations of cardiac biomarker NT-proBNP with ARRY-371797 (PF-07265803) treatment. The trial demonstrates that inhibition of p38 MAPK with a selective p38α inhibitor (ARRY-371797 [PF-07265803]) may provide a novel therapeutic approach with the potential to fill an unmet medical need for the treatment of LMNA-related DCM. ARRY-371797 (PF-07265803) has the potential to improve functional capacity and quality of life in these patients and is currently being tested as the first therapeutic approach specifically targeted to treat patients with LMNA-related DCM.
Article Information
Acknowledgments
Medical writing support was provided by Caitlin Watson, PhD, of Engage Scientific Solutions, and funded by Pfizer. We thank Lance Wollenberg, Pfizer, for assistance with statistical analysis. We also thank Mieke Ptaszynski, Pfizer, for assistance with data interpretation and article drafting.
All authors had access to study data, contributed to the design, and conduct of the analysis; interpretation of the data; preparation, review, and approval of the article; and have approved the final version of the article. Dr Lee had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Sources of Funding
This study was sponsored by Array BioPharma Inc, which was acquired by Pfizer in July, 2019. Pfizer contributed to the study design and management and collection of data. In their role as authors, employees of Pfizer were involved in the interpretation of data; preparation, review, and approval of the article; and the decision to submit for publication, along with their coauthors. The study sponsor approved the article from an intellectual property perspective but had no right to veto the publication.
Disclosures
Dr MacRae has consulted for Affinia, Array BioPharma, AstraZeneca, Bayer, Bristol Myers Squibb, Design Therapeutics, Dewpoint Therapeutics, DiNAQOR, Merck, MyoKardia, Novartis, and Pfizer; and has received grant support from Apple, Astra Zeneca, Bayer, Merck, Novartis, Quest Diagnostics, Sanofi and Verily. Dr Taylor has consulted for Array Biopharma, Rocket Pharmaceuticals, and 4D Molecular Therapeutics. Dr Mestroni has consulted for Array Biopharma and Pfizer. Dr Moses has no relevant conflicts. Dr Ashley is a founder of Deepcell, Personalis, and Silicon Valley Exercise Analytics; a founding advisor for Nuevocor, an advisor for Apple, Cathay Capital, Disney, Foresite Capital, Genome Medical, Medical Excellence Capital, Novartis, Sequence Bio, and Third Rock Ventures; and a nonexecutive director at AstraZeneca. He has also received grants from Google and Verily, academic grants from Bristol Myers Squibb and Takeda, and collaborative support in kind from Analog Devices, Illumina, Oxford Nanopore Technologies, PacBio, and Samsung. Dr Wheeler has received modest consulting fees from Array BioPharma, MyoKardia, Bayer, BioTelemetry, and Verily; research funding from Array BioPharma, Pfizer, MyoKardia, Bristol Myers Squibb, Novartis, and Salubris Biopharma; and in-kind writing support from Bristol Myers Squibb and Pfizer. Dr Lakdawala receives modest consulting incomes from MyoKardia, Bristol Myers Squibb, Tenaya Therapeutics, Array BioPharma, and Pfizer. Dr Hershberger has no relevant conflicts. At the time of this study Drs Ptaszynski, Sandor, Saunders, Oliver, and Lee were full-time employees of Array BioPharma Inc, which was acquired by Pfizer in July, 2019. Dr Judge has received consultancy fees from 4D Molecular Therapeutics, ADRx, Cytokinetics, Pfizer, and Tenaya Therapeutics. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Table S1
Figures S1–S3
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 6MWT
- 6-minute walk test
- AE
- adverse event
- BID
- twice daily
- DCM
- dilated cardiomyopathy
- HF
- heart failure
- LMNA
- lamin A/C gene
- MAPK
- mitogen-activated protein kinase
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCGEN.122.003730.
For Sources of Funding and Disclosures, see page 18.
Contributor Information
Matthew R.G. Taylor, Email: matthew.taylor@cuanschutz.edu.
Luisa Mestroni, Email: LUISA.MESTRONI@CUANSCHUTZ.EDU.
John Moses, Email: mosesj848@gmail.com.
Euan A. Ashley, Email: euan@stanford.edu.
Matthew T. Wheeler, Email: wheelerm@stanford.edu.
Neal K. Lakdawala, Email: nlakdawala@bwh.harvard.edu.
Ray E. Hershberger, Email: ray.hershberger@osumc.edu.
Victor Sandor, Email: vsan@hotmail.com.
Michael E. Saunders, Email: mikesaundersmd@aol.com.
Colleen Oliver, Email: COLLEEN.OLIVER@PFIZER.COM.
Patrice A. Lee, Email: Patrice.Lee@pfizer.com.
Daniel P. Judge, Email: judged@musc.edu.
References
- 1.Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Jr, Spudich S, De Girolami U, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302 [DOI] [PubMed] [Google Scholar]
- 2.Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat Rev Cardiol. 2013;10:531–547. doi: 10.1038/nrcardio.2013.105 [DOI] [PubMed] [Google Scholar]
- 3.McKenna WJ, Judge DP. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol. 2021;18:22–36. doi: 10.1038/s41569-020-0428-2 [DOI] [PubMed] [Google Scholar]
- 4.Hasselberg NE, Haland TF, Saberniak J, Brekke PH, Berge KE, Leren TP, Edvardsen T, Haugaa KH. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur Heart J. 2018;39:853–860. doi: 10.1093/eurheartj/ehx596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sidhu K, Han L, Picard KCI, Tedrow UB, Lakdawala NK. Ventricular tachycardia in cardiolaminopathy: characteristics and considerations for device programming. Heart Rhythm. 2020;17:1704–1710. doi: 10.1016/j.hrthm.2020.05.023 [DOI] [PubMed] [Google Scholar]
- 6.Wahbi K, Ben Yaou R, Gandjbakhch E, Anselme F, Gossios T, Lakdawala NK, Stalens C, Sacher F, Babuty D, Trochu JN, et al. Development and validation of a new risk prediction score for life-threatening ventricular tachyarrhythmias in laminopathies. Circulation. 2019;140:293–302. doi: 10.1161/CIRCULATIONAHA.118.039410 [DOI] [PubMed] [Google Scholar]
- 7.Priori SG, Blomström-Lundqvist C. 2015 European society of cardiology guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death summarized by co-chairs. Eur Heart J. 2015;36:2757–2759. doi: 10.1093/eurheartj/ehv445 [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Baldinger SH, Gandjbakhch E, Maury P, Sellal JM, Androulakis AF, Waintraub X, Charron P, Rollin A, Richard P, et al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J Am Coll Cardiol. 2016;68:2299–2307. doi: 10.1016/j.jacc.2016.08.058 [DOI] [PubMed] [Google Scholar]
- 9.Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E, Di Lenarda A, Bohlmeyer TJ, Ferguson DA, Brodsky GL, et al.; Familial Dilated Cardiomyopathy Registry Research Group. Natural history of dilated cardiomyopathy due to lamin A/C gene mutations. J Am Coll Cardiol. 2003;41:771–780. doi: 10.1016/s0735-1097(02)02954-6 [DOI] [PubMed] [Google Scholar]
- 10.Milan DJ, MacRae CA. Animal models for arrhythmias. Cardiovasc Res. 2005;67:426–437. doi: 10.1016/j.cardiores.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 11.Muchir A, Wu W, Choi JC, Iwata S, Morrow J, Homma S, Worman HJ. Abnormal p38alpha mitogen-activated protein kinase signaling in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum Mol Genet. 2012;21:4325–4333. doi: 10.1093/hmg/dds265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: Angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323 [DOI] [PubMed] [Google Scholar]
- 14.Liang F, Gardner DG. Mechanical strain activates BNP gene transcription through a p38/NF-kappaB-dependent mechanism. J Clin Invest. 1999;104:1603–1612. doi: 10.1172/JCI7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao RP, Kass DA, et al. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Huang S, Sah VP, Ross J, Jr, Brown JH, Han J, Chien KR. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161 [DOI] [PubMed] [Google Scholar]
- 17.Kan H, Birkle D, Jain AC, Failinger C, Xie S, Finkel MS. p38 MAP kinase inhibitor reverses stress-induced cardiac myocyte dysfunction. J Appl Physiol (1985). 2005;98:77–82. doi: 10.1152/japplphysiol.00171.2004 [DOI] [PubMed] [Google Scholar]
- 18.Olsson LG, Swedberg K, Clark AL, Witte KK, Cleland JG. Six minute corridor walk test as an outcome measure for the assessment of treatment in randomized, blinded intervention trials of chronic heart failure: a systematic review. Eur Heart J. 2005;26:778–793. doi: 10.1093/eurheartj/ehi162 [DOI] [PubMed] [Google Scholar]
- 19.Zugck C, Kruger C, Durr S, Gerber SH, Haunstetter A, Hornig K, Kubler W, Haass M. Is the 6-minute walk test a reliable substitute for peak oxygen uptake in patients with dilated cardiomyopathy?. Eur Heart J. 2000;21:540–549. doi: 10.1053/euhj.1999.1861 [DOI] [PubMed] [Google Scholar]
- 20.Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999;14:270–274. doi: 10.1034/j.1399-3003.1999.14b06.x [DOI] [PubMed] [Google Scholar]
- 21.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 22.Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, DeVore AD, Albert NM, Duffy CI, Patterson JH, Thomas L, et al. Comparison of New York Heart Association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:522–531. doi: 10.1001/jamacardio.2021.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodsky GL, Muntoni F, Miocic S, Sinagra G, Sewry C, Mestroni L. Lamin A/C gene mutation associated with dilated cardiomyopathy with variable skeletal muscle involvement. Circulation. 2000;101:473–476. doi: 10.1161/01.cir.101.5.473 [DOI] [PubMed] [Google Scholar]
- 24.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, Erdos MR, Robbins CM, Moses TY, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigouroux C, Magre J, Vantyghem MC, Bourut C, Lascols O, Shackleton S, Lloyd DJ, Guerci B, Padova G, Valensi P, et al. Lamin A/C gene: sex-determined expression of mutations in Dunnigan-type familial partial lipodystrophy and absence of coding mutations in congenital and acquired generalized lipoatrophy. Diabetes. 2000;49:1958–1962. doi: 10.2337/diabetes.49.11.1958 [DOI] [PubMed] [Google Scholar]
- 26.Carboni N, Politano L, Floris M, Mateddu A, Solla E, Olla S, Maggi L, Antonietta Maioli M, Piras R, Cocco E, et al. Overlapping syndromes in laminopathies: a meta-analysis of the reported literature. Acta Myol. 2013;32:7–17. Available at https://pubmed.ncbi.nlm.nih.gov/23853504/ [PMC free article] [PubMed] [Google Scholar]
- 27.Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B). Hum Mol Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453 [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Sanderson B, Bittner V. The 6-minute walk test: how important is the learning effect?. Am Heart J. 2003;146:129–133. doi: 10.1016/S0002-8703(03)00119-4 [DOI] [PubMed] [Google Scholar]
- 29.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. Available at https://pubmed.ncbi.nlm.nih.gov/3978515/ [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.