Abstract
Background and Objective
To investigate the pathway-specific correspondence between structural and functional changes resulting from focal subcortical stroke and their causal influence on clinical symptom.
Methods
In this retrospective, cross-sectional study, we mainly focused on patients with unilateral subcortical chronic stroke with moderate-severe motor impairment assessed by Fugl-Meyer Assessment (upper extremity) and healthy controls. All participants underwent both resting-state fMRI and diffusion tensor imaging. To parse the pathway-specific structure-function covariation, we performed association analyses between the fine-grained corticospinal tracts (CSTs) originating from 6 subareas of the sensorimotor cortex and functional connectivity (FC) of the corresponding subarea, along with the refined corpus callosum (CC) sections and interhemispheric FC. A mediation analysis with FC as the mediator was used to further assess the pathway-specific effects of structural damage on motor impairment.
Results
Thirty-five patients (mean age 52.7 ± 10.2 years, 27 men) and 43 healthy controls (mean age 56.2 ± 9.3 years, 21 men) were enrolled. Among the 6 CSTs, we identified 9 structurally and functionally covaried pathways, originating from the ipsilesional primary motor area (M1), dorsal premotor area (PMd), and primary somatosensory cortex (p < 0.05, corrected). FC for the bilateral M1, PMd, and ventral premotor cortex covaried with secondary degeneration of the corresponding CC sections (p < 0.05, corrected). Moreover, these covarying structures and functions were significantly correlated with the Fugl-Meyer Assessment (upper extremity) scores (p < 0.05, uncorrected). In particular, FC between the ipsilesional PMd and contralesional cerebellum (β = −0.141, p < 0.05, CI = [−0.319 to −0.015]) and interhemispheric FC of the PMd (β = 0.169, p < 0.05, CI = [0.015–0.391]) showed significant mediation effects in the prediction of motor impairment with structural damage of the CST and CC.
Discussions
This study reveals causal influence of structural and functional pathways on motor impairment after subcortical stroke and provides a promising way to investigate pathway-specific structure-function coupling. Clinically, our findings may offer a circuit-based evidence for the PMd as a critical neuromodulation target in more impaired patients with stroke and also suggest the cerebellum as a potential target.
Motor impairment is the most common domain of functional deficits after stroke,1 which is attributed to a high prevalence of subcortical motor pathway damage (typically caused by basal ganglia stroke).2 The extent of lesions, the integrity of descending motor pathways (e.g., corticospinal tracts [CSTs]),3,4 and the secondary degeneration remote from lesions (e.g., corpus callosum [CC])5,6 have the potential to predict motor function after stroke. Moreover, these focal lesions can lead to a wide range of functional reorganization, such as overactivation of the contralesional areas during motor execution,5,7 and the alterations of resting-state functional connectivity (FC) pattern8-10 or network topology,11 some of which also showed association with the degree of motor dysfunction. Although extensive neuroimaging studies have investigated the correlations among structural damage, functional abnormalities, and motor impairment, few studies have uncovered the causal effect of structural and functional pathways on motor impairment after stroke.
Structure is well considered to be the basis of function; uncovering the structure-function relationship is a fundamental concern of systems neuroscience.12,13 In healthy populations, previous studies have suggested that FC is primarily shaped by structural connectivity.14-16 Yet, experimentally examining how direct perturbations of white matter pathways are reflected in the functional connectome remains scarce in human subjects. Importantly, focal brain lesions induced by stroke may provide a unique opportunity to infer causal influence between structural and functional pathways. Previous studies have shown correlations between the damage degree of the CST or interhemispheric anatomic connectivity and distributed FC disruptions in subcortical stroke.5,17,18 A recent study on a large sample further indicates that widespread FC disruptions are primarily explained by structural disconnections after stroke.19 However, a model directly testing whether structural pathway damage produces motor impairment through a mediating effect of disrupted FC is still lacking.
Moreover, stroke-induced changes in refined pathways originating from different subareas of the sensorimotor cortex (SMC) have rarely been investigated. Previous studies have mainly focused on the functional and structural pathways arising from the primary motor cortex (M1) in subcortical stroke,20,21 although roughly 50% of CST inputs are from the higher-order motor areas such as the premotor cortex and supplementary motor area (SMA).22 Emerging evidence has found that the CST fibers originating from the premotor cortex help to accurately predict poststroke functional deficits.23,24 Recently, a study have created a fine-grained map of the CST with distinct cortical origins and reported that the integrity of CSTs arising from the M1 and SMA is closely in assessing and predicting long-term motor outcome in patients with subcortical stroke.25 In addition to CSTs, degeneration of the transcallosal fibers connecting the bilateral M1 and higher-order motor areas also plays a crucial role in influencing cortical functional reorganization and motor outcome after subcortical stroke.5,26,27 Therefore, using the fine-grained CST and CC maps arising from distinct SMC subareas may help to clarify the structure-function relationship and discern the roles of different cortical motor areas in functional deficits after stroke, thereby which will enlighten interventions for clinical recovery.
A promising approach to enhance poststroke motor recovery has been applied to directly modulate cortical excitability using noninvasive brain stimulation such as transcranial magnetic stimulation (TMS) and transcranial current stimulation (tDCS). Numerous studies have found that the beneficial effects of stimulation targets vary with the clinical severity and/or the extent of structural damage after stroke. Specifically, the standard approach to excitation of ipsilesional M1 or inhibition of contralesional M1 has been effective in improving upper limb function in mildly-impaired individuals, whereas stimulation of higher-order motor areas (e.g., premotor cortex) may subserve motor recovery for those with more severe impairment.28,29 Although several cortical targets have been tested (e.g., M1 and premotor cortex), the circuitry mechanism underlying the difference in clinical efficacy after stimulation of different motor areas is largely unclear. Previous work from healthy individuals has suggested that greater white matter coherence allows to promote the transmission of functional signals in specific projection target regions, which might then influence behavior.30 Accordingly, we expected that cortical areas with functionally mediating effect on the relationship between structural damage and motor impairment are likely effective intervention targets for patients with stroke.
In this study, we aimed (1) to systematically explore the pathway-specific structure-function covariations based on the fine-grained CST and CC maps for the patients with unilateral subcortical stroke and (2) to further test the causal effect of structural and functional pathways on motor impairment using a mediation model. We hypothesized that (1) structural damage to the subpathways of the CST/CC caused by stroke may be associated with functional reorganization of their respective origins of the SMC, and (2) except for the M1 pathway, the covaried pathways of the higher-order SMC may also contribute to poststroke motor deficits, and the specific FC abnormalities of the higher-order SMC (e.g., premotor cortex) induced by structure damage (CST/CC) would be the mediator to predict motor impairment, especially in more patients with severe stroke.
Methods
Participants
Forty patients with subcortical stroke were recruited from the Department of Rehabilitation Medicine at Huashan Hospital affiliated with Fudan University. Five patients were excluded because of severe head motion during scanning, leaving 35 patients for the final analysis (see eMethods: Controlling for Head Motion, links.lww.com/WNL/C459). The inclusion criteria were as follows: (1) age 18–80 years31, (2) first-onset stroke with an ischemic infarct or hemorrhage in the subcortical regions, (3) poststroke time interval >3 months, (4) moderate-severe motor dysfunctions for the hemiplegic upper extremity assessed by Fugl-Meyer Assessment–Upper Extremity (FM-UE scores ≤37),32 and (5) right handed prior to stroke as determined by the Edinburgh Handedness Scale.33 The exclusion criteria were as follows: (1) any contraindications to MRI scanning, (2) Mini-Mental State Examination (MMSE) score <27, (3) severe hand spasticity, (4) severe aphasia, neglect, and sensory disturbances, and history of alcohol, drug abuse, or epilepsy, (5) participation in any experimental rehabilitation or drug studies, and (6) bilateral lesions or involvement of the cerebral cortex. The detailed demographic and clinical data of the patients are listed in eTable 1 (links.lww.com/WNL/C459). For healthy controls, the inclusion criteria were as follows: (1) age 18–80 years, (2) right handed, and (3) no history of neurologic or psychiatric disorders. The exclusion criteria were as follows: (1) any contraindications to MRI scanning and (2) MMSE score <27.
Standard Protocol Approvals, Registrations, and Patient Consents
This retrospective study was approved by the Ethics Committee of East China Normal University (HR 017-2020), which determined that participant consent was waived.
MRI Data Acquisition
All participants underwent MRI at the Shanghai Key Laboratory of Magnetic Resonance, East China Normal University (Shanghai, China). High-resolution 3D T1-weighted anatomic images, resting-state fMRI data of the whole brain, T2-weighted images, and diffusion tensor imaging (DTI) data were acquired. The detailed parameters of each pulse sequence are shown in the eMethods (links.lww.com/WNL/C459).
Lesion Map and Image Flip
The lesion of each patient was drawn on the respective high-resolution T1-weighted images using MRIcron (nitrc.org/projects/mricron),34 and then, the binary lesion masks were spatially normalized to a Montreal Neurological Institute (MNI) brain template. Finally, the lesion masks were summed and overlapped on the MNI template. Prior to data analysis, the MRI volumes of 17 patients with right hemispheric lesions were flipped along the midsagittal plane.7 To avoid differences caused by interhemispheric asymmetry, the data of an equivalent proportion of healthy controls (n = 20) were processed in the same manner.35
DTI Analysis for Identifying Structural Damage in Patients With Stroke
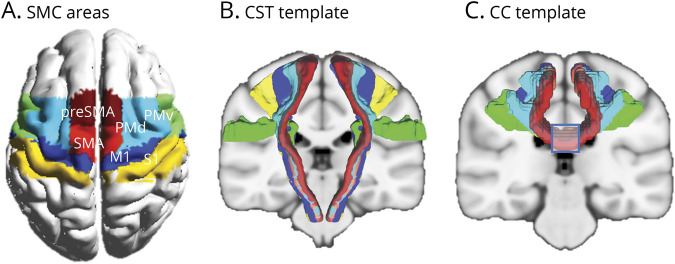
The preprocessing procedures for DTI data are provided in the eMethods (links.lww.com/WNL/C459). To identify pathway-specific structural damage in patients with stroke, we mapped the distinct CST and CC pathways according to their different origins in the SMC. Thus, 12 subareas of the SMC including the bilateral M1, dorsal premotor area (PMd), ventral premotor areas (PMv), primary somatosensory cortex (S1), SMA, and presupplementary motor area (preSMA) (Figure 1A), were defined using the human motor area template (HMAT) parcellation proposed in a previous study.36 Moreover, to overcome the potential shortcomings of fiber tracking, especially for brains with lesions, we adopted fine-grained CSTs from the sensorimotor area tract template (SMATT)37 (Figure 1B, individual CSTs are shown in eFigure 1) and CC sections from the transcallosal tract template (TCATT) frontal tracts38 (Figure 1C, individual CC sections are shown in eFigure 2) with different sensorimotor origins. Specifically, CC sections were identified by bilateral 10-mm parallel planes on the coronal MRI image. We considered the CC sections instead of whole transcallosal tracts they have a relatively coherent arrangement and do not overlap with CSTs.20 SMATT and TCATT templates were created using the diffusion spectral imaging data of 100 healthy participants from the Human Connectome Project (humanconnectomeproject.org)39 with a probabilistic fiber tracking approach. The mean fractional anisotropy (FA) values of 12 CSTs from bilateral hemispheres and 6 CC sections were extracted from CST and CC templates for both patients with stroke and healthy controls. Notably, we used FA value to assess the damage of the CST, instead of the involvement of lesion. It is because there would be a reduction of lesion extent from acute to chronic stages partly due to spontaneous absorption. Thus, the CST probably had been affected, despite lesion was not directly involved at chronic stage.
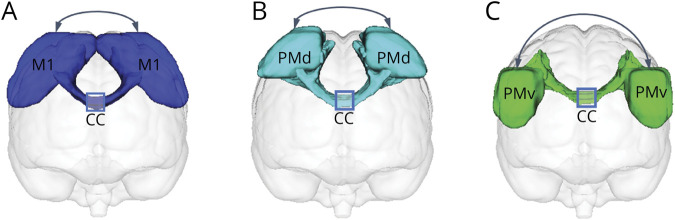
Figure 1. Sensorimotor Areas of Interest and White Matter Fibers of Interest.
(A) Subareas of the sensorimotor cortex. These subareas were identified by human motor area template (HMAT) in which they were used as seed regions for the probabilistic tractography of distinct corticospinal tracts (CSTs) and corpus callosum (CC) fibers. (B) The template of distinct CSTs obtained from the sensorimotor area tract template (SMATT). Six individual CSTs are shown in eFigure 1 (links.lww.com/WNL/C459). (C) The template of CC sections (as illustrated by the blue square, 6 individual CC sections are shown in eFigure 2) obtained from the transcallosal tract template (TCATT) frontal tracts. M1 = primary motor cortex; preSMA = presupplementary motor area; PMd = dorsal premotor cortex; PMv = ventral premotor cortex; S1 = primary somatosensory cortex; SMA = supplementary motor area; SMC = sensorimotor cortex.
To reduce the effect of individual difference, we calculated the asymmetry of the FA values between the ipsilesional and contralesional CSTs for evaluating the structural damage of each CST as follows:4
 |
where the asymmetry values of FA range from −1 to 1. Larger values indicate a higher degree of structural damage (lower structural integrity) to the ipsilesional CST pathways. For healthy control group where no lesion occurs, the absolute FAasymmetry were calculated with contralesional and ipsilesional side corresponding to right and left side, respectively. Regarding the structural integrity of the CC sections, the mean FA value of each CC section was calculated for each participant.
To detect the structural damage of each CST and CC section in patients with stroke, intergroup comparisons for FAasymmetry of the CST and FA of the CC were performed using the general linear model, with age and sex controlled (we set p < 0.05, Bonferroni corrected as statistically significant). This statistical analysis was conducted using Statistical Package for the Social Sciences version 23.0 (SPSS, IBM Corporation, Armonk, NY).
Resting-State fMRI Analysis for Identifying Abnormalities of FC in Patients With Stroke
The preprocessing procedures for resting-state fMRI data are provided in the eMethods (links.lww.com/WNL/C459). Consistent with the subareas of the SMC used in structural pathway analyses, resting-state FC maps of the ipsilesional M1, PMd, PMv, S1, SMA, and preSMA were obtained by computing Pearson correlations of the fMRI signals in a voxelwise manner in the whole brain. To ensure normality, we transformed all correlation coefficients into Fisher Z-scores.40 Thus, Z-scored FC maps were obtained and used for further statistical analysis. To identify abnormalities in FC related to each subarea of the SMC, between-group comparisons were performed using 2-sample t tests, with sex, age, and mean framewise displacement as covariates. We set a voxel-level p < 0.001 and cluster p < 0.05, corrected for multiple comparisons using the familywise error (FWE) method, as statistically significant.
In addition, FC of the bilateral M1, PMd, PMv, S1, SMA, and preSMA was calculated using Pearson correlation analysis. The FC values were also transformed into Z-scores for between-group analysis. Between-group comparisons were performed using the general linear model, with sex, age, and mean framewise displacement as covariates. We considered p < 0.05, Bonferroni corrected, as statistically significant.
Identification of Covaried Structural-Functional Pathways in Patients
We identified the covaried structural-functional pathways from 2 perspectives. First, to explore the brain-wide FC abnormalities of SMC subareas that might be directly affected by the damage of refined CSTs, association analyses between damage to each CST and abnormalities in FC of the corresponding SMC subarea were conducted using a multiple regression model, with age, sex, and poststroke time intervals as covariates. To control for multiple comparisons, a combined voxelwise and cluster extent threshold, corresponding to an FWE rate of p < 0.05, was determined using SPM's threshold-free cluster enhancement (TFCE) approach (dbm.neuro.uni-jena.de/tfce/).41 In addition, the FC values in the covaried structural-functional pathways were extracted and used to perform partial correlation analysis with the corresponding damage of CSTs while controlling for age, sex, and poststroke time intervals to obtain the r (partial correlation coefficient) and p values. Second, we focused on the relationship between the FC connecting bilateral SMC subareas and microstructural integration of the corresponding CC sections. Thus, partial correlation analysis was performed between the interhemispheric FC values and FA values of the corresponding CC sections while controlling for age, sex, and poststroke time intervals. Finally, after the covaried structural-functional pathways were defined, we explored the relationship between the covaried structural-functional pathways and motor deficit. The association analysis between structural (FAasymmetry of the CST and FA values of the CC) and functional (zFC values) metrics of covaried pathways and FM-UE scores were assessed with partial correlation analyses.
Analysis of the Mediation Effect
In the current study, our main hypothesis is that specific FC abnormality may play a mediating effect on the relationship between structural damage and motor impairment in terms of common motor cortex origins. Accordingly, we chose the commonly used30,42 simple mediation model, which only includes an estimator (structure damage), a mediator (function alteration), and an outcome (motor impairment). This model requires few assumptions and can be explained clearly. Specifically, among the identified pathways of structural-functional covariation, the simple mediating model (i.e., model 4) was applied using PROCESS in SPSS.43 The significance of the indirect or mediated effect was assessed using 10,000 bootstrapping to obtain estimates (β value) and CIs. The indirect effect was considered significant if the 95% CI did not include zero. The detailed information of the mediation model is provided in the eMethods (links.lww.com/WNL/C459).
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Participant Characteristics
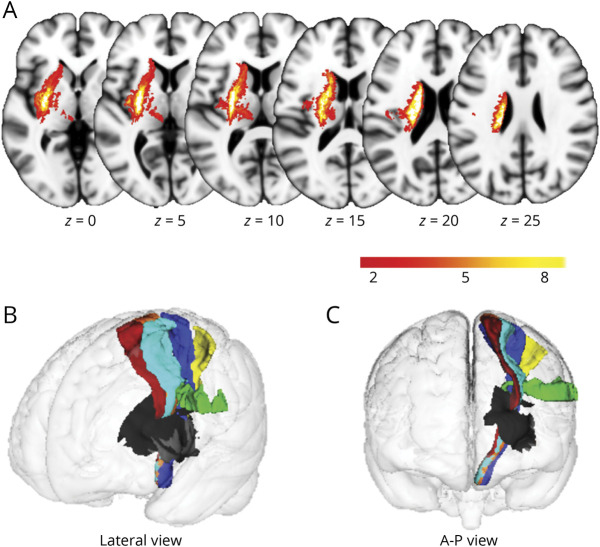
Participants included 35 patients with unilateral subcortical stroke and 43 healthy controls. The group mean scores of FM-UE were 16.29 ± 10.96, and the mean time from stroke onset was 10.90 ± 7.94 months. There was no significant difference in age between the 2 groups (52.74 ± 10.21 years for patients; 56.23 ± 9.57 years for controls; p = 0.125), whereas sex showed a significant difference (27 males and 8 females for patients; 21 males and 22 females for controls; p = 0.019). Demographic and clinical characteristics of the participants are presented in Table 1. The lesion incidence map of the 37 patients with stroke is shown in Figure 2A. The stroke lesions were primarily located in the basal ganglia, internal capsule, thalamus, and corona radiata. The positional relationship between the lesions and subpathways of the CSTs is shown in Figure 2B and C.
Table 1.
Demographic and Clinical Characteristics of the Participants
Figure 2. Lesion Map.
(A) Overlapped lesion map of 35 patients. The color bar represents the number of patients with a lesion in a specific voxel. Left side indicates the left hemisphere. Positional relationship between the lesions and CSTs from lateral view (B) and from anterior to posterior (A-P) view (C) is also shown. The black region represents lesions, and the colored fibers represent the CSTs originating from different sensorimotor areas. Blue, primary motor cortex; cyan, dorsal premotor cortex; green, ventral premotor cortex; yellow, primary somatosensory cortex; orange, supplementary motor area; red, presupplementary motor area. Z values denote MNI coordinates. CST = corticospinal tract; MNI = Montreal Neurological Institute.
Structural Damage of the Specific Pathways of the CST (CC) in Patients With Stroke
Compared with the healthy controls, FAasymmetry of all 6 subpathways of the CST originating from the M1, PMd, PMv, S1, SMA, and preSMA was significantly increased in the stroke group (p < 0.05, Bonferroni corrected). The higher FAasymmetry meant more serious CST structural damage (eResults, eFigure 3A, and eTable 2, links.lww.com/WNL/C459).
FA values of 6 CC sections connecting the bilateral subareas of the SMC were significantly reduced after stroke compared with the healthy controls (p < 0.05, Bonferroni corrected). The higher FA values, the better the structural integrity of the CC (eResults, eFigure 3B, and eTable 3, links.lww.com/WNL/C459).
FC Abnormalities Related to the Specific Pathways of the CST (CC) in Patients With Stroke
FC of the 6 ipsilesional SMC subareas changed in multiple regions after stroke, including motor and nonmotor cortices, basal ganglia regions, and the cerebellum in patients with stroke (eResults, eFigure 3C, and eFigure 4, links.lww.com/WNL/C459). The detailed information on the involved brain regions is summarized in eTable 4 and eTable 5. Meanwhile, interhemispheric FC between bilateral homotopic SMC subareas (M1, PMd, PMv, S1, and SMA) was also disrupted (eResults, eFigure 3D, and eTable 6).
Pathway-Specific Covariations of CSTs and FC in Patients With Stroke
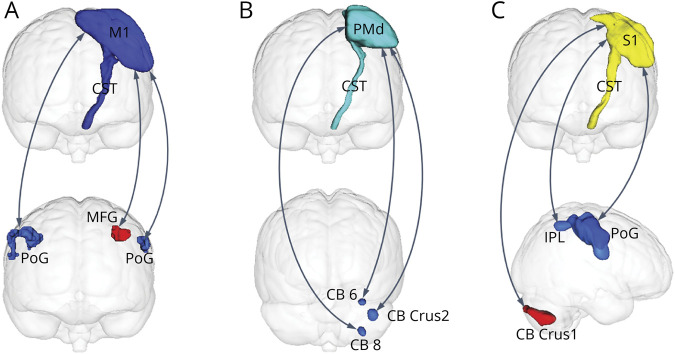
The covaried structural-functional pathways related to the M1, S1, and PMd were discovered in patients with stroke (pFWE < 0.05, TFCE). Specifically, the decreased FC of the ipsilesional M1 with the bilateral postcentral gyrus (for the contralesional postcentral gyrus, r = −0.530, p = 0.002; for the ipsilesional postcentral gyrus, r = −0.661, p = 3.8 × 10−5) and the increased FC of the ipsilesional M1 with the ipsilesional middle frontal gyrus (r = 0.502, p = 0.003) showed significant correlations with the damage degree of the CST originating from the M1; the decreased FC of the ipsilesional PMd with the contralesional cerebellum crust 2 (r = −0.517, p = 0.002), cerebellum 6 (r = −0.522, p = −0.002), and cerebellum 8 (r = −0.437, p = 0.006) exhibited significant correlations with the damage degree of the CST originating from the PMd; the decreased FC of the ipsilesional S1 with the contralesional postcentral gyrus (r = −0.556, p = 0.002), inferior parietal lobule (r = −0.522, p = 0.002), and increased FC of the ipsilesional S1 with the contralesional cerebellum crus 1 (r = 0.614, p = 1.9 × 10−4) displayed significant correlations with the damage degree of the CST originating from the S1 (Figure 3 and eFigure 5, links.lww.com/WNL/C459). The detailed information of altered FC is shown in eTable 7.
Figure 3. Pathway-Specific Covariations of CSTs and FC in Patients With Stroke.
Pathway-specific covariations of CSTs and FC originating from the ipsilesional (left) M1 (A), PMd (B), and S1 (C), respectively (p < 0.05, corrected). The double arrow lines denote altered functional connections. Blue clusters indicate with which regions the FC decreased, and red clusters indicate with which regions the FC increased. CB = cerebellum; CST = corticospinal tract; FC = functional connectivity; IPL = inferior parietal lobe; M1 = primary motor cortex; MFG = middle frontal gyrus; PoG = postcentral gyrus; PMd = dorsal premotor cortex; S1 = primary somatosensory cortex; IPL = inferior parietal lobe.
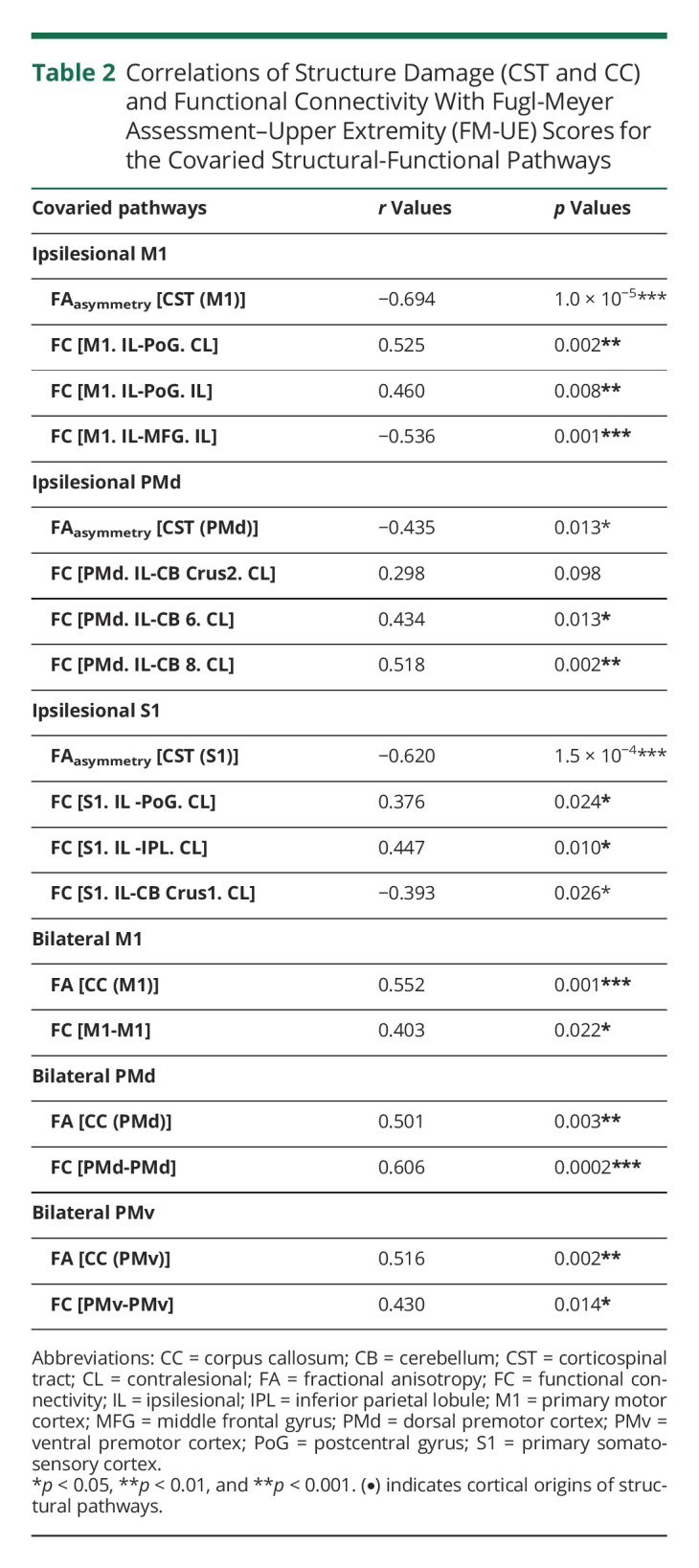
The damage degree of the CST and abnormalities of FC in the covaried structural-functional pathways related to the ipsilesional M1, PMd, and S1 significantly correlated with the FM-UE scores. The associations between the structural-functional pathways of CSTs and FM-UE are provided in Table 2.
Table 2.
Correlations of Structure Damage (CST and CC) and Functional Connectivity With Fugl-Meyer Assessment–Upper Extremity (FM-UE) Scores for the Covaried Structural-Functional Pathways
Pathway-Specific Covariations of CC Sections and Interhemispheric FC in Patients With Stroke
The microstructural integrity of the CC and FC between the bilateral M1 (r = 0.476, p = 0.006), PMd (r = 0.491, p = 0.004), and PMv (r = 0.528, p = 0.002) exhibited significantly positive correlations, whereas the integrity of the CC and FC of the bilateral S1 and bilateral SMA was not significant (Figure 4 and eFigure 6, links.lww.com/WNL/C459). We set the significance threshold at p < 0.05, Bonferroni corrected for 6 comparisons.
Figure 4. Pathway-Specific Covariations of CC Sections and Interhemispheric FC in Patients With Stroke.
Pathway-specific covariations of CC sections and FC between the bilateral M1 (A), PMd (B), and PMv (C), respectively (p < 0.05, corrected). The double arrow lines denote altered functional connections. The blue squares indicate the target areas of the CC sections that were extracted to describe the integrity of the CC. CC = corpus callosum; M1 = primary motor cortex; PMd = dorsal premotor cortex; PMv = ventral premotor cortex.
The integrity of the CC and the interhemispheric FC connecting the bilateral M1, PMd, and PMv was positively correlated with the FM-UE scores. The correlations of the structural-functional pathways of the CC and the FM-UE scores are provided in Table 2.
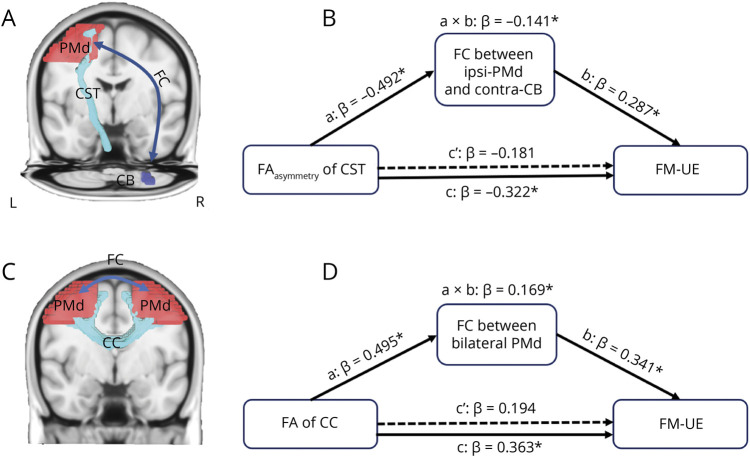
Pathway-Specific Mediation Effect Between Structural Damage, FC Abnormality, and Motor Impairment
For the identified structural-functional covaried pathways, which showed significant correlation with motor impairment, the indirect effect of the structural damage of the CST to FM-UE through the FC abnormality was tested. We found that only the indirect coefficient for the FC between the ipsilesional PMd and contralesional cerebellum 8 was significant (β = −0.141, p < 0.05, CI = [−0.319 to −0.015]) (changes of CI do not contain 0), with total effect c (β = −0.322, p = 0.013, CI = [−0.571 to −0.074]) being significant and direct effect c’ not being significant (β = −0.181, p = 0.172, CI = [−0.446 to 0.083]), which means that when the intermediary variable of the FC is placed, the direct predictive effect of the structural integrity of the CST originating from the PMd on the FM-UE scores is no longer significant, whereas the intermediary effect of the FC is significant. This indicates that the structural damage of the CST originating from the PMd predicts FM-UE scores through the mediating effect of the FC between the ipsilesional PMd and contralesional cerebellum 8 area (Figure 5 A and B). Similar to the CST pathway, we also found that the indirect coefficient for the FC between the bilateral PMd was significant (β = 0.169, p < 0.05, CI = [0.015–0.391]), with significant total effect for the integrity of the CC connecting the bilateral PMd (β = 0.363, p = 0.003, CI = [0.129–0.596]) and no significant direct effect c’(β = 0.194, p = 0.108, CI = [−0.046 to 0.434]) (Figure 5, C–D).
Figure 5. Pathway-Specific Mediation Effects.
(A and B) FC between the ipsilesional PMd and contralesional cerebellum 8 area significantly mediates the relationship between structural damage of the CST and motor impairment. Structural damage of the CST was estimated by FAasymmetry of the CST. (C and D) FC between the bilateral PMd significantly mediates the relationship between the structural integrity of the CC and motor impairment. The structural integrity of the CC was estimated using the FA value of the CC. Path a: the effect of structural damage on altered functional connectivity; Path b: the effect of structural damage on motor impairment; Path c’: the direct effect of structural damage on the outcome after controlling for mediator; a × b effect, referred to as the indirect effect of mediator. The total effect (c) is the sum of the indirect effect (a × b) and the direct effect (c’). β denotes the beta value. *p < 0.05, dashed lines indicate nonsignificant paths. CC = corpus callosum; contra-CB = contralesional cerebellum 8; CST = corticospinal tract; FA = fractional anisotropy; FC = functional connectivity; FM-UE = Fugl-Meyer Assessment–Upper Extremity; ipsi-PMd = ipsilesional dorsal premotor cortex.
Discussion
One of the main challenges in systems neuroscience is to understand the relationship between structure and function. Clinically, knowledge of the correspondence between structural and functional changes resulting from brain diseases and their causal influence on clinical symptom remains scarce. To better parse these relationships, in this study, we systematically examined pathway-specific covariations of structure and function induced by focal subcortical stroke based on fine-grained CST/CC maps. Furthermore, the pathway-specific mediation effect among structural damage, functional abnormality, and motor impairment was tested.
We found 4 subregions of the SMC (i.e., M1, S1, PMd, and PMv) showing pathway-specific structure-function covariations in patients with stroke. Furthermore, only the M1 and PMd had both functionally relevant covarying CST and CC pathways. More importantly, the FC between the ipsilesional PMd and contralesional cerebellum and the interhemispheric FC between the bilateral PMd showed significant mediation effects on motor impairment. Accumulating evidence has indicated that higher-order motor areas, especially the contralesional PMd, play an important compensatory role in the motor recovery of patients with stroke with severe impairment.44,45 It is because that the M1 becomes less effective in motor execution with increasing CST damage. The PMd is reciprocally connected with the ipsilateral and contralateral cortical motor areas, and the contralesional PMd likely exerts an increasing influence on the ipsilesional SMC to support motor function in patients with stroke with more impairment.28 Clinically, recent studies have consistently suggested that neuromodulation to the PMd may subserve motor recovery for patients with stroke with more severe impairment,28,46 whereas the M1 has been shown to be effective for mildly impaired individuals.29,31 Therefore, our findings drawn from mediation analysis may offer a circuit-based evidence for explaining the PMd as a critical neuromodulation target for more impaired patients with stroke.
In addition, previous studies have indicated that the cerebellum has vast connections to interact with cortical motor areas and is involved in monitoring and optimizing movements through sensory feedback.47,48 Convergent evidence from healthy subjects emphasizes the potential of cerebellar noninvasive neuromodulation for enhancement of motor functions.47,49 Consistently, our result further suggests that the contralesional cerebellum might be an alternative promising therapeutic target for patients with stroke. Based on the understanding of the underlying circuit mechanism, in the future, novel stimulation protocols such as multifocal brain stimulation can be designed toward stratified intervention and maximization of functional recovery for patients with stroke.
In addition, although noninvasive brain stimulation has been shown to be effective for various neuropsychiatric diseases including stroke, therapeutic mechanisms remain largely unclear. One major obstacle may come from the complicated brain connections, especially widespread polysynaptic connections. Fortunately, convergent evidence has indicated that effective targets used to treat the same disease most often are nodes in the same brain network identified by resting-state FC.50 Specifically, a previous study of focal pontine lesions has provided direct evidence that cerebrocerebellar FC reflects polysynaptic anatomic pathways.51 Moreover, emerging evidence has indicated that neurostimulation of the cerebellum can modulate cerebellar cortical interactions.47 Therefore, noninvasive brain stimulation (TMS/tDCS) would be effective for polysynaptic connections, and stimulation-induced change of specific FC probably severs as a biomarker of therapeutic efficacy.
However, it should be noted that low spatial resolution (on the order of centimeters) is a common drawback for the noninvasive brain stimulation such as TMS and tDCS. In general, localization of the M1 is relatively easy, which can be verified through the evoked instantaneous movement. Although it is challengeable to precisely localize the subregions of the SMC, several studies have tested the target of the PMd using TMS.28,29,31 To further improve the localization of SMC subregions and validate previous findings, a real-time navigation with high-resolution structural MRI52 or an alternative promising noninvasive neurostimulation technique (i.e., transcranial focused ultrasound, on the order of millimeters) with relatively high spatial resolution53 can be considered for future research.
Although many pathway-specific structure-function covariations (i.e., originating from the M1, S1, and PMv) did not show a causal effect on motor impairment, our findings provided a complete, fine-grained relationship between structural and functional pathways disrupted by stroke. A previous study of patients with stroke with good motor outcomes has exclusively focused on the M1 and reported that the structural integrity of the affected CST positively correlated with anatomic connectivity between the bilateral M1 and negatively correlated with FC between the bilateral M1.20 In line with the previous study, our result revealed that the structural integrity of CSTs and CC sections was positively correlated for all SMC origins including the M1 (eTable 8, links.lww.com/WNL/C459). Differently, we calculated the FC of the M1 with the rest of the brain in a voxelwise manner, rather than the FC between the bilateral M1 exclusively. Accordingly, we found that the structural integrity of the affected CST fibers originating from the M1 was positively correlated with the FC of the M1 and the bilateral postcentral gyrus and negatively correlated with the FC of the M1 and the middle frontal gyrus. We speculate that the inconsistency of covaried FC may be due to the varying degree of impairment in the patients included. The patients enrolled in the current study were more impaired showing a breakdown of FC within the sensorimotor network and an increase of FC between the M1 and higher-order frontal area, whereas well-recovered patients might be supported by enhanced FC between the bilateral M1. Together, these pathway-specific structure-function covariations may allow to explain why the M1 is not an ideal target for more impaired patients with stroke with greater CST disruption. However, whether these pathway-specific structure-function covariations would selectively show a mediation effect on motor deficits in patients with varying damage degree needs to be verified in future studies.
There are several limitations to the present study that should be noted. First, a cross-sectional design was adopted to reveal the mediation effect between damaged structure, abnormal function, and motor outcome. To ensure the stabilities of the mediation effects and variability following recovery, longitudinal studies are warranted in the future. In addition, although other more complicated mediation models are possible, such as moderated mediating model (with other moderator variables) and multiple-step multiple mediator model (mediators are allowed to causally affect other mediators), they do not fit our hypothesis very well. If other moderators or potential interactions between different mediators can be proposed reasonably, corresponding models would be tested in future studies. Second, only patients with moderate-severe stroke were enrolled in this study. It would be possible to compare the functional and structural relationships with different levels of motor deficits, which would be conducive to more effective rehabilitation of patients with severe stroke. Third, to reduce the mixed effect, we only selected patients with stroke with focal lesions in the subcortical ganglia. Future studies should also investigate patients with different lesion locations to discern the diversities of the functional mediation effects and to explore more structure-function covariations of specific pathways.
In conclusion, this work revealed pathway-specific structure-function covariations based on fine-grained CST/CC maps. Moreover, the causal effects of functional connections between the ipsilesional PMd and contralateral cerebellum and between the bilateral PMd on the relationship between structural damage and motor were identified through the mediation model. This study provided a promising way to investigate how specific FC disruption mediates the association between structural damage and clinical symptom and further deepened our understanding of the neuroreorganization mechanism of stroke. Clinically, the cortex with functionally mediating effect possibly serves as effective intervention targets for patients with chronic stroke, especially for those with severe structural pathway injury.
Glossary
- CC
corpus callosum
- contra-CB
contralesional cerebellum 8
- CST
corticospinal tract
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FC
functional connectivity
- FM-UE
Fugl-Meyer Assessment–Upper Extremity
- FWE
familywise error
- IPL
inferior parietal lobe
- ipsi-PMd
ipsilesional dorsal premotor cortex
- M1
primary motor cortex
- MFG
middle frontal gyrus
- MNI
Montreal Neurological Institute
- PMd
dorsal premotor cortex
- PMv
ventral premotor cortex
- PoG
postcentral gyrus
- preSMA
presupplementary motor area
- S1
primary somatosensory cortex
- SMA
supplementary motor area
- SMATT
sensorimotor area tract template
- SMC
sensorimotor cortex
- TFCE
threshold-free cluster enhancement
- TMS
transcranial magnetic stimulation
- tDCS
transcranial current stimulation
Appendix. Authors
Footnotes
Editorial, page 271
Study Funding
This work was supported by the National Natural Science Foundation of China (81471651, 11835003, and 31600869).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Krakauer JW. Arm function after stroke: from physiology to recovery. Semin Neurol. 2005;25(4):384-395. doi: 10.1055/s-2005-923533 [DOI] [PubMed] [Google Scholar]
- 2.Li J, Wei XH, Liu YK, et al. Evidence of motor injury due to damaged corticospinal tract following acute hemorrhage in the basal ganglia region. Sci Rep. 2020;10(1):16346. doi: 10.1038/s41598-020-73305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeng J, Zheng P, Xu J, et al. Prediction of motor function by diffusion tensor tractography in patients with basal ganglion haemorrhage. Arch Med Sci. 2011;2(2):310-314. doi: 10.5114/aoms.2011.22083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindenberg R, Renga V, Zhu LL, Betzler F, Alsop D, Schlaug G. Structural integrity of corticospinal motor fibers predicts motor impairment in chronic stroke. Neurology. 2010;74(4):280-287. doi: 10.1212/wnl.0b013e3181ccc6d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang LE, Tittgemeyer M, Imperati D, et al. Degeneration of corpus callosum and recovery of motor function after stroke: a multimodal magnetic resonance imaging study. Hum Brain Mapp. 2012;33(12):2941-2956. doi: 10.1002/hbm.21417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhadelia RA, Price LL, Tedesco KL, et al. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke. 2009;40(12):3816-3820. doi: 10.1161/strokeaha.109.564765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236-246. doi: 10.1002/ana.21228 [DOI] [PubMed] [Google Scholar]
- 8.Zheng X, Sun L, Yin D, et al. The plasticity of intrinsic functional connectivity patterns associated with rehabilitation intervention in chronic stroke patients. Neuroradiology. 2016;58(4):417-427. doi: 10.1007/s00234-016-1647-4 [DOI] [PubMed] [Google Scholar]
- 9.Tang C, Zhao Z, Chen C, et al. Decreased functional connectivity of homotopic brain regions in chronic stroke patients: a resting state fMRI study. PLoS One. 2016;11(4):e0152875. doi: 10.1371/journal.pone.0152875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Z, Wu J, Fan M, et al. Altered intra- and inter-network functional coupling of resting-state networks associated with motor dysfunction in stroke. Hum Brain Mapp. 2018;39(8):3388-3397. doi: 10.1002/hbm.24183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin D, Song F, Xu D, et al. Altered topological properties of the cortical motor-related network in patients with subcortical stroke revealed by graph theoretical analysis. Hum Brain Mapp. 2014;35(7):3343-3359. doi: 10.1002/hbm.22406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misic B, Betzel RF, de Reus MA, et al. Network-level structure-function relationships in human neocortex. Cereb Cortex. 2016;26(7):3285-3296. doi: 10.1093/cercor/bhw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342(6158):1238411. doi: 10.1126/science.1238411 [DOI] [PubMed] [Google Scholar]
- 14.Goni J, van den Heuvel MP, Avena-Koenigsberger A, et al. Resting-brain functional connectivity predicted by analytic measures of network communication. Proc Natl Acad Sci USA. 2014;111(2):833-838. doi: 10.1073/pnas.1315529111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72-78. doi: 10.1093/cercor/bhn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Dai Z, Gong G, Zhou C, He Y. Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist. 2015;21(3):290-305. doi: 10.1177/1073858414537560 [DOI] [PubMed] [Google Scholar]
- 17.Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage. 2008;39(3):1370-1382. doi: 10.1016/j.neuroimage.2007.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newton JM, Ward NS, Parker GJM, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas--relevance to stroke recovery. Brain. 2006;129(7):1844-1858. doi: 10.1093/brain/awl106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffis JC, Metcalf NV, Corbetta M, Shulman GL. Structural disconnections explain brain network dysfunction after stroke. Cel Rep. 2019;28(10):2527-2540 e9. doi: 10.1016/j.celrep.2019.07.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Qin W, Zhang J, Zhang X, Yu C. Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke. 2015;46(4):1045-1051. doi: 10.1161/strokeaha.114.007044 [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Liu J, Hua M, Liang M, Yu C. Enhanced effective connectivity from ipsilesional to contralesional M1 in well-recovered subcortical stroke patients. Front Neurol. 2019;10:909. doi: 10.3389/fneur.2019.00909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11(3):667-689. doi: 10.1523/jneurosci.11-03-00667.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito KL, Kim B, Liu JC, et al. Corticospinal tract lesion load originating from both ventral premotor and primary motor cortices are associated with post-stroke motor severity. Neurorehabil Neural Repair. 2022;36(3):179-182. doi: 10.1177/15459683211068441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boccuni L, Meyer S, D'Cruz N, et al. Premotor dorsal white matter integrity for the prediction of upper limb motor impairment after stroke. Sci Rep. 2019;9(1):19712. doi: 10.1038/s41598-019-56334-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Wang C, Qin W, et al. Corticospinal fibers with different origins impact motor outcome and brain after subcortical stroke. Stroke. 2020;51(7):2170-2178. doi: 10.1161/strokeaha.120.029508 [DOI] [PubMed] [Google Scholar]
- 26.Tang Q, Li G, Liu T, et al. Modulation of interhemispheric activation balance in motor-related areas of stroke patients with motor recovery: systematic review and meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2015;57:392-400. doi: 10.1016/j.neubiorev.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 27.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions onMotor function inChronic stroke. Ann Neurol. 2004;55(3):400-409. doi: 10.1002/ana.10848 [DOI] [PubMed] [Google Scholar]
- 28.Bestmann S, Swayne O, Blankenburg F, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci. 2010;30(36):11926-11937. doi: 10.1523/jneurosci.5642-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Lin YL, Cunningham DA, et al. Repetitive transcranial magnetic stimulation of the contralesional dorsal premotor cortex for upper extremity motor improvement in severe stroke: study protocol for a pilot randomized clinical trial. Cerebrovasc Dis. 2022;51(5):557-564. doi: 10.1159/000521514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leong JK, Pestilli F, Wu CC, Samanez-Larkin GR, Knutson B. White-matter tract connecting anterior insula to nucleus accumbens correlates with reduced preference for positively skewed gambles. Neuron. 2016;89(1):63-69. doi: 10.1016/j.neuron.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Wang H, Yuan Y, et al. Effects of high frequency rTMS of contralesional dorsal premotor cortex in severe subcortical chronic stroke: protocol of a randomized controlled trial with multimodal neuroimaging assessments. BMC Neurol. 2022;22(1):125. doi: 10.1186/s12883-022-02629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woytowicz EJ, Rietschel JC, Goodman RN, et al. Determining levels of upper extremity movement impairment by applying a cluster Analysis to the Fugl-Meyer Assessment of the upper extremity in chronic stroke. Arch Phys Med Rehabil. 2017;98(3):456-462. doi: 10.1016/j.apmr.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97-113. doi: 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 34.NeuroImaging Teams & Resources Collaboratory. MRIcron. nitrc.org/projects/mricron
- 35.Nijboer TCW, Buma FE, Winters C, et al. No changes in functional connectivity during motor recovery beyond 5 weeks after stroke; A longitudinal resting-state fMRI study. PLoS One. 2017;12(6):e0178017. doi: 10.1371/journal.pone.0178017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31(4):1453-1474. doi: 10.1016/j.neuroimage.2006.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Archer DB, Vaillancourt DE, Coombes SA. A template and probabilistic atlas of the human sensorimotor tracts using diffusion MRI. Cereb Cortex. 2018;28(5):1685-1699. doi: 10.1093/cercor/bhx066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Archer DB, Coombes SA, McFarland NR, DeKosky ST, Vaillancourt DE. Development of a transcallosal tractography template and its application to dementia. Neuroimage. 2019;200:302-312. doi: 10.1016/j.neuroimage.2019.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Human Connectome Project website. humanconnectomeproject.org
- 40.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537-541. doi: 10.1002/mrm.1910340409 [DOI] [PubMed] [Google Scholar]
- 41.Index of /tfce. dbm.neuro.uni-jena.de/tfce/
- 42.van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J Neurosci. 2014;34(31):10298-10310. doi: 10.1523/jneurosci.4105-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach . : Guilford Press; 2013. [Google Scholar]
- 44.Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99(22):14518-14523. doi: 10.1073/pnas.222536799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plow EB, Cunningham DA, Varnerin N, Machado A. Rethinking stimulation of the brain in stroke rehabilitation: why higher motor areas might be better alternatives for patients with greater impairments. Neuroscientist. 2015;21(3):225-240. doi: 10.1177/1073858414537381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunningham DA, Varnerin N, Machado A, et al. Stimulation targeting higher motor areas in stroke rehabilitation: a proof-of-concept, randomized, double-blinded placebo-controlled study of effectiveness and underlying mechanisms. Restorative Neurol Neurosci. 2015;33(6):911-926. doi: 10.3233/rnn-150574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wessel MJ, Hummel FC. Non-invasive cerebellar stimulation: a promising approach for stroke recovery? Cerebellum. 2018;17(3):359-371. doi: 10.1007/s12311-017-0906-1 [DOI] [PubMed] [Google Scholar]
- 48.Jueptner M, Weiller C. A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain. 1998;121(8):1437-1449. doi: 10.1093/brain/121.8.1437 [DOI] [PubMed] [Google Scholar]
- 49.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29(28):9115-9122. doi: 10.1523/jneurosci.2184-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA. 2014;111(41):E4367-E4375. doi: 10.1073/pnas.1405003111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu J, Liu H, Zhang M, et al. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci. 2011;31(42):15065-15071. doi: 10.1523/jneurosci.2364-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58(4):208-213. doi: 10.1016/j.rehab.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 53.Kubanek J. Neuromodulation with transcranial focused ultrasound. Neurosurg Focus. 2018;44(2):E14. doi: 10.3171/2017.11.focus17621 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.