Abstract
Background and Objectives
Antitumor necrosis factor α (TNFα) agents are a class of biologic drugs used for the treatment of several immune-mediated conditions. An increased risk of multiple sclerosis (MS) with their use has been suggested, but studies have been limited. Relevant population-based epidemiologic data linking anti-TNFα to MS are scarce. The objective was to compare the risk of MS in anti-TNFα users with nonusers among patients with rheumatic disease (RD) or inflammatory bowel disease (IBD).
Methods
A nested case-control study was conducted. Population-based health care–linked databases from 4 Canadian provinces were used. All patients with RD or IBD residing within a participating province between January 2000 and March 2018 were identified by validated case definitions. Any anti-TNFα dispensation in the 2 years before the index date (MS onset) was identified. Incident onset MS cases were ascertained using a validated algorithm. Up to 5 controls were matched to each MS case based on birth year ±3 years, disease duration, and health authority (based on region of residence). Conditional logistic regressions were used to calculate the incidence rate ratio (IRR) after adjusting for potential confounders. A meta-analysis was conducted to provide pooled estimates across provinces using random-effects models.
Results
Among 296,918 patients with RD patients, 462 MS cases (80.1% female, mean [SD] age, 47.4 [14.6] years) were matched with 2,296 controls (59.5% female, mean [SD] age, 47.4 [14.5] years). Exposure to anti-TNFα occurred in 18 MS cases and 42 controls. After adjusting for matching variables, sex, and the Charlson Comorbidity Index, the pooled IRR was 2.05 (95% CI, 1.13–3.72). Among 84,458 patients with IBD, 190 MS cases (69.5% female, mean [SD] age, 44.3 [12.3] years) were matched with 943 controls (54.1% female, mean [SD] age, 44.2 [12.2] years). Exposure to anti-TNFα occurred in 23 MS cases and 98 controls. The pooled adjusted IRR was 1.35 (95% CI, 0.70–2.59).
Discussion
The use of anti-TNFα was associated with an increased risk of MS compared with nonusers, especially among patients with RD. These findings could help clinicians and patients with RD to make more informed treatment decisions. Further studies are needed to validate these results for patients with IBD.
Multiple sclerosis (MS) is one of the world's most common neurologic disorders,1 affecting an estimated 2.8 million people worldwide in 2020.2 Antitumor necrosis factor α (TNFα) agents are a class of biologic drugs used for the treatment of several chronic immune-mediated inflammatory diseases such as moderate to severe rheumatic diseases (RDs) [rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis] and inflammatory bowel diseases (IBDs). Although anti-TNFα agents are generally well-tolerated and have been shown to significantly improve patients' quality of life,3-6 an increased risk of MS has been suspected after their use.7-11
One of the first studies that postulated an association with MS pathogenesis was a randomized controlled trial that was stopped early because of an increased MS exacerbation rate among lenercept-treated patients.12 Epidemiologic data suggesting anti-TNFα use may trigger new onset MS are scarce and contradictory,13-17 mainly because of potential biases such as sparse data bias.16 A 2020 study found that the use of anti-TNFα was associated with an increased risk of overall inflammatory demyelinating events among patients with RD or IBD receiving medical care at 3 Mayo Clinics, USA.13 However, MS was not studied specifically because of the study's small sample size. The use of data from a tertiary referral center also limits its generalizability to the general population.
With the increase in the incidence and prevalence of both RD and IBD worldwide,18-21 the number of anti-TNFα users is also expected to increase. A potential risk of MS among users of anti-TNFα, a drug class for which there might be other safer alternatives (e.g., emerging biologics), could further increase the burden of disease in patients already afflicted with a moderate to severe chronic disease. We aimed to quantify the risk of MS in anti-TNFα users with RD and IBD using population-based health administrative databases from 4 Canadian provinces.
Methods
Data Sources
This study was undertaken in 4 western Canadian provinces: British Columbia, Alberta, Saskatchewan, and Manitoba, which collectively encompassed over 11 million people, nearly one-third of Canada's population. Each province captures nearly 100% of registered residents and their health-related information through comprehensive population-based linked databases. Personal identifiers are used to link records belonging to the same individual across files and over time. Canadian provinces administer publicly funded, universally available health care systems and maintain computerized records related to the provision of these services. These records capture all physician visits,22 hospitalizations,23 demographic data,24 and all outpatient or community dispensations of prescription medication.25 Numerous population-based studies have been successfully conducted using these data sources.26-29 An overview of each province's databases is presented in eTable 1 (links.lww.com/WNL/C454). Data for RD and IBD cohorts within each province are saved in different server and can only be analyzed in-house.
Study Design
We undertook 2 nested case-control (NCC) studies for patients with RD and IBD age18 years or older, separately, in each province between January 2000 and March 2018. The NCC has been deemed the ideal design for drug safety studies in large populations with long follow-ups because it mitigates some of the complexities that might arise when large cohorts are followed for a long period.30 Cohort studies require much larger sample sizes when the outcome is rare.30
Cohort Definition
Owing to data availability, RD cohorts were available in 2 provinces, British Columbia and Manitoba; each province used its internally validated case definition that has been used previously to identify persons with rheumatoid arthritis (ICD-9 714.X),26,28 ankylosing spondylitis (ICD-9 720.X; ICD-10 M45.X),31 and psoriatic diseases (ICD-9 696.X; ICD-10 L40.X).32 Case definitions for RD have a positive predictive value (PPV) of up to 82%26,28 and are presented in eTable2 (links.lww.com/WNL/C454).
IBD cohorts were identified in the 4 provinces, selected within each province by internally validated case definitions, using ICD-9 555, 556; ICD-10 K50, K51 (see eTable 2 for details, links.lww.com/WNL/C454). Case definitions for IBD have a PPV of up to 97%.33,34
The date after meeting the validated case definition of RD or IBD was defined as the cohort entry date. After identifying the disease cohorts, patients with previous diagnostic codes for MS or any demyelinating events were excluded (the relevant codes are listed below under “Case and Control Definition”). Patients were followed from the cohort entry date to (1) death, (2) MS onset, (3) termination of health coverage, or (4) last date of available data.
Case and Control Definition
Cases were identified separately for the RD and IBD cohorts in each province between January 2000 and March 2018 and were ascertained using a previously validated and successfully applied algorithm using health administrative data.29 A MS case was defined as a subject who had at least 3 records related to MS from physician visits (ICD-9 340), hospitalizations (ICD-9 340 or ICD10 G35), or prescription claims specific for MS (eTable 3, links.lww.com/WNL/C454) in any combination using all available data. This algorithm has a PPV of 99.5%.29 The date of the first ICD-9/10 code for MS or the first code for a demyelinating event (optic neuritis [377.3/H46], acute transverse myelitis [323.82/G37], acute disseminated encephalomyelitis [323/G36.9], demyelinating disease of CNS unspecified [341.9/G37.8], acute disseminated demyelination [G36], or neuromyelitis optica [341.0/G36.0]) was deemed the index date (MS onset). To ensure MS cases were incident, we required all newly diagnosed MS individuals to have at least 5 years of prior registration in the databases (i.e., “run-in” period) before the index date. The case definition also required patients without having a diagnosis of MS or demyelinating event ever before the index date.
Controls were selected from the RD or IBD cohorts using a density-based sampling algorithm.30 First, a risk set of all patients with RD or IBD with new onset MS (cases) and their corresponding controls was created. For each case, a pool of controls was identified as all with RD or IBD who had no prior record of MS or a related demyelinating event or prescription filled for an MS drug (eTable 3, links.lww.com/WNL/C454) at the index date. Because there is usually little marginal increase in precision from increasing the ratio of controls to cases beyond 4,35 from the potential pool of controls, each MS case was matched to up to 5 controls based on the following criteria: (1) birth year ± 3 years; (2) the same RD or IBD disease duration, thereby controlling for the calendar time bias36; and (3) the same health authority based on each individual's place of residence to ensure that a specific geographic location does not differentially affect anti-TNFα prescribing between cases and controls. Controls were assigned the same index date as their matched case. The density-based sampling approach for control selection has been shown to generate an OR that closely approximates the incidence rate ratio (IRR) derived from a cohort study.30
Exposure Assessment
All anti-TNFα drugs approved by Health Canada for the treatment of RD or IBD and dispensed in 2 years before the index date (MS onset) were identified including, adalimumab, certolizumab, etanercept, infliximab, and golimumab (eTable 4, links.lww.com/WNL/C454). Because the risk of MS with anti-TNFα has been reported to occur across a variable time frame (between 2 to 24 months between the initiation of anti-TNFα and onset of MS),11 we considered different risk periods in relation to anti-TNFα use. For the main analysis, a 2-year exposure assessment period of anti-TNFα use was examined. The use of 2 years of exposure assessment period may better capture the latency of MS onset. We also examined the risk of anti-TNFα use on MS during a 1-year period as a sensitivity analysis because most patients developed MS within 1 year or less after the therapy initiation.11 The use of anti-TNFα during the exposure assessment period was represented as a binary variable (exposed/unexposed). Specifically, users were defined as individuals with at least 1 prescription filled for an anti-TNFα agent during the exposure assessment period. The reference comparison group was patients who have not filled a prescription for an anti-TNFα agent in any of the risk periods.
Covariates
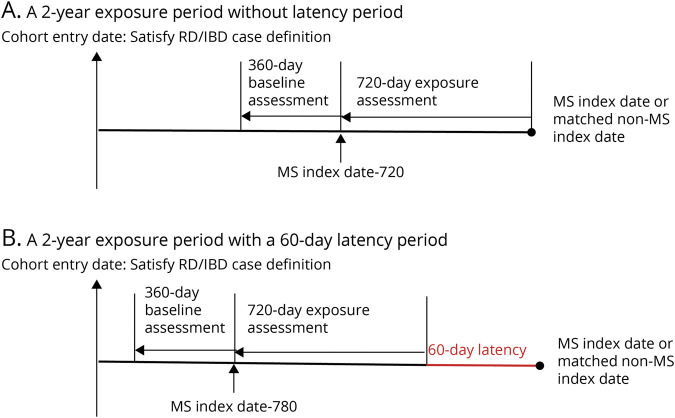
All baseline covariates were measured during 360 days preceding the 2 years exposure assessment period (Figure 1A) to avoid overadjustment bias (i.e., adjusting for mediator variables).37 In addition to matching variables, the following covariates were considered: sex, number of physician visits and hospitalizations, the Charlson Comorbidity Index (CCI),38 and any dispensations for oral glucocorticoids, prescribed nonsteroidal anti-inflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), or immunosuppressant drugs. Causal direct acyclic graphs (cDAGs) were used to select confounding variables for model adjustments (eFigure 1, links.lww.com/WNL/C454).
Figure 1. Schematic Representation of the Study Design.
After satisfying the case definition of rheumatic diseases (RDs) or inflammatory bowel diseases (IBDs), patients were followed until (1) death, (2) multiple sclerosis (MS) onset, (3) termination of health coverage, or (4) last date of available data. Among cases and matched general population controls, all antitumor necrosis factor α (TNFα) drugs approved by Health Canada for the treatment of RD or IBD and dispensed in 2 years before the index date were identified (A). To account for the latency of MS, a 60-day latency period was applied where the exposure assessment period was pushed back by 60 days (B). Baseline covariates were measured during the 360 days preceding the 2-year exposure assessment period.
Statistical Analysis
Baseline covariates between patients with MS and controls were compared using descriptive statistics. A conditional logistic regression model was used to obtain IRRs of MS among users and nonusers of anti-TNFα. Based on the cDAGs (eFigure 1, links.lww.com/WNL/C454), in addition to matching variables, sex and CCI were further adjusted. Interaction effect of sex was evaluated by adding an interaction term anti-TNFα × sex in the conditional logistic regression model.
Owing to provincial data privacy mandates, we analyzed RD and IBD cohorts, separately, in each province. Then, a meta-analysis was conducted to obtain the pooled estimates across provinces using random-effects models. A test of heterogeneity using Cochrane Q statistic was performed,39 which describes the percentage of total variation across effect sizes because of heterogeneity rather than chance. An alpha level of p ≤ 0.05 was used to reject the null hypothesis that the IRRs were statistically the same across all provinces. We calculated the number needed to harm (NNH), which represents the number of patients needed to be treated for 1 additional patient to be harmed for case-control studies using equation 1/[(OR-1) × unexposed event rate].
Sensitivity Analyses
To further test the robustness of our results, besides the analysis on the risk of anti-TNFα use during a 1-year period outlined earlier, 2 additional sensitivity analyses were performed. First, to account for possible reverse causality bias (which refers to a situation where early symptoms of the outcome could affect anti-TNFα use), a 60-day latency period was applied where the exposure assessment period was pushed back by 60 days (Figure 1B). Specifically, the first 60 days before the index date were disregarded. Second, to estimate the effect of unmeasured confounders (e.g., smoking), an E-value was calculated.41,42 Specifically, an estimation of the minimum strength of association was calculated that an unmeasured confounder would need to have with both exposure and outcome to fully explain away a specific exposure-outcome association.41,42
SAS V.9.4 was used. Meta-analyses were performed with HEpiMA V.2.3.
Standard Protocol Approvals, Registrations, and Patient Consents
No personally identifying information was made available as part of this study. Procedures used were in compliance with British Columbia's Freedom of Information and Privacy Protection Act. Ethics approval was obtained from the University of British Columbia's Clinical Research Ethics Board (H15-00887), the University of Calgary's Conjoint Health Research Ethics Board (REB16-2375), the University of Saskatchewan Biomedical Research Ethics Board (Bio-REB 2298), and the University of Manitoba Health Research Ethics Board (HS24393), which granted a waiver of informed consent because data are deidentified.
Data Availability
Data for this study reside in limited-access secure research environments. The data cannot leave this secure research environment for legal and ethical reasons.
Results
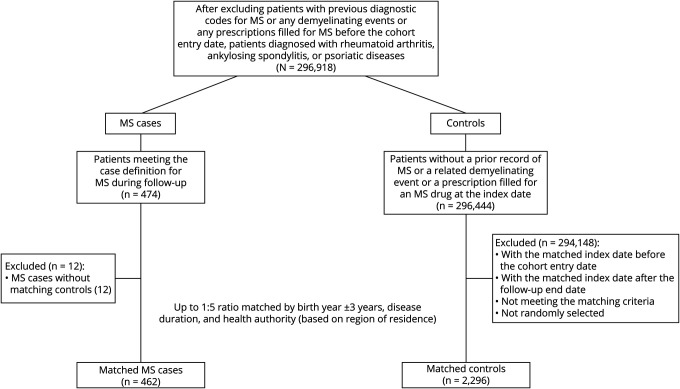
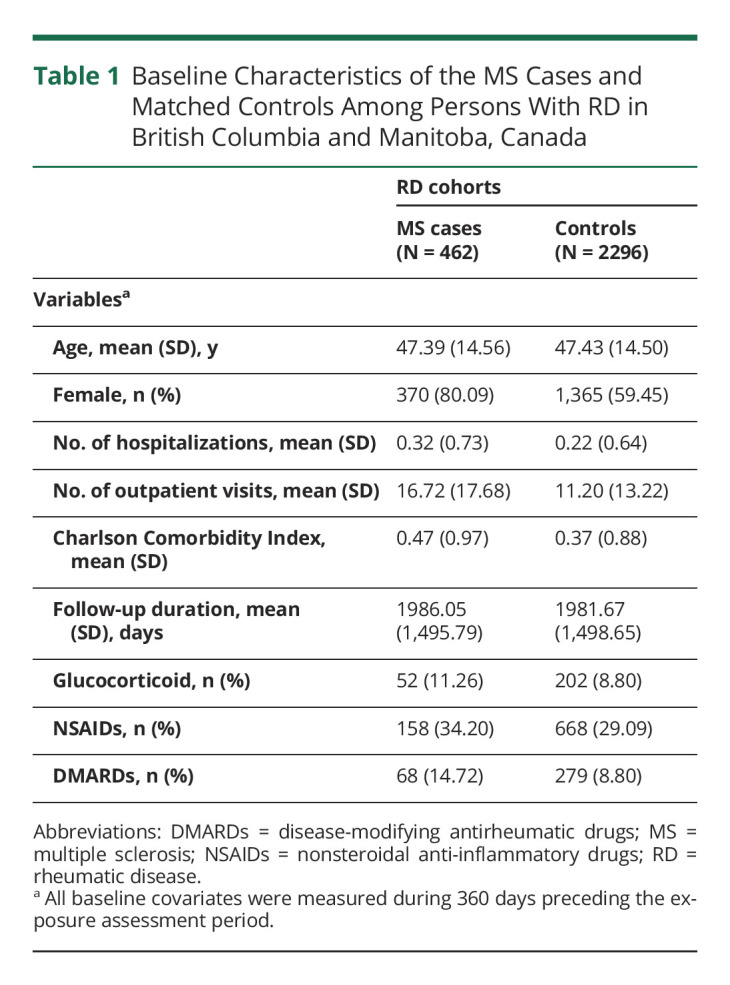
After excluding individuals with MS or any demyelinating events before the cohort entry date, in total, we identified 296,918 patients with RD in British Columbia and Manitoba combined (Figure 2). During follow-up, a total of 462 patients developed MS (80.1% female, mean [SD] age, 47.4 [14.6] years) and were matched with 2,296 controls with RD (59.5% female, mean [SD] age, 47.4 [14.5] years). Table 1 summarizes the baseline characteristics of the combined RD cohorts. Compared with controls, MS cases had a higher number of physician visits and hospitalizations; higher use of glucocorticoids, NSAIDs, and DMARDs; and higher CCI scores at baseline.
Figure 2. Nested Case-Control Inclusion Criteria for the Rheumatic Diseases (RD) Cohorts Among the 4 Canadian Provinces.
Abbreviation: MS = multiple sclerosis.
Table 1.
Baseline Characteristics of the MS Cases and Matched Controls Among Persons With RD in British Columbia and Manitoba, Canada
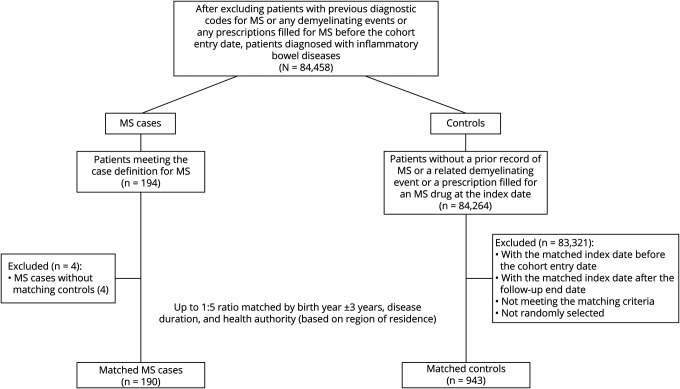
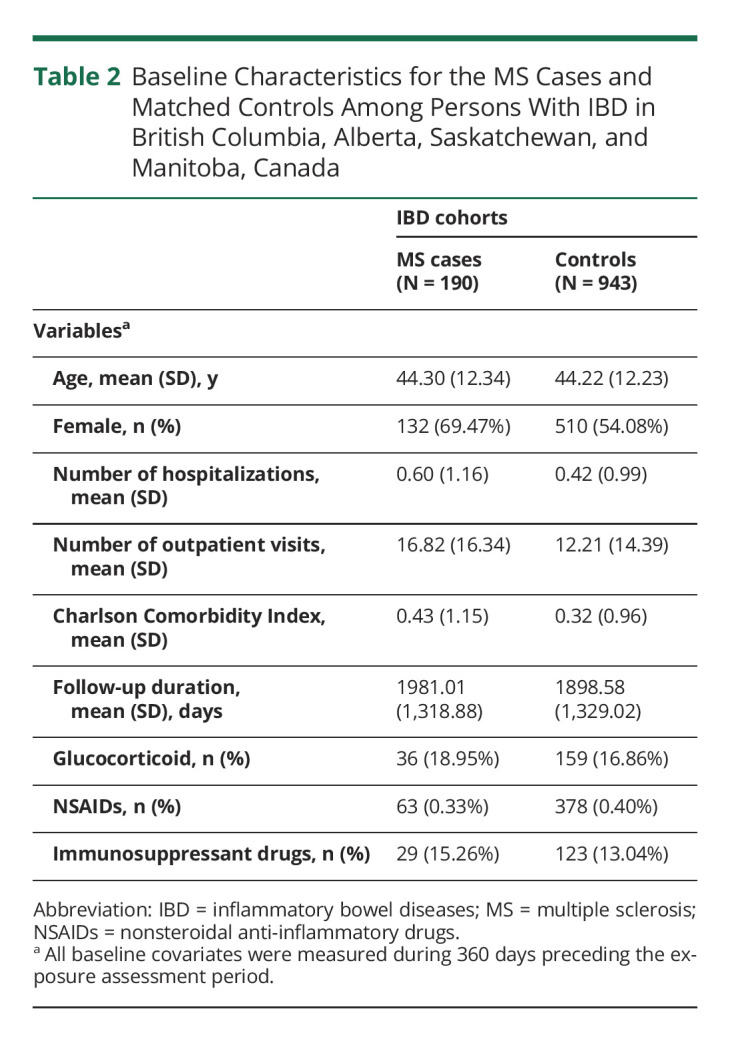
Among the 84,458 patients with IBD from the 4 provinces combined (Figure 3), 190 patients developed MS (69.5% female, mean [SD] age, 44.3 [12.3] years) during follow-up and were matched with 943 controls with IBD (54.1% female, mean [SD] age, 44.2 [12.2] years). Table 2 summarizes the baseline characteristics of the IBD cohorts for the 4 provinces combined. Like the RD cohorts, MS cases had a higher number of physician visits and hospitalizations, higher use of glucocorticoids and immunosuppressant drugs, and higher CCI scores when compared with controls at baseline.
Figure 3. Nested Case-Control Inclusion Criteria for Inflammatory Bowel Diseases (IBD) Cohorts Among the 4 Canadian Provinces.
Abbreviation: MS = multiple sclerosis.
Table 2.
Baseline Characteristics for the MS Cases and Matched Controls Among Persons With IBD in British Columbia, Alberta, Saskatchewan, and Manitoba, Canada
We computed the crude incidence rate of MS among incident users of anti-TNFα with respect to MS in each province for the patients with RD and IBD separately, during the entire follow-up period. For RD, the crude incidence rate (95% CI) was 0.29 (0.16–0.48)/1,000 person-years for British Columbia and 0.15 (0.02–0.54)/1,000 person-years for Manitoba. For IBD, the crude incidence rate (95% CI) was 0.26 (0.09–0.61)/1,000 person-years for British Columbia, 0.43 (0.25–0.68)/1,000 person-years for Alberta, 0.41 (0.11–1.05)/1,000 person-years for Saskatchewan, and 0.30 (0.04–1.08)/1,000 person-years for Manitoba.
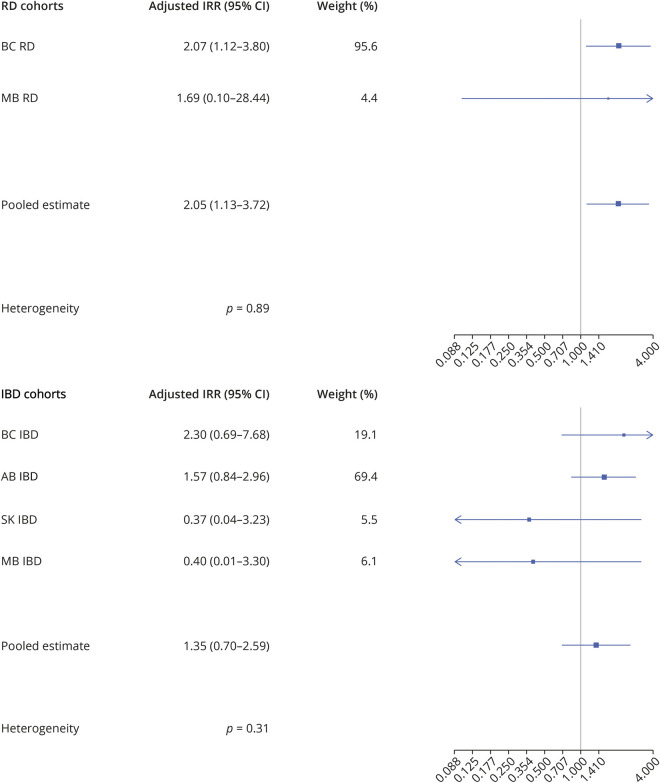
In the RD cohorts and across all provinces combined, 18 anti-TNFα users were observed among MS cases compared with 42 anti-TNFα users among controls in the 2 years before the index date (MS onset) (Table 3). After adjusting for sex and CCI, the corresponding fully adjusted IRR (95% CI) was 2.07 (1.12–3.80) for RD in British Columbia and 1.69 (0.10–28.44) in Manitoba, resulting in a pooled matched IRR (95% CI) of 2.05 (1.13–3.72) for both RD cohorts combined (Table 3 and Figure 4). The p value of the interaction term (anti-TNFα × sex) was not statistically significant. The pooled 2-year fully adjusted NNH was 2,268 for RD, meaning that 2,268 patients needed to be treated for 1 additional patient to be harmed.
Table 3.
Pooled Associations Between Anti-TNFα and Subsequent MS During the 2-Year Exposure Assessment Period in British Columbia, Alberta, Saskatchewan, and Manitoba, Canada
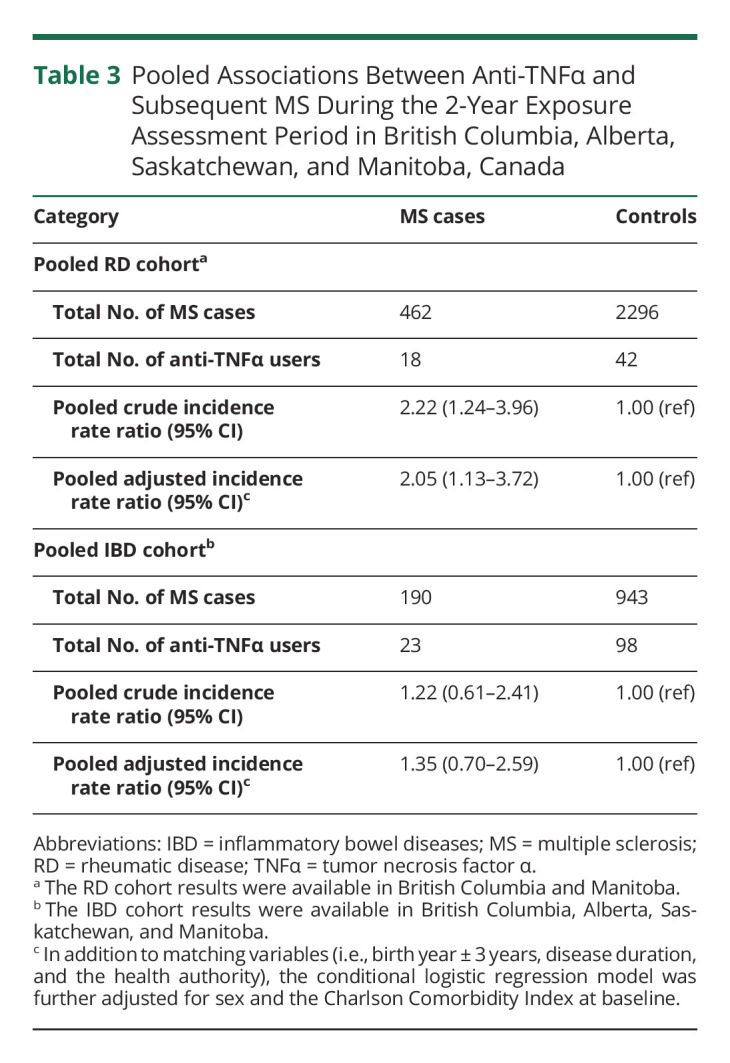
Figure 4. Association Between Antitumor Necrosis Factor α and Multiple Sclerosis Among 4 Canadian Provinces.
Adjusted IRR and pooled estimates for the association between antitumor necrosis factor α (TNFα) and multiple sclerosis (MS) in the rheumatic disease (RD) and inflammatory bowel disease (IBD) cohorts in British Columbia (BC), Alberta (AB), Saskatchewan (SK), and Manitoba (MB), Canada.
In the IBD cohorts and across all 4 provinces, 23 anti-TNFα users were observed among MS cases compared with 98 anti-TNFα users among controls (Table 3). After adjusting for sex and CCI, the corresponding fully adjusted IRR (95% CI) was 2.30 (0.69–7.68) for IBD in British Columbia, 1.57 (0.84–2.96) in Alberta, 0.37 (0.04–3.23) in Saskatchewan, and 0.40 (0.01–3.30) in Manitoba, resulting in a pooled fully adjusted IRR (95% CI) of 1.35 (0.70–2.59) (Table 3 and Figure 4). The p value of the interaction term (anti-TNFα*sex) was not statistically significant. Heterogeneity was not found between individual provinces with values of the Cochrane Q statistic all larger than 0.05 among the RD and IBD cohorts across all provinces (Figure 4).
Similar results were found in the sensitivity analyses (eTable 5 and eTable 6, links.lww.com/WNL/C454). Using the E-value metric, the observed IRR for RD would be explained away by an unmeasured confounder that was associated with both anti-TNFα and MS by a risk ratio of at least 3.52-fold each, after adjusting for potential confounders.
Discussion
In this multiprovincial Canadian population-based study, we found that the use of anti-TNFα was associated with an increased risk of MS compared with nonusers for RD. The finding of an increased MS risk could help clinicians and patients with RD when considering the use of anti-TNFα to make more informed treatment decisions. We also found an increased risk of MS among patients with IBD, but given the observational nature of this study and the wide confidence intervals, further studies are needed to validate these results.
Previous studies linking anti-TNFα use to MS risk have been somewhat mixed. For example, in a double-blind, placebo-controlled trial, the drug lenercept was administrated to 168 patients with clinically definite or laboratory supported definite MS. After 24 weeks, lenercept users reported more MS-related exacerbations than the placebo group (p = 0.007), resulting in the manufacturer's decision to terminate the study early.12 A NCC study that used medical records of patients with RD or IBD treated at 3 Mayo Clinics in the United States (2003–2019)13 found that the odds of inflammatory demyelinating events were 3 times higher among anti-TNFα users when compared with nonusers among chronic immune-mediated diseases. The authors did not specifically examine the association between anti-TNFα and MS, but instead looked at all types of inflammatory demyelinating events, and combined RD and IBD, because of the relatively small number of individuals included (N = 212). Furthermore, because the Mayo Clinic is a tertiary referral clinic center and only includes insured patients, this study may be subject to referral bias which may also reduce the generalizability of findings. In the same year, others suggested that no significantly increased risk of MS in users of anti-TNFα compared with nonusers among patients with RD (RR = 1.02, 95% CI, 0.23–4.46) in a cohort study using the nationwide clinical rheumatology registers in Sweden and Denmark (2000–2017).15 However, only 6 MS cases were identified among anti-TNFα users in Sweden and 4 in Denmark, resulting in rather wide confidence intervals. The heterogeneity from previous studies can make the results challenging for clinicians and patients to interpret. Thus, our large, population-based, multiprovince study, which used a common protocol to combine regions, contributes substantially to the understanding of MS risk in persons using anti-TNFα.
The mechanism for anti-TNFα potentially causing MS in persons with RD (and possibly IBD) is not fully understood. Current hypothesized mechanisms involve11 (1) the increased demyelination through increased ingress of peripheral autoreactive T cells into the CNS related to anti-TNFα; (2) the aggravation effect of anti-TNFα on CNS demyelination by decreasing TNF receptor 2 which is important for the myelin repair; (3) anti-TNFα can also downregulate interleukin-10 and upregulate interleukin-12 and interferon γ, which can demonstrate a profile like MS; (4) anti-TNFα may not deactivate TNFα in the CNS, facilitating a relatively high concentration of TNFα; (5) patients with MS may demonstrate increased serum neutralization capacity of TNFα; and (6) anti-TNFα may also increase the risk of an underlying latent infection, which could lead to demyelination.
The strengths of this study were the use of large Canadian administrative data sets that included the entire RD and IBD cohorts from up to 4 provinces, limiting selection bias and maximizing the generalizability of findings. Selecting confounding variables based on cDAGs enabled our study to be less prone to confounding by indication. Moreover, we used a highly accurate case definition for MS. Finally, implementation of a MS latency period in the analysis may help to control for reverse causality bias.
The limitations deserve comment. The main contribution to these results was from British Columbia and Alberta. Data from the provinces of Saskatchewan and Manitoba had lower weights mainly because of smaller sample sizes, hence relatively lower precision (Figure 4). The association between anti-TNFα and MS risk was not consistent in the IBD cohort, possibly because of the smaller cohort size or different disease states. As with all pharmacoepidemiologic studies using health administrative databases, we only have information on dispensation of prescription drugs and not their actual intake. However, because anti-TNFα agents are administered intravenously or subcutaneously and require special approvals for government-funded access, misclassification of anti-TNFα use is highly unlikely. We did not have information on lifestyle-related factors such as smoking. However, through our sensitivity analysis using the E-value, the observed IRR for RD would be hard to explain away. Health administrative databases capture information on diagnosis, but not necessarily on MS symptoms (which could indicate early disease onset). A previous study has shown that the prodromal phase to MS might present 5 or more years before the typical symptoms of demyelinating disease.43 As such, subjects exhibiting early MS symptoms or MS prodrome who have not yet been diagnosed might be misclassified as controls. However, this type of misclassification which is nondifferential usually leads to an underestimation of the risk of MS with the use of anti-TNFα. It is also possible that our results may be subject to ascertainment bias. Specifically, there might be more screening and surveillance of demyelination among users of anti-TNFα compared with nonusers. However, because of the concern that anti-TNFα use might lead to MS, clinicians might be withholding anti-TNFα to patients with symptoms that might resemble MS or those with a family history of MS. Thus, confounding by contraindication might also mean that our estimates are conservative and our results are potentially an underestimation of the true risk.
In conclusion, this is the largest population-based study to date using health administrative data sets from 4 Canadian provinces to demonstrate that the use of anti-TNFα was associated with an increased risk of incident MS among patients with RD when compared with nonusers. The finding of the increased MS risk could help clinicians and patients with RD who requires anti-TNFα to make better informed decisions regarding treatment. Specifically, clinicians and patients can weigh the risk quality of life trade-offs between using an anti-TNFα drug or choosing other available alternative medications that are comparable in efficacy but have not been associated with MS.44,45
Acknowledgment
Access to data provided by the Data Steward(s) is subject to approval but can be requested for research projects through the Data Steward(s) or their designated service providers. All inferences, opinions, and conclusions drawn in this publication are those of the author(s) and do not reflect the opinions or policies of the Data Steward(s). This study is based in part on data provided by Alberta Health. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Governments of Alberta. Neither the Government of Alberta nor Alberta Health expressed any opinion in relation to this study. This study is based in part on deidentified data provided by the Saskatchewan Ministry of Health and eHealth Saskatchewan. The interpretation and conclusions contained herein do not necessarily represent those of the Government of Saskatchewan, the Saskatchewan Ministry of Health, or eHealth Saskatchewan. The authors acknowledge the Manitoba Centre for Health Policy for use of the Manitoba Population Research Data Repository under project #2020-069 (HIPC #2020/2021-58). The results and conclusions presented are those of the authors, and no official endorsement by the Manitoba Centre for Health Policy, Manitoba Health, or other data providers is intended or should be inferred. The authors thank Dr. Stephanie Coward, Jessica Osei, Fernando Maldonado Daza, and Shelby Marozoff for the coordination of this work.
Glossary
- CCI
Charlson Comorbidity Index
- cDAG
causal direct acyclic graph
- DMARD
disease-modifying antirheumatic drug
- IBD
inflammatory bowel disease
- IRR
incidence rate ratio
- MS
multiple sclerosis
- NCC
nested case control
- NSAID
nonsteroidal anti-inflammatory drug
- RD
rheumatic disease
- TNFα
tumor necrosis factor α
Appendix. Authors
Footnotes
Editorial, page 267
Study Funding
Canadian Institutes of Health Research (CIHR Grant Numbers THC-135235 and PJT-166183).
Disclosure
L. Li is supported by the Canadian Institutes of Health Research Fred Frederick Banting and Charles Best Canada Graduate Scholarships Doctoral Awards. J.A.Aviña-Zubieta is supported by the BC Lupus Society Scholar Award and is the Walter & Marilyn Booth Research Scholar. C. Bernstein reports no disclosures relevant to the manuscript. G.G. Kaplan has received honoraria for speaking or consultancy from AbbVie, Janssen, Pfizer, Amgen, and Takeda. He has received research support from Ferring, Janssen, AbbVie, GlaxoSmith Kline, Merck, and Shire. He has been a consultant for Gilead. He shares ownership of a patent:treatment of inflammatory disorders, autoimmune disease, and PBC. UTI Limited Partnership, assignee. Patent WO2019046959A1. PCT/CA2018/051098. September 7, 2018. H. Tremlett reports no disclosures relevant to the manuscript. H. Xie reports no disclosures relevant to the manuscript. J.N. Peña-Sánchez reports no disclosures relevant to the manuscript. R.A. Marrie reports no disclosures relevant to the manuscript. M. Etminan reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325(8):765-779. doi: 10.1001/jama.2020.26858 [DOI] [PubMed] [Google Scholar]
- 2.Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816-1821. doi: 10.1177/135245852097084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feagan BG, Patel H, Colombel JF, et al. Effects of vedolizumab on health-related quality of life in patients with ulcerative colitis: results from the randomised GEMINI 1 trial. Aliment Pharmacol Ther. 2017;45(2):264-275. doi: 10.1111/apt.13852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled. Arthritis Rheum. 2004;50(5):1400-1411. doi: 10.1002/art.20217 [DOI] [PubMed] [Google Scholar]
- 5.Loftus EV, Feagan BG, Colombel JF, et al. Effects of adalimumab maintenance therapy on health-related quality of life of patients with Crohn's disease: patient-reported outcomes of the CHARM trial. Am J Gastroenterol. 2008;103(12):3132-3141. doi: 10.1111/j.1572-0241.2008.02175.x [DOI] [PubMed] [Google Scholar]
- 6.Holdam ASK, Bager P, Dahlerup JF. Biological therapy increases the health-related quality of life in patients with inflammatory bowel disease in a clinical setting. Scand J Gastroenterol. 2016;51(6):706-711. doi: 10.3109/00365521.2015.1136352 [DOI] [PubMed] [Google Scholar]
- 7.Boggs JME, Barnes L. Demyelination during anti-tumour necrosis factor therapy for psoriasis. Clin Exp Dermatol. 2018;43(5):577-578. doi: 10.1111/ced.13412 [DOI] [PubMed] [Google Scholar]
- 8.Theibich A, Dreyer L, Magyari M, Locht H. Demyelinizing neurological disease after treatment with tumor necrosis factor alpha-inhibiting agents in a rheumatological outpatient clinic: description of six cases. Clin Rheumatol. 2014;33(5):719-723. doi: 10.1007/s10067-013-2419-8 [DOI] [PubMed] [Google Scholar]
- 9.Chey SY, Kermode AG. Central nervous system demyelination related to tumour necrosis factor Alpha inhibitor. Mult Scler J Exp Transl Clin. 2022;8(1):20552173211070750. doi: 10.1177/20552173211070750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutto SK, Rice DR, Mateen FJ. CNS demyelination with TNFα inhibitor exposure: a retrospective cohort study. J Neuroimmunol. 2021;356:577587. doi: 10.1016/j.jneuroim.2021.577587 [DOI] [PubMed] [Google Scholar]
- 11.Kemanetzoglou E, Andreadou E. CNS demyelination with TNF-α blockers. Curr Neurol Neurosci Rep. 2017;17(4):36. doi: 10.1007/s11910-017-0742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. The lenercept multiple sclerosis study group and the university of British Columbia MS/MRI analysis group. Neurology. 1999;53(3):457-465. [PubMed] [Google Scholar]
- 13.Kunchok A, Aksamit AJ, Davis JM, et al. Association between tumor necrosis factor inhibitor exposure and inflammatory central nervous system events. JAMA Neurol. 2020;77(8):937-946. doi: 10.1001/jamaneurol.2020.1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernatsky S, Renoux C, Suissa S. Demyelinating events in rheumatoid arthritis after drug exposures. Ann Rheum Dis. 2010;69(9):1691-1693. doi: 10.1136/ard.2009.111500 [DOI] [PubMed] [Google Scholar]
- 15.Kopp TI, Delcoigne B, Arkema EV, et al. Risk of neuroinflammatory events in arthritis patients treated with tumour necrosis factor alpha inhibitors: a collaborative population-based cohort study from Denmark and Sweden. Ann Rheum Dis. 2020;79(5):566-572. doi: 10.1136/annrheumdis-2019-216693 [DOI] [PubMed] [Google Scholar]
- 16.Dreyer L, Magyari M, Laursen B, Cordtz R, Sellebjerg F, Locht H. Risk of multiple sclerosis during tumour necrosis factor inhibitor treatment for arthritis: a population-based study from DANBIO and the Danish Multiple Sclerosis Registry. Ann Rheum Dis. 2016;75(4):785-786. doi: 10.1136/annrheumdis-2015-208490 [DOI] [PubMed] [Google Scholar]
- 17.Taylor TRP, Galloway J, Davies R, Hyrich K, Dobson R. Demyelinating events following initiation of anti-TNFα therapy in the British Society for Rheumatology biologics registry in rheumatoid arthritis. Neurol Neuroimmunol Neuroinflammation. 2021;8(3):e992. doi: 10.1212/NXI.0000000000000992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis—comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59(5):566-571. doi: 10.1111/ijd.14864 [DOI] [PubMed] [Google Scholar]
- 19.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769-2778. doi: 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 20.Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019;78(11):1463-1471. doi: 10.1136/annrheumdis-2019-215920 [DOI] [PubMed] [Google Scholar]
- 21.Crossfield SSR, Marzo-Ortega H, Kingsbury SR, Pujades-Rodriguez M, Conaghan PG. Changes in ankylosing spondylitis incidence, prevalence and time to diagnosis over two decades. RMD Open. 2021;7(3):1-8. doi: 10.1136/rmdopen-2021-001888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.British Columbia Ministry of Health [creator]: Medical services plan (MSP) payment information file. Population Data BC [publisher]. Data extract. MOH; 2017. popdata.bc.ca/data/health/msp. Accessed April 06, 2022.
- 23.Canadian Institute for Health Information [creator](2017): Discharge Abstract Database (Hospital Separations). Population Data BC [publisher]. Data Extract. MOH; 2017). popdata.bc.ca/data/health/dad. Accessed April 06, 2022.
- 24.British Columbia Ministry of Health [creator]: Consolidation file (MSP registration & premium billing). Population Data BC [publisher]. Data Extract. MOH; 2017. popdata.bc.ca/data/population/consolidationfile. Accessed April 06, 2022.
- 25.British Columbia Ministry of Health [creator]. 2018. PharmaNet. Population Data BC [publisher]. Data Extract. Data Stewardship Committee; 2017. popdata.bc.ca/data/health/PharmaNet. Accessed April 06, 2022.
- 26.Li L, Lu N, Avina-Galindo AM, et al. The risk and trend of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a general population-based study. Rheumatology (Oxford). 2021;60(1):188-195. doi: 10.1093/rheumatology/keaa262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. 2019;156(5):1345-1353.e4. doi: 10.1053/j.gastro.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 28.Hitchon CA, Khan S, Elias B, Lix LM, Peschken CA. Prevalence and incidence of rheumatoid arthritis in canadian first nations and non-first nations people: a population-based study. J Clin Rheumatol. 2020;26(5):169-175. doi: 10.1097/RHU.0000000000001006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Sakran LH, Marrie RA, Blackburn DF, Knox KB, Evans CD. Establishing the incidence and prevalence of multiple sclerosis in saskatchewan. Can J Neurol Sci. 2018;45(3):295-303. doi: 10.1017/cjn.2017.301 [DOI] [PubMed] [Google Scholar]
- 30.Essebag V, Genest J, Suissa S, Pilote L. The nested case-control study in cardiology. Am Heart J. 2003;146(4):581-590. doi: 10.1016/S0002-8703(03)00512-X [DOI] [PubMed] [Google Scholar]
- 31.Aviña-Zubieta JA, Chan J, De Vera M, Sayre EC, Choi H, Esdaile J. Risk of venous thromboembolism in ankylosing spondylitis: a general population-based study. Ann Rheum Dis. 2019;78(4):480-485. doi: 10.1136/annrheumdis-2018-214388 [DOI] [PubMed] [Google Scholar]
- 32.Tan J, Avina-Zubieta JA, Dominique A, Tavakoli H, Simon TA. Risk of cancer in patients with psoriasis/psoriatic arthritis: a population-based study in the province of British Columbia [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). acrabstracts.org/abstract/risk-of-cancer-in-patients-with-psoriasis-psoriatic-arthritis-a-population-based-study-in-the-province-of-british-columbia/. Accessed April 06, 2022. [Google Scholar]
- 33.Rezaie A, Quan H, Fedorak RN, Panaccione R, Hilsden RJ. Development and validation of an administrative case definition for inflammatory bowel diseases. Can J Gastroenterol. 2012;26(10):711-717. doi: 10.1155/2012/278495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn's disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol. 1999;149(10):916-924. [DOI] [PubMed] [Google Scholar]
- 35.Wacholder S, Silverman DT, Mclaughlin JK, Mandel JS. Selection of controls in case-control studies: II. Types of controls. Am J Epidemiol. 1992;135(9):1029-1041. doi: 10.1093/oxfordjournals.aje.a116397 [DOI] [PubMed] [Google Scholar]
- 36.Etminan M, Sodhi M, Li L. Methodological considerations for the case-control study of metformin and age-related macular degeneration. JAMA Ophthalmol. 2021;139(8):918-919. doi: 10.1001/jamaophthalmol.2021.2129 [DOI] [PubMed] [Google Scholar]
- 37.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488-495. doi: 10.1097/EDE.0b013e3181a819a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Cin Epidemiol. 1993;46(10):1075-1079. doi: 10.1016/0895-4356(93)90103-8 [DOI] [PubMed] [Google Scholar]
- 39.Costa-Bouzas J, Takkouche B, Cadarso-Suárez C, Spiegelman D. HEpiMA: software for the identification of heterogeneity in meta-analysis. Comput Methods Programs Biomed. 2001;64(2):101-107. doi: 10.1016/S0169-2607(00)00087-0 [DOI] [PubMed] [Google Scholar]
- 40.Bjerre LM, LeLorier J. Expressing the magnitude of adverse effects in case-control studies: “The number of patients needed to be treated for one additional patient to be harmed. BM J. 2000;320(7233):503-506. doi: 10.1136/bmj.320.7233.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Der Weele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 42.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Website and R Package for computing E-values. Epidemiology. 2018;29(5):e45–e47. doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tremlett H, Munger KL, Makhani N. The multiple sclerosis prodrome: evidence to action. Front Neurol. 2022;12:761408. doi: 10.3389/fneur.2021.761408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor α antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther. 2019;49(7):873-879. doi: 10.1111/apt.15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emery P, Rondon J, Parrino J, et al. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58(5):849-858. doi: 10.1093/rheumatology/key36 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this study reside in limited-access secure research environments. The data cannot leave this secure research environment for legal and ethical reasons.