Abstract
Although the prognostic value of blood creatinine levels in patients with paraquat (PQ) poisoning has been studied for a long time, the results are still controversial. Therefore, we performed the first meta-analysis to comprehensively assess the value of blood creatinine in predicting the prognosis of patients with PQ poisoning. We searched PubMed, EMBase, Web of Science, ScienceDirect, Cochrane Library, China National Knowledge Infrastructure, China Science and Technology Journal Database, and China Online Journals to identify all relevant papers published up to June 2022. Data were extracted for pooled analysis, heterogeneity testing, sensitivity analysis, publication bias analysis, and subgroup analysis. Ultimately, 10 studies involving 862 patients were included. The I2 of diagnostic odds ratio (DOR), sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of this study were all greater than 50%, which showed the existence of heterogeneity in this study, and a random effects model was used for the combination of the above five effect sizes. Pooled analysis showed a high predictive value of blood creatinine for prognosis of PQ poisoning [pooled DOR:22.92, 95% confidence interval (CI):15.62–33.65, P < 0.001]. The combined sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were 86% (95% CI: 0.79–0.91), 78% (95% CI: 0.69–0.86), 4.01 (95% CI: 2.81–5.71), and 0.17 (95% CI: 0.12–0.25), respectively. Deeks publication bias test revealed there was publication bias. Sensitivity analysis showed no significant differences in the estimates of impact. Serum creatinine is an effective predictor of mortality in patients with PQ poisoning.
Introduction
Paraquat (PQ), an organic heterocyclic pesticide, is highly toxic to humans [1, 2]. Many people ingest PQ as a suicide agent, since PQ is readily available and inexpensive in most countries and has a very high mortality rate after poisoning, especially in Asian countries where PQ poisoning is a serious public health problem [3, 4]. PQ poisoning can lead to multi-organ failure, with the main organs damaged being the kidneys, liver, and lungs [5], but the mechanism of poisoning has not been fully determined. The main treatment modalities for PQ poisoning are in vitro removal, immunosuppression, and antioxidants, and although many treatments have been developed, the efficacy of these treatments is uncertain [6, 7]. Because of the extremely high mortality rate from PQ poisoning, researchers have developed a number of survival predictors.
Some studies have shown that plasma PQ concentration and severity index of PQ poisoning (SIPP) can be used as reliable predictors of mortality from PQ poisoning [2, 7], but plasma PQ concentration is expensive and difficult to detect. Some studies have shown that sequential organ failure assessment (SOFA) score and acute physiology and chronic health evaluation II (APACHE II) score can be used as prognostic indicators of PQ poisoning [6–11], but they are comprehensive indicators that have challenges in calculation as well as limited usability and reliability in the early stages of poisoning, and are hardly representative of the patient’s early condition. In addition, the toxic dose can be used as a prognostic indicator [12], but it is unable to evaluate an accurate value. Thus, there is a need for a better prognostic indicator that is simple and accurately reflects the true condition of the body. The kidney is the main organ for excreting PQ, and the early stage of PQ poisoning can lead to kidney damage [13, 14]. Meanwhile, the blood creatinine level of the organism reflects the renal function [15]. Several studies have concluded that blood creatinine levels can be used as a prognostic indicator of PQ intoxication. However, the sample size of a single study was small and the statistical efficacy was low, we used meta-analysis to improve the statistical efficacy and to investigate the predictive value of blood creatinine on mortality from PQ poisoning in a comprehensive manner.
Methods
As a meta-analysis, this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist. Since we only review previously published research, there are no ethical issues involved in our study. In June 2022, the study protocol was registered in the International Prospective Systematic Review under protocol number: CRD42022335006.
Search strategy
The following 8 databases were used for this study: PubMed, EMBase, Web of Science, ScienceDirect, Cochrane Library, China National Knowledge Infrastructure, China Science and Technology Journal Database, and China Online Journals. The literature prior to 2022.6.1 was searched using the terms PQ and creatinine; see the Appendix for specific search strategies (S1 Table).
Inclusion and exclusion criteria
The inclusion criteria were as follows: studies covering serum creatinine in patients with PQ poisoning in both the survival and death groups; patients of any age diagnosed with oral paraquat poisoning (although inhalation or contact with the skin were also routes of poisoning, these were not the focus of the study); studies focused on people with moderate to severe poisoning, as typically in clinical practice, mild paraquat poisoning (which tends to resolve on its own) and extreme severe poisoning patients often did not receive treatment; studies with sample sizes ≥ 30 cases; studies covering mortality within 90 days of paraquat poisoning; data related to serum creatinine could be calculated as true positive, false positive, true negative, and false negative. The exclusion criteria were as follows: reviews, commentaries, conferences, letters, editorial abstracts, meta-analyses, animal studies, informal publications, and case reports; literature with insufficient data; literature with implications for the stability of the combined results. In case of duplicate data publication, studies with the most recent year or larger sample size were selected.
Data extraction
The required content was extracted independently by two researchers, and the extracted content included: first author, year of publication, cut-off value, blood creatinine level, sample size, number of survivors and deaths, cut-off value, sensitivity and specificity of blood creatinine to predict PQ mortality and study type. Discrepancies were resolved through third-party coordination.
Risk of bias
We used quality assessment of diagnostic accuracy study 2 tool (QUADAS-2) separately to assess the quality of this study, and the risk of bias was graded as low, unclear, or high, with any inconsistencies resolved through discussion.
Statistical analysis
Commonly used software for diagnostic accuracy meta-analysis include Stata, R, and meta disc. The packages that can do diagnostic accuracy test meta-analysis in R are "meta4diag, bamdit, hierarchical summary receiver operating characteristic (HSROC), metamisc, mada, CopulaREMADA, and Metatron", and the first three packages are based on Bayesian theory. The effect sizes in meta-analysis of combined diagnostic accuracy tests with these R packages above have certain drawbacks, for example, the meta4diag package cannot perform heterogeneity tests, the HSROC package cannot draw forest plots, and although heterogeneity tests can be performed with the mada package, it cannot calculate the combined data. The study used stata17 for combining all effect sizes and sensitivity analysis, analyzed Deeks publication bias test and summary ROC (SROC) curves, also processed Review Manager 5.3 for quality assessment graphs. I2<50%, considered low heterogeneity, was used to combine effect sizes using a fixed-effects model; I2>50%, considered high heterogeneity, was used to combine effect sizes using a random-effects model.
Results
Literature search
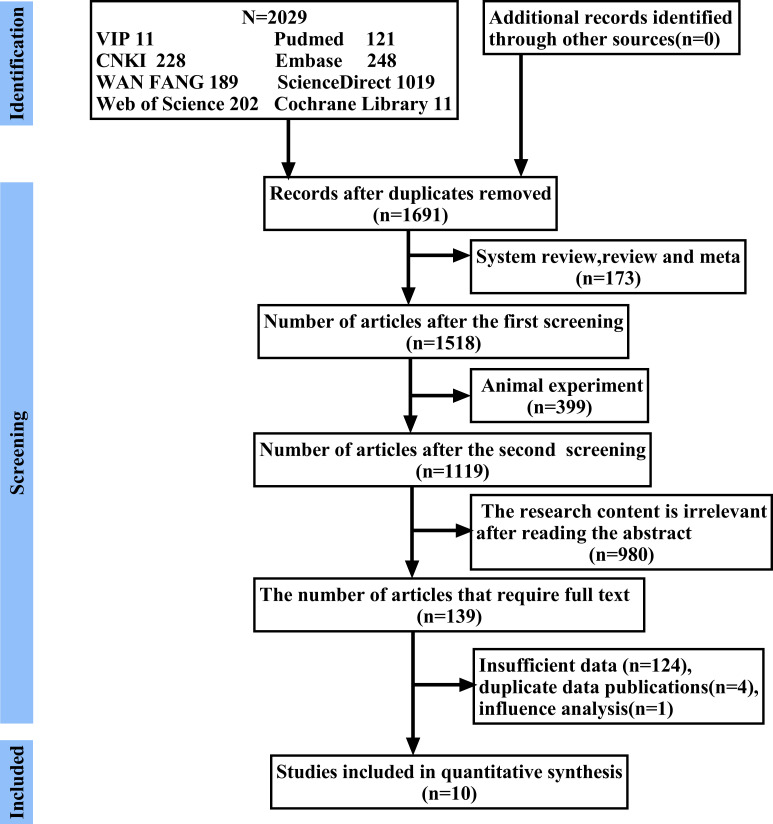
According to the search strategy, a total of 2,029 publications were found in PubMed, EMBase, Web of Science, ScienceDirect, Cochrane Library, China National Knowledge Infrastructure, China Science and Technology Journal Database, and China Online Journals. Duplicates, animal experiments, reviews, and meta-analyses were excluded successively, followed by reading the titles and abstracts to exclude irrelevant studies. Finally, after reading the full text, literature with duplicate publications, insufficient data, and influence on the stability of the results were excluded in turn, and 10 papers [16–25] with a total sample size of 862 were finally selected. The search process is shown in Fig 1.
Fig 1. Flow chart of search process.
Characteristics of the included studies
The baseline characteristics are shown in Table 1. The 10 studies were published in the years 2011–2020, all retrospective, with a total sample size of 862 and a mean sample size of 86.2. Sample sizes ranged from 40 to 190 cases. Mortality rates ranged from 40.0% to 65.12%, with nine studies conducted in China and one in India. Five studies were published in English and the other five studies were published in Chinese.
Table 1. Characteristics of included studies.
| First author | Publicatio-n year | Region | Study Design | Sample size | Morta-lity (%) | Cut-off (μmol/L) | Serum creatinine (μmol/L) | Study period | |
|---|---|---|---|---|---|---|---|---|---|
| Survivor | Non-survivor | ||||||||
| Song | 2020 | China | Retrospective | 114 | 45.61 | 122 | 93.2 (73.0–124.2; 35.7–725.0) | 277.0 (213.3–382.4; 67.8–1105.0) | 2018–2020 |
| Zhao | 2020 | China | Retrospective | 108 | 59.26 | 74.5 | 62.50 (22.00) | 104.00 (69.50) | 2019–2020 |
| Wan | 2018 | China | Retrospective | 40 | 40 | NA | NA | NA | 2017–2018 |
| Su | 2020 | China | Retrospective | 190 | 62.63 | 102.1 | 82.71 + 42.55 | 88.94 + 112.72 | 2019–2020 |
| Chen | 2011 | China | Retrospective | 45 | 62.22 | NA | 54± 12 | 148± 24 | 2009–2011 |
| Xiao | 2017 | China | Retrospective | 58 | 43.10 | 110.7 | 64.03 ±23.52 | 149.34 ±86.03 | 2016–2017 |
| Wan | 2018 | China | Retrospective | 162 | 37.04 | NA | 75.06±50.81 | 130.54±126.16 | 2017–2018 |
| Wan | 2011 | China | Retrospective | 57 | 49.12 | 80.85 | 67.54±17.67 | 144.21±76.98 | 2010–2011 |
| Li | 2014 | China | Retrospective | 43 | 65.12 | 90.70 | 70.89±13.38 | 106.1±45.07 | 2013–2014 |
| Kavousi | 2017 | India | Retrospective | 104 | 43.27 | 172.38 | NA | NA | 2015–2017 |
NA: not available; Continuous variable is presented as mean ± standard deviation, median (interquartile range) or numbers.
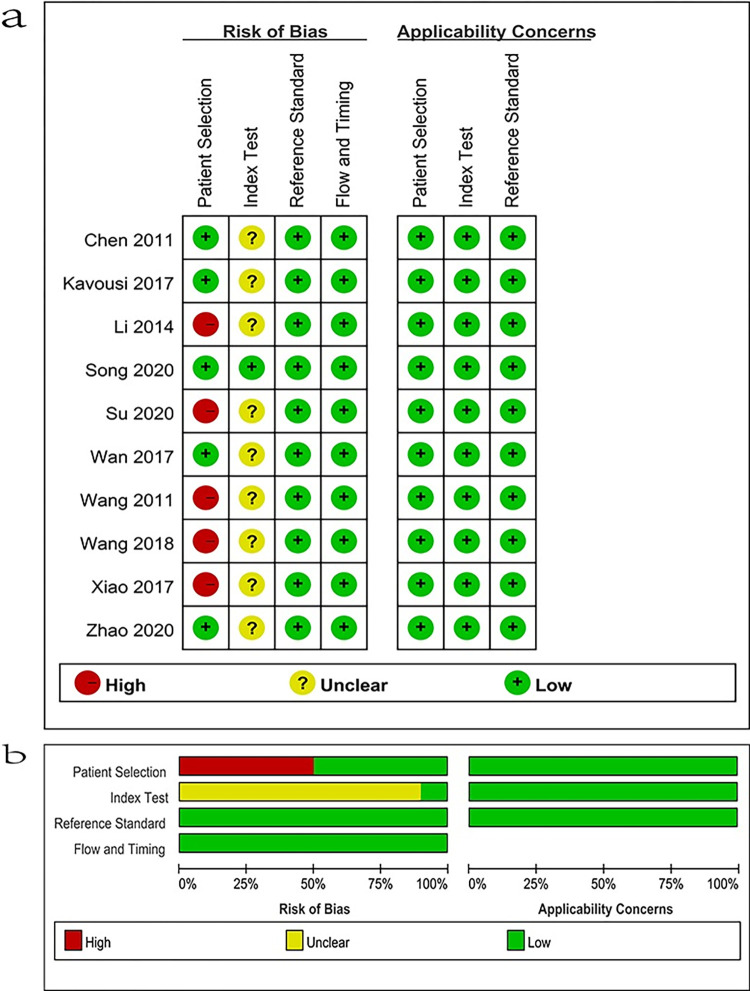
Quality assessment
The S2 Table presents the distribution of QUADAS-2 scores for the methodological quality of these 10 studies. All 10 studies showed moderate to high quality according to QUADAS-2 for quality assessment, so these studies are suitable for this meta-analysis (Fig 2).
Fig 2. Risk of bias and applicability concerns.
(+) denotes low risk, (−) denotes high risk.
Meta-analysis of mortality
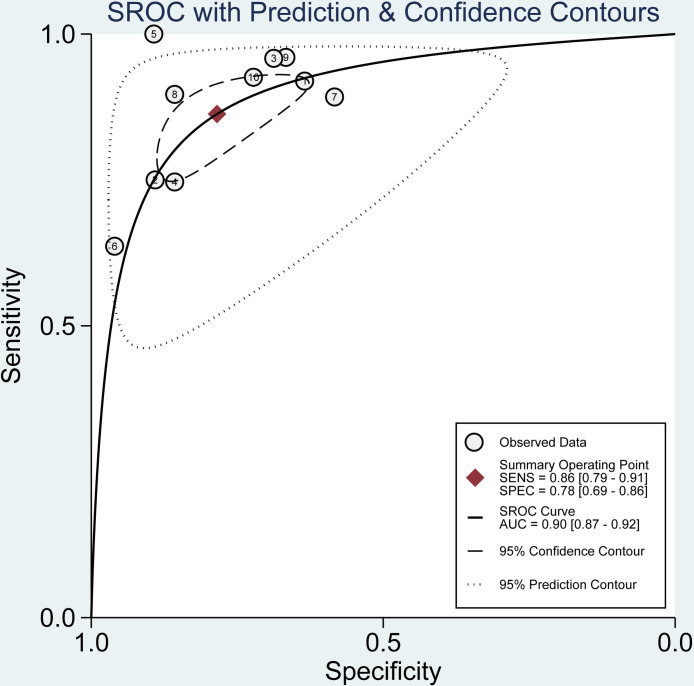
The data were imported into meta disc1.4 software for analysis, and the spearman correlation coefficient between the log of sensitivity and the log of (1-specificity) was 0.365 (P = 0.30), which was not significant, implying that there was no heterogeneity due to threshold effect in this study. Furthermore, by plotting the SROC curves, there was no "shoulder arm shape", which further indicated that there was no threshold effect in this study (Fig 3).
Fig 3. Summary ROC curve for the 6 included studies.
Numbers in brackets are 95% CIs. ROC: receiver operating characteristic, AUC: area under curve, SENS: sensitivity, SPEC: specificity, CI: confidence interval.
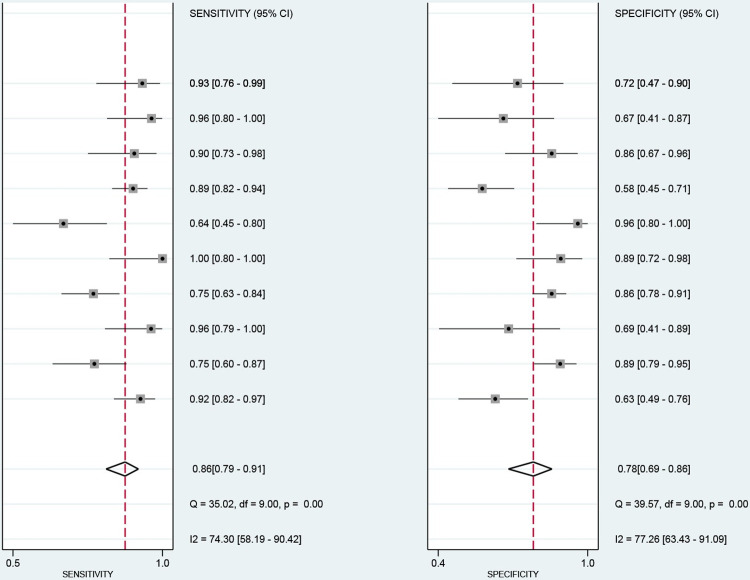
The data from this study were imported into stata17, and the Cochran-Q test for the diagnostic odds ratio (DOR) yielded Cochran-Q = 35.10, I2 = 74.36, P<0.01 (Fig 4), implying the existence of heterogeneity caused by non-threshold effects in this study. In addition, the I2 of sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of this study were all greater than 50%, which showed the existence of heterogeneity in this study, and the random effects model was used for the combination of the above five effect sizes. The combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and DOR were 86% (95% CI: 0.79–0.91; Fig 5), 78% (95% CI: 0.69–0.86; Fig 5), 4.01 (95% CI: 2.81–5.71; S1 Fig), 0.17 (95% CI: 0.12–0.25; S1 Fig), and 22.92 (95% CI: 15.62–33.65; Fig 4). The area under the curve (AUC) of the blood creatinine test was 0.90 (95% CI: 0.87–0.92; Fig 3), which meant that the diagnostic accuracy was relatively high.
Fig 4. Forest plot for the association of serum creatinine and mortality of paraquat poisoning.
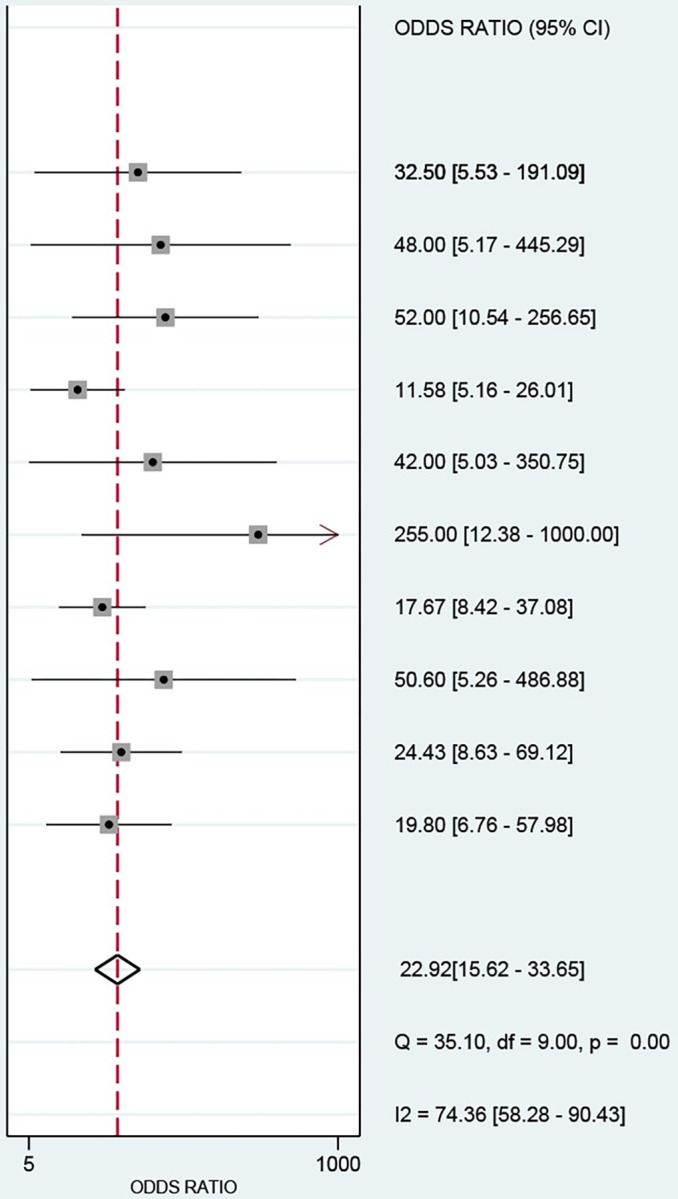
Fig 5. Forest plots of sensitivity and specificity of Serum creatinine in predicting mortality after paraquat poisoning.
The diamond indicates the combined estimate from the included studies.
Heterogeneity, sensitivity analysis, and publication bias assessment
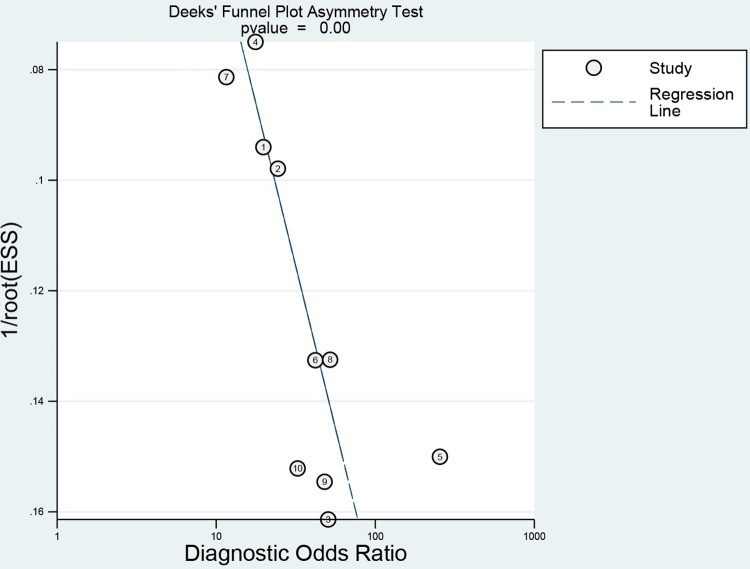
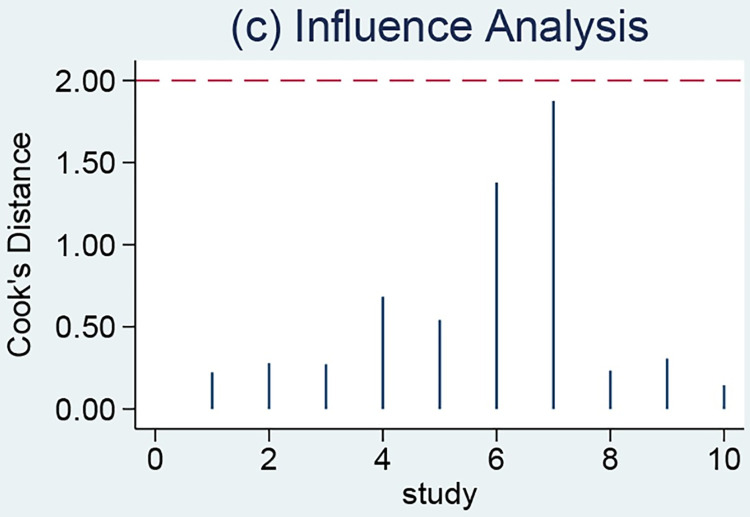
A meta-regression analysis was performed based on several covariates, including sample size, year of publication, percentage of men, mortality rate, and study area; however, the meta-regression results did not identify sources of heterogeneity (Table 2). P<0.001 in Fig 6 implied that the funnel plot was asymmetric and there was a publication bias in this study. In addition, one article with strong sensitivity was excluded by sensitivity analysis, and the remaining original study did not cause sensitivity in the arithmetic results (Fig 7). Taken together, the results of this study are relatively stable.
Table 2. Meta-regression analysis of potential sources of heterogeneity.
| Heterogeneity factors | N | LRTChi2 | Pvalue | I2 | I2lo | I2hi |
|---|---|---|---|---|---|---|
| Study Region | ||||||
| India | 1 | 0.69 | 0.71 | 0 | 0 | 100 |
| China | 9 | |||||
| Mortality% | ||||||
| ≥50% | 4 | 1.30 | 0.52 | 0 | 0 | 100 |
| <50% | 6 | |||||
| Sample size | ||||||
| ≥100 | 5 | 5.48 | 0.06 | 64 | 18 | 100 |
| <100 | 5 | |||||
| Publication year | ||||||
| >2017 | 5 | 5.09 | 0.08 | 61 | 11 | 100 |
| ≤2017 | 5 | |||||
| Male% | ||||||
| ≥50% | 4 | 0.13 | 0.94 | 0 | 0 | 100 |
| <50% | 6 |
Fig 6. Deeks publication bias test.
Fig 7. Sensitivity analysis of the relationship between serum creatinine and mortality of paraquat poisoning.
Translation to clinical practice
Fagan plots showed that when the pre-test probability of death from acute PQ poisoning by blood creatinine were 25%, 50%, and 75%, positive post-test probability were 57%, 80%, and 92%; and negative post-test probability was 6%, 15%, and 34%, respectively (S2 Fig).
Discussion
This study aimed to find a simple and accurate prognostic indicator that reflected the true condition of the organism. This meta-analysis showed an association between blood creatinine levels and clinical prognosis in patients with PQ intoxication, which meant that the patient with PQ intoxication who had an elevated blood creatinine level had a significantly increased risk of death.
After PQ poisoning, it is mainly distributed in the lung, kidney, liver, muscle, and other tissues, while the absorbed PQ is mainly excreted through the kidney, and the renal PQ concentration is extremely high [26]. Reactive oxygen species produced by PQ poisoning cause the onset of cellular damage in the kidney proximal tubule, followed by lipid peroxidation, which disrupts membrane integrity and causes cell death [27]. This is a process that cells undergo swelling, degeneration and partial necrosis, interstitial congestion and edema, so that PQ poisoning often results in acute kidney injury, which in turn leads to renal failure, manifested by proteinuria and oliguria, and then progresses to acute tubular necrosis [28]. Direct oxidative damage to renal tubules by PQ can induce elevated blood creatinine [14]. The level of serum creatinine is mainly determined by glomerular filtration capacity, and a decrease in glomerular filtration capacity increases blood creatinine concentration, indicating that elevated blood creatinine levels are closely related to acute kidney injury and are a direct reflection of progressive kidney damage [29, 30]. As a result, blood creatinine concentrations can be monitored to detect renal failures and predict long-term outcomes [30–33]. From this study, the predictive value of blood creatinine for PQ poisoning mortality is high by DOR analysis. The PQ absorbed in the body is mainly excreted in its original form through the kidney, and if the kidney function is damaged at an early stage, it will greatly reduce the excretion rate of PQ, which will aggravate the accumulation and damage of PQ in lung tissues and organs, thus affecting the prognosis [34].
The sensitivity of blood creatinine to predict the prognosis of PQ poisoning was 0.86, which was higher than SOFA (sensitivity 0.72) [35], white blood cell count (sensitivity 0.75) [36], APACHE II scores (sensitivity 0.75) [8], arterial lactate (sensitivity 0.77) [37], base excess (sensitivity 0.78) [38], severity index (sensitivity of 0.84) [39], and plasma PQ concentration (sensitivity of 0.86) [39]. The AUC of the SROC curve was 0.90, indicating that blood creatinine had a high predictive value for the prognosis of PQ poisoning, and the results of the Fagan curve showed that when the pretest probability was 25%, the negative likelihood ratio was 0.17, and the posttest probability was 6%, indicating that the probability of having a certain disease was only 6%; when the pretest probability was 75%, the positive likelihood ratio was 4, and the posttest probability was 92%, indicating that the probability was 94%. It further confirmed that serum creatinine had a good predictive value for acute PQ poisoning.
There was significant heterogeneity in the accuracy assessment of this study, and meta-regression to explore some of the variables that might have contributed to the heterogeneity showed that none of these factors were the source of heterogeneity in this study. Therefore, the source of heterogeneity remained to be determined. In addition, there are some limitations of this study. First, the included literatures were retrospective and mostly case-control studies, which were susceptible to selection bias and information bias. Second, only 10 studies were included and some of them had small sample sizes. These might cause the limited generalizability and differences in race, region, disease stage, and treatment regimen across studies, which also led to bias. Third, there was a publication bias in this study, which might be related to the fact that most of the enrolled studies were conducted in China and the observational studies were more prone to publication bias. Finally, no unpublished studies were identified in our study, and no attempt was made to include articles in languages other than English.
In conclusion, this meta-analysis demonstrates that blood creatinine may be an independent prognostic indicator for patients with PQ poisoning. Therefore, clinicians should consider blood creatinine levels after PQ poisoning. However, a more adequate reason needs to be confirmed by clinical trials.
Supporting information
Recent queries in Databases on July 1 2022.
(PDF)
(PDF)
(PDF)
The diamond indicates the combined estimate from the included studies.
(PDF)
(A) Pre-test probability = 25%; (B) Pre-test probability = 50%; (C) Pre-test probability = 75%. Each Fagan plot contains a vertical axis on the left for the pre-test probability, an axis in the middle represents the likelihood ratio, and a vertical axis on the right represents the post-test probability. NLR: negative likelihood ratio, PLR: positive likelihood ratio.
(PDF)
(PDF)
(XLSX)
Acknowledgments
The authors would like to thank Zhang Zheng for assistance in statistical analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72(5):745–57. doi: 10.1111/j.1365-2125.2011.04026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila). 2008;46(6):515–8. doi: 10.1080/15563650701549403 [DOI] [PubMed] [Google Scholar]
- 3.Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health. 2007;7:357. doi: 10.1186/1471-2458-7-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee HL, Lin HJ, Yeh ST, Chi CH, Guo HR. Presentations of patients of poisoning and predictors of poisoning-related fatality: findings from a hospital-based prospective study. BMC Public Health. 2008;8:7. doi: 10.1186/1471-2458-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Bai L, Xue X, Peng L, Jiang J, Zhang X. Hyperamylasemia as an early predictor of mortality in patients with acute paraquat poisoning. J Int Med Res. 2020;48(3):300060520910037. doi: 10.1177/0300060520910037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Lee JH, Seong AJ, Hong CK, Lee HJ, Shin DH, et al. Arterial lactate as a predictor of mortality in emergency department patients with paraquat intoxication. Clin Toxicol (Phila). 2012;50(1):52–6. doi: 10.3109/15563650.2011.639716 [DOI] [PubMed] [Google Scholar]
- 7.Min YG, Ahn JH, Chan YC, Ng SH, Tse ML, Lau FL, et al. Prediction of prognosis in acute paraquat poisoning using severity scoring system in emergency department. Clin Toxicol (Phila). 2011;49(9):840–5. doi: 10.3109/15563650.2011.619137 [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Xuan D, Li X, Ma L, Zhou Y, Zou H. The value of APACHE II in predicting mortality after paraquat poisoning in Chinese and Korean population: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(30):e6838. doi: 10.1097/MD.0000000000006838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XW, Ma T, Li LL, Qu B, Liu Z. Predictive values of urine paraquat concentration, dose of poison, arterial blood lactate and APACHE II score in the prognosis of patients with acute paraquat poisoning. Exp Ther Med. 2017;14(1):79–86. doi: 10.3892/etm.2017.4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weng CH, Hu CC, Lin JL, Lin-Tan DT, Hsu CW, Yen TH. Predictors of acute respiratory distress syndrome in patients with paraquat intoxication. PLoS One. 2013;8(12):e82695. doi: 10.1371/journal.pone.0082695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng CH, Hu CC, Lin JL, Lin-Tan DT, Huang WH, Hsu CW, et al. Sequential organ failure assessment score can predict mortality in patients with paraquat intoxication. PLoS One. 2012;7(12):e51743. doi: 10.1371/journal.pone.0051743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Liu L, Li T, Yuan D, Wang Y, Xu Z, et al. A novel simple risk model to predict the prognosis of patients with paraquat poisoning. Sci Rep. 2021;11(1):237. doi: 10.1038/s41598-020-80371-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila). 2009;47(9):870–5. doi: 10.3109/15563650903306651 [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24(4):1226–32. doi: 10.1093/ndt/gfn615 [DOI] [PubMed] [Google Scholar]
- 15.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–93. doi: 10.1146/annurev.pharmtox.48.113006.094615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen XF, Li XM, Liu KX, Wang YL, Chen XB. Prognostic values of serum creatinine in patients with acute paraquat intoxication in 24 hours. Chin Gen Pract. 2011;14(30):3511–2. doi: 10.3969/j.issn.1007-9572.2011.30.030 (2011). [DOI] [Google Scholar]
- 17.Li J, Li Y, Tian JY., Shou ST., Li T. The changes and clinical significance of renal function in patients with acute paraquat poisoning. Chin Comm Doc. 2014;30(14):69–70+2. doi: 10.3969/j.issn.1007-614x.2014.14.40 (2014). [DOI] [Google Scholar]
- 18.Wang JL., Cao SH., Wang YQ., Chang WX. Analysis of prognostic factors of paraquat poisoning. Chin J of Prevent Control of Chronic Dis. 2011;19(02):201–2. doi: 10.16386/j.cjpccd.issn.1004-6194.2011.02.033 [DOI] [Google Scholar]
- 19.Wang L, Cai X, Zhao M. Analysis of risk factors for mortality from acute paraquat poisoning. J China Med Univ. 2018;47(03):237–9+43. doi: 10.12007/j.issn.0258-4646.2018.03.010 (2018). [DOI] [Google Scholar]
- 20.Xiao FX, Peng K, Zhang JL, Indima N, Xu TT, Tang GY. The prognostic value of volume of ground glass opacity with in 24 hours and its early dynamic change in paraquat poisoning. J Clin Radiol. 2017;36(07):976–80. doi: 10.13437/j.cnki.jcr.2017.07.015 [DOI] [Google Scholar]
- 21.Kavousi-Gharbi S, Jalli R, Rasekhi-Kazerouni A, Habibagahi Z, Marashi SM. Discernment scheme for paraquat poisoning: A five-year experience in Shiraz, Iran. World J Exp Med. 2017;7(1):31–9. doi: 10.5493/wjem.v7.i1.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song YX, Fan SL, Peng A, Shen S, Cheng JF, Chen GQ, et al. A retrospective analysis reveals a predictor of survival for the patient with paraquat intoxication. Clin Chim Acta. 2020;511:269–77. doi: 10.1016/j.cca.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 23.Su Y, Liu W, Dong G, Qian Z, Gurram N, Liu E, et al. Investigation of simple, objective, and effective indicators for predicting acute paraquat poisoning outcomes. Toxicol Ind Health. 2020;36(6):417–26. doi: 10.1177/0748233720933522 [DOI] [PubMed] [Google Scholar]
- 24.Wan X, Zhou C, Kang X, Hu D, Xue W, Li X, et al. Metabolic Profiling of Amino Acids Associated with Mortality in Patients with Acute Paraquat Poisoning. Med Sci Monit. 2018;24:1397–407. doi: 10.12659/msm.905843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Feng SY, Li Y. Serum anion gap at admission as a predictor of the survival of patients with paraquat poisoning: A retrospective analysis. Medicine (Baltimore). 2020;99(31):e21351. doi: 10.1097/MD.0000000000021351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Jiang X, Lu G, Zhou J, Kang J, Zhang JS. Identify the Early Predictor of Mortality in Patients with Acute Paraquat Poisoning. Biomed Res Int. 2020;2020:8894180. doi: 10.1155/2020/8894180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Song YQ, Gao J, Feng SY, Li Y. Monocytes as an Early Predictor for Patients with Acute Paraquat Poisoning: A Retrospective Analysis. Biomed Res Int. 2019;2019:6360459. doi: 10.1155/2019/6360459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180(1):65–77. doi: 10.1016/s0300-483x(02)00382-7 [DOI] [PubMed] [Google Scholar]
- 29.Mohamed F, Buckley NA, Jayamanne S, Pickering JW, Peake P, Palangasinghe C, et al. Kidney damage biomarkers detect acute kidney injury but only functional markers predict mortality after paraquat ingestion. Toxicol Lett. 2015;237(2):140–50. doi: 10.1016/j.toxlet.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 30.Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, et al. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011;202(1):69–74. doi: 10.1016/j.toxlet.2011.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang C, Kim SC, Lee SH, Jeong JH, Kim DS, Kim DH. Absolute lymphocyte count as a predictor of mortality in emergency department patients with paraquat poisoning. PLoS One. 2013;8(10):e78160. doi: 10.1371/journal.pone.0078160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scherrmann JM, Houze P, Bismuth C, Bourdon R. Prognostic value of plasma and urine paraquat concentration. Hum Toxicol. 1987;6(1):91–3. doi: 10.1177/096032718700600116 [DOI] [PubMed] [Google Scholar]
- 33.Wu WP, Lai MN, Lin CH, Li YF, Lin CY, Wu MJ. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: a nationwide study. PLoS One. 2014;9(1):e87568. doi: 10.1371/journal.pone.0087568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34(2):368–73. doi: 10.1097/01.ccm.0000195013.47004.a8 [DOI] [PubMed] [Google Scholar]
- 35.Wang WJ, Zhang LW, Feng SY, Gao J, Li Y. Sequential organ failure assessment in predicting mortality after paraquat poisoning: A meta-analysis. PLoS One. 2018;13(11):e0207725. doi: 10.1371/journal.pone.0207725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Zhang H, Zhang G. [Early white blood cell count in predicting mortality after acute paraquat poisoning: a Meta-analysis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31(8):1013–7. doi: 10.3760/cma.j.issn.2095-4352.2019.08.020 [DOI] [PubMed] [Google Scholar]
- 37.Li S, Zhao D, Li Y, Gao J, Feng S. Arterial lactate in predicting mortality after paraquat poisoning: A meta-analysis. Medicine (Baltimore). 2018;97(34):e11751. doi: 10.1097/MD.0000000000011751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang FW, Gao J, Zhang SL, Wu CP, Li Y, Bai WJ, et al. Base excess in predicting the prognosis of patients with paraquat poisoning: A meta-analysis. Medicine (Baltimore). 2019;98(23):e15973. doi: 10.1097/MD.0000000000015973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao ZX, Zhao Y, Gao J, Feng SY, Wu CP, Zhai YZ, et al. Comparison of severity index and plasma paraquat concentration for predicting survival after paraquat poisoning: A meta-analysis. Medicine (Baltimore). 2020;99(6):e19063. doi: 10.1097/MD.0000000000019063 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Recent queries in Databases on July 1 2022.
(PDF)
(PDF)
(PDF)
The diamond indicates the combined estimate from the included studies.
(PDF)
(A) Pre-test probability = 25%; (B) Pre-test probability = 50%; (C) Pre-test probability = 75%. Each Fagan plot contains a vertical axis on the left for the pre-test probability, an axis in the middle represents the likelihood ratio, and a vertical axis on the right represents the post-test probability. NLR: negative likelihood ratio, PLR: positive likelihood ratio.
(PDF)
(PDF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.