Abstract
Background
This study evaluated of clinical characteristics, outcomes, and mortality risk factors of a severe multisystem inflammatory syndrome in children admitted to a the pediatric intensive care unit.
Methods
A retrospective multicenter cohort study was conducted between March 2020 and April 2021 at 41 PICUs in Turkey. The study population comprised 322 children diagnosed with multisystem inflammatory syndrome.
Results
The organ systems most commonly involved were the cardiovascular and hematological systems. Intravenous immunoglobulin was used in 294 (91.3%) patients and corticosteroids in 266 (82.6%). Seventy-five (23.3%) children received therapeutic plasma exchange treatment. Patients with a longer duration of the PICU stay had more frequent respiratory, hematological, or renal involvement, and also had higher D-dimer, CK-MB, and procalcitonin levels. A total of 16 patients died, with mortality higher in patients with renal, respiratory, or neurological involvement, with severe cardiac impairment or shock. The non-surviving group also had higher leukocyte counts, lactate and ferritin levels, and a need for mechanical ventilation.
Conclusions
In cases of MIS-C, high levels of D-dimer and CK-MB are associated with a longer duration of PICU stay. Non-survival correlates with elevated leukocyte counts and lactate and ferritin levels. We were unable to show any positive effect of therapeutic plasma exchange therapy on mortality.
Impact
MIS-C is a life-threatening condition.
Patients need to be followed up in the intensive care unit.
Early detection of factors associated with mortality can improve outcomes.
Determining the factors associated with mortality and length of stay will help clinicians in patient management.
High D-dimer and CK-MB levels were associated with longer PICU stay, and higher leukocyte counts, ferritin and lactate levels, and mechanical ventilation were associated with mortality in MIS-C patients.
We were unable to show any positive effect of therapeutic plasma exchange therapy on mortality.
Introduction
Coronavirus disease 2019 (COVID-19) was first defined in China at the end of 2019 and spread around the world within months, resulting in high rates of mortality and morbidity. On March 11, 2020, the World Health Organization (WHO) declared it a pandemic.1,2 The rate of severe acute respiratory syndrome among children identified as infected with coronavirus 2 (SARS-CoV-2) at that time was 2–6%.3,4 From April 2020, cases of pediatric patients epidemiologically related to SARS-CoV-2 were reported in Europe and the USA presenting with fever, severe systemic hyperinflammation, and cardiovascular shock.5–8 Fever, rash, hyperinflammation, gastrointestinal symptoms, myocardial dysfunction, shock, and serologic evidence for SARS-CoV-2 became common characteristics of the emerging disease.
Although the course of COVID-19 was milder in children than in adults, this pediatric inflammatory disease frequently resulted in severe illness with multiorgan failure and shock and the need for pediatric intensive care unit (PICU) admission. With the increase in cases, on May 14, 2020, the Centers for Disease Control and Prevention (CDC) issued a national health advisory to report on cases meeting the criteria for multisystem inflammatory syndrome in children (MIS-C).9
Children with MIS often improve rapidly with intensive monitoring and supportive care.10,11 Some, however, deteriorate rapidly, requiring cardiac or respiratory support, with poor prognosis related to involvement of the cardiovascular system accompanied by systemic inflammation, severe cardiac disease, shock, and disease of the coronary arteries.
Our aim in this study was to describe the demographic characteristics, presenting symptoms, clinical course, laboratory findings, and the therapies received in case of MIS-C. We also aimed to identify clinical and biological markers that predicting severe disease and mortality among children and adolescents meeting the CDC case definition of MIS-C.
Methods
Study design
This was a retrospective multicenter study conducted on cases in 41 PICUs in Turkey. The patients included in the study had all been diagnosed as having MIS-C according to the CDC criteria and admitted to PICUs between March 2020 and March 2021. Demographic characteristics, clinical and laboratory data, immunomodulatory therapies, respiratory and cardiovascular support modalities (vasoactive drugs and/or extracorporeal membrane oxygenation [ECMO]), and extracorporeal therapies, such as renal replacement therapy and therapeutic plasma exchange (TPE), were all recorded. All laboratory examinations and treatment decisions were made by the attending physicians in each unit.
Case definition
The CDC case definition of MIS-C was used.9 Cardiovascular involvement was defined as follows: need for vasopressors or vasoactive support to maintain blood pressure (BP) within normal limits for age, ejection fraction (EF) by echocardiographic of below 55%, dilated coronary arteries, pericarditis or pericardial effusion, and high troponin or N-Terminal pro-brain natriuretic peptide (NT-proBNP) levels or cardiac arrhythmia. Left ventricular EF (LVEF) measurement was based on the modified Simpson’s method and categorized as either normal (≥55%), or mild (45–54%), moderate (30–44%), or severe impairment (<30%).12 Patients were clinically diagnosed as being in shock when their BP was lower than the fifth percentile of the normal values for age, they needed for vasoactive medication to maintain normal BP, or they showed symptoms of hypoperfusion despite adequate fluid resuscitation.12,13 Patients with clinical presentations of Kawasaki-like disease (KLD) were categorized as such according to the 2017 KD criteria of the American Heart Association (AHA).13
Elevation of creatinine levels more than twice the normal by age was the criterion for renal dysfunction; coagulation abnormality or thrombocytopenia (<100,000/mm3) was regarded as hematological dysfunction; tachypnea, dyspnea, pneumonia, and acute respiratory distress syndrome (ARDS) or pleural effusion were regarded as respiratory disease. Illness severity was estimated using the Pediatric Risk of Mortality III (PRISM III) and Pediatric Logistic Organ Dysfunction 2 (PELOD-2) scores.14,15 For the PRISM III score, various variables were recorded at 24 h of admission (heart rate, systolic BP, temperature, mental status, pupillary response, acidosis, pH, pCO2, total CO2, PaO2, glucose, potassium, creatinine, blood urea nitrogen [BUN], white blood cell [WBC] count, platelet count, and prothrombin and partial thromboplastin time [PT and PTT]). The Pediatric Logistic Organ Dysfunction (PELOD) score consists of ten variables used to represent five organ dysfunctions (neurological, cardiovascular, renal, respiratory, and hematologic).
Study approval
This study was approved by the Scientific Research Platform of the Turkish Republic Ministry of Health and ethically approved by Acıbadem Mehmet Ali Aydınlar University Medical Research Evaluation board (2021-02/16).
Statistical analyses
The Number Cruncher Statistical System (NCSS) 2007 (Kaysville, Utah) program was used for statistical analyses. Descriptive statistical methods were used for demographic data (mean, standard deviation, median, first and third quartiles, frequency, percentage, and minimum and maximum). The normality of quantitative data distributions was tested using the Shapiro–Wilk test and graphics. Comparisons between groups of quantitative data were made using the independent samples t test if distributed normally or the Mann–Whitney U-test if skewed. Pearson’s Chi-square and Fisher’s exact test were used for comparisons of qualitative data. Diagnostic screening tests and ROC analysis were used to determine the cut-off point for leukocyte, ferritin, and lactate measurements according to mortality. p Values of <0.05 were accepted as significant.
Results
Demographic and clinical characteristics
Between March 1, 2020 and March 31, 2021, 322 children met the criteria for confirmed MIS-C in 41 PICUs in Turkey and were included in the analyses; demographics and baseline clinical characteristics, clinical presentation, and comorbidities are presented in Table 1. The total number of patients admitted to the PICUs during this period was 17,423 (mortality rate 9%, n = 1569). The patient medians for age and weight were 119.4 ± 53.8 months and 38.9 ± 22.1 kg. Of the 322 patients, 185 (57%) were male, and 275 (85.4%) had no previous disease. The largest number of patients were admitted in December 2020 (39.1%, n = 126).
Table 1.
Demographic and clinical characteristics of patients.
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 137 (43) |
| Male | 185 (57) |
| Age, months | 119.44 ± 53.8 |
| PRISM | 9 (4–15) |
| PELOD | 10 (6–14) |
| VIS | 15 (10–30) |
| Mean fever time (days) | 4.5 (2–6) |
| SARS-CoV-2 antigen PCR positive | 30 (9) |
| SARS-CoV-2 serology positive | 240 (74.5) |
| SARS-CoV-2 antigen PCR and serology positive | 13 (4) |
| Length of stay PICU, days | 6 (3–9) |
| Clinical presenting features | |
| Fever | 322 (100) |
| Shock | 215 (66.8) |
| Tachypnea or dyspnea | 196 (60.8) |
| Skin rash | 182 (57) |
| Abdominal pain | 176 (54.6) |
| Conjunctival changes | 168 (52.1) |
| Nausea/vomiting | 150 (46.6) |
| Comorbidities | 47 (14.6) |
| Obesity | 22 (6.8) |
| Congenital heart disease | 6 (1.9) |
| Neuromuscular disease | 6 (1.9) |
| Malignancy | 8 (2.5) |
| Rheumatological disease | 3 (0.9) |
| Asthma | 2 (0.6) |
| The most commonly involved organ systems | |
| Cardiovascular | 274 (85) |
| Hematological | 267 (82.9) |
| Gastrointestinal | 201 (62.4) |
| Mucocutaneous | 190 (59) |
| Respiratory | 163 (50.6) |
| Renal | 68 (21.1) |
| Neurological | 88 (27.3) |
| Therapy | |
| Mechanical ventilation | 55 (17) |
| Non-invasive MV | 68 (21.1) |
| HFNC | 97 (30.1) |
| ECMO | 11 (3.4) |
| TPE | 75 (23.3) |
| CRRT | 19 (5.9) |
| Vasopressor support | 228 (70.8) |
| Milrinone | 129 (40) |
| Epinephrine | 117 (36.3) |
| Norepinephrine | 102 (31.7) |
| Dopamine | 27 (8.4) |
| IVIG | 294 (91.3) |
| Systemic glucocorticoids | 266 (82.6) |
| Low dose (2 mg/kg/day) | 139 (43.2) |
| Medium dose (10 mg/kg/day) | 22 (6.8) |
| High dose (30 mg/kg/day) | 105 (32.6) |
| IVIG+ glucocorticoids | 255 (79.2) |
| Interleukin-1Ra inhibitor | 72 (22.4) |
| Interleukin-6 inhibitors | 11 (3.4) |
| Anticoagulation (LMWH) | 225 (69.9) |
| Aspirin | 127 (39.4) |
PRISM pediatric risk of mortality, PELOD pediatric logistic organ dysfunction, VIS vasoactive inotropic score, PCR polymerase chain reaction, MV mechanical ventilation, HFNC high flow nasal cannula, ECMO extracorporeal membrane oxygenation, TPE therapeutic plasma exchange, CRRT continuous renal replacement treatment, IVIG intravenous immunoglobulin, LMWH low molecular weight heparin.
RT-PCR and antibody status by clinical subphenotype
A total of 249 (77.3%) children had microbiological or serological evidence of SARS-CoV-2 infection; 36 (11.1%) had a positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR), 230 (71.4%) were positive for SARS-CoV-2 immunoglobulin (Ig)-M or IgG, and 17 (5.2%) patients tested positive for both RT-PCR and antibodies. All patients without serological evidence had evidence of infection in the previous 4 weeks or a history of contact with a positive family member. The duration of PICU stay was longer in patients who were PCR-positive than in those with seropositivity. Respiratory disease was more common in PCR-positive patients, and cardiovascular disease was more common in seropositive patients.
Laboratory and echocardiographic findings
One hundred forty-three (44.4%) children had thrombocytopenia and lymphopenia was found in 255 (79.2%). Overall, the majority of cases had markedly elevated inflammatory markers: C-reactive protein (CRP) (97.8%), ferritin (83.5%), procalcitonin (68.6%), and erythrocyte sedimentation rate (ESR) (51.2%). Elevated levels of fibrinogen were found in 74.8% of patients, and hypoalbuminemia was identified in 215 patients (66.8%). The majority of patients had elevated levels of NT-proBNP (60.2%), and 66.1% had elevated troponin levels. The laboratory parameters are shown in Table 2.
Table 2.
Admission and peak laboratory test results.
| Median (IQR) | |
|---|---|
| WBC (×103/L) | 10.9 (7.1–15.9) |
| Peak WBC (×103/L) | 6.4 (4.4–9.1) |
| ALC (×103/L) | 0.9 (0.6–1.6) |
| Minimum ALC (×103/L) | 0.7 (0.4–0.9) |
| Platelets (×109/L) | 160 (108.6–250) |
| D-dimer (μg/mL) | 3.2 (1.4–5.9) |
| Lactate, mmol/L | 2 (1.5–3) |
| Peak lactate, mmol/L | 2.9 (2.01–4.4) |
| Serum creatinine, mg/dL | 0.6 (0.4–0.8) |
| Sodium meq/L | 133.3 (131–137) |
| Albumin, g/dL | 3.1 (2.8–3.7) |
| AST, U/L | 35.5 (24–58) |
| ALT (U/L) | 27 (17–50) |
| Ferritin ng/mL | 513.3 (282–974) |
| Peak ferritin ng/mL | 673 (400–1325) |
| Troponin, ng/mL | 0.1 (0.02–0.3) |
| CK-MB, ng/mL | 3.3 (1.1–14) |
| NT-pro-BNP pg/mL | 3077 (624–12802.5) |
| Peak NT-pro-BNP pg/mL | 5000 (1124–16054) |
| CRP mg/dL | 23.2 (11.9–99.2) |
| Peak CRP mg/dL | 27.8 (16.6–156.5) |
| Procalcitonin ng/mL | 4.2 (1.1–22.4) |
| Peak procalcitonin ng/mL | 8.1 (2.3–33) |
| IL-6 pg/mL | 70.5 (20.1–355) |
| Peak IL-6 pg/mL | 107 (28.8–585) |
| ESR mm/h | 45 (23–76) |
| Fibrinogen ng/mL | 455 (258.2–622.5) |
| Peak fibrinogen ng/mL | 500 (361.5–662) |
WBC white blood cell count, ALC absolute lymphocyte count, AST aspartate aminotransferase, ALT alanine aminotransferase, CK-MB creatine kinase isoenzyme, NT-proBNP N-terminal pro-brain natriuretic peptide, CRP C-reactive protein, IL-6 interleukin 6, ESR erythrocyte sedimentation rate.
During hospitalization, at least one echocardiogram was obtained for 300 (93.2%) patients, of whom 134 (41.6%) had decreased LVEF (<55%), and 84 (28%) had pericardial effusion. Of the patients with decreased LVEF, 36 (11%) had an EF of <30%, and 98 (30%) had an EF of between 30 and 55%. In total, 28 patients had cardiac arrhythmias and 2 had intracardiac thrombosis.
Among the 322 children with MIS-C, 32 (10%) had overlapping features with KD and were defined as having KLD. Shock was seen in 11 children. Twenty-two children (6.8%) with a diagnosis of KLD were found to have coronary artery abnormalities on echocardiography during PICU admission, including 6 with z scores >2.5. Giant coronary artery aneurysms (z score >10) were documented in one patient. All 32 patients with a KLD diagnosis received high-dose intravenous immunoglobulin (IVIG) (2 g/kg). Patients with KLD were younger than the patients without KLD (8.5 years [IQR 4–12] vs. 10.1 years (IQR 6.5–14), p = 0.047). Nevertheless, there were no significant differences between the two groups in terms of laboratory parameters, duration of stay, mechanical ventilation, or mortality.
Management and clinical outcomes
Treatments and outcomes are shown in Table 1. Seventy-five (23.3%) children were treated with TPE, and 19 (5.9%) received continuous renal replacement therapy (CRRT). Several parameters were significantly higher in the TPE group (Table 3).
Table 3.
Comparison between the TPE (−) and TPE (+) groups.
| TPE (−) (n = 247) | TPE (+) (n = 75) | ||
|---|---|---|---|
| Mean ± SD (median) | Mean ± SD (median) | ||
| Age (months) | 114.33 ± 52.32 | 136.27 ± 55.52 | 0.001* |
| PICU duration | 5 (3–8) | 7 (5–12) | 0.002* |
| PRISM III score | 9 (6–16) | 15 (8–28) | <0.001* |
| PELOD score | 10 (5–11) | 15 (11–29) | 0.002* |
| Number of organ system disease | 4 (3–5) | 4 (3–6) | a0.001* |
| VIS | 15 (10–25) | 20 (12–55) | 0.001* |
| Presence of shock | 156 (63.2%) | 59 (78.7%) | b0.013* |
| Severe cardiac impairment EF <30% | 7 (2.8%) | 9 (12%) | c0.004* |
| Mechanical ventilation | 24 (9.7%) | 31 (41.3%) | b<0.001* |
| Immunomodulatory treatment—anakinra | 42 (17%) | 30 (40%) | b<0.001* |
| Immunomodulatory treatment—tocilizumab | 4 (1.6%) | 7 (9.3%) | c0.004* |
TPE therapeutic plasma exchange, PRISM pediatric risk of mortality, PELOD pediatric logistic organ dysfunction, VIS vasoactive inotropic score, EF ejection fraction.
*p < 0.05.
aMann–Whitney U-test; shown in the table as the median (Q1–Q3).
bPearson chi-square test.
cFisher’s exact test.
The median length of PICU stay was 6 (3–9) days. Patients with longer duration of PICU stay (defined as >6 days) had more frequent respiratory, hematologic, or renal involvement and also had higher D-dimer, CK-MB, and procalcitonin levels. Logistic regression analysis showed that elevated D-dimer (odds ratio [OR] = 1.041, 95% confidence interval [CI]: [1.008–1.074]; p = 0.015) and CK-MB (OR = 2.264, 95% CI: [1.060–4.835]; p = 0.035) levels were significantly associated with longer duration of PICU stay.
Among the 16 (5%) patients who died, eight were male (50%), and the median age was 127.4 ± 61.1 months. Eight of these patients had diagnoses of underlying conditions (acute lymphocytic leukemia, malignancy, congenital heart diseases, cerebral palsy), and five received ECMO support. All of them received systemic glucocorticoids, 14 received IVIG, and 8 received immunomodulators.
The percentages of patients with neurological or respiratory symptoms were higher among non-survivors, and there was an increased incidence of severe cardiac impairment (EF < 30%) compared with survivors. The presence of renal involvement, need for mechanical ventilation, and shock were also significantly more common in non-survivors. The serum levels of D-dimer, ferritin, lactate, and CRP were significantly raised, and lymphopenia was more common in non-survivors than survivors. A comparison of laboratory parameters of survivors and non-survivors is presented in Table 4. Logistic regression analysis showed that the non-surviving group had higher leukocyte counts, lactate levels, ferritin levels, and a need for mechanical ventilation. The cut-off value was calculated for the factors associated with mortality (leukocyte, lactate, ferritin) (Table 5). ROC curve for leukocyte, lactate and ferritin have shown in Figs. 1 and 2.
Table 4.
Comparison of the survivor and non-survivor groups.
| Survivor (n = 306) | Non-survivor (n = 16) | ap | |
|---|---|---|---|
| Median (Q1, Q3) | Median (Q1, Q3) | ||
| Age | 110.2 ± 43.4 | 127.4 ± 61.1 | 0.145 |
| PRISM score | 9 (4–15) | 24 (17–33) | <0.001* |
| PELOD | 10 (5–12) | 30 (15–40) | <0.001* |
| Organ system involvement | 4 (3–5) | 6 (4.5–6) | <0.001* |
| VIS | 15 (10–25) | 105 (55–144) | <0.001* |
| Leukocyte (on admittance) | 10.92 (6.86–15.51) | 16.06 (9.49–27.05) | 0.019* |
| Lymphocyte (on admittance) | 2.83 (0.9–4.42) | 0.9 (0.6–1.44) | 0.001* |
| D-dimer (Ug/mL) (on admittance) | 3.24 (1.38–4.53) | 9 (1.54–16) | 0.024* |
| Lactate (on admittance) | 2 (1.4–2.96) | 4.5 (2.5–13) | <0.001* |
| Lactate (peak) | 2.8 (2–4.1) | 12.7 (5.2–20) | <0.001* |
| Ferritin (on admittance) | 503 (278.45–900.5) | 2216 (1084–18,090) | <0.001* |
| Ferritin (peak) | 659 (400–1211) | 10,956 (2834–21,320) | <0.001* |
| Troponin (on admittance) | 0.07 (0.02–0.27) | 0.24 (0.04–0.47) | 0.173 |
| CK-MB (on admittance) | 3.25 (1.12–12.93) | 5.52 (0.96–21.51) | 0.639 |
| NT-proBNP (on admittance) | 3077 (634–12,405) | 2676 (256–14,000) | 0.739 |
| CRP (on admittance) | 14.1 (2.18–20.4) | 23.76 (12.3–105.39) | 0.008* |
| CRP (peak) | 20.25 (15.15–35.1) | 28.4 (16.68–160.6) | 0.196 |
| PCT (on admittance) | 4.85 (1.31–22.36) | 0.74 (0.43–58.3) | 0.247 |
| PCT (peak) | 8.22 (2.41–31.8) | 3.77 (1.7–58.3) | 0.796 |
| IL-6 (on admittance) | 70 (19–355) | 285 (35.85–652.5) | 0.220 |
| Sedimentation (on admittance) | 45 (23–76) | 60 (26.5–65) | 0.964 |
| Fibrinogen (on admittance) | 461 (273–630) | 309.5 (94–391) | 0.028* |
| n (%) | n (%) | p | |
| Cardiac disease | 259 (84.6) | 15 (93.7) | b0.483 |
| Renal disease | 59 (19.3) | 11 (68.7) | b<0.001* |
| Respiratory disease | 151 (49.3) | 12 (75) | c0.045* |
| Hematological disease | 251 (82) | 16 (100) | b0.084 |
| Neurological disease | 79 (25.8) | 9 (56.2) | b0.017* |
| Severe cardiac impairment EF < 30% | 27 (8.2) | 9 (56.2) | b0.005* |
| Mechanical ventilation | 42 (13.7) | 13 (81.2) | b<0.001* |
PRISM pediatric risk of mortality, PELOD pediatric logistic organ dysfunction, VIS vasoactive inotropic score, CK-MB creatine kinase isoenzyme, NT-proBNP N-terminal pro-brain natriuretic peptide, CRP C-reactive protein, PCT procalcitonin, IL-6 interleukin 6, EF ejection fraction.
*p < 0.05.
aMann–Whitney U-test.
bFisher’s exact test.
cPearson chi-square test.
Table 5.
Mortality screening tests and ROC curve results.
| Mortality scan | ROC curve | p | ||||||
|---|---|---|---|---|---|---|---|---|
| Cut off | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Area | 95% confidence interval | ||
| Leukocyte on admittance | ≥17.6 | 50.0 | 82.62 | 13.11 | 96.92 | 0.746 | 0.619–0.874 | 0.002** |
| Lactate on admittance | ≥2.34 | 86.67 | 61.73 | 10.92 | 98.84 | 0.823 | 0.731–0.945 | 0.001** |
| Lactate max | ≥4,6 | 100.0 | 80.59 | 22.05 | 100.0 | 0.936 | 0.894–0.978 | 0.001** |
| Ferritin on admittance | ≥1082 | 80.0 | 79.79 | 16.90 | 98.73 | 0.803 | 0.660–0.946 | 0.001** |
| Ferritin max | ≥2790 | 78.57 | 92.34 | 35.48 | 98.77 | 0.795 | 0.604–0.986 | 0.001** |
**p < 0.01.
Fig. 1. Leukocyte, lactate and ferritine levels on admission.
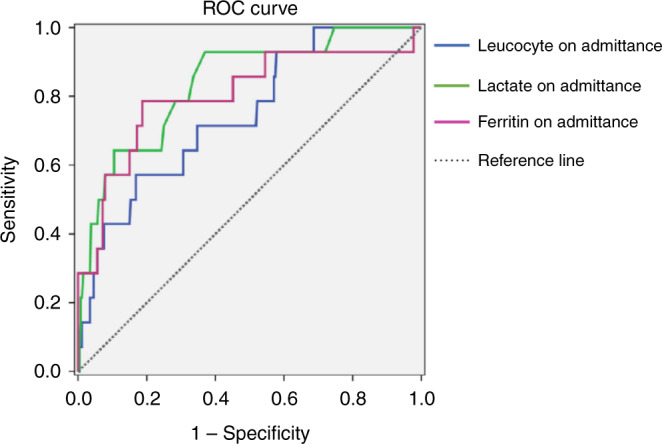
ROC curves of leukocyte, lactate and ferritin on admission.
Fig. 2. Max lactate and ferritin levels.
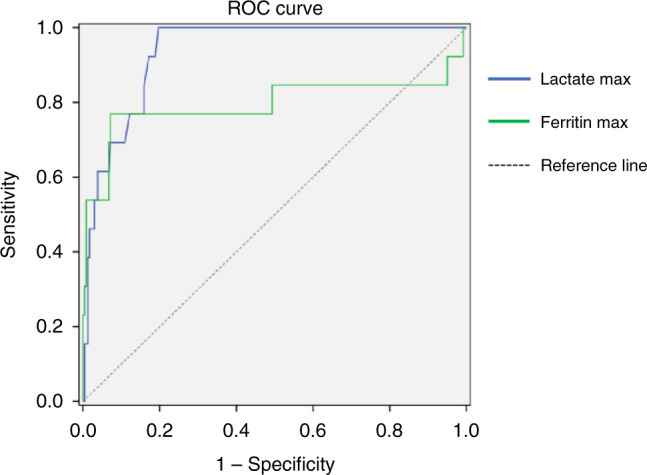
ROC curves of max lactate and ferritin levels.
Discussion
Our study of pediatric patients with MIS-C in Turkey, describes a febrile hyperinflammatory syndrome currently seen worldwide that has gastrointestinal, dermatologic, mucocutaneous, hematological, and respiratory manifestations associated with cardiac dysfunction. Although multiple reports have been published internationally on MIS-C, there are a limited number of studies evaluating patients with severe disease admitted to the PICU.
Similar to other reports, most of the patients in our study were male (57%).16–18 This higher frequency of males can be understood as an effect of the biological differences between the sexes of sensitivity to infections, adaptive immune response, immune regulation, inflammation, and tissue repair.19
Because the case definition is nonspecific and confirmatory laboratory testing does not exist, it may be difficult to distinguish MIS-C from other conditions with overlapping clinical manifestations such as severe acute COVID-19. In a study by Godfred et al.20 in which the patients were classified into three groups according to clinical characteristics, in class 1 patients, cardiovascular system disease was prominent, and SARS-CoV-2 serology was positive in 98%, while class 2 patients had mainly respiratory system disease and the highest rate of SARS-CoV-2 positivity in PCR (84%). These class 2 patients also had a longer duration of ICU stay. Similarly, the rate of cardiovascular system findings in our study was higher in patients with positive SARS-CoV-2 serology, and respiratory system disease was more frequent in patients testing positive for SARS-CoV-2, and the latter had a longer duration of PICU stay.
The rates of patients diagnosed with MIS-C and being admitted to the ICU have been reported at between 68 and 80%,10,17,21 and mortality rates are 1.7–2.6%.7,17,18,20,21 In a study by Son et al.,22 385 out of 518 patients with MIS-C were admitted to the ICU, and 9 (2.3%) died (although there are studies reporting mortality rates as high as 27% and even 66%).23,24 The mortality rate was 5% in our study. This relatively higher rate can be explained by the study population being comprised of patients admitted to the ICU, but it may also be the result of possible challenges in diagnosis and referral for a relatively new syndrome resulting in delays in treatment. Some patients were transferred from other cities, while others were transferred to the PICU only when they deteriorated clinically and displayed an emerging need for mechanical ventilation or ECMO, both of which would affect the prognosis negatively. Mortality increases with delayed admittance to the PICU and delayed referral to centers with ICUs.
Although there are numerous studies on MIS-C, factors associated with mortality rates are rarely commented on. Maheshwari et al. reported that non-survivors had more neurocognitive and respiratory symptoms and an increased incidence of myocarditis than survivors. Furthermore, the presence of acute kidney injury, need for ventilation, and shock was significantly more common in non-survivors.23 Similarly, in our study, non-survivors had more neurologic and respiratory symptoms and an increased incidence of severe cardiac impairment compared with survivors. The presence of renal involvement and the need for mechanical ventilation were significantly more common in non-survivors.
In proinflammatory states, including MIS-C, inflammatory markers levels, such as CRP, ESR, procalcitonin, D-dimer, and ferritin, may be raised.5,24–26 Patients with severe MIS‐C also have higher levels of WBCs, absolute neutrophil count (ANC), and ferritin, as well as CRP and D‐dimer, and lower levels of absolute lymphocyte count (ALC) and fibrinogen than patients with non‐severe MIS‐C.26–28 In our study, serum levels of D-dimer, CRP, lactate, and ferritin were significantly raised in non-survivors as compared with survivors. Also, non-survivors had lower ALC and low fibrinogen levels. This indicates a more severe inflammatory response in non-survivors. One of the distinctive features of our study was the definition of factors that associated with mortality and duration of stay in PICU. According to our data, high levels of D-dimer and CK-MB are related to longer duration of PICU stay; high leukocyte count, ferritin and lactate levels, and mechanical ventilation are all related to mortality.
In other reports of patients with MIS-C, the rates of shock at presentation were 50–84%,7,29–31 inotropic support 25–77%,7,16,28,30,32 and mechanical ventilation 4–43%7,28,32 in patients with MIS-C. Notable findings were the high prevalence of cardiac dysfunction and shock, in contrast with most cases of acute COVID-19 among children in the PICU. Our results are consistent with those of other studies to date, which have been limited to case reports, short reports, and case series.10,17,25,27,33
Both NT-proBNP and cardiac troponin levels are extremely high in patients with MIS-C. In our study, the majority of patients had elevated levels of NT-proBNP and troponin. Whittaker et al. found that NT-proBNP levels were elevated in 83% of patients, and troponins were increased in 68% of patients.7 We have reported 85% of all patients as evidencing of cardiac involvement based on biochemical, electrocardiogram (ECG), and echocardiogram data. This is a significantly high rate when compared with a study from Spain, which reported cardiologic complications in 61% and myocardial dysfunction in 48% of MIS-C patients.31 A study by Garcia-Salido et al.30 described cardiac dysfunction in 53.3% of patients, an Italian study reported cardiac involvement with ECG abnormalities in 60%,6 and Torres et al.34 found abnormal ECG in 31%. All MIS-C patients should be evaluated through ECG in a sequential protocol because the rate of cardiac involvement is high with myocardial dysfunction and changes in coronary arteries and the long-term prognosis is not definitive.
TPE is a well-known immunomodulatory technique in pediatrics that removes high-molecular-weight substances, including cytokines and autoantibodies. In adult patients with severe COVID-19 infection, good results with TPE have been reported attributed to the control of the hyperinflammatory state, removing cytokines and chemical mediators, decreasing the viral load, and targeting the effects of endothelial dysfunction and coagulopathy with TPE.35 Although the data are insufficient, there are case series reporting the benefit of TPE as salvage therapy in situations where LV dysfunction, a high vasoactive inotropic score or PELOD or PRISM scores with high levels of laboratory markers (ferritin, CRP, and NT-proBNP) are present.11 Our study data show that mortality rate was not associated with TPE in high-risk patients (those with high lactate and ferritin levels and high VIS and PRISM scores, presence of shock, LV dysfunction, mechanical ventilation).
The strength of our study lies in its presentation of detailed clinical and laboratory data from multiple centers about MIS-C in a specific patient population (PICU patients). The main limitations are the retrospective design and that the tests and treatments were not made according to a single common protocol but were determined independently in the different centers by the attending physician. Extrapolations of our findings to the current pandemic with viral variants and rising vaccination rates may be challenging because our study ended in March 2021. Lastly, we do not have data following discharge to capture late mortality or delayed complications.
In conclusion, we have defined laboratory and clinical factors that can affect severe disease, PICU stay, and mortality in these 322 patients admitted to the PICUs in Turkey for the new inflammatory phenomenon known as MIS-C. Although long-term results are not known, most of the patients had cardiac involvement. Most (93.2%) of the patients were evaluated using ECG, but echocardiography should be ordered for all the children presenting with MIS-C because the cardiac involvement rate is high. According to our data, high levels of D-dimer and CK-MB are related to longer stay; high leukocyte count and ferritin and lactate levels, and mechanical ventilation are related to mortality. Although there is inadequate evidence to support any specific treatment, most patients received immunomodulatory treatment; some were treated with TPE but we were unable to show any positive effect of this therapy on mortality could be defined. This lack of evidence on useful treatment modalities are useful underlines the need for further clinical studies.
Author contributions
G.S., A.I., A.C., I.B., and A.A. conceptualized and designed the study, collected data, drafted the initial manuscript, and reviewed and revised the manuscript. N.A., S.K., F.A., T.K., G.A., O.S., F.V., P.Y.O., M.D., A.Z.B., S.O., G.A., M.K., S.B., U.A., A.B.A., M.H., A.F.Y., T.D., N.Z., A.O., H.S.K., F.İ.G., L.T., D.Y., N.Y., U.Y., M.A., M.A.K., M.C., A.D., F.B., F.S., M.O., S.T., C.K., A.Y., N.A., C.O., A.Y., E.S., B.K., C.D., E.G., S.E., P.S., E.S., H.F.A., M.B., F.D., S.E., G.O., M.D., M.N.T., G.O.Y., D.L., S.H., E.Z.B., M.M., A.B., N.K., E.A.O., M.N.O., F.E., M.U., A.E.A., N.O.K., A.B., S.Ö., T.C., Y.O., and A.O.K. collected data and designed the data collection instruments. T.K., H.S.K., D.Y., N.Y., and E.S. collected data, critically reviewed the manuscript for important intellectual content, and revised the manuscript.
Data availability
The datasets generated during and/or analyzed in the current study are not publicly available as the patients were in intensive care, but they available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Scientific Research Platform of the Turkish Republic Ministry of Health and was ethically approved by Acıbadem Mehmet Ali Aydınlar University Medical Research Evaluation board (2021-02/16). Since it was a retrospective study, ethics committee approval was obtained while patient consent was not required.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Guntulu Sık, Email: drguntulu@hotmail.com.
Turkish MIS-C Study Group:
Ibrahim Bıngol, Agageldi Annayev, Esra Sevketoglu, Banu Katlan, Cansu Durak, Emrah Gun, Seher Erdogan, Pınar Seven, Ebru Sahın, Hatice Feray Arı, Merve Boyraz, Fatih Durak, Serhat Emeksız, Göktug Ozdemır, Murat Duman, Mehmet Nur Talay, Gülcin Otar Yener, Doga Luleyap, Sezer Harmanogulları, Evic Zeynep Başar, Mehmet Mercan, Alkan Bal, Nevin Kılıc, Ebru Atike Ongun, Makbule Nilufer Ozturk, Faruk Ekıncı, Muhammed Udurgucu, Ali Ertug Arslankoylu, Nurettin Onur Kutlu, Aysegul Bukulmez, Serkan Özsoylu, Taylan Celık, Yasemin Ozkale, and Ahmet Osman Kılıc
References
- 1.Rosenberg ES, et al. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State—March 2020. Clin. Infect. Dis. 2020;71:1953–1959. doi: 10.1093/cid/ciaa549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus diseases (COVID-19) situation report–2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200527-covid-19-sitrep-128.pdf?sfvrsn=11720c0a_2 (2020).
- 3.Castagnoli R, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 4.Yonker LM, Shen K, Kinane TB. Lessons unfolding from pe- diatric cases of COVID-19 disease caused by SARS-CoV-2 infection. Pediatr. Pulmonol. 2020;55:1085–1086. doi: 10.1002/ppul.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdoni L, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;6736:1–8. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittaker E, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belhadjer Z, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). CDC Health Alert Network. https://emergency.cdc.gov/han/2020/han00432.asp (2020).
- 10.Radia T, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021;38:51–57. doi: 10.1016/j.prrv.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emeksiz, S. et al. Therapeutic plasma exchange: a potential management strategy for critically ill MIS-C patients in the pediatric intensive care unit. Transfus. Apher. Sci. 60, 103119 (2021). [DOI] [PubMed]
- 12.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the european association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 13.McCrindle BW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 14.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit. Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Leteurtre S, et al. PELOD-2: an update of the Pediatric logistic organ dysfunction score. Crit. Care Med. 2013;41:1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 16.Lima-Setta F, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: a multicenter, prospective cohort study. J. Pediatr. 2021;97:354–361. doi: 10.1016/j.jped.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldstein LR, et al. Multisystem inflammatory syndrome in US children and adolescents. N. Engl. J. Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies P, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc. Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020;20:442–447. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfred-Cato S, et al. COVID-19–associated multisystem inflammatory syndrome in children—United States, March–July 2020. Morb. Mortal. Wkly. Rep. 2020;69:1074. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed M, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. doi: 10.1016/j.eclinm.2020.100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Son MBF, et al. Multisystem inflammatory syndrome in children—initial therapy and outcomes. N. Engl. J. Med. 2021;385:23–34. doi: 10.1056/NEJMoa2102605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maheshwari A, et al. Comparison of clinical and laboratory profile of survivors and non‐survivors of SARS‐CoV‐2‐related multisystem inflammatory syndrome of childhood in India: an observational study. J. Paediatr. Child Health. 2022;58:136–140. doi: 10.1111/jpc.15675. [DOI] [PubMed] [Google Scholar]
- 24.Pereira MFB, et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics. 2020;75:e2209. doi: 10.6061/clinics/2020/e2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shekerdemian L, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Yin L, Patel J, Tang L, Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS‐C) and adolescents associated with COVID‐19: a meta‐analysis. J. Med. Virol. 2021;93:4358–4369. doi: 10.1002/jmv.26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dufort EM, et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bautista-Rodriguez C, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147:e2020024554. doi: 10.1542/peds.2020-024554. [DOI] [PubMed] [Google Scholar]
- 29.Capone CA, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J. Pediatr. 2020;224:141–145. doi: 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Salido A, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit. Care. 2020;24:1–13.. doi: 10.1186/s13054-020-03332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moraleda C, et al. Multi-inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin. Infect. Dis. 2021;72:e397–e401. doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YP, et al. Distinct clinical and immunological features of SARS– CoV-2–induced multisystem inflammatory syndrome in children. J. Clin. Investig. 2020;130:5942–5950. doi: 10.1172/JCI141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toubiana J, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres JP, et al. Multisystem inflammatory syndrome in children (MIS-C): Report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int. J. Infect. Dis. 2020;100:75–81. doi: 10.1016/j.ijid.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keith P, et al. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit. Care. 2020;24:128. doi: 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are not publicly available as the patients were in intensive care, but they available from the corresponding author upon reasonable request.


