Abstract
In March of 2020, with the full magnitude of the COVID-19 pandemic yet to be seen, Costa and Martin released a report through the Economic Policy Institute noting that “To prevent infections and the spread of COVID-19 on farms, farm employers should be planning and implementing safety measures to protect their employees” (Costa D, Martin P, Coronavirus and farmworkers: farm employment, safety issues, and the H-2A guestworker program, Economic Policy Institute, https://www.epi.org/publication/coronavirus-and-farmworkers-h-2a/, 2020). The report goes on to provide multiple observations recognizing the seasonal nature of farm work, effects increased unemployment may have on the workforce, industry dependence on H-2A visa farm workers, impact school closings would have on worker availability, and includes recommendations for safety equipment, social distancing, as well as worker housing and transportation. This paper focuses on the worker housing component of those recommendations and describes an effort to rapidly develop and deploy a computationally efficient, web-based, low-fidelity mathematical model of COVID-19 spread in dormitory style housing to support education and mitigation strategies for the historically underserved farmworker community.
Keywords: COVID-19, Coronavirus, Farmworker health, Modeling and simulation
Introduction
Agriculture, Forestry, and Fishing workers consistently have the highest fatal injury rate amongst US workers. The farmworker community, and particularly the migrant work force, is chronically medically underserved due to a variety of barriers including language, cost, lack of insurance, transportation, sparsity of rural clinics, and long work hours [1]. In an effort to support research, education, and injury prevention activities in the United States, the National Institute for Occupational Safety and Health (NIOSH), under the Centers for Disease Control (CDC), established the Agricultural Health and Safety Initiative in 1990 [2]. In the present day, there are 11 NIOSH-funded Centers throughout the United States with a focus on Agricultural Safety and Health. When the highly contagious novel coronavirus (COVID-19) was identified, these centers by and large quickly took up the charge of developing education and mitigation strategies to support the farmworker community.
On December 31, 2019, the WHO China Country Office was notified of cases of pneumonia, later identified as COVID-19 [3]. As of June 21, 2021, more than 180 million cases of COVID-19 have been confirmed with nearly 4 million deaths globally [4]. An early estimate of the reproductive value (R0) of the virus was 2.28 [5]. Subsequent studies have demonstrated transmission rates ranging from 1.4 to 6.49, with an average reproductive value of 3.28 [6]. These studies indicate that transmissibility of the virus, is slightly higher than SARS-CoV (R0 = 3, with a range of 2–5) and significantly higher than MERS (R0 < 1) [7, 8].
As a result, efforts to mitigate the spread of COVID-19 led to strict and widespread measures to reduce transmission at the national, state and local level. Prevention directives included bans on large gatherings, “shelter-in-place” orders for non-essential personnel, and social distancing, whenever possible. However, on March 19th, 2020, the U.S. Department of Homeland Security declared agriculture critical infrastructure [9]; thus, deeming farmworkers essential personnel. However, public health directives aimed at the general public and conventional living arrangements posed many challenges for agricultural employers and farmworkers given the unique nature of their working and living arrangements.
In particular, the U.S. is highly reliant on temporary and immigrant workers who come from a variety of countries and regions of the U.S. Roughly 50% of farmworkers are undocumented [10], while 10% work under the H-2A visa program, which allows them to seek temporary employment in the U.S. agricultural industry [11]. The health of H-2A farmworkers is a concern because they travel from their home countries to the US in large groups and are more likely to live in employer-provided housing than other farmworkers. For H-2A workers, employers are obligated to provide housing, but due to the lack of rural housing options, as well as the advantages of having workers close to the worksite [12], housing is also occasionally provided for farmworkers that are not part of a guest worker program. The Housing Assistance Council reports that 25% of housing units occupied by farmworkers are employer-owned [12].
However, looking at the agricultural worker population as a whole (which includes undocumented immigrant workers), the National Agricultural Workers Survey (NAWS) states, “Fifty-four percent of farmworkers interviewed in 2015–2016 reported that they lived in housing they rented from someone other than their employer, 28% of workers said they lived in a home owned by themselves or a family member, and 1% said they paid rent for housing provided by the government, a charity, or other organization. Four percent lived in various other types of housing including duplexes or triplexes, dormitories or barracks, and motels or hotels” [13].
Housing is typically crowded, with workers sharing bedrooms or stacked in bunks to increase capacity. According to a study conducted in North Carolina, 25% of migrant camps violate regulations for bedroom occupancy limits [14, 15]. The 2015–2016 National Agricultural Workers Survey (NAWS) [13] provides further cause for concern. According to the survey “Thirty-three percent of farmworkers lived in “crowded” dwellings, defined as housing units in which the number of persons per room was greater than one”. As a result, farmworker housing, as well as transportation to and from the worksite, present unique risks for disease exposure [12].
Given these factors, practical solutions and tools for reducing the spread of COVID-19 amongst farmworkers was deemed crucial for containing the virus, protecting vulnerable workers and maintaining a reliable workforce for agricultural producers. Initial recommendations for the agricultural workforce largely resembled the sanitation and personal protective equipment (PPE) recommendations from the CDC in response to COVID-19 [16] and for poultry workers during the Avian Influenza crisis [17]; similarly, workplace social distancing guidelines largely draw from prior experience with influenza pandemics [18]. These recommendations include early reporting of suspected cases, worker training to help them accurately identify symptoms of the disease, access to personal protective equipment (PPE), training on effective use of PPE, prophylactic use of antiviral drugs, and vaccination when it became available. However, in early stages of the pandemic, PPE and testing availability was highly limited, and as noted previously, financial constraints, lack of insurance, rural clinic availability, and immigration status all present barriers to access to healthcare for farmworker populations.
With this background as motivation, the authors sought to develop a computationally efficient, user-friendly, online tool geared specifically toward education and COVID-19 spread mitigation for the agricultural workforce living in dormitory style housing. That is, we sought to use simple mathematical modeling in a novel manner to address specific needs of the underserved farmworker community. This paper summarizes both the model, and the breadth of expertise required to develop an effective tool, to serve as a case study for future interventions of this sort.
Modeling
Early in the COVID-19 pandemic, the rallying cry of “flatten the curve” in many ways provided the public with a general introduction to S-I-R models. In an S-I-R model, individuals are classified as either susceptible, infected, or removed/recovered. Individuals are able to “move” from the susceptible state to the infected state, and from the infected state to the removed state, and individuals in the removed state are immune to the virus [19, 20]. Flattening the curve refers to slowing the rate at which individuals move from susceptible to infected. These compartmental epidemiological models have existed since the early twentieth century, with various nuances having been introduced to capture vaccination, reinfection, maternal immunity, age effects, etc. Early in the COVID-19 pandemic, the University of Pennsylvania launched the Penn COVID-19 Hospital Impact Model for Epidemics (CHIME) website using a modified S-I-R model to support hospital capacity planning [21]. Furthermore, NetLogo, a widely used agent-based modeling tool, which looks at individuals as discrete autonomous decision makers, has a viral spread model available via their website [22].
It was our goal to create a simple hybrid of a S-I-R and a pseudo agent-based model that could emulate a farm’s dormitory-style sleep and work environment, and deliver the model in a manner that is accessible to users without a medical or modeling background. The simulation program, entitled “Room for Improvement: An Online Tool for Preventing the Spread of COVID-19 in Farmworker Housing”, was created to help farm owners and farmworkers assess various strategies for mitigating the risk of spreading COVID-19 in a multi-occupant, housing environment. The intent of the web-based tool, available at https://www.nycamh.org/covid-19/housing-simulator/, is largely educational. User-inputs demonstrate how the spread of COVID-19 in farmworker housing can be considerably altered by utilizing several simple prevention strategies. Farm owners and farmworkers can assess their current practices and compare the efficacy of various interventions and strategies to reduce contagion, as well as lower the risk of lost lives, productivity, and wages. As such, the tool is designed to educate farm owners and workers on potential risks, as well as strategies to mitigate the spread of COVID-19.
Assumptions and Initial Conditions
The website tool is a hybrid of a S-I-R model, emulating daytime virus transmission, and a proximity-based model for transmission in a dormitory-style housing arrangement during the evening. Each scenario modeled starts with an initial condition of a single sick individual in the multi-occupant bedroom for an initial night. Over that evening, they conceivably infect none, one or many of their susceptible roommates. The sick worker is presumed to be identified as ill upon arrival at work, then sent away from work and removed from the housing in order to avoid further infection. Notably, the U. S. Department of Labor provided guidance early in the pandemic to enable employers to pursue alternative housing arrangements to slow the spread of the virus [23]. Within the simulation, individuals who are newly infected, however, are contagious and may infect others while asymptomatic as described in further detail in the sections to follow. Recognizing that there are various stochastic variables entering into consideration, such as the number of individuals sleeping in the space, the baseline health of each individual (described below), location of the susceptible and infected individuals while sleeping, and the duration of time an infected individual is asymptomatic, 10,000 Monte Carlo simulations are conducted prior to returning values to the user. With the use of a low-fidelity and computationally efficient model, predicting the number of residents who will become infected over the allocated time can be calculated within seconds using JavaScript in the user’s browser.
User Inputs
To customize the results to an environment closest to that of the farm in question, the user is asked to provide information on the configuration of their sleeping environment, specifically:
Room width.
Room length.
- Single beds or single bunk beds.
- Arrangement of sleepers in bunk beds (e.g. if people sleep with their heads toward the walls or if they sleep alternating direction between top and bottom bunk).
Number of people that usually sleep in the room.
With this information, a floor plan is displayed. It is worth noting, the user can enter a number of residents that exceeds the Code of Federal Regulations 20 CFR §654.407 [24] limits, which is intended to demonstrate the adverse effects of overcrowding. The user is then asked to characterize the farm’s approach to the use of PPE, social distancing, and hand sanitization. An updated floorplan is then displayed illustrating the scenario with text describing most common and worst outcomes in terms of number infected found via 10,000 Monte Carlo simulations.
The First Night—Pseudo Agent-Based Model
With the user provided inputs, the simulation begins by randomly assigning each resident to a specific bed. Beds are evenly spaced throughout the room on alternating sides of the room. Each bed is taken to be 40 in. wide and 75 in. long (nominally twin bed size). When bunks are selected, the upper bunk is located 30 in. above the lower bunk. The location of each individual’s head is assumed to be 9 in. from the end of the bed. The location of the sick resident is also randomized with each simulation.
A parameter referred to as ‘baseline health’ is introduced as an effort to capture the ordinary variability in health seen by the public at large. With recognition that pre-existing conditions may increase susceptibility to viral infection, each resident is also assigned a random baseline health value between 0.5 and 1.0. One resident is marked as sick initially and is assigned a baseline health of 0. Drawing off the kernel function concept of the meshfree, Lagrangian, computational fluids approach of Smoothed Particle Hydrodynamics (SPH) [25], in which the interaction between particles is governed by a function which is stronger with close proximity, and weaker as distance increases, a kernel function is defined per Eq. 1 to govern the health effect of a sick or infected individual upon another.
| 1 |
In Eq. 1, r is the distance between any given resident and a sick or infected resident and h is a parameter governing the likely infection radius. In this model, the infection radius is taken as 6 feet.
The health level of each resident is then calculated as their baseline health multiplied by this kernel function (Eq. 1). This kernel function results in a small value when the sick or infected residents are close together and tends toward 1 as distance from sick or infected residents increases, thus resulting in a strong lowering effect on the health level of residents who are near a sick or infected resident, with little effect on more distant residents. If the resultant health level of a resident falls below a threshold value, 0.3 for purposes of these simulations, the resident is deemed to be infected. In short, the further one’s bed is from the sick worker, the less likely that individual is to become infected. Once a resident is deemed infected, they are assumed to be asymptomatic for a certain number of days, determined by a Gaussian distribution with a mean of 5.1 days and standard deviation of 0.35 [26], and the infected resident’s health is set to 0. During this time the resident is considered contagious.
Subsequent Days—Modified S-I-R Model
The daytime model is predicated on the assumption that once an individual is identified as sick (health level ≤ 0.3 and asymptomatic period concluded), they are removed from both the workplace and the housing environment. As such, on the hypothetical initial day of the simulation, the worker who was sick on the initial evening is removed upon arrival to work. No new resident is assigned to the sick individual’s bed or bunk.
The propensity for asymptomatic individuals to spread the virus during the daytime work environment is modeled using a modified S-I-R model [19, 20] as described in Eqs. 2–4. In Eqs. 2–4, S denotes the number of individuals who are susceptible, I provides the number who are infected, R indicates the number who have been removed, and N is the total number of individuals. The time step is always 1 day and m represents the given day of the simulation. Notably, S-I-R models are intended for large sample sizes; due to the small sample sizes likely to be used with this tool, rounding is utilized to ensure that changes to the susceptible, infected, and removed population occur as integer values and Monte Carlo simulation is used to capture rare but not improbable events that would otherwise have been detected with large sample sizes. Furthermore, it is worth reiterating that the modeling used is intentionally simplified for computational efficiency, online delivery, and clarity as an educational tool for farmworkers.
| 2 |
| 3 |
| 4 |
In this simulation tool, the parameter β correlates to the rate at which infected individuals spread infection to susceptible individuals and is based upon user input. The user is asked to select from amongst five options (see Fig. 1) to indicate the level of social distancing and sanitization employed on the farm, or indicate that no preventative practices are used. Based on how aggressive the farm’s mitigation approach is, as reflected by user inputs, a value of β = 0.8, 0.68, 0.56, 0.44, 0.32 or 0.2 is entered into the simulation. The discrete change in number of residents removed, ∆R(m), is calculated by monitoring the number of days each infected individual is asymptomatic (randomly generated following a Gaussian distribution as described previously), and then removing the infected individual after that number of days.
Fig. 1.
Illustration of connections between daytime and evening models
The average number of secondary infections from one infected individual, R0, does not explicitly appear in the model given by Eqs. 2–4. Rather, should one wish to estimate the R0 value from this model, it would be calculated as the ratio of β/γ with β based on user input as described previously, and γ being the removal rate, which in this model would be 1 divided by the number of asymptomatic days. Because the asymptomatic period is randomized as described previously, γ is not a fixed value. With these sources of variability in both β and γ, R0 values for this model are nominally in the range of 1.02 to 4.08. In summary, Eqs. 2–4 represent a discretization of a more classic S-I-R model given by Eqs. 5–7. The values of β referred to in the prior paragraph were selected to yield the approximate, and wide, range of R0 values expected of COVID-19 at the time of the tool’s development [5, 6, 27], and to reflect how use of multiple intervention strategies reduces spread.
| 5 |
| 6 |
| 7 |
To maintain continuity with the evening model, after each time step (1 day), bed locations are randomly assigned for the number of newly infected individuals, and similarly the newly infected individuals are randomly assigned an asymptomatic period based on a random Gaussian distribution as described previously. The health level of the newly infected individuals is set to 0.
Subsequent Nights—Pseudo Agent-Based Model
On each subsequent evening, the process utilized for the first night is repeated, with the only variation being that proximity to newly infected individuals, rather than the original (now removed) sick individual, is the driving factor for decreasing the health level of other residents. At any point, if a resident’s health level falls below the threshold value of 0.3, that resident is deemed infected. For consistency with the daytime model, if an individual should become newly infected over an evening simulation cycle, the S-I-R model parameters are updated to reflect the increase in I (infected) and decrease in S (susceptible). In this manner, the model consistently maintains integer values within the S-I-R model, and S+I+R = N at all time steps. To visualize this model flow, refer to Fig. 1.
An example S-I-R plot in Fig. 2, generated using a MATLAB formulation of this model, shows how these daytime and evening modeling components intersect over the course of a single simulation. In Fig. 2, the initial condition of one sick individual results in two individuals becoming infected on the original evening (Evening 0) from the proximity-based model. Though the sick individual is removed at the start of the first work day, the asymptomatic, infected individuals remain on the farm and in the multi-occupant bedroom. By Day 2, there is one more infection from daytime S-I-R-modeled interactions on Day 1. By Day 3, a fourth individual is infected due to nighttime proximity spread. On Day 7, two previously asymptomatic, infected individuals become symptomatic and are removed. On Day 9, the last two residents become symptomatic, and are removed.
Fig. 2.

Sample S-I-R plot. 12′ × 12′ room layout with 5 residents, sleeping in a head-to-toe bunkbed configuration. β = 0.8. One resident is initially sick, causing two to become infected the first evening, leading to two more infections over the course of the simulation
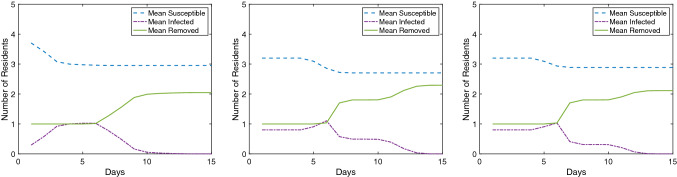
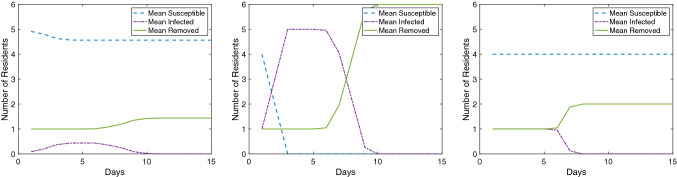
To illustrate the impact of initial conditions upon simulation result, Figs. 3 and 4 present susceptible, infected, and removed curves averaged over 10,000 simulations for a 12′ × 12′ room layout with 5 residents and a 15′ × 15′ room layout with 6 residents respectively. In both figure sets, generated using the aforementioned MATLAB formulation of this model, sleep orientation and β are varied. It is worth reinforcing that this model is intended to be educational, not predictive, and as such these figures are provided to demonstrate impact (subtle in Fig. 3, pronounced in Fig. 4) rather than to be interpreted as quantitative findings.
Fig. 3.
Average S-I-R plots over 10,000 simulations. 12′ × 12′ room layout with 5 residents. Left: head-to-toe bunkbed configuration with β = 0.8. Middle: heads at walls bunkbed configuration with β = 0.8. Right: heads at walls bunkbed configuration with β = 0.2
Fig. 4.
Average S-I-R plots over 10,000 simulations. 15′ × 15′ room layout with 6 residents. Left: head-to-toe bunkbed configuration with β = 0.8. Middle: heads at walls bunkbed configuration with β = 0.8. Right: heads at walls bunkbed configuration with β = 0.2
Browser-Based Implementation
The fundamental goal of the tool is to provide farm owners and workers the ability to assess and compare risk of COVID-19 transmission and implement calculated mitigation strategies to reduce infection. According to a study that investigated factors influencing farmers’ use of decision support tools, farmers suggested that a tool “has got to be really simple and user-friendly to be able to understand” and must provide “instantaneous information” [28]. With this in mind, for ease of distribution of this tool to the farming workforce at large, a JavaScript based website was developed with simplicity and a variety of devices in mind. That is to say, the website was implemented as a single stand-alone page with all scripts embedded on the page to eliminate the need for use of additional libraries, and to simplify distribution. The simplicity of design also lends itself to operation on a number of platforms including mobile devices. Furthermore, the phrasing used on the website was honed for clarity and educational purposes, and delivered in both English and Spanish. Rather than providing users S-I-R plots, a simple floor plan of the hypothetical scenario is given, and an assessment of risks is provided based upon the Monte–Carlo simulation described previously. The tool is provided through a NIOSH agriculture center’s website at [redacted], which serves as a primary source for occupational health and safety information for farmers, foresters and fishermen.
Multi-disciplinary Collaboration and Dissemination
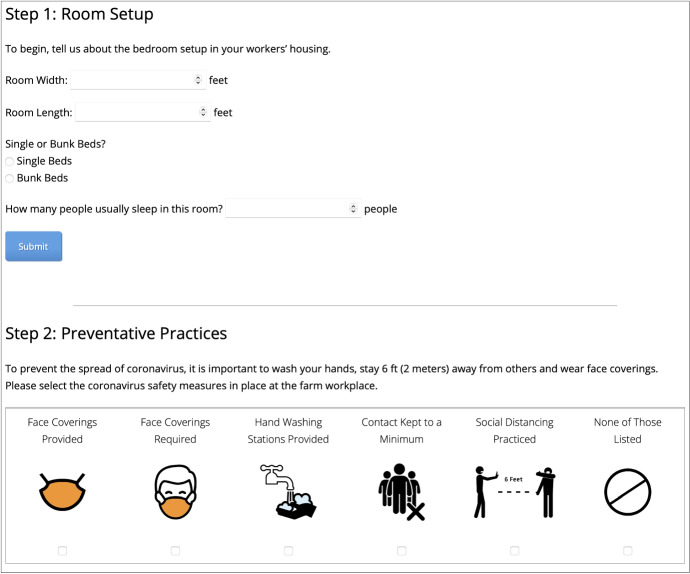
This occupational safety intervention relied upon the collective expertise from partners with backgrounds in modeling and simulation, bilingual communications, statistics, epidemiology, and user interface. The evolution of the tool from its initial release (Fig. 5) to final design (Fig. 6) illustrates how multiple disciplinary perspectives were leveraged to develop a tool intended for the target audience of farmworkers, rather than a scientific or regulatory audience. Beyond the team directly involved in tool development, a key aspect to adoption was opportunities to elicit informal feedback on the tool from the target population and key informants. In this regard, partnership between NIOSH-supported agricultural centers proved invaluable. Given the rapidly evolving nature of the pandemic, getting information out quickly was of the utmost importance. The first English version of this farmworker housing tool was released in April of 2020, with a Spanish version following in early May. The tool continued to be refined into August of 2020. A sister-NIOSH agricultural center hosted a “State of the Science” meeting on “Global Pandemics and the Agricultural Workforce: Research and Policy Implications” which provided an opportunity to discuss this work with researchers, scientists, and agriculture extension agents during a virtual conference format in September 2020, webinar in December 2020, and white paper in March of 2021. We observed a measurable increase in site traffic following these activities, demonstrating that the sister-center’s partnership was key to increasing information dissemination. In the time frame between site launch in April of 2020 to draft paper submission in June of 2021, the English language website was visited 1012 times, and the Spanish language site 48 times.
Fig. 5.
Data entry steps for early version of web-based intervention
Fig. 6.
Data entry steps for final version of web-based intervention
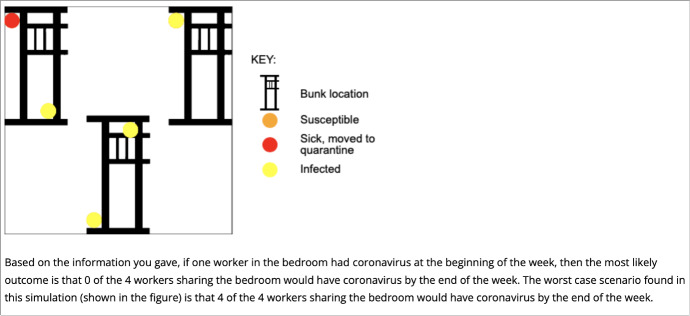
User outputs provide an interpretation of the findings as shown in Fig. 7, along with general recommendations for improved safety and a simplified explanation of the model as shown in Fig. 8. While early versions of the website reported statistical insights for users, it was concluded that instead, emphasizing the educational aspects with high level text and graphical descriptions would be more impactful to the end user.
Fig. 7.
Summary of findings presented to website user
Fig. 8.
Generalized recommendations provided to end user
Discussion
This tool was developed rapidly with an eye toward supporting farmworkers in the midst of an evolving crisis that uniquely affects the agricultural sector [29]. As documented in prior studies of farm laborers, these individuals typically experience increased barriers to accessing healthcare due to lack of health insurance and concerns about leaving the farm due to the potential for deportation if they are undocumented [30–33]. Language, literacy and transportation barriers provide additional challenges for farmworkers attempting to navigate the healthcare system. According to the 2015–2016 NAWS report [13], 77% of workers were Spanish speaking and almost half reported that they were not able to read English at all. Survey responses also indicated that only 18% have access to health insurance through their employer. With an income range of $17,500–$19,999, health care is largely unaffordable [13] and often under-reported [31].
Underlying health conditions also increase the potential for severe complications from COVID-19. Studies detailing farmworker health status indicate that farmworkers are six times more likely to have tuberculosis, and report significantly higher rates of HIV infection and respiratory conditions resulting from work exposures, as compared to the general population [31–34]. Migrant workers are also at increased risk for severe hypertension and obesity. Diabetes in migrant and seasonal farmworkers poses additional challenges for this population as diabetes often requires follow-up care [31]. These chronic conditions have been listed by the CDC as significantly increasing the risk of severe complications from COVID-19 [35]. A 2003 study of farmworker death certificates mirrors these findings, listing elevated rates of mortality due to tuberculosis, cerebrovascular disease, respiratory diseases and hypertension [32]. Forty percent of farmworkers reported not living with their family, which means these workers are also less likely to have the support of family members if they do become ill [13].
With these pandemic control challenges, the research team identified a critical need for an educational tool to increase the safety of the work environment. Rather than being designed to predict the exact number of COVID-19 infections that will occur in farmworker housing, the model is intended to help the user understand how changes in safety practices may slow the spread of the virus. While sophisticated agent-based models of this sort exist [22, 36–38], and are in use for modeling COVID-19 at large, these models are computationally intensive and/or require training or expertise on the part of the user. Instead, the model described in this paper focuses on certain key inputs that the farmer owner or farmworker may be able to modify, in order to increase the safety of the work environment. Underlying assumptions, such as removing a worker from housing once they have been identified as sick, is intended to mimic best practice in infection control, which is reinforced on the simulator webpage.
The simplified nature of the mathematical modeling is at the core of designing this effective intervention. Being able to describe the two phases of the model to users via accessible examples was beneficial for generating buy-in. The S-I-R component of the model allowed us to leverage the “flattening the curve” language that became so ubiquitous early in the pandemic. The pseudo agent-based piece to the model permitted analogy to individuals’ past experiences with airplane [39] or bus seating (e.g. how reducing seat usage reduces likelihood of spread). The multi-disciplinary team, coupled with the collaborative nature of the NIOSH agriculture centers, resulted in this effort serving as a unique and effective case study showcasing collaboration between engineering, statistics, epidemiology, and communications experts to rapidly develop and deploy an occupational safety intervention for an underserved community.
Conclusion and Future Study
Traditional epidemiological models of virus transmission through a population are derived from so-called S-I-R models [19, 20], short for susceptible-infected-recovered or removed. S-I-R models study disease transmission through a population at large driven by key parameters that correspond to the probability of spreading the disease and the recovery rate, from which the commonly referred to basic reproduction number, R0, for an epidemic is derived [40]. As such, S-I-R models look at viral spread on a holistic level. Agent-based models treat individuals as autonomous decision makers and can capture stochasticity of individual decisions [41]. Coupling S-I-R and agent-based modeling is a technique that has been used to demonstrate the spread of disease and has been specifically used to try to predict the course of the COVID-19 pandemic [37]. This paper focused on development of a deliberately simplified model designed for use as an educational tool for a historically underserved community, and describes the modeling, website deployment, and teaming structure used to launch this intervention.
As demonstrated in a variety of national media publications, COVID-19 spread rapidly on some farms. The farmworker population faces considerable barriers to healthcare and reports higher rates of underlying health conditions, which emphasized the considerable need to heavily invest in COVID-19 prevention efforts for this population of essential workers [42]. Prevention is often more cost-effective than treatment of disease and has been highlighted in prior farm labor health studies as an effective tool for protecting this particularly vulnerable population of workers [31].
With challenges in access to testing, and vaccines only having become available after a full harvest season of pandemic, it was crucial to provide tools that demonstrate how simple prevention strategies can markedly improve outcomes or how ignoring these outcomes can lead to the rapid spread of contagion. The tool and process are shared in this paper as a case study for future multi-disciplinary collaborations in modeling-inspired safety interventions. Funding for additional research focused on evaluation of worker or farmer-owner utilization of the simulation tool, as well as predictive accuracy would greatly enhance the viability of future predictive models. Evaluation activities could include worker or farm-owner focus groups or interviews designed to elicit user experiences, as well as review of retrospective records on COVID transmission among workers.
Such a study may prompt refinement towards more sophisticated agent-based modeling to capture movements within a living space using open source or web-based tools such as NetLogo [22]. More sophisticated modeling could also quantify impacts of ventilation and other modifications to the living environment. In focus group activities it would be worthwhile to assess primary drivers for adaptation by farmworkers. That is to say, such a study should seek to evaluate if farmworkers prefer simplified educational models such as the one presented here, or if farmworkers would prefer models designed for predictive use, and if so, what corresponding training opportunities on use of the model would be appropriate.
Acknowledgements
The authors gratefully acknowledge the assistance of Van Jones and Dhawal Bhanderi for their input on the layout of the described website, Tristan VanValkenburgh for her assistance with graphic design of the website user interface, and Anna Meyerhoff for support of the site translation.
Funding
The research activities described in this manuscript were made possible through funding provided by the National Institute of Occupational Safety and Health (NIOSH), Grant Number 5U54OH007542-20. The authors bear sole responsibility for the manuscript content and do not necessarily reflect the views of NIOSH or the Centers for Disease Control (CDC).
Data Availability
N/A.
Code Availability
The developed code is publicly available on the described website.
Declarations
Conflict of interest
The authors of this paper have no potential conflicts to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Leigh McCue-Weil, Email: lmccuewe@gmu.edu.
Margaret Knight, Email: miknight@ucdavis.edu.
Maryellen Driscoll, Email: maryellen.driscoll@bassett.org.
Paul Jenkins, Email: paul.jenkins@bassett.org.
Julie Sorensen, Email: julie.sorensen@bassett.org.
References
- 1.Farmworker Justice: The Role of Community Health Centers in Promoting Health Care Access and COVID-19 Prevention for Agricultural Workers (2020). https://www.farmworkerjustice.org/blog-post/the-role-of-community-health-centers-in-promoting-health-care-access-and-covid-19-prevention-for-agricultural-workers/
- 2.Centers for Disease Control and Prevention: Centers for Agricultural Safety and Health (2014). https://www.cdc.gov/niosh/oep/agctrhom.html
- 3.World Health Organization: Novel Coronavirus (2019-nCoV) Situation Report—1 (2020). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf
- 4.World Health Organization: COVID-19 Weekly Epidemiological Update Edition 46 (2021). https://www.who.int/docs/default-source/coronaviruse/situation-reports/20210629_weekly_epi_update_46.pdf
- 5.Zhang S, Diao M, Yu W, Pei L, Lin Z, Chen D. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship: a data-driven analysis. Int. J. Infect. Dis. 2020;93:201–204. doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020;27(2):021. doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19) Treasure Island: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 8.Chowell G, Blumberg S, Simonsen L, Miller MA, Viboud C. Synthesizing data and models for the spread of MERS-CoV, 2013: key role of index cases and hospital transmission. Epidemics. 2014;9:40–51. doi: 10.1016/j.epidem.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Homeland Security, Cybersecurity & Infrastructure Security: Memorandum on Identification of Essential Critical Infrastructure Workers During COVID-19 Response (2020). https://www.cisa.gov/sites/default/files/publications/CISA-Guidance-on-Essential-Critical-Infrastructure-Workers-1-20-508c.pdf
- 10.US Department of Agriculture, Economic Research Service: Legal Status and Migration Practices of Hired Crop Farmworkers (2020). https://www.ers.usda.gov/topics/farm-economy/farm-labor/#legalstatus
- 11.Costa, D., Martin, P.: Coronavirus and Farmworkers: Farm Employment, Safety Issues, and the H-2A Guestworker Program. Economic Policy Institute (2020). https://www.epi.org/publication/coronavirus-and-farmworkers-h-2a/
- 12.Holden, C., George, L., Smith, A.: No Refuge from the Fields: Findings from a Survey of Farmworker Housing Conditions in the United States. Housing Assistance Council, Washington, DC (2001). https://www.innovations.harvard.edu/sites/default/files/hac_farmworker_report.pdf
- 13.Hernandez, T., Gabbard, S.: Findings from the National Agricultural Workers Survey (NAWS) 2015–2016, JBS International, Research Report Number 13 (2018). https://www.dol.gov/sites/dolgov/files/ETA/naws/pdfs/NAWS_Research_Report_13.pdf
- 14.Arcury TA, Quandt SA. Living and working safely: challenges for migrant and seasonal farmworkers. N. C. Med. J. 2011;72(6):466–470. [PMC free article] [PubMed] [Google Scholar]
- 15.Vallejos QM, Quandt SA, Grzywacz JG, Isom S, Chen H, Galván L, Whalley L, Chatterjee AB, Arcury TA. Migrant farmworkers' housing conditions across an agricultural season in North Carolina. Am. J. Ind. Med. 2011;54(7):533–544. doi: 10.1002/ajim.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control (CDC): Cleaning and Disinfection for Community Facilities—Interim Recommendations for U.S. Community Facilities with Suspected/Confirmed Coronavirus Disease 2019 (COVID-19) (2020). https://www.cdc.gov/coronavirus/2019-ncov/community/organizations/cleaning-disinfection.html?deliveryName=USCDC_10_4-DM24118. Accessed Apr 2020
- 17.MacMahon KL, Delaney LJ, Kullman G, Gibbins JD, Decker J, Kiefer MJ. Protecting poultry workers from exposure to avian influenza viruses. Public Health Rep. 2008;123(3):316–322. doi: 10.1177/003335490812300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed F, Zviedrite N, Uzicanin A. Effectiveness of workplace social distancing measures in reducing influenza transmission: a systematic review. BMC Public Health. 2018;18(1):518. doi: 10.1186/s12889-018-5446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kermack W, McKendrick AG. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A. 1927;115:700–721. doi: 10.1098/rspa.1927.0118. [DOI] [Google Scholar]
- 20.Intellectual Ventures Management LI: SIR and SIRS Models. http://www.idmod.org/docs/hiv/model-sir.html. Accessed 10 Apr 2020
- 21.The Trustees of the University of Pennsylvania (Penn): COVID-19 Hospital Impact Model for Epidemics (CHIME) (2020). https://penn-chime.phl.io
- 22.Wilensky, U.: NetLogo. Center for Connected Learning and Computer-Based Modeling. Northwestern University, Evanston (1999). http://ccl.northwestern.edu/netlogo/.
- 23.U.S. Department of Labor, Employment and Training Administration, Office of Foreign Labor Certification: COVID-19 Frequently Asked Questions—Round 3 (2020). https://www.dol.gov/sites/dolgov/files/ETA/oflc/pdfs/DOL-OFLC_COVID-19_FAQs_Round%203.pdf
- 24.Code of Federal Regulations (eCFR): Electronic Code of Federal Regulations (2021). https://www.ecfr.gov/cgi-bin/text-idx?mc=true&node=pt20.3.654&rgn=div5#se20.3.654_1407
- 25.Liu G, Liu M. Smoothed Particle Hydrodynamics: A Meshfree Particle Method. Singapore: World Scientific; 2002. [Google Scholar]
- 26.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Center for Disease Control and Prevention: COVID-19 Pandemic Planning Scenarios (2021). https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
- 28.Rose DC, Sutherland WJ, Parker C, Lobley M, Winter M, Morris C, Twining S, Ffoulkes C, Amano T, Dicks LV. Decision support tools for agriculture: towards effective design and delivery. Agric. Syst. 2016;149:165–174. doi: 10.1016/j.agsy.2016.09.009. [DOI] [Google Scholar]
- 29.Shoichet, C.E.: The farmworkers putting food on America's tables are facing their own coronavirus crisis. CNN (2020). https://www.cnn.com/2020/04/11/us/farmworkers-coronavirus/index.html
- 30.Slesinger DP. Health status and needs of migrant farm workers in the United States: a literature review. J. Rural Health. 1992;8(3):227–234. doi: 10.1111/j.1748-0361.1992.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 31.Hansen E, Donohoe M. Health issues of migrant and seasonal farmworkers. J. Health Care Poor Underserved. 2003;14(2):153–164. doi: 10.1353/hpu.2010.0790. [DOI] [PubMed] [Google Scholar]
- 32.Villarejo D. The health of U.S. hired farm workers. Annu. Rev. Public Health. 2003;24:175–193. doi: 10.1146/annurev.publhealth.24.100901.140901. [DOI] [PubMed] [Google Scholar]
- 33.Villarejo D, Baron SL. The occupational health status of hired farm workers. Occup. Med. (Philadelphia) 1999;14(3):613–635. [PubMed] [Google Scholar]
- 34.Mobed K, Gold EB, Schenker MB. Occupational health problems among migrant and seasonal farm workers. West. J. Med. 1992;157(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention: People at Increased Risk (2021). https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html
- 36.Cuevas E. An agent-based model to evaluate the COVID-19 transmission risks in facilities. Comput. Biol. Med. 2020;121:103827. doi: 10.1016/j.compbiomed.2020.103827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alzu’bi AA, Alasal SIA, Watzlaf VJM. A simulation study of coronavirus as an epidemic disease using agent-based modeling. J. AHIMA. 2020;18:1. [PMC free article] [PubMed] [Google Scholar]
- 38.Staffini A, Svensson A, Chung U, Svensson T. An agent-based model of the local spread of SARS-CoV-2: Modeling study. JMIR Med. Inform. 2021;9(4):e24192. doi: 10.2196/24192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCue L. A low-fidelity stochastic model of viral spread in aircraft to assess risk mitigation strategies. ASME J. Risk Uncertain. B. 2021;7(3):031002. doi: 10.1115/1.4050040. [DOI] [Google Scholar]
- 40.van den Driessche P. Reproduction numbers of infectious disease models. Infect. Dis. Modell. 2017;2(3):288–303. doi: 10.1016/j.idm.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonabeau E. Agent-based modeling: methods and techniques for simulating human systems. Proc. Natl. Acad. Sci. 2002 doi: 10.1073/pnas.082080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liem A, Wang C, Wariyanti Y, Latkin CA, Hall BJ. The neglected health of international migrant workers in the COVID-19 epidemic. Lancet Psychiatry. 2020;7(4):e20. doi: 10.1016/S2215-0366(20)30076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.
The developed code is publicly available on the described website.