ABSTRACT
Background: Stressful events during a pandemic are a major cause of serious health problems, such as burnout, depression and posttraumatic stress disorder (PTSD) among health care workers (HCWs). During three years, HCWs, on the frontline to fight the COVID-19 pandemic, have been at an increased risk of high levels of stress, anxiety, depression, burnout and PTSD. Regarding potential psychological interventions, Eye Movement Desensitization & Reprocessing (EMDR) is a structured, strongly recommended therapy based on its well-known efficacy in reducing PTSD symptoms and anxiety.
Objectives: This study, designed as a trial within a cohort (TwiC), aims to 1) estimate the prevalence of depression, burnout and PTSD in a sample of HCWs after experiencing the COVID-19 emergency (cohort part) and 2) assess the efficacy and acceptability of ‘EMDR + usual care’ for HCWs from the cohort who report significant psychological symptoms (trial part).
Methods: The study, designed as a TwiC, consists of a prospective cohort study (n = 3000) with an embedded, pragmatic, randomized open-label superiority trial with two groups (n = 900). Participants included in the trial part are HCWs recruited for the cohort with significant symptoms on at least one psychological dimension (depression, burnout, PTSD) at baseline, 3 months or 6 months, determined by using the Patient Health Questionnaire (PHQ-9), Professional Quality of Life (ProQOL) scale, and PTSD Checklist for the DSM-5 (PCL-5). The intervention consists of 12 separate EMDR sessions with a certified therapist. The control group receives usual care. The trial has three primary outcomes: changes in depression, burnout and PTSD scores from randomization to 6 months. All participants are followed up for 12 months.
Conclusions: This study provides empirical evidence about the impact of the COVID-19 pandemic and the mental health burden it places on HCWs and assesses the effectiveness of EMDR as a psychological intervention.
Trial registration NCT04570202
KEYWORDS: EMDR, depression, stress, burnout, health care workers, COVID-19, study protocol, trial within a cohort study, TwiC
HIGHLIGHTS
Health care workers are at increased risk of stress, anxiety, depression, burnout and PTSD following the COVID-19 pandemic.
In this study, the effectiveness of EMDR in reducing depression, burnout and PTSD in health care workers exposed to COVID-19 is investigated.
In this study, an original ‘trial within a cohort’ (TwiC) design that consists of a cohort study with an embedded pragmatic randomized trial is used.
The study is fully web-based, including online screening, consent and assessments.
Abstract
Antecedentes: Los eventos estresantes durante una pandemia son una causa importante de problemas de salud graves, como el burnout, depresión y trastorno de estrés postraumatico (TEPT), junto con el deterioro del funcionamiento entre los trabajadores de la salud (HCWs por sus siglas en inglés). Por casi tres años, los HCWs, en la primera línea para luchar contra la pandemia COVID-19, han tenido un mayor riesgo de sufrir altos niveles de estrés, ansiedad, depresión, burnout y TEPT. En relación a las intervenciones psicológicas potenciales, la Desensibilización y Reprocesamiento por Movimientos Oculares (EMDR) es una terapia estructurada y muy recomendada, basada en su eficacia bien conocida en reducir síntomas de TEPT y ansiedad.
Objetivos: Este estudio, diseñado como un ensayo dentro de una cohorte (TwiC por sus siglas en inglés), tiene como objetivos 1) estimar la prevalencia de depresión, burnout y TEPT en una muestra de HCWs después de experimentar la emergencia de COVID-19 (parte de la cohorte) y 2) evaluar la eficacia y aceptabilidad de ‘EMDR + tratamiento habitual’ para los HCWs de la cohorte que informan síntomas psicológicos importantes (parte del ensayo).
Método: Este estudio diseñado como un TwiC, consiste en un estudio de cohorte prospectivo (n = 3,000) con un ensayo de superioridad integrado, pragmático, aleatorizado y abierto en dos grupos (n = 900). Los participantes incluidos en la parte del ensayo son HCWs reclutados para la cohorte con síntomas significativos de al menos una dimensión psicológica (depresión, burnout y TEPT) al inicio, a los 3 meses o 6 meses, determinados mediante el Cuestionario de Salud del Paciente (PHQ-9), Escala de Calidad de Vida Profesional (ProQOL) y la Lista de Chequeo de Trastorno de Estrés Postraumático para el DSM-5 (PCL-5). La intervención consiste en 12 sesiones separadas de EMDR con un terapeuta certificado en EMDR. El grupo control recibe el tratamiento habitual. El ensayo tiene tres resultados primarios: cambios en los puntajes de depresión, burnout y TEPT desde la aleatorización hasta los 6 meses. Todos los participantes son seguidos durante 12 meses.
Conclusiones: Este estudio proporciona evidencia empírica sobre el impacto de la pandemia de COVID-19 y la carga de salud mental que impone a los trabajadores de la salud y evalúa la efectividad de EMDR como una intervención psicológica.
PALABRAS CLAVE: EMDR, depresión, estrés, burnout, profesionales de la salud, COVID-19, protocolo de estudio, ensayo dentro de un estudio de cohorte, TwiC
Abstract
背景:疫情期间的压力事件是严重健康问题的主要原因,例如倦怠、抑郁和创伤后应激障碍 (PTSD),以及医护人员 (HCW) 的功能受损。 近三年来,居于抗击 COVID-19 疫情第一线HCW承受高压力、焦虑、抑郁、倦怠和创伤后应激障碍的风险不断增加。 关于潜在的心理干预,由于在减少 PTSD 症状和焦虑方面具有众所周知的功效,眼动脱敏和再加工 (EMDR) 是一种结构化的、强烈推荐的疗法。
目的:本研究设计为队列内试验 (TwiC),旨在 1) 评估经历过 COVID-19 紧急情况后HCW样本中抑郁、倦怠和 PTSD 的流行率(队列部分),以及 2) 评估疗效和 ‘EMDR + 常规护理’对于队列中报告有显著心理症状的HCW的可接受性(试验部分)。
方法:该研究设计为 TwiC,由一项前瞻性队列研究 (n = 3000) 和一项包含两组 (n = 900) 的嵌入式、实用、随机、开放标签的优效性试验组成。 试验部分的参与者是为队列招募的医护人员,他们在基线、3个月或 6个月时至少有一个心理维度(抑郁、倦怠和创伤后应激障碍)有显著症状,分别使用患者健康问卷 (PHQ-9)、职业生活质量量表(ProQOL)和 DSM-5创伤后应激障碍检查表(PCL-5)确定。 干预包括与经过认证的 EMDR 治疗师进行的 12次独立 EMDR 疗程。 对照组接受常规护理。 该试验具有三个主要
结果:从随机化到 6个月,抑郁、倦怠和 PTSD 评分的变化。 对所有参与者进行 12个月的随访。
结论: 本研究提供了 COVID-19 疫情的影响及其对HCW造成的心理健康负担的实证证据,并评估了 EMDR 作为心理干预的有效性。
1. Background
Stressful and critical events during a pandemic are major causes of serious mental health problems, such as burnout, depression and posttraumatic stress disorder (PTSD), alongside impaired functioning and loss of productivity among health care workers (HCWs) (El-Hage et al., 2020; Kachadourian et al., 2022; Li et al., 2021; Vancappel, Jansen, et al., 2021). COVID-19 emerged in 2019 and spread exponentially worldwide due to exposure to the new coronavirus SARS-CoV-2, for which humanity had no immunity (Dong et al., 2020). The COVID-19 pandemic is also the longest pandemic ever experienced by modern health systems across the world, leading to major public health consequences due to high infectiousness, morbidity and mortality (Khachfe et al., 2020). For almost three years, HCWs have been on the front line to fight this pandemic.
Data from the literature suggest that frontline and nonfrontline HCWs may suffer from different types of psychological distress (Li et al., 2020). Many studies have reported that HCWs experience anxiety and depression (Cai et al., 2020; Chew et al., 2020; Dosil Santamaría et al., 2021; Lai et al., 2020; Que et al., 2020; Shechter et al., 2020; Tan et al., 2020), PTSD (Chew et al., 2020; Dosil Santamaría et al., 2021); insomnia (Dosil Santamaría et al., 2021; Shechter et al., 2020), burnout (Barello et al., 2020; Vancappel, Jansen, et al., 2021), and other psychological symptoms (Huang et al., 2020; Lai et al., 2020; Wang et al., 2020). A recent narrative review reported that the current pandemic has triggered a generalized climate of wariness and uncertainty worldwide, particularly among HCWs (El-Hage et al., 2020). Furthermore, a meta-analysis investigating the psychological impact of COVID-19 among HCWs indicated a high prevalence of anxiety (34.4%), depression (31.8%), posttraumatic stress (11.4%), insomnia (27.8%), psychological distress (46.1%) and burnout (37.4%). Additionally, they described a higher prevalence of anxiety (39.3–46.9% vs. 27.1–44.2%) and depression (23.6–43.4% vs. 19.6–40.9%) among females, nurses, and frontline responders than males, doctors, and second-line HCWs (Batra et al., 2020). Another review and meta-analysis revealed a high prevalence of anxiety (30.0%), depression (31.1%), acute stress (56.5%), potential PTSD (20.2%) and sleep disorders (44.0%) among HCWs (Marvaldi et al., 2021). Overall, there is a consensus in the literature that HCWs are at an increased risk of high levels of stress, anxiety, depression, burnout and PTSD, which could have long-term psychological implications. Thus, there is a critical need during pandemics and other emergency situations for screening for psychological distress early and investigating long-term psychological impacts to promote evidence-based, effective psychological interventions to improve the psychological wellbeing of HCWs (Batra et al., 2020).
Regarding potential psychological interventions, eye movement desensitization and reprocessing (EMDR) and cognitive behavioural therapy (CBT) are effective treatment options for symptoms of stress, depression and PTSD (Bisson et al., 2019; Haerizadeh et al., 2020; Khan et al., 2019). In particular, EMDR has proven to be a remarkably effective therapeutic tool to prevent and treat the long-term effects of memories of critical and traumatic events, such as direct exposure to COVID-19. EMDR appears to be one of the first choice therapies based on its well-known efficacy in reducing PTSD symptoms and anxiety (Khan et al., 2018), its cost-effectiveness, and the fact that it is manualized (Mavranezouli et al., 2020), supporting an easy implementation strategy across a health care system. A recent case series pilot study assessed the effectiveness of remote EMDR treatment for HCWs working with COVID-19 patients who suffer from anxiety and depression. This study revealed that the URG-EMDR (emergency EMDR) protocol led to a decrease in anxiety and depression scores in all 17 participants (Tarquinio et al., 2021).
However, although the efficacy of EMDR in alleviating symptoms of different psychiatric disorders after exposure to distressing events is well supported (with varying levels of evidence), its efficacy has never been formally tested in a large-scale randomized trial in the context of a major health crisis. Here, we report the design and study protocol of an ongoing large-scale study aiming to evaluate EMDR as a psychological intervention to improve the psychological well-being of HCWs exposed to COVID-19. The ‘trial within a cohort’ (TwiC) study design allows for the long-term assessment of the psychological well-being of HCWs involved in COVID-19 patient care.
2. Objectives
This observational cohort study aims to estimate the prevalence of symptoms of mental disorders (depression, burnout, PTSD, and anxiety) in a sample of HCWs after experiencing a public health emergency (COVID-19) and to identify the baseline and follow-up variables associated with psychological outcomes at 1 year.
The trial aims to assess the efficacy and acceptability of a 12-session therapeutic intervention including ‘EMDR + usual care’ compared to ‘usual care’ alone for HCWs involved in COVID-19 patient care who report clinically significant symptoms on at least one psychological dimension (depression, burnout, PTSD).
3. Methods
3.1. Study design
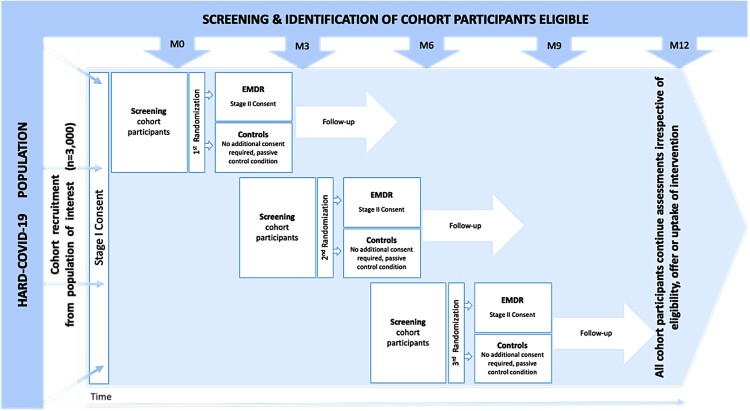
The HealthcAre woRkers coviD-19 (HARD) study is planned as a TwiC (Relton et al., 2010) that consists of a prospective observational cohort study with an embedded, pragmatic, randomized, open-label superiority trial with two groups. See the TwiC design in Figure 1.
Figure 1.
Randomized controlled trial within a cohort (TWIC) design.
3.2. Study setting and participants
3.2.1. Study setting
HCWs who were directly involved in the coronavirus outbreak are recruited throughout the country from metropolitan public or private hospitals (e.g. emergency units, intensive care units, dedicated COVID units) or nursing homes for elderly individuals. Recruitment is conducted using a communication plan to target personnel through different communication channels (hospital newsletters, emails, and internet advertisements).
3.2.2. Eligibility criteria
To be eligible to participate in the cohort study, an adult must meet all of the following criteria: be a health care worker, speak French, work in metropolitan public or private hospitals or in nursing homes for elderly individuals, have taken care of patients with COVID-19, be covered by or entitled to health insurance coverage, be able to comply with the requirements of the study and have signed an informed consent form. There are no exclusion criteria for this study.
For the trial part, the HCWs recruited for the cohort are screened using electronic, web-based, self-administered questionnaires to identify those with significant symptoms on at least one psychological dimension (depression, burnout, and PTSD), at M0 (baseline), M3 (3 months) or M6 (6 months), by using the Patient Health Questionnaire (PHQ-9) (Carballeira et al., 2007; Kroenke et al., 2001; Spitzer et al., 1999), Professional Quality of Life (ProQOL) scale (www.proqol.org), and Posttraumatic Stress Disorder Checklist for the DSM-5 (PCL-5) (Ashbaugh et al., 2016). The inclusion criteria for the trial are participation in the cohort study and significant symptoms of PTSD (i.e. an overall score ≥40 on the PCL-5), depression (i.e. a score ≥15 on the PHQ-9) or burnout (i.e. scores of ≤22 on the compassion satisfaction dimension, ≥42 on the burnout dimension and/or ≥42 on the secondary trauma dimension of the ProQOL) at either M0, M3 or M6. There were no exclusion criteria for this study, but the included participants were not allowed to be involved in pharmacological studies at inclusion or during the entire follow-up period (12 months).
3.3. Randomization
Once eligible for inclusion in the trial (i.e. with significant symptoms of PTSD, depression, or burnout), cohort participants are randomly allocated to either the EMDR + usual care group (intervention) or the usual care group (control) in a 1:1 ratio using an online central randomization procedure. The randomization list is computer-generated using permuted blocks of random sizes and stratification by condition (PTSD, burnout, or depression). If a participant has significant symptoms of two or three conditions, we first consider scores of PTSD, then those of burnout, and finally those of depression (considered less specific). The block sizes are not disclosed to study investigators, and the allocation sequence is generated by a statistician who is not involved in the recruitment or follow-up of the participants.
3.4. Blinding
No blinding is used in this pragmatic study. Participants randomized to the intervention group are aware that they are included in a randomized trial and can benefit from EMDR if they accept the treatment. Participants randomized to the control group are not aware that they are part of the trial. Nevertheless, information received at inclusion in the cohort study states that they consent to the use of their data as a control group in a future trial. EMDR therapists are not blinded. Outcomes of participants in the cohort study are collected in the same way as those of participants included in the randomized trial.
3.5. Interventions
All participants in the cohort study receive written personalized feedback on their scores after each measurement. They receive written explanations of the clinical significance of their personal scores along with short recommendations. In case of significant symptoms, the participants are invited to discuss their symptoms with their general practitioner or be referred to a study professional. They can also contact the study coordinator for advice. Thus, participants can decide to see their care provider and receive usual medical care. Participants are free of their usual care.
In addition to usual care, participants allocated to the intervention group receive EMDR therapy, which requires attending up to 12 separate sessions with an EMDR therapist located close to where they live. One EMDR session is approximately 45 minutes in duration.
3.6. Study procedures
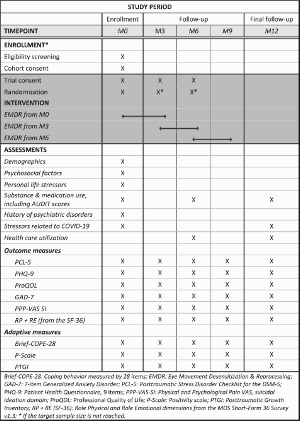
All patients are followed for a 12-month period after inclusion (Table 1): a visit at baseline or Day 1 for randomization (M0) followed by a visit every 3 months, which amounts to a visit at the 3rd (M3), 6th (M6), and 9th months (M9), in addition to the final follow-up visit at 12 months (M12). Patients can be randomized at M0, M3 and M6 until the target sample size is reached. The study started in November 2020.
Table 1.
Schedule of enrollment, interventions and assessments.
 |
3.7. Outcomes
3.7.1. Primary outcome measures
The trial has three primary endpoints, all evaluated from randomization to 6 months (corresponding to 3 months after the end of therapy in the EMDR group):
Changes over a 6-month period in symptoms of PTSD as assessed by the PTSD Checklist for the DSM-5 (PCL-5) (Ashbaugh et al., 2016), a 20-item self-administered questionnaire that assesses symptoms using a Likert-type scale for each symptom with scores ranging from 0 (not at all) to 4 (extremely). The PCL-5 allows us to assess overall PTSD severity (score of 0–80) as well as subscales of re-experiencing (0–20), avoidance (0–8), negative alterations in cognition and mood (0–24) and arousal (0–28). In this study, we decided that a score of 40 or above is associated with PTSD. The primary outcome is the change in the overall score; changes in subscale scores and in the proportion of participants with an overall score of 40 or above are secondary outcomes.
Changes over a 6-month period in symptoms of depression, as assessed by the Patient Health Questionnaire (PHQ-9) (Carballeira et al., 2007; Kroenke et al., 2001; Spitzer et al., 1999), a 9-item questionnaire designed to screen for depression in primary care and other medical settings. The cutoff score for severe depression symptoms is 15 or above (range 0–27; normal 0–4, mild 5–9, moderate 10–14, severe 15–21). The primary outcome is the change in the total score; the change in the proportion of participants with a score of 15 or above is the secondary outcome.
Changes over a 6-month period in symptoms of burnout as assessed by the Professional Quality of Life (ProQOL) scale, a validated extension of the Compassion Fatigue Test. The ProQOL scale is a 30-item self-reported measure to assess dimensions of compassion satisfaction (CS), burnout (BO) and secondary trauma (ST) (www.proqol.org). The CS dimension (Items 3, 6, 12, 16, 18, 20, 22, 24, 27, and 30) measures pleasure derived from being able to do work well; high scores represent greater satisfaction related to the ability to be an effective caregiver (range 10–50; low ≤22, moderate 23–41, high ≥42). The BO dimension (Items 1, 4, 8, 10, 15, 17, 19, 21, 26, and 29) is associated with feelings of hopelessness and difficulties in dealing with work; higher scores are related to a higher risk for BO (range 10–50; low ≤22, moderate 23–41, high ≥42). The ST dimension (Items 2, 5, 7, 9, 11, 13, 14, 23, 25, and 28) relates to work-related secondary exposure to extremely stressful events (range 10–50; low ≤22, moderate 23–41, high ≥42); high scores indicate work exposure to frightening experiences (Lauvrud et al., 2009). The primary outcome is the change in the BO dimension; changes in the CS and ST dimensions and in the proportion of participants with extreme scores for each dimension are the secondary outcomes.
3.7.2. Secondary outcome measures
In addition to those mentioned above, the secondary outcomes in the trial part are as follows:
Changes in PTSD, burnout and depression symptoms over a 3-month period measured by the PCL-5, ProQOL and PHQ-9, respectively.
Changes in PTSD, burnout and depression symptoms over a 12-month period for the participants enrolled in the trial at M0.
Changes in anxiety over a 6-month period from baseline measured by the GAD-7.
Changes in anxiety over a 12-month period from baseline measured by the GAD-7 for participants enrolled in the trial at M0.
Self-reported number of suicide attempts over a 6-month period from baseline.
The Generalized Anxiety Disorder scale (GAD-7) is a 7-item, self-rated scale developed by Spitzer et al. (2006) as a screening tool and severity indicator for GAD (Micoulaud-Franchi et al., 2016; Spitzer et al., 2006). The cutoff score for detecting severe symptoms of anxiety is 15 (range 0–21; normal 0–4, mild 5–9, moderate 10–14, severe 15–21).
Exploratory outcomes are also planned in the trial, such as:
Changes in the level of suicidal ideation over a 6- and 12-month period from baseline.
Changes in the impact of health on functioning (evaluated by the SF-36) over a 6- and 12-month period from baseline.
Changes in substance use over 12 months.
Changes in self-reported medication use and health care utilization over 6 months.
Suicidal ideation levels were evaluated using the Physical and Psychological Pain Visual Analog Scale (PPP-VAS) (Jollant et al., 2019). The PPP-VAS is a brief questionnaire with 3 VASs assessing the intensity of suicidal ideations (at the time of assessment, as the mean of the last 15 days, at the worst moment of the last 15 days).
The MOS Short-Form 36 survey v1.3 (SF-36) is a generic health-related quality of life questionnaire composed of 36 items. Out of the 8 domains assessed by the questionnaire, two are especially related to the impact of health status on emotional and physical functioning: the RE (role emotional) and RP (role physical) domains (Leplege et al., 2001; Leplège et al., 1998; McHorney et al., 1993). Higher scores represent better health and functioning.
Adaptive strategies to stressors are evaluated using the Brief COPE-28, the P-Scale and the Posttraumatic Growth Inventory (PTGI).
The Brief COPE-28 is a 28-item scale that assesses the degree to which a respondent utilizes a specific coping strategy. The 28 items have been categorized into the following 14 subscales (2–8), all assessing different coping strategies: active coping, planning, positive reframing, acceptance, humour, religion, the use of emotional support, the use of instrumental support, self-distraction, denial, venting, substance use, behavioural disengagement and self-blame (Carver, 1997). Respondents rate items on a 4-point Likert scale, ranging from 1 ‘I haven't been doing this at all’ to 4 ‘I’ve been doing this a lot’. The scores provide information regarding which coping strategy the respondent is more engaged in. A higher score indicates a higher level of coping using the strategy.
Positivity is measured by the P-Scale (Caprara et al., 2012; Vancappel, Courtois, et al., 2021): It is an 8-item questionnaire that evaluates positivity, which is an individual's tendency to see their life and experiences with a positive orientation. For instance, one item is ‘I have great faith in the future’. Respondents rate their level of agreement with each item using a 5-point scale from 1 (strongly disagree) to 5 (strongly agree), with higher scores reflecting greater positivity.
The Posttraumatic Growth Inventory (PTGI) comprises 21 positively formulated statements describing various changes that can occur as a result of an experienced traumatic event (Dubuy et al., 2022; Tedeschi & Calhoun, 1996). The PTGI is scored by adding all the responses (0–105). Individual factors are scored by adding responses to items on each factor. Each item (0–5) falls under one of the five individual factors, namely, personal strength (0–20), new possibilities (0–25), improved relationships (0–35), spiritual growth (0–10) and appreciation for life (0–15). A high total score implies that a person has undergone a positive transformation.
We also evaluate licit and illicit psychoactive substance use. Alcohol use is assessed using the Alcohol Use Disorder Identification Test (AUDIT) (Gache et al., 2005), which was developed in collaboration with the World Health Organization (WHO). The AUDIT includes 10 questions about the level of consumption, symptoms of dependence and alcohol-related consequences. The AUDIT total score (0–40) allows alcohol use severity to be assessed. See Table 1 for an overview of enrollment, interventions and assessments.
3.8. Data collection and methods
Data collection of the self-reported outcomes is carried out using online electronic case report forms (eCRFs). Participants receive an email (reminder) two days before the scheduled date to ease the completion of the questionnaires. In case of nonresponse, a maximum of 3 reminders is sent 7, 14 and 21 days after the scheduled date. Each participant is offered 10€ for time compensation for each completed visit.
3.9. Data handling
Data are entered directly by each participant through a secure website on the study-specific eCRF. To maintain participants’ anonymity, eCRFs are identified only by a patient number. All transmitted personal nominal data are stored separately from the study data records.
The hosting of the study's data is provided by the company OVH, the provider of Ennov and publisher of the software Ennov Clinical, which was used for the creation of the study database. This hosting solution is located in a secure Datacenter. Backups of software data are scheduled daily. The Ennov Clinical solution complies with all international requirements for human health (FDA, Health Canada, EMA, ICH) and with the general data protection regulations (GDPRs).
3.10. Sample size
To reach 90% power with α = 0.05 (two-sided) for a 0.245 relevant effect size maximum on each of the primary endpoints, we need, among the eligible participants, a maximum of 450 participants per trial group. This means that a maximum of 450 participants will be included in the EMDR + usual care group, and 450 participants will be allocated to the usual care group (control group). The Family Wise Type I Error Rate will be controlled using Holm's procedure to account for the presence of three primary endpoints (Holm, 1979). Therefore, it is expected that 900 participants will be recruited from the cohort for the trial. If the sample size of 900 participants included in the randomized trial is not achieved after the baseline assessment (M0), we will expand randomization at the 3-month (M3) and 6-month (M6) follow-up visits.
The sample size of the study was primarily defined for assessing the efficacy on primary outcomes for the trial, and no formal calculations were made to determine the sample size for the cohort study. However, if 3000 participants are recruited within the cohort as expected, cohort analysis will plausibly be performed with a sufficient level of precision and power.
3.11. Statistical methods
All analyses will be performed according to the intention-to-treat (ITT) principle. The objective is to assess the effect of the offer of EMDR in combination with usual care compared to usual care alone for all patients with analyzable data. Patients will remain in their randomization group, irrespective of whether they accept and receive the EMDR intervention.
As the ITT analysis of the trial can dilute the intervention effect if there is low acceptance of or compliance with EMDR, we will also consider a possible complier average causal effect (CACE) analysis. CACE analysis consists of comparing participants in the EMDR group who accept the offer of EMDR to those in the control group who would have accepted the offer if they had received it.
The primary outcomes of the trial will be analysed as continuous variables in the primary analysis, and statistical comparison between the treatment groups will be performed using linear mixed models, considering the correlation of repeated measures in participants. The treatment group, visit and treatment-by-visit interaction will be considered fixed effects. The model will include random individual intercepts and slopes over time. The effect of the treatment group will be assessed by the significance of the treatment-by-visit interaction.
We will also perform subgroup analyses according to the stratification variable used for randomization (i.e. significant PTSD, burnout, or depression symptoms).
For the secondary outcomes of the trial, statistical comparison between the treatment groups for changes in PTSD, burnout and depression symptoms over a 3- or 12-month period will be performed using a two independent sample t test for the observed differences. To analyse the change in anxiety over a 6- and 12-month period from baseline, we will use either a mixed linear model or a two independent sample Student's t test as appropriate according to the number of measures per participant. The number of suicide attempts over a 6-month period from baseline will be compared between the two groups with a Wilcoxon rank sum test. To control the Family Wise Type I Error Rate, the Holms procedure will be used to account for multiple primary endpoints.
To handle missing data, we will use a two-step framework. First, before any statistical analyses, we will perform the imputation of missing items by the Personal Mean Score (PMS) (Leplege et al., 2001). Second, when we conduct a specific analysis, we will first describe the typology of the missing sum scores and formulate a hypothesis on the plausible mechanism of missingness: missing completely at random (MCAR), missing at random (MAR), or missing not at random (MNAR), according to Little and Rubin's classification (Little and Rubin, 2019). If missingness can be considered MCAR, a simple imputation by the sample mean will compensate for the loss in power. In other cases, Multiple Imputation by Chained Equations (MICE) (van Buuren, 2007) will be performed.
Regarding the cohort study, first, a descriptive analysis of the baseline characteristics of the cohort will be performed. Then, for all the variables measured at least two times within the study period (trial outcomes: scores on the PCL-5, PHQ-9, ProQOL, GAD-7, SF-36, and PPP-VAS-SI, substance use, medication use, health care utilization) and adaptive measures to stressors (Brief Cope-28 score, P-Scale score, PTGI scores), the change over time will be estimated and tested using linear mixed models for quantitative variables and the McNemar χ2 test or Cochran's Q test for qualitative variables.
The prevalence and incident rate of psychiatric conditions (PTSD, depression, burnout), substance use, and suicidal attempts for the study period will be estimated within the cohort. For each continuous outcome (PCL-5, PHQ-9, ProQOL, SF-36, and PPP-VAS scores, as well as the number of suicide attempts), we will explain the change in intensity of symptoms over time and its association with covariates using linear mixed models for repeated measurements.
3.12. Ethics and dissemination
The study protocol was approved by the Sud-Ouest et Outremer I Ethical Committee (No. 1-20-046 ID 48680). TwiC studies have specificity in informed consent procedures. Such a design has been used several times for psychiatric conditions (Grace et al., 2012; Singh et al., 2017; Viksveen et al., 2017). All cohort participants will sign a written informed consent form that describes the study cohort and provides sufficient information to make an informed decision about participation. Consent will be obtained from participants before they undergo any study procedure. Those joining the cohort will initially grant permission for their data to be used for intervention research. For the EMDR randomized trial, half of the eligible participants will be randomly selected to be offered the intervention, and their outcomes will be compared to those who are not offered the intervention. Participants randomized to the group receiving this offer of EMDR will be told (after randomization) that they have been randomly selected, and trial consent will be obtained to receive the intervention. Participants allocated to the control group of the randomized trial will not have to sign a trial consent form, as participation in the cohort will explicitly state that they could be randomized to receive usual care and that their data could be used as controls in a trial. Participants’ care will not be affected by their decision to participate or not in the cohort and in the trial. Participants may withdraw from the study at any time during the clinical trial without any impact on their care. In this event, data collected prior to participant withdrawal will be used in the trial analysis.
The research findings will be submitted for publication in peer-reviewed journals regardless of whether they are statistically significant. The authors will be individuals who have made key contributions to the study's design and conduct. The trial findings will also be submitted for presentation at scientific meetings. The study findings will also be presented at relevant national and international conferences.
3.13. Safety reporting
This research is part of the research involving human persons at minimal risks and constraints, and in accordance with the regulations in force, the investigator is responsible for declaring any serious undesirable event occurring during the investigations (article R1413-67 of the CSP) according to the internal procedure for reporting a serious adverse event associated with the care of its establishment.
4. Discussion
This is the first randomized control trial to investigate the effectiveness of EMDR as a psychological intervention on long-term control over a 12-month follow-up of HCWs exposed to COVID-19. This trial has an original research design on at least three different levels. First, the TwiC design allows participants to perform a self-administered evaluation for common psychiatric symptoms at multiple time points to obtain feedback on their symptoms (at the very least, they are invited to seek help from the health care system if they are considered at risk), and some of them will benefit from therapy within the study protocol. Second, the TwiC design also offers the possibility to embed several trials in the same cohort, assessing different interventions or including participants with different eligibility criteria (e.g. psychological distress) and thus answering several research questions. This design is more pragmatic than the usual randomized controlled trial, as it is closer to common practice. In this case, the observational cohort provides the opportunity to understand the long-term evolution of the psychological well-being of HCWs exposed to COVID-19 in France. Third, the study is fully web-based, including online screening, consent and assessments. Only randomized participants allocated to the EMDR intervention group will benefit from face-to-face EMDR therapy near their home. It should be noted that such a study design is used for the first time in psychiatry. Additionally, it is worth noting that such a study design is consistent with a large current effort to shift from efficacy trials to real-world effectiveness trials. For instance, this movement is sustained by the Patient-Centered Outcomes Research Institute (PCORI), which promotes comparative clinical effectiveness research and is in line with the dissemination and implementation strategies.
One major limitation of this study is the open-label design. Since participants will know which treatment arm they are assigned to, they cannot be blinded to the intervention. However, this is a pragmatic trial, focusing more on real-world effectiveness than efficacy. As the study is online-based, another major limitation is the use of only self-administered questionnaires, which may result in lower response rates and the absence of control for the validity of the responses by a clinician. One additional difficulty is controlling the impact of isolation and social segregation periods. Even if we did not specifically control for these factors, we administered the Holmes-Rahe Life Stress Inventory, in which we have one item about ‘major changes in social activities’. In addition, we will be able to create a parallel confinement between dates and the evolution of the scores. In the trial part, there should be no impact as participants will be randomly allocated in a 1:1 ratio.
This longitudinal study will better help to understand the prevalence changes and awareness of mental disorders among HCWs following the novel coronavirus disease 2019 outbreak. This study will also provide mental health care to HCWs who are severely impacted by exposure to COVID-19. The results should provide empirical evidence about the impact of the COVID-19 pandemic and the mental health burden it places on health care workers to provide appropriate responses to mental health intervention programming for health care workers confronted with urgent and exceptional situations.
Funding Statement
The study was supported by the French Ministry of Health funding no. 20208025.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article, as data were not included.
References
- Ashbaugh, A. R., Houle-Johnson, S., Herbert, C., El-Hage, W., & Brunet, A. (2016). Psychometric validation of the English and French versions of the posttraumatic stress disorder checklist for DSM-5 (PCL-5). PloS One, 11(10), e0161645. doi: 10.1371/journal.pone.0161645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barello, S., Palamenghi, L., & Graffigna, G. (2020). Burnout and somatic symptoms among frontline healthcare professionals at the peak of the Italian COVID-19 pandemic. Psychiatry Research, 290, 113129. doi: 10.1016/j.psychres.2020.113129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra, K., Singh, T. P., Sharma, M., Batra, R., & Schvaneveldt, N. (2020). Investigating the psychological impact of COVID-19 among healthcare workers: A meta-analysis. International Journal of Environmental Research and Public Health, 17(23), 9096. doi: 10.3390/ijerph17239096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson, J. I., Berliner, L., Cloitre, M., Forbes, D., Jensen, T. K., Lewis, C., Monson, C. M., Olff, M., Pilling, S., Riggs, D. S., Roberts, N. P., Shapiro, F. (2019). The international society for traumatic stress studies new guidelines for the prevention and treatment of posttraumatic stress disorder: Methodology and development process. Journal of Traumatic Stress, 32(4), 475–483. doi: 10.1002/jts.22421 [DOI] [PubMed] [Google Scholar]
- Cai, W., Lian, B., Song, X., Hou, T., Deng, G., & Li, H. (2020). A cross-sectional study on mental health among health care workers during the outbreak of Corona Virus Disease 2019. Asian Journal of Psychiatry, 51, 102111. doi: 10.1016/j.ajp.2020.102111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara, G. V., Alessandri, G., Eisenberg, N., Kupfer, A., Steca, P., Caprara, M. G., Yamaguchi, S., Fukuzawa, A., Abela, J. (2012). The positivity scale. Psychological Assessment, 24(3), 701–712. doi: 10.1037/a0026681 [DOI] [PubMed] [Google Scholar]
- Carballeira, Y., Dumont, P., Borgacci, S., Rentsch, D., de Tonnac, N., Archinard, M., Andreoli, A. (2007). Criterion validity of the French version of Patient Health Questionnaire (PHQ) in a hospital department of internal medicine. Psychology and Psychotherapy: Theory, Research and Practice, 80(Pt 1), 69–77. doi: 10.1348/147608306X103641 [DOI] [PubMed] [Google Scholar]
- Carver, C. S. (1997). You want to measure coping but your protocol’s too long: Consider the brief COPE. International Journal of Behavioral Medicine, 4(1), 92–100. doi: 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- Chew, N. W. S., Lee, G. K. H., Tan, B. Y. Q., Jing, M., Goh, Y., Ngiam, N. J. H., Yeo, L. L. L., Ahmad, A., Ahmed Khan, F., Napolean Shanmugam, G., Sharma, A. K., Komalkumar, R. N., Meenakshi, P. V., Shah, K., Patel, B., Chan, B. P. L., Sunny, S., Chandra, B., Ong, J. J. Y., … Sharma, V. K. (2020). A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain, Behavior, and Immunity, 88, 559–565. doi: 10.1016/j.bbi.2020.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, E., Du, H., & Gardner, L. (2020). An interactive web-based dashboard to track COVID-19 in real time. The Lancet Infectious Diseases, 20(5), 533–534. doi: 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosil Santamaría, M., Ozamiz-Etxebarria, N., Redondo Rodríguez, I., Jaureguizar Alboniga-Mayor, J., & Picaza Gorrotxategi, M. (2021). Psychological impact of COVID-19 on a sample of Spanish health professionals. Revista de Psiquiatría y Salud Mental, 14(2), 106–112. doi: 10.1016/j.rpsm.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuy, Y., Sébille, V., Bourdon, M., Hardouin, J. B., & Blanchin, M. (2022). Posttraumatic growth inventory: Challenges with its validation among French cancer patients. BMC Medical Research Methodology, 22(1), 246. doi: 10.1186/s12874-022-01722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hage, W., Hingray, C., Lemogne, C., Yrondi, A., Brunault, P., Bienvenu, T., Etain, B., Paquet, C., Gohier, B., Bennabi, D., Birmes, P., Sauvaget, A., Fakra, E., Prieto, N., Bulteau, S., Vidailhet, P., Camus, V., Leboyer, M., Krebs, M.-O., & Aouizerate, B. (2020). [Health professionals facing the coronavirus disease 2019 (COVID-19) pandemic: What are the mental health risks?]. Encephale, 46(3S), S73–S80. doi: 10.1016/j.encep.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gache, P., Michaud, P., Landry, U., Accietto, C., Arfaoui, S., Wenger, O., Daeppen, J.-B. (2005). The Alcohol Use Disorders Identification Test (AUDIT) as a screening tool for excessive drinking in primary care: Reliability and validity of a French version. Alcoholism: Clinical & Experimental Research, 29(11), 2001–2007. doi: 10.1097/01.alc.0000187034.58955.64 [DOI] [PubMed] [Google Scholar]
- Grace, J. B., Schoolmaster, D. R., Guntenspergen, G. R., Little, A. M., Mitchell, B. R., Miller, K. M., Schweiger, E. W. (2012). Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere, 3(8), art73. doi: 10.1890/ES12-00048.1 [DOI] [Google Scholar]
- Haerizadeh, M., Sumner, J. A., Birk, J. L., Gonzalez, C., Heyman-Kantor, R., Falzon, L., Gershengoren, L., Shapiro, P., Kronish, I. M. (2020). Interventions for posttraumatic stress disorder symptoms induced by medical events: A systematic review. Journal of Psychosomatic Research, 129, 109908. doi: 10.1016/j.jpsychores.2019.109908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scand J Stat, 6(2), 65–70. [Google Scholar]
- Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant, F., Voegeli, G., Kordsmeier, N. C., Carbajal, J. M., Richard-Devantoy, S., Turecki, G., Cáceda, R. (2019). A visual analog scale to measure psychological and physical pain: A preliminary validation of the PPP-VAS in two independent samples of depressed patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 90, 55–61. doi: 10.1016/j.pnpbp.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachadourian, L., Murrough, J., Kaplan, C., Kaplan, S., Feingold, J., Feder, A., Charney, D., Southwick, S., Peccoralo, L., DePierro, J., Ripp, J., & Pietrzak, R. (2022). A prospective study of transdiagnostic psychiatric symptoms associated with burnout and functional difficulties in COVID-19 frontline healthcare workers. Journal of Psychiatric Research, 152, 219–224. doi: 10.1016/j.jpsychires.2022.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachfe, H. H., Chahrour, M., Sammouri, J., Salhab, H., Makki, B. E., & Fares, M. (2020). An epidemiological study on COVID-19: A rapidly spreading disease. Cureus, 12(3), e7313. doi: 10.7759/cureus.7313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A., Khan, S., & Shah, S. T. (2019). Efficacy of eye movement desensitization & reprocessing versus cognitive behavioral therapy in post-traumatic stress and depressive symptoms: Study protocol for a randomized controlled trial. Contemporary Clinical Trials Communications, 16, 100439. doi: 10.1016/j.conctc.2019.100439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, A. M., Dar, S., Ahmed, R., Bachu, R., Adnan, M., & Kotapati, V. P. (2018). Cognitive behavioral therapy versus eye movement desensitization and reprocessing in patients with post-traumatic stress disorder: Systematic review and meta-analysis of randomized clinical trials. Cureus, 10(9), e3250. doi: 10.7759/cureus.3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke, K., Spitzer, R. L., & Williams, J. B. (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, J., Ma, S., Wang, Y., Cai, Z., Hu, J., Wei, N., Wu, J., Du, H., Chen, T., Li, R., Tan, H., Kang, L., Yao, L., Huang, M., Wang, H., Wang, G., Liu, Z., Hu, S. (2020). Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Network Open, 3(3), e203976. doi: 10.1001/jamanetworkopen.2020.3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauvrud, C., Nonstad, K., & Palmstierna, T. (2009). Occurrence of post traumatic stress symptoms and their relationship to professional quality of life (ProQoL) in nursing staff at a forensic psychiatric security unit: A cross-sectional study. Health and Quality of Life Outcomes, 7(1), 31. doi: 10.1186/1477-7525-7-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leplege, A., Ecosse, E., Pouchot, J., Coste, J., & Perneger, T. (2001). The MOS SF-36 Questionnaire: User Manual and Score Interpretation Guide. Editions Estem. [Google Scholar]
- Leplège, A., Ecosse, E., Verdier, A., & Perneger, T. (1998). The French SF-36 Health Survey: Translation, cultural adaptation and preliminary psychometric evaluation. Journal of Clinical Epidemiology, 51(11), 156. doi: 10.1016/S0895-4356(98)00093-6 [DOI] [PubMed] [Google Scholar]
- Li, J., Su, Q., Li, X., Peng, Y., & Liu, Y. (2021). COVID-19 negatively impacts on psychological and somatic status in frontline nurses. Journal of Affective Disorders, 294, 279–285. doi: 10.1016/j.jad.2021.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z., Ge, J., Yang, M., Feng, J., Qiao, M., Jiang, R., Bi, J., Zhan, G., Xu, X., Wang, L., Zhou, Q., Zhou, C., Pan, Y., Liu, S., Zhang, H., Yang, J., Zhu, B., Hu, Y., Hashimoto, K., … Yang, C. (2020). Vicarious traumatization in the general public, members, and non-members of medical teams aiding in COVID-19 control. Brain, Behavior, and Immunity, 88, 916–919. doi: 10.1016/j.bbi.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, R. J. A., & Rubin, D. B. (2019). Statistical analysis with missing data (3rd ed.). John Wiley & Sons, Ltd. [Google Scholar]
- Marvaldi, M., Mallet, J., Dubertret, C., Moro, M. R., & Guessoum, S. B. (2021). Anxiety, depression, trauma-related, and sleep disorders among healthcare workers during the COVID-19 pandemic: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 126, 252–264. doi: 10.1016/j.neubiorev.2021.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavranezouli, I., Megnin-Viggars, O., Grey, N., Bhutani, G., Leach, J., Daly, C., Dias, S., Welton, N. J., Katona, C., El-Leithy, S., Greenberg, N., Stockton, S., Pilling, S. (2020). Cost-effectiveness of psychological treatments for post-traumatic stress disorder in adults. PloS One, 15(4), e0232245. doi: 10.1371/journal.pone.0232245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney, C. A., Ware, J. E., & Raczek, A. E. (1993). The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care, 31(3), 247–263. doi: 10.1097/00005650-199303000-00006 [DOI] [PubMed] [Google Scholar]
- Micoulaud-Franchi, J. A., Lagarde, S., Barkate, G., Dufournet, B., Besancon, C., Trébuchon-Da Fonseca, A., Gavaret, M., Bartolomei, F., Bonini, F., McGonigal, A. (2016). Rapid detection of generalized anxiety disorder and major depression in epilepsy: Validation of the GAD-7 as a complementary tool to the NDDI-E in a French sample. Epilepsy & Behavior, 57(Pt A), 211–216. doi: 10.1016/j.yebeh.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Que, J., Shi, L., Deng, J., Liu, J., Zhang, L., Wu, S., Gong, Y., Huang, W., Yuan, K., Yan, W., Sun, Y., Ran, M., Bao, Y., & Lu, L. (2020). Psychological impact of the COVID-19 pandemic on healthcare workers: A cross-sectional study in China. General Psychiatry, 33(3), e100259. doi: 10.1136/gpsych-2020-100259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relton, C., Torgerson, D., O’Cathain, A., & Nicholl, J. (2010). Rethinking pragmatic randomised controlled trials: Introducing the “cohort multiple randomised controlled trial” design. BMJ, 340(mar19 1), c1066. doi: 10.1136/bmj.c1066 [DOI] [PubMed] [Google Scholar]
- Shechter, A., Diaz, F., Moise, N., Anstey, D. E., Ye, S., Agarwal, S., Birk, J. L., Brodie, D., Cannone, D. E., Chang, B., Claassen, J., Cornelius, T., Derby, L., Dong, M., Givens, R. C., Hochman, B., Homma, S., Kronish, I. M., Lee, S. A. J., … Abdalla, M. (2020). Psychological distress, coping behaviors, and preferences for support among New York healthcare workers during the COVID-19 pandemic. General Hospital Psychiatry, 66, 1–8. doi: 10.1016/j.genhosppsych.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. P., Tuomainen, H., Girolamo, G. d., Maras, A., Santosh, P., McNicholas, F., Schulze, U., Purper-Ouakil, D., Tremmery, S., Franić, T., Madan, J., Paul, M., Verhulst, F.C., Dieleman, G.C., Warwick, J., Wolke, D., Street, C., Daffern, C., Tah, P., … Walker, L. (2017). Protocol for a cohort study of adolescent mental health service users with a nested cluster randomised controlled trial to assess the clinical and cost-effectiveness of managed transition in improving transitions from child to adult mental health services (the MILESTONE study). BMJ Open, 7(10), e016055. doi: 10.1136/bmjopen-2017-018424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer, R. L., Kroenke, K., & Williams, J. B. (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders. Patient health questionnaire. JAMA, 282(18), 1737–1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- Spitzer, R. L., Kroenke, K., Williams, J. B. W., & Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166(10), 1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Tan, B. Y. Q., Chew, N. W. S., Lee, G. K. H., Jing, M., Goh, Y., Yeo, L. L. L., Zhang, K., Chin, H.-K., Ahmad, A., Khan, F. A., Shanmugam, G. N., Chan, B. P. L., Sunny, S., Chandra, B., Ong, J. J. Y., Paliwal, P. R., Wong, L. Y. H., Sagayanathan, R., Chen, J. T., … Sharma, V. K. (2020). Psychological impact of the COVID-19 pandemic on health care workers in Singapore. Annals of Internal Medicine, 173(4), 317–320. doi: 10.7326/M20-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarquinio, C., Brennstuhl, M. J., Rydberg, J. A., Bassan, F., Peter, L., Tarquinio, C. L., Auxéméry, Y., Rotonda, C., Tarquinio, P. (2021). EMDR in telemental health counseling for healthcare workers caring for COVID-19 patients: A pilot Study. Issues in Mental Health Nursing, 42(1), 3–14. doi: 10.1080/01612840.2020.1818014 [DOI] [PubMed] [Google Scholar]
- Tedeschi, R. G., & Calhoun, L. G. (1996). The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. Journal of Traumatic Stress, 9(3), 455–471. doi: 10.1002/jts.2490090305 [DOI] [PubMed] [Google Scholar]
- van Buuren, S. (2007). Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research, 16(3), 219–242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- Vancappel, A., Courtois, R., Siragusa, M., Hingray, C., Réveillère, C., Caprara, G., Belzung, C., El-Hage, W. (2021). Validation of the French Version of the Positivity Scale (P Scale). Frontiers in Psychology, 12, 724253. doi: 10.3389/fpsyg.2021.724253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancappel, A., Jansen, E., Ouhmad, N., Desmidt, T., Etain, B., Bergey, C., d'Ussel, M., Krebs, M.-O., Paquet, C., Réveillère, C., Hingray, C., El-Hage, W. (2021). Psychological impact of exposure to the COVID-19 sanitary crisis on French healthcare workers: Risk factors and coping strategies. Frontiers in Psychiatry, 12, 701127. doi: 10.3389/fpsyt.2021.701127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viksveen, P., Relton, C., & Nicholl, J. (2017). Depressed patients treated by homeopaths: A randomised controlled trial using the “cohort multiple randomised controlled trial” (cmRCT) design. Trials, 18(1), 299. doi: 10.1186/s13063-017-2040-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., Pan, R., Wan, X., Tan, Y., Xu, L., Ho, C. S., & Ho, R. C. (2020). Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. International Journal of Environmental Research and Public Health, 17(5), 1729. doi: 10.3390/ijerph17051729 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as data were not included.