Abstract
The integration of large-scale gene expression mapping into a multifaceted larval zebrafish brain atlas accelerates the characterization of neurons in behaviorally relevant circuits.
Nervous systems can be viewed from a variety of perspectives. How scientists define the elements of the brain depends on their approach to studying neurons, whether analyzing their connectivity, the genes they express, or their activity in different behavioral contexts. These are just some of the many ways to describe nervous systems, but none are complete on their own. The multifaceted and interdependent features of nervous systems demand approaches to bridge these perspectives and merge different types of data into a common framework. Neurobiologists have made substantial strides toward these goals in a variety of animals, by constructing multimodal brain atlases that integrate information about neuronal structure, connectivity, physiological properties, molecular characteristics, and activity into a shared anatomical space. The application of these methods to two genetic model systems—the mouse and the fly—have been particularly successful. In these cases, multimodal atlases and shared resources have prompted rapid progress in the discovery and characterization of neural circuits relevant for behavior (1, 2).
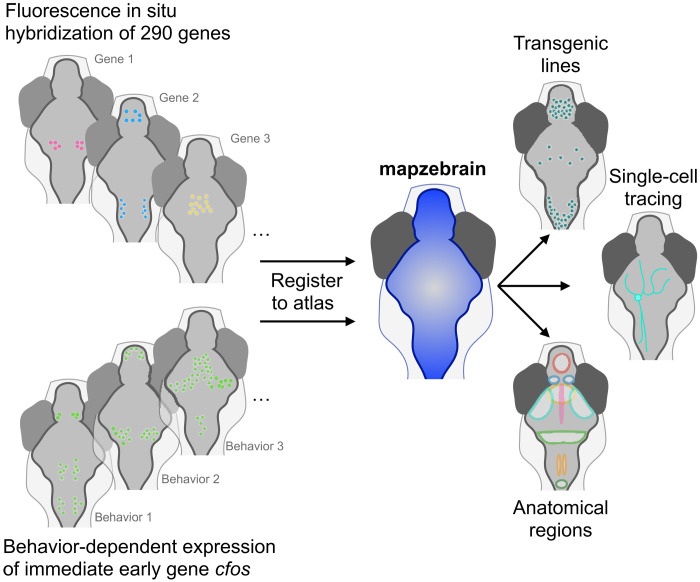
These efforts are now gaining traction in another important genetic model system for neuroscience—the larval zebrafish. Herwig Baier and colleagues at the Max Planck Institute for Biological Intelligence in Munich have developed mapzebrain (mapzebrain.org), a multimodal digital atlas of the larval zebrafish that allows for registration of whole-brain imaging volumes for comparison across individuals (3). During the larval stage at 6 days old, the small freshwater teleost is almost transparent and well-suited for optical investigations of its approximately 100,000 neurons, while showing relatively complex sensorimotor behaviors. The mapzebrain atlas provides a central resource of standardized larval zebrafish brain volumes at this age, comprising a library of transgenic lines, antibody stains, more than 4000 single neuron morphological reconstructions, and the annotation of hundreds of brain areas—all registered to a common spatial reference. Most recently, this framework includes an electron microscopy volume suitable for morphological tracing and synaptic connectivity mapping (4). Other established zebrafish brain atlases can also be registered in this framework, including the Z-Brain atlas (5).
Now, Baier’s group has added another important resource to the multimodal atlas. In this issue of Science Advances, Shainer and colleagues use cellular-level whole-brain imaging of fluorescence in situ hybridization to introduce a spatial map of gene expression in the larval zebrafish brain (6). Shainer et al. used hybridization chain reaction (HCR)—a next generation RNA fluorescence in situ hybridization approach (7)—to label 290 genes that are crucial for cell-cell interaction, cell fate regulation, neurotransmission, and neuromodulation. They recorded cellular-resolution fluorescence with volumetric confocal microscopy and registered every dataset to a standard reference brain.
This resource is a modern update and improvement of a prior dataset of larval zebrafish gene expression (8)—a pioneering but imperfect record that was not at cellular resolution, was not registered to an atlas, and was restricted to very early stage larvae. In contrast, by integrating their high-resolution data into the standardized mapzebrain framework, Shainer et al. enable the identification of multiple features of neurons classified by gene expression. Furthermore, Shainer et al. use HCR for brain-wide mapping of the immediate early gene cfos after fish engage with a range of behavioral contexts, to identify the distribution of active neurons. While cfos labeling is unable to measure the temporal dynamics of active neurons, immediate early gene labeling with HCR can be applied to larvae performing any freely moving behavior and is not limited by the constraints of head-tethered preparations commonly used for faster time-scale calcium imaging.
A key feature of the mapzebrain framework is a query tool that allows users to search neurons in a brain region based on one feature, such as gene expression, and identify other features of the cells within this region (Fig. 1). Shainer et al. exhibit these functions with two representative examples. First, the authors start with a gene expression pattern and search for a Gal4 transgenic zebrafish line that may label these same neurons; the authors demonstrate that aldh1a2 expression overlaps with the mpn321Gt transgenic enhancer trap line, allowing for further characterization of these neurons by detailed observation and perturbation with the Gal4/UAS system. Second, the authors show the ability to start with functional identification of active neurons and go on to characterize their genetic and morphological properties. Using cfos expression to identify neurons active after exposure to prey, the authors found labeled cells in the secondary gustatory nucleus (SGN)—a hindbrain region that had not been previously identified in studies of virtual prey capture in tethered larvae. By further examining the SGN in the context of the multimodal brain atlas, the authors link this region to the expression of calb2a, npy1r, and of cells with distinct projections to other brain regions, including retinal arborization field 7, pretectum, tectum, hindbrain, and hypothalamus. These regions are known to contain neurons involved in visual perception, action, and hunger—all aspects of hunting and feeding. These two examples demonstrate the benefits of an integrated multimodal brain atlas.
Despite the advantages of this gene expression map and the broader mapzebrain resource, some questions about the link between cellular structure and function cannot be fully answered with this resource alone. Owing to the heterogeneity of neural circuits in vertebrates, observation of common cellular morphology, gene expression, and function within an anatomical region may not necessarily indicate that these features are all shared by the same cells. Claiming this kind of a link requires direct correspondence between such features in the same cells of individual animals, as demonstrated through within-subject cellular-level registration of in vivo calcium dynamics and either gene expression (9) or cellular morphology (10). Furthermore, some gene expression patterns may not be associated with neurons at all, but rather are present in glia or other cell types in the brain.
Another limitation of the Shainer et al. dataset is the narrow focus on the 6-day-old larva. While this stage is well-represented in the zebrafish literature and certainly deserves detailed and extensive documentation, the resources in Shainer et al. may not necessarily be applicable to fish at other ages. For instance, late-stage larvae and juvenile zebrafish are becoming more established for studying complex behaviors such as learning, decision-making, and social engagement, suggesting the potential benefits of expanding brain atlas efforts to include a diversity of ages. Future studies can also apply similar efforts to establish community resources for emerging genetic model systems such as Danionella cerebrum (11) whose small size and transparency may allow for comparable brain-wide atlases across the entire life span.
Overall, this paper is an important contribution to the growing project of zebrafish brain atlases, and integrative neuroscience in general. The fact that the gene expression data presented by Shainer et al. and the broader mapzebrain atlas are freely available is critical for its adoption, success, and continued growth, allowing scientists to come together and compare diverse classes of biological data on common ground.
Fig. 1. Identifying multiple features of neurons classified by gene expression.
Adding whole-brain in situ hybridization maps of 290 genes and activity-dependent gene expression to the mapzebrain larval zebrafish brain atlas produces a standardized and searchable resource for linking gene expression to multiple features of neurons across the brain. Credit: Matthew Lovett-Barron.
REFERENCES
- 1.A. S. Bates, J. Janssens, G. S. Jefferis, S. Aerts, Neuronal cell types in the fly: Single-cell anatomy meets single-cell genomics. Curr. Opin. Neurobiol. 56, 125–134 (2019). [DOI] [PubMed] [Google Scholar]
- 2.H. Zeng, What is a cell type and how to define it? Cell 185, 2739–2755 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.M. Kunst, E. Laurell, N. Mokayes, A. Kramer, F. Kubo, A. M. Fernandes, H. Baier, A cellular-resolution atlas of the larval zebrafish brain. Neuron 103, 21–38.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 4.F. Svara, D. Förster, F. Kubo, M. Januszewski, M. Dal Maschio, P. J. Schubert, H. Baier, Automated synapse-level reconstruction of neural circuits in the larval zebrafish brain. Nat. Methods 19, 1357–1366 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O. Randlett, C. L. Wee, E. A. Naumann, O. Nnaemeka, D. Schoppik, J. E. Fitzgerald, A. F. Schier, Whole-brain activity mapping onto a zebrafish brain atlas. Nat. Methods 12, 1039–1046 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.I. Shainer, E. Kuehn, E. Laurell, M. Al Kassar, N. Mokayes, S. Sherman, J. Larsch, M. Kunst, H. Baier, A single-cell resolution gene expression atlas of the larval zebrafish brain. Sci. Adv. 9, eade9909 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.H. M. Choi, M. Schwarzkopf, M. E. Fornace, A. Acharya, G. Artavanis, J. Stegmaier, N. A. Pierce, Third-generation in situ hybridization chain reaction: Multiplexed, quantitative, sensitive, versatile, robust. Development 145, dev165753 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.B. Thisse, C. Thisse, Fast release clones: A high throughput expression analysis. ZFIN Direct Data Submiss. 2004 (2004).
- 9.M. Lovett-Barron, R. Chen, S. Bradbury, A. S. Andalman, M. Wagle, S. Guo, K. Deisseroth, Multiple convergent hypothalamus–brainstem circuits drive defensive behavior. Nat. Neurosci. 23, 959–967 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A. Kramer, Y. Wu, H. Baier, F. Kubo, Neuronal architecture of a visual center that processes optic flow. Neuron 103, 118–132.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 11.L. Schulze, J. Henninger, M. Kadobianskyi, T. Chaigne, A. I. Faustino, N. Hakiy, B. Judkewitz, Transparent Danionella translucida as a genetically tractable vertebrate brain model. Nat. Methods 15, 977–983 (2018). [DOI] [PubMed] [Google Scholar]