ABSTRACT
Tumor necrosis factor-α inhibitors are monoclonal antibodies that are commonly used in the treatment of inflammatory bowel disease. A rare side effect of these biological agents is chronic inflammatory demyelinating polyneuropathy, which is a debilitating disease characterized by weakness, sensory dysfunction, and diminished or absent reflexes. We present the first reported case of chronic inflammatory demyelinating polyneuropathy after treatment with the tumor necrosis factor-α inhibitor biosimilar, infliximab-dyyp (Inflectra).
KEYWORDS: biosimilar, Crohn's disease, demyelination, infliximab, tumor necrosis factor alpha inhibitor
INTRODUCTION
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a disorder of the peripheral nervous system characterized by progressive symmetrical polyradiculoneuropathy with both proximal and distal weakness and sensory dysfunction in all extremities.1 Patients can have either reduced or absent tendon reflexes throughout along with evidence of demyelination on nerve biopsy.1 Although the pathophysiology of CIDP remains unknown, it is presumed to be autoimmune with both T-cell and antibody involvement as supported by improvement in symptoms after treatment with steroids, intravenous immunoglobulin (IVIg), and plasma exchange.2
Inflammatory bowel disease (IBD) has been reported to be a rare independent risk factor of development of CIDP.3–5 In addition, tumor necrosis factor-α inhibitors (TNFis) have also been linked to the development of CIDP, although this is reported to occur in <1% of cases.6–10 TNFis are some of the most prescribed medications in the treatment of autoimmune diseases such as IBD, rheumatoid arthritis, psoriasis, and ankylosing spondylitis. The most commonly implicated TNFis are infliximab, adalimumab, and etanercept. CIDP has been known to arise at any time throughout the treatment course.9 We present the first reported case of CIDP after administration of the infliximab biosimilar, infliximab-dyyp (Inflectra).
CASE REPORT
A 67-year-old man with a history of Crohn's disease (CD) of the terminal ileum (Montreal classification A3, L1, B1) presented to the hospital with 3 months of progressive bilateral motor and sensory deficits in the upper and lower extremities. His medical history was significant for tobacco use, nephrolithiasis, and monoclonal gammopathy of undetermined significance (MGUS). He had no history of alcohol abuse and denied recent toxin exposure, infection, or vaccination. His family history was negative for demyelinating disease.
CD was diagnosed 9 months before hospitalization. He was initially treated with budesonide and mesalamine, per patient preference, before being treated with Inflectra, 5 mg/kg every 8 weeks. He had been receiving Inflectra infusions for 6 months before symptom onset, with his last dose 8 weeks before hospitalization. He was in clinical remission at the onset of neurologic symptoms, and a colonoscopy at that time confirmed endoscopic remission.
He initially developed paresthesias in the distal upper and lower extremities that progressed to involve the trunk. As the paresthesias progressed, he developed muscle weakness to the extent that he lost grip strength and the ability to walk independently. On presentation, he used a wheelchair because of severe muscle weakness and recurrent falls.
Physical examination was significant for decreased strength in his bilateral upper and lower extremities (3/5 strength), impaired vibratory sensation, and decreased or absent reflexes (0) throughout. Neurology was consulted, and an extensive workup revealed normal computed tomography and magnetic resonance imaging of his brain and spinal cord (Figure 1). Inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein, were normal. Infectious workup including fungal cultures, venereal disease research laboratory tests, Borrelia burgdorferi antibodies, and West Nile virus antibodies were all negative. The vitamin B12 level was normal. He had a mildly elevated lead level of 5.8 μg/dL on heavy metal screen. His anti-nuclear antibody titer was positive at 1:160 and of a speckled pattern. Toxicology and rheumatology were consulted given these findings, but both the mildly elevated lead level and the anti-nuclear antibody titer were determined to be clinically insignificant. Electromyography showed evidence of severe active acute denervation in proximaland distal muscles, but was unable to assess whether polyradiculoneuropathy was demyelinating or axonal (Tables 1 and 2). Sural nerve biopsy revealed a severe decrease in myelinated nerve fibers (Figure 2) and epineural collections of inflammatory cells (Figure 3), suggesting an inflammatory demyelinating process. Lumbar puncture revealed a normal opening pressure with <1 white blood cell/mm3, protein 40 mg/dL, and glucose 61 mg/dL. He had matching oligoclonal bands in both cerebrospinal fluid and serum, suggestive of albuminocytologic dissociation. Neurology ultimately diagnosed the patient with CIDP.
Figure 1.
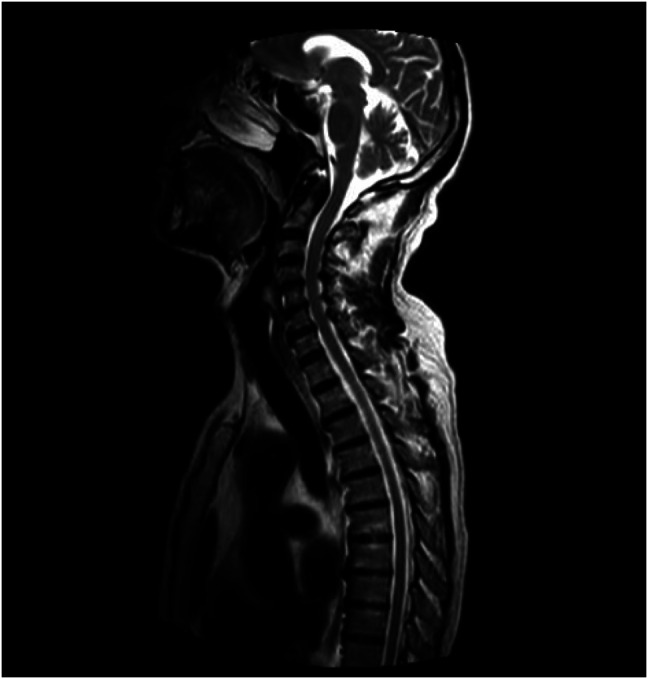
Magnetic resonance imaging showing no demyelination within the head, cervical spine, or thoracic spine.
Table 1.
Electromyographic results of motor nerve conduction with no response throughout
| Motor nerve conduction | ||||||||
| Nerve | Muscle | Latency observed (ms) | Latency normal (ms) | Amplitude observed (mV) | Amplitude normal (mV) | Distance (mm) | Velocity observed (m/s) | Velocity normal (m/s) |
| Right peroneal | Extensor digitorum brevis | NR | <6.6 | NR | >2 | 80 | NR | >41 |
| Right peroneal | Tibialis anterior | NR | <6.8 | NR | >5 | 100 | NR | >43 |
| Right tibial | Abductor hallucis | NR | <6.1 | NR | >4 | 70 | NR | >40 |
| Right median | Abductor pollicis brevis | NR | <4.5 | NR | >4 | 70 | NR | >48 |
| Right ulnar | Abductor digiti minimi | NR | <3.6 | NR | >6 | 60 | NR | >51 |
NR, no response.
Table 2.
Electromyographic results of sensory nerve conduction with no response throughout
| Sensory nerve conduction | |||||||||
| Nerve | Recording site | Onset latency (ms) | Peak latency observed (ms) | Peak latency normal (ms) | Amplitude observed (μV) | Amplitude normal (μV) | Distance (mm) | Velocity observed (m/s) | Velocity normal (m/s) |
| Right superficial peroneal | Ankle | NR | NR | n/a | NR | n/a | 100 | NR | n/a |
| Right sural | Ankle | NR | NR | <4.5 | NR | >6 | 120 | NR | >40 |
| Right median | Digit II | NR | NR | <3.6 | NR | >15 | 14 | NR | >50 |
| Right ulnar | Digit V | NR | NR | <3.1 | NR | >10 | 120 | NR | >50 |
| Right radial | Wrist | NR | NR | <2.9 | NR | >20 | 100 | NR | >50 |
n/a, not available; NR, no response.
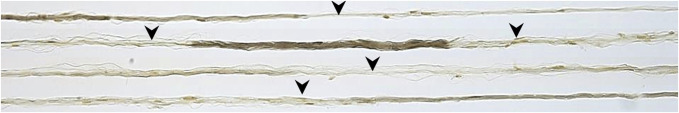
Figure 2.
Teased nerve fibers from sural nerve biopsy with arrowheads pointing to sites of active demyelination.
Figure 3.
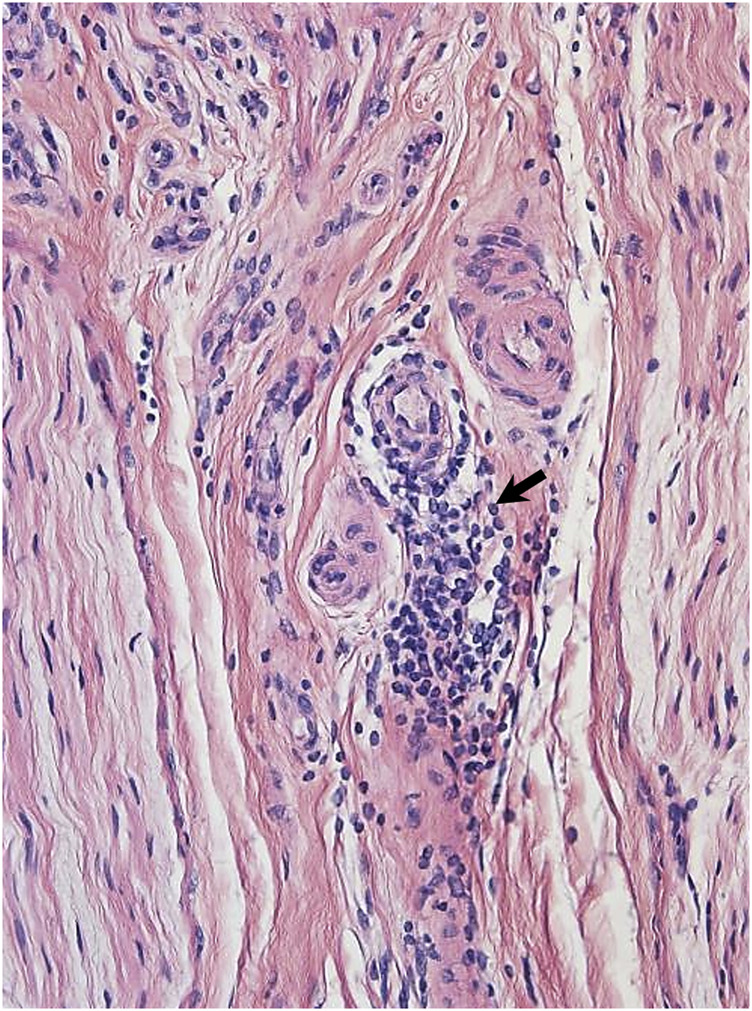
Hemotoxylin and eosin stain from sural nerve biopsy with arrows pointing to inflammatory cell infiltrate.
Inflectra was discontinued as a possible etiology of his CIDP, and the patient was started on IVIg with slow improvement in strength. C-reactive protein and fecal calprotectin were collected after stopping Inflectra and resulted as 0.3 mg/dL and 726 μg/mL, respectively. Two months later, the patient required rehospitalization for recurrence of his neurological symptoms. His strength had diminished to 1–2 of 5 in all extremities. IVIg was stopped, and the patient was transitioned to intravenous steroid injections and biweekly plasma exchange. He started to show improvement in strength after the fourth treatment and continued to receive steroids and plasma exchange in the outpatient setting.
Over the next 6 months, the patient's neurological symptoms significantly improved, and he now has only mild residual weakness in his hands. He was able to be weaned off weekly intravenous steroid infusions and started treatment with mycophenolate mofetil to sustain CIDP remission alongside continued plasma exchange. He remains in clinical remission on budesonide therapy for CD with plans to transition to vedolizumab after completion of plasma exchange.
DISCUSSION
To the best of our knowledge, this is the first reported case of the development of CIDP after administration of the infliximab biosimilar, Inflectra. Other possible causes of CIDP in our case included his underlying IBD or MGUS. However, owing to the temporal relationship between the biosimilar infusions and the onset of symptoms, improvement after discontinuation, and both clinical and endoscopic remission of CD, it was suspected that Inflectra may be related to his presentation of CIDP. MGUS is considered a risk factor of the development of CIDP and may have also increased the patient's likelihood of developing this rare adverse effect, but likely was not a primary culprit given that his MGUS had remained stable for several years.11 The overall incidence of CIDP in the general population is low at 2.81 per 100,000 persons.12 Case series have described the onset of neurological symptoms anywhere between 2 weeks and 32 months after initiation of TNFis.8,9,13 The average time to symptom onset was 13.7 months.
TNFis are associated with demyelination in both the central nervous system (CNS) and the peripheral nervous system, with the CNS being more commonly affected.8 In patients treated with TNFis for IBD, CNS demyelination (mostly multiple sclerosis or optic neuritis) accounts for 19.8% of neurological adverse events, whereas CIDP accounts for only 5.1%.14 Although there are several case reports documenting the association of CIDP with the use of TNFis for various rheumatological conditions, there are only 4 cases related to IBD (Table 3).15–18 Unfortunately, discontinuation of the affecting agent alone has not reliably been shown to have long-term effectiveness. Neurological symptoms often recur, and patients require treatment with steroids, IVIg, or plasma exchange.9,16 Despite adequate treatment, some patients continue to have a relapsing and remitting disease course as seen in our patient.2,9,16,19
Table 3.
Literature review of cases of patients with IBD treated with TNFis who developed CIDP
| Reference | Disease | TNFi | Time to symptom onset |
| Almuntashri et al15 | Crohn's disease | Infliximab | 12 months |
| Kamel et al16 | Crohn's disease | Infliximab | 3.5 months |
| Ohyagi et al17 | Crohn's disease | Infliximab | Unknown |
| Yao et al18 | Crohn's disease | Adalimumab | 8 months |
CIDP, chronic inflammatory demyelinating polyneuropathy; IBD, inflammatory bowel disease; TNFi, tumor necrosis factor-α inhibitor.
With the advent of biosimilars for treating autoimmune diseases such as CD, it is essential that clinicians are aware of how these drugs differ from generic medications. Biosimilars are biological products that are manufactured to resemble a reference medication or product. Biosimilars receive US Food and Drug Administration approval if they maintain the same primary amino acid sequence and mechanism of action as the reference medication.20 However, because each medication's manufacturing process is proprietary information, the biosimilar product always differs slightly to the reference medication. As a result, there are similarities between generic and biosimilar medications, but they do not have the identical biologic signature that is seen with brand name and generic medications.
Before starting treatment with any TNFi, including biosimilars, clinicians should inquire about the history of demyelinating diseases and TNFi should be avoided or used with extreme caution in patients with a history of these conditions. Patients should be closely monitored for the development of peripheral neuropathies while receiving treatment with TNFis because CIDP is known to arise at any time during treatment.9
DISCLOSURES
Author contributions: All authors made substantial contributions to the conception and design of this article. All authors edited and revised this work and have agreed on its accuracy. J. Larson is the article guarantor.
Acknowledgment: The authors acknowledge P. James B. Dyck, head of the peripheral neurology department at Mayo Clinic, for providing sural nerve biopsy images.
Financial disclosure: None to report.
Previous presentation: This case was presented at the 2022 Internal Medicine House Officer Poster Session on May 26, 2022, at the University of Nebraska Medical Center in Omaha, NE.
Informed consent was obtained for this case report.
Contributor Information
Patrick Twohig, Email: Patrick.twohig@unmc.edu.
Kathryn Hutchins, Email: Katie.hutchins@unmc.edu.
REFERENCES
- 1.Van den Bergh PYK, Hadden RDM, Bouche P, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. Eur J Neurol. 2010;17(3):356–63. [DOI] [PubMed] [Google Scholar]
- 2.Hughes RAC, Allen D, Makowska A, Gregson NA. Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2006;11(1):30–46. [DOI] [PubMed] [Google Scholar]
- 3.Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Arch Neurol. 1989;46(8):878–84. [DOI] [PubMed] [Google Scholar]
- 4.Gondim FAA, Brannagan TH, Sander HW, Chin RL, Latov N. Peripheral neuropathy in patients with inflammatory bowel disease. Brain. 2005;128:867–79. [DOI] [PubMed] [Google Scholar]
- 5.Ariatti A, Ficarra G, Girolami F, Pentore R, Galassi G. Chronic inflammatory demyelinating polyradiculoneuropathy associated with inflammatory bowel diseases: Questioning the autoimmunity hypothesis. Int J Colorectal Dis. 2009;24(5):603–4. [DOI] [PubMed] [Google Scholar]
- 6.Zhu TH, Nakamura M, Abrouk M, Farahnik B, Koo J, Bhutani T. Demyelinating disorders secondary to TNF-inhibitor therapy for the treatment of psoriasis: A review. J Dermatol Treat. 2016;27(5):406–13. [DOI] [PubMed] [Google Scholar]
- 7.Kaltsonoudis E, Voulgari PV, Konitsiotis S, Drosos AA. Demyelination and other neurological adverse events after anti-TNF therapy. Autoimmun Rev. 2014;13(1):54–8. [DOI] [PubMed] [Google Scholar]
- 8.Seror R, Richez C, Sordet C, et al. Pattern of demyelination occurring during anti-TNF-α therapy: A French national survey. Rheumatology. 2013;52(5):868–74. [DOI] [PubMed] [Google Scholar]
- 9.Alshekhlee A, Basiri K, Miles JD, Ahmad SA, Katirji B. Chronic inflammatory demyelinating polyneuropathy associated with tumor necrosis factor-α antagonists. Muscle Nerve. 2010;41(5):723–7. [DOI] [PubMed] [Google Scholar]
- 10.Stübgen J. Tumor necrosis factor-alpha antagonists and neuropathy. Muscle Nerve. 2008;37(3):281–92. [DOI] [PubMed] [Google Scholar]
- 11.Doneddu PE, Cocito D, Manganelli F, et al. Frequency of diabetes and other comorbidities in chronic inflammatory demyelinating polyradiculoneuropathy and their impact on clinical presentation and response to therapy. J Neurol Neurosurg Psychiatry. 2020;91(10):1092–9. [DOI] [PubMed] [Google Scholar]
- 12.Broers MC, Bunschoten C, Nieboer D, Lingsma HF, Jacobs BC. Incidence and prevalence of chronic inflammatory demyelinating polyradiculoneuropathy: A systematic review and meta-analysis. Neuroepidemiology. 2019;52(3–4):161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richez C, Blanco P, Lagueny A, Schaeverbeke T, Dehais J. Neuropathy resembling CIDP in patients receiving tumor necrosis factor-alpha blockers. Neurology. 2005;64(8):1468–70. [DOI] [PubMed] [Google Scholar]
- 14.Deepak P, Stobaugh DJ, Sherid M, Sifuentes H, Ehrenpreis ED. Neurological events with tumour necrosis factor alpha inhibitors reported to the food and drug administration adverse event reporting system. Aliment Pharmacol Ther. 2013;38(4):388–96. [DOI] [PubMed] [Google Scholar]
- 15.Almuntashri F, Binyaseen K, Alkhotani A. Chronic inflammatory demyelinating polyneuropathy in patients with Crohn's disease on infliximab therapy. Curēus. 2021;13(10):e19041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamel AY, Concepcion O, Schlachterman A, Glover S, Forsmark CY. Chronic inflammatory demyelinating polyneuropathy following anti-TNF-α therapy with infliximab for Crohn's disease. ACG Case Rep J. 2016;3(3):187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohyagi M, Ohkubo T, Yagi Y, et al. Chronic inflammatory demyelinating polyradiculoneuropathy in a patient with Crohn's disease. Intern Med. 2013;52(1):125–8. [DOI] [PubMed] [Google Scholar]
- 18.Yao A, Chan H, Macdonell RAL, Shuey N, Khong JJ. Bilateral facial nerve palsies secondary to chronic inflammatory demyelinating polyneuropathy following adalimumab treatment. Clin Neurol Neurosurg. 2018;164:64–6. [DOI] [PubMed] [Google Scholar]
- 19.Karantali E, Katsikaki G, Chatzikonstantinou S, Papagiannopoulos S. Infliximab induced chronic inflammatory demyelinating polyneuropathy: A case report. Hippokratia. 2019;23(4):179–80. [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Sabbagh A, Olech E, McClellan JE, Kirchhoff CF. Development of biosimilars. Semin Arthritis Rheum. 2016;45(5):S11–8. [DOI] [PubMed] [Google Scholar]