Abstract
We present the case of a young woman admitted for diabetic ketoacidosis with persistent, asymptomatic lactic acid (LA) elevation during the evolving COVID-19 pandemic. Cognitive biases in interpreting an elevated LA in this patient’s care resulted in an extensive infectious workup instead of the low-cost and potentially diagnostic provision of empiric thiamine. We discuss clinical patterns and etiologies of LA elevation and the role of thiamine deficiency. We also address cognitive biases potentially affecting the interpretation of elevated lactate levels and provide guidance for clinicians to determine appropriate patients for empiric thiamine administration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08091-w.
CASE
A 21-year-old female with uncontrolled (HbA1c 13.6% at most recent hospitalization for ketoacidosis) type 1 diabetes mellitus (T1DM) presented with a four-day history of progressive nausea, multiple episodes of non-bloody emesis, and limited oral intake in November 2020, during the first wave of the SARS-CoV-2 pandemic. Associated symptoms included upper abdominal pain, cough, diarrhea, shortness of breath, myalgias, and sore throat. On initial assessment, she was found to be in diabetic ketoacidosis (DKA), with labs notable for a glucose of 748 (70–100) mmol/L, an anion gap of 28 (7–17) mmol/L, an arterial pH of 7.29 (7.35–7.45), a lactate of 4.9 (0.5–2.2) mmol/L, and white blood cell count (WBC) of 8.7 (4–11 K/μL). Liver transaminases were mildly elevated (ALT 75 (10–35) U/L, AST 85 (10–35) U/L) and right upper quadrant ultrasound was unremarkable. Chest X-ray was normal and SARS-CoV-2 RNA PCR testing was negative. Urinalysis showed 4 + glucose, 3 + ketones, and 19 WBC (0–5/hpf) without bacteria.
The initial treatment regimen included an insulin drip, dextrose, anti-emetics, and ceftriaxone for a possible urinary tract infection, in addition to potassium repletion and intravenous fluids. Within the next 12 h, the serum glucose level (126 mmol/L) and anion gap normalized with significant improvement in symptoms. The insulin drip was discontinued, and her outpatient subcutaneous insulin dose was resumed.
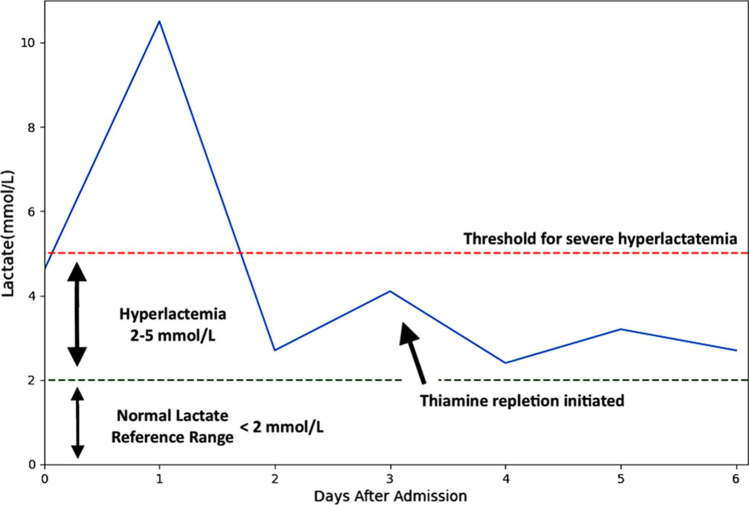
The initial elevation of lactate, or lactic acidosis (LA), was attributed to volume depletion (type A LA), a common finding in DKA.1 Over the next 24 h, her blood glucose levels were labile, peaking at 270 mg/dL, and her lactate increased to 10.5 mmol/L. After adequate fluid resuscitation, the lactate decreased to 2.4 mmol/L on day 2 of admission. She remained asymptomatic and clinically well-appearing for the remainder of her hospitalization, with blood glucose levels between 90 and 150 mmol/L. However, her lactate continued to fluctuate, spiking to 4.1 mmol/L on hospital day 3 (Fig. 1). Anion gap remained within normal limits and transaminases normalized. There were no apparent signs of new infection or gut ischemia. Abdominal examination was repeatedly benign. Blood cultures were not drawn given very low clinical suspicion for bacteremia in the setting of hemodynamic stability, and lack of fever or peripheral leukocytosis. Testing for stool pathogens, sexually transmitted diseases, respiratory viruses, repeat SARS-CoV-2, and acute hepatitis were all negative.
Figure 1.
Graph of prolonged elevated lactate throughout hospital course.
Once this comprehensive evaluation was negative, type B LA was suspected. She was not on commonly implicated LA-inducing drugs including metformin, nucleoside reverse transcriptase inhibitors, or acetaminophen (Table 1). Empiric thiamine (vitamin B1) repletion was provided, and the lactate level stabilized under 3 mmol/L (Fig. 1). The patient was discharged after eight days of hospitalization on daily oral vitamin B1 supplementation and her previously established insulin regimen. Following discharge, the thiamine level from earlier in her admission resulted at 7 nmol/L (8–30 nmol/L).
Table 1.
| Type A | Type B |
|---|---|
|
- Hypoxemia - Hypoperfusion states - Severe vasodilation - Heart failure - Respiratory failure - Sepsis |
- Medications (acetaminophen overdose, nucleoside reverse transcriptase inhibitors, phenformin, metformin) - Acquired co-factor deficiency (thiamine deficiency) - Congenital enzyme deficiency (PDH deficiency) - Congenital or acquired respiratory chain mitochondrial dysfunctions - Malignancy, most commonly hematological malignancies and advanced-stage solid tumors - Toxins that induce mitochondrial respiratory dysfunction (ex. cyanide) - Carbon monoxide poisoning - Alcohols (ethanol, methanol, propylene glycol, ethylene glycol) |
DISCUSSION
This case illustrates the cognitive biases in which an overreliance on heuristics resulted in an extensive workup for lactic acidosis in a clinically well-appearing, asymptomatic patient for whom thiamine repletion was a safe, cost-effective treatment (Fig. 2). We discuss the pathophysiology of lactic acidosis and thiamine (B1) deficiency, and explore the cognitive biases particular to interpretation of elevated lactate levels during inpatient clinical management, including anchoring, availability and base rate biases, and heuristics and illness scripts.
Figure 2.
Hospital course overview of patient case.
Lactate can be generated by routine activities such as aerobic exercise, as well as by pathological states that increase energy expenditure (seizures), reduce oxygen supply to the tissue (shock, respiratory failure, or tissue ischemia), or impair the ability of glucose to be metabolized aerobically (thiamine deficiency). Lactate accumulates due to competing enzyme processes required for pyruvate metabolism and utilization (type B), or due to a lack of substrates (oxygen) needed for the tricarboxylic acid cycle (TCA) and electron transport chain (type A). Lactate is rapidly cleared by the liver, and partially excreted by the kidneys.2,3
Type A is the most common cause of LA and the most clinically pressing due to the hypoxemia and hypoperfusion states as seen in sepsis, severe vasodilation, heart failure, or respiratory failure.2 Lactate measurements are often used as a prognostic factor in shock, correlating with increased mortality.2,4 Type A LA is often initially present in patients with DKA. This is the result of intravascular volume contraction which is exacerbated by macrovascular disease and higher glycosylated hemoglobin levels, which increase susceptibility to hypoxemia and anaerobic glycolysis.5 One study of ED patients with DKA found that 68% had LA (lactate >2.5 mmol/L) mostly attributed to hypovolemia, hypotension, and hyperventilation.1
There is a broader range of etiologies causing elevated lactate in type B LA, such as enzyme or co-factor deficiencies (Table 1).2,6 In addition to the etiologies of type A LA in DKA, type B etiologies include metabolic derangements and thiamine deficiency. Data suggest up to a quarter of patients presenting with DKA are thiamine deficient, with an inverse correlation between thiamine and lactate levels, and significantly elevated rates of vomiting and abdominal pain in thiamine-deficient patients.7–12 In vitro studies have shown that insulin deficiency leads to both poor small intestine thiamine absorption and renal reuptake in the proximal tubules, which may explain why patients with diabetes are often thiamine deficient.7,8 The term “gastrointestinal beriberi” has been used to reference nausea, emesis, and abdominal pain that resolve with thiamine administration10,11 and could have been a contributory factor to our patient’s gastrointestinal symptoms on presentation.
Thiamine has proven to be a safe, inexpensive, and readily available treatment that quickly reverses LA secondary to thiamine deficiency. Risk factors for thiamine deficiency include gastrointestinal dysfunction such as persistent vomiting (e.g., hyperemesis gravidarum) or malabsorption, poor nutritional status, diuretic use, and hemodialysis.13 In addition to understanding drivers of lactate acidosis, knowledge of thiamine and risk factors for thiamine deficiency could have improved this patient’s care.14–16
Our case illustrates several biases in the context of DKA and LA (Table 2). Reliance on lactate levels as a marker of sepsis, despite other potential drivers of lactic acidosis, has increased since the onset of the SARS-CoV-2 pandemic.17–20 Dependence on heuristics has increased in the COVID-19 era given the frequency of rapid patient decompensation, healthcare provider burnout, and limited resources.21,22 Hence, accurate interpretation of LA levels within the appropriate clinical context and ability to recognize the pitfalls of cognitive biases have become more challenging.
Table 2.
Types of Cognitive Biases and Tools to Address Them
| Cognitive bias | Definition | Example | Tools to address bias |
|---|---|---|---|
| Anchoring bias | Tendency to fixate and base decisions on initial evidence 23 | Fixating on a type A septic etiology of LA and overlooking type B LA |
Utilize a stepwise schema as a tool for redirection For example, in working up lactic acidosis 2: (1) Evaluate for adequate perfusion (2) Systemic work up for local ischemia (3) Remove potential agents (4) Check for thiamine deficiency (5) Consider other metabolic etiologies |
| Availability bias | Recent events amplified by an emotional component “prime” the physician to focus on a diagnosis 23 | The preponderance of patients with sepsis and type A LA during the COVID-19 pandemic increase might justify focus on type A LA | |
| Confirmation bias | Tendency to interpret new data to fit the presumed diagnosis 23 | Excessive infectious workup to support type A LA causes despite scant clinical evidence of infection | |
| Conjunction fallacy | The belief that multiple events are more probable than a single process 14 | The persistent search for a cause of sepsis in addition to DKA, with less emphasis on how DKA could drive LA | Bias-specific teaching sessions to prompt clinicians to prioritize a unifying diagnosis over concurrent separate processes 14 |
| Diagnostic momentum | Tendency to maintain a clinical course of action initiated by the prior care team 23 | Continued emphasis on a type A LA etiology throughout her hospitalization despite transferring of services | Diagnostic time-outs to review and synthesize all data and consider alternative disease process 14 |
| Base rate bias | Failure to consider underlying incidence rates of conditions 23 | Very low likelihood that a clinically well-appearing, hemodynamically stable 21-year-old patient was in occult shock | Consider pre-test probability as well as magnitude of abnormal test to maximize clinical utility 24 |
| Heuristics and illness scripts | Mental shortcuts in which an illness/diagnosis becomes associated with a pattern of presenting symptoms and/or a particular demographic 21,22 |
Thiamine deficiency is primarily associated with older men with alcohol use disorder 25–28 Gaps in knowledge about the prevalence of thiamine deficiency in patients with DKA further exacerbates this bias |
Practicing metacognition and questioning one’s own thinking process, as well as continuous medication education to fill in gaps 14 |
Exclusive focus on a type A etiology of LA and overlooking type B LA is an example of anchoring bias, which is the tendency to fixate and base decisions on initial evidence,23 and in this case reflects a focus on the tissue hypoperfusion with which our patient was admitted. Availability bias, in which recent events amplified by an emotional component “prime” the physician to focus on a diagnosis, was another likely contributor given the preponderance of patients with sepsis during the SARS-CoV-2 pandemic.14 The continued emphasis on a type A LA throughout our patient’s hospitalization despite transferring of services from emergency department (ED), hospitalist admission triaging, and inpatient medicine floor care is representative of diagnostic momentum, in which a clinical course of action by the prior care team is maintained.14 In this case, a working diagnosis of type A lactic acidosis was inconsistent with hemodynamic stability and a comprehensive negative infectious evaluation. Moreover, the persistent search for a cause of sepsis in addition to her DKA, with less emphasis on how DKA could drive LA, represents a conjunction fallacy, which is the belief that multiple events are more probable than a single process.
The base rate bias may have also been pertinent, in which clinicians fail to consider underlying incidence rates of conditions. The pre-test probability that a clinically well-appearing, hemodynamically stable young adult patient is in occult shock was low, and thus an alternative diagnosis like type B LA could have been considered earlier and reduced hospital costs.14 Some providers may consider continuous laboratory values as dichotomous instead of considering the magnitude of the abnormal test to maximize clinical utility.24 Badaushvili et al. illustrate how interpreting ROC curves and likelihood ratios can help mitigate this overestimation and determine post-test probability for improved clinical judgement.24
Finally, heuristics and illness scripts, while core parts of clinical reasoning, have contributed to the association of thiamine deficiency primarily with older men with alcohol use disorder25–28 compared to our patient who was a young woman with T1DM.
Suggested methods of cognitive de-biasing for clinicians include participating in bias-specific teaching sessions, diagnostic timeouts, practicing metacognition, and focusing on what data cannot be accounted for by a diagnosis among other methods.14–16 Moreover, gaps in knowledge surrounding type B LA reinforce the importance of continuous medical education as a powerful de-biasing tool. A diagnostic timeout could have helped redirect clinicians away from sepsis-driven LA to considering her LA and GI upset in the context of her DKA and underlying thiamine deficiency. Schemas can be helpful tools to enable redirection; one such schema for working up LA proposed by Andersen et al. starts with focused evaluation for adequate perfusion, followed by systematic workup for any local ischemia, removing any potentially offending agents, checking for thiamine deficiency, and finally considering other metabolic etiologies such as liver dysfunction.2
CONCLUSION
In this case of a young woman admitted for DKA and manifesting persistent, asymptomatic LA elevation during the evolving COVID-19 pandemic, cognitive biases resulted in an extensive infectious workup in lieu of the low-cost and potentially diagnostic provision of empiric thiamine. The delay in considering thiamine deficiency resulted in a prolonged eight-day hospitalization, at least four days longer than was indicated given the patient’s clinical stability and resolution of DKA. Several factors likely contributed to the LA including contracted intravascular volume, metabolic derangements, and thiamine deficiency. The potential for thiamine deficiency supports a low clinical threshold for thiamine infusion in patients who are critically ill, exhibit any mental status changes or unspecific neurologic symptoms, or have any form of malnutrition.29 Commensurately, the risk of cognitive biases should drive clinician vigilance to identify bias and fill gaps in knowledge with the ultimate goal to deliver more efficient, effective care.
Key Learning Points for the Case:
Thiamine deficiency can be the primary precipitant of a type B LA, especially in patients with diabetes/DKA.
Several cognitive biases affect interpretation of elevated lactate levels, including anchoring bias, availability bias, confirmation bias, diagnostic momentum, and base rate bias. Gaps in clinical knowledge exacerbate these biases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All authors had access to the data and a role in writing the manuscript.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cox K, et al. Prevalence and significance of lactic acidosis in diabetic ketoacidosis. J Crit Care 2012;27(2):132-7. [DOI] [PMC free article] [PubMed]
- 2.Andersen LW, et al. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88(10):1127-40. [DOI] [PMC free article] [PubMed]
- 3.Connor H, et al. A model of L(+)-lactate metabolism in normal man. Ann Nutr Metab. 1982;26(4):254-63. [DOI] [PubMed]
- 4.Howell MD, et al. Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med. 2007;33(11):1892-9. [DOI] [PubMed]
- 5.Feenstra RA, et al. Lactic acidosis in diabetic ketoacidosis. BMJ Case Rep. 2014. 2014. [DOI] [PMC free article] [PubMed]
- 6.Adeva-Andany M, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014;17:76-100. [DOI] [PubMed]
- 7.Moskowitz A. et al. The relationship between lactate and thiamine levels in patients with diabetic ketoacidosis. J Crit Care. 2014;29(1):182 e5–8. [DOI] [PMC free article] [PubMed]
- 8.Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr Diabetes Rev. 2005;1(3):287-98. [DOI] [PubMed]
- 9.Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65(6):684-90. [DOI] [PubMed]
- 10.De WH, Lennox B. Cerebral beriberi (Wernicke's encephalopathy); review of 52 cases in a Singapore prisoner-of-war hospital. Lancet 1947;1(6436):11-7. [PubMed]
- 11.Donnino M. Gastrointestinal beriberi: a previously unrecognized syndrome. Ann Intern Med. 2004;141(11):898-9. [DOI] [PubMed]
- 12.Hibbs RE. Beriberi in Japanese prison camp. Ann Intern Med. 1946;25:270-82. [DOI] [PubMed]
- 13.Attaluri P, et al. Thiamine Deficiency: An Important Consideration in Critically Ill Patients. Am J Med Sci. 2018;356(4):382-390. [DOI] [PubMed]
- 14.O'Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48(3):225-232. [DOI] [PubMed]
- 15.Royce CS, Hayes MM, Schwartzstein RM. Teaching Critical Thinking: A Case for Instruction in Cognitive Biases to Reduce Diagnostic Errors and Improve Patient Safety. Acad Med. 2019;94(2):187-194. [DOI] [PubMed]
- 16.Trowbridge RL. Twelve tips for teaching avoidance of diagnostic errors. Med Teach. 2008;30(5):496-500. [DOI] [PubMed]
- 17.Kocak Tufan Z, Kayaaslan B, Mer M. COVID-19 and Sepsis. Turk J Med Sci. 2021;51(SI-1):3301-3311. [DOI] [PMC free article] [PubMed]
- 18.Vincent JL. COVID-19: it's all about sepsis. Future Microbiol. 2021;16:131-133. [DOI] [PMC free article] [PubMed]
- 19.Vassiliou AG, et al. Lactate Kinetics Reflect Organ Dysfunction and Are Associated with Adverse Outcomes in Intensive Care Unit Patients with COVID-19 Pneumonia: Preliminary Results from a GREEK Single-Centre Study. Metabolites 2020;10(10). [DOI] [PMC free article] [PubMed]
- 20.Price-Haywood EG, et al. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N Engl J Med. 2020;382(26):2534-2543. [DOI] [PMC free article] [PubMed]
- 21.DiMaria CN, et al. Cognitive Bias in the COVID-19 Pandemic. Cureus 2020;12(7):e9019. [DOI] [PMC free article] [PubMed]
- 22.Raudenska J, et al. Occupational burnout syndrome and post-traumatic stress among healthcare professionals during the novel coronavirus disease 2019 (COVID-19) pandemic. Best Pract Res Clin Anaesthesiol. 2020;34(3):553-560. [DOI] [PMC free article] [PubMed]
- 23.Tversky A, Kahneman D. Judgment under Uncertainty: Heuristics and Biases. Science 1974;185(4157):1124-31. [DOI] [PubMed]
- 24.Baduashvili A, Guyatt G, Evans AT. ROC Anatomy-Getting the Most Out of Your Diagnostic Test. J Gen Intern Med. 2019;34(9):1892-1898. [DOI] [PMC free article] [PubMed]
- 25.Lubarsky S, et al. Using script theory to cultivate illness script formation and clinical reasoning in health professions education. Can Med Educ J. 2015;6(2):e61-70. [PMC free article] [PubMed]
- 26.Trimble M, Hamilton P. The thinking doctor: clinical decision making in contemporary medicine. Clin Med (Lond), 2016;16(4):343-6. [DOI] [PMC free article] [PubMed]
- 27.Marewski JN, Gigerenzer G. Heuristic decision making in medicine. Dialogues Clin Neurosci. 2012;14(1):77-89. [DOI] [PMC free article] [PubMed]
- 28.Blumenthal-Barby JS, Krieger H. Cognitive biases and heuristics in medical decision making: a critical review using a systematic search strategy. Med Decis Making. 2015;35(4):539-57. [DOI] [PubMed]
- 29.Amrein K, et al. Severe lactic acidosis reversed by thiamine within 24 hours. Crit Care. 2011;15(6):457. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.