Abstract
Aims:
To investigate in-hospital and long-term prognosis in T2DM patients presenting with acute myocardial infarction (AMI) treated with SGLT2-I versus other oral anti-diabetic agents (non-SGLT2-I users).
Methods:
In this multicenter international registry all consecutive diabetic AMI patients undergoing percutaneous coronary intervention between 2018 and 2021 were enrolled and, based on the admission anti-diabetic therapy, divided into SGLT-I users versus non-SGLT2-I users. The primary endpoint was defined as a composite of cardiovascular death, recurrent AMI, and hospitalization for HF (MACE). Secondary outcomes included i) in-hospital cardiovascular death, recurrent AMI, occurrence of arrhythmias, and contrast-induced acute kidney injury (CI-AKI); ii) long-term cardiovascular mortality, recurrent AMI, heart failure (HF) hospitalization.
Results:
The study population consisted of 646 AMI patients (with or without ST-segment elevation): 111 SGLT2-I users and 535 non-SGLT-I users. The use of SGLT2-I was associated with a significantly lower in-hospital cardiovascular death, arrhythmic burden, and occurrence of CI-AKI (all p < 0.05). During a median follow-up of 24 ± 13 months, the primaiy composite endpoint, as well as cardiovascular mortality and HF hospitalization were lower for SGLT2-I users compared to non-SGLT2-I patients (p < 0.04 for all). After adjusting for confounding factors, the use of SGLT2-I was identified as independent predictor of reduced MACE occurrence (HR=0.57; 95%CI:0.33–0.99; p = 0.039) and HF hospitalization (HR=0.46; 95%CI:0.21–0.98; p = 0.041).
Conclusions:
In T2DM AMI patients, the use of SGLT2-I was associated with a lower risk of adverse cardiovascular outcomes during index hospitalization and long-term follow-up. Our findings provide new insights into the cardioprotective effects of SGLT2-I in the setting of AMI.
Registration:
Data are part of the observational international registry: SGLT2-I AMI PROTECT. ClinicalTrials.gov Identifier: NCT05261867.
Keywords: SGLT2-I, Acute myocardial infarction, Outcomes, Arrhythmias, HF hospitalization
1. Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2-I) are oral anti-diabetic (OAD) agents that exert beneficial effects on glycemic control in type 2 diabetes mellitus (T2DM). In large, randomized trials, SGLT2-I significantly improved cardiovascular and renal outcomes in diabetic patients, extending benefits to non-diabetic patients with heart failure (HF) [1–5]. Pre-clinical studies have also shown that SGLT2-I mitigates acute myocardial I/R injury, attenuating cardiac infarct size, increasing left ventricular function, and reducing arrhythmias [6,7]. There are some ongoing trials, compounded by the first published results of the EMMY Trial, which did not find any difference in acute troponin values between the SGLT2-I treated and untreated cohorts [8,9]. However, the EMMY trial included only a minority of diabetic patients, and all patients were randomized to the treatment at the time of the AMI admission. Thus, the actual efficacy and safety of SGLT2-I chronic therapy in diabetic patients with AMI remain an under-studied topic. On the clinical ground, we recently demonstrated that T2DM patients hospitalized for AMI and receiving SGLT2-I exhibited a significantly reduced inflammatory and arrhythmic burden and infarct size compared to non-SGLT2-I users, independently of glucose-metabolic control [10,11].
Based on these observations, we hypothesized that SGLT2-I might have acute and long-term cardioprotective effects with favorable prognostic impact, on top of their anti-hyperglycemic properties [12]. To test this hypothesis, we investigated the in-hospital and long-term prognosis in T2DM patients with AMI receiving SGLT2-I compared to other OAD agents (non-SGLT-I users).
2. Methods
2.1. Study population
In this multicenter international observational registry (SGLT2-I AMI PROTECT, ClinicalTrials.gov Identifier: NCT05261867), we included consecutive diabetic patients admitted with AMI, both ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI), undergoing percutaneous coronary intervention (PCI), between January 2018 and November 2021 (Fig. 1). The definition of STEMI and NSTEMI and patient management followed current guidelines [13,14]. Based on admission antidiabetic therapy, patients were divided into SGLT2-I users, if they were admitted on chronic SGLT2-I therapy (started at least 3 months before hospitalization), and non-SGLT2-I users, if they received other OAD strategies. Patients on insulin therapy or with incomplete information on medical therapy were excluded. Further exclusion criteria were coronary artery bypass graft surgery (CABG) as revascularization treatment, severe valvular heart disease, prosthetic heart valves, severe anemia, history of bleeding, pulmonary embolism, glomerular filtration rate < 30 ml/min/1.73 m2, malignancies, and follow-up data shorter than 3 months. Patients with more than 20 % of missing values in the collected data were excluded due to potential bias. The present study was conducted according to the principles of the Declaration of Helsinki; all patients were informed about their participation in the registry and provided informed consent for the anonymous publication of scientific data.
Fig. 1.
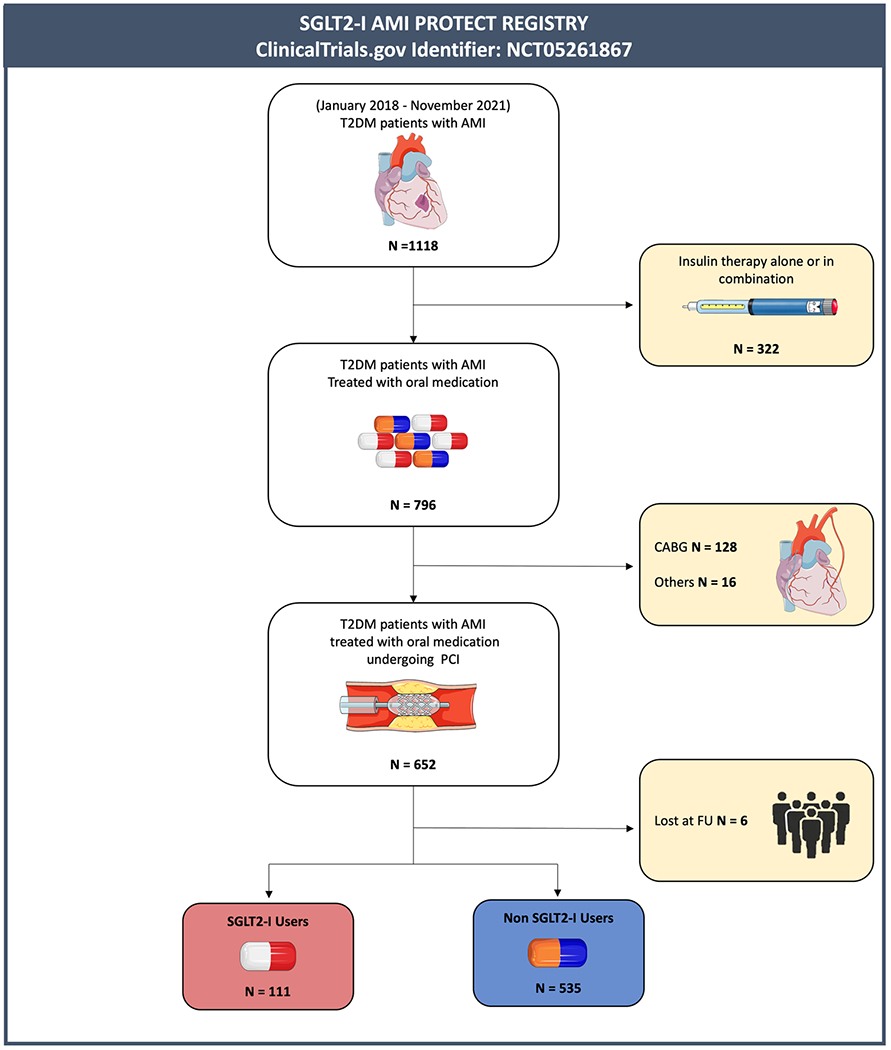
Study flow chart. Abbreviations: T2DM = type 2 diabetes mellitus; AMI = acute myocardial infarction; CABG = coronary artery bypass graft; PCI = Percutaneous coronary intervention; SGLT2-I = Sodium-glucose co-transporter 2 inhibitors.
2.2. Clinical endpoints and follow-up
Patients were followed over time with outpatient visits and telephone contacts using a standard questionnaire. Clinical outcomes were defined according to the current standards [15]. The primary endpoint of our study was defined as a composite of cardiovascular death, recurrent AMI, and hospitalization for HF (MACE). Secondary in-hospital outcomes included length of hospital stay, in-hospital cardiovascular death, recurrent AMI, the occurrence of major arrhythmias, and contrast-induced acute kidney injury. Secondary long-term outcomes were cardiovascular mortality, recurrent AMI, any coronary revascularization, and hospitalization for HF. The definition of clinical endpoints is reported in the Supplementary File.
2.3. Statistical analysis
Normal distribution of continuous variables was assessed by histograms and q-plot; the Shapiro-Wilk test was used when required. Continuous variables with normal distribution were expressed as the mean ± standard deviation and non-normally distributed variables as median and interquartile range. Normal ranges were presented as the 5th and 95th percentiles. Categorical variables were expressed as counts and percentages. Differences between groups were analyzed using the t-test or the Mann–Whitney U-test for continuous variables and the chi-square test or the Fisher’s exact test for categorical variables, as appropriate. To compare paired data a Wilcoxon signed-test or a Paired sample T-test were performed as appropriate. Univariate analysis was performed to identify variables associated with cardiovascular death, hospitalization, and MACE. Significant variables were then entered into a multivariable analysis using the Cox regression model to determine the independent association of each risk factor with outcomes occurrence. The hazard ratio (HR) and the associated 95 % confidence interval (CI) for each variable were determined. The final list of covariates was also determined by removing variables that caused high collinearity, as accessed by variance inflation factors. Kaplan-Meier analysis and Logrank test were used to compare the cumulative incidence of clinical events between groups. In addition, linear and polynomial regression models were fit to evaluate the relationship between continuous variables. P-values < 0.05 is considered statistically significant. All analyses were performed using R statistical software version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria), Statistical Package for Social Sciences, version 25.0 (SPSS, PC version, Chicago, IL, USA) and GraphPad Prism (GraphPad Software, Inc., CA, US).
3. Results
3.1. Study population
Out of 1118 diabetic patients with AMI screened, 322 were excluded due to insulin therapy, 128 because they underwent CABG, 16 for all the other exclusion criteria. and 6 due to a clinical follow-up either unavailable or shorter than 3 months. The final study population consisted of 646 diabetic patients with AMI treated with PCI, divided into SGLT2-I (n = 111) or non-SGLT2-I users (n = 535) (Fig. 1).
3.2. Baseline and procedure characteristics
Baseline characteristics, cardiovascular risk factors, and comorbidities are reported in Table 1. The mean age of the overall study population was 70 [61–79] years, and more than 77% were males. The mean T2DM duration was similar for both groups (6.9±2.9 years for SGLT2-I users and 7.1±1.5 years for non-SGLT2-I users, p = 0.123). SGLT2-I patients were younger and with better renal function at admission compared to non-SGLT2-I users. The mean time of SGLT2-I therapy duration was 7.3 ± 3 months. At variance, gender, body mass index/surface area, main cardiovascular risk factors, glucose-metabolic control, and comorbidities were similar in the two groups. Regarding admission medical therapy, no differences were found, except for a lower intake of sulfonylureas in SGLT2-I users (Table 2).
Table 1.
Study population baseline eharaeteristies and clinical presentation.
| Tota (N = 646) |
SGLT2-I users (N = 111) |
Non-SGLT2-I users (N = 535) |
P-value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, years | 70 [61–79] | 66 [59–73] | 72 [62–80] | <0.001 |
| Male Sex, n (%) | 498 (77.1) | 90 (81.1) | 405 (75.7) | 0.222 |
| BMI, kg/m2 | 27.7 [25 – 31.3] | 27.1 [24.6–30] | 27.7 [25–31.4] | 0.245 |
| BSA, m2 | 1.94 [1.8–2.1] | 1.96 [1.8 – 2.03] | 1.93 [1.78 – 2.1] | 0.261 |
| Smoking, n (%) | 370 (57.3) | 67 (60.4) | 303 (56.6) | 0.470 |
| Hypertension, n (%) | 541 (83.7) | 98 (88.3) | 443 (82.8) | 0.154 |
| Dyslipidemia, n (%) | 508 (78.6) | 90 (81.1) | 418 (78.1) | 0.490 |
| PAD, n (%) | 82 (12.7) | 16 (14.4) | 66 (12.3) | 0.550 |
| COPD, n (%) | 90 (13.9) | 15 (13.5) | 75 (14) | 0.889 |
| CKD, n(%) | 58 (9) | 10 (9) | 47 (8.8) | 0.886 |
| Previous TIA/CVA, n (%) | 52 (8) | 10 (9) | 42 (7.9) | 0.683 |
| Previous AMI, n (%) | 169 (26.2) | 30 (27) | 136 (25.4) | 0.724 |
| Previous PCI, n (%) | 183 (28.3) | 35 (31.5) | 144 (26.9) | 0.322 |
| Clinical presentation | ||||
| STEMI, n (%) | 309 (47.8) | 52 (46.8) | 257 [48] | 0.819 |
| Time symptoms–balloon (STEMI), hours | 3 [2–5] | 3 [2–6] | 3 [2–5] | 0.648 |
| Time symptoms–balloon < 24 h (NSTEMI) | 207 (61.4) | 39 (66.1) | 175 (62.9) | 0.647 |
| SBP, mmHg | 140 [125–160] | 140 [125–155] | 140 [125–160] | 0.639 |
| DBP, mmHg | 80 [70–90] | 83 [70–90] | 80 [70–90] | 0.551 |
| HR, bpm | 81 [70–94] | 75 [68–86] | 83 [72–95] | <0.001 |
| Angina, n (%) | 466 (72.1) | 80 (72.1) | 386 (72.1) | 0.987 |
| NYHA> 2, n (%) | 113 (17.5) | 16 (14.4) | 101 (18.9) | 0.266 |
| Killip Class ≥ 2, n (%) | 135 (20.9) | 18 (16.2) | 117 (21.9) | 0.183 |
| VT/VF, n (%) | 21 (3.3) | 2 (1.8) | 19 (3.6) | 0.344 |
| AF, n (%) | 58 (9) | 9 (8.1) | 49 (9.2) | 0.725 |
Continuous variables are presented as mean±SD or as median [IQR]; while categorical variables as number (%).
Abbreviations: BMI=Body Mass Index; BSA=Body Surface Area; CKD=chronic kidney disease with 30 <GFR< 60 ml/min; PCI=Pereutaneous Coronary Intervention; AF=atrial fibrillation; ACEI=Angiotensin-converting enzyme; ARB = Angiotensin II Receptor Blockers; CCB=Caleium Channel Blockers; BB=B-bloekers; GFR=Glomerular Filtration Rate. STEMI=ST-segment Elevation Myocardial Infarction; NSTEMI=non-ST segment Elevation Myocardial Infarction; SBP=systolie blood pressure; DBP=diastolie blood pressure; HR=heart rate; NYHA=New York Heart Association; VT=Ventrieular Tachycardia; VF=Ventricular Fibrillation; AF=Atrial Fibrillation.
Table 2.
Admission, in-hospital and discharge medical therapy.
| Total (N = 646) |
SGLT2-I users (N = 111) |
Non-SGLT2-I users (N = 535) |
P value | |
|---|---|---|---|---|
| Admission medical therapy | ||||
| Anti platelets, n (%) | 321 (49.7) | 60 (54.1) | 261 (48.8) | 0.312 |
| Anti coagulation, n (%) | 55 (8.5) | 6 (5.4) | 49 (9.2) | 0.197 |
| RAAS inhibitor, n (%) | 378 (58.5) | 69 (62.2) | 309 (57.8) | 0.391 |
| Diuretics, n (%) | 196 (30.3) | 31 (27.9) | 165 (30.8) | 0.543 |
| B-blockers, n (%) | 296 (45.8) | 55 (49.5) | 241 (45) | 0.386 |
| CCB, n (%) | 197 (30.5) | 35 (31.5) | 162 (30.3) | 0.794 |
| Statins, n (%) | 329 (50.9) | 61 [55] | 268 (50.1) | 0.351 |
| Low/moderate intensity | 238 (72.3) | 39 (63.9) | 199 (74.3) | 0.104 |
| High intensity | 91 (27.7) | 22 (36.1) | 69 (25.7) | |
| Ezetimibe, n (%) | 78 (12.1) | 15 (13.5) | 63 (11.8) | 0.609 |
| Admission glucose-lowering agents | ||||
| Metformin, n (%) | 467 (72.3) | 80 (72.1) | 387 (72.3) | 0.955 |
| Sulfonylureas, n (%) | 166 (25.7) | 13 (11.7) | 153 (28.6) | 0.001 |
| DPP-4 Inhibitors, n (%) | 54 (8.4) | 8 (7.2) | 46 (8.6) | 0.630 |
| GLP-1 Agonist, n (%) | 19 (2.9) | 5 (4.5) | 14 (2.6) | 0.284 |
| In-hospital glucose-lowering strategy | ||||
| Insulin sc., n (%) | 430 (66.6) | 57 (51.4) | 394 (73.6) | <0.001 |
| Insulin iv., n (%) | 65 (10.1) | 17 (15.3) | 144 (26.9) | 0.010 |
| Discharge medical therapy (*) | ||||
| Anti platelets, n (%) | 621 (99.4) | 110 (99.1) | 511 (99.4) | 0.704 |
| DAPT, n (%) | 609 (97.4) | 109 (98.4) | 500 (97.3) | 0.577 |
| Anti coagulation, n (%) | 81 (12.5) | 10 (9) | 71 (13.3) | 0.217 |
| SRAA, n (%) | 416 (66.6) | 89 (80.2) | 409 (79.6) | 0.885 |
| Diuretics, n (%) | 271 (43.4) | 38 (34.2) | 233 (45.3) | 0.032 |
| B-blockers, n (%) | 545 (87.2) | 98 (88.3) | 445 (86.6) | 0.315 |
| CCB, n (%) | 147 (23.5) | 34 (30.6) | 113 (22) | 0.053 |
| Statins, n (%) | 587 (93.9) | 109 (98.2) | 495 (96.3) | 0.315 |
| Ezetimibe, n (%) | 118 (18.9) | 44 (39.6) | 210 (40.9) | 0.812 |
| Discharge glucose-lowering agents (*) | ||||
| Metformin, n (%) | 404 (64.6) | 83 (74.8) | 321 (62.5) | 0.014 |
| Sulfonylureas, n (%) | 137 (21.9) | 9 (8.1) | 128 (24.9) | <0.001 |
| DPP-4 Inhibitors, n (%) | 83 (13.3) | 13 (11.7) | 70 (13.6) | 0.591 |
| GLP-1 Agonist, n (%) | 26 (4.2) | 8 (7.2) | 18 (3.5) | 0.081 |
| Insulin sc., n (%) | 96 (15.4) | 8 (7.2) | 78 (15.2) | 0.027 |
RAAS = Renin-angiotensin-aldosterone system; CCB = Calcium channel blockers; DPP-4 = Dipeptidyl peptidase-4; GLP-1 = Glucagon-like peptide-1; sc. = subcutaneous; iv. = intravenous; DAPT = Dual Antiplatelet Therapy.
Percentages calculated on the number of patients discharged alive.
The two study groups exhibited similar admission characteristics, including Killip Class, the occurrence of angina, AF, and VT/VF presentation (Table 1). The rate of STEMI was similar between the two study groups and the median times from symptoms to diagnostic coronary angiography did not differ between groups for both STEMI and NSTEMI (Table 1). The main angiographic characteristics were also similar between the two study groups (Supplementary Table 1), except for the higher number of stents implanted in the SGLT2-I group (p = 0.041). Vascular access and contrast dosage did not differ between the 2 cohorts. Finally, a similar rate of complete revascularization, staged procedure and complex PCI was observed between the study groups. On admission and after 24 h, non-SGLT2-I users exhibited a higher inflammatory burden compared to the SGLT2-I group. Stress hyperglycemia was significantly lower in SGLT2-I patients compared to the non-SGLT2-I group (p = 0.007), even though HbA1c did not differ between groups (Supplementary Table 2). Discharge medical therapy, as well as in-hospital glucose-lowering strategies, are provided in Table 2. Due to the lower stress admission hyperglycemia, insulin therapy (both s.c. and i.v.) and hypoglycemic episodes were significantly lower in SGLT2-I users (p < 0.01 for all). In the latter cohort, no patient had to discontinue SGLT2-I for hypoglycemic episodes that occurred during hospitalization.
3.3. Impact of SGLT2-I on left ventricular function
Troponin values were significantly lower in SGLT2-I users than in non-SGLT2-I patients (p ≥ 0.003 for all, Table 3). Consistently, ST-segment resolution post-PCI was more frequently observed in the SGLT2-I group (p = 0.001). On admission, left ventricular volume, ejection fraction (LVEF) and regional wall motion abnormalities (RWMA) were similar between the two study groups. In both study cohorts, the LVEF increased significantly after the revascularization, between admission and discharge (p < 0.001 in both cohorts). However, the increase was significantly higher in the SGLT2-I users compared to non-SGLT2-I users (p < 0.001, Table 3 and Fig. 2). In addition, at discharge, RWMA were significantly reduced in the SGLT2-I users (81.1 % versus 62.2 %, p = 0.003), but not in the non-SGLT2-I cohort (83.6 % versus 79.8 %, p = 0.133). As a result, a lower rate of discharge moderate-to-severe mitral regurgitation was detected in SGLT2-I users than in the non-SGLT2-I cohort, compared to hospital admission (Table 3 and Fig. 2).
Table 3.
LV remodeling in SGLT2-I users versus non-SGLT2-users.
| Total (N = 646) |
SGLT2-I users (N = 111) |
Non-SGLT2-I users (N = 535) |
P-value | |
|---|---|---|---|---|
| Hospital Admission | ||||
| Q wave, n (%) | 160 (24.8) | 24 (21.6) | 136 (25.4) | 0.399 |
| LV-EDV, ml | 108 ± 33 | 107 ± 35 | 108 ± 33 | 0.627 |
| LV-EF, % | 47 ± 11 | 48 ± 10 | 47 ± 11 | 0.183 |
| RWMA, n (%) | 537 (83.1) | 90 (81.1) | 447 (83.6) | 0.527 |
| Mitral regurgitation, n (%) | ||||
| Moderate | 52 (8.7) | 8 (7.2) | 44 (9.1) | 0.014 |
| Severe | 11 (1.8) | 0 (0) | 11 (2.3) | |
| I hs-TnI, ng/L | 233 [47–1450] | 158 [35–730] | 245 [53 – 1959] | 0.003 |
| II hs-TnI, ng/L | 1397 [341–9224] | 652 [170–1998] | 1740 [373 – 9223] | <0.001 |
| III hs-TnI, ng/L | 1328 [420–9224] | 485 [155–1308] | 2316 [576 – 9223] | <0.001 |
| hs-TnI peak, ng/L | 2368 [625–9224] | 903 [278–2438] | 3155 [731 – 9223] | <0.001 |
| Hospital Discharge | ||||
| ST resolution, n (%) | 206 (66.7) | 44 (84.6) | 162 (63) | 0.003 |
| LV-EDV, ml | 109 ± 36 | 103 ± 29 | 110 ± 38 | 0.267 |
| LV-EF, % | 49 ± 10 | 53 ± 9 | 48 ± 10 | <0.001 |
| RWMA, n (%) | 496 (76.8) | 69 (62.2) | 427 (79.8) | 0.001 |
| Mitral regurgitation, n (%) | ||||
| Moderate | 40 (6.4) | 3 (2.7) | 37 (7.2) | <0.001 |
| Severe | 12 (1.9) | 0 (0) | 2 (2.3) |
Continuous variables are presented as meanαSD or as median [IQR]; while categorical variables as number (%).
Abbreviations: LV-EDV=Left Ventricular End Diastolic Volume; LVEF=left ventricular ejection fraction; RWMA=regional wall motion abnormalities; Hs-TnI=high sensitivity Troponin I.
Fig. 2.
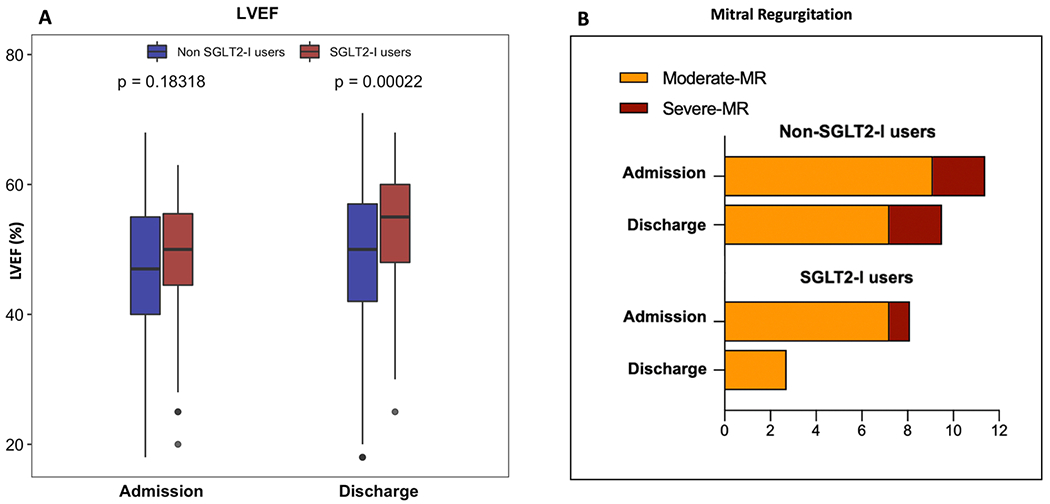
Comparison of the LVEF values (panel A) and mitral regurgitation degree (panel B) in SGLT2-I users versus non-SGLT2-I users at hospital admission versus hospital discharge. Abbreviations: LVEF = left ventricular ejection fraction; MR = mitral regurgitation; SGLT2-I = Sodium-glucose co-transporter 2 inhibitors.
3.4. Impact of SGLT2-I on in-hospital endpoints
Overall, 19 patients died during hospitalization due to cardiovascular causes. The in-hospital mortality was significantly higher in non-SGLT2-I users (3.6 % vs 0 %, p = 0.041). SGLT2-I users patients exhibited a lower arrhythmic burden during hospitalization - ventricular arrhythmias and atrial fibrillation - compared to non-SGLT2-I patients (p = 0.010, Table 4). No significant differences were noticed for mechanical circulatory support with an intra-aortic balloon pump, re-AMI, and days of hospital stay between the 2 study groups (Table 4). Interestingly, SGLT2-I users experienced a lower occurrence of contrast-induced acute kidney injury (p = 0.022).
Table 4.
Outcomes of SGLT2-I users versus non-SGLT2-users.
| Total (N = 646) |
SGLT2-I users (N = 111) |
Non-SGLT2-I users (N = 535) |
P-value | |
|---|---|---|---|---|
| In-hospital outcomes | ||||
| Cardiovascular-death, n (%) | 19 (2.9) | 0 (0) | 19 (3.6) | 0.041 |
| Arrhythmia, n (%) | 91 (14.1) | 7 (6.3) | 84 (15.7) | 0.010 |
| New-onset AF, n (%) | 56 (8.7) | 5 (4.5) | 51 (9.5) | |
| VT/VF, n (%) | 35 (5.4) | 2 (1.8) | 33 (6.2) | |
| Re-AMI, n (%) | 7 (1.1) | 1 (0.9) | 6 (1.1) | 0.838 |
| Re-PCI, n (%) | 13 (2.0) | 4 (3.6) | 9 (1.7) | 0.190 |
| LABP, n (%) | 23 (3.6) | 4 (3.6) | 19 (3.6) | 0.978 |
| CI-AKI, n (%) | 68 (10.5) | 6 (5.4) | 70 (13.1) | 0.022 |
| Hospital stay, days | 5 [4–8] | 5 [4–8] | 5 [4–8] | 0.526 |
| Long-term outcomes (*) | ||||
| All-cause deaths, n (%) | 76 (12.2) | 7 (6.3) | 69 (13.4) | 0.037 |
| Cardiovascular-death, n (%) | 54 (8.6) | 4 (3.6) | 50 (9.7) | 0.036 |
| Re-AMI, n (%) | 39 (6.2) | 6 (5.4) | 33 (6.4) | 0.759 |
| Re-PCI, n (%) | 53 (8.5) | 11 (9.9) | 42 (8.2) | 0.551 |
| HF Hospitalization, n (%) | 104 (16.6) | 7 (6.3) | 97 (18.9) | 0.001 |
| MACE, n (%) | 160 (25.6) | 14 (12.6) | 146 (28.4) | <0.001 |
| ICD, n (%) | 44 (6.8) | 7 (1.1) | 37 (5.7) | 0.817 |
Long term outcomes (*): total numbers of patients discharge alive (N = 625): SGLT2-I users (N = 111) and non-SGLT2-I users (N = 514).
Abbreviations: AF=Atrial Fibrillation; VT=Ventricular Tachycardia; VF=Ventricular Fibrillation; AMI=Acute Myocardial Infarction, PCI=Primaiy Percutaneous Coronary Intervention; IABP=Intra-Aortic Balloon Pump; CI-AKI=Contrast-Induced Acute Kidney Injury; HF=Heart Failure; MACE=major adverse cardiovascular events; ICD=Implantable-Cardioverter-Defibrillator.
3.5. Impact of SGLT2-I on endpoints at the follow-up
The median follow-up duration after discharge was 24 ± 13 months. Over this period, 76 (12.2 %) deaths were recorded, 8.6 % related to cardiovascular causes. Thirty-nine (6.2 %) patients had re-AMI, 53 (8.5 %) any revascularization, 104 patients (16.6 %) were hospitalized for HF, while 160 (25.6 %) experienced the composite endpoint. Kaplan-Meier estimates along with 3 years are shown in Fig. 3. The composite endpoint (MACE) was higher for the non-SGLT2-I patients compared to SGLT2-I users (p < 0.001, Table 4 and Fig. 3), without any gender difference in both cohorts (11.1 % vs 19.4%, p = 0.753 % and 28.4% vs 23.8 % p = 0.368). Among SGLT2-I users, cardiovascular mortality and HF hospitalization occurred less frequently than in no-SGLT2-I patients (p < 0.04 for both, Table 4 and Fig. 3). During the follow-up, the 2 study groups exhibited a similar rate of re-AMI, any coronary revascularization, and implantable-cardioverter-defibrillator (ICD) implantation. In the multivariable Cox regression model, after adjusting for all confounding factors, the use of SGLT2-I was identified as an independent predictor of reduced MACE occurrence (HR=0.57; 95 %CI 0.33–0.99; p = 0.039), together with complete revascularization, lower discharge moderate-to-severe mitral regurgitation, and lower creatine values. Similarly, SGLT2-I therapy appeared to be an independent predictor of reduced HF hospitalization (HR=0.46; 95 %CI 0.21–0.98; p = 0.041), together with complete revascularization (Table 5).
Fig. 3.
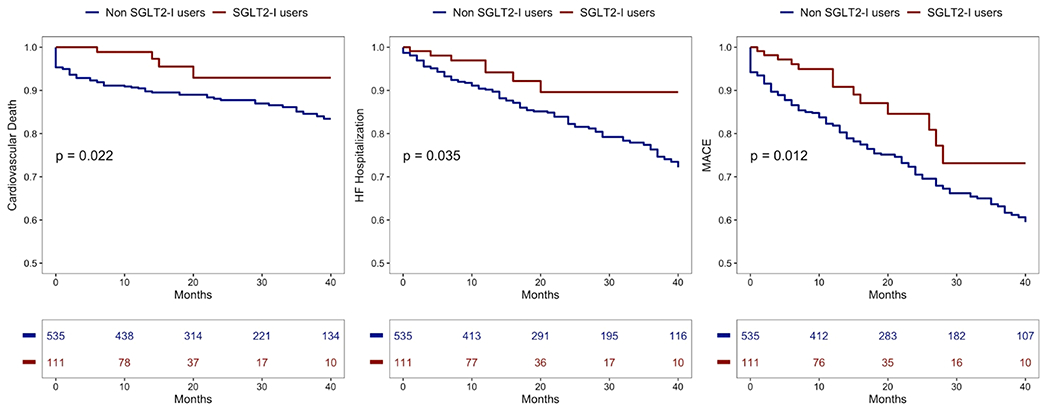
Kaplan-Meier survival curves in SGLT2-I users (red curve) versus non-SGLT2-I users (blue curve). Panel A: cardiovascular mortality. Panel B: heart failure hospitalization. Panel C: MACE. Abbreviations: SGLT2-I = Sodium-glucose co-transporter 2 inhibitors; MACE = major adverse cardiovascular events.
Table 5.
Univariate and multivariable analysis. Predictors of cardiovascular death, HF hospitalization, and MACE.
| Cardiovascular Death |
HF Hospitalization |
MACE |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
Univariate analysis |
Multivariate analysis |
|||||||||||||
| Variables | HR | 95 %CI | p-value | HR | 95 %CI | p-value | HR | 95 %CI | p-value | HR | 95 % CI | p-value | HR | 95 % CI | p-value | HR | 95 % CI | p-value |
| Age, years | 1.03 | 1.01–1.05 | 0.004 | 1.02 | 0.99 – 1.05 | 0.085 | 1.01 | 0.98–1.02 | 0.863 | – | – | – | 1.02 | 0.99–1.03 | 0.053 | – | – | – |
| Gender, male | 1.42 | 0.86–2.34 | 0.167 | – | – | – | 0.80 | 0.49–1.29 | 0.357 | – | – | – | 1.13 | 0.81–1.59 | 0.467 | – | – | – |
| Admission BGL | 1.01 | 1.01–1.02 | 0.009 | 1.01 | 0.99 – 1.01 | 0.776 | 1.01 | 0.99–1.01 | 0.623 | – | – | – | 1.01 | 0.99–1.02 | 0.098 | – | – | – |
| Admission CRP | 1.01 | 0.99–1.01 | 0.292 | – | – | – | 0.99 | 0.97–1.02 | 0.606 | – | – | – | 1.01 | 0.99–1.02 | 0.094 | – | – | – |
| Peak Hs-TnI, ng/L | 1.01 | 1.01–1.03 | <0.001 | 1.01 | 1.01 – 1.03 | 0.018 | 1.01 | 1.01–1.03 | 0.023 | 1.01 | 1.01–1.03 | 0.068 | 1.01 | 1.01–1.02 | 0.026 | 1.01 | 0.99–1.00 | 0.238 |
| NSTEMI | 0.63 | 0.40–1.02 | 0.054 | – | – | – | 0.86 | 0.59–1.26 | 0.441 | – | – | – | 0.90 | 0.67–1.21 | 0.482 | – | – | – |
| Complex PCI | 1.15 | 0.65–2.04 | 0.620 | – | – | – | 1.35 | 0.85–2.15 | 0.200 | – | – | – | 1.34 | 0.94–1.91 | 0.101 | – | – | – |
| Complete Rev. | 0.32 | 0.20–0.51 | <0.001 | 0.51 | 0.30–0.89 | 0.017 | 0.39 | 0.26–0.58 | <0.001 | 0.38 | 0.25–0.57 | <0.001 | 0.30 | 0.22–0.40 | <0.001 | 0.37 | 0.26–0.51 | 0.001 |
| Discharge moderate-to-severe MR | 2.07 | 1.55–2.77 | <0.001 | 1.48 | 1.05–2.09 | 0.025 | 1.28 | 1.06–1.69 | 0.040 | 1.17 | 0.89–1.55 | 0.251 | 1.48 | 1.21–1.82 | <0.001 | 1.29 | 1.04–1.59 | 0.018 |
| Discharge crea. | 1.24 | 1.14–1.35 | <0.001 | 1.33 | 1.17–1.52 | <0.001 | 1.11 | 0.99–1.26 | 0.061 | – | – | – | 1.22 | 1.14–1.30 | <0.001 | 1.13 | 1.04–1.22 | 0.003 |
| SGLT2-I | 0.33 | 0.12–0.90 | 0.031 | 0.53 | 0.19–1.52 | 0.237 | 0.45 | 0.21–0.97 | 0.038 | 0.46 | 0.21 – 0.98 | 0.041 | 0.51 | 0.29–0.87 | 0.014 | 0.57 | 0.33–0.99 | 0.039 |
Abbreviations: BGL=blood glucose level; CRP=C-reactive Protein; Hs-TnI=high sensitivity Troponin I; NSTEMI=non-ST segment Elevation Myocardial Infarction; PCI=Primary Percutaneous Coronary Intervention; Complete Rev.=complete revascularization. MR=mitral regurgitation; HF=Heart Failure; MACE=major adverse cardiovascular events.
4. Discussion
Our study is the first report investigating the in-hospital and longterm outcomes in a cohort of T2DM patients admitted with AMI, comparing chronic SGLT2-I therapy versus non-SGLT2-I users. The main findings include: i) a mitigated negative LV remodeling was detected in patients receiving SGLT2-I compared to non-SGLT2-I ones; ii) the use of SGLT2-I was associated with a lower in-hospital cardiovascular death, arrhythmic burden and occurrence of contrast-induced acute kidney injury; iii) in SGLT2-I users the composite endpoint (MACE), as well as, cardiovascular mortality and HF-hospitalization were significantly lower compared to no-SGLT2-I patients; iv) after adjusting for all confounding factors, the use of SGLT2-I was identified as an independent predictor of reduced MACE occurrence and HF-hospitalization.
In the last years, SGLT2-I gained an intense interest in searching for the mechanisms responsible for their beneficial effects in patients with and without DM [3,16,17]. More recently, SGLT2-I revealed cardioprotective effects in HF patients, independently of their diabetic status [2,5]. Since the expression of SGLT2 in human cardiomyocytes is still doubtful, it is intriguing how SGLT2-I might display beneficial off-target effects on the cardiovascular system [18]. SGLT2-I might reduce ischemia/reperfusion injury and affect cell ionic homeostasis, resulting in mitigation of the infarct size, LV remodeling, and arrhythmic burden. The attenuated myocardial necrosis and arrhythmic burden point out a novel mechanism underlying the significant reduction of cardiovascular mortality found in our study [4,19]. In addition, a reduction of myocardial necrosis might improve both the AMI-related in-hospital and long-term outcomes and reduce the progression to HF. SGLT2-I also directly affect the arrhythmic burden, particularly acting on sodium and calcium homeostasis. Taken together, these cardioprotective properties might favorably impact the in-hospital and long-term outcomes in AMI T2DM patients treated with SGLT2-I.
4.1. Impact of SGLT2-I on left ventricle remodeling
Infarct size and left ventricular remodeling following AMI increase the risk for HF and significantly decrease survival [20,21]. Earlier treatment strategies sought to reverse mechanical changes after AMI, reducing pre, after, and volume load. Current therapeutic strategies mostly improve cardiovascular mortality but occasionally fail to prevent the progression toward HF [22,23]. This aspect suggests that current therapeutic approaches miss further key pathophysiological mechanisms like inflammation, cardiac energy metabolism, and myocardial fibrosis, which also contribute to the extent of infarct size and adverse LV remodeling. Interestingly, many of the proposed actions of SGLT2-I coincide with known mechanisms recognized to mitigate infarct size extension and LV remodeling after AMI [3,24]. Clinical and in vitro data demonstrated that SGLT2-I exhibit favorable properties against inflammation, ischemia/reperfusion injury, and generation of reactive oxygen species, thereby improving cardiac energy metabolism and metabolic flexibility, myocardial hypertrophy and fibrosis, myocardial regeneration and proliferation, as well as neurohormonal activation and cardio-renal interplay [3,25,26]. The SGLT2-I-related lower inflammatory burden might be pivotal in explaining infarct size attenuation [10, 27]. Inflammation is an essential contributor of infarct size severity, and pro-inflammatory biomarkers correlate with the prognosis of AMI [28–30]. In our recent study, inflammatory indices on admission and after 24 h were significantly higher in non-SGLT2-I users, with a significant increase in neutrophil levels at 24 h observed in non-SGLT2-I patients but not in the SGLT2-I group [10]. The in vitro evidence that SGLT2-I might inhibit the nucleotide-binding domain-like receptor protein-3 (NLRP3) inflammasome, thus reducing the secretion of inflammatory markers, further strengthens our hypothesis [31]. Alternative explanations for the smaller infarct size in diabetic patients receiving SGLT2-I include improving cardiomyocyte energy metabolism and metabolic flexibility with a shift towards ketone bodies as the metabolic substrate for the cardiomyocytes, with a larger cardiac ATP production [3,32,33]. Finally, stress admission hyperglycemia was more frequently observed in non-SGLT2-I users than in those receiving SGLT2-I, confirming the effect of ameliorating glycemic parameters when used alone or in combination in T2DM patients [34].
In pre-clinical studies, SGLT2-I provided evidence for a reduction in acute myocardial I/R injury, infarct size, and arrhythmias, decreasing myofibroblast infiltration and myocardial fibrosis, both key pathophysiological mechanisms related to LV remodeling, with a parallel increase in the left ventricular function, independent of diabetic status [6,7,35–39]. On the clinical ground, in line with these studies, our results showed significantly lower troponin values, with a concomitant higher rate of post-PCI ST-resolution, a higher increase of LVEF with a lower rate of RWMA after the revascularization in patients treated with SGLT2-I. As a result, a lower rate of discharge moderate-to-severe mitral regurgitation was detected in SGLT2-I users than in the non-SGLT2-I cohort, compared to hospital admission. The latter finding becomes even more important considering that ischemic MR, as a consequence of LV remodeling, has been recognized as an important predictor of an adverse prognosis after AMI and is known to worsen patients’ prognoses even if its degree is moderate [40]. Interestingly, lower troponin peak levels were documented as an independent predictor of improvement in ischemic MR after primary PCI in the chronic phase, further emphasizing the lower troponin values found in SGLT2-I users in our study [41]. Although troponin values, LVEF, and RWMAs do not represent the current gold standard for assessing infarct size, our results provide new insights into the possible cardioprotective properties of chronic SGLT2-I therapy in type 2 diabetic patients hospitalized for AMI, exhibiting a significantly mitigated LV adverse remodeling with reduced moderate-to-severe MR, compared to non-SGLT2-I users.
Remarkably, most of these effects discussed previously could be related to persistent molecular and metabolic changes since all patients had been treated with SGLT2-I for at least 3 months before the AMI. Indeed, the recently published EMMY trial did not find any difference in acute troponin values between the SGLT2-I treated and untreated cohorts [9]. However, the EMMY trial included only a minority of diabetic patients, and all patients were randomized to the treatment at the time of the AMI admission, for only 3 days, rather than receiving SGLT2-I some months earlier as in our study.
4.2. Impact of SGLT2-I on the arrhythmic burden
Our study demonstrated that in diabetic AMI patients, SGLT2-I significantly reduced the AF and ventricular arrhythmias episodes that occur in the acute phase of AMI. The anti-arrhythmic effects of SGLT2-I remain to be better explored. It might be partly related to the reduction in inflammatory burden, admission stress hyperglycemia, and LV infarct size. Previous reports hypothesized that SGLT2-I might induce changes in calcium ion currents, reducing calcium-related arrhythmogenesis. [42–44]. Another beneficial effect of SGLT2-I is the protection against hyperglycemia-induced sympathetic overstimulation slowing the action potential duration [45]. Accordingly, our patients treated with SGLT2-I exhibited a lower heart rate and admission blood glucose level than patients treated with other OAD agents. Moreover, the lower number of hypoglycemic episodes associated with reduced insulin therapy (both s. c. and i.v.), resulting from minor stress admission hyperglycemia, further corroborates the reduced in-hospital occurrence of arrhythmias in SGLT2-I users [46].
4.3. Study limitations
Our results should be interpreted considering some limitations. First, the sample size was powered to evaluate only a “class effect” but not the “doses effect.” However, a recent analysis of a nationwide real-world dataset suggested that the risk of cardiovascular events including HF, MI, stroke, and AF would be comparable between individual SGLT2 inhibitors, supporting our hypothesis of “class effects”[47]. Second, the observational study design represents a methodological limitation concerning the applicability of the study results that should be considered as hypothesis-generating. Third, our results could not be extended to patients revascularized with CABG strategy, on insulin therapy, with GFR < 30 ml/min and severe VHD.
5. Conclusions
In T2DM patients with AMI, the use of SGLT2 inhibitors was associated with a lower risk of adverse cardiovascular outcomes during index hospitalization and long-term follow-up. Our findings are hypothesis-generating and provide new insights into the cardioprotective role of SGLT2-I in the setting of CAD pointing out the potential clinical impact of these drugs in improving cardiovascular outcomes after AMI.
Supplementary Material
Funding
Dr. Paolisso and Dr. Esposito report receiving a research grant from the CardioPaTh PhD Program.
Abbreviations:
- AMI
acute myocardial infarction
- SGLT2-I
Sodium-glucose cotransporter 2 inhibitors
- OAD
oral antidiabetic
- T2DM
type 2 diabetes mellitus
- HF
heart failure
- STEMI
ST-segment elevation myocardial infarction
- NSTEMI
non-ST-segment elevation myocardial infarction
- PCI
percutaneous coronary intervention
- CABG
coronary artery bypass graft surgery
- RWMA
regional wall motion abnormalities
Footnotes
Ethics approval and consent to participate
Data were collected as part of an approved international multicenter observational study. The present study was conducted according to the principles of the Declaration of Helsinki; all patients were informed about their participation in the registry and provided informed consent for the anonymous publication of scientific data.
Statement of guarantor
C.P. and E.B. are the guarantors of the research and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Permissions information
The authors do hereby declare that all illustrations and figures in the manuscript are entirely original and do not require reprint permission.
Competing interests
The authors declare that they have no competing interests.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2022.106597.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- [1].Udell JA, Jones WS, Petrie MC, et al. , Sodium glucose cotransporter-2 inhibition for acute myocardial infarction: JACC review topic of the week, J. Am. Coll. Cardiol 79 (2022) 2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anker SD, Butler J, Filippatos G, et al. , Empagliflozin in heart failure with a preserved ejection fraction, N. Engl. J. Med 385 (2021) 1451–1461. [DOI] [PubMed] [Google Scholar]
- [3].Varzideh F, Kansakar U, Santulli G, SGLT2 inhibitors in cardiovascular medicine, Eur. Heart J. Cardiovasc. Pharmacother 7 (2021) e67–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zinman B, Wanner C, Lachin JM, et al. , Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes, N. Engl. J. Med 373 (2015) 2117–2128. [DOI] [PubMed] [Google Scholar]
- [5].McMurray JJV, Solomon SD, Inzucchi SE, et al. , Dapagliflozin in patients with heart failure and reduced ejection fraction, N. Engl. J. Med 381 (2019) 1995–2008. [DOI] [PubMed] [Google Scholar]
- [6].Andreadou I, Bell RM, Bøtker HE, Zuurbier CJ, SGLT2 inhibitors reduce infarct size in reperfused ischemic heart and improve cardiac function during ischemic episodes in preclinical models, Biochim Biophys. Acta Mol. Basis Dis 1866 (2020), 165770. [DOI] [PubMed] [Google Scholar]
- [7].Lahnwong S, Palee S, Apaijai N, et al. , Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury, Cardiovasc Diabetol 19 (2020) 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harrington J, Udell JA, Jones WS, et al. , Empagliflozin in patients post myocardial infarction rationale and design of the EMPACT-MI trial, Am. Heart J 253 (2022) 86–98. [DOI] [PubMed] [Google Scholar]
- [9].von Lewinski D, Kolesnik E, Tripolt NJ, et al. , Empagliflozin in acute Myocardial Infarction: the EMMY trial, Eur. Heart J (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Paolisso P, Bergamaschi L, Santulli G, et al. , Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry, Cardiovasc Diabetol. 21 (2022) 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cesaro A, Gragnano F, Paolisso P, et al. , In-hospital arrhythmic burden reduction in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: insights from the SGLT2-I AMI PROTECT study, Front. Cardiovasc. Med 9 (2022) 1012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marfella R, D’Onofrio N, Trotta MC, et al. , Sodium/glucose cotransporter 2 (SGLT2) inhibitors improve cardiac function by reducing JunD expression in human diabetic hearts, Metabolism 127 (2022), 154936. [DOI] [PubMed] [Google Scholar]
- [13].Collet JP, Thiele H, Barbato E, et al. , ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation, Eur. Heart J 2021 (42) (2020) 1289–1367. [DOI] [PubMed] [Google Scholar]
- [14].Ibanez B, James S, Agewall S, et al. , ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC), Eur. Heart J 2018 (39) (2017) 119–177. [DOI] [PubMed] [Google Scholar]
- [15].Hicks KA, Mahaffey KW, Mehran R, et al. , Cardiovascular and stroke endpoint definitions for clinical trials, Circulation 2018 (137) (2017) 961–972. [DOI] [PubMed] [Google Scholar]
- [16].Mascolo A, Scavone C, Scisciola L, Chiodini P, Capuano A, Paolisso G, SGLT-2 inhibitors reduce the risk of cerebrovascular/cardiovascular outcomes and mortality: a systematic review and meta-analysis of retrospective cohort studies, Pharmacol. Res 172 (2021), 105836. [DOI] [PubMed] [Google Scholar]
- [17].Ciardullo S, Trevisan R, Perseghin G, Sodium-glucose transporter 2 inhibitors for renal and cardiovascular protection in US adults with type 2 diabetes: impact of the 2020 KDIGO clinical practice guidelines, Pharmacol. Res 166 (2021), 105530. [DOI] [PubMed] [Google Scholar]
- [18].Marfella R, Scisciola L, D’Onofrio N, et al. , Sodium-glucose cotransporter-2 (SGLT2) expression in diabetic and non-diabetic failing human cardiomyocytes, Pharmacol. Res 184 (2022), 106448. [DOI] [PubMed] [Google Scholar]
- [19].Neal B, Perkovic V, Mahaffey KW, et al. , Canagliflozin and cardiovascular and renal events in type 2 diabetes, N. Engl. J. Med 377 (2017) 644–657. [DOI] [PubMed] [Google Scholar]
- [20].Torabi A, Cleland JG, Khan NK, et al. , The timing of development and subsequent clinical course of heart failure after a myocardial infarction, Eur. Heart J 29 (2008) 859–870. [DOI] [PubMed] [Google Scholar]
- [21].Stone GW, Selker HP, Thiele H, et al. , Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials, J. Am. Coll. Cardiol 67 (2016) 1674–1683. [DOI] [PubMed] [Google Scholar]
- [22].Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ, Myocardial reperfusion injury: looking beyond primary PCI, Eur. Heart J 34 (2013) 1714–1722. [DOI] [PubMed] [Google Scholar]
- [23].Jelani A, Jugdutt BI, STEMI and heart failure in the elderly: role of adverse remodeling, Heart Fail. Rev 15 (2010) 513–521. [DOI] [PubMed] [Google Scholar]
- [24].Theofilis P, Antonopoulos AS, Katsimichas T, et al. , The impact of SGLT2 inhibition on imaging markers of cardiac function: a systematic review and metaanalysis, Pharmacol. Res 180 (2022), 106243. [DOI] [PubMed] [Google Scholar]
- [25].Frantz S, Hundertmark MJ, Schulz-Menger J, Bengel FM, Bauersachs J, Left ventricular remodelling post-myocardial infarction: pathophysiology, imaging, and novel therapies, Eur. Heart J (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mone P, Varzideh F, Jankauskas SS et al. , SGLT2 inhibition via empagliflozin Improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients, Hypertension 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koyani CN, Plastira I, Sourij H, et al. , Empagliflozin protects heart from inflammation and energy depletion via AMPK activation, Pharmacol. Res 158 (2020), 104870. [DOI] [PubMed] [Google Scholar]
- [28].Paolisso P, Foà A, Bergamaschi L, et al. , Impact of admission hyperglycemia on short and long-term prognosis in acute myocardial infarction: MINOCA versus MIOCA, Cardiovasc Diabetol. 20 (2021) 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paolisso P, Foà A, Bergamaschi L, et al. , Hyperglycemia, inflammatory response and infarct size in obstructive acute myocardial infarction and MINOCA, Cardiovasc. Diabetol 20 (2021) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhong Y, Yu X, Li X, Zhou H, Wang Y, Augmented early aged neutrophil infiltration contributes to late remodeling post myocardial infarction, Microvasc. Res 139 (2022), 104268. [DOI] [PubMed] [Google Scholar]
- [31].Kim SR, Lee SG, Kim SH, et al. , SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease, Nat. Commun 11 (2020) 2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ferrannini E, Baldi S, Frascerra S, et al. , Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2, Diabetes Diabetes 65 (2016) 1190–1195. [DOI] [PubMed] [Google Scholar]
- [33].Zelniker TA, Braunwald E, Mechanisms of cardiorenal effects of sodium-glucose cotransporter 2 inhibitors: JACC state-of-the-art review, J. Am. Coll. Cardiol 75 (2020) 422–434. [DOI] [PubMed] [Google Scholar]
- [34].Buse JB, Wexler DJ, Tsapas A, et al. , Update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD), Diabetologia 2020 (63) (2019) 221–228. [DOI] [PubMed] [Google Scholar]
- [35].Tanajak P, Sa-Nguanmoo P, Sivasinprasasn S, et al. , Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury, J. Endocrinol 236 (2018) 69–84. [DOI] [PubMed] [Google Scholar]
- [36].Sayour AA, Celeng C, Oláh A, Ruppert M, Merkely B, Radovits T, Sodium-glucose cotransporter 2 inhibitors reduce myocardial infarct size in preclinical animal models of myocardial ischaemia-reperfusion injury: a meta-analysis, Diabetologia 64 (2021) 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee TM, Chang NC, Lin SZ, Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts, Free Radic. Biol. Med 104 (2017) 298–310. [DOI] [PubMed] [Google Scholar]
- [38].Lim VG, Bell RM, Arjun S, Kolatsi-Joannou M, Long DA, Yellon DM, SGLT2 inhibitor, canagliflozin, attenuates myocardial infarction in the diabetic and nondiabetic heart, JACC Basic Transl. Sci 4 (2019) 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. , Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics, J. Am. Coll. Cardiol 73 (2019) 1931–1944. [DOI] [PubMed] [Google Scholar]
- [40].Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ, Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment, Circulation 103 (2001) 1759–1764. [DOI] [PubMed] [Google Scholar]
- [41].Nishino S, Watanabe N, Kimura T, et al. , The course of ischemic mitral regurgitation in acute myocardial infarction after primary percutaneous coronary intervention: from emergency room to long-term follow-up, Circ. Cardiovasc Imaging 9 (2016), e004841. [DOI] [PubMed] [Google Scholar]
- [42].Manolis AA, Manolis TA, Melita H, Manolis AS, Sodium-glucose cotransporter type 2 inhibitors and cardiac arrhythmias, Trends Cardiovasc. Med (2022). [DOI] [PubMed] [Google Scholar]
- [43].Attachaipanich T, Chattipakorn SC, Chattipakorn N, Potential roles of sodium-glucose co-transporter 2 inhibitors in attenuating cardiac arrhythmias in diabetes and heart failure, J. Cell Physiol 237 (2022) 2404–2419. [DOI] [PubMed] [Google Scholar]
- [44].Philippaert K, Kalyaanamoorthy S, Fatehi M, et al. , Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin, Circulation 143 (2021) 2188–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shimizu W, Kubota Y, Hoshika Y, et al. , Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial, Cardiovasc. Diabetol 19 (2020) 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Andersen A, Jørgensen PG, Knop FK, Vilsbøll T, Hypoglycaemia and cardiac arrhythmias in diabetes, Ther. Adv. Endocrinol. Metab 11 (2020), 2042018820911803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Suzuki Y, Kaneko H, Okada A, et al. , Comparison of cardiovascular outcomes between SGLT2 inhibitors in diabetes mellitus, Cardiovasc. Diabetol 21 (2022) 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


