Abstract
Objective
The present study aimed to evaluate the relationship between all-cause mortality and the neutrophil percentage-to-albumin ratio (NPAR) in patients with atrial fibrillation (AF).
Methods
We obtained clinical information from patients with AF from the Medical Information Mart for Intensive Care-IV version 2.0 (MIMIC-IV) database and the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (WMU). The clinical endpoints were all-cause death measured at 30-day, 90-day, and one-year intervals. For endpoints associated with the NPAR, logistic regression models were used to calculate odds ratios (OR) with 95% confidence intervals (CI). Receiver operating characteristic (ROC) curves and area under the curve (AUC) were developed to compare the ability of different inflammatory biomarkers to predict 90-day mortality in patients with AF.
Results
Higher NPAR was associated with a higher risk of 30-day (OR 2.08, 95% CI 1.58–2.75), 90-day (OR 2.07, 95% CI 1.61–2.67), and one-year mortality (OR 1.60, 95% CI 1.26–2.04) in patients with AF in 2813 patients from MIMIC-IV. The predictive performance of NPAR (AUC = 0.609) for 90-day mortality was better than that of neutrophil-to-lymphocyte ratio (NLR) (AUC = 0.565, P < 0.001), and platelet-to-lymphocyte ratio (PLR) (AUC = 0.528, P < 0.001). When NPAR and sequential organ failure assessment (SOFA) were combined, the AUC increased from 0.609 to 0.674 (P < 0.001). Higher NPAR was associated with a higher risk of 30-day mortality (OR 2.54, 95% CI 1.02–6.30) and 90-day mortality (OR 2.76, 95% CI 1.09–7.01) in 283 patients from WMU.
Conclusion
An increased 30-day, 90-day, and one-year mortality risk among patients with AF were linked to a higher NPAR in MIMIC-IV. NPAR was thought to be a good predictor of 90-day all-cause mortality. Higher NPAR was associated with a higher risk of 30-day and 90-day mortality in WMU.
Keywords: atrial fibrillation, neutrophil percentage-to-albumin ratio, mortality, inflammatory biomarker
Introduction
Clinicians frequently encounter atrial fibrillation (AF) as the most common and widespread cardiac arrhythmia. AF is associated with high morbidity and mortality.1 The global burden of AF has increased so rapidly in the last 20 years that disability-adjusted life years (DALYs) for AF have almost linearly increased, with its incidence and prevalence rising by 63% and 66% after 30 years.2 Patients with AF experience longer periods of mechanical ventilation support, prolonged Intensive Care Unit (ICU) stays, and higher mortality than patients without AF.3 Studies have indicated a causal association between sterile low-grade inflammation and the outcome of AF patients.4–6
Multiple studies have recently demonstrated that the neutrophil percentage-to-albumin ratio (NPAR) has a qualified predictive power for their prognosis in patients with cardiovascular events including acute myocardial infarction, coronary artery disease, heart failure, and cardiogenic shock.7–10 The NPAR is a novel, integrated inflammatory biomarker based on neutrophils and albumin. NPAR is currently thought to reflect inflammation levels accurately. The rising neutrophil is considered to have sinister prognostic implications and a factor in unfavorable outcomes because it plays a crucial role in the inflammatory response to injury.11,12 As a negative acute phase reactant, albumin has an inverse relationship with oxidative stress and inflammation. A higher incidence of AF was associated with decreased albumin levels.13
Although NPAR is a reliable indicator of mortality in many diseases based on systemic inflammation, there is insufficient evidence that NPAR has predictive value for prognosis in AF patients. The present study aimed to explore the association between NPAR and mortality risk in patients with AF.
Materials and Methods
Data Source
We first extracted the data from the Medical Information Mart for Intensive Care IV (MIMIC-IV, Version 2.0), an extensive, freely accessible database containing deidentified health-related information linked to a large number of patients residing in the ICU at the Beth Israel Deaconess Medical Center (Boston, MA) between 2008 and 2019.14 The database includes demographic information, vital signs taken at the bedside, laboratory metrics, interventions, drugs, notes from caregivers, imaging, and mortality. The Institutional Review Boards (IRB) at Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology approved the project.
We also collected data from AF patients admitted to the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (WMU) between June 1st, 2015 and December 31st, 2020. Personal information, disease course during hospitalization, laboratory reports, imaging examination results, treatments, and diagnoses were all stored in a non-public Electronic Medical Records System. The Medical Ethics Committee of WMU approved the current project (Approval number: 2021-K-71-01). The experiment did not affect clinical care, and all protected health information was deidentified. Therefore, informed consent was not required.
Study Subjects
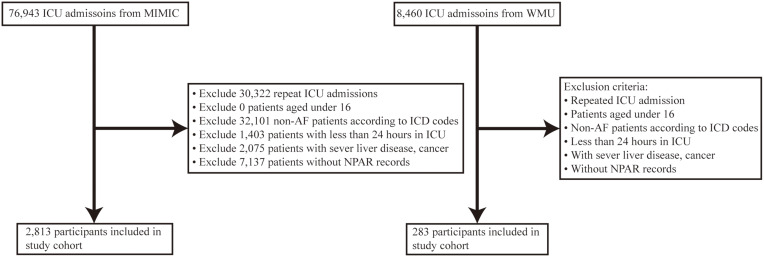
Participants diagnosed with AF and admitted to ICU were selected for the present study. The diagnosis of AF was based on the International Classification of Diseases (ICD) codes (Table S1 in Supplementary Appendix). If a patient met one of the following criteria, they were excluded: (1) age under 16; (2) admitted and discharged from the ICU within 24 hours; (3) concurrently diagnosed with cancer or severe liver disease on admission; and (4) the first record of neutrophil percentage and serum albumin was missing. We only include the initial admission if a patient had more than one (Figure 1). Esophageal varices, gastric varices, hepatic encephalopathy, portal hypertension, hepatorenal syndrome, and hepatic failure were the most severe liver diseases (Table S2 in Supplementary Appendix).
Figure 1.
Flow chart of the study population.
Abbreviations: MIMIC, Medical Information Mart for Intensive Care IV; WMU, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University; ICU, intensive care unit; AF, atrial fibrillation; ICD, international classification of diseases; NPAR, neutrophil percentage-to-albumin ratio.
Demographical and Laboratory Variables
The MIMIC-IV database was enquired for patient information using a structured query language. WMU data were derived from an electronic medical record. Population statistics, vital signs, comorbidities, laboratory tests, scoring system, pharmacological treatments and survival data were all extracted. Population statistics included age and gender. The vital signs of the patients included their heart rate, systolic and diastolic blood pressure, respiration rate, and temperature. Coronary artery disease, valvular disease, heart failure, hypertension, diabetes mellitus, and stroke were among the comorbidities. Albumin, creatinine, white blood cell, hemoglobin, neutrophil, platelet, and lymphocyte levels were all measured in the laboratory. Pharmacological treatments included anticoagulants, rate control drugs, and amiodarone. The sequential organ failure assessment score (SOFA) was used as the scoring tool. The variables mentioned above were extracted on the first day of admission. The initial measured value was used when there were multiple records for an indicator. Death after discharge was included in the Survival data (30-day, 90-day, and one-year mortality).
The NPAR was calculated by dividing the neutrophil percentage numerator serum albumin concentration. The neutrophil-to-lymphocyte ratio (NLR) was calculated using the absolute counts of neutrophils and lymphocytes. The platelet counts-to-lymphocyte counts ratio (PLR) was determined by dividing the total number of platelets by the total number of lymphocytes. The primary endpoint was 90-day mortality. In contrast, secondary outcomes were 30-day and one-year mortality.
Statistical Analysis
The study participants were divided into tertiles based on their NPAR value, ranging from small to large. Continuous variables were expressed as mean [standard deviation (SD)] or median [interquartile range (IQR)]. In contrast, categorical variables were expressed as count and proportion. Logistic regression models were developed to investigate the relationship between NPAR and each outcome. NPAR was considered both a continuous and a categorical variable. The first tertile group of the NPAR was selected as the reference group, and the results were presented as an odds ratio (OR) with 95% confidence intervals (CIs). Each covariate was added to the model one by one. If factors influenced NPAR estimates of all-cause mortality by more than 10% or were significantly related to all-cause mortality, they were included as potential confounders in the final models. Age, gender, heart failure, hypertension, stroke, temperature, respiration rate, systolic and diastolic blood pressure, white blood cell, lymphocyte, hemoglobin, creatinine, SOFA score, anticoagulant, rate control drugs and amiodarone were all predetermined and assessed based on available connections or plausible biological relationships. Subgroup analyses were performed to confirm the independent effect of NPAR on 90-day mortality and their interactions. Receiver Operating Characteristic (ROC) curve was used to compare the predictive value of different inflammatory biomarkers for 90-day mortality. The area under the curve (AUC) with corresponding 95% CIs for the anticipated survival was calculated to estimate the predictive performance. To determine the best risk prediction indicator, compare the AUCs of different models (NPAR, NLR, PLR, SOFA, SOFA plus NPAR).
All statistical analyses were performed using R software version 3.4.3. P < 0.05 was considered statistically significant.
Results
Subject Characteristics
After screenings, the presented study included 2813 MIMIC-IV patients with AF and 283 WMU patients. The baseline characteristics were classified according to NPAR tertiles (Table 1). In MIMIC-IV, there were 937 patients in tertile I (<22.8), 938 patients in tertile II (22.8–28.4), and 938 patients in tertile III (≥28.4). There were 1217 females and 1596 males among these patients. Patients in the high tertile had lower systolic and diastolic blood pressure, lymphocyte, hemoglobin, and albumin levels and a higher heart rate, respiration rate, NLR, PLR, white blood cell, neutrophil, platelet, and SOFA score. There were 102 patients in tertile I, 97 in tertile II, and 84 in tertile III at WMU. Fewer patients in the higher NPAR group had coronary artery disease, valvular disease, hypertension, lymphocyte, hemoglobin, and albumin. Patients had higher age, heart rate, NLR, PLR, white blood cell, neutrophil, and SOFA core. Both cohorts indicated that patients with higher tertile had higher 30-day, 90-day, and one-year mortality rates.
Table 1.
Baseline Characteristics of Participants
| Characteristics | MIMIC Cohort | WMU Cohort | ||||
|---|---|---|---|---|---|---|
| NPAR | NPAR | |||||
| <22.8 | 22.8–28.4 | ≥28.4 | <22.8 | 22.8–28.4 | ≥28.4 | |
| Number | 937 | 938 | 938 | 102 | 97 | 84 |
| Age, mean (SD), years | 74.4 (12.4) | 75.4 (11.9) | 74.1 (12.6) | 74.9 (9.4) | 78.0 (10.7) | 79.8 (9.0) |
| Gender, n (%) | ||||||
| Female | 387 (41.3) | 405 (43.2) | 425 (45.3) | 45 (44.1) | 33 (34.0) | 37 (44.0) |
| Male | 550 (58.7) | 533 (56.8) | 513 (54.7) | 57 (55.9) | 64 (66.0) | 47 (56.0) |
| Comorbidities, n (%) | ||||||
| Coronary artery disease | 372 (39.7) | 372 (39.7) | 344 (36.7) | 24 (23.5) | 22 (22.7) | 16 (19.0) |
| Valvular disease | 132 (14.1) | 129 (13.8) | 133 (14.2) | 48 (47.1) | 40 (41.2) | 31 (36.9) |
| Heart failure | 538 (57.4) | 605 (64.5) | 533 (56.8) | 40 (39.2) | 32 (33.0) | 36 (42.9) |
| Hypertension | 357 (38.1) | 351 (37.4) | 380 (40.5) | 78 (76.5) | 63 (64.9) | 58 (69.0) |
| Diabetes mellitus | 347 (37.0) | 394 (42.0) | 371 (39.6) | 30 (29.4) | 31 (32.0) | 24 (28.6) |
| Stroke | 64 (6.8) | 57 (6.1) | 57 (6.1) | 44 (43.1) | 55 (56.7) | 44 (52.4) |
| Vital signs | ||||||
| Temperature, °C | 36.8 (0.6) | 36.8 (0.6) | 36.8 (0.6) | 36.8 (0.8) | 37.0 (1.0) | 37.0 (1.1) |
| Heart rate, beats/min | 85.4 (17.4) | 88.6 (18.4) | 91.0 (17.8) | 97.7 (29.7) | 105.4 (28.4) | 105.7 (27.5) |
| Respiration rate, beats/min | 20.1 (3.6) | 20.7 (3.9) | 20.9 (4.2) | 20.7 (4.5) | 22.5 (7.0) | 21.8 (5.7) |
| Systolic blood pressure, mmHg | 116.4 (16.2) | 113.9 (15.8) | 110.7 (13.5) | 140.8 (24.1) | 137.6 (28.7) | 132.0 (32.4) |
| Diastolic blood pressure, mmHg | 62.8 (11.4) | 61.4 (10.4) | 59.1 (9.5) | 81.8 (15.9) | 77.1 (14.4) | 72.9 (18.1) |
| Compound biomarkers | ||||||
| NPAR | 19.3 (3.0) | 25.4 (1.6) | 34.4 (6.5) | 19.3 (2.8) | 25.3 (1.6) | 33.2 (5.6) |
| NLR | 5.4 (3.2–9.3) | 11.4 (7.1–19.1) | 15.5 (9.5–28.7) | 4.9 (3.0–8.1) | 11.1 (7.5–18.7) | 18.8 (11.8–33.1) |
| PLR | 157.1 (101.4–236.1) | 235.5 (149.2–371.3) | 279.4 (169.7–467.8) | 137.2 (114.0–201.5) | 228.9 (161.5–337.5) | 289.7 (181.6–650.7) |
| Laboratory parameters | ||||||
| White blood cell, 109/L | 11.3 (6.4) | 13.6 (6.8) | 14.4 16.0 (8.4) | 9.0 (4.6) | 11.1 (4.7) | 13.6 (7.0) |
| Neutrophil, % | 71.9 (13.7) | 83.1 (7.7) | 86.1 (6.7) | 74.8 (11.5) | 85.8 (5.8) | 89.9 (5.4) |
| Lymphocyte, % | 15.9 (10.2) | 8.5 (5.4) | 6.5 (4.4) | 17.3 (10.0) | 8.0 (4.4) | 5.4 (4.2) |
| Hemoglobin, g/dl | 11.5 (2.4) | 10.9 (2.3) | 10.1 (2.1) | 13.2 (2.0) | 11.8 (2.4) | 10.9 (2.5) |
| Platelet, 109/L | 218.8 (105.7) | 223.8 (116.8) | 238.4 (137.8) | 188.6 (76.3) | 169.3 (63.2) | 176.2 (88.2) |
| Albumin, g/dl | 3.7 (0.5) | 3.3 (0.3) | 2.6 (0.4) | 3.9 (0.5) | 3.4 (0.3) | 2.8 (0.3) |
| Creatinine, mg/dl | 1.3 (0.9–2.1) | 1.5 (1.0–2.4) | 1.4 (0.9–2.5) | 0.9 (0.7–1.2) | 0.97 (0.7–1.5) | 1.1 (0.8–1.7) |
| Scoring system | ||||||
| SOFA score | 6.0 (4.0–9.0) | 7.0 (4.0–10.0) | 8.0 (5.0–11.0) | 5.0 (2.0–8.0) | 7.5 (5.0–12.0) | 10.0 (6.8–12.2) |
| Pharmacological treatments | ||||||
| Anticoagulanta | 568 (60.6) | 573 (61.1) | 587 (62.6) | 44 (43.1) | 37 (38.1) | 31 (36.9) |
| Rate control drugsb | 387 (41.3) | 346 (36.9) | 389 (41.5) | 46 (45.1) | 28 (28.9) | 29 (34.5) |
| Amiodarone | 178 (19.0) | 171 (18.2) | 205 (21.9) | 25 (24.5) | 28 (28.9) | 28 (33.3) |
| Outcomes | ||||||
| 30-day mortality, n (%) | 182 (19.4) | 240 (25.6) | 339 (36.1) | 40 (39.2) | 58 (59.8) | 63 (75.0) |
| 90-day mortality, n (%) | 255 (27.2) | 335 (35.7) | 440 (46.9) | 41 (40.2) | 61 (62.9) | 66 (78.6) |
| One-year mortality, n (%) | 392 (41.8) | 449 (47.9) | 530 (56.5) | 47 (48.0) | 64 (69.6) | 69 (82.1) |
Notes: Data were presented as n (%), mean (SD) and median (IQR). aAnticoagulant contains enoxaparin, heparin and warfarin. bRate control drugs contains metoprolol, esmolol, diltiazem and digoxin.
Abbreviations: SD, standard deviation; IQR, interquartile range; MIMIC, Medical Information Mart for Intensive Care; WMU, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University; NPAR, neutrophil percentage-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SOFA, sequential organ failure assessment.
Associations Between NPAR and Mortality
Multiple analyses were performed to investigate the association between NPAR and all-cause mortality in AF patients (Table 2). When considered as a continuous variable, higher NPAR was significantly correlated with 30-day, 90-day, and one-year mortality in all MIMIV-IV models. We used tertile I as the reference group when dividing into tertiles. Higher NPAR was associated with 30-day mortality (OR 2.43, 95% CI 1.96–3.00), 90-day mortality (OR 2.49, 95% CI 2.04–3.04), and one-year mortality (OR 1.90, 95% CI 1.57–2.29) in Model I after adjusting for gender and age. In model II, higher NPAR was linked to higher 30-day (OR, 95% CI: 2.08, 1.58–2.75), 90-day (OR, 95% CI: 2.07, 1.61–2.67), and one-year all-cause mortality (OR, 95% CI: 1.60, 1.26–2.04) after adjusting for age, gender, heart failure, hypertension, stroke, temperature, respiration rate, systolic and diastolic blood pressure, white blood cell, lymphocyte, hemoglobin, creatinine, SOFA score, anticoagulant, rate control drugs and amiodarone. After total adjustment in Model II, higher NPAR was significantly associated with 30-day and 90-day mortality in WMU (OR, 95% CI: 2.54, 1.02–6.30; 2.76, 1.09–7.01) (Table 3).
Table 2.
Association Between NPAR and All-Cause Mortality in MIMIC Cohort
| Non-Adjusted OR (95% CIs) | P value | Model I OR (95% CIs) | P value | Model II OR (95% CIs) | P value | |
|---|---|---|---|---|---|---|
| 30-day mortality | ||||||
| NPAR | 1.05 (1.04, 1.06) | <0.001 | 1.05 (1.04, 1.07) | <0.001 | 1.05 (1.03, 1.06) | <0.001 |
| NPAR tertiles | ||||||
| <22.8 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | |||
| 22.8–28.4 | 1.43 (1.15, 1.77) | <0.001 | 1.40 (1.12, 1.75) | 0.003 | 1.29 (1.00, 1.67) | 0.055 |
| ≥28.4 | 2.35 (1.90, 2.90) | <0.001 | 2.43 (1.96, 3.00) | <0.001 | 2.08 (1.58, 2.75) | <0.001 |
| 90-day mortality | ||||||
| NPAR | 1.06 (1.05, 1.07) | <0.001 | 1.06 (1.05, 1.07) | <0.001 | 1.06 (1.04, 1.07) | <0.001 |
| NPAR tertiles | ||||||
| <22.8 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | |||
| 22.8–28.4 | 1.49 (1.22, 1.81) | <0.001 | 1.46 (1.20, 1.79) | <0.001 | 1.32 (1.05, 1.67) | 0.020 |
| ≥28.4 | 2.36 (1.95, 2.87) | <0.001 | 2.49 (2.04, 3.04) | <0.001 | 2.07 (1.61, 2.67) | <0.001 |
| One-year mortality | ||||||
| NPAR | 1.04 (1.03, 1.05) | <0.001 | 1.05 (1.04, 1.06) | <0.001 | 1.05 (1.03, 1.06) | <0.001 |
| NPAR tertiles | ||||||
| <22.8 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | |||
| 22.8–28.4 | 1.28 (1.06, 1.53) | 0.009 | 1.25 (1.04, 1.51) | 0.020 | 1.15 (0.93, 1.44) | 0.197 |
| ≥28.4 | 1.81 (1.50, 2.17) | <0.001 | 1.90 (1.57, 2.29) | <0.001 | 1.60 (1.26, 2.04) | <0.001 |
Notes: Models were all derived from logistic regression models. Non-adjusted model adjusted for none. Model I adjusted for age and gender. Model II adjusted for age, gender, heart failure, hypertension, stroke, temperature, respiration rate, systolic blood pressure, diastolic blood pressure, white blood cell, lymphocyte, hemoglobin, creatinine, SOFA score, anticoagulant, rate control drugs and amiodarone.
Abbreviations: MIMIC, Medical Information Mart for Intensive Care; NPAR, neutrophil percentage-to-albumin ratio; OR, odds ratio; CI, confidence interval; Ref, reference; SOFA, sequential organ failure assessment.
Table 3.
Association Between NPAR and All-Cause Mortality in WMU Cohort
| Non-Adjusted OR (95% CIs) | P value | Model I OR (95% CIs) | P value | Model II OR (95% CIs) | P value | |
|---|---|---|---|---|---|---|
| 30-day mortality | ||||||
| NPAR | 1.14 (1.09, 1.19) | <0.001 | 1.13 (1.08, 1.19) | <0.001 | 1.11 (1.03, 1.19) | 0.005 |
| NPAR tertiles | ||||||
| <22.8 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | |||
| 22.8–28.4 | 2.31 (1.31, 4.07) | 0.004 | 2.18 (1.23, 3.88) | 0.008 | 1.35 (0.63, 2.87) | 0.445 |
| ≥28.4 | 4.65 (2.47, 8.77) | <0.001 | 4.38 (2.30, 8.33) | <0.001 | 2.54 (1.02, 6.30) | 0.044 |
| 90-day mortality | ||||||
| NPAR | 1.15 (1.10, 1.21) | <0.001 | 1.15 (1.09, 1.21) | <0.001 | 1.11 (1.03, 1.19) | 0.007 |
| NPAR tertiles | ||||||
| <22.8 | 1.0 (ref.) | 1.0 (ref.) | 1.0 (ref.) | |||
| 22.8–28.4 | 2.52 (1.42, 4.46) | 0.002 | 2.42 (1.36, 4.31) | 0.003 | 1.38 (0.64, 2.97) | 0.409 |
| ≥28.4 | 5.46 (2.84, 10.50) | <0.001 | 5.17 (2.66, 10.04) | <0.001 | 2.76 (1.09, 7.01) | 0.033 |
Notes: Models were all derived from Logistic regression models. Non-adjusted model adjusted for none. Model I adjusted for age and gender. Model II adjusted for age, gender, stroke, white blood cell, lymphocyte, hemoglobin, creatinine, anticoagulant, rate control drugs and amiodarone.
Abbreviations: WMU, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University; NPAR, neutrophil percentage-to-albumin ratio; OR, odds ratio; CI, confidence interval; Ref, reference.
Subgroup Analysis
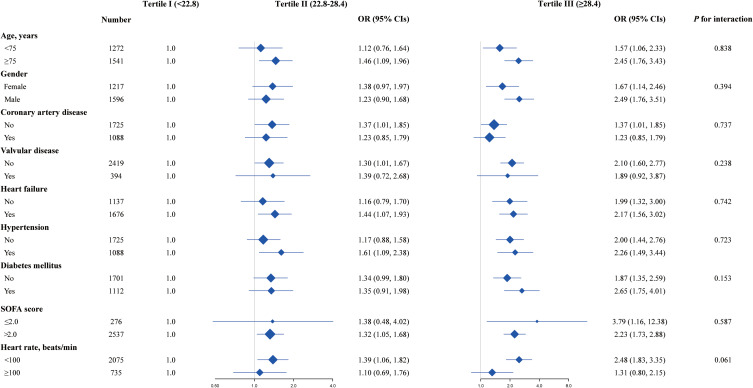
In subgroup analyses, this positive effect of NPAR on 90-day mortality was evident in all subgroups studied and after careful adjustments. No discernible interaction confirmed the independent association between NPAR and the risk of 90-day mortality in MIMIC-IV (Figure 2).
Figure 2.
Subgroup analysis of the association between NPAR and 90-day mortality Logistic regression models were used to calculate OR with 95% CIs. Plots showed adjusted ORs for high versus low NPAR tertile for 90-day mortality in different patient subgroups, tertile I was set as the reference group. Each stratification adjusted for age, gender, heart failure, hypertension, stroke, temperature, respiration rate, systolic blood pressure, diastolic blood pressure, white blood cell, lymphocyte, hemoglobin, creatinine, and SOFA score except for the stratification factor itself.
Abbreviations: OR, odds ratio; CI, confidence interval; NPAR, neutrophil percentage-to-albumin ratio; SOFA, sequential organ failure assessment.
Predictive Performance of NPAR
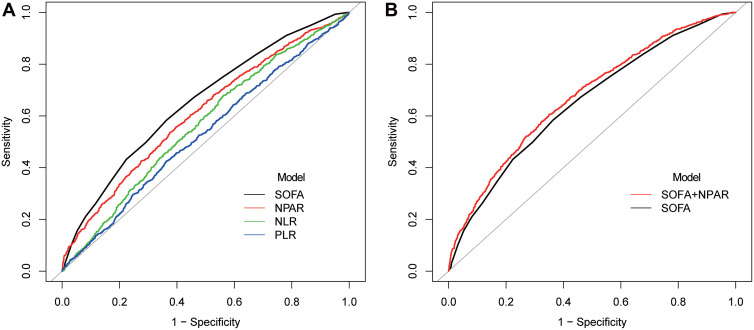
We performed ROC analysis to predict the 90-day all-cause mortality of NPAR in MIMIC-IV (Figure 3). The AUC of PLR, NLR, NPAR, and SOFA for 90-day mortality was 0.528, 0.565, 0.609, and 0.652 respectively (Table 4). NPAR was superior to PLR or NLR alone (P < 0.001) but inferior to SOFA (P = 0.003) compared to the respective AUCs. When SOFA and NPAR were combined, prediction power was significantly higher than SOFA alone (0.674 vs 0.652, P < 0.001).
Figure 3.
ROC analysis for the prediction of 90-day mortality (A) Comparisons of ROC curves of different models; (B) ROC curves of SOFA and SOFA plus NPAR for 90-day mortality.
Abbreviations: ROC, receiver operating characteristic; NPAR, neutrophil percentage-to-albumin ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; SOFA, sequential organ failure assessment.
Table 4.
Predictive Performance of Inflammatory Biomarkers and SOFA Score for 90-Day Mortality
| Model | AUC | 95% CIs | P value |
|---|---|---|---|
| NPAR | 0.609 | 0.587, 0.630 | Ref. |
| PLR | 0.528 | 0.506, 0.550 | <0.001 |
| NLR | 0.565 | 0.544, 0.587 | <0.001 |
| SOFA | 0.652 | 0.631, 0.672 | 0.003 |
| SOFA+NPAR | 0.674 | 0.654, 0.695 | <0.001 |
Abbreviations: AUC, area under curve; CI, confidence interval; Ref, reference; NPAR, neutrophil percentage-to-albumin ratio; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; SOFA, sequential organ failure assessment.
Discussion
Even after correcting for potential confounding factors, the present study found that NPAR was independently associated with death at 30-day, 90-day, and one-year mortality in patients with AF. ROC curves indicated that NPAR had a moderate ability to predict 90-day mortality in ICU patients with AF. The subgroup analysis revealed no clear interaction in all subgroups. This positive effect was evident in all subgroups studied and after careful adjustments.
The remodeling of the atria causes connective tissue, deposition, fibroblast activation, inflammation, fibrosis, and dilatation, all of which induce AF. This procedure modifies the conduction channels of atria, enabling targeted reentry of electrical impulses. AF promotes this situation, which may help to explain the phenomenon that AF breeds AF.15 Acute events during severe illness rapidly produce the sensitive atrial stroma, opening up the potential for developing AF in response to several critical arrhythmic triggers.16 Systemic inflammatory responses and other events can set off AF in critically ill patients. The direct arrhythmogenic impact of inflammatory cytokines on the atrial myocardium has been associated with the onset of AF.17–20 Inflammation may contribute to the development of arrhythmias because atrial myocytes are affected by inflammatory cell infiltration and oxidative damage.21,22 As mentioned previously, inflammation is crucial to the development of AF, whether or not it is accompanied by concurrent infection. Higher inflammatory markers have been linked to an increased risk of developing AF in patients with sepsis and those who have just undergone surgery.23 Earlier research suggests anti-inflammatory drugs like glucocorticoids and statins may reduce the risk of developing AF.24 Patients with AF with low-grade inflammation and elevated serum proinflammatory cytokines may experience inflammation through various mechanisms.25
Neutrophils are one of the most well-known cellular effectors and have been crucial in mediating inflammatory responses as a vital component of white blood cells.26,27 Regarding the general populace and several pathological situations, hypoalbuminemia is a powerful prognostic marker, primarily as an indicator of malnutrition and inflammation.28,29 Low serum albumin levels are associated with various cardiovascular diseases including AF, venous thromboembolism, stroke, and ischemic heart disease.30–32 The NPAR computation exacerbates variations in these two indicators because they are a mixture of two inflammatory markers.
NLR and PLR were both composite inflammatory markers that were shown to predict the outcomes of AF patients.33,34 The findings of present study revealed that NPAR was not only a reliable indicator of all-cause death, but it also had a limited ability to predict the 90-day mortality of ICU patients with AF. It was worth noting that while the AUC of NPAR was smaller than that of SOFA, the predictive ability improved with the addition of NPAR. At least it served as a reminder that NPAR might be able to help direct our therapeutic practice in situations where the SOFA score cannot be calculated. Expecting NPAR to be as effective as more established classic scores like SOFA was unrealistic. Despite the difficulty of calculating SOFA scores, NPAR may be clinically valuable for critically ill patients with AF because of its availability, affordability, simplicity, and ability to predict mortality.
The present study had some strengths. First, it is the first and largest retrospective study of NPAR in AF patients using two data sources. We could conclude from the large study population that the link between AF and death was not simply due to a more severe illness among AF patients. Second, we identified that NPAR better predicted mortality than NLR or PLR, indicating that it should be used in clinical work.
There were several limitations of the present study. First, our findings were drawn from AF patients and cannot be extrapolated to other populations. Second, the sample size of WMU was small, which may influence its statistical effectiveness. Third, more key variables are generally required to control confounding factors, but some relevant clinical factors such as C-reactive protein were either unavailable or only infrequently present (Table S3 in Supplementary Appendix). When CRP was included in Multiple analyses as a confounding factor, the final results were similar to the present study (Tables S4 and S5 in Supplementary Appendix). So lacking CRP did not affect our conclusions. Fourth, while logistic regression analysis successfully balanced many covariates, variables that could affect mortality and AF risk were not investigated in the present study. Furthermore, due to the lack of albumin data, the sample size of the present study was significantly reduced. Finally, because it was challenging to distinguish whether one person had a previous history of AF or a new onset when we chose the research sample, our conclusion may not apply to both sides due to potential differences in the pathophysiological process and treatment. Well-designed randomized controlled trials would be ideal for validating the study’s findings. Considering the predictive ability of NPAR demonstrated in the present study, it was worthwhile to develop a multivariable model or scoring system that included NPAR, to predict AF patients’ prognoses.
Conclusions
The present study revealed that NPAR was independently associated with mortality in patients with AF from MIMIC-IV, including 30-day, 90-day, and one-year all-cause mortality. Moreover, NPAR was a reliable predictor of 90-day all-cause mortality. Meanwhile, NPAR was associated with 30-day and 90-day mortality in WMU patients with AF. As an inflammatory biomarker, NPAR could help clinicians determine the prognosis of patients with AF earlier and more effectively, as well as immediate treatment.
Acknowledgments
We thank the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University for supporting our work.
Funding Statement
This work was supported by the Major Science and Technology Special Project of Wenzhou (2018ZY018).
Data Sharing Statement
The corresponding author will provide the datasets used and analyzed during the current work upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–1468. doi: 10.1161/CIRCRESAHA.114.303211 [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217–221. doi: 10.1177/1747493019897870 [DOI] [PubMed] [Google Scholar]
- 3.Gupta S, Tiruvoipati R, Green C. Atrial fibrillation and mortality in critically ill patients: a retrospective study. Am J Crit Care. 2015;24(4):336–341. doi: 10.4037/ajcc2015319 [DOI] [PubMed] [Google Scholar]
- 4.Liuba I, Ahlmroth H, Jonasson L, et al. Source of inflammatory markers in patients with atrial fibrillation. Europace. 2008;10(7):848–853. doi: 10.1093/europace/eun111 [DOI] [PubMed] [Google Scholar]
- 5.Charitakis E, Barmano N, Walfridsson U, Walfridsson H. Factors predicting arrhythmia-related symptoms and health-related quality of life in patients referred for radiofrequency ablation of atrial fibrillation: an observational study (the SMURF study). JACC Clin Electrophysiol. 2017;3(5):494–502. doi: 10.1016/j.jacep.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Adamsson Eryd S, Smith JG, Melander O, Hedblad B, Engstrom G. Inflammation-sensitive proteins and risk of atrial fibrillation: a population-based cohort study. Eur J Epidemiol. 2011;26(6):449–455. doi: 10.1007/s10654-011-9565-6 [DOI] [PubMed] [Google Scholar]
- 7.Sun T, Shen H, Guo Q, et al. Association between neutrophil percentage-to-albumin ratio and all-cause mortality in critically ill patients with coronary artery disease. Biomed Res Int. 2020;2020:8137576. doi: 10.1155/2020/8137576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Liu Y, Ling X, et al. The neutrophil percentage-to-albumin ratio as a new predictor of all-cause mortality in patients with cardiogenic shock. Biomed Res Int. 2020;2020:7458451. doi: 10.1155/2020/7458451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Z, Wang J, Xue Y, et al. The neutrophil-to-albumin ratio as a new predictor of all-cause mortality in patients with heart failure. J Inflamm Res. 2022;15:701–713. doi: 10.2147/JIR.S349996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Y, Lin Y, Yue J, Zou Q. The neutrophil percentage-to-albumin ratio is associated with all-cause mortality in critically ill patients with acute myocardial infarction. BMC Cardiovasc Disord. 2022;22(1):115. doi: 10.1186/s12872-022-02559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guasti L, Dentali F, Castiglioni L, et al. Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects. Thromb Haemost. 2011;106(10):591–599. doi: 10.1160/TH11-02-0096 [DOI] [PubMed] [Google Scholar]
- 12.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. doi: 10.1016/j.jacc.2005.02.054 [DOI] [PubMed] [Google Scholar]
- 13.Mukamal KJ, Tolstrup JS, Friberg J, Gronbaek M, Jensen G. Fibrinogen and albumin levels and risk of atrial fibrillation in men and women (the copenhagen city heart study). Am J Cardiol. 2006;98(1):75–81. doi: 10.1016/j.amjcard.2006.01.067 [DOI] [PubMed] [Google Scholar]
- 14.Isaka Y, Hayashi H, Aonuma K, et al. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Clin Exp Nephrol. 2020;24(1):1–44. doi: 10.1007/s10157-019-01750-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–809. doi: 10.1016/j.jacc.2007.09.064 [DOI] [PubMed] [Google Scholar]
- 16.Bosch NA, Cimini J, Walkey AJ. Atrial fibrillation in the ICU. Chest. 2018;154(6):1424–1434. doi: 10.1016/j.chest.2018.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinz G. Atrial fibrillation in the intensive care unit. Intensive Care Med. 2006;32(3):345–348. doi: 10.1007/s00134-005-0033-1 [DOI] [PubMed] [Google Scholar]
- 18.Seguin P, Laviolle B, Maurice A, Leclercq C, Mallédant Y. Atrial fibrillation in trauma patients requiring intensive care. Intensive Care Med. 2006;32(3):398–404. doi: 10.1007/s00134-005-0032-2 [DOI] [PubMed] [Google Scholar]
- 19.Tselentakis EV, Woodford E, Chandy J, Gaudette GR, Saltman AE. Inflammation effects on the electrical properties of atrial tissue and inducibility of postoperative atrial fibrillation. J Surg Res. 2006;135(1):68–75. doi: 10.1016/j.jss.2006.03.024 [DOI] [PubMed] [Google Scholar]
- 20.Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157(2):243–252. doi: 10.1016/j.ahj.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 21.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–1184. doi: 10.1161/01.cir.96.4.1180 [DOI] [PubMed] [Google Scholar]
- 22.Mihm MJ, Yu F, Carnes CA, et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation. 2001;104(2):174–180. doi: 10.1161/01.cir.104.2.174 [DOI] [PubMed] [Google Scholar]
- 23.Klein Klouwenberg PM, Frencken JF, Kuipers S, et al. Incidence, predictors, and outcomes of new-onset atrial fibrillation in critically ill patients with sepsis. A cohort study. Am J Respir Crit Care Med. 2017;195(2):205–211. doi: 10.1164/rccm.201603-0618OC [DOI] [PubMed] [Google Scholar]
- 24.Jacob KA, Nathoe HM, Dieleman JM, van Osch D, Kluin J, van Dijk D. Inflammation in new-onset atrial fibrillation after cardiac surgery: a systematic review. Eur J Clin Invest. 2014;44(4):402–428. doi: 10.1111/eci.12237 [DOI] [PubMed] [Google Scholar]
- 25.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. doi: 10.1038/nrcardio.2015.2 [DOI] [PubMed] [Google Scholar]
- 26.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430 [DOI] [PubMed] [Google Scholar]
- 27.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–2051. doi: 10.1161/atvbaha.108.179705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuzuya M, Izawa S, Enoki H, Okada K, Iguchi A. Is serum albumin a good marker for malnutrition in the physically impaired elderly? Clin Nutr. 2007;26(1):84–90. doi: 10.1016/j.clnu.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 29.Don BR, Kaysen G. Poor nutritional status and inflammation: serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17(6):432–437. doi: 10.1111/j.0894-0959.2004.17603.x [DOI] [PubMed] [Google Scholar]
- 30.Takayoshi K, Kusaba H, Aikawa T, et al. Hypoalbuminemia for the prediction of venous thromboembolism and treatment of direct oral anticoagulants in metastatic gastric cancer patients. Gastric Cancer. 2019;22(5):988–998. doi: 10.1007/s10120-019-00930-2 [DOI] [PubMed] [Google Scholar]
- 31.Shaper AG, Wannamethee SG, Whincup PH. Serum albumin and risk of stroke, coronary heart disease, and mortality: the role of cigarette smoking. J Clin Epidemiol. 2004;57(2):195–202. doi: 10.1016/j.jclinepi.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Nelson JJ, Liao D, Sharrett AR, et al. Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol. 2000;151(5):468–477. doi: 10.1093/oxfordjournals.aje.a010232 [DOI] [PubMed] [Google Scholar]
- 33.Ertaş G, Sönmez O, Turfan M, et al. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324(1–2):49–52. doi: 10.1016/j.jns.2012.09.032 [DOI] [PubMed] [Google Scholar]
- 34.Gungor H, Babu AS, Zencir C, et al. Association of preoperative platelet-to-lymphocyte ratio with atrial fibrillation after coronary artery bypass graft surgery. Med Princ Pract. 2017;26(2):164–168. doi: 10.1159/000453614 [DOI] [PMC free article] [PubMed] [Google Scholar]