Abstract
Background
Previous interim data from a phase I study of AKS-452, a subunit vaccine comprising an Fc fusion of the respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein receptor binding domain (SP/RBD) emulsified in the water-in-oil adjuvant, Montanide™ ISA 720, suggested a good safety and immunogenicity profile in healthy adults. This phase I study was completed and two dosing regimens were further evaluated in this phase II study.
Methods
This phase II randomized, open-labelled, parallel group study was conducted at a single site in The Netherlands with 52 healthy adults (18 – 72 years) receiving AKS-452 subcutaneously at one 90 µg dose (cohort 1, 26 subjects) or two 45 µg doses 28 days apart (cohort 2, 26 subjects). Serum samples were collected at the first dose (day 0) and at days 28, 56, 90, and 180. Safety and immunogenicity endpoints were assessed, along with induction of IgG isotypes, cross-reactive immunity against viral variants, and IFN-γ T cell responses.
Results
All AEs were mild/moderate (grades 1 or 2), and no SAEs were attributable to AKS-452. Seroconversion rates reached 100% in both cohorts, although cohort 2 showed greater geometric mean IgG titers that were stable through day 180 and associated with enhanced potencies of SP/RBD-ACE2 binding inhibition and live virus neutralization. AKS-452-induced IgG titers strongly bound mutant SP/RBD from several SARS-CoV-2 variants (including Omicrons) that were predominantly of the favorable IgG1/3 isotype and IFN-γ-producing T cell phenotype.
Conclusion
These favorable safety and immunogenicity profiles of the candidate vaccine as demonstrated in this phase II study are consistent with those of the phase I study (ClinicalTrials.gov: NCT04681092) and suggest that a total of 90 µg received in 2 doses may offer a greater duration of cross-reactive neutralizing titers than when given in a single dose.
Keywords: Infectious disease, Coronavirus, Prophylaxis, Pandemic, COVID-19, Fc-fusion, SARS-CoV-2, AKS-452, Vaccine, Phase 2
1. Background
Significant hospitalization and death rates of the global COVID-19 pandemic due to SARS-CoV-2 viral infection have triggered the development of a variety of vaccines [1], [2]. While some vaccines with acceptable protective efficacy and safety profiles have gained Emergency Use Authorization or regulatory approval [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], there remain challenges with global distribution and access to COVID-19 vaccines that can be addressed via improved costs and speed of manufacturing, along with reasonable non-cold-chain vaccine storage [12], [13]. We are developing such a stable recombinant subunit vaccine, AKS-452, that is an Fc fusion protein comprised of the SARS-CoV-2 SP/RBD and the human IgG1 Fc region formulated in the water-in-oil adjuvant, Montanide™ ISA 720 [14], [15]. Advantages of the Fc moiety are facilitation of correct protein folding and rapid purification, in addition to enhanced thermostability, in vivo half-life, and immunogenicity of target antigens via Fc receptor-mediated binding to antigen-presenting cells, which has been demonstrated with other subunit vaccines against coronaviruses and HIV [16], [17], [18], [19], [20], [21], [22]. We have demonstrated enhanced production efficiency, thermostability, and immunogenicity attributed to the Fc moiety in AKS-452, in addition to the need for emulsification in Montanide™ ISA 720 to achieve high IgG titers in unprimed animals of several species that allows for dose sparing [14]. AKS-452 has recently been evaluated in the dose-ranging phase I portion of a combined phase I/II clinical trial (ClinicalTrials.gov: NCT04681092) that demonstrated acceptable safety and immunogenicity profiles at the day-56 interim analysis, in which the 90 µg dose level induced the highest IgG neutralizing titers [15]. Therefore, this dose level was selected for further evaluation at a single 90 µg dose or two 45 µg doses administered 28 days apart for safety, reactogenicity, and immunogenicity in this phase II trial involving COVID-19-naïve healthy adults between 18 and 72 years of age at a single-center, University Medical Center Groningen (UMCG) in The Netherlands. Results through the 180-day completion for this phase II study, along with those of the phase I study through the 180-day follow-up (included as Supplemental Information) are presented here.
2. Methods
2.1. Vaccine components
AKS-452 is a recombinant fusion protein comprising SP/RBD and an Fc fragment containing a portion of the hinge region, in which the full CH2 and CH3 domains of the human IgG1 Fc fragment are connected via a covalent peptide linker sequence, all encoded by a single nucleic acid molecule expressed in CHO-K1 cells as previously described [14], [15] (#MDS0002, 586 µg/ml; Akston Biosciences, Beverly, MA; see PCT/US21/26577 for details). AKS-452 drug product was released for clinical use from ICON (Groningen, The Netherlands) after passing established criteria as previously described [15] and was stored at −80 °C. Data from stability studies support storage at −80 °C, 2–8 °C, and 25 °C for at least twelve months (see Table S1 ). This sterile aqueous solution of AKS-452 was thawed and emulsified in the water-in-oil adjuvant, Montanide™ ISA 720 (#2587851 Seppic S.A., Paris, France; 30%/70% aqueous antigen/adjuvant emulsification) [23], [24] and administered to subjects within 24 h of preparation as previously described [15].
2.2. Study design and procedures
This phase II study was designed as part of a combined phase I/II study (ClinicalTrials.gov, NCT04681092; see Clinical Protocol in Supplementary Material s). Potentially eligible subjects were recruited via announcements on social media and local and regional newspapers. Screening visits occurred in June-July 2021 during the second wave of the COVID-19 pandemic that began in July 2021 as the contagious Delta variant began to circulate. During the screening visit, subjects were provided a questionnaire (with inclusion and exclusion questions) and informed about study aims, possible adverse events, procedures, possible hazards, and maintenance of confidentiality of their data. Subjects were then contacted by phone, mail, or e-mail by a research physician (study investigator) to confirm subject’s interest in study participation, after which eligibility was approved by the research physician in consultation with the treating physician (i.e., principal investigator) by checking inclusion and exclusion criteria. The first subject was enrolled on 15 July 2021 and the last subject’s final visit occurred on 14 February 2022. Main exclusion criteria were use of corticosteroids (excluding topical preparations for cutaneous or nasal use) or other immunosuppressive drugs within 30 days prior to the first vaccination dose, in addition to previous SARS-CoV-2 infection or vaccination assessed via questionnaire. Note that testing of SARS-CoV-2 infection was both passively and actively surveilled in which subjects were assessed at screening and monitored after enrolment at days 0, 28, and 56 via an EUA-approved PCR test (AlinityM SARS-CoV-2 Assay, Abbott Molecular, Inc.; positive result of infection was cycle number ≤ 36) in addition to being prompted to take the test at any time to confirm suspected COVID-19-related symptoms, after which any positive test result was reported.
Enrolled subjects were randomized to one of the two cohorts receiving either a single 500 μL dose of 90 µg (cohort 1) or two 250 μL doses of 45 µg 28 days apart (cohort 2) via subcutaneous injection in the deltoid region of the upper arm. Each subject was observed during a 15 min post-vaccination period before being released. Safety reviews and immunogenicity assessments were scheduled for study days 0, 28, 56, 90 and 180, at which time blood samples were obtained for preparation of serum and PBMCs and stored frozen until analysis. An informed consent form was signed voluntarily before any study-related procedure was performed, indicating that the subject understood the purpose and procedures required for the study and was willing to participate. Subjects were given an emergency call card at the day-0 visit prior to vaccination and instructed to report, in an unsolicited manner, every change in health or well-being after vaccination at the day-1 visit. After the opportunity to report and discuss unsolicited reactions at this day-1 visit, subjects were given a symptom questionnaire and diary card after which they were instructed to report (i.e., solicited) any adverse events (AEs) and serious adverse events (SAEs) at any time during the trial. During all follow-up appointments, subjects reported any symptoms in an open unsolicited manner followed by a solicited symptom questionnaire discussion. Participants could also contact clinical trial researchers (via contact information on the emergency card) to report symptoms at any moment between follow-up appointments. (See [15] for details of the clinical study procedures in the Research Protocol, # 900452-CT-20-001.)
2.3. Trial oversight
The trial was reviewed and approved by the Central Committee on Research involving Humans (CCMO) in The Hague, together with a review by the Ministry of Health, Welfare and Sport (VWS). Local feasibility was assessed and approved by the UMCG Institutional Review Board. For release of the trial at the clinical site, the UMCG was the regulatory sponsor and TRACER BV (The Netherlands) was the contract research organization that managed and was responsible for the entire clinical project. The decision to submit the manuscript for publication was made by all authors who vouch for the accuracy and completeness of the reported data and for the fidelity of trial operations to the protocol. No one who is not an author contributed to the preparation of the manuscript.
2.4. Primary and secondary endpoints
Primary endpoints were local and systemic (general) AEs and SAEs of each dose schedule, and secondary endpoints were serum anti-SP/RBD-specific IgG titers and inhibitory/neutralization potencies. Research objectives included IgG titers against mutant SP/RBD of different SARS-CoV-2 variant strains (via enzyme-linked immunosorbent assays, ELISA) and the neutralization of such live virus variants (via Plaque Reduction Neutralization Test; PRNT), in addition to serum IgG isotyping and peripheral blood mononuclear cell (PBMC) IFN-γ production (via ELISPOT). AEs and SAEs were graded by a numerical scoring system defined by the NCI Common Terminology Criteria for Adverse Events (NCI CTCAE; version number V4.03; i.e., Grade 1, Mild; Grade 2, Moderate; Grade 3, Severe or medically significant but not immediately life threatening; Grade 4, Life threatening consequences; Grade 5, Death related to the adverse event).
2.5. Laboratory analyses
Different types of ELISAs were used to measure SARS-CoV-2 SP/RBD-specific binding IgG titers (see details in Supplemental Methods). A semi-quantitative screening ELISA (SARS-CoV-2 IgG, ARCHITECT I System, Abbott, Sligo, Ireland) was performed at the UMCG that detected anti-SARS-CoV-2 SP IgG titers in which validated negative and positive cutoff values were used as inclusion and exclusion enrollment criteria, respectively, during screening. A quantitative anti-SP/RBD IgG titer ELISA (AntiCoV-ID™ IgG ELISA, Akston Biosciences, Beverly, MA) was used to assess titers at baseline (day 0) and on days 28, 56, 90, and 180 post-vaccination in which seropositivity was defined as a titer >4.5 standard deviations above the value obtained using sera from 80 COVID-19-naïve subjects [i.e., 2.42 µg/mL, data not shown]. The seroconversion rate of a cohort was defined as the percentage of subjects that became seropositive after receiving vaccination. The capacity of anti-SP/RBD antibodies (and associated IgG isotypes) to bind a series of SP/RBD mutant proteins from known SARS-CoV-2 variants was assessed by ELISA (see Supplemental Methods for details). The potency of serum to inhibit binding of recombinant SP/RBD to recombinant angiotensin-converting enzyme II (ACE2) were expressed as % inhibition at 40-fold dilution and inhibitory dilution 50% (ID50) values, and were performed at Akston Biosciences (Beverly, MA) as previously described [14]. A PRNT was performed with VERO E6 cells to define the % inhibition values at 40-fold serum dilution; the three viral strains evaluated were Original Washington (USA-WA1/2020; World Reference Center for Emerging Viruses and Arboviruses, University of Texas Medical Branch, TX, USA; GenBank accession no. MN985325.1), Alpha (NR-54011, BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2, Isolate USA/CA_CDC_5574/2020), and Delta (NR-55611, BEI Resources, NIAID, NIH: SARS-Related Coronavirus 2, Isolate hCoV-19/USA/PHC658/2021 Lineage B.1.617.2; contributed by Dr. Richard Webby and Dr. Anami Patel). SP/RBD-specific T cell responses in PBMC samples were evaluated via an IFN-γ ELISPOT assay using SP/RBD peptide pools of the Original Washington, Alpha, and Omicron B.1 strains and performed at the UMCG. All assays were conducted in a blinded fashion and performed as previously described [14], [25] or included in Supplementary Methods. HCS samples from unvaccinated subjects (i.e., n = 32) known to have acquired COVID-19 (confirmed via PCR test) that recovered to an asymptomatic state for at least 14 days prior to serum collection during the first wave of COVID-19 (i.e., from February through December 2020) were purchased or obtained from BioIVT (Westbury, NY), Invent Diagnostica (Hennigsdorf, Germany), and locally sourced donors under informed consent. [The mean ± SD age at time of sampling was 42.7 ± 13.7 years (range, 21–77 years), mean ± SD days after COVID-19 symptoms at which samples were obtained was 26.4 ± 5.1 (range, 19–34 days)].
The IFN-γ ELISPOT assay was used to quantitate the frequency of SP/RBD-specific IFN-γ-producing T cells in PBMC samples collected at days 0, 28 (for cohort 1), 56 (for cohort 2), 90, and 180. A subject’s response to vaccination was reported as the ratio (Stimulation Index; SI) of the mean IFN-γ net-Spot-Forming Cells (net-SFC) from each post-vaccination day dividing by the mean net-SFC of the respective day 0 for each subject. In addition to the SI, each post-vaccination PBMC sample was scored in a binary manner as a positive or negative responder sample defined as having a SI of at least 2 in addition to having at least 25 net-SFCs greater than that of the day 0 sample. Reported here are the SIs of all samples in which “negative responder” samples were assigned a value of “1”, in addition to the proportion (and percentage in parentheses) of “positive responders” per cohort and timepoint. Note that additional PBMC samples were obtained from an external cohort of subjects who participated in the Vaccination Against COVID-19 in Cancer (VOICE) clinical trial (Cohort A: individuals without cancer) who received two injections, 21-days apart, of the mRNA-1273 SARS-CoV-2 vaccine (ClinicalTrials.gov Identifier: NCT04715438) [26], in which blood samples were collected 28 days after the second injection.
2.6. Statistical analysis
As described in the Study Protocol (see Supplemental Information), statistical derivation of the dosing regimen and number of subjects for this phase II study were based on the phase I interim safety assessment conducted on each cohort considering a minimum projected seroconversion rate of 7/10 for each cohort (a true positive was based on the quantitative SP/RBD IgG ELISA positive/negative cutoff criteria). While each of the six phase I cohorts passed the safety criteria (i.e., no AEs > grade 3 and no SAEs due to vaccine) and immunogenicity criteria (≥70% seroconversion rate) to advance to phase II, only cohorts that received the higher dose level of 90 µg (either in a single dose or two doses) achieved a 100% conversion rate, which was selected for phase II. Accordingly, statistical analysis of phase I immunogenicity data (following methods of the Study Protocol) derived a total of 52 participants in phase II sufficient to provide a descriptive safety and immunogenicity assessment. Subject demographic characteristics (such as age, sex, race, ethnicity, Body Mass Index, medical history, and morbidity) were displayed as geometric means with standard deviations or as medians with range and frequencies. Continuous variables were tested for normal distribution and if non-normally distributed, data were log10-transformed to obtain a normal distribution. Immunogenicity data were log-transformed (except for PRNT data) before performing 1-way or 2-way analysis of variance (ANOVA) comparisons of least squares means, as appropriate, using SAS® for WindowsTM Version 9.4 Proc Mixed (SAS Institute, Inc.). Tukey adjustment for multiple comparisons or Dunnett adjustment for one control group comparison were used. Specific statistical analyses and results are described in detail in each figure legend. As a per-protocol criterion, any subject showing a positive SARS-CoV-2 infection via PCR testing was allowed to continue in the study, but all data collected after infection diagnosis was evaluated separately from the statistical study analyses. Such data was not handled as an Intent-To-Treat analysis because of the known specific safety and immunogenicity parameters affected by such an infection that would confound the effects of vaccination.
3. Results
3.1. Subject demographics and safety assessment
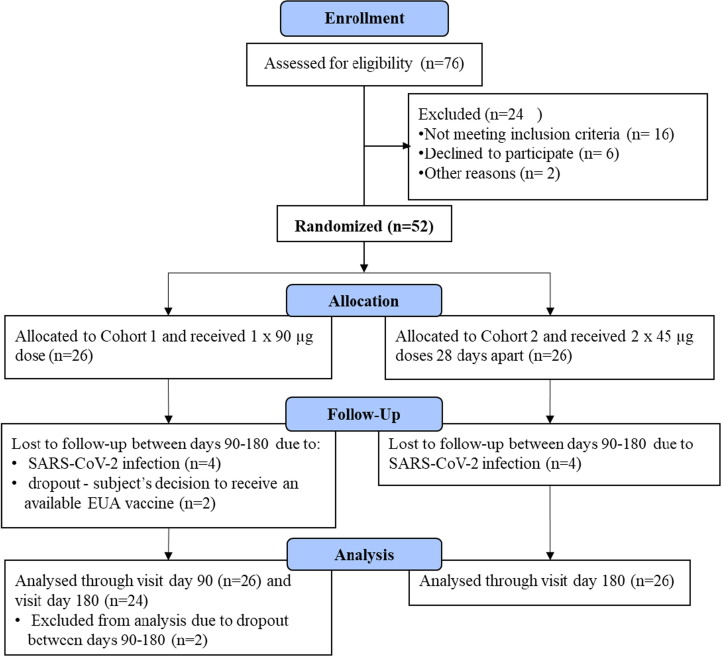
Seventy-six subjects were screened, resulting in enrolment of 52 subjects (Fig. 1 ) comprised of 31 men (59.6%) and 21 women (40.4%) with a mean age of 41.5 years (range 18 to 72 years; see Table 1 for demographics). None of the 52 subjects in either dosing cohort reported an AE ≥ grade 3 or an SAE. In fact, all AEs were Grade 1 (mild) except for two transient “chest pain” AEs that were deemed Grade 2 (moderate) (Table 2 ). There were 56 local AEs (all related to vaccine dosing) reported by 39 subjects after the first dose that were collected in a solicited manner, in which 47 of these AEs appeared within 7 days of injection (Table 2). Forty-six of these local AEs resolved within 7 days whereas 10 resolved between 7 and 71 days. Note that 8 subjects showed an Injection Site Nodule AE after the first dose that is expected from use of the Montanide ISA 720 adjuvant [23], in which 3 of these resolved within 7 days while the other 5 resolved between 30 and 105 days. There were 21 local site AEs after the second injection of similar nature to those after the first dose, in which 6 of 7 Injection Site Nodule AEs showed a protracted resolution (Table 2). Unsolicited general AEs reported after the first and second doses were mostly unrelated to dosing, and those that were related to dosing tended to appear and resolve within 7 days (Table 2). Note that 8 subjects (4 from cohort 1 and 4 from cohort 2) acquired SARS-CoV-2 infection with mild to moderate symptoms after day 90 and before their final visit at day 180 that occurred between 12 Nov 2021 and 24 Jan 2022 (Table 2) during the European Omicron infection wave first announced 24 November 2021 [27]. No irregularities were observed in laboratory analyses in any subject during the entire 180-day trial (Table S2). For comparison, the complete 6-month laboratory data and AE results of the predecessor phase I trial are reported in Table S3 and Table S4, respectively (i.e., including days 90 and 180 that were not reported in the previously published interim day-56 analyses [15]), which demonstrated a similar safety profile, showing that none of the 60 subjects in any of the six dosing cohorts had an AE ≥ grade 3 or an SAE attributable to AKS-452 dosing.
Fig. 1.
AKS-452 Phase II clinical study design. Phase II was a 2 × 26 design; i.e., Cohort 1 was a single-dose (90 µg) and Cohort 2 was a two-dose (45 µg) regimen in which subjects were enrolled and dosed concurrently. The dosing regimens were based on safety and immunogenicity outcomes of the interim results of the phase I study [15].
Table 1.
AKS-452 Phase II characteristics of subjects at baseline.
| Characteristic | Cohort 1 (1 × 90 ug) | Cohort 2 (2 × 45 ug) | Total |
|---|---|---|---|
| No. of Subjects | 26 | 26 | 52 |
| Sex -N (%) | |||
| Male | 16 | 15 | 31 (59.6%) |
| Female | 10 | 11 | 21 (40.4%) |
| Age - yr | |||
| Mean | 43.3 | 39.7 | 41.5 |
| Range | 18–72 | 20–67 | 18–72 |
| Race/Ethnicity - N (%) | |||
| Caucasian | 26 | 24 | 50 (96.2%) |
| Black | 0 | 1 | 1(1.9%) |
| Other | 0 | 1 | 1 (1.9%) |
| Body Mass Index [Median (range)] | 24.69 (19.66–29.13) | 24.71 (19.31–29.99) | 24.70 (19.31 – 29.99) |
| SARS-Cov-2 seronegative - N (%) | 26 | 26 | 52 (100%) |
| Congenital abnormalities - N (%) | |||
| Cardiac abnormalities | 1 | 0 | 1 (1.9%) |
| Non-cardiac abnormalities | 0 | 0 | 0 |
| No abnormalities | 25 | 26 | 51 (98.1%) |
| Charlson Comorbidity Index (CCI) - N (%) | |||
| 0 | 26 | 24 | 50 (96.2%) |
| 1 | 0 | 2 | 2 (3.8%) |
| Use of medication in month prior to screening - N (%) | 11 | 10 | 21 (40.4%) |
| Antibiotics/antiviral therapy | 1 | 0 | 1 (1.9%) |
| Statins | 1 | 1 | 2 (3.8%) |
| Hormone treatment | 0 | 1 | 1 (1.9%) |
| Antihypertensive drugs | 0 | 2 | 2 (3.8%) |
| Benzodiazepines | 0 | 1 | 1 (1.9%) |
| Opiates | 0 | 1 | 1 (1.9%) |
| Other | 9 | 6 | 15 (28.8%) |
Table 2.
AKS-452 Phase II overview of AEs per cohort.
|
Cohort 1 (1 × 90 µg) |
Cohort 2 (2 × 45 µg) |
Total AE's |
|||
|---|---|---|---|---|---|
| Symptoms | days 0–7† | days 8–71‡ | days 0–7† | days 8–71‡ | |
| AEs after FIRST dose | |||||
| Local | |||||
| Injection site induration | 1 | 1 | |||
| Injection site nodule | 2 + 1* | 1* | 2* | 1 + 1* | 8 |
| Injection site reaction⁑ | 4 | 3 + 1* | 5 + 1* | 1 | 15 |
| Injection site swelling | 3 | 1 | 3 | 7 | |
| Injection site tenderness | 9 | 11 + 1* | 21 | ||
| Injection site muscle pain | 2 | 2 | |||
| Itchy skin | 1* | 1 | |||
| Muscle tension | 1* | 1 | |||
| General | |||||
| Dizziness | 1 | 1 | |||
| Chest Pain | 1* (ULR) | 1 | |||
| Coughing | 1* (ULR) | 1 (ULR) | 2 | ||
| Fever | 1 (ULR) | 1 (ULR) | 2 | ||
| Gastroenteritis | 1 (ULR) | 1 | |||
| General malaise | 1 (ULR) | 1 (ULR) | 2 | ||
| Headache | 5 | 1 (ULR) + 1* | 7 | ||
| Menorrhagia | 1 (ULR) | 1 | |||
| Menstruation delayed | 1 | 1* | 2 | ||
| Nausea | 2 (1ULR) | 2 | |||
| Neck pain | 1 | 1 | |||
| Palpitations | 1 | 1 | |||
| Tiredness | 2 (+1*) | 1 (ULR) | 5 | ||
| Burn-out syndrome | 1* (ULR) | 1 | |||
| Eye damage | 1* (ULR) | 1 | |||
| Shoulder bursitis | 1* (ULR) | 1 | |||
| SARS-CoV-2 Infection | 4 (between days 90–180)§ | 4 (between days 90–180)§ | 8 | ||
| AEs after SECOND dose | |||||
| Chest Pain | N/A | N/A | 1 | 1 | |
| Neck pain | N/A | N/A | 1 | 1 | |
| Tiredness | N/A | N/A | 1 | 1 | |
| Muscle tension | N/A | N/A | 1 | 1 | |
| Injection site nodule | N/A | N/A | 1 + 6* | 7 | |
| Injection site reaction | N/A | N/A | 9 + 3* | 12 | |
| Injection site muscle pain | N/A | N/A | 1 | 1 | |
“Injection site reaction” is defined by at least two or more of the following symptoms: swelling, redness, itching or pain at the injection site.
All AEs were deemed “mild” (except the 2 Chest Pain AEs that were “moderate”) and all were “Possibly, Probably, or Definitely” Related to Vaccination unless designated with ULR (i.e., Unlikely or Definitely Not Related to Vaccination). There were no SAE’s reported in the study. All Local AEs were collected in a solicited manner, while most General AEs were unsolicited, especially those with ULR.
AEs reported within 7 days of injection and resolved within 7 days of appearance unless designated with “*” in which case the AE resolved > 7 and < 71 days. Note that 3 “Injection Site Nodule” AEs that occurred after the Second Dose showed an extended presence beyond 71 days.
AEs reported between 8 and 72 days after injection and resolved within 7 days of appearance unless designated with “*” in which case the AE resolved > 7 and < 71 days.
Subjects dropped out of study between days 90 and 180 due to a positive PCR result for SARS-CoV-2 infection.
3.2. Humoral immunogenicity
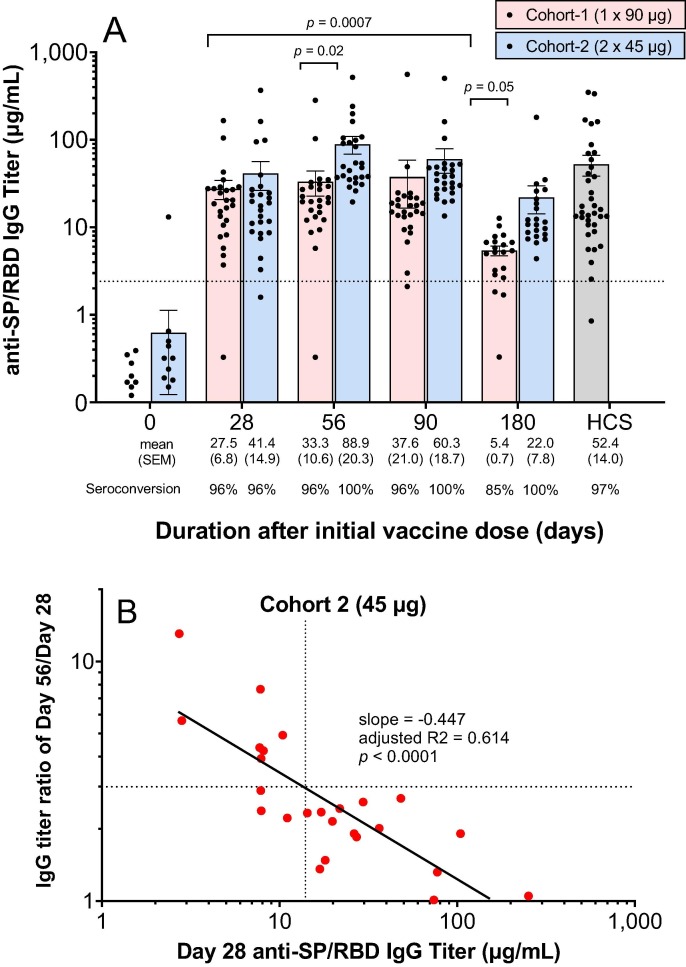
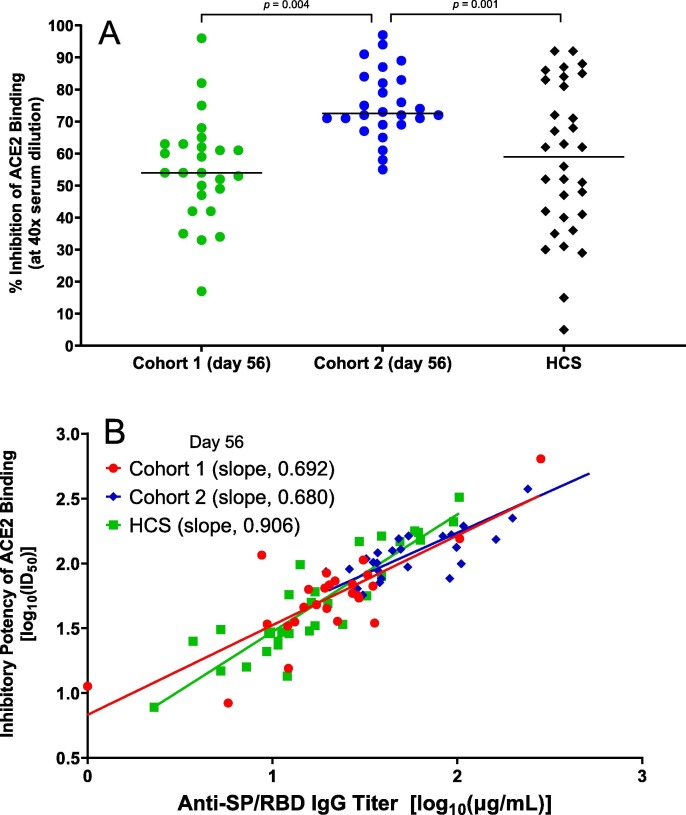
A single administration of 90 µg (cohort 1) of AKS-452 resulted in a 96% (25 of 26 subjects) anti-SP/RBD IgG titer seroconversion rate at days 28, 56, and 90 and declined to 85% by day 180 (Fig. 2 A). In contrast, administration of two 45 µg doses 28 days apart (cohort 2) resulted in 100% seroconversion from days 56 to 180 (Fig. 2 A). Mean anti-SP/RBD IgG titers were similar between cohorts 1 and 2 at day 28 (i.e., after a single dose), but were significantly higher on days 56 and 180 in cohort 2 after the subjects received the second 45 µg dose on day 28 (Fig. 2 A). The mean titer on day 180 of cohort 1, but not that of cohort 2, showed a significant decline relative to the respective day 28 mean titer. Thus, two doses of the lower dose level, 45 µg, achieved greater titers for longer duration than did a single dose of the full dose level, 90 µg. Similar results in the two-dose cohorts in the phase I study were observed (Fig. S1). With respect to titers induced by the first dose in Cohort 2, there was an inverse correlation between the titer on day 28 and the degree of response to the second dose (i.e., the titer ratio of day 56/day 28) such that an anti-SP/RBD IgG titer of 14 µg/mL was an approximate cut-off that defined a 3-fold responsiveness (Fig. 2 B). A similar inverse correlation was also observed in the two-dose cohorts of phase I (Fig. S2). All phase II subjects showed IgG responses that strongly inhibited SP/RBD binding to ACE2 at day 56 in which the two-dose cohort 2 achieved significantly greater inhibitory IgG titers than those of the single-dose cohort 1 (Fig. 3 A). A strong correlation was observed between individual subjects’ respective day-56 IgG titers of SP/RBD binding (µg/mL) and ACE2-SP/RBD inhibition (inhibitory dilution 50%; ID50) from both cohorts (Fig. 3 B). Note that mean IgG binding (Fig. 2 A) and ACE2-SP/RBD inhibition (Fig. 3 A) titers from both cohorts were similar to or greater than those of the HCS group, demonstrating that AKS-452 vaccination is as effective at inducing titers against SP/RBD of the Original SARS-CoV-2 strain as those induced via infection of that strain (i.e., HCS sera were obtained during the first COVID-19 wave of the Original strain).
Fig. 2.
AKS-452 immunogenicity: IgG titers. (A) Serum samples were obtained at Days 0, 28, 56, 90 and 180 of the initial vaccine dose and assessed for anti-SP/RBD IgG binding titers via ELISA and presented per subject (all Day 0 samples were < lower limit of quantitation; not shown). Seroconversion was defined as > 2.42 µg/mL IgG (dotted line; derived from validation studies with COVID-19 naïve subject samples; see Methods). HCS titers were used as a comparator for samples from vaccinated subjects. Statistical comparisons between mean values of study day within each cohort and between cohorts were performed using a model with “cohort” and “day” values as fixed effects and a “random subject effect” in which p values were adjusted for multiplicity (Tukey). (B) Correlation between subject’s responsiveness to the second dose (day 56 titer divided by day 28 titer) and the titer on the day of the second dose (i.e., day 28). The dotted lines delineate the approximate day 28 titer that is correlated with a 3-fold response to the second dose at day 56. The solid black line is the linear regression of log-transformed values.
Fig. 3.
AKS-452 immunogenicity:inhibitory potency and IgG Isotype titers. (A) Serum samples were obtained at Day 56 after the initial vaccine dose and assessed at a 1:40 dilution for % Inhibition of recombinant human ACE2 binding to SP/RBD via ELISA and presented per subject (all Day 0 samples were < lower limit of quantitation; not shown). Statistical comparisons between cohorts were performed using a model with “cohort” as a fixed effect in which p-values were not adjusted for multiplicity. (B) Comparison of IgG titer vs. inhibitory potency (inhibitory dilution 50% in ACE2 binding assay, ID50) of Day 56 sera for vaccinated subjects and HCS. Linear regression was performed on log-transformed data; adjusted r2 0.638, p < 0.0001 (cohort 1), adjusted r2 0.680, p ≤ 0.0001 (cohort 2), adjusted r2 0.803, p ≤ 0.0001 (HCS).
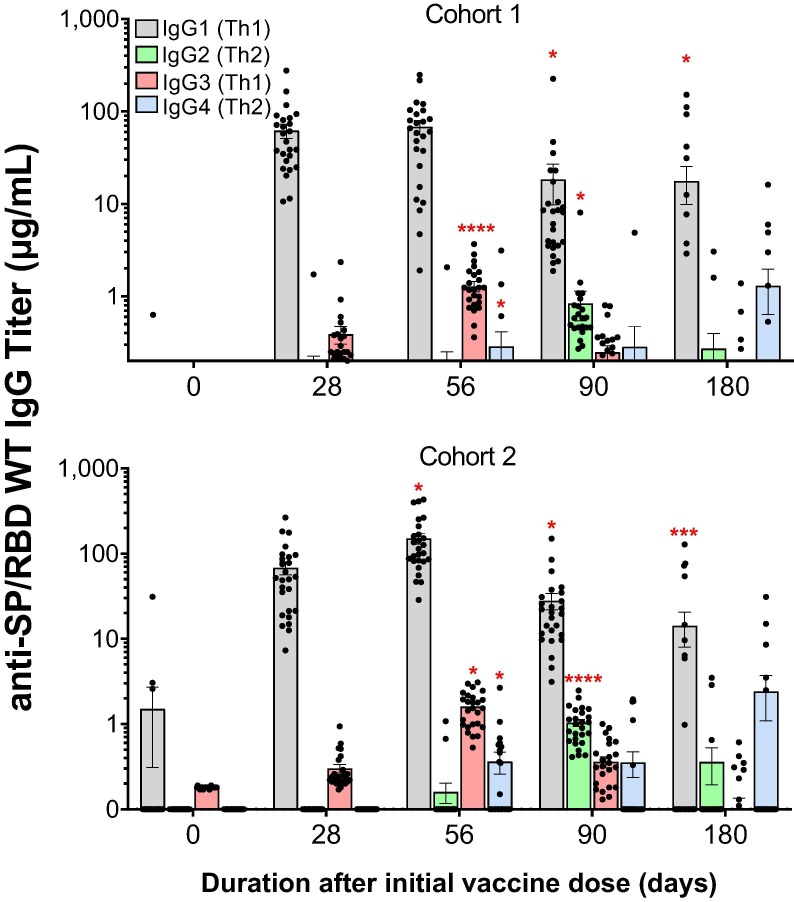
IgG titer isotyping demonstrated the favored effector isotype responses, IgG1 and IgG3, to vaccination in both cohorts throughout the trial (Fig. 4 ; similar results were observed in the phase I study, Fig. S2). While the IgG1 and IgG3 responses peaked on day 56, different kinetics were observed for the other isotype responses that peaked on day 90 (i.e., IgG2) or day 180 (i.e., IgG4) for both cohorts.
Fig. 4.
AKS-452 immunogenicity kinetics and IgG Isotype titers. Serum samples were obtained at Days, 0, 28, 56, 90, and 180 from the initial vaccine dose from all subjects in cohort 1 (1 × 90 µg) and cohort 2 (2 × 45 µg) and assessed for anti-SP/RBD IgG isotype titers via isotype-specific ELISAs (mean µg/mL ± SEM). *, p ≤ 0.05; ***, p ≤ 0.001; ****, p ≤ 0.0001; 2-tailed t-test (GraphPad Prism).
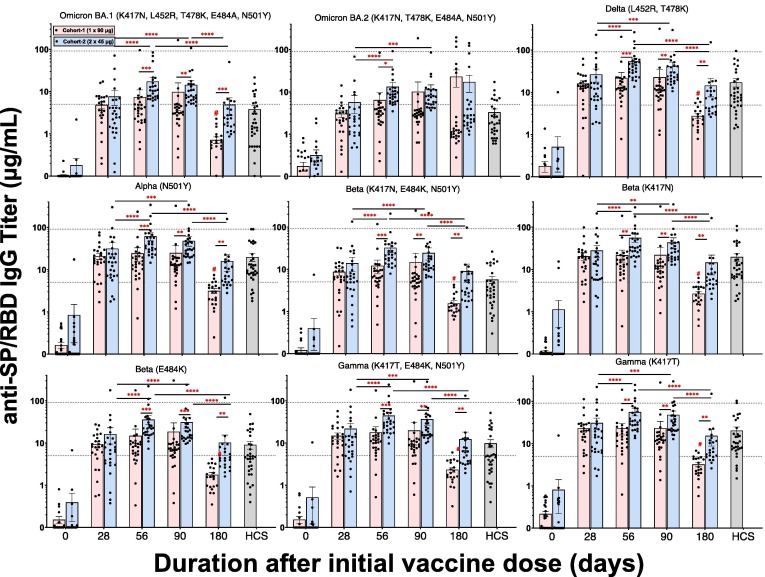
A series of mutant SP/RBD ELISAs were used to address whether the Original Washington strain (WT) SP/RBD antigenic sequence of AKS-452 induced IgG titers that bound mutant SP/RBD epitopes of currently known SARS-CoV-2 variant strains (Fig. 5 ). Similar to the kinetic profiles of IgG titers against the Original Washington SP/RBD (see Fig. 2), the second dose in cohort 2 significantly enhanced mean IgG titers against all mutant SP/RBDs by day 56 and were significantly greater than those of the respective cohort 1 means (Fig. 5). Day-180 titers against mutant SP/RBDs (except for Omicron BA.2) showed a substantial and significant decline in both cohorts relative to the respective day-56 titers, although cohort 1 showed a more substantial decline than cohort 2. Similar results after the second dose were also observed in phase I (Fig. S4; note that Omicron variant SP/RBDs were not available at the time of Phase I analyses). Mean IgG titers to Omicron BA.1 and Omicron BA.2 SP/RBD variants were consistently lower than those of other variants, including the Original Washington WT strain.
Fig. 5.
AKS-452 IgG titers against SARS-Cov-2 viral variants. Serum samples were obtained at Day 0, 28, 56, 90, and 180 from the initial vaccine dose from Cohorts 1 and 2 and assessed for IgG binding titers against SP/RBD from different SARS-CoV-2 variants via ELISA and presented per subject with mean ± SEM per cohort. For comparison, the dotted reference lines denote the highest and lowest mean titers from the Original Washington WT strain (see Fig. 2). HCS from unvaccinated individuals during the first wave of SARS-CoV-2 infection (i.e., Original Washington strain) was used as a comparator to samples from vaccinated subjects. For each viral variant, statistical comparisons between days “within a cohort” and “between cohorts” were performed using a model entailing “cohort” and “day” values as fixed effects and a “random subject effect” using log-transformed values in which p values were adjusted for multiplicity (Tukey-Kramer). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001; #, p ≤ 0.0001 vs. Cohort 1 days 28, 56, or 90.
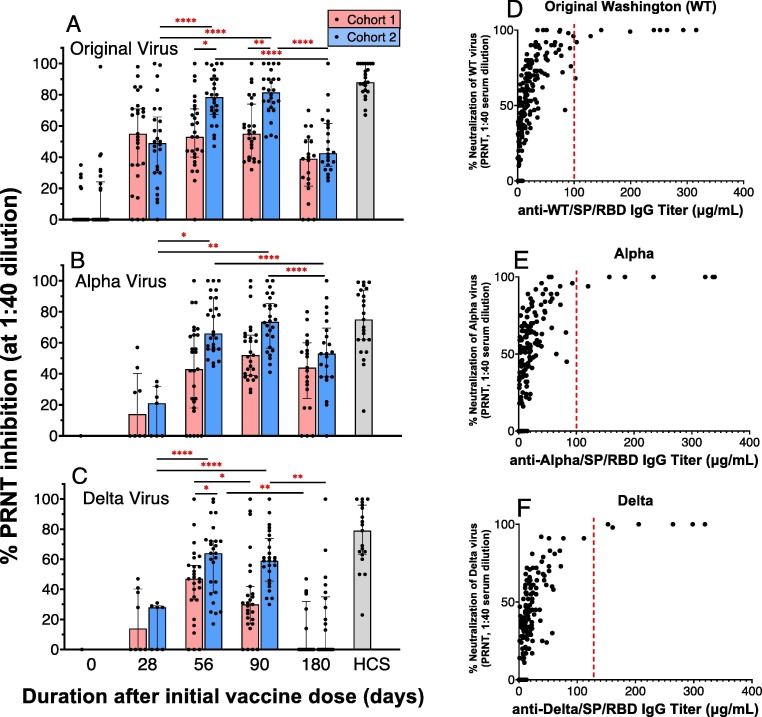
3.3. Live virus neutralization assay
Mean percent neutralization (at 1:40 dilution of serum) of the Original Washington WT, alpha variant, and delta variant viruses at days 28, 56, 90, and 180 of AKS-452 vaccination was assessed via the PRNT (Fig. 6 ). Consistent with IgG titer kinetic profiles (see Fig. 2, Fig. 3, Fig. 5), mean percent inhibition of the three viruses at days 56 and 90 after the second dose in cohort 2 were significantly elevated relative to the respective cohort 2 day-28 mean and were similar to HCS means (Fig. 6). While there was a trend towards greater mean neutralization values at day-56 and day-90 in cohort 2 relative to cohort 1, these differences were significant only in the Original strain at both days (Fig. 6 A) and on day 56 in the Delta strain (Fig. 6 C). Day-180 neutralization mean values of the Original (Fig. 6 A), Alpha (Fig. 6 B), Delta (Fig. 6 C) strains significantly declined in cohort 2 (but not in cohort 1) relative to the respective day-56 and day-90 values. In addition, cohort 1 and 2 neutralization potencies against the Original virus at day 28 were significantly greater (p < 0.01, 2-tailed t-test) than those of the Alpha and Delta viruses. A similar difference in neutralization potency at day 28 between the Original strain relative to the Alpha and Delta strains was observed in phase I (Fig. S5). While there was not a significant correlation between IgG titer and PRNT values (via linear regression analysis), there was a clear IgG titer cutoff of approximately 100 µg/mL above which viral neutralization was consistently 100% at the 1:40 dilution (red dotted lines in Fig. 6 D, E, and F).
Fig. 6.
AKS-452 serum neutralization of live virus, Plaque Reduction Neutralization Test (PRNT). Serum samples from Cohorts 1 (one 90 µg dose) and 2 (two 45 µg doses) obtained on Days, 0, 28, 56, 90, and 180 of initial vaccine dose were assessed for % neutralization (at 1:40 dilution of serum) of the Original Washington (A), Alpha (B) and Delta (C) live virus strains to infect live VERO E6 cells via the PRNT. For each viral variant (panels A, B, C), statistical comparisons between days “within a cohort” and “between cohorts” were performed using model entailing “cohort” and “day” values as fixed effects and a “random subject effect” using untransformed values in which p values were adjusted for multiplicity (Tukey-Kramer). *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. PRNT potency values were compared to IgG titers against the respective viral strain SP/RBD (D, E, F) to determine the IgG titer cutoff (∼100 µg/mL) that defined a consistent 100% viral neutralization (red dotted line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
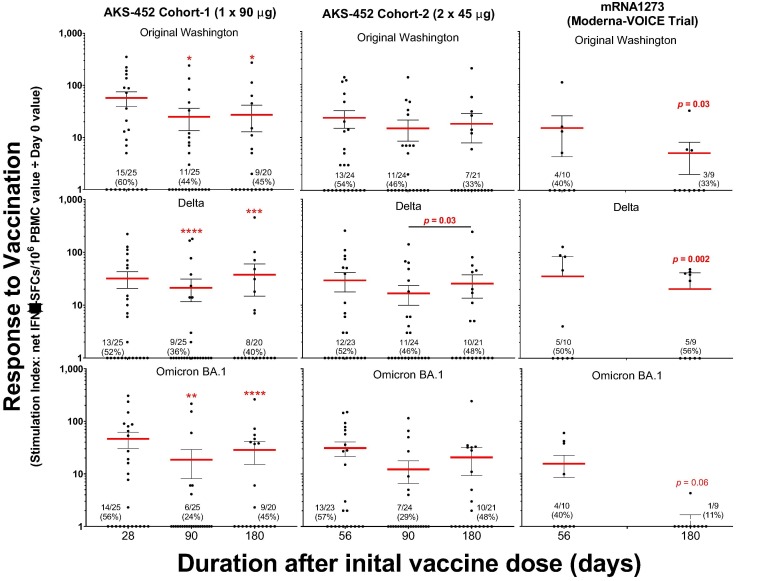
3.4. Cellular immunogenicity
IFN-γ-producing T cell responses specific to the SP/RBD antigens of the Original, Delta, and Omicron BA.1 strains were induced by AKS-452 vaccination in both cohorts (Fig. 7 ). The kinetic response pattern of mean SIs and proportion of positive responders by each of the three SP/RBD strain antigens demonstrated that the IFN-γ-producing T cell response was maintained throughout the study to day 180 with slight but significant declines in mean SIs after day 28 in cohort 1. In contrast, T cell IFN-γ responses induced by the mRNA1273 vaccine significantly declined by day 180, especially in response to the Omicron BA.1 SP/RBD antigen.
Fig. 7.
AKS-452 PBMC assessment of SP/RBD-specific T cell responses via IFN-γ ELISPOT assay. The IFN-γ ELISPOT assay was used to quantitate the frequency of SP/RBD-specific IFN-γ-producing T cells in PBMC samples stimulated by the SP/RBD of Original Washington, Delta, or Omicron SARS-CoV-2 strains. PBMC samples were obtained from subjects who received AKS-452 (cohorts 1 and 2) or mRNA 1273 (Moderna; 2 doses 28 days apart) vaccines collected at days 0, 28 (for cohort 1), 56 (for cohort 2), 90 and 180. For each subject’s sample, the mean of triplicate spot-forming cells (SFCs) of negative control DMSO cultures was subtracted from the mean of triplicate SFCs of SP/RBD-stimulated cultures to generate the net-SFC value. Reported is the ratio (Stimulation Index; SI) of the mean net-SFCs from each post-vaccination day divided by the mean net-SFC of the respective day 0 for each subject’s sample. A “positive responder” sample was defined as having an SI of at least 2 and an increase in the total number of net-SFCs ≥ 25 per 106 PBMCs, and the proportion (and percentage) are reported per cohort per timepoint. For each viral variant, log-transformed SI values of responders (log10 SI values > 1) were subjected to an ANOVA model with “cohort” and “day” as fixed effects and a “random subject effect” in which p values were adjusted for multiplicity (Tukey-Kramer between days within a cohort); *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; ****, p ≤ 0.0001. Comparisons for day 180 mean SI values among Cohorts 1, 2, and mRNA1273 were evaluated using a correction for multiplicity (Dunnett) that showed no significant differences.
3.5. Stimulation of IgG titers by SARS-CoV-2 Omicron variant infection after AKS-452 vaccination
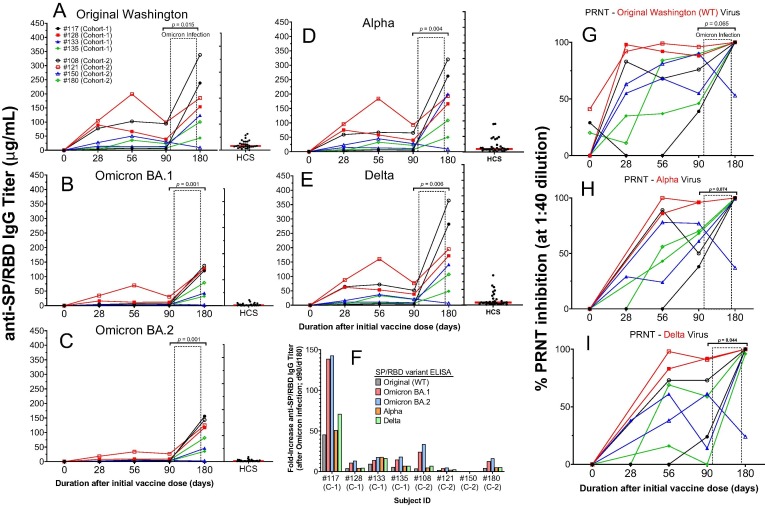
A total of eight subjects from phase II (four from cohort 1 and four from cohort 2) acquired a mild-moderate symptomatic SARS-CoV-2 infection after day 90 and before their final visit at day 180 during the recent European Omicron infection wave first announced 24 November 2021 [27]. Therefore, these subjects dropped out of the study after day 90 but before day 180, although each subject donated their day-180 blood sample for this analysis. While such day-180 serum and PBMC samples from these subjects were excluded from all other analyses, presentation of these data in a longitudinal context per subject demonstrated substantial induction of SP/RBD-specific IgG titers against Original Washington, Alpha, and Delta strains after infection in 7 of the 8 subjects (Fig. 8 A-E). These day-180 titers increased from the respective day-90 titers by 2- to 45-fold (Fig. 8 F). Infection also strongly elevated potency to neutralize each virus to 100% effectiveness in 7 of the 8 subjects via PRNT (Fig. 8 G, H, I). These data demonstrate that infection by SARS-CoV-2 virus after full vaccination strongly induced IgG titers well beyond those stimulated by vaccination alone, suggesting that vaccination can prime the immune system for such strong reactivity to infection. Indeed, the relatively low IgG titers of HCS from subjects who were infected (with the Original strain) without prior vaccination (Fig. 8 A-E) supports such a priming concept of vaccination.
Fig. 8.
Modulation of immunogenicity (IgG titers and neutralization potency) by SARS-CoV-2 Omicron variant infection after day 90 of 8 subjects in the AKS-452 Phase II study. Serum samples were obtained at Day 0, 28, 56, 90, and 180 after the initial vaccine dose from four cohort 1 subjects and four cohort 2 subjects who had acquired SARS-CoV-2 Omicron infection between their day 90 and 180 follow-up visits. Serum samples were assessed for IgG binding titers against SP/RBD from the Original Washington (A), Omicron B.1 (B), Omicron BA.2 (C), Alpha (D), and Delta (E) SARS-CoV-2 strains via ELISA and presented as the mean µg/mL of duplicate tests. The fold-change from day-90 to day-180 titers associated with infection is presented in panel F. Sera were also evaluated for potency to neutralize the Original (G), Alpha (H), and Delta (I) live virus strains from infecting VERO E6 cells via the PRNT. HCS from unvaccinated individuals during the first wave of the SARS-CoV-2 infection (i.e., Original Washington strain) was used as a comparator to samples from vaccinated subjects. For each viral variant and each parameter (IgG titer and PRNT), untransformed values of days 90 and 180 were compared (p values) using an ANOVA model with “day” as a fixed effect and a “random subject effect”.
To further understand the priming and boosting potential of AKS-452, there was an opportunity in phase I to offer subjects in the single dosing cohorts (i.e., cohort 1, 3, and 5 receiving 22.5, 45, and 90 µg, respectively) to receive a non-adjuvanted booster dose after their completion of the study on day 180. Five such subjects agreed to receive a second non-adjuvanted dose on day 180 and responded to a similar degree as those subjects receiving their second adjuvanted dose at day 28 during the trial (i.e., cohorts, 2, 4, and 6 [15]) in both IgG titers (Fig. S6 A, B, C) and live virus neutralization (Fig. S6 D, E, F), in addition to a dominant IgG1 response (Fig. S7). Subject #52 showed an abnormally high increase in titer only between 28 and 56 days after the booster dose (i.e., study days 208 to 236), which was correlated with a positive test of SARS-CoV-2 infection during that time. Also, the high titer of Subject #67 on the day of the booster dose and the non-responsiveness to this dose is consistent with the inverse correlation observed of subjects in the phase II cohort 2 (see Fig. 2 B).
4. Discussion
The overall safety assessment of AKS-452 in this 52-subject phase II clinical study (and of the predecessor phase I trial) showed limited side-effects in which no SAEs were reported, and the expected site injection symptoms were mild, self-resolving, and comparable to or less than those of other registered COVID-19 vaccines [28], [29]. The expected Injection Site Nodule AEs are attributed to the use of the water-in-oil adjuvant, Montanide™ ISA 720, necessary for AKS-452 to induce strong IFN-γ and IgG titer responses, as we have previously demonstrated in mice [14]. Although “oil-in-water” adjuvants (typically 50%/50% oil/water) have demonstrated major safety concerns with severe injection site reactions, the Montanide “water-in-oil” adjuvant contains the highly immunogenic squalene oil that allows less oil content in the formulation (i.e., 30%/70% oil/water) known to dramatically reduce reactogenicity in humans that has demonstrated a safe use during clinical trials conducted over 25 years [23], [24], [30], [31], [32], [33], [34].
Limitations of this study were the lack of a control groups receiving placebo, an active control vaccine, or a non-adjuvanted formulation of AKS-452. However, inclusion of a negative control placebo group was discouraged by regulatory authorities due to the ethical consideration of preventing subjects access to available EUA vaccines. Also, the objective of this study was not to address non-inferiority or superiority of AKS-452 in a comparator vaccine study, nor was it to merely demonstrate the immunogenic values of the Montanide adjuvant that has been demonstrated in animal studies with AKS-452 and in a vast number of other clinical studies, as mentioned above. Another limitation of this study is that the full kinetic profile of immunogenicity was not established beyond 180 days; i.e., while mean IgG titers trended towards a decline at 180 days, the seroconversion rate was still 100% within the 2-dose cohort 2. Additional studies are planned to address the extended duration of immunogenicity and efficacy of AKS-452.
Levels of anti-SP/RBD IgG titers and inhibitory potencies were higher in the two-dose regimen relative to those after the single-dose regimen, although all were within the range of convalescent sera. Indeed, HCS titers and potencies have been strongly associated with protection from SARS-CoV-2 infection [29]. Such IgG titers correlated well with ACE2-SP/RBD binding titers and neutralization with live virus. Importantly, the IgG1 isotype and IFN-γ-producing T cell responses induced by AKS-452 are consistent with the protective responses of the currently registered Emergency Use Authorization and regulatory-approved vaccines [35], [36]. While the SP/RBD-specific IgG1 and IgG3 titers were orders of magnitude greater than the IgG2 and IgG4 responses at all timepoints, the IgG2 and IgG4 isotype responses increased at day 90 despite the decrease in IgG1 and IgG3 isotype responses which appears to be a qualitative shift in the immune response to vaccination, a phenomenon that must be further investigated.
Although the Omicron viral strains were not tested in the PRNT due to the unavailability of laboratory stocks at the time of publication, strong anti-Omicron SP/RBD responses were apparent as measured by IgG titers via ELISAs and by IFN-γ-producing T cell responses via ELISPOT. While AKS-452-induced IgG titers to Omicron SP/RBD were approximately two- to three-fold less than those to the Original strain, such AKS-452-induced IgG titer responses are a significant improvement relative to those of the mRNA- and adenovirus-based vaccines that showed 33– to 44-fold decreases relative to the Original strain, i.e., BNT162b2 (Pfizer-BioNTech) and ChAdOx1 (AstraZeneca) [37], or completely lost reactivity to Omicron, i.e., Ad26.COV2.S (J&J Janssen) [38], and these IgG titers induced by SPs of the Original strain are known to have significantly reduced affinities to bind and neutralize Omicron virus from infection or re-infection due to SP/RBD mutations [27], [39]. Accordingly, Omicron SP/RBD-specific IFN-γ-producing T cell responses induced by AKS-452 were maintained during the full 6 months of the study among cohorts 1 and 2, but those induced by the mRNA1273 vaccine in healthy subjects (who participated in the VOICE trial) sharply declined during a similar period (see Fig. 7). Note, however, additional booster administrations of mRNA- and adenovirus-based vaccines have demonstrated increased neutralization toward Omicron, suggesting that activation of B cells against mutant SP/RBD epitopes could be accomplished by multiple vaccination doses [27], [40], [41]. Perhaps the underlying mechanism of greater reactivity to omicron with SP/RBD-based AKS-452 or with increased SP-based vaccine dosing may be related to the “original antigenic sin” phenomenon [42]. That is, an initial B cell antibody response during first exposure to viral antigen, whether from natural infection or vaccination, is known to dominate during subsequent exposures to mutated epitope(s) of the same virus. A consequence of this initial exposure is that the high-affinity memory B cells against the original antigenic epitopes can suppress development of new mutant epitope-specific B cells necessary for protection against the new viral variant, presumably via mechanisms of immunologic competition [42]. Such suppression might be more efficiently overcome via vaccination with very focused antigenic epitopes such as the RBD of AKS-452 rather than whole SP used in other vaccines. Consistent with this idea are clinical outcomes of an Original-specific SP/RBD-based vaccine that demonstrated equivalent titers against omicron BA5 and the original strain [43]. Indeed, others are evaluating the booster potency of an Omicron-specific SP/RBD-Fc antigen vaccine formulated in Alum [19], [20].
While cohort 1 and 2 IgG titers against most SARS-CoV-2 SP/RBD variants tended to decline after day 90 (i.e., at day 180), IFN-γ-producing T cell responses were maintained at day 180, supporting that the dynamics of IgG titers may not accurately reflect the true protective immune status of subjects receiving AKS-452. This protracted T cell response relative to IgG titers has been observed with other COVID vaccines in combination with natural immunity after SARS-CoV-2 infection [44]. Moreover, humoral (i.e., IgG) responses are associated with extracellular neutralization of viruses crucial in preventing infection whereas T cell responses recognize and eliminate virus-infected cells and therefore are associated with reduced disease severity, including reductions in hospitalizations and deaths [44]. T cell responses to the multitude of peptide epitopes of SARS-CoV-2 antigens are much more variable and numerous than those of B cell neutralizing antibodies such that protective T cell responses are not as sensitive to mutations of SARS-CoV-2 variants (reviewed in [44]). This may explain why T cell responses are associated with less severe disease even in subjects that were seronegative at the time of diagnosis (reviewed in [44]).
Results of these AKS-452 phase I/II studies support a strong booster capacity of AKS-452, whether in combination with itself or other vaccines, or with natural immunity after infection. The concept of boosting is based on a primed immune status caused by prior infection or vaccination. The Omicron infection acquired > day 90 after priming with one or two doses of AKS-452 caused a dramatic increase in IgG titers by day 180 in 7 of 8 subjects. Accordingly, the optional second non-adjuvanted AKS-452 dose given at day 180 to 5 voluntary “single-dose” subjects from phase I (cohorts, 1, 3, and 5) induced very rapid and robust responses 28 days later (day 208), demonstrating that a single dose of AKS-452 given 6 months earlier provided very strong priming. In fact, these day-180 booster responses induced without adjuvant were of similar magnitude to those induced at the scheduled day-28 “adjuvanted” dose of subjects in the phase I two-dose cohorts 2, 4, and 6, suggesting that boosting does not necessarily require adjuvant or need to occur within 28 days of the first (i.e., priming) injection to elicit a maximal booster response. Consistent with these observations are preclinical results demonstrating very strong responsiveness to non-adjuvanted AKS-452 of a non-human primate that had prior, but unnoticed, SARS-CoV-2 infection [14]. In addition, the enhanced vaccine immunogenicity with a protracted period prior to a booster dose has been demonstrated with approved mRNA- and adenovirus-based vaccines in which administration of a third booster dose 12 weeks after the last dose was significantly more effective than when given 2 to 4 weeks after the last dose [45]. Collectively, the non-adjuvanted AKS-452 vaccine may enable repeated booster injections to amplify the immune response against the SARS CoV-2 antigen to combat waning immunity, especially against more infective variants (e.g., delta and omicron) with higher affinity neutralizing antibodies.
In further understanding the boosting capacity of AKS-452, the possible reason for subject #67 in the phase I trial not responding to the booster (second) dose may be that the pre-existing high IgG titers (induced by the priming dose) bound and neutralized the SP/RBD-Fc antigenic component of AKS-452 that dampened its boosting potential. This was further explored via response correlations using IgG titers of the two-dose cohorts in phase I (cohort 2, 4, and 6) and phase II (cohort 2) that showed an inverse correlation between the titers on the day of the second dose (day 28) and the degree of response to such second dose (i.e., the ratio of day 56 titer to day 28 titer). An anti-SP/RBD IgG titer of 14 µg/mL was associated with a 3-fold responsiveness to the second dose that perhaps could be used to gauge when to administer the second dose such that optimal protective neutralizing titers are maintained. Collectively, these observations support that boosting should not occur during sufficiently high titers from prior dosing of vaccination. Boosting studies are currently in progress in AKS-452 clinical studies to better quantify the effect in a larger cohort and in people that received authorized vaccines (ClinicalTrials.gov Identifier: NCT05124483).
The acceptable safety profile, the 96% to 100% seroconversion rates, and inhibitory potencies consistent with that of HCS are successful outcomes of this phase I/II trial series. In addition, a sufficient scale-up of the GMP manufacturing process will enable production of a sufficiently large quantity of doses for larger clinical studies and future commercialization of AKS-452. Akston’s current estimates indicate that a single 2,000 L bioreactor production train run could yield enough material for such an expected 45 µg dose of drug substance (at approximately $1 production cost per dose) to treat approximately 100 million people receiving a single dose. A 2,000L bioreactor production train running ten times per year would therefore supply over 1 billion doses of AKS-452 at 45 µg per dose, creating an abundant vaccine resource world-wide including low- and middle-income countries. This manufacturing capacity is extremely significant and far surpasses the production throughput and costs of the other viral-based, nucleic acid-based, and full-length recombinant SP subunit-based vaccines. Therefore, potency, manufacturability, stability at easily achievable temperatures, and mechanism-of-action of the AKS-452 vaccine offer an opportunity to immunize a significant number of people globally to maintain high levels of immunity throughout the population regardless of COVID-19 status.
Funding statement
This trial was funded by Akston Biosciences Corp. (Beverly, MA), and not funded by any specific grant from funding agencies in the public or not-for-profit sectors.
CRediT authorship contribution statement
Eline A. Feitsma: Investigation, Methodology, Formal analysis, Writing – review & editing. Yester F. Janssen: Investigation, Methodology, Formal analysis, Writing – review & editing. Hendrikus H. Boersma: Investigation, Methodology, Writing – review & editing. Yannick van Sleen: Investigation, Formal analysis. Debbie van Baarle: Data curation. David G. Alleva: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing, Formal analysis, Data curation. Thomas M. Lancaster: Conceptualization, Investigation, Methodology. Thillainaygam Sathiyaseelan: Conceptualization, Investigation, Methodology. Sylaja Murikipudi: Conceptualization, Investigation, Methodology. Andrea R. Delpero: Investigation. Melanie M. Scully: Investigation. Ramya Ragupathy: Investigation. Sravya Kotha: Investigation. Jeffrey R. Haworth: Investigation. Nishit J. Shah: Investigation. Vidhya Rao: Investigation. Shashikant Nagre: Investigation. Shannon E. Ronca: Investigation, Methodology. Freedom M. Green: Investigation. Ari Aminetzah: Writing – review & editing. Frans Sollie: Formal analysis, Writing – review & editing. Schelto Kruijff: Investigation, Writing – review & editing. Maarten Brom: Writing – review & editing. Gooitzen M. van Dam: Conceptualization, Writing – review & editing. Todd C. Zion: Conceptualization, Writing – review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The infrastructure and material support from the Department of Nuclear Medicine and Molecular Imaging as well as the pharmaceutical support from the staff at the Department of Clinical Pharmacy and Pharmacology (UMCG) are greatly acknowledged. We thank, in particular, Professor Rudi Dierckx, Mrs. Gerda Bakker, Professor Bert Niesters, Mr. Roelof Bekkema, Mrs. Anouk Bos, and Mrs. Janneke Holthuis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.02.057.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Heaton P.M. The Covid-19 vaccine-development multiverse. N Engl J Med. 2020;383(20):1986–1988. doi: 10.1056/NEJMe2025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karnik M., et al. A review on SARS-CoV-2-induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Mol Neurobiol. 2021;58(9):4535–4563. doi: 10.1007/s12035-021-02399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos R., et al. Ad26 vector-based COVID-19 vaccine encoding a prefusion-stabilized SARS-CoV-2 Spike immunogen induces potent humoral and cellular immune responses. NPJ Vaccines. 2020;5:91. doi: 10.1038/s41541-020-00243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q., et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369(6499):77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization, W.H., Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. WHO Guidance Document, 2021. May 18th, 2021.
- 7.Thompson MG et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers - Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep 2021;70(13): 495-500. [DOI] [PMC free article] [PubMed]
- 8.Verbeke R., et al. The dawn of mRNA vaccines: the COVID-19 case. J Control Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia S., et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu F.C., et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramasamy M.N., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ewer K., et al. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum Vaccin Immunother. 2017;13(12):3020–3032. doi: 10.1080/21645515.2017.1383575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park K.S., et al. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alleva D.G., et al. Development of an IgG-Fc fusion COVID-19 subunit vaccine, AKS-452. Vaccine. 2021;39(45):6601–6613. doi: 10.1016/j.vaccine.2021.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen Y.F., et al. Phase I interim results of a phase I/II study of the IgG-Fc fusion COVID-19 subunit vaccine, AKS-452. Vaccine. 2022;40(9):1253–1260. doi: 10.1016/j.vaccine.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du L., et al. A truncated receptor-binding domain of MERS-CoV spike protein potently inhibits MERS-CoV infection and induces strong neutralizing antibody responses: implication for developing therapeutics and vaccines. PLoS One. 2013;8(12):e81587. doi: 10.1371/journal.pone.0081587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Y., et al. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J Immunol. 2005;174(8):4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- 18.Loureiro S et al. Virus Glycoproteins Tagged with the Human Fc Domain as Second Generation Vaccine Candidates. Innovation in Vaccinology: From Design, Through to Delivery and Testing, 2012. Chapter 3.
- 19.Luo D., et al. An updated RBD-Fc fusion vaccine booster increases neutralization of SARS-CoV-2 Omicron variants. Signal Transduct Target Ther. 2022;7(1):327. doi: 10.1038/s41392-022-01185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S., et al. Recombinant vaccine containing an RBD-Fc fusion induced protection against SARS-CoV-2 in nonhuman primates and mice. Cell Mol Immunol. 2021;18(4):1070–1073. doi: 10.1038/s41423-021-00658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M.Y., et al. Cross-reactive HIV-1-neutralizing activity of serum IgG from a rabbit immunized with gp41 fused to IgG1 Fc: possible role of the prolonged half-life of the immunogen. Vaccine. 2009;27(6):857–863. doi: 10.1016/j.vaccine.2008.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang N., et al. Identification of an ideal adjuvant for receptor-binding domain-based subunit vaccines against Middle East respiratory syndrome coronavirus. Cell Mol Immunol. 2016;13(2):180–190. doi: 10.1038/cmi.2015.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aucouturier J., et al. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1(1):111–118. doi: 10.1586/14760584.1.1.111. [DOI] [PubMed] [Google Scholar]
- 24.Ko J.H., et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 25.Park J.G., et al. Rapid in vitro assays for screening neutralizing antibodies and antivirals against SARS-CoV-2. J Virol Methods. 2021;287 doi: 10.1016/j.jviromet.2020.113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oosting S.F., et al. mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol. 2021;22(12):1681–1691. doi: 10.1016/S1470-2045(21)00574-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann M., et al. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447–456 e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q., et al. Evaluation of the safety profile of COVID-19 vaccines: a rapid review. BMC Med. 2021;19(1):173. doi: 10.1186/s12916-021-02059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khoury D.S., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 30.Herrera S., et al. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with montanide ISA 720 or montanide ISA 51. Am J Trop Med Hyg. 2011;84(2 Suppl):12–20. doi: 10.4269/ajtmh.2011.09-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Q., et al. Induction of multispecific Th-1 type immune response against HCV in mice by protein immunization using CpG and Montanide ISA 720 as adjuvants. Vaccine. 2008;26(43):5527–5534. doi: 10.1016/j.vaccine.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira G.A., et al. Safety and enhanced immunogenicity of a hepatitis B core particle Plasmodium falciparum malaria vaccine formulated in adjuvant Montanide ISA 720 in a phase I trial. Infect Immun. 2005;73(6):3587–3597. doi: 10.1128/IAI.73.6.3587-3597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles A.P., et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine. 2005;23(19):2530–2539. doi: 10.1016/j.vaccine.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence G.W., et al. Phase I trial in humans of an oil-based adjuvant SEPPIC MONTANIDE ISA 720. Vaccine. 1997;15(2):176–178. doi: 10.1016/s0264-410x(96)00150-8. [DOI] [PubMed] [Google Scholar]
- 35.Kober C., et al. IgG3 and IgM identified as key to SARS-CoV-2 neutralization in convalescent plasma pools. PLoS One. 2022;17(1):e0262162. doi: 10.1371/journal.pone.0262162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gray G., et al. Effectiveness of Ad26.COV2.S and BNT162b2 Vaccines against Omicron Variant in South Africa. N Engl J Med. 2022 doi: 10.1056/NEJMc2202061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt F., et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med. 2022;386(6):599–601. doi: 10.1056/NEJMc2119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M., Arora P., Pohlmann S. Understanding Omicron: transmissibility, immune evasion and antiviral intervention. Clin Transl Med. 2022;12(5):e839. doi: 10.1002/ctm2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abu-Raddad L.J., et al. Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–1816. doi: 10.1056/NEJMoa2200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarebska-Michaluk D., et al. COVID-19 vaccine booster strategies for Omicron SARS-CoV-2 variant: effectiveness and future prospects. Vaccines (MDPI) 2022;10:1–16. doi: 10.3390/vaccines10081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noori M., Nejadghaderi S.A., Rezaei N. “Original antigenic sin”: a potential threat beyond the development of booster vaccination against novel SARS-CoV-2 variants. Infect Control Hosp Epidemiol. 2022;43(8):1091–1092. doi: 10.1017/ice.2021.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C.Y., et al. A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against Delta and Omicron variants. J Clin Invest. 2022;132(10) doi: 10.1172/JCI157707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- 45.Belik M., et al. Comparative analysis of COVID-19 vaccine responses and third booster dose-induced neutralizing antibodies against Delta and Omicron variants. Nat Commun. 2022;13(1):2476. doi: 10.1038/s41467-022-30162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.