Abstract
Background
Commensal bacteria play an important role in the pathogenesis of inflammatory bowel disease (IBD) and probiotics have been used as treatment options. We aimed to explore the current use of probiotics and factors associated with their prescription in patients with IBD.
Methods
This cross-sectional study was conducted on a single hospital-based cohort. Patients were eligible if they were ≥18 years old, visited the IBD clinic as an outpatient more than twice during the study period, and had a confirmed diagnosis of IBD. Patients were divided into two groups based on the prescription of probiotics. Clinical assessments were compared between the two groups.
Results
In total, 217 patients were enrolled in this study. In patients with Crohn disease (CD), moderate or severe abdominal pain; prior use of methotrexate (MTX), iron, thiopurines, or biologics; history of IBD-related surgery; and stool frequency were independently associated with the prescription of probiotics. In patients with ulcerative colitis (UC), moderate or severe abdominal pain, hematochezia, stool frequency, and moderate or severe physician global assessment score were independently associated with the prescription of probiotics.
Conclusion
Increased disease activity may be associated with fewer prescriptions of probiotics in patients with IBD. However, physicians prescribed probiotics to control symptoms, such as abdominal pain and increased stool frequency in patients with UC and CD, and hematochezia in patients with UC. Additionally, the use of MTX and iron, and a history of IBD-related surgeries were associated with more frequent probiotic prescriptions in patients with CD.
Keywords: Crohn disease, Inflammatory bowel diseases, Prescriptions, Probiotics, Ulcerative colitis
Introduction
Inflammatory bowel disease (IBD), including Crohn disease (CD) and ulcerative colitis (UC), is a chronic relapsing disorder that causes immune-mediated gastrointestinal tract inflammation. Although the etiology remains unclear, it has been proposed that the pathogenesis of IBD results from interactions between genetic, environmental, intestinal barrier, and immune response factors [1]. Among these, intestinal luminal flora and related aberrant immunological responses are considered central factors in the development of IBD [2,3]. Evidence supports the hypothesis that the intestinal microbiota is involved in the pathogenesis of IBD. Animal models manipulated under germ-free conditions, defined as the absence of commensal flora, do not develop intestinal inflammation regardless of the strain, genetic background, or method used to induce inflammation [4]. In patients with IBD, serum antibodies against microorganisms have been used as parameters to diagnose or differentiate between CD and UC [5]. In patients with CD, high-level immune responses to microbial antigens are associated with severe disease activity or the occurrence of disease-related complications [6]. These findings highlight that commensal bacteria are essential for the initiation of intestinal inflammatory conditions and are closely associated with IBD pathogenesis.
Based on this evidence, many attempts have been made to use probiotics as a treatment option for IBD. Most studies have focused on the role of probiotics in the induction or maintenance of remission. Although results regarding the effectiveness of probiotics are controversial, most studies have not indicated that probiotics are harmful [7]. According to the Korean guidelines for CD and UC, probiotics are considered to play a minor role in the management of IBD. In the UC guideline, Escherichia coli Nissle 1917 is considered an alternative therapy when a patient is intolerant to 5-aminosalicylic acids during induction therapy [8]. In contrast, the use of probiotics was not mentioned in the CD guideline [9]. However, in real-life practice, probiotics are frequently prescribed to patients with IBD. However, few studies have evaluated the patterns, factors, or outcomes of probiotics prescribed by physicians. This study aimed to explore the current use of probiotics and factors associated with the prescription of probiotics in patients with IBD.
Methods
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Yonsei University Health Systems, Severance Hospital (IRB No: 4-2021-0539). There was no requirement for consent because this study involved only a retrospective medical chart review of anonymous patient data.
1. Study participants
This study was a cross-sectional, single hospital-based cohort study. Patients with CD and UC were recruited from outpatients at the IBD clinic in Severance Hospital in Seoul, Korea. The inclusion criteria were as follows: (1) age of ≥18 years; (2) attended the IBD clinic as an outpatient more than twice between December 2019 and November 2020; and (3) confirmed diagnosis established by clinical, radiological, endoscopic, and histopathologic criteria [10]. We excluded patients who procured probiotics without a prescription. The patients were divided into two groups according to the timing of the probiotic prescription. The probiotics group comprised patients who were prescribed probiotics for the first time during the study period after the diagnosis of IBD. The control group included patients with IBD who were not prescribed probiotics during the study period.
2. Probiotic formulation
The four probiotics reviewed in this study were as follows: (1) Lacidofil (Pharmbio Korea Co., Seoul, Korea), a powder of a mixed bacterial culture of Lactobacillus rhamnosus R0011 and Lactobacillus helveticus R0052 20 mg; (2) Medilac (Hanmi Pharma Co., Seoul, Korea), Enterococcus faecium and Bacillus subtilis culture 250 mg; (3) Ramnos (Hanwha Pharma Co., Seoul, Korea), freeze-dried Lactobacillus casei variety rhamnosus culture 250 mg; and (4) Bioflor (Kuhnil Pharma Co., Seoul, Korea), Saccharomyces boulardii 250 mg.
3. Clinical assessments
All patients visited the IBD clinic regularly based on a physician’s medical judgment. At each visit, the patients underwent clinical examinations, including the determination of physician global assessment (PGA), Crohn disease activity index (CDAI), partial Mayo score, drug compliance, gastrointestinal symptoms, extraintestinal manifestations, systemic symptoms, and laboratory findings. PGA and drug compliance were assessed by a physician based on his/her subjective judgment. PGA is one of the items comprising the UC disease activity index [11]. In this study, in addition to the CDAI and partial Mayo score, PGA was used to assess the disease activity of both CD and UC and was assessed based on a 4-point scale as follows: 0, remission; 1, mild; 2, moderate; and 3, severe. Laboratory tests included complete blood count, blood chemistry, C-reactive protein level, and erythrocyte sedimentation rate (ESR). In addition, we counted the number of days that patients consumed probiotics in the probiotics group. We reviewed the clinical assessments mentioned above at the initiation of probiotics in the probiotics group and at the first visit during the study period in the control group. The history of previous medications was defined as the history of medication use before the prescription of probiotics in the probiotics group and before the end of the study period in the control group. Biologic or small-molecule agents included infliximab, vedolizumab, adalimumab, ustekinumab, tofacitinib, and golimumab. Immunomodulators included methotrexate (MTX) and thiopurines, such as azathioprine (AZA) and 6-mercaptopurine (6-MP).
4. Statistical analysis
Descriptive analyses were performed to assess the differences in baseline characteristics between the probiotics and control groups. Quantitative data are expressed as arithmetic means±standard deviation, where appropriate. Categorical data are presented as total percentages. The chi-square test, Fisher exact test, and linear-by-linear association are used for categorical variables. The Student t-tests were used to compare continuous variables. In addition, we performed a binary logistic regression test to determine factors associated with the prescription of probiotics. Kaplan-Meier analysis was used to assess the proportion of patients prescribed probiotics, and the log-rank test was used to compare the Kaplan-Meier curves for CD and UC. All p-values are two-sided, and the results were considered statistically significant if the p-value was <0.05. Statistical analyses were conducted using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA).
Results
During the 1-year study period, 217 patients were enrolled in this study, of which 52 (24.0%) were included in the probiotics group. The baseline characteristics of the enrolled patients according to probiotic prescriptions are shown in Table 1. Lacidofil was the most frequently prescribed probiotic (40.4%), followed by Medilac (28.8%), Bioflor (25.0%), and Ramnos (5.8%). The probiotics group had a longer disease duration (p=0.020) and higher stool frequency (p=0.014) than the control group did. The proportion of patients who were diagnosed with CD (p<0.001), moderate or severe abdominal pain (p<0.001), and a history of IBD-related surgeries (p=0.002) was higher in the probiotics group than in the control group.
Table 1.
Comparison of baseline clinical characteristics between the probiotics and control groups
| Characteristic | Probiotics group | Control group | p-value |
|---|---|---|---|
| No. of patients | 52 | 165 | |
| Age (yr) | 34.5±15.7 | 38.5±13.1 | 0.069 |
| Sex | |||
| Male | 33 (63.5) | 108 (65.5) | 0.793 |
| Female | 19 (36.5) | 57 (34.5) | |
| Disease duration (yr) | 22.3±40.3 | 8.7±13.5 | 0.020 |
| Body mass index (kg/m2) | 22.2±3.9 | 22.6±3.6 | 0.492 |
| Duration of probiotics prescription (day) | 180.2±127.3 | ||
| Type of probiotics | |||
| Lacidofil | 21 (40.4) | ||
| Medilac | 15 (28.8) | ||
| Bioflor | 13 (25.0) | ||
| Ramnos | 3 (5.8) | ||
| Type of IBD | |||
| Crohn disease | 33 (63.5) | 54 (32.7) | <0.001 |
| Ulcerative colitis | 19 (36.5) | 111 (67.3) | |
| Physician global assessment | |||
| Remission or mild | 46 (88.5) | 153 (92.7) | 0.331 |
| Moderate or severe | 6 (11.5) | 12 (7.3) | |
| Stool frequency (/day) | 2.93±2.15 | 2.13±1.48 | 0.014 |
| Stool consistency | |||
| Normal | 31 (59.6) | 117 (70.9) | 0.127 |
| Loose stool or diarrhea | 21 (40.4) | 48 (29.1) | |
| Abdominal pain | |||
| Absence or mild | 38 (73.1) | 157 (95.2) | <0.001 |
| Moderate or severe | 14 (26.9) | 8 (4.8) | |
| Hematocheziaa) | |||
| Absence | 43 (84.3) | 127 (77.0) | 0.263 |
| Presence | 8 (15.7) | 38 (23.0) | |
| History of IBD-related surgeries | |||
| Presence | 18 (34.6) | 25 (15.2) | 0.002 |
| Absence | 34 (65.4) | 140 (84.8) | |
| Drug complianceb) | |||
| Good | 45 (90.0) | 146 (89.0) | 0.845 |
| Poor | 5 (10.0) | 18 (11.0) | |
| C-reactive protein (mg/L) | 10.5±17.31 | 4.17±12.3 | 0.027 |
| ESR (mm/hr) | 18.7±22.4 | 15.8±16.7 | 0.451 |
| Hemoglobin (g/dL) | 13.7±2.3 | 13.8±1.6 | 0.788 |
| Total protein (g/dL) | 7.2±0.6 | 11.6±55.5 | 0.599 |
Values are presented as number only, mean±standard deviation, or number (%).
IBD, inflammatory bowel disease; ESR, erythrocyte sedimentation rate.
Lacidofil: Pharmbio Korea Co., Seoul, Korea; Medilac: Hanmi Pharma Co., Seoul, Korea; Bioflor: Kuhnil Pharma Co., Seoul, Korea; Ramnos, Hanwha Pharma Co., Seoul, Korea.
One case was not reported in probiotics group.
Two cases and one case were not reported in probiotics group and control group, respectively.
1. Comparison of clinical characteristics between the probiotics and control groups in each IBD type
As each IBD type can have different clinical characteristics and treatment options, we analyzed these variables individually for each IBD type. Of the 217 enrolled patients, 87 (40.1%) were patients with CD, of whom 33 (37.9% of the total patients with CD) were prescribed probiotics (Table 2). The most frequently prescribed probiotics in patients with CD were Lacidofil (36.4%), followed by Ramnos (30.3%), Medilac (24.2%), and Bioflor (9.1%). In the univariate analysis, the patients with CD in the probiotics group showed greater stool frequency (p=0.002) and moderate or severe abdominal pain (p=0.001) than those in the control group. In terms of medications, the proportion of patients who had previously used thiopurines (p=0.010), MTX (p=0.018), or iron (p=0.027) was higher in the probiotics group than in the control group. There were 130 patients with UC (59.9%), and 19 of them (14.6% of the total patients with UC) were administered probiotics (Table 3). Similar to that observed in the patients with CD, Lacidofil (47.4%) was the most often prescribed probiotic in the patients with UC. Abdominal pain (p=0.002) was the only variable that showed a significant difference between the probiotics and control groups among the patients with UC.
Table 2.
Comparison of baseline clinical characteristics between the probiotics and control groups in patients with Crohn disease
| Characteristic | Probiotics group (n=33) | Control group (n=54) | p-valuea) | OR (95% CI) | p-valueb) |
|---|---|---|---|---|---|
| Age (yr) | 31.5±12.6 | 33.5±11.2 | 0.461 | ||
| Sex | |||||
| Male | 24 (72.7) | 42 (77.8) | 0.593 | ||
| Female | 9 (27.3) | 12 (22.2) | |||
| Disease duration (yr) | 22.6±40.1 | 9.1±15.8 | 0.074 | ||
| Body mass index (kg/m2) | 22.0±4.1 | 21.8±3.1 | 0.804 | ||
| Disease location | |||||
| Small bowel | 16 (48.5) | 14 (25.9) | 0.034 | ||
| Colon | 1 (3.0) | 2 (3.7) | |||
| Small bowel and colon | 16 (48.5) | 38 (70.4) | |||
| Disease behavior | |||||
| Inflammatory | 18 (54.5) | 33 (61.1) | 0.810 | ||
| Fistulating | 11 (33.3) | 13 (24.1) | |||
| Stenosis | 4 (12.1) | 8 (14.8) | |||
| Duration of probiotics prescription (day) | 196.1±134.5 | ||||
| Types of probiotics | |||||
| Lacidofil | 12 (36.4) | ||||
| Medilac | 8 (24.2) | ||||
| Bioflor | 3 (9.1) | ||||
| Ramnos | 10 (30.3) | ||||
| Fistula | |||||
| Presence | 18 (54.5) | 29 (53.7) | 0.939 | ||
| Absence | 15 (45.5) | 25 (46.3) | |||
| Physician global assessment | |||||
| Remission or mild | 29 (87.9) | 52 (96.3) | 0.133 | ||
| Moderate or severe | 4 (12.1) | 2 (3.7) | |||
| CDAI | |||||
| <150 | 29 (87.9) | 52 (96.3) | 0.133 | ||
| Mildc) | 4 (12.1) | 2 (3.7) | |||
| Stool frequency (/day) | 2.8±1.8 | 1.7±1.0 | 0.002 | 2.458 (1.381–4.374) | 0.002 |
| Stool consistency | |||||
| Normal | 21 (63.6) | 39 (72.2) | 0.401 | ||
| Loose stool or diarrhea | 12 (36.4) | 15 (27.8) | |||
| Abdominal pain | |||||
| Absence or mild | 24 (72.7) | 52 (96.3) | 0.001 | 1 | |
| Moderate or severe | 9 (27.3) | 2 (3.7) | 81.846 (5.707–173.788) | 0.001 | |
| History of IBD-related surgeries | |||||
| Presence | 18 (54.5) | 19 (35.2) | 0.076 | 4.588 (1.233–7.068) | 0.023 |
| Absence | 15 (45.5) | 35 (64.8) | 1 | ||
| Drug complianced) | |||||
| Good | 28 (87.5) | 48 (88.9) | 0.846 | ||
| Poor | 4 (12.5) | 6 (11.1) | |||
| Prior biologics use | |||||
| Presence | 12 (36.4) | 20 (37.0) | 0.950 | 0.158 (0.030–0.825) | 0.029 |
| Absence | 21 (63.6) | 34 (63.0) | 1 | ||
| Prior oral 5-ASA use | |||||
| Presence | 33 (100) | 50 (92.6) | 0.109 | ||
| Absence | 0 (0) | 4 (7.4) | |||
| Prior topical 5-ASA use | |||||
| Presence | 3 (9.1) | 2 (3.7) | 0.295 | ||
| Absence | 30 (90.9) | 52 (96.3) | |||
| Prior thiopurine use | |||||
| Presence | 21 (63.6) | 47 (87.0) | 0.010 | 0.206 (0.048–0.891) | 0.035 |
| Absence | 12 (36.4) | 7 (13.0) | 1 | ||
| Prior corticosteroid use | |||||
| Presence | 15 (45.5) | 31 (57.4) | 0.278 | ||
| Absence | 18 (54.5) | 23 (42.6) | |||
| Prior methotrexate use | |||||
| Presence | 5 (15.2) | 1 (1.9) | 0.018 | 31.702 (2.016–498.540) | 0.014 |
| Absence | 28 (84.8) | 53 (98.1) | 1 | ||
| Prior iron use | |||||
| Presence | 14 (42.4) | 11 (20.4) | 0.027 | 15.054 (2.963–76.475) | 0.001 |
| Absence | 19 (57.6) | 43 (79.6) | 1 | ||
| Prior psychotropic drug use | |||||
| Presence | 3 (9.1) | 1 (1.9) | 0.118 | ||
| Absence | 30 (90.9) | 53 (98.1) | |||
| C-reactive protein (mg/L) | 11.9±18.3 | 7.7±19.4 | 0.319 | ||
| ESR (mm/hr) | 17.3±22.7 | 18.1±21.1 | 0.870 | ||
| Hemoglobin (g/dL) | 13.7±2.5 | 13.7±1.8 | 0.938 | ||
| Total protein (g/dL) | 7.0±0.6 | 7.1±0.5 | 0.683 |
Values are presented as mean±standard deviation or number (%) unless otherwise specified.
OR, odds ratio; CI, confidence interval; CDAI, Crohn disease activity index; IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylic acid; ESR, erythrocyte sedimentation rate.
Lacidofil: Pharmbio Korea Co., Seoul, Korea; Medilac: Hanmi Pharma Co., Seoul, Korea; Bioflor: Kuhnil Pharma Co., Seoul, Korea; Ramnos, Hanwha Pharma Co., Seoul, Korea.
Mild CDAI was defined as CDAI of 150–220.
Logistic regression.
Mild CDAI was defined as CDAI of 150-220.
One case was not reported in probiotics group of Crohn disease.
Table 3.
Comparison of baseline clinical characteristics between the probiotics and control groups in patients with ulcerative colitis
| Characteristic | Probiotics group (n=19) | Control group (n=111) | p-valuea) | OR (95% CI) | p-valueb) |
|---|---|---|---|---|---|
| Age (yr) | 39.6±19.2 | 40.9±13.3 | 0.778 | ||
| Sex | |||||
| Male | 9 (47.4) | 66 (59.5) | 0.324 | ||
| Female | 10 (52.6) | 45 (40.5) | |||
| Disease duration (yr) | 21.8±41.7 | 8.5±12.4 | 0.183 | ||
| Body mass index (kg/m2) | 22.6±3.6 | 23.0±3.7 | 0.685 | ||
| Disease location | |||||
| Proctosigmoiditis | 11(57.9) | 51 (45.9) | 0.328 | ||
| Left-sided | 1 (5.3) | 16 (14.4) | |||
| Extensive | 7 (36.8) | 44 (39.6) | |||
| Duration of probiotics prescription (day) | 152.6±111.7 | ||||
| Types of probiotics | |||||
| Lacidofil | 9 (47.4) | ||||
| Medilac | 7 (36.8) | ||||
| Bioflor | 3 (15.8) | ||||
| Ramnos | 0 (0) | ||||
| Physician global assessment | |||||
| Remission or mild | 17 (89.5) | 101 (91.0) | 0.688 | 1 | |
| Moderate or severe | 2 (10.5) | 10 (9.0) | 0.006 (0.000–0.291) | 0.010 | |
| Partial Mayo score | |||||
| Remission | 8 (42.1) | 75 (67.6) | 0.156 | ||
| Mild | 9 (47.4) | 26 (23.4) | |||
| Moderate | 1 (5.3) | 6 (5.4) | |||
| Severe | 1 (5.3) | 4 (3.6) | |||
| Stool frequency (/day) | 3.2±2.7 | 2.3±1.6 | 0.180 | 2.069 (1.256–3.409) | 0.004 |
| Stool consistency | |||||
| Normal | 10 (52.6) | 78 (70.3) | 0.129 | 1 | |
| Loose stool or diarrhea | 9 (47.4) | 33 (29.7) | 0.088 (0.007–1.137) | 0.063 | |
| Abdominal pain | |||||
| Absence or mild | 14 (73.7) | 105 (94.6) | 0.002 | 1 | |
| Moderate or severe | 5 (26.3) | 6 (5.4) | 44.705 (3.683–542.598) | 0.003 | |
| Hematocheziac) | |||||
| Absence | 11 (61.1) | 83 (74.8) | 0.227 | 1 | |
| Presence | 7 (38.9) | 28 (25.2) | 10.479 (1.042–105.376) | 0.046 | |
| History of IBD-related surgeries | |||||
| Presence | 0 (0) | 6 (5.4) | 0.299 | ||
| Absence | 19 (100) | 105 (94.6) | |||
| Drug complianced) | |||||
| Good | 17 (94.4) | 98 (89.1) | 0.486 | ||
| Poor | 1 (5.6) | 12 (10.9) | |||
| Prior biologics use | |||||
| Presence | 3 (15.8) | 18 (16.2) | 0.963 | ||
| Absence | 16 (84.2) | 93 (83.8) | |||
| Prior oral 5-ASA use | |||||
| Presence | 18 (94.7) | 106 (95.5) | 0.884 | ||
| Absence | 1 (5.3) | 5 (4.5) | |||
| Prior topical 5-ASA use | |||||
| Presence | 15 (78.9) | 90 (81.1) | 0.827 | ||
| Absence | 4 (21.1) | 21 (18.9) | |||
| Prior thiopurine use | |||||
| Presence | 3 (15.8) | 33 (29.7) | 0.210 | 0.214 (0.035–1.301) | 0.094 |
| Absence | 16 (84.2) | 78 (70.3) | 1 | ||
| Prior corticosteroid use | |||||
| Presence | 11 (57.9) | 51 (45.9) | 0.335 | ||
| Absence | 8 (42.1) | 60 (54.1) | |||
| Prior iron use | |||||
| Presence | 1 (5.3) | 9 (8.1) | 0.667 | ||
| Absence | 18 (94.7) | 102 (91.9) | |||
| Prior psychotropic drug use | |||||
| Presence | 2 (10.5) | 5 (4.5) | 0.283 | ||
| Absence | 17 (89.5) | 106 (95.5) | |||
| C-reactive protein (mg/L) | 6.2±13.8 | 2.2±4.6 | 0.367 | ||
| ESR (mm/hr) | 22.8±22.0 | 14.5±13.6 | 0.088 | ||
| Hemoglobin (g/dL) | 13.8±1.9 | 13.9±1.5 | 0.810 | ||
| Total protein (g/dL) | 7.5±0.7 | 13.9±68.0 | 0.756 |
Values are presented as mean±standard deviation or number (%) unless otherwise specified.
OR, odds ratio; CI, confidence interval; IBD, inflammatory bowel disease; 5-ASA, 5-aminosalicylic acid; ESR, erythrocyte sedimentation rate.
Lacidofil: Pharmbio Korea Co., Seoul, Korea; Medilac: Hanmi Pharma Co., Seoul, Korea; Bioflor: Kuhnil Pharma Co., Seoul, Korea; Ramnos, Hanwha Pharma Co., Seoul, Korea.
Chi-square test.
Logistic regression.
One case was not reported in probiotics group of ulcerative colitis.
One case was not reported in both probiotics and control group.
2. Factors associated with probiotic prescriptions in patients with IBD
Multivariate analysis using a binary logistic regression model was performed to determine the factors associated with probiotic prescriptions. We included all variables that demonstrated either a statistically significant difference in the univariate analysis or were clinically related to probiotic prescriptions.
In patients with CD, logistic regression revealed that a moderate or severe abdominal pain (odds ratio [OR], 81.846; 95% confidence interval [CI], 5.707–1173.788; p=0.001), prior MTX use (OR, 31.702; 95% CI, 2.016–498.540; p=0.014), prior iron use (OR, 15.054; 95% CI, 2.963–76.475; p=0.001), history of IBD-related surgeries (OR, 4.588; 95% CI, 1.233–17.068; p=0.023), stool frequency (OR, 2.458; 95% CI, 1.381–4.374; p=0.002), prior thiopurine use (OR, 0.206; 95% CI, 0.048–0.891; p=0.035), and prior biologics use (OR, 0.158; 95% CI, 0.030–0.825; p=0.029) were independently associated with prescribing probiotics (Table 2).
Moderate or severe abdominal pain (OR, 44.705; 95% CI, 3.683–542.598; p=0.003), hematochezia (OR, 10.479; 95% CI, 1.042–105.376; p=0.046), stool frequency (OR, 2.069; 95% CI, 1.256–3.409; p=0.004), and moderate or severe PGA (OR, 0.006; 95% CI, 0.000–0.291; p=0.010) were independently associated with prescribing probiotics in patients with UC (Table 3).
3. Proportion of patients who were prescribed probiotics
After plotting Kaplan-Meier curves, we compared the proportion of patients who continued probiotic use, among those who were prescribed probiotics, between the different IBD types by log-rank test. There was no significant difference (p=0.474) between the two curves (Fig. 1).
Fig. 1.
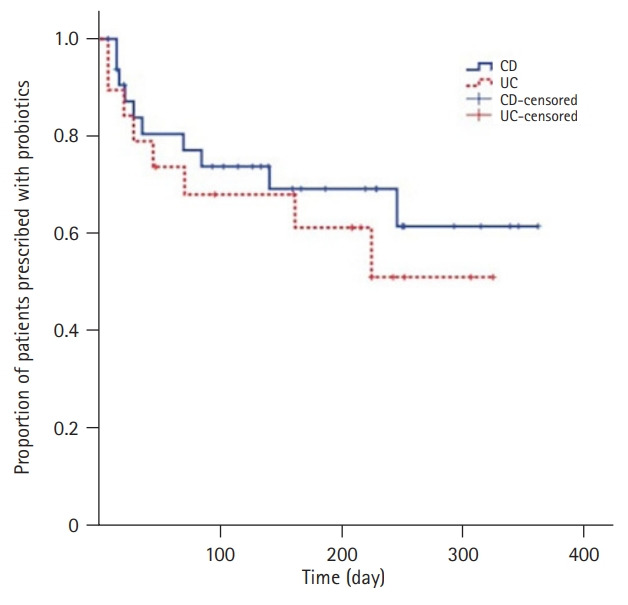
Proportion of patients who maintained use of probiotics among those prescribed probiotics stratified by the type of inflammatory bowel disease. CD, Crohn disease; UC, ulcerative colitis.
Discussion
Few studies have reported the factors associated with the prescription of probiotics to patients with IBD by physicians in clinical practice. Most previous studies have focused on either the effects of probiotics on disease activity or the evaluation of their efficacy in inducing or maintaining remission. Although some studies have demonstrated beneficial effects, others have not shown any positive effects of probiotics in reducing disease activity [7]. Because of these inconsistent results, the Korean guidelines for UC recommend only a certain probiotic strain for maintaining, but not inducing, remission in patients intolerant to 5-aminosalicylic acid [8]. However, the Korean guidelines for CD do not mention the role of probiotics in this disease [9]. However, probiotics are frequently consumed by patients with IBD [12]. Thus, we evaluated the current status of the use of probiotics and the factors associated with their prescription in clinical practice.
In this study, probiotics were more frequently prescribed to patients with CD than to those with UC. Although some studies showed contrasting results, anxiety episodes were more prevalent [13], and the proportion of complementary and alternative medicine users was higher among patients with CD than among those with UC [14]. The differences in probiotic prescriptions according to disease type may reflect the characteristics of patients with CD. However, the proportion of patients who maintained probiotic use among those who were prescribed probiotics did not differ between the patients with CD and those with UC.
Both moderate or severe abdominal pain and stool frequency were associated with a high likelihood of probiotic prescription in patients with CD and in those with UC. In addition, hematochezia was associated with a high likelihood of probiotic prescription in patients with UC. Some studies have demonstrated the positive effects of probiotics on such gastrointestinal symptoms in UC. Patients receiving Bifidobacterium longum 536 or VSL#3 as an adjunct to standard treatment showed a significant decrease in rectal bleeding [15,16]. Probiotic users who were administered a multispecies probiotic including Bifidobacterium and Lactobacillus were more likely to experience a reduction in stool frequency and abdominal pain as well as an improvement in stool texture [17]. When combined with mesalazine (i.e., 5-aminosalicylic acid), probiotics also led to a significant improvement in rectal bleeding and stool frequency compared with that of mesalazine alone [18]. Although data on the role of probiotics in CD are scarce, some studies have shown improvements in gastrointestinal symptoms in patients with CD who consume probiotics. A yeast preparation of S. boulardii was shown to reduce the frequency of bowel movements [19]. Probiotic and prebiotic combined therapy, consisting of Bifidobacterium breve, L. casei, B. longum, and psyllium (Plantago ovata), significantly reduced the daily incidence of diarrhea and the abdominal pain index [20]. In contrast, Bifidobacterium in the form of fermented milk products did not show any effects on abdominal symptoms, stool frequency, or rectal bleeding [21]. Some studies have reported side effects of probiotics, such as abdominal pain, in patients with IBD [22]. Although probiotics may not be effective for some gastrointestinal symptoms or they may show side effects, physicians can expect some positive effects of probiotics and prescribe them to improve symptoms.
A high PGA score did not lead to increased prescription of probiotics by physicians. Moderate or high PGA scores were negatively associated with prescribing probiotics to patients with UC. PGA is an important component in evaluating disease activity, such as the UC disease activity index, and correlates well with disease activity [23]. Physicians may prescribe probiotics to patients with UC to relieve gastrointestinal symptoms, such as abdominal pain, hematochezia, or increased stool frequency, but not to control disease activity. Abdominal pain, increased stool frequency, and hematochezia are related to disease activity; however, they can occur even if disease activity does not increase. For example, the disease activity of IBD can remain unchanged, but the above symptoms may occur with gastroenteritis, infectious colitis, or irritable bowel syndrome. Therefore, it can be inferred that probiotics tend to be used when symptoms, which are not related to increased disease activity, worsen. In addition, even if objective indicators such as endoscopic findings are useful for assessing disease activity, patients with IBD often complain of subjective gastrointestinal or irritable bowel syndrome symptoms. Controlling these subjective symptoms as well as disease activity is important. Physicians tend to take this into account when prescribing probiotics.
Surgical treatment is often considered in patients with IBD. In patients with CD, the 10-year surgery risk was 46.6% [24], and surgery was indicated in cases of bowel perforation, abscesses, massive hemorrhage, cancer development, or bowel obstruction not alleviated by medical therapies [25]. In the present study, a history of IBD-related surgery was associated with probiotic prescriptions in patients with CD. Previous studies have focused on the role of probiotics in the prophylaxis of postoperative CD recurrence with contradictory results. Treatment with VSL#3 within 30 days after ileal resection and reanastomosis resulted in a reduction in severe endoscopic recurrence by day 90 [26]. In addition, when combined with antibiotics, probiotics could be efficacious in the prophylaxis of postoperative recurrence of CD [27]. In contrast, some studies showed the ineffectiveness of probiotics in preventing recurrence after surgery for CD [28,29]. Although there was no information on the temporal relationship between the administration of probiotics, surgery, and concomitant medications in this study, probiotics were used to prevent recurrence after surgery. From a psychiatric point of view, patients with CD who underwent IBD-related surgery had a risk of anxiety [30,31]. Physicians may prescribe probiotics to reduce such neurotic symptoms.
The past use of certain medications was associated with the prescription of probiotics in patients with CD. Patients with prior MTX or iron prescriptions were prescribed a significantly higher number of probiotics than patients without these prior medications. MTX is an anti-metabolite known to induce intestinal mucositis. MTX can induce or maintain remission in CD [9]. However, its use is limited by low efficacy and common side effects such as abdominal pain, diarrhea, nausea, and vomiting [32]. Although no prior studies have evaluated the effects of probiotics in MTX-treated patients with CD, some studies have examined the impact of probiotics in animal models. In rats with MTX-induced intestinal mucositis, probiotics helped maintain the mucosal barrier and improve intestinal permeability and tissue architecture following MTX-induced small intestinal mucositis [33]. Contrary to the effect of MTX on disease activity, iron-deficiency anemia, a common hematologic complication in CD, is often treated with iron supplementation. However, oral iron intake may intensify tissue damage and disease activity of the underlying IBD due to oxidative stress [34]. In addition, oral iron supplementation may aggravate inflammation in IBD by altering gut microbial composition, which plays an important role in IBD [35]. Another study that used an animal model indicated the possibility of a protective effect of probiotic administered in combination with iron supplementation in IBD. Specifically, the probiotic E. coli Nissle 1917 outcompeted pathogenic bacteria in a dextran sodium sulfate-induced colitis model [35]. The absorption of iron can also be increased in combination with probiotics. In a previous study, freeze-dried Lactobacillus plantarum 299v was shown to enhance iron absorption when administered with a meal catered to ensure high iron bioavailability [36]. In this context, physicians may prescribe probiotics in combination with iron supplementation. The development of iron-deficiency anemia is related to disease activity, as blood loss is triggered by intestinal inflammation [37]. MTX may be used in patients with moderate-to-severe CD [9]. In other words, iron and MTX were used more often in patients with severe inflammation. Physicians should consider prescribing probiotics to reduce inflammation in patients with severe symptoms.
A history of biologics and thiopurine use, including AZA or 6-MP, was associated with lower probiotic prescriptions in patients with CD. Infliximab and adalimumab are generally used to induce and maintain remission in moderate-to-severe CD [9]. Vedolizumab and ustekinumab also induce response and remission in patients with moderately to severely active CD who are unresponsive to either tumor necrosis factor antagonists or conventional therapy [38,39]. Thiopurine alone is not recommended as an induction therapy but can be used with infliximab in moderate-to-severe CD for induction [9]. It can also be used as a maintenance therapy when induction is achieved with systemic steroids [9]. In other words, biologic drugs and thiopurines are indicated for moderate-to-severe CD. These findings reveal that probiotics are not generally prescribed to patients with moderate-to-severe CD. This tendency can be consistent with UC, in which moderate or severe PGA had a negative relationship with probiotic prescriptions in the present study. The use of biologics could also eliminate the need for probiotics. Patients receiving biologics reported a high degree of satisfaction with their therapy and appeared physically and emotionally healthier than their counterparts who did not receive biologics [40].
This study had some limitations. First, regarding the medication data, previously used medications were considered and not concomitant prescriptions. Thus, it is unclear whether these medications were associated with the prescription of probiotics in the probiotics group. Second, over-the-counter use of probiotics was not considered in this study. Probiotics are often considered over-the-counter drugs and do not require prescriptions. Thus, physicians may not have prescribed probiotics to patients who were already consuming them. Third, the factors associated with probiotic prescriptions were assessed retrospectively based on medical charts. Since this study was retrospectively designed, we were not able to follow up on changes in gastrointestinal symptoms after using probiotics. Fourth, the probiotics were prescribed based on the subjective symptoms of the patients and at the discretion of the physician, which might have affected the results.
In conclusion, increased disease activity might be associated with lower probiotic prescriptions in both patients with UC and those with CD. In contrast, physicians prescribe probiotics more frequently in patients who complain of abdominal pain and increased stool frequency in both UC and CD, as well as in UC patients experiencing hematochezia. Some medications, such as MTX and iron supplements, and a history of IBD-related surgeries were also associated with more probiotic prescriptions in patients with CD. Although there have been studies that have shown the usefulness of probiotics in patients with IBD, there is still a lack of consistent opinions concerning their effectiveness. Thus, rather than determining the effectiveness of probiotics in patients with IBD, we believe that it is important to identify the factors for which patients are more likely to be prescribed probiotics. In particular, the results of this study are meaningful considering that probiotics are prescribed when patients have subjective symptoms, even though the disease activity of IBD is well controlled.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization, Validation, Supervision: JHC; Methodology, Formal analysis, Investigation: JHC, JKK; Software, Data curation: JKK; Writing-original draft: JKK; Writing-review & editing: JHC; Approval of final manuscript: all authors.
References
- 1.Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–65. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizoguchi E, Low D, Ezaki Y, Okada T. Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models. Intest Res. 2020;18:151–67. doi: 10.5217/ir.2019.09154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 5.Norouzinia M, Chaleshi V, Alizadeh AH, Zali MR. Biomarkers in inflammatory bowel diseases: insight into diagnosis, prognosis and treatment. Gastroenterol Hepatol Bed Bench. 2017;10:155–67. [PMC free article] [PubMed] [Google Scholar]
- 6.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Jakubczyk D, Leszczyńska K, Górska S. The effectiveness of probiotics in the treatment of inflammatory bowel disease (IBD): a critical review. Nutrients. 2020;12:1973. doi: 10.3390/nu12071973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi CH, Moon W, Kim YS, Kim ES, Lee BI, Jung Y, et al. Second Korean guidelines for the management of ulcerative colitis. Intest Res. 2017;15:7–37. doi: 10.5217/ir.2017.15.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JJ, Yang SK, Ye BD, Kim JW, Park DI, Yoon H, et al. Second Korean guidelines for the management of Crohn’s disease. Intest Res. 2017;15:38–67. doi: 10.5217/ir.2017.15.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13:144–64. doi: 10.1093/ecco-jcc/jjy113. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland LR, Martin F, Greer S, Robinson M, Greenberger N, Saibil F, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–8. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim SB, Park SJ, Chung SH, Hahn KY, Moon DC, Hong SP, et al. Vaccination and complementary and alternative medicine in patients with inflammatory bowel disease. Intest Res. 2014;12:124–30. doi: 10.5217/ir.2014.12.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irving P, Barrett K, Nijher M, de Lusignan S. Prevalence of depression and anxiety in people with inflammatory bowel disease and associated healthcare use: population-based cohort study. Evid Based Ment Health. 2021;24:102–9. doi: 10.1136/ebmental-2020-300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opheim R, Hoivik ML, Solberg IC, Moum B; IBSEN Study Group. Complementary and alternative medicine in patients with inflammatory bowel disease: the results of a population-based inception cohort study (IBSEN) J Crohns Colitis. 2012;6:345–53. doi: 10.1016/j.crohns.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, et al. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. 2016;28:67–74. doi: 10.1111/den.12553. [DOI] [PubMed] [Google Scholar]
- 16.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–27. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Waal MB, Flach J, Browne PD, Besseling-van der Vaart I, Claassen E, van de Burgwal LH. Probiotics for improving quality of life in ulcerative colitis: exploring the patient perspective. Pharmanutrition. 2019;7:100139. [Google Scholar]
- 18.Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P, Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:372–7. doi: 10.5507/bp.2016.044. [DOI] [PubMed] [Google Scholar]
- 19.Plein K, Hotz J. Therapeutic effects of Saccharomyces boulardii on mild residual symptoms in a stable phase of Crohn’s disease with special respect to chronic diarrhea: a pilot study. Z Gastroenterol. 1993;31:129–34. [PubMed] [Google Scholar]
- 20.Fujimori S, Tatsuguchi A, Gudis K, Kishida T, Mitsui K, Ehara A, et al. High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. J Gastroenterol Hepatol. 2007;22:1199–204. doi: 10.1111/j.1440-1746.2006.04535.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsuoka K, Uemura Y, Kanai T, Kunisaki R, Suzuki Y, Yokoyama K, et al. Efficacy of bifidobacterium breve fermented milk in maintaining remission of ulcerative colitis. Dig Dis Sci. 2018;63:1910–9. doi: 10.1007/s10620-018-4946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dore MP, Bibbò S, Fresi G, Bassotti G, Pes GM. Side effects associated with probiotic use in adult patients with inflammatory bowel disease: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2019;11:2913. doi: 10.3390/nu11122913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jitsumura M, Kokelaar RF, Harris DA. Remission endpoints in ulcerative colitis: a systematic review. World J Metaanal. 2017;5:85–102. [Google Scholar]
- 24.Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Ueno F, Matsui T, Matsumoto T, Matsuoka K, Watanabe M, Hibi T, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48:31–72. doi: 10.1007/s00535-012-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clin Gastroenterol Hepatol. 2015;13:928–35. doi: 10.1016/j.cgh.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Campieri M, Rizzello F, Venturi A, Poggioli G, Ugolini F. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn’s disease: a randomized controlled study vs mesalamine. Gastroenterology. 2000;118:A781. [Google Scholar]
- 28.Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn’s disease: a randomised controlled trial with Lactobacillus GG. Gut. 2002;51:405–9. doi: 10.1136/gut.51.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: a randomised, double blind, placebo controlled GETAID trial. Gut. 2006;55:842–7. doi: 10.1136/gut.2005.076604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao X, Tang Y, Lei N, Luo Y, Chen P, Liang C, et al. Symptoms of anxiety/depression is associated with more aggressive inflammatory bowel disease. Sci Rep. 2021;11:1440. doi: 10.1038/s41598-021-81213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maconi G, Gridavilla D, Viganò C, Sciurti R, Asthana AK, Furfaro F, et al. Perianal disease is associated with psychiatric co-morbidity in Crohn’s disease in remission. Int J Colorectal Dis. 2014;29:1285–90. doi: 10.1007/s00384-014-1935-6. [DOI] [PubMed] [Google Scholar]
- 32.McDonald JW, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn’s disease. Cochrane Database Syst Rev. 2014;2014:CD003459. doi: 10.1002/14651858.CD003459.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Southcott E, Tooley KL, Howarth GS, Davidson GP, Butler RN. Yoghurts containing probiotics reduce disruption of the small intestinal barrier in methotrexate-treated rats. Dig Dis Sci. 2008;53:1837–41. doi: 10.1007/s10620-008-0275-1. [DOI] [PubMed] [Google Scholar]
- 34.Kulnigg S, Gasche C. Systematic review: managing anaemia in Crohn’s disease. Aliment Pharmacol Ther. 2006;24:1507–23. doi: 10.1111/j.1365-2036.2006.03146.x. [DOI] [PubMed] [Google Scholar]
- 35.Constante M, Fragoso G, Lupien-Meilleur J, Calvé A, Santos MM. Iron supplements modulate colon microbiota composition and potentiate the protective effects of probiotics in dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2017;23:753–66. doi: 10.1097/MIB.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 36.Hoppe M, Önning G, Hulthén L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females: double isotope sequential single-blind studies in menstruating women. PLoS One. 2017;12:e0189141. doi: 10.1371/journal.pone.0189141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dignass AU, Gasche C, Bettenworth D, Birgegård G, Danese S, Gisbert JP, et al. European consensus on the diagnosis and management of iron deficiency and anaemia in inflammatory bowel diseases. J Crohns Colitis. 2015;9:211–22. doi: 10.1093/ecco-jcc/jju009. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee R, Chuah SW, Hilmi IN, Wu DC, Yang SK, Demuth D, et al. Efficacy and safety of vedolizumab in Crohn’s disease in patients from Asian countries in the GEMINI 2 study. Intest Res. 2021;19:83–94. doi: 10.5217/ir.2019.09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–60. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 40.Norton BA, Thomas R, Lomax KG, Dudley-Brown S. Patient perspectives on the impact of Crohn’s disease: results from group interviews. Patient Prefer Adherence. 2012;6:509–20. doi: 10.2147/PPA.S32690. [DOI] [PMC free article] [PubMed] [Google Scholar]


