Abstract
Mitochondria are organelles that serve as a central hub for physiological processes in eukaryotes, including production of ATP, regulation of calcium dependent signaling, generation of ROS, and regulation of apoptosis. Cancer cells undergo metabolic reprogramming in an effort to support their increasing requirements for cell survival, growth, and proliferation, and mitochondria have primary roles in these processes. Because of their central function in survival of cancer cells and drug resistance, mitochondria are an important target in cancer therapy and many drugs targeting mitochondria that target the TCA cycle, apoptosis, metabolic pathway, and generation of ROS have been developed. Continued use of mitochondrial-targeting drugs can lead to resistance due to development of new somatic mutations. Use of drugs is limited due to these mutations, which have been detected in mitochondrial proteins. In this review, we will focus on genetic mutations in mitochondrial target proteins and their function in induction of drug-resistance.
Keywords: Mitochondria targeting drugs, Mutations in mitochondrial proteins, Drug resistance, Chemotherapy
Introduction
Mitochondria consist of double membranes, the outer mitochondrial membrane (OMM) and the inner mitochondrial membrane (IMM), with intermembrane space in between. Invagination of IMM into the mitochondrial matrix results in formation of cristae, a structure that is essential for the function of mitochondria. Mitochondria serve as a central hub for physiological processes in eukaryotes, including production of adenosine triphosphate (ATP), regulation of calcium dependent signaling, generation of reactive oxygen species (ROS), and regulation of apoptosis (Han et al. 2019).
Mitochondria are dysfunctional in various cancers due to somatic mutations of mitochondrial DNA (mtDNA) and defects of mitochondrial enzyme leading to tumorigenesis and tumor progression due to abnormalities of the metabolic pathway or resistance to apoptosis (Hsu et al. 2016). Generation of ROS, oxidative stress, and other mutations in mtDNA responsible for continued induction of tumorigenesis are exacerbated by mutations of mtDNA (Hahn and Zuryn 2019). Hypoxia-inducible factor 1α (HIF1α), whose overexpression occurs in many cancers as an adaptive regulator of hypoxia for tumorigenesis, is stabilized by defects in metabolic enzymes such as succinate dehydrogenase (SDH) and fumarate hydratase (FH) (Talks et al. 2000; Sharp and Bernaudin 2004; Pollard et al. 2005; Yee Koh et al. 2008). Furthermore, mitochondria have a capacity for rapid sensing and adaptation to stress stimuli, thus management of drug-induced stress can lead to resistance to chemotherapy (Mizutani et al. 2009; Eisner et al. 2018).
Cancer cells undergo metabolic reprogramming in an effort to support their increasing requirements for cell survival, growth, and proliferation, and mitochondria have primary functions in these processes. Because of their central function in survival of cancer cells and drug resistance, mitochondria are an important target in cancer therapy (Ghosh et al. 2020; Vasan et al. 2020). Many drugs target mitochondria. However, development of new somatic mutations, leading to resistance, can occur with continued use of mitochondrial-targeting drugs (Aminuddin et al. 2020). These mutations, which have been detected in mitochondrial proteins, limit the use of drugs (Tanaka et al. 2021; Xu and Ye 2022).
In this review, we will focus on genetic mutations in mitochondrial target proteins and their function in induction of drug-resistance.
Mitochondrial targets for cancer therapy
Development of cancer therapies targeting dysfunctional mitochondria in cancer has been reported (Horton et al. 2008; Kuznetsov et al. 2011; Thomas et al. 2017; Kim et al. 2020). Binding of chemotherapeutic drugs such as doxorubicin, trastuzumab, and sunitinib to mtDNA leads to induction of apoptosis through generation of ROS and loss of mitochondrial function (Gorini et al. 2018). However, these chemotherapeutic drugs exhibit a high level of toxicity and serious adverse effects on heart function have been reported (Khakoo et al. 2008; Chatterjee et al. 2010; Huszno et al. 2013).
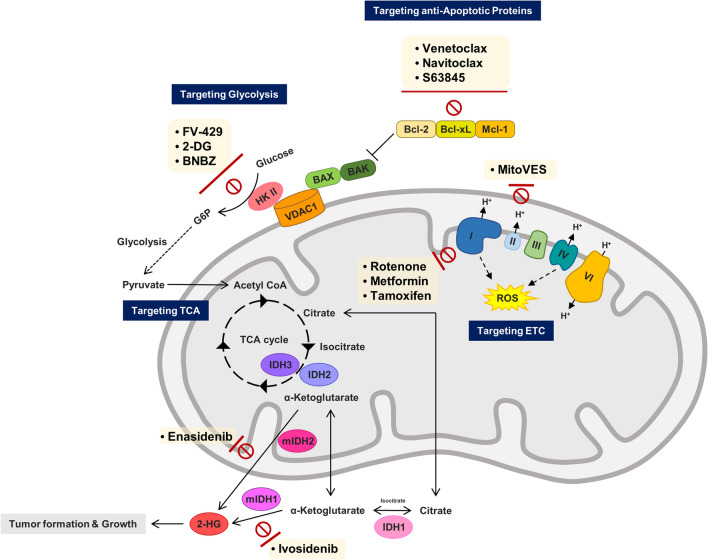
Alternatively, compared to the conventional chemotherapy, utilization of drugs targeting mitochondrial proteins (Table 1) that induce growth of cancer cells can minimize side effects through selective removal of cancer cells (Dong et al. 2008; McGee et al. 2011; Zamberlan et al. 2022). Oligomerization of pro-apoptotic proteins, BAX/BAK is inhibited by members of the anti-apoptotic B cell CLL/lymphoma 2 (BCL-2) family of proteins located in the inner mitochondrial membrane, which block the release of mitochondrial cytochrome c, consequently resulting in inhibition of apoptosis. Overexpression of these proteins, which are associated with chemotherapy resistance, occurs in many cancer cells (Kang and Reynolds 2009). Use of anti-apoptotic BCL-2 family protein inhibitors such as venetoclax, navitoclax, Obatoclax, TW-37, BM-1197, S63845, and AZD-5991 for induction of apoptosis in cancer cells has been reported (Neuzil et al. 2013; Ashkenazi et al. 2017; Kotschy et al. 2016; Tron et al. 2018; Cournoyer et al. 2019; Ahn et al. 2019; Sun et al. 2019). Glycolytic proteins can be targeted using 3-bromopyruvate (3BP), Mito-CP, Mito-Q, and 2-Deoxyglucose (2-DG) to inhibit energy metabolism for induction of apoptosis in cancer cells (Cheng et al. 2012). Mutated isocitrate dehydrogenases (mIDH) is inhibited by AGI-5198, AGI-6780, AG-120, and AG-221, which are detected in a variety of cancers and are regarded as prime targets for chemotherapy through regulation of the tricarboxylic acid (TCA) cycle (Zong et al. 2016; Golub et al. 2019). Induction of ROS and destruction of cancer cells are induced by alpha-tocopheryl succinate (α-TOS), metformin, rotenone, and Mitochondrially targeted vitamin E succinate (MitoVES) through inhibition of the function of proteins of the electron transport chain (ETC) in oxidative phosphorylation (OXPHOS) activity and generation of ATP (Zhang and Fariss 2002; Li et al. 2003; Dong et al. 2008; Kalyanaraman et al. 2018; Fontaine 2018).
Table 1.
Drugs of targeting mitochondrial proteins
| Mitochondria protein-target drug | Target protein | Cell death mechanism | Reference |
|---|---|---|---|
| AZD-5991 | BCL-2 family | Apoptosis induction | Tron et al. (2018) |
| BM-1197 | Sun et al. (2019) | ||
| Obatoclax (GX15-070) | Cournoyer et al. (2019) | ||
| S63945 | Kotschy et al. (2016) | ||
| TW-37 | Ahn et al. (2019) | ||
| Navitoclax (ABT-263) | Ashkenazi et al. (2017) | ||
| Venetoclax (ABT-199) | |||
| 2-deoxyglucose (2-DG) | Hexokinase II | Inhibition of cell metabolism | Zhao et al. (2019) |
| 3-bromopyruvate (3BP) | Lis et al. (2016) | ||
| Benitrobenrazide | Zheng et al. (2021) | ||
| FV-429 | Zhou et al. (2016) | ||
| AG-120 | Isocitrate dehydrogenase I, II | Inhibition of cell metabolism | Golub et al. (2019) |
| AG-221 | |||
| AGI-5198 | |||
| AGI-7680 | |||
| Enasidenib (AG-221) | |||
| Ivosidenib (AG-120) | |||
| Metformin | Complex I | ROS accumulation | Fontaine et al. 2018 |
| Rotenone | Li et al. (2003) | ||
| Alpha-tocopheryl succinate (α-TOS) | Complex II | ROS accumulation | Dong et al. (2008) |
| Atpenin A5 | Kluckova et al. (2013) | ||
| MitoVES | Yan et al. (2015) | ||
| Thenoyltrifluoroacetone | Zhang and Fariss (2002) |
Despite their strong efficacy against cancer with low side effects, modulation of the efficacy of these drugs can occur in various ways in cancer cells, leading to treatment resistance. Resistance to mitochondrial protein-targeted anticancer therapies can be caused by various genetic factors in various carcinomas (Xu et al. 2018; Çoku et al. 2022). In addition, induction of apoptosis can be avoided due to involvement of the mitochondrial regulatory protein that induces apoptosis in interactions with various proteins rather than a single protein (Lopez and Tait 2015).
Cases of resistance to mitochondrial-targeted anti-cancer drugs due to genetic factors
Drugs targeting anti-apoptotic proteins to induce intrinsic apoptosis
The intrinsic apoptotic pathway is controlled by the BCL-2 family, a family of proteins sharing BCL-2 homology (BH) domains, through control of mitochondrial outer membrane permeabilization (MOMP). Among the members of the BCL-2 family, anti-apoptotic proteins such as BCL-2, BCL-XL, and MCL-1 induce inhibition of apoptosis leading to tumor promotion (Youle and Strasser 2008). These proteins of the BCL-2 family are important in several carcinomas as targets for cancer therapy, including prostate cancer, breast cancer, and blood cancer (Emi et al. 2005; Yoshino et al. 2006; Soderquist et al. 2016).
Of the BH1 through BH4 domains, BH3 is a key domain for induction of anti-apoptosis in proteins of the anti-apoptotic BCL-2 family (Kelekar and Thompson 1998). Direct activation of BAX/BAK resulting in induction of apoptosis occurs by way of BH3-only proteins, such as the BCL-2 interacting apoptosis mediator (BIM) (O'Connor et al. 1998), and the activities of BAX/BAK are inhibited by BIM binding to the BH3 domain of the BCL-2 family proteins, which results in anti-apoptosis (Ewings et al. 2007). In the effort to maintain the activity of BIM, many studies on cancer therapies using “BH3 mimics” that bind to this domain of BCL-2 family proteins have been reported (Souers et al. 2013; Wang et al. 2016; Konopleva et al. 2016; Campos and Pinto 2019; Fleischmann et al. 2022; Calis et al. 2022). Venetoclax (ABT-199), a representative BH3 mimic, is used for chemotherapy of hematologic malignancies including acute myeloid leukemia (AML) that shows high expression of BCL-2 (Souers et al. 2013; Konopleva et al. 2016; Campos et al. 2018; Fleischmann et al. 2022).
Genetic mutations responsible for resistance to venetoclax were recently detected in patients with progressive Chronic Lymphocytic Leukemia (CLL) with G101V, F104C, F104L, and D103Y mutations in the BCL-2 gene. These mutations, which reduced the binding affinity of venetoclax to the BCL-2 protein due to the presence of a bulkier sidechain within the interior of globular BCL-2 protein, were responsible for resistance to venetoclax (Birkinshaw et al. 2019; Tausch et al. 2019). BCL-2 and BCL-XL were inhibited by the use of navitoclax (ABT-263) in hepatocellular carcinoma (HCC) cells, however, MCL-1 mRNA and protein were stabilized, resulting in a limited effect (Wang et al. 2014). Because venetoclax and navitoclax are selective drugs against BCL-2, activation of other members of the BCL-2 family such as MCL-1 has been reported as the primary cause of resistance (Van Delft et al. 2006; Souers et al. 2013). In an effort to address this limitation, a combination of S63845 (MCL-1 inhibitor) and venetoclax were applied, resulting in induction of apoptosis in venetoclax-resistant AML cells (Hormi et al. 2020).
In the case of MCL-1 with L267V mutation detected in myeloma patients, the mutation does not interfere with binding of MCL-1 inhibitors such as S63845 and AZD-5991 to MCL-1, rather it prevents displacement of pro-apoptotic proteins by the drug, resulting in disruption of the process of apoptosis (Chen et al. 2018).
In addition, a decrease in BAX, a pro-apoptotic protein, also led to induction of resistance to venetoclax. Interaction of BAX with Voltage Dependent Anion Channel (VDAC) occurs upon induction of apoptosis, leading to an increase of MOMP, resulting in loss of membrane potential and release of cytochrome c (Adachi et al. 2004). Significantly reduced efficacy of venetoclax was reported in BAX-deficient CLL patients with C-terminal BAX mutations who received long-term treatment with venetoclax (Blombery et al. 2020), resulting in elimination of external mitochondrial membrane localization of BAX and induction of resistance to venetoclax (Blombery et al. 2022).
Drugs targeting glycolysis for inhibition of mitochondrial metabolic pathways
Most cancer cells undergo metabolic reprogramming in an effort to adapt to unfavorable conditions such as hypoxia and a low supply of nutrients up to 60% of ATP is derived from the “Warburg effect” and the remaining ATP from OXPHOS. The Warburg effect, a high level of glycolysis over OXPHOS even in the presence of oxygen in cancer cells, enables acquisition of oxygen-independent metabolism in cells (Alfarouk et al. 2014; Lis et al. 2016).
Intracellular fixation of glucose catalyzed by hexokinase (HK) is the first step of glycolysis. ATP-dependent phosphorylation of glucose is catalyzed by HK for generation of glucose-6-phosphate (G6P) which is also utilized in OXPHOS and the pentose-phosphate pathway (Rosano et al. 1999).
The mitochondrial bound form of hexokinase 2 (HK2) most likely has an anti-apoptotic function. which might be responsible for its overexpression in most cancers. HK2 is bound to the outer membrane protein VDAC in mitochondria and probably gives HK2 an advantage regarding access to ATP generated during OXPHOS (Nakashima et al. 1986; Arora and Pedersen 1988). HK2 improves the rate of glycolysis and is required for initiation and maintenance of tumors. Therefore, development of anti-cancer drugs that target HK2 for inhibition of glycolysis and induction of apoptosis has been reported (Zhou et al. 2016; Zheng et al. 2021).
2-DG, a glucose analogue, competes with glucose and binds to HK2, leading to inhibition of glycolysis (Zhao et al. 2019). No cases of resistance to 2-DG, an HK2 inhibitor, in cancer have been reported. However, one case of 2-DG resistance with a novel mutation in the yeast gene Hkk2 has been reported (Zhao et al. 2019). No interaction was observed between the Hkk2 G238V mutation and either glucose or 2-DG, however, its potential to affect binding and catalysis of hexose through an allosteric mechanism was demonstrated (Hellemann et al. 2022), suggesting a clinical relevance to 2-DG resistance.
Drugs targeting the TCA cycle to inhibit mitochondrial production of ATP
The TCA cycle, also known as the citric acid cycle or the Krebs cycle, is a mediator that assists in production of ATP through supply of electrons to the ETC. The TCA cycle, which occurs in mitochondria, is composed of eight chemical reactions for production of electron reservoirs in the form of NADH and FADH2 from acetyl-CoA derived from carbohydrates, proteins, and fats. In turn, NADH and FADH2 are utilized in the OXPHOS pathway for production of chemical energy in the form of ATP. Both storage of electrons and generation of amino acid precursors occur during this cycle.
Although utilization of aerobic glycolysis is known to be a hallmark of cancer cell metabolism, cancer cells also rely on the TCA cycle for production of energy and macro-molecular synthesis (Anderson et al. 2018; Eniafe and Jiang 2021). Catalysis of isocitrate to α-ketoglutarate (α-KG) and reduction of NAD(P) + to NAD(P)H are induced by Isocitrate dehydrogenases 1 and 2 (IDH1 and IDH2), metabolic enzymes in the TCA cycle (Reitman and Yan 2010). In particular, IDH2, located in mitochondria, is involved in regulation of oxidative respiration, and thus plays an important role in tumorigenesis (Nekrutenko et al. 1998; Kalyanaraman et al. 2018; Qiao et al. 2021). Mutation of IDH1 and IDH2 has been reported in several carcinomas including AML, and solid tumors, including glioma, chondrosarcoma, and cholangiocarcinoma (Wouters 2021). The conversion of α-KG to 2-hydroxyglutarate (2-HG), an oncometabolite, is catalyzed by mutant IDH1 and IDH2 (mIDH1 and mIDH2), which also mediate most of the carcinogenic potential (Dong and Neuzil 2019). Accumulation of 2-HG is a factor in tumor formation and growth of malignant tumors (Dang et al. 2009). Therefore, high efficacy of anticancer drugs, including AGI-5198, AGI-6780, AG-120, and AG-221 in targeting mIDH1 and mIDH2 has been reported in a wide range of cancer types (Wouters et al. 2021).
Enasidenib (AG-221), a selective inhibitor of mIDH2, displays a potent inhibition of 2-HG production in the context of the IDH2 R140Q/WT heterodimer or the IDH2 R140Q homodimer. Suppressed production of 2-HG and induction of cellular differentiation caused by AG 221 in primary human mIDH2–positive AML cells has been demonstrated (Yen et al. 2017). A recent study reported on the secondary mutations in IDH2, Q316E and I319M, detected in two AML patients who developed resistance to Enasidenib and tumor relapsed. The Q316E mutation caused a reduction of hydrogen bonding with Enasidenib, and the I319M mutation conveyed steric hindrance to the bulky side chain (Zhuang et al. 2022). Resistance to Ivosidenib (AG-120), an inhibitor of mIDH1, was also reported in the AML patients with the secondary mutation, S280F of IDH1. IDH1 S280F mutation was expected to result in a steric hindrance caused by substituted phenylalanine near the binding site of Ivosidenib and mIDH1 (Oltvai et al. 2021).
Drugs targeting the mitochondrial ETC protein to apoptosis via generation of ROS
ROS, a byproduct of normal metabolism of oxygen in cells, are highly reactive oxygen-containing molecules that react readily with other molecules in cells. Production of ROS occurs through recurring biochemical reactions during OXPHOS in the ETC with passage of electrons through a series of proteins in order to finally reach oxygen in mitochondria. Increased levels of ROS detected in cancer cells has long been regarded as tumorigenic through promotion of genomic instability. A moderately increased level of ROS in cancer cells can promote tumorigenesis and progression through activation of signaling pathways responsible for regulation of cellular proliferation, metabolic alterations, and angiogenesis as well as induction of DNA mutation (Sullivan and Chandel 2014; Perillo et al. 2020).
However, a continuous increase in the level of ROS leads to inactivation of BCL-2 and BCL-XL via activation of c-Jun N-terminal Kinase (JNK) and induction of apoptosis by release of cytochrome c by BAX/BAK (Redza-Dutordoir and Averill-Bates 2016). Therefore, wide use of cancer treatment that induces excessive production of ROS has been reported (Perillo et al. 2020). NADH generated through the TCA cycle in the mitochondrial matrix is oxidized by respiratory complex I (Complex I), which also causes reduction of ubiquinone to ubiquinol using two electrons (Sharma et al. 2009). Catalysis of the oxidation of succinate to fumarate and transfer of electrons to ubiquinone are induced by SDH (Complex II) (Bandara et al. 2021). They donate electrons to ETC and ultimately play an important role in generation of ATP (Nolfi-Donegan et al. 2020). Production of ATP is hindered by inhibition of Complex I and Complex II, which also causes excessive production of ROS (Chen et al. 2007), which is a target of cancer treatment (Yoshida et al. 2021; Kluckova et al. 2013).
Complex I is composed of 45 subunits; seven of these, ND1-6 and ND4L, are encoded by mitochondrial DNA (Sharma et al. 2009). Binding of rotenone, an inhibitor of Complex I, to the ND4 site, results in induction of apoptosis, inhibiting proliferation of cells in various carcinomas including lung cancer, colon cancer, and breast cancer (Heinz et al. 2017; Kampjut and Sazanov 2020). The G11778A mutation in ND4 has been reported to induce resistance to rotenone in patients with Leber's hereditary optic neuropathy (Degli Esposti et al. 1994; Musiani et al. 2022). Although no clinical cases in cancer patients have been reported, it is expected that cancer patients with the G11778A mutation of ND4 will have an equal likelihood of developing resistance to rotenone. In addition, resistance to rotenone was reported in hypoxia-tolerant human glioma cells (M010b) harboring the T14634C mutation of ND6 that showed no change in expression of ND6 (DeHaan et al. 2004).
Ubiquinone obtains electrons through binding to the ubiquinone binding site (Qp) of complex II. Upon gaining electrons, ubiquinone is reduced to ubiquonol in order to supply electrons to complexes III and IV (Cecchini 2003; Sun et al. 2005). Interaction of Atpenin and MitoVES with Qp in complex II, which inhibited the reduction of ubiquinone and the oxidation of succinate, has been reported (Miyadera et al. 2003; Yan et al. 2015). According to the result, ROS was produced by Complex II, leading to saturation of the succinate concentration (Siebels and Dröse 2013). However, interaction of Complex II with inhibitors such as thenoyltrifluoroacetone (TTFA), Atpenin A5, and MitoVES was inhibited by mutations at the Qp-binding site of Complex II, leading to development of drug resistance (Kluckova et al. 2015).
Conclusion
Mitochondrial dysfunction is a major cause of tumorigenesis and tumor progression in many cells. Many anticancer drugs that target dysfunctional mitochondrial metabolism and apoptosis pathways have been developed (Fig. 1). However, intrinsic genetic mutations and mutations in the target protein that are induced by continuous administration of anti-cancer drugs can cause induction of resistance. Administration of multi-drug therapy is recommended as a method for overcoming resistance to anti-cancer drugs. Resistance was overcome with use of a combination of mitochondrial-targeting drugs (Cheng et al. 2019). However, there are both advantages and disadvantages associated with overcoming drug-resistance. The primary risk is side effects. A better effect was not achieved by combination of valproic acid with all-trans retinoic acid, and valproic acid-related hematologic toxicity and higher mortality were observed with co-administration of idarubicin in patients with AML (Tassara et al. 2014). In addition, patients may show separate side effects for each drug at the same time, and determining which drug caused the side effect can be difficult (Mokhtari et al. 2017). Thus, the significance of targeted therapy to minimize toxicity may be undermined by the side effects of multi-drug therapy. In addition, increased drug costs due to increased usage of drugs and improper multi-drug use can impose a cost burden.
Fig. 1.
Cancer drugs that inhibit protein function involved in mitochondrial metabolism and anti-apoptosis
Positive combination therapy can have a synergistic effect on efficacy (Duarte and Vale 2022), however, use of sequential monotherapy may enable greater dose intensity as well use of a treatment approach that enables attainment of the maximum time and benefit from each agent (Dear et al. 2013).
Therefore, selection of an optimal mitochondria-target in order to minimize drug toxicity, identification of patients who show resistance to the drug to be administered, and design of an alternative strategy for treatment in patients who show resistance are important. Combination drug therapy or another single drug capable of evading resistance might be an alternative strategy. Finally, as various anti-cancer drug therapies have been developed, cases of resistance to the drug have also been reported; thus, conduct of many clinical studies is still required in various cases in order to achieve a successful treatment outcome for cancer patients.
Acknowledgements
This work was supported by the Basic Science Research Progream through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2021R1F1A1060935, 2022R1F1A1066987).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adachi M, Higuchi H, Miura S, Azuma T, Inokuchi S, Saito H, Kato S, Ishii H. Bax interacts with the voltage-dependent anion channel and mediates ethanol-induced apoptosis in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G695–705. doi: 10.1152/ajpgi.00415.2003. [DOI] [PubMed] [Google Scholar]
- Ahn CH, Lee WW, Jung YC, Shin JA, Hong KO, Choi S, Swarup N, Kim J, Ahn MH, Jung M, Cho SD, Jin B. Antitumor effect of TW-37, a BH3 mimetic in human oral cancer. Lab Anim Res. 2019;4(35):27. doi: 10.1186/s42826-019-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfarouk KO, Verduzco D, Rauch C, Muddathir AK, Adil HH, Elhassan GO, Ibrahim ME, David Polo Orozco J, Cardone RA, Reshkin SJ, Harguindey S. Glycolysis, tumor metabolism, cancer growth and dissemination. A new pH-based etiopathogenic perspective and therapeutic approach to an old cancer question. Oncoscience. 2014;1(12):777–802. doi: 10.18632/oncoscience.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminuddin A, Ng PY, Leong CO, Chua EW. Mitochondrial DNA alterations may influence the cisplatin responsiveness of oral squamous cell carcinoma. Sci Rep. 2020;10(1):7885. doi: 10.1038/s41598-020-64664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NM, Mucka P, Kern JG, Feng H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell. 2018;9(2):216–237. doi: 10.1007/s13238-017-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988;263(33):17422–17428. doi: 10.1016/S0021-9258(19)77853-3. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A, Fairbrother WJ, Leverson JD, Souers AJ. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat Rev Drug Discov. 2017;16(4):273–284. doi: 10.1038/nrd.2016.253. [DOI] [PubMed] [Google Scholar]
- Bandara AB, Drake JC, Brown DA. Complex II subunit SDHD is critical for cell growth and metabolism, which can be partially restored with a synthetic ubiquinone analog. BMC Mol Cell Biol. 2021;22(1):35. doi: 10.1186/s12860-021-00370-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkinshaw RW, Gong JN, Luo CS, Lio D, White CA, Anderson MA, Blombery P, Lessene G, Majewski IJ, Thijssen R, Roberts AW, Huang DCS, Colman PM, Czabotar PE. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat Commun. 2019;10(1):2385. doi: 10.1038/s41467-019-10363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blombery P, Thompson ER, Chen X, Nguyen T, Anderson MA, Westerman DA, Seymour JF, Tam CS, Dengler MA, Huang DCS, Wei A, Roberts AW. BAX-mutated clonal hematopoiesis in patients on long-term venetoclax for relapsed/refractory chronic lymphocytic leukemia. Blood. 2020;136(Supplement 1):9–10. doi: 10.1182/blood-2020-137775. [DOI] [Google Scholar]
- Blombery P, Lew TE, Dengler MA, Thompson ER, Lin VS, Chen X, Nguyen T, Panigrahi A, Handunnetti SM, Carney DA, Westerman DA, Tam CS, Adams JM, Wei AH, Huang DCS, Seymour JF, Roberts AW, Anderson MA. Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL. Blood. 2022;139(8):1198–1207. doi: 10.1182/blood.2021012775. [DOI] [PubMed] [Google Scholar]
- Calis S, Dogan B, Durdagi S, Celebi A, Yapicier O, Kilic T, Turanli ET, Avsar T. A novel BH3 mimetic Bcl-2 inhibitor promotes autophagic cell death and reduces in vivo Glioblastoma tumor growth. Cell Death Discov. 2022;8(1):433. doi: 10.1038/s41420-022-01225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos EDV, Pinto R. Targeted therapy with a selective BCL-2 inhibitor in older patients with acute myeloid leukemia. Hematol Transfus Cell Ther. 2019;41(2):169–177. doi: 10.1016/j.htct.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini G. Function and structure of complex II of the respiratory chain. Annu Rev Biochem. 2003;72:77–109. doi: 10.1146/annurev.biochem.72.121801.161700. [DOI] [PubMed] [Google Scholar]
- Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115(2):155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120(Pt 23):4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- Chen B, Pamela W, Auclair D, Keats JJ, Secrist P, Cidado J, Tron AE, Dunham CM, Lonial S, Opferman J, Matulis SM, Boise LH. Myeloma Patient-Derived MCL1 Point Mutations Can Influence MCL1-Inhibitor Function. Blood. 2018;132(Supplement 1):951. doi: 10.1182/blood-2018-99-113444. [DOI] [Google Scholar]
- Cheng G, Zielonka J, Dranka BP, McAllister D, Mackinnon AC, Jr, Joseph J, Kalyanaraman B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72(10):2634–2644. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Zielonka J, Hardy M, Ouari O, Chitambar CR, Dwinell MB, Kalyanaraman B. Synergistic inhibition of tumor cell proliferation by metformin and mito-metformin in the presence of iron chelators. Oncotarget. 2019;10(37):3518–3532. doi: 10.18632/oncotarget.26943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çoku J, Booth DM, Skoda J, Pedrotty MC, Vogel J, Liu K, Vu A, Carpenter EL, Ye JC, Chen MA, Dunbar P, Scadden E, Yun TD, Nakamaru-Ogiso E, Area-Gomez E, Li Y, Goldsmith KC, Reynolds CP, Hajnoczky G, Hogarty MD. Reduced ER-mitochondria connectivity promotes neuroblastoma multidrug resistance. EMBO J. 2022;41(8):e108272. doi: 10.15252/embj.2021108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournoyer S, Addioui A, Belounis A, Beaunoyer M, Nyalendo C, Le Gall R, Teira P, Haddad E, Vassal G, Sartelet H. GX15-070 (Obatoclax), a Bcl-2 family proteins inhibitor engenders apoptosis and pro-survival autophagy and increases Chemosensitivity in neuroblastoma. BMC Cancer. 2019;19(1):1018. doi: 10.1186/s12885-019-6195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dear RF, McGeechan K, Jenkins MC, Barratt A, Tattersall MH, Wilcken N (2013) Combination versus sequential single agent chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2013(12):CD008792. doi:10.1002/14651858.CD008792 [DOI] [PMC free article] [PubMed]
- Degli Esposti M, Carelli V, Ghelli A, Ratta M, Crimi M, Sangiorgi S, Montagna P, Lenaz G, Lugaresi E, Cortelli P. Functional alterations of the mitochondrially encoded ND4 subunit associated with Leber's hereditary optic neuropathy. FEBS Lett. 1994;352(3):375–379. doi: 10.1016/0014-5793(94)00971-6. [DOI] [PubMed] [Google Scholar]
- DeHaan C, Habibi-Nazhad B, Yan E, Salloum N, Parliament M, Allalunis-Turner J. Mutation in mitochondrial complex I ND6 subunit is associated with defective response to hypoxia in human glioma cells. Mol Cancer. 2004;12(3):19. doi: 10.1186/1476-4598-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Neuzil J. Targeting mitochondria as an anticancer strategy. Cancer Commun (lond) 2019;39(1):63. doi: 10.1186/s40880-019-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LF, Low P, Dyason JC, Wang XF, Prochazka L, Witting PK, Freeman R, Swettenham E, Valis K, Liu J, Zobalova R, Turanek J, Spitz DR, Domann FE, Scheffler IE, Ralph SJ, Neuzil J. Alpha-tocopheryl succinate induces apoptosis by targeting ubiquinone-binding sites in mitochondrial respiratory complex II. Oncogene. 2008;27(31):4324–4335. doi: 10.1038/onc.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte D, Vale N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr Res Pharmacol Drug Discov. 2022;3:100110. doi: 10.1016/j.crphar.2022.100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner V, Picard M, Hajnóczky G. Mitochondrial dynamics in adaptive and maladaptive cellular stress responses. Nat Cell Biol. 2018;20(7):755–765. doi: 10.1038/s41556-018-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emi M, Kim R, Tanabe K, Uchida Y, Toge T. Targeted therapy against Bcl-2-related proteins in breast cancer cells. Breast Cancer Res. 2005;7(6):R940–R952. doi: 10.1186/bcr1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eniafe J, Jiang S. The functional roles of TCA cycle metabolites in cancer. Oncogene. 2021;40:3351–3363. doi: 10.1038/s41388-020-01639-8. [DOI] [PubMed] [Google Scholar]
- Ewings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract. ERK Repels Cell Cycle. 2007;6(18):2236–2240. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- Fleischmann M, Scholl S, Frietsch JJ, Hilgendorf I, Schrenk K, Hammersen J, Prims F, Thiede C, Hochhaus A, Schnetzke U. Clinical experience with venetoclax in patients with newly diagnosed, relapsed, or refractory acute myeloid leukemia. J Cancer Res Clin Oncol. 2022;148(11):3191–3202. doi: 10.4161/cc.6.18.4728.10.1007/s00432-022-03930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front Endocrinol (lausanne) 2018;17(9):753. doi: 10.4161/cc.6.18.4728.10.3389/fendo.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Vidal C, Dey S, Zhang L. Mitochondria targeting as an effective strategy for cancer therapy. Int J Mol Sci. 2020;21(9):3363. doi: 10.3390/ijms21093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub D, Iyengar N, Dogra S, Wong T, Bready D, Tang K, Modrek AS, Placantonakis DG. Mutant Isocitrate Dehydrogenase Inhibitors as Targeted Cancer Therapeutics. Front Oncol. 2019;17(9):417. doi: 10.3389/fonc.2019.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic Drugs and Mitochondrial Dysfunction: Focus on Doxorubicin, Trastuzumab, and Sunitinib. Oxid Med Cell Longev. 2018;18(2018):7582730. doi: 10.1155/2018/7582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Zuryn S. Mitochondrial Genome (mtDNA) Mutations that Generate Reactive Oxygen Species. Antioxidants (basel) 2019;8(9):392. doi: 10.3390/antiox8090392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y-S, Jegal M-E, Kim Y-J. Mitochondrial Dysfunction and Cancer. J Life Sci. 2019;29(9):1034–1046. doi: 10.5352/JLS.2019.29.9.1034. [DOI] [Google Scholar]
- Heinz S, Freyberger A, Lawrenz B, Schladt L, Schmuck G, Ellinger-Ziegelbauer H. Mechanistic Investigations of the Mitochondrial Complex I Inhibitor Rotenone in the Context of Pharmacological and Safety Evaluation. Sci Rep. 2017;4(7):45465. doi: 10.1038/srep45465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemann E, Walker JL, Lesko MA, Chandrashekarappa DG, Schmidt MC, O'Donnell AF, Durrant JD. Novel mutation in hexokinase 2 confers resistance to 2-deoxyglucose by altering protein dynamics. PLoS Comput Biol. 2022;18(3):e1009929. doi: 10.1371/journal.pcbi.1009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormi M, Birsen R, Belhadj M, Huynh T, Cantero Aguilar L, Grignano E, Haddaoui L, Guillonneau F, Mayeux P, Hunault M, Tamburini J, Kosmider O, Fontenay M, Bouscary D, Chapuis N. Pairing MCL-1 inhibition with venetoclax improves therapeutic efficiency of BH3-mimetics in AML. Eur J Haematol. 2020;105(5):588–596. doi: 10.1111/ejh.13492. [DOI] [PubMed] [Google Scholar]
- Horton KL, Stewart KM, Fonseca SB, Guo Q, Kelley SO. Mitochondria-penetrating peptides. Chem Biol. 2008;15(4):375–382. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Tseng LM, Lee HC. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (maywood) 2016;241(12):1281–1295. doi: 10.1177/1535370216641787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszno J, Leś D, Sarzyczny-Słota D, Nowara E. Cardiac side effects of trastuzumab in breast cancer patien–s - single centere experiences. Contemp Oncol (pozn) 2013;17(2):190–195. doi: 10.5114/wo.2013.34624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman B, Cheng G, Hardy M, Ouari O, Lopez M, Joseph J, Zielonka J, Dwinell MB. A review of the basics of mitochondrial bioenergetics, metabolism, and related signaling pathways in cancer cells: Therapeutic targeting of tumor mitochondria with lipophilic cationic compounds. Redox Biol. 2018;14:316–327. doi: 10.1016/j.redox.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampjut D, Sazanov LA. The coupling mechanism of mammalian respiratory complex I. Science. 2020;370(6516):eabc4209. doi: 10.1126/science.abc4209. [DOI] [PubMed] [Google Scholar]
- Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15(4):1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8(8):324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Kassiotis CM, Tannir N, Plana JC, Halushka M, Bickford C, Trent J, 2nd, Champion JC, Durand JB, Lenihan DJ. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112(11):2500–2508. doi: 10.1002/cncr.23460. [DOI] [PubMed] [Google Scholar]
- Kim MS, Gernapudi R, Cedeño YC, Polster BM, Martinez R, Shapiro P, Kesari S, Nurmemmedov E, Passaniti A. Targeting breast cancer metabolism with a novel inhibitor of mitochondrial ATP synthesis. Oncotarget. 2020;11(43):3863–3885. doi: 10.18632/oncotarget.27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluckova K, Bezawork-Geleta A, Rohlena J, Dong L, Neuzil J. Mitochondrial complex II, a novel target for anti-cancer agents. Biochim Biophys Acta. 2013;1827(5):552–564. doi: 10.1016/j.bbabio.2012.10.015. [DOI] [PubMed] [Google Scholar]
- Kluckova K, Sticha M, Cerny J, Mracek T, Dong L, Drahota Z, Gottlieb E, Neuzil J, Rohlena J. Ubiquinone-binding site mutagenesis reveals the role of mitochondrial complex II in cell death initiation. Cell Death Dis. 2015;6(5):e1749. doi: 10.1038/cddis.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, McKeegan E, Salem AH, Zhu M, Ricker JL, Blum W, DiNardo CD, Kadia T, Dunbar M, Kirby R, Falotico N, Leverson J, Humerickhouse R, Mabry M, Stone R, Kantarjian H, Letai A. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016;6(10):1106–1117. doi: 10.1158/2159-8290.CD-16-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, Chanrion M, Kelly GL, Gong JN, Moujalled DM, Bruno A, Csekei M, Paczal A, Szabo ZB, Sipos S, Radics G, Proszenyak A, Balint B, Ondi L, Blasko G, Robertson A, Surgenor A, Dokurno P, Chen I, Matassova N, Smith J, Pedder C, Graham C, Studeny A, Lysiak-Auvity G, Girard AM, Gravé F, Segal D, Riffkin CD, Pomilio G, Galbraith LC, Aubrey BJ, Brennan MS, Herold MJ, Chang C, Guasconi G, Cauquil N, Melchiore F, Guigal-Stephan N, Lockhart B, Colland F, Hickman JA, Roberts AW, Huang DC, Wei AH, Strasser A, Lessene G, Geneste O. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Margreiter R, Amberger A, Saks V, Grimm M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim Biophys Acta. 2011;1813(6):1144–1152. doi: 10.1016/j.bbamcr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, Robinson JP. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278(10):8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Lis P, Dyląg M, Niedźwiecka K, Ko YH, Pedersen PL, Goffeau A, Ułaszewski S. The HK2 Dependent "Warburg Effect" and Mitochondrial Oxidative Phosphorylation in Cancer: Targets for Effective Therapy with 3-Bromopyruvate. Molecules. 2016;21(12):1730. doi: 10.3390/molecules21121730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112(6):957–962. doi: 10.1038/bjc.2015.85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AM, Douglas DL, Liang Y, Hyder SM, Baines CP. The mitochondrial protein C1qbp promotes cell proliferation, migration and resistance to cell death. Cell Cycle. 2011;10(23):4119–4127. doi: 10.4161/cc.10.23.18287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadera H, Shiomi K, Ui H, Yamaguchi Y, Masuma R, Tomoda H, Miyoshi H, Osanai A, Kita K, Omura S. Atpenins, potent and specific inhibitors of mitochondrial complex II (succinate-ubiquinone oxidoreductase) Proc Natl Acad Sci U S A. 2003;100(2):473–477. doi: 10.1073/pnas.0237315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S, Miyato Y, Shidara Y, Asoh S, Tokunaga A, Tajiri T, Ohta S. Mutations in the mitochondrial genome confer resistance of cancer cells to anticancer drugs. Cancer Sci. 2009;100(9):1680–1687. doi: 10.1111/j.1349-7006.2009.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhtari RB, Homayouni TS, Baluch N, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043. doi: 10.18632/oncotarget.16723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiani F, Rigobello L, Iommarini L, Carelli V, Degli Esposti M, Ghelli AM. New insights on rotenone resistance of complex I induced by the m.11778G>A/MT-ND4 mutation associated with leber's hereditary optic neuropathy. Molecules. 2022;27(4):1341. doi: 10.3390/molecules27041341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima RA, Mangan PS, Colombini M, Pedersen PL. Hexokinase receptor complex in hepatoma mitochondria: evidence from N, N'-dicyclohexylcarbodiimide-labeling studies for the involvement of the pore-forming protein VDAC. Biochemistry. 1986;25(5):1015–1021. doi: 10.1021/bi00353a010. [DOI] [PubMed] [Google Scholar]
- Nekrutenko A, Hillis DM, Patton JC, Bradley RD, Baker RJ. Cytosolic isocitrate dehydrogenase in humans, mice, and voles and phylogenetic analysis of the enzyme family. Mol Biol Evol. 1998;15(12):1674–1684. doi: 10.1093/oxfordjournals.molbev.a025894. [DOI] [PubMed] [Google Scholar]
- Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13(3):199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020;37:101674. doi: 10.1016/j.redox.2020.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltvai ZN, Harley SE, Koes D, Michel S, Warlick ED, Nelson AC, Yohe S, Mroz P. Assessing acquired resistance to IDH1 inhibitor therapy by full-exon IDH1 sequencing and structural modeling. Cold Spring Harb Mol Case Stud. 2021;7(2):a006007. doi: 10.1101/mcs.a006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perillo B, Di Donato M, Pezone A, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard PJ, Brière JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, Hargreaves IP, Heales SJ, Chung YL, Griffiths JR, Dalgleish A, McGrath JA, Gleeson MJ, Hodgson SV, Poulsom R, Rustin P, Tomlinson IP. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- Qiao S, Lu W, Glorieux C, Li J, Zeng P, Meng N, Zhang H, Wen S, Huang P. Wild-type IDH2 protects nuclear DNA from oxidative damage and is a potential therapeutic target in colorectal cancer. Oncogene. 2021;40(39):5880–5892. doi: 10.1038/s41388-021-01968-2. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M, Averill-Bates DA. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta. 2016;1863(12):2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Reitman ZJ, Yan H. Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. J Natl Cancer Inst. 2010;102(13):932–941. doi: 10.1093/jnci/djq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Sabini E, Rizzi M, Deriu D, Murshudov G, Bianchi M, Serafini G, Magnani M, Bolognesi M. Binding of non-catalytic ATP to human hexokinase I highlights the structural components for enzyme-membrane association control. Structure. 1999;7(11):1427–1437. doi: 10.1016/s0969-2126(00)80032-5. [DOI] [PubMed] [Google Scholar]
- Sharma LK, Lu J, Bai Y. Mitochondrial respiratory complex I: structure, function and implication in human diseases. Curr Med Chem. 2009;16(10):1266–1277. doi: 10.2174/092986709787846578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5(6):437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Siebels I, Dröse S. Q-site inhibitor induced ROS production of mitochondrial complex II is attenuated by TCA cycle dicarboxylates. Biochim Biophys Acta. 2013;1827(10):1156–1164. doi: 10.1016/j.bbabio.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Soderquist S, Eastman A. BCL2 Inhibitors as Anticancer Drugs: A Plethora of Misleading BH3 Mimetics. Mol Cancer Ther. 2016;15(9):2011–2017. doi: 10.1158/1535-7163.MCT-16-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;28(2):17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121(7):1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Sun YL, Jiang WQ, Luo QY, Yang DJ, Cai YC, Huang HQ, Sun J. A novel Bcl-2 inhibitor, BM-1197, induces apoptosis in malignant lymphoma cells through the endogenous apoptotic pathway. BMC Cancer. 2019;20(1):1. doi: 10.1186/s12885-019-6169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157(2):411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Lin JJ, Li C, Ryan MB, Zhang J, Kiedrowski LA, Michel AG, Syed MU, Fella KA, Sakhi M, Baiev I, Juric D, Gainor JF, Klempner SJ, Lennerz JK, Siravegna G, Bar-Peled L, Hata AN, Heist RS, Corcoran RB. Clinical Acquired Resistance to KRASG12C Inhibition through a Novel KRAS Switch-II Pocket Mutation and Polyclonal Alterations Converging on RAS-MAPK Reactivation. Cancer Discov. 2021;11(8):1913–1922. doi: 10.1158/2159-8290.CD-21-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassara M, Döhner K, Brossart P, Held G, Götze K, Horst H-A, Ringhoffer M, Köhne C-H, Kremers S, Raghavachar A, Wulf G, Kirchen H, Nachbaur D, Derigs HG, Wattad M, Koller E, Brugger W, Matzdorff A, Greil R, Heil G, Paschka P, Gaidzik VI, Göttlicher M, Döhner H, Schlenk RF. Valproic acid in combination with all-trans retinoic acid and intensive therapy for acute myeloid leukemia in older patients. Blood. 2014;123(26):4027–4036. doi: 10.1182/blood-2013-12-546283. [DOI] [PubMed] [Google Scholar]
- Tausch E, Close W, Dolnik A, Bloehdorn J, Chyla B, Bullinger L, Döhner H, Mertens D, Stilgenbauer S. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica. 2019;104(9):e434–e437. doi: 10.3324/haematol.2019.222588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AP, Palanikumar L, Jeena MT, Kim K, Ryu JH. Cancer-mitochondria-targeted photodynamic therapy with supramolecular assembly of HA and a water soluble NIR cyanine dye. Chem Sci. 2017;8(12):8351–8356. doi: 10.1039/c7sc03169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron AE, Belmonte MA, Adam A, Aquila BM, Boise LH, Chiarparin E, Cidado J, Embrey KJ, Gangl E, Gibbons FD, Gregory GP, Hargreaves D, Hendricks JA, Johannes JW, Johnstone RW, Kazmirski SL, Kettle JG, Lamb ML, Matulis SM, Nooka AK, Packer MJ, Peng B, Rawlins PB, Robbins DW, Schuller AG, Su N, Yang W, Ye Q, Zheng X, Secrist JP, Clark EA, Wilson DM, Fawell SE, Hird AW. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat Commun. 2018;9(1):5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10(5):389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasan K, Werner M, Chandel NS. Mitochondrial Metabolism as a Target for Cancer Therapy. Cell Metab. 2020;32(3):341–352. doi: 10.1016/j.cmet.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Ni Z, Dai X, et al. The Bcl-2/xL inhibitor ABT-263 increases the stability of Mcl-1 mRNA and protein in hepatocellular carcinoma cells. Mol Cancer. 2014;13:98. doi: 10.1186/1476-4598-13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang C, Yan X, Lan B, Wang J, Wei C, Cao X, Wang R, Yao J, Zhou T, Zhou M, Liu Q, Jiang B, Jiang P, Kesari S, Lin X, Guo F. A novel bioavailable BH3 mimetic efficiently inhibits colon cancer via cascade effects of mitochondria. Clin Cancer Res. 2016;22(6):1445–1458. doi: 10.1158/1078-0432.CCR-15-0732. [DOI] [PubMed] [Google Scholar]
- Wouters BJ. Targeting IDH1 and IDH2 mutations in acute myeloid leukemia: emerging options and pending questions. Hemasphere. 2021;5(6):e583. doi: 10.1097/HS9.0000000000000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Ye H. Progress in understanding the mechanisms of resistance to BCL-2 inhibitors. Exp Hematol Oncol. 2022;11(1):31. doi: 10.1186/s40164-022-00283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Chen S, Yang W, Cheng X, Ye Y, Mao J, Wu X, Huang L, Ji J. FGFR4 Links Glucose Metabolism and Chemotherapy Resistance in Breast Cancer. Cell Physiol Biochem. 2018;47(1):151–160. doi: 10.1159/000489759. [DOI] [PubMed] [Google Scholar]
- Yan B, Stantic M, Zobalova R, Bezawork-Geleta A, Stapelberg M, Stursa J, Prokopova K, Dong L, Neuzil J. Mitochondrially targeted vitamin E succinate efficiently kills breast tumour-initiating cells in a complex II-dependent manner. BMC Cancer. 2015;13(15):401. doi: 10.1186/s12885-015-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33(11):526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Yen K, Travins J, Wang F, David MD, Artin E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, Chen Y, Nagaraja R, Choe S, Jin L, Konteatis Z, Cianchetta G, Saunders JO, Salituro FG, Quivoron C, Opolon P, Bawa O, Saada V, Paci A, Broutin S, Bernard OA, de Botton S, Marteyn BS, Pilichowska M, Xu Y, Fang C, Jiang F, Wei W, Jin S, Silverman L, Liu W, Yang H, Dang L, Dorsch M, Penard-Lacronique V, Biller SA, Su SM. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring Oncogenic IDH2 Mutations. Cancer Discov. 2017;7(5):478–493. doi: 10.1158/2159-8290.CD-16-1034. [DOI] [PubMed] [Google Scholar]
- Yoshida J, Ohishi T, Abe H, Ohba SI, Inoue H, Usami I, Amemiya M, Oriez R, Sakashita C, Dan S, Sugawara M, Kawaguchi T, Ueno J, Asano Y, Ikeda A, Takamatsu M, Amori G, Kondoh Y, Honda K, Osada H, Noda T, Watanabe T, Shimizu T, Shibasaki M, Kawada M. Mitochondrial complex I inhibitors suppress tumor growth through concomitant acidification of the intra- and extracellular environment. iScience. 2021;24(12):103497. doi: 10.1016/j.isci.2021.103497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino T, Shiina H, Urakami S, Kikuno N, Yoneda T, Shigeno K, Igawa M. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res. 2006;12(20 Pt 1):6116–6124. doi: 10.1158/1078-0432.CCR-06-0147. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. TheBCL-2proteinfamily:opposingactivitiesthatmediatecelldeath. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zamberlan M, Boeckx A, Muller F, Vinelli F, Ek O, Vianello C, Coart E, Shibata K, Christian A, Grespi F, Giacomello M, Struman I, Scorrano L, Herkenne S. Inhibition of the mitochondrial protein Opa1 curtails breast cancer growth. J Exp Clin Cancer Res. 2022;41(1):95. doi: 10.1186/s13046-022-02304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JG, Fariss MW. Thenoyltrifluoroacetone, a potent inhibitor of carboxylesterase activity. Biochem Pharmacol. 2002;63(4):751–754. doi: 10.1016/s0006-2952(01)00871-1. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ma Y, Zhang Y, Fu B, Wu X, Li Q, Cai G, Chen X, Bai XY. Low-dose 2-deoxyglucose and metformin synergically inhibit proliferation of human polycystic kidney cells by modulating glucose metabolism. Cell Death Discov. 2019;11(5):76. doi: 10.1038/s41420-019-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Wu C, Yang K, Yang Y, Liu Y, Gao S, Wang Q, Li C, Chen L, Li H. Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol Res. 2021;164:105367. doi: 10.1016/j.phrs.2020.105367. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lu N, Qiao C, Ni T, Li Z, Yu B, Guo Q, Wei L. FV-429 induces apoptosis and inhibits glycolysis by inhibiting Akt-mediated phosphorylation of hexokinase II in MDA-MB-231 cells. Mol Carcinog. 2016;55(9):1317–1328. doi: 10.1002/mc.22374. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Pei HZ, Li T, Huang J, Guo Y, Zhao Y, Yang M, Zhang D, Chang Z, Zhang Q, Yu L, He C, Zhang L, Pan Y, Chen C, Chen Y. The molecular mechanisms of resistance to idh inhibitors in acute myeloid leukemia. Front Oncol. 2022;12:931462. doi: 10.3389/fonc.2022.931462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Rabinowitz JD, White E. Mitochondria and Cancer. Mol Cell. 2016;61(5):667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]