Abstract
Purpose
The ASCENT trial demonstrated the efficacy of sacituzumab govitecan for the treatment of advanced or metastatic triple-negative breast cancer (TNBC). The current study evaluated the cost-effectiveness of receiving sacituzumab govitecan compared with standard of care chemotherapy from the United States payer perspective.
Methods
A partitioned survival approach was used to project the disease course of advanced or metastatic TNBC. Two survival modes were applied to analyze two groups of patients. The survival data were gathered from the ASCENT trial. Direct medical costs were derived from the data of Centers for Medicare & Medicaid Services. Utility data was collected from the published literature. The incremental cost-utility ratio (ICUR) was the primary outcome that measured the cost-effectiveness of therapy regimen. One-way sensitivity and probabilistic sensitivity analysis were implemented to explore the uncertainty and validate the stability of results.
Results
In the base-case, the ICUR of sacituzumab govitecan versus chemotherapy is $ 778,771.9/QALY and $ 702,281/QALY for full population group and brain metastatic-negative (BMN) group with the setting of classic survival mode. And in the setting of cure survival mode, the ICUR is $ 506,504.5/QALY for the full population group and $ 274,232.0/QALY for BMN population group. One-way sensitivity analyses revealed that the unit cost of sacituzumab govitecan and body weight were key roles that lower the ICUR value. Probabilistic sensitivity analyses also showed that reducing the unit price of sacituzumab govitecan can improve the likelihood of becoming cost-effective.
Conclusion
The cost-effectiveness analysis suggested that from a US payer perspective, sacituzumab govitecan at current price is unlikely to be a preferred option for patients with advanced or metastatic TNBC at a threshold of $ 150,000/QALY.
Keywords: Cost-effectiveness, Triple-negative breast cancer, Sacituzumab govitecan, Cure model, Partitioned survival approach
Highlights
-
•
Sacituzumab govitecan was better in the brain metastases-negative group than in the full population.
-
•
The survival benefit is more significant in the cure mode than in the classic mode.
-
•
The unit price of sacituzumab govitecan and the body weight could have a significant impact on the reduction in ICUR.
-
•
From a US payer perspective, sacituzumab govitecan is unlikely to be a preferred option for patients with advanced or metastatic TNBC.
1. Introduction
Breast cancer is the most common malignancy among women worldwide and GLOBOCAN data from the World Health Organization estimates that 2.26 million new breast cancer cases were diagnosed in 2020, which accounts for 11.7% of new diagnosed cancers across the world [1]. And the Global Burden of Disease (GBD) reported that the disability-adjusted life-years (DALYs) of breast cancer ranked first and accounted for 17.06% in female malignancies [2]. Breast cancer is classified into 4 types according to the expression of relevant receptors such as estrogen receptors (ERs), progesterone receptors (PRs) and human epidermal growth factors receptor 2 (HER2). These subtypes include luminal A-like, Luminal B-like, HER2-positive and triple-negative breast cancer [3]. Triple-negative breast cancer (TNBC) is defined as a breast cancer that does not express the ER, PR, or HER2, which accounts for 15–20% of all breast cancers. Due to the poor response to endocrine therapy and targeted therapy, treatment strategies are very limited and the prognosis of TNBC is poor. Among patients with recurrent or metastatic disease TNBC, the median overall survival (OS) is 9–13 months compared with 20 months in patients with non-TNBC [4]. The U.S. Food and Drug Administration (FDA) approved the poly ADP-ribose polymerase (PARP) inhibitors olaparib and talazoparib for patients with TNBC of germline BRCA mutations [5,6], and the programmed cell death protein receptor-1 (PD-1) inhibitor pembrolizumab in combination with chemotherapy was recommended for TNBC patients whose tumors express programmed cell death ligand 1 (PD-L1) [7]. However, only 15% of patients with TNBC have germline BRCA mutations and 40% express PD-L1 [8,9]. Therefore, although the response rate of chemotherapy regimen is only 10%–15% and the median progression-free survival (PFS) is only 2–3 months [10,11], chemotherapy is still the standard of care for patients with TNBC. However, the approval of sacituzumab govitecan by FDA offers new therapeutic options for TNBC [12]. Sacituzumab govitecan, as an antibody-drug conjugate (ADC), is a new antineoplastic agent. It is composed of monoclonal antibody (mAb) targeting trophoblast cell-surface antigen 2 (Trop-2) coupled by a hydrolysable linker to SN-38, which is the active metabolite of irinotecan [13]. So, in addition to the powerful killing effect of traditional chemotherapy drugs, sacituzumab govitecan also has the tumor-targeting property of antibody drugs, which can significantly enhance the efficacy and safety of the drug [14]. The ASCENT trial revealed that the median PFS was 5.6 months with sacituzumab govitecan and 1.7 months with chemotherapy (hazard ratio (HR) of 0.41, P < 0.001) for the treatment of patients with TNBC. And the median OS was 12.1 months with sacituzumab govitecan and 6.7 months with chemotherapy (HR of 0.48, P < 0.001) [15]. The results showed the significant survival benefit of sacituzumab govitecan compared with chemotherapy regimen. However, the use of sacituzumab govitecan to the treatment of TNBC could cause economic burden. Whether the survival benefit of sacituzumab govitecan reaches the expected value that matches the pricing needs to be further explored. More economic analyses were needed urgently. In this study, a cost-effectiveness analysis of sacituzumab govitecan versus chemotherapy for the treatment of TNBC from the payer perspective of the United States was conducted. It could provide some pharmacoeconomics data for oncologists or policy-makers when determining the therapy regimen or allocating limited healthcare resources.
2. Material and methods
2.1. Model structure
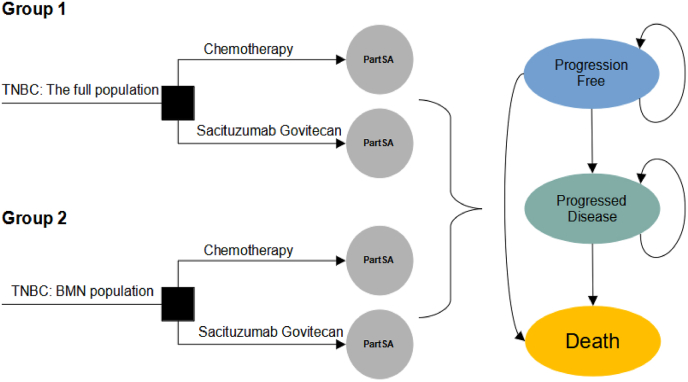
A decision analytic model was designed for sacituzumab govitecan and chemotherapy to analyze the cost-effectiveness from the US payer perspective. A partitioned survival approach (PartSA) was applied to simulate disease developing of patients with TNBC. The simulated population of the PartSA is patients were at least 18 years of age with relapsed or refractory to two or more previous standard chemotherapy regimens for unresectable, locally advanced or metastatic triple-negative breast cancer, which is consistent with the ASCENT trial [15]. In this PartSA, there are three mutually exclusive health states including progression free (PF) survival, progressed disease (PD) and death. It is assumed that the patients entered the model with a default of progression-free survival state, which could develop into progressed disease state or death state based on the clinical survival data.
In this analysis, the patients with TNBC receive the following therapy regimens: (1) chemotherapy; (2) sacituzumab govitecan. The two regimens and the doses strategies are kept with the ASCENT trial. The chemotherapy regimen is composed of eribulin, vinorelbine, capecitabine and gemcitabine, which is determined based on physician's choice. Therein, the eribulin was administrated at a dose of 1.4 mg/m2 body surface area (BSA) intravenously on days 1 and 8 of each 21-day cycle, vinorelbine was used at a dose of 25 mg/m2 intravenously on day 1 each 7-day cycle, capecitabine was administrated at a dose of 1000–1250 mg/m2 orally twice daily on days 1–14 of each 21-day cycle, or gemcitabine was used at a dose of 800–1200 mg/m2 intravenously on days 1, 8 and 15 of each 28-day cycle. The sacituzumab govitecan regimen was administrated at a dose of 10 mg/kg intravenously on days 1 and 8 of each 21-day cycle. Treatment was continued unless encountering the disease progression, unacceptable toxic effects or death. Based on the administration cycle, the one-week model cycle length was set to facilitate cost estimates. The 10-year time horizon is determined in the analysis. Two groups were included in the analysis. The group 1 is linked to the analysis for the full population (all randomized patients including with and without brain metastases) and the group 2 is for the population without brain metastatic. The decision problem and model structure can see Fig. 1.
Fig. 1.
The decision tree and model structure overview. TNBC triple-negative breast cancer; PartSA partitioned survival approach; BMN brain metastatic-negative.
2.2. Clinical data
The ASCENT trial reported the PFS and OS data and safety data. However, the observable survival time is not sufficient for the analysis of the whole model time horizon. Therefore, appropriate extrapolation beyond the follow-up time is needed. The PFS and OS curves of ASCENT trial were digitized to get the time-to-survival data. And then, in order to get the time-to-event data, an algorithm that can obtain pseudo individual participant data (IPD) by Guyot was adopted in this study [16]. The generated time-to-event data was applied to fit a range of parametric distributions, including Weibull, Gompertz, exponential, log-normal, log-logistic distribution. Additionally, mixture and non-mixture cure mode of above five parametric distributions were also used to test the long-term survival outcomes of two treatment regimens when the cure state was considered. In parametric cure models, it is assumed that a proportion of subjects will not experience the event. This ‘cured’ and ‘uncured’ group is separated in a mixture cure model, with cured subjects assuming no excess risk and uncured subjects assuming excess risk. Non-mixture models scale parametric survival distributions so that survival approaches cure fraction asymptotically. The Akaike information criterion (AIC) value of each distribution for all arms was calculated and the best fitted distribution was determined according to the AIC values. As the long-term survival prognosis of cure mode differed from the classic parametric distribution, we built two scenarios to explore the difference of two treatment regimens in survival outcome and cost-effectiveness. In scenario 1, classic parametric distribution was applied to the survival fit. And in scenario 2, the cure mode of classic parametric distribution was used in the fitting of survival. The best fitted distributions and curve parameters were shown in Table 1.
Table 1.
Key clinical data.
| Parameters | Scenario 1 |
Scenario 2 |
|||
|---|---|---|---|---|---|
| Classic mode | values | Cure mode | values | ||
| Full population | PFS: chemotherapy | Log-logistic | Shape = 2.339 Scale = 9.146 |
Log-logistic mixture cure | theta = 0.0426 shape = 2.5569 scale = 8.6537 |
| PFS: sacituzumab govitecan | Log-normal | Meanlog = 2.9889 Sdlog = 1.0169 |
Log-normal mixture cure | theta = 0.0569 meanlog = 2.8824 sdlog = 0.9411 |
|
| OS: chemotherapy | Log-logistic | Shape = 1.904 Scale = 28.350 |
Log-logistic non-mixture cure | theta = 0.0243 shape = 1.74 scale = 67 |
|
| OS: sacituzumab govitecan | Weibull | Shape = 1.424 Scale = 67.278 |
Weibull mixture cure | theta = 0.1174 shape = 1.5381 scale = 57.0151 |
|
| BMN population | PFS: Chemotherapy | Log-logistic | Shape = 2.569 Scale = 8.972 |
Log-logis mixture cure | theta = 0.00241 shape = 2.59 scale = 8.95 |
| PFS: sacituzumab govitecan | Log-logistic | Shape = 1.770 Scale = 22.097 |
Weibull mixture cure | theta = 0.0721 shape = 1.4158 scale = 27.8427 |
|
| OS: chemotherapy | Log-logistic | Shape = 1.839 Scale = 28.903 |
Log-logistic mixture cure | theta = 0.00415 shape = 1.85 scale = 28.8 |
|
| OS: sacituzumab govitecan | Weibull | Shape = 1.411 Scale = 72.222 |
Gompertz non-mixture cure | theta = 0.24201 shape = 0.03646 rate = 0.00385 |
|
| Grade 3 or 4 AEs in the chemotherapy arm | Incidence | Range | Distribution | ||
| Fatigue | 5.40% | (4.05%–6.75%) | Beta | ||
| Leukopenia | 5.40% | (4.05%–6.75%) | Beta | ||
| Anemia | 4.90% | (3.68%–6.13%) | Beta | ||
| Neutropenia | 33% | (24.75%–41.25%) | Beta | ||
| Diarrhea | 0.40% | (0.3%–0.5%) | Beta | ||
| Febrile neutropenia | 2.20% | (1.65%–2.75%) | Beta | ||
| Grade 3 or 4 AEs in the sacituzumab govitecan arm | |||||
| Fatigue | 3.10% | (2.33%–3.88%) | Beta | ||
| Leukopenia | 10.10% | (7.58%–12.63%) | Beta | ||
| Anemia | 7.80% | (5.85%–9.75%) | Beta | ||
| Neutropenia | 51.20% | (38.4%–64%) | Beta | ||
| Diarrhea | 10.50% | (7.88%–13.13%) | Beta | ||
| Febrile neutropenia | 5.80% | (4.35%–7.25%) | Beta | ||
| PFS progression-free survival. OS overall survival. BMN brain metastatic-negative. AEs adverse events. | |||||
As the grade 1 to 2 treatment-related adverse events (AEs) can be managed well, only the grade 3 and 4 AEs were included in the analysis. The incidence of adverse events derived from the ASCENT trial can be seen in Table 1.
2.3. Costs and utilities
The United States payer perspective was adopted in this analysis. Therefore, direct medical expenditures were included in the cost estimates, which covered the therapy drugs, administration for intravenous injection, management of severe AEs, follow-up and palliative care. The drugs costs were collected from the Centers for Medicare & Medicaid Services (CMS) [17]. The average sales price (ASP) that the manufacturer reported was adopted. The average weight of 77.5 kg and height of 1.61 m in women, derived from the National Center for Health Statistics (NCHS) data, were used to calculate the average BSA and the dosage of drugs. A 1.86 m2 of BSA was applied [18]. The overall drug costs were calculated according to the predetermined dosing strategy. In the chemotherapy regimen, the eribulin, vinorelbine, capecitabine and gemcitabine single agent were assigned according to the proportion of 54%, 20%, 13% and 12%, respectively [15]. The cost of chemotherapy regimen is estimated based on the proportions. The cost of administration for intravenous injection was taken from the 2021 Physician's Fee Schedule [19]. As for the cost data of palliative care, follow-up and best supportive care, we referred to a pharmacoeconomic study based on the US perspective and extracted the cost data with appropriate model cycle adjustments to adapt the current study [20]. The expenditure of management of severe (grade 3 and 4) AEs was gathered from open accessed database [21]. All costs presented for years prior to 2021 are updated to 2021 US dollars (USD) using Consumer Price Index (CPI).
Each health state in the PartSA model should be assigned a health utility value that reflects its stage of progression. However, the direct utility data of ASCENT trial was not reported. Therefore, highly relevant and robust data is extremely crucial. We assumed the quality of life is related to the progressive stage, the utility value for PF state was estimated to be 0.86 and the PD state was 0.60 according to relevant published studies [22]. More detailed values of inputs are summarized in Table 2.
Table 2.
Model Costs, Utility estimates and other parameters.
| Parameter | Distribution | The US | |
|---|---|---|---|
| Treatment costs | Values (Range), USD | Reference | |
| Sacituzumab govitecan (per 2.5 mg) | Gamma | 30.354 (22.766–37.943) | [17] |
| Eribulin (per 0.1 mg) | Gamma | 123.930 (92.948–154.913) | [17] |
| Capecitabine (per 500 mg) | Gamma | 0.922 (0.692–1.153) | [17] |
| Vinorelbine (per 10 mg) | Gamma | 10.012 (7.509–12.515) | [17] |
| Gemcitabine (per 200 mg) | Gamma | 3.816 (2.862–4.770) | [17] |
| Administration (first hour) | Gamma | 148.30 (111.23–185.38) | [19] |
| Administration (additional hour) | Gamma | 31.40 (23.55–39.25) | [19] |
| Follow-up (per cycle) | Gamma | 299.96 (224.97–374.95) | [20] |
| Best supportive care (per cycle) | Gamma | 1207.69 (905.77–1509.61) | [20] |
| Palliative care | Gamma | 10,023.77 (7517.83 to 12,529.71) | [20] |
| Expenditure of AEs management | Values (Range), USD | Reference | |
| Fatigue | Gamma | 28,725 (21,544–35,906) | [21] |
| Leukopenia | Gamma | 30,434 (22,826–38,043) | [21] |
| Anemia | Gamma | 33,585 (25,189–41,981) | [21] |
| Neutropenia | Gamma | 51,418 (38,564–64,273) | [21] |
| Diarrhea | Gamma | 31,805 (23,854–39,756) | [21] |
| Febrile neutropenia | Gamma | 51,384 (38,538–64,230) | [21] |
| Utility estimates | Values (Range) | Reference | |
| Progression-Free Disease | Beta | 0.86 (0.645–1) | [22] |
| Progressive Disease | Beta | 0.60 (0.45–0.75) | [22] |
| Utility decrements | Values (Range) | Reference | |
| Fatigue | Beta | 0.09 (0.0675–0.1125) | [23] |
| Leukopenia | Beta | 0.09 (0.0675–0.1125) | [24] |
| Anemia | Beta | 0.12 (0.09–0.15) | [23] |
| Neutropenia | Beta | 0.09 (0.0675–0.1125) | [23] |
| Diarrhea | Beta | 0.12 (0.09–0.15) | [23] |
| Febrile neutropenia | Beta | 0.09 (0.0675–0.1125) | [23] |
| Other parameters | Values (Range) | Reference | |
| Body surface area, m2 | Normal | 1.86 (1.40–2.33) | [18] |
The costs of AEs in this table were presented on a per-event basis, and the costs and disutilities of AEs were calculated only once at the beginning of running the analysis model. All costs reported for years prior to 2021 are updated to 2021 USD using the American CPI. All costs sourced from China in this study were converted into US dollars ($1 = RMB 6.4512, Average exchange rate for 2021). Abbreviations: AEs adverse events, CPI Consumer Price Index, USD US dollars, CMS Centers for Medicare and Medicaid Services.
2.4. Analyses
In the base-case analysis, incremental cost per additional life-year (LY) gained between the two regimens was assessed using the incremental cost-effectiveness ratio (ICER). The incremental cost-utility ratio (ICUR) was used to assess incremental cost per quality-adjusted life-year (QALY). All QALYs and costs were discounted at an annual rate of 3%. It indicates that the regimen is “cost-effective” if the ICUR is below the willingness-to-pay (WTP) threshold. A systematic review summarized the WTP threshold in the health economic studies, and threshold of $100,000–150,000/QALY usually was used in the setting of the United States [25]. In this analysis, we adopted the threshold of $150,000/QALY to assess the ICUR in the context of the United States.
In order to assess the robustness of our findings and determine which variable had a significant impact on them, we conducted both one-way and probabilistic sensitivity analysis (PSA) for all model inputs. In one-way sensitivity analyses, the range of annual discount rate is from 0 to 8% and other inputs of that were assumed a variation by ± 25% of the base-case value. And Monte Carlo simulations of 1000 iterations were used for the PSA. Each of the inputs was sampled simultaneously based on specific probability distributions. Gamma distribution was used for the cost inputs, and Beta distribution is for health utilities and probabilities of adverse events [26]. The cost-effectiveness acceptability curve (CEAC) was created to clearly present the likelihood that treatment strategy was cost-effective at a range of WTP threshold. R (version 4.1.2, http://www.r-project.org) was used to create and programmed the PartSA model and cost-effectiveness analysis model.
3. Results
3.1. Replicated Kaplan-Meier survival curve and the predicted survival curve
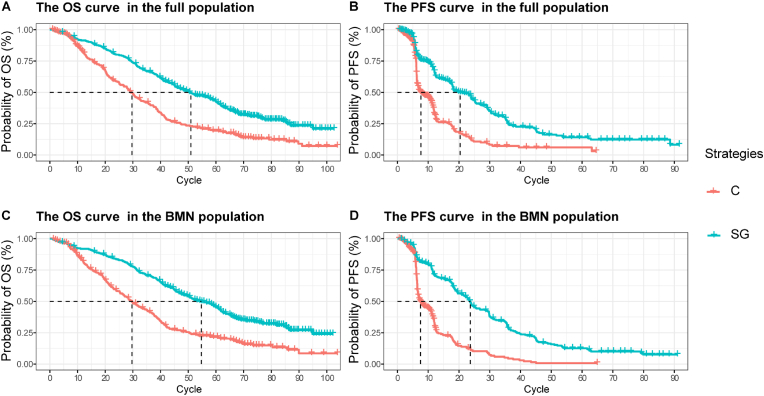
As shown in Fig. 2, replicated Kaplan-Meier survival curves were generated. Additionally, the predicted PFS and OS curves of every treatment regimen also were simulated (see Supplemental Fig. 1). The selected distribution of projected curve could be seen in Table 1. All estimated parameters and AIC value from each survival model were listed in Supplemental Table 1.
Fig. 2.
Replicated Kaplan-Meier survival curve in different regimens. A: Output of OS curve for the full population; B: Output of PFS curve for the full population; C: Output of OS curve for the BMN population; D: Output of PFS curve for the BMN population. Each cycle of the x-axis is one week. PFS progression-free survival, OS overall survival, BMN brain metastatic-negative, SG sacituzumab govitecan, C chemotherapy.
3.2. Base-case analysis
The outputs of base-case analysis varied large across the classic mode and cure mode. The value of ICER or ICUR in the setting of cure mode was lower than that in the classic mode. All base-case results were summarized in Table 3.
Table 3.
Results of the base-case analysis.
| Fit mode | Population | Regimen | LYs | QALYs | Cost, US$ | ICER($/LY) | ICUR ($/QALY) |
|---|---|---|---|---|---|---|---|
| Classic mode | Full population | chemotherapy | 0.8680559 | 0.519220 | 76,793.6 | – | – |
| sacituzumab govitecan | 1.2051341 | 0.781556 | 281,093.5 | 606,090.52 | 778,771.9 | ||
| BMN population | chemotherapy | 0.9095444 | 0.537392 | 75,508.2 | – | – | |
| sacituzumab govitecan | 1.3223683 | 0.865926 | 306,231.4 | 558,890.12 | 702,281.0 | ||
| Cure mode | Full population | chemotherapy | 1.085719 | 0.712345 | 102,898.7 | – | – |
| sacituzumab govitecan | 2.056067 | 1.299555 | 400,323.2 | 306,513.23 | 506,504.5 | ||
| BMN population | chemotherapy | 0.9400396 | 0.557619 | 76,980.2 | – | – | |
| sacituzumab govitecan | 3.075471 | 1.836158 | 427,596.5 | 164,189.91 | 274,232.0 |
BMN brain metastatic-negative, LY life-year, QALY quality-adjusted life-year, ICER incremental cost-effectiveness ratio, ICUR incremental cost-utility ratio.
3.2.1. Scenario 1: classic mode
3.2.1.1. Full population group
In this group, patients with TNBC received chemotherapy regimen gained 0.8681 LY, 0.5192 QALYs and expended $ 76,793. Receiving sacituzumab govitecan regimen resulted in 1.2051 LY, 0.7815 QALYs gained and $ 281,093 expended. Compared with the chemotherapy regimen, the sacituzumab govitecan regimen increased the overall cost by $ 204,300. For effectiveness, sacituzumab govitecan regimen showed an increase of 0.337 LY, 0.2623 QALYs compared with chemotherapy regimen. The ICER and ICUR of Sacituzumab govitecan versus chemotherapy is $ 606,090.5/LY and $ 778,771.9/QALY, respectively.
3.2.1.2. BMN population group
TNBC patients without brain metastases received chemotherapy regimen gained 0.9095 LY, 0.5373 QALYs and expended $ 75,508. Receiving sacituzumab govitecan regimen resulted in 1.3224 LY, 0.865 QALYs gained and $ 306,231 expended. Compared with the chemotherapy regimen, the sacituzumab govitecan regimen increased the overall cost by $ 230,723.2. For effectiveness, sacituzumab govitecan regimen showed an increase of 0.4129 LY, 0.3285 QALYs compared with chemotherapy regimen. The ICER and ICUR of sacituzumab govitecan versus chemotherapy is $ 558,890.1/LY and $ 702,281.0/QALY, respectively.
3.2.2. Scenario 2: cure mode
3.2.2.1. Full population group
In this group, patients with TNBC received chemotherapy regimen gained 1.0857 LY, 0.7123 QALYs and expended $ 102,898. Receiving sacituzumab govitecan regimen resulted in 2.0561 LY, 1.2995 QALYs gained and $ 400,323 expended. Compared with the chemotherapy regimen, the sacituzumab govitecan regimen increased the overall cost by $ 297,425. For effectiveness, sacituzumab govitecan regimen showed an increase of 0.9704 LY, 0.5872 QALYs compared with chemotherapy regimen. The ICER and ICUR of sacituzumab govitecan versus chemotherapy is $ 306,513.2/LY and $ 506,504.5/QALY, respectively.
3.2.2.2. BMN population group
TNBC patients without brain metastases received chemotherapy regimen gained 0.9400 LY, 0.5576 QALYs and expended $ 76,980. Receiving sacituzumab govitecan regimen resulted in 3.0755 LY, 1.8361 QALYs gained and $ 427,596 expended. Compared with the chemotherapy regimen, the sacituzumab govitecan regimen increased the overall cost by $ 350,616. For effectiveness, sacituzumab govitecan regimen showed an increase of 2.1355 LY, 1.2785 QALYs compared with chemotherapy regimen. The ICER and ICUR of sacituzumab govitecan versus chemotherapy is $ 164,189.9/LY and $ 274,232.0/QALY, respectively.
3.3. One-way sensitivity analysis
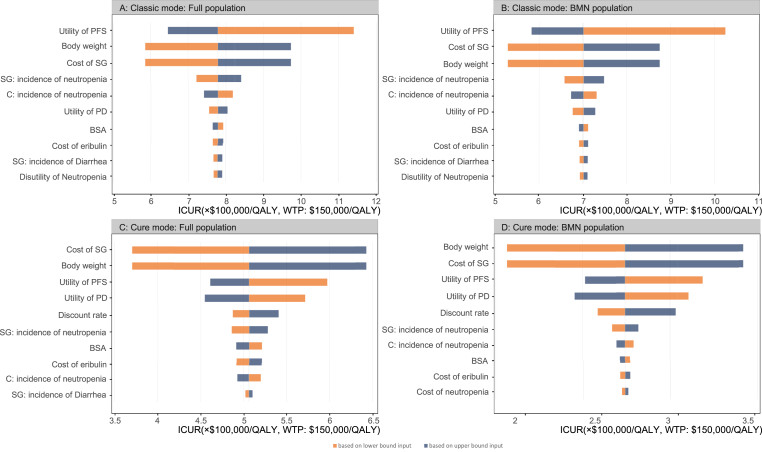
The one-way sensitivity analyses were conducted to test sensitivity of output to key parameters. The analysis outcomes, represented in the form of a tornado diagrams (Fig. 3), can illustrate the impact of changing input parameters on the ICUR while holding all other parameters constant.
Fig. 3.
Tornado diagram of the one-way sensitivity analysis results. A The output of full population in the classic mode. B The output of BMN population in the classic mode. C The output of full population in the cure mode. D The output of BMN population in the cure mode. BMN brain metastatic-negative, SG sacituzumab govitecan, C chemotherapy, BSA body surface area, PFS progression-free survival, PD progressed disease, ICUR incremental cost-utility ratio, QALY quality-adjusted life-year, WTP willingness to pay.
3.3.1. Scenario 1: Classic mode
In the full population group, we can find that, from the diagram of tornado (Fig. 3A), the utility of PFS, the unit price of sacituzumab govitecan and the body weight were the key driving factors that have a significant impact on ICUR between sacituzumab govitecan and chemotherapy regimen. The range of the ICUR was from $ 585,269.8/QALY to $ 1,140,339.1/QALY.
In the brain metastatic-negative (BMN) population group, the tornado diagram of sacituzumab govitecan versus chemotherapy regimen (Fig. 3B) showed that the utility of PFS, the unit price of sacituzumab govitecan and the body weight could yield significant effects on the ICUR. The range of ICUR was from $ 528,928.4/QALY to $ 1,024,781.6/QALY. The unit price of the sacituzumab govitecan and the weight, as key variables affecting the total cost in sacituzumab govitecan regimen, can minimize the ICUR. The impact of other variables on the ICUR was not prominent.
3.3.2. Scenario 2: Cure mode
In full population, the limit of outputs of one-way sensitivity analysis ranged from $ 370,257/QALY to $ 642,761/QALY. The tornado diagram (Fig. 3C) revealed that the unit price of sacituzumab govitecan and body weight were the key variables affecting the ICUR. Lowering the values of these two variables can significantly decrease the cost difference across the sacituzumab govitecan and chemotherapy regimens. The ICUR would decrease accordingly.
In the BMN population, the range of outputs was from $ 206,371.1/QALY to $ 342,097.3/QALY. Similar to the full population group, the two variables that contribute most to the reduction of ICUR are still the unit price of sacituzumab govitecan and body weight. Other variables had little impact on the reduction of ICUR.
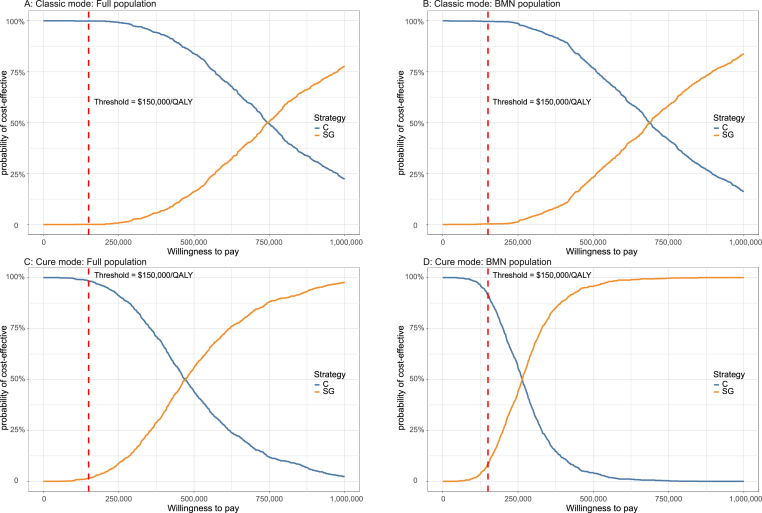
3.4. Probabilistic sensitivity analysis
A total of 1000 iterations were run to sample all model parameters from probability distributions simultaneously. The average results of the probabilistic sensitivity analysis generally align with the base-case results (see Supplemental Table 2). The CEACs (Fig. 4) were built to assess the likelihood of each treatment regimen would be regarded as cost-effective at various thresholds of WTP.
Fig. 4.
Cost-effectiveness acceptable curve. The y-axis indicates the likelihood that a regimen is cost-effective across the willingness-to-pay threshold (x-axis). BMN brain metastatic-negative, QALY quality-adjusted life-year, SG sacituzumab govitecan, C chemotherapy.
3.4.1. Scenario 1: Classic mode
Whether in the full population or BMN population, the CEAC showed sacituzumab govitecan regimen was almost 0% of being cost-effective at the $150,000/QALY threshold. And the chemotherapy regimen was close to 100% of being cost-effective at the same threshold. As the price of sacituzumab govitecan is a potential variable that can reduce the ICUR, an additional probabilistic sensitivity analyses of setting its price to 75%, 50% and 25% of the base-value were conducted. The further CEACs could be seen in Supplemental Fig. 2. In the full population, the likelihood of sacituzumab govitecan in the price reduction setting was 0.1%, 1.4% and 29.7% of being cost-effective respectively. And in the BMN population, the likelihood of sacituzumab govitecan was 0.3%, 4.3% and 32.6%, respectively. More outputs were summarized in Supplemental Table 3.
3.4.2. Scenario 2: Cure mode
In full population, at the threshold of $150,000/QALY, sacituzumab govitecan regimen have 1.7% likelihood of being cost-effective, the chemotherapy regimen was 98.3% of being cost-effective. In the BMN population, the likelihood of sacituzumab govitecan regimen being cost-effective reached 8.5%, the chemotherapy regimen was 91.5%. The CEAC (Supplemental Fig. 2B) also showed the trend of the probability when the price of sacituzumab govitecan was reduced to 75%, 50% and 25%. We summarized the probabilities of each regimen becoming a cost-effective regimen. For detailed data, please see Supplemental Table 3. In the full population, the sacituzumab govitecan show a 4.5%, 21.5% and 83% of being cost-effective strategy, respectively. And in the BMN population, the probability that sacituzumab govitecan regimen would be deemed as cost-effective was 22.6%, 61% and 98.7%, respectively.
4. Discussion
Sacituzumab govitecan is a first-in-class, Trop-2-directed antibody-drug conjugate that has shown significant benefits in terms of PFS and OS compared to standard of care chemotherapy. Its efficacy was recognized and the approval provided a new option for treatment of TNBC. However, economic evaluation is lacking, which motivates the current study. This study examined the cost-effectiveness of sacituzumab govitecan for TNBC patients with or without brain metastases compared to standard of care chemotherapy. Also, exploratory scenario analyses for long-term outcomes were established based on the survival mode. According to the base-case analyses, the ICUR of sacituzumab govitecan versus chemotherapy in all groups and scenarios exceed the prespecified threshold of $150,000/QALY. The ICUR value in the BMN group is lower than that in the full population group. The average output of probabilistic sensitivity analyses is generally consistent with the base-case results, which also indicated the robustness of this analysis. And the one-way sensitivity analyses illustrated that the unit price of sacituzumab govitecan and body weight have significant effect on the reduction of the ICUR across two therapy regimens. Since weight affects dosage, the overall cost can be affected by both unit price and dosage. These analyses implied that the price of sacituzumab govitecan exceed the value matched its efficacy.
In the absence of the long-term follow-up data, two modes of extrapolating the survival data were adopted. Cure mode was designed to fit Kaplan-Meier curve when the tail of curve has shown a long period of flatness. Although the cure is an unlikely situation at present, the cure mode is still used for exploratory purposes. The use of classical mode seems to be more in line with the progressing trend of advanced cancer. The outputs of two modes can provide valuable economic information when long-term survival is included in decision-making. From the results of base-case analysis, the application of cure mode improved the QALY significantly, especially in the sacituzumab govitecan regimen. But the ICUR of full population group ($ 506,504.5/QALY) or BMN group ($ 274,232.0/QALY) still exceeds the threshold. Once again, it is implied that its pricing of sacituzumab govitecan exceeds the value that matches its efficacy. It is, therefore, important to strike a balance between the costs and the added value of clinical efficacy. For this purpose, we have hypothetically analyzed several scenarios of the probability of the sacituzumab govitecan becoming the preferred strategy with a 25%, 50%, and 75% price reduction, respectively (see Supplemental Tables 2–3). In the setting of classic mode, the ICUR ($ 199,875.6/QALY for full population group vs. $185,025/QALY for BMN group) decreased to close to $150,000/QALY when the unit cost of sacituzumab govitecan decreased by 75%. In the setting of cure mode, the ICUR for BMN group was $ 139,406.4/QALY when the unit cost of sacituzumab govitecan decreased by 50%. It is indicated that sacituzumab govitecan regimen would be cost-effective. And with the 75% reduction of unit price of sacituzumab govitecan, the value of ICUR for both the full population and BMN group would be lower than $ 100,000/QALY. The results suggested that sacituzumab govitecan needs to become more affordable to enhance the use of this regimen as a preferred treatment. Considering these results, it is clear that reducing the price of sacituzumab govitecan is essential to make it a preferred regimen. Additionally, a strategy known as “reduced doses” can be used well for clinical setting to control the expenditure, in which the minimal doses necessary are taken to reach a good response with a potential reduction of adverse events. Several studies on optimal dosing strategy identified advantages in terms of drug-exposure risk and cost saving [[27], [28], [29]]. For example, the use of fractionated lower doses of gemtuzumab ozogamicin (an ADC targeting CD33 for acute myelogenous leukemia) allows the safe delivery of higher cumulative doses and substantially improves outcomes [29]. Lowering the dosage of sacituzumab govitecan can decrease the overall drug expenditure. However, there is a lack of evidence and clinical trial to verify the effectiveness of sacituzumab govitecan's “reduced doses” strategy for TNBC. More studies on dose intensity or dose strategy of sacituzumab govitecan should be conducted.
This study has several potential weaknesses as well. Firstly, since patient survival data were taken from the ASCENT trial, only the utility values derived from the ASCENT trial were closest to the true values. The outcomes about health-related utility values without disease progression and progressed disease derived from the ASCENT trial weren't reported. Therefore, we had to extract utility data from published literature. The tornado diagram showed utility values do not contribute significantly to ICUR reduction. Secondly, in the progress of digitizing, gathering the data points from the PFS and OS curves resulted in uncertainty. And the individual patient data used in the model was generated through algorithm rather than the real individual patient data. It was validated that the replicated Kaplan-Meier survival curves based on pseudo IPD matched well with the original Kaplan-Meier curves. Thirdly, the application of cure mode also resulted in uncertainty. As the prognosis of advanced or metastatic breast cancer is poor, the probability of cure state is little [30]. Although a small proportion of patients in the cohort may have a potentially curative condition, cure still belongs to an ideal state and the setting of cure mode was conducted for exploratory purposes only.
The cost-effectiveness analysis suggested that from a US payer perspective, sacituzumab govitecan at current price is unlikely to be a preferred option for patients with advanced or metastatic TNBC at a WTP threshold of $ 150,000/QALY. Some measures, such as price strategy, dose strategy, payment strategy, etc., need to be taken to make it a cost-effective option for the treatment of TNBC.
Author contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; All authors took part in drafting the article or revising it critically for important intellectual content and agreed to submit to the current journal; All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Ethics approval and informed consent
This study is based on a literature review and modeling techniques, so it doesn't require the approval by an institutional research ethics board.
Funding
This work was supported by authors' organization and the Wu Jieping Medical Foundation (grant number 320.6750.2021-10-28).
Data availability
All data generated or analyzed during this study are included in this article/Supplementary Material.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2023.02.003.
Contributor Information
Xiaoyan Liu, Email: liuxiaoyanrj@sjtu.edu.cn.
Bin Wu, Email: scilwsjtu-wb@yahoo.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Kyu H.H., Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N., et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tarantino P., Hamilton E., Tolaney S.M., Cortes J., Morganti S., Ferraro E., et al. HER2-Low breast cancer: pathological and clinical landscape. J Clin Orthod. 2020;38:1951–1962. doi: 10.1200/JCO.19.02488. [DOI] [PubMed] [Google Scholar]
- 4.Tray N., Adams S., Esteva F.J. Antibody–drug conjugates in triple negative breast cancer. Future Oncol. 2018;14:2651–2661. doi: 10.2217/fon-2018-0131. [DOI] [PubMed] [Google Scholar]
- 5.Robson M.E., Tung N., Conte P., Im S.-A., Senkus E., Xu B., et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558–566. doi: 10.1093/annonc/mdz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes J., Cescon D.W., Rugo H.S., Nowecki Z., Im S.-A., Yusof M.M., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–1828. doi: 10.1016/S0140-6736(20)32531-9. [DOI] [PubMed] [Google Scholar]
- 8.Bardia A., Tolaney S.M., Punie K., Loirat D., Oliveira M., Kalinsky K., et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32:1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Weiss J., Glode A., Messersmith W.A., Diamond J. Sacituzumab govitecan: breakthrough targeted therapy for triple-negative breast cancer. Expet Rev Anticancer Ther. 2019;19:673–679. doi: 10.1080/14737140.2019.1654378. [DOI] [PubMed] [Google Scholar]
- 10.Plasilova M.L., Hayse B., Killelea B.K., Horowitz N.R., Chagpar A.B., Lannin D.R. Features of triple-negative breast cancer: analysis of 38,813 cases from the national cancer database. Medicine. 2016;95 doi: 10.1097/MD.0000000000004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin L., Duan J.-J., Bian X.-W., Yu S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22:61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Syed Y.Y. Sacituzumab govitecan: first approval. Drugs. 2020;80:1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGuinness J.E., Kalinsky K. Antibody-drug conjugates in metastatic triple negative breast cancer: a spotlight on sacituzumab govitecan, ladiratuzumab vedotin, and trastuzumab deruxtecan. Expet Opin Biol Ther. 2021;21:903–913. doi: 10.1080/14712598.2021.1840547. [DOI] [PubMed] [Google Scholar]
- 14.Khongorzul P., Ling C.J., Khan F.U., Ihsan A.U., Zhang J. Antibody–drug conjugates: a comprehensive review. Mol Cancer Res. 2020;18:3–19. doi: 10.1158/1541-7786.MCR-19-0582. [DOI] [PubMed] [Google Scholar]
- 15.Bardia A., Hurvitz S.A., Tolaney S.M., Loirat D., Punie K., Oliveira M., et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–1541. doi: 10.1056/NEJMoa2028485. [DOI] [PubMed] [Google Scholar]
- 16.Guyot P., Ades A., Ouwens M.J., Welton N.J. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Medicare&Medicaid Services . 2021. https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2022-asp-drug-pricing-files ASP Drug Pricing Files n.d. accessed. [PubMed] [Google Scholar]
- 18.Centers for Disease control and Prevention Measured average height, weight, and waist circumference for adults aged 20 and over. https://www.cdc.gov/nchs/fastats/body-measurements.htm National Center for Health Statistics: Body Measurements n.d. accessed.
- 19.Centers for Medicare and Medicaid Services Search the physician fee Schedule n.d. https://www.cms.gov/medicare/physician-fee-schedule/search accessed.
- 20.Wu B., Ma F. Cost-effectiveness of adding atezolizumab to first-line chemotherapy in patients with advanced triple-negative breast cancer. Ther Adv Med Oncol. 2020;12 doi: 10.1177/1758835920916000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality Healthcare cost and utilization project n.d. https://hcupnet.ahrq.gov/#setup
- 22.Paracha N., Thuresson P.-O., Moreno S.G., MacGilchrist K.S. Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:549–559. doi: 10.1080/14737167.2016.1222907. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler S.B., Rotter J., Gogate A., Reeder-Hayes K.E., Drier S.W., Ekwueme D.U., et al. Cost-effectiveness of pharmacologic treatment options for women with endocrine-refractory or triple-negative metastatic breast cancer. J Clin Orthod. 2022 doi: 10.1200/JCO.21.02473. JCO.21.02473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding D., Hu H., Li S., Zhu Y., Shi Y., Liao M., et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Cancer Netw. 2021:jnccn20454. doi: 10.6004/jnccn.2020.7796. [DOI] [PubMed] [Google Scholar]
- 25.Verma V., Sprave T., Haque W., Simone C.B., Chang J.Y., Welsh J.W., et al. A systematic review of the cost and cost-effectiveness studies of immune checkpoint inhibitors. J Immunotherapy Cancer. 2018;6:128. doi: 10.1186/s40425-018-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs A.H., Weinstein M.C., Fenwick E.A.L., Karnon J., Sculpher M.J., Paltiel A.D., et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32:722–732. doi: 10.1177/0272989X12458348. [DOI] [PubMed] [Google Scholar]
- 27.Amadori S., Suciu S., Selleslag D., Aversa F., Gaidano G., Musso M., et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Orthod. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 28.Petersdorf S.H., Kopecky K.J., Slovak M., Willman C., Nevill T., Brandwein J., et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castaigne S., Pautas C., Terré C., Raffoux E., Bordessoule D., Bastie J.-N., et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 30.Liu C.-T., Hsieh M.-C., Su Y.-L., Hung C.-M., Pei S.-N., Liao C.-K., et al. Metronomic vinorelbine is an excellent and safe treatment for advanced breast cancer: a retrospective, observational study. J Cancer. 2021;12:5355–5364. doi: 10.7150/jca.60682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article/Supplementary Material.