Abstract
Introduction
Many psychiatric illnesses have been linked to the gut microbiome, with supplements such as probiotics showing some efficacy in alleviating the symptoms of some psychiatric illnesses. The aim of this review is to evaluate the current literature investigating the effects of adjuvant probiotic or synbiotic administration in combination with first-line treatments for psychiatric illnesses.
Method
A systematic search of four databases was conducted using key terms related to treatments for psychiatric illnesses, the gut microbiome, and probiotics. All results were then evaluated based on specific eligibility criteria.
Results
Eight studies met eligibility criteria and were analyzed for reported changes in outcome measures used to assess the symptoms of psychiatric illness and the tolerability of treatment. All Major Depressive Disorder (MDD) (n = 5) and Generalized Anxiety Disorder (GAD) (n = 1) studies found adjuvant probiotic or synbiotic treatment to be more efficacious in improving the symptoms of psychiatric illness than the first-line treatment alone or with placebo. The schizophrenia studies (n = 2) found adjuvant probiotic treatment to have no significant difference in clinical outcomes, but it was found to improve the tolerability of first-line antipsychotics.
Discussion and conclusion
The findings of the studies included in this review suggest the use of adjuvant probiotic treatment with selective serotonin reuptake inhibitors (SSRIs) for MDD and GAD to be superior to SSRI treatment alone. Probiotic adjuvant treatment with antipsychotics could be beneficial for improving the tolerability of the antipsychotics, but these findings do not suggest that adjuvant probiotic treatment would result in improved clinical outcomes for symptoms of schizophrenia.
Keywords: probiotics, psychiatric illness, psychotropics, adjuvant therapy, gut-brain-axis probiotics, gut-brain-axis, Major Depressive Disorder
Introduction
The human gastrointestinal (GI) tract houses trillions of microorganisms, which have co-evolved with their host and collectively contain over 100 times as many genes as the human genome (Bermon et al., 2015). Colonization of the gut begins at birth, being influenced by the mode of delivery and breastfeeding (Martin et al., 2016) and through the gut’s microbial composition somewhat stabilizes throughout adulthood, factors such as the environment, diet, medication, genetics, and age continue to shape microbiota composition and function throughout one’s life (Li et al., 2014; Heiman and Greenway, 2016; Odamaki et al., 2016; Cussotto et al., 2019). There is a well-established, bidirectional connection between the gut microbiome and the brain, known as the gut-brain-axis (GBA). This communication includes portions of the sympathetic and the parasympathetic nervous system, the enteric nervous system, as well as both neuroimmune and neuroendocrine signaling (Cryan et al., 2019; Morais et al., 2021; Liu et al., 2022). Research suggests that the GBA may influence a variety of neurological functions, including the pathology of psychiatric disorders.
Major Depressive Disorder (MDD), Generalized Anxiety Disorder (GAD) and Schizophrenia (SZ) are widely known and severe psychiatric disorders. MDD is characterized by pervasive depressed mood and/or loss of interest or pleasure, along with an array of other possible psychiatric and physiological symptoms, and is the leading cause of disability worldwide (Evans-Lacko et al., 2018). GAD has a similarly significant impairment in daily functioning (American Psychiatric Association [APA], 2013), and is characterized by a persistent, exaggerated worry about everyday events. MDD and GAD are also somewhat gendered illnesses, with the prevalence in women being reported as 1.5 to 3 times that of men (Vesga-López et al., 2008; Sabic et al., 2021). Antidepressant medications such as selective serotonin reuptake inhibitors (SSRIs) are considered a first-line therapy for MDD and GAD (Gelenberg et al., 2010; Baldwin et al., 2011). SZ is a highly heterogenous psychotic disorder, characterized by continuous or relapsing episodes of positive symptoms like delusions, hallucinations and irrational thoughts or actions, and negative symptoms like lethargy, apathy, and social withdrawal. Second-generation antipsychotics are considered the first-line treatment for SZ (Patel et al., 2014). Gender differences found in schizophrenia are less consistent, with some reports of equal prevalence between men and women, and some reports of increased prevalence among men (Ochoa et al., 2012).
Various studies have found the microbiota composition of patients with these psychiatric disorders to be significantly different from those of healthy controls (Zhu et al., 2020; Nikolova et al., 2021). Interestingly, fecal microbiota transplants from psychiatric patients to germ-free rodents have been shown to induce symptoms similar to those associated with the disorders of the donors (Bercik et al., 2011; Neufeld et al., 2011). Certain probiotics or fecal microbiota transplants from healthy patients have also helped alleviate symptoms and induced positive outcomes in patients with psychiatric disorders (Meyyappan et al., 2020; Johnson et al., 2021). As such, there is emerging evidence to suggest that the gut microbiome and the GBA play a crucial role in inducing and modulating psychiatric disorders.
A significant subset of patients affected by these disorders are treatment-resistant or experience adverse effects when taking antidepressants or antipsychotics. Antipsychotic usage is commonly associated with adverse metabolic and endocrine effects such as weight gain and insulin resistance (De Hert et al., 2011), while SSRIs frequently induce unpleasant side effects such as nausea, insomnia, drowsiness and agitation (Hirsch and Birnbaum, 2017). Such adverse effects contribute to low treatment compliance and tolerability. Low compliance and inconsistent efficacy indicate that there is a need to explore alternative treatments, or approaches to counteract these unwanted side effects.
Interestingly, both antipsychotics and antidepressants have been found to have antimicrobial properties (Munoz-Bellido et al., 2000; Maier et al., 2018). It is thought that such psychotropic medications can modulate the gut microbiome, and consequently influence the GBA. Whether the therapeutic benefits or the adverse effects of these medications are influenced, in part, by their impact on the GBA remains to be determined, however, several recent studies have indicated that the gut microbiome composition could be used as a biomarker to predict pharmacological treatment outcomes (responders versus treatment resistance) in MDD and SZ (Fontana et al., 2020; Ciocan et al., 2021; Yuan et al., 2021). This suggests that the GBA could play a significant role in the efficacy and tolerability of psychotropic medication. This evidence, combined with the aforementioned therapeutic benefits of microbiome modulation on psychiatric disorders, and the proven ability of probiotics to normalize metabolic issues (Le Barz et al., 2015), suggest that combining psychotropic medication with gut microbiome targeting treatments could have beneficial results. The aim of this systematic review is to evaluate the current literature investigating the effect of adjuvant probiotic or synbiotic (a combination of probiotics and prebiotics) treatment on clinical outcomes and tolerability of first-line psychotropic treatments. We conducted this systematic review as a means to gather scientific evidence and provide a comprehensive and current overview of this topic.
Methods
Literature search strategy
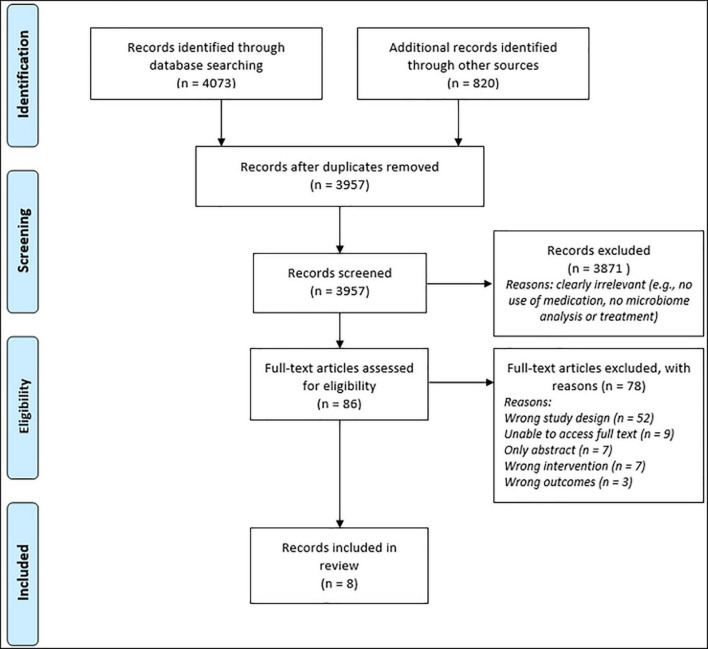
This review was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1; Moher et al., 2009). A systematic search was conducted using 4 databases (MEDLINE, EMBASE, PsycINFO, and Web of Science) to identify relevant studies using the following search terms:(antidepressant OR selective serotonin reuptake inhibitors, SSRI OR SNRI OR TCA OR MAOI OR anti-anxiety drugs OR anxiolytics OR benzodiazepines OR beta-blockers OR antipsychotic medication OR mood stabilizer) AND (microbiome OR microbiota OR gut bacteria OR intestinal bacteria OR dysbiosis OR bacteriostatic OR bactericidal OR antibiotic OR bacterial therapy OR bacteriotherapy OR psychobiotic OR microbial therapy OR fecal microbiota transplant OR probiotic) AND (depression OR depressive disorder OR major depression OR bipolar OR mood disorders OR affective disorders OR stress, psychological OR anxiety OR anxiety disorder OR generalized anxiety disorder OR social anxiety disorder OR PTSD OR OCD OR mania OR panic OR phobia OR psychiatric illness). The database searches were supplemented by retrieval of any additional papers meeting eligibility criteria that were cited in reference lists of relevant review articles yielding 820 additional articles. Searches were conducted in January and February 2022 and yielded 3957 studies after duplicates were removed. Studies that were excluded during full-text screening were rejected due to wrong study design, including the article being a review article or abstract only, and wrong study outcomes. Articles rejected due to “wrong study outcome” did not measure changes in psychiatric symptoms in response to the use of a microbiome-targeted therapeutic as an adjuvant to medication for psychiatric illness.
FIGURE 1.
Flow chart showing literature search and screening process using PRISMA guidelines.
Eligibility criteria
Eligible articles were restricted to those that were published in peer-reviewed journals and were written in English. Studies eligible for inclusion involved clinical samples that assessed changes in psychiatric wellbeing after standard treatment indicated for psychiatric illness and an adjuvant therapeutic targeting the microbiome.
Study selection
Two authors (BB and AS) completed the initial search of the databases, adhering to the search strategy as described above. Two authors (EF and one of BB or AS) independently assessed the titles and abstracts of records retrieved from a systematic search according to the identified inclusion and exclusion criteria. Two authors (BB and EF) completed the full-text review. Any disagreements were resolved by a fourth author (AC). Quality assessment of eligible articles was completed by a fifth author (CS).
Study quality
Quality assessment of articles was completed using Covidence’s built-in, Cochrane Handbook for Systematic Reviews of Interventions, Risk of Bias (RoB) template. The Cochrane RoB tool assesses the risk of bias for the following domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and “other” sources of bias. Most studies presented with a low level of bias. Studies where there was no mention of blinding to participants, personnel, outcome assessors, or allocation of treatment, were assigned a “high” judgment in risk of bias. In studies where there was no given description or details regarding one of the RoB domains (i.e., sequence generation), the risk of bias was assigned as “unsure.” A detailed summary of the quality assessment can be found in Table 1.
TABLE 1.
Summary of quality assessment details and judgment for risk of bias of each study.
| Study | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | |||||
| Judgment | Comments | Judgment | Comments | Judgment | Comments | Judgment | Comments | Judgment | Comments | |
| Dickerson et al., 2014 | Unsure | No mention of sequence generation. | Unsure | No mention of allocation concealment for placebo vs. treatment groups. | Low | Study was double-blind, but there was no mention of how this was maintained. | Low | Study was double-blind, but there was no mention of how this was maintained. | Low | Subjects that were excluded were documented with reasoning. |
| Kazemi et al., 2019 | Low | Patients were randomly assigned to experimental groups (1:1:1) in blocks of 6. | Low | Participants, clinicians, and raters remained blind to the allocated group of each participant. | Low | Participants, clinicians, and raters remained blind to the allocated group of each participant. | Low | Participants, clinicians, and raters remained blind to the allocated group of each participant. | Low | Subjects that were excluded were documented with reasoning. |
| Ghorbani et al., 2018 | Unsure | No mention of treatment group sequencing. | Low | Double-blind study with 1:1 ratio for treatment vs. placebo randomization. | Low | Double-blind study. Throughout the study, the psychiatrist, the rater (study researchers), and the patients were all blind to allocation. | Low | The raters (study researchers) were blind to allocation. | Low | Subjects that were excluded were documented with reasoning. |
| Eskandarzadeh et al., 2021 | Low | Patients were randomly assigned using a random numbers table to either treatment or placebo groups. | Low | Patients were randomly assigned using a random numbers table to either treatment or placebo groups. | Low | The study is double-blind, but there was no mention as to how that was maintained. | Low | The study is double-blind, but there was no mention as to if raters were blinded. | Low | Subjects who were excluded were documented with reasoning. |
| Arifdjanova et al., 2021 | Low | Participants were randomized to either experimental or control group, but no mention as to how. | High | No concealment of treatment group. | High | No mention of blinding of staff. | High | No mention of blinding of raters. | Low | Some subjects were not included at the beginning, but criteria for which they were not included was not disclosed. |
| Miyaoka et al., 2018 | Unsure | No mention of allocation sequence. | Unsure | No mention of allocation concealment. | High | No mention of blinding. | High | No mention of blinding. | Low | All data was reported and subjects that were excluded were documented with reasoning. |
| Rudzki et al., 2019 | Low | Patients were randomly assigned to placebo or probiotic group using computer generated randomization list. | Low | The study was blinded at group allocator, participant, and assessor levels. | Low | The study was blinded at group allocator, participant, and assessor levels. | Low | The study was blinded at group allocator, participants, and assessor levels. | Low | Subjects that were excluded were documented with reasoning. |
| Yang et al., 2021 | Unsure | No mention of how the participants were randomized. | High | The blind method was not used in this study, and the researchers were fully aware of the medication. | High | Researchers were fully unblinded and aware of the medication. No mention of unblinding to participants. | High | The blind method was not used in this study, and the researchers were fully aware of the medication. | Low | Subjects that were excluded were documented with reasoning. |
Results
Search results
Following the removal of 1072 duplicates, the search yielded 3,957 results. Subsequent abstract screening and full-text screening, according to the search criteria highlighted earlier (shown in Figure 1) resulted in 8 papers with direct relevance to the research question.
Study characteristics
Our findings can be grouped into studies examining three major categories of psychological disorders. For one, 370 patients across 5 trials were categorized and treated as patients with MDD (Ghorbani et al., 2018; Miyaoka et al., 2018; Kazemi et al., 2019; Rudzki et al., 2019; Arifdjanova et al., 2021). A significant majority of these patients received traditional antidepressant medication in the form of SSRIs. The sole trial examining 48 patients with GAD (Eskandarzadeh et al., 2021) assessed the use of sertraline, a common SSRI, as its psychotropic agent. The remaining two trials examined 132 patients with SZ or schizoaffective disorder (SZA) (Dickerson et al., 2014; Yang et al., 2021), one in which participants received the atypical antipsychotic Olanzapine, and the other in which participants continued taking whichever antipsychotic they were prescribed prior to enrolling in the study. As can be seen in Table 2, the studies were conducted across 6 different countries and on predominantly female populations. Furthermore, while the majority of studies used an SSRI or atypical antipsychotic, the makeup and the quantity of probiotic administered varied greatly across trials, reducing the generalizability of conclusions.
TABLE 2.
Summary of key study characteristics and outcomes.
| References | Study population | Study design | Sample size | Mean age (%F) | Country | Intervention type | Duration | Probiotic | Outcome measures | Conclusion |
| Arifdjanova et al., 2021 | Mild-moderate MDD (ICD-10), 18–45yo | Placebo, double-blind RCT | n = 149 | 32.9 (62.2%) | Russia | Cipralex (SSRI) + Placebo or Probiotic | 6 weeks | Bac-Set Forte* 3 capsules/day (1010 CFU) | HAM-D for depression severity, ELISA for cortisol and cytokines, HPLC for blood plasma | Found decreased levels of cortisol, dopamine, IL-6, TNF-a and nitric oxide, and a bigger reduction in depressive symptoms in the adjuvant PB group compared to standard therapy. |
| Rudzki et al., 2019 | Moderate MDD (DSM-IV-R) | Placebo, double-blind RCT | n = 60 | 39 (71%) | Poland | Antidepressant (various SSRIs) + Probiotic or Placebo | 8 weeks | 2 capsules/day (10 × 109 CFU of Lactobacillus Plantarum 299v each) | Symptom Severity: HAM-D 17, SCL-90, PSS-10, cognitive function, biochemical parameters also assessed | PB correlated with increased cognitive performance and decreased kynurenine concentration in MDD patients, no significant effect on symptom severity. |
| Ghorbani et al., 2018 | Moderate MDD (DSM-V), 18–55yo | Placebo, double-blind RCT | n = 40 | 34.8 (70%) | Iran | Fluoxetine (SSRI, 20 mg/day - 4W) then Fluoxetine + synbiotic capsule or Placebo (6W) | 6 weeks | 1 capsule/day (MS probiotic**, 500 mg + prebiotic, 100 mg) | HAM-D primary outcome | Found a greater reduction in HAM-D scores in synbiotic treated patients compared to the placebo group. |
| Kazemi et al., 2019 | Mild-moderate MDD (ICD-10) on medication, 18–50yo | Three-arm placebo, double-blind RCT | n = 81 | 36.5 (70.9%) | Iran | Antidepressant (sertraline, fluoxetine, citalopram, amitriptyline) + Probiotic or Prebiotic or Placebo | 8 weeks | 1 sachet/day - probiotic (≥10 × 109 CFU Lactobacillus helveticus and Bifidobacterium longum), or prebiotic (galactooligosaccharide) | BDI primary outcome, HPLC for serum tryptophan and branched chain amino acids, ELISA for kynurenine | PB resulted in a decrease in BDI score and increased tryptophan/isoleucine ratio compared to placebo and prebiotic. No significant results for prebiotic and placebo groups |
| Miyaoka et al., 2018 | Treatment Resistant MDD (DSM-IV) | Prospective open label randomized | n = 40 | 43.5, (60%) | Japan | Antidepressant (fluvoxamine, paroxetine, escitalopram, duloxetine, and sertraline) with or without (control) Probiotic | 8 weeks | 60 mg/day (Clostridium butyricum MIYAIRI (CBM588)–10 CFU/gram) | HAM-D, BDI and the Beck Anxiety Inventory | PB correlated to significant improvement in depression regardless of antidepressant type; well tolerated. |
| Eskandarzadeh et al., 2021 | Drug-free patients with GAD (DSM-V), 18–65yo | Placebo, double-blind RCT | n = 48 | 33.9 (81.2%) | Iran | Sertraline (SSRI, 25 mg/day) + Placebo or Probiotic | 8 weeks | 1 capsule/day (18*109 CFU Bifidobacterium longum, Bifidobacterium bifidum, Bifidobacterium lactis and Lactobacillus acidophilus) | HAM-A scale for anxiety, Beck Anxiety Inventory, State-Trait Anxiety Inventory | Found Sertraline + PB group to have improved clinical outcome measures as opposed to Sertraline + placebo. Significance varied depending on scale used. |
| Dickerson et al., 2014 | Mild-moderate SZ (DSM-IV, PANSS), 18–65yo | Placebo, double-blind RCT | n = 65 | 46.2 (35.4%) | U.S. | Antipsychotic (various) + Placebo or Probiotic | 14 weeks | 1 Capsule/day (109 CFU combined Lactobacillus rhamnosus strain and Bifidobacterium animalis subsp. lactis strain Bb12) | PANSS to measure psychiatric symptoms + difficulty of bowel movement scale | No significant difference in psychiatric scores, PB well tolerated, PB group had less bowel problems associated w/treatment. |
| Yang et al., 2021 | First-episode SZ or SZA (DSM-V), 18–55yo | Open-label, RCT | n = 67 | 43.2 (67.7%) | China | Olanzapine with or without (control) Bifidobacterium group | 12 weeks | 3 capsules/day (live combined Bifidobacterium, Lactobacillus, and Enterococcus capsules; 1 × 109 CFU each) | Body weight, BMI, appetite, latency to increased appetite, and baseline weight increase of more than 7%, PANSS to measure psychiatric symptoms |
No significant differences in PANSS scores. In the first 4 weeks there was reduced weight change and BMI for PB group, but this difference disappeared after 4 weeks. There were no overall differences in appetite. |
*Contents of Bac-Set Forte: Streptococcus thermophilus; Bifidobacterium ssp; Lactobacillus ssp. among others. **Contents of MS probiotic: L. casei = 3 × 108, L. acidophilus = 2 × 108, L. bulgaricus = 2 × 109, L. rhamnosus = 3 × 108, B. breve = 2 × 108, B. longum = 1 × 109, S. thermophilus = 3 × 108.
In all studies, a subset of patients received their psychotropic medication in conjunction with a type of probiotic supplementation. Most of these studies were conducted in the form of a double-blinded, randomized clinical trial. Two studies, however, (Miyaoka et al., 2018; Yang et al., 2021) lacked a placebo arm, following an open-label randomized format.
Efficacy of probiotics as an adjuvant therapy
In the eight studies examined (shown in Table 2), the medications to treat psychiatric illness were either antidepressants (n = 6), or antipsychotics (n = 2). All studies included measures for clinical outcomes and symptom severity. In the depression studies, the majority used the Hamilton Depression Rating Scale (Hamilton, 1960) (HAM-D) (n = 4) or the Beck Depression Inventory (Beck et al., 1996) (BDI) (n = 2) to measure symptoms of mood, anhedonia, sleep, anxiety, appetite, and other symptoms associated with depression. The anxiety study used the Hamilton Anxiety Rating Scale (Hamilton, 1959) (HAM-A) (n = 1) to assess symptoms of anxiety such as mood, tension, insomnia, physiological symptoms. The schizophrenia studies used the Positive and Negative Syndrome Scale (Kay et al., 1987) (PANSS) (n = 2) to assess positive and negative symptoms associated with schizophrenia such as delusions, hallucinations, blunted affect, and social withdrawal. Higher scores on these scales correspond to an increased severity of the illness.
When examining the effects on symptom severity in patients with MDD or GAD, five of the six studies found that patients who received adjuvant probiotic treatment had significant reductions in symptom severity on the majority of the scales used. One study (Rudzki et al., 2019) did not find any significant effects on symptom severity in patients with MDD receiving adjuvant probiotic therapy using the HAM-D, Symptom Checklist-90 (Derogatis and Savitz, 1999) (SCL-90), and Perceived Stress Scale-10 (Cohen et al., 1983) (PSS-10), but did find that adjuvant probiotic treatment was correlated with increased cognitive performance. Neither of the two studies using patients with schizophrenia or schizoaffective disorder taking antipsychotics found adjuvant probiotic treatment to have any effect on the psychiatric symptoms. That being said, both studies found adjuvant probiotic treatment to reduce the adverse events and side effects associated with antipsychotic treatment, with Dickerson et al. finding fewer reports of bowel difficulties, and Yang et al. finding a reduced weight gain in the first 4 weeks in the adjuvant probiotic groups. Though the reduced weight gain in the study conducted by Yang et al. was transient, with no differences between the adjuvant and monotherapy groups by weeks 8 and 12, adjuvant probiotic therapy was found to eliminate the observed sex-based differences in weight gain seen in the olanzapine monotherapy group. There was no significant difference in body weight change between men and women in the adjuvant probiotic group, whereas there were significantly higher increases in the body weight of women compared to men in the olanzapine monotherapy group.
Discussion
The clinical outcome findings from the studies included in this review suggest probiotic and synbiotic adjuvant treatment with SSRIs for MDD and GAD to be more effective in decreasing depressive and anxious symptomology, respectively, than SSRI treatment alone. In the one study included in this review that used included a prebiotic group, prebiotics alone were not found to have a significant effect on clinical symptoms. The improved clinical outcomes of probiotic adjuvant treatment for MDD and GAD were found to be persistent throughout the course of the treatment, but further long-term follow-up assessments would be needed to investigate the persistence of this effect when treatment is discontinued. For individuals with schizophrenia, adjuvant probiotic treatment was not found to be more effective in reducing clinical symptom severity than standard antipsychotic treatment alone. Some potential limitations that could explain this lack of effect on clinical symptoms and outcomes are discussed in the conclusion. Though there was no significance in clinical findings for the schizophrenia groups, adjuvant probiotic treatment was associated with a decrease in treatment associated adverse events and side effects. The findings of these studies suggest adjuvant probiotic treatment to have an alleviative effect on some of the gastrointestinal adverse events associated with antipsychotic treatment, such as weight gain and bowel problems. Some of these beneficial effects were found to be fairly long lasting, as is the case in the trial by Dickerson et al. (2014), but some effects were found to be transient, with Yang et al. finding a reduced weight gain for only the first 4 weeks of adjuvant probiotic treatment (Yang et al., 2021).
As mentioned in the introduction, some antipsychotics and antidepressants have been found to have antibacterial properties, as such, it is important to consider the specific medications used in each study. All MDD and GAD studies used participants taking SSRIs, with Miyoaka et al. including participants taking the SNRIs duloxetine (n = 9) and milnacipran (n = 3) and Kazemi et al. including participants taking the tricyclic antidepressant amitriptyline (n not reported). The SSRIs involved in the studies included escitalopram, citalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. It has been found SSRIs vary in the degree to which the inhibit bacterial growth, with sertraline and fluoxetine having the strongest antimicrobial activity, followed by paroxetine and fluvoxamine, and then escitalopram and citalopram (McGovern et al., 2019). Amitriptyline has also been found to have antimicrobial effects to around the same degree as paroxetine (Mandal et al., 2010). The antimicrobial effect of SNRIs is less clear, with studies finding venlafaxine to have no effect (Ait Chait et al., 2020), and others finding the clinical effects of duloxetine to be reduced by the bacteria Ruminococcus flavefaciens (Lukić et al., 2019), suggesting an interaction between duloxetine and the bacteria. Despite the differences in medications used and their degree of antimicrobial activity, all the MDD and GAD studies found results suggesting probiotics combined with antidepressants improved clinical outcomes when compared to antidepressants alone.
For the schizophrenia studies, all participants in the study conducted by Yang et al. took olanzapine, whereas participants in the Dickerson et al. study took a variety of antipsychotics. The antipsychotics used in the Dickerson et al. study were clozapine (n = 17), olanzapine (n = 15), risperidone (n = 15), aripiprazole (n = 11), quetiapine (n = 9), haloperidol (n = 7), ziprasidone (n = 5), and asenapine (n = 1), including some participants that took more than one antipsychotic. Though a variety of antipsychotics were used in the Dickerson et al. study, all fall under the category of atypical antipsychotics except for haloperidol which is a butyrophenone derivative. Olanzapine has been found to shift the fecal microbiota in mice toward an “obesogenic” profile (Morgan et al., 2014), and all of the atypical antipsychotics used in the Dickerson et al. study have been found to be associated with significant changes in the gut microbiome and a decrease in species diversity in females (Flowers et al., 2017). Haloperidol has also been found to have some bacteria inhibiting effects (Korbee et al., 2018). Taking that into consideration, though the two studies used different antipsychotics, they both used primarily atypical antipsychotics, but the exact degree in which each individual antipsychotic effects the gut microbiome is not certain. As such, it is possible that the use of differing antipsychotics could have contributed to the difference in longevity of the observed beneficial effects.
The exact mechanisms of action for these beneficial effects of probiotic adjuvant treatment are not fully understood. Though the exact pathway and importance of the various pathways by which probiotic adjuvant treatment may exert its effect is not known, multiple pathways have been described for ways in which the microbiome affects the brain and central nervous system. Biomarker data collected in the MDD studies suggests that the probiotic adjuvant treatment exerts its therapeutic effect through effects on the immune system, the hypothalamic pituitary adrenal axis (HPA-axis), and the tryptophan system. Decreased concentrations of immune markers such as interleukin-6, tumor necrosis factor-a, and nitric oxide suggests a decrease in the activity of the immune system in response to probiotic adjuvant treatment. The immune system has long been known to be intimately linked to MDD and depressive symptomology, with an over-active immune system often being observed in individuals with MDD (Leonard, 2010). One proposed mechanism of action for microbiome targeting treatment for MDD is a reduction in immune system activity through a decrease in gut permeability. It is thought that individuals with MDD and/or other illnesses have increased gastrointestinal permeability, allowing for microorganisms and other potentially harmful toxins from the gut to pass into the body, resulting in an increase in inflammation and immune system activity. Repopulation of the gut microbiome and/or the introduction of beneficial bacteria through probiotic treatment is thought to alleviate this increased gut permeability, and thus reduce inflammation and immune system activity, leading to a decrease in depressive symptomology. Biomarker data from the study conducted by Arifdjanova et al. (2021) also found cortisol levels to be decreased in the probiotic adjuvant treatment group. Cortisol is often associated with stress and is a major indicator HPA-axis activity. Individuals with MDD and/or GAD often are found to have an overactive HPA-axis and increased levels of cortisol. Probiotic and other gut microbiome targeting treatments have been frequently found to have an inhibitory effect on HPA-axis activity and has been associated with decreased cortisol levels. Influencing the HPA-axis and cortisol production and availability seems to be another pathway by which probiotic adjuvant treatment results in improved clinical outcomes. Another potential mechanism for the improved clinical outcome observed in the probiotic adjuvant treatment groups is through effects on the tryptophan system. Kazemi et al. (2019) and Rudzki et al. (2019) found an increased tryptophan/isoleucine ratio and decreased kynurenine (a tryptophan metabolite) concentration in the probiotic treatment groups of their respective studies. Tryptophan is a precursor to serotonin, a neurotransmitter that has long been associated with MDD and GAD. SSRIs exert their therapeutic action by inhibiting serotonin reuptake transporter proteins, leading to an increase in the relative abundance and concentration of serotonin in the brain. Though serotonin is often associated with the brain and psychiatric illnesses, up to 90% of the body’s serotonin is produced in the gut. The mechanism by which the gut microbiome affects serotonin production and availability is thought to be through interactions with microbiome metabolites and enterochromaffin cells (EC cells) in the gut. The microbiome produces long and short chain fatty acids (SCFA) through the fermentation of non-digestible carbohydrates. Long chain fatty acids influence serotonin production indirectly through interactions with glucagon-like protein-1 (GLP-1) cells leading to increased GLP-1 which interacts with EC cells to increase serotonin production and availability. Short chain fatty acids interact directly with EC cells to increase serotonin production and availability. In addition to these interactions with EC cells, short chain fatty acids have the ability to cross the gut-blood and blood-brain barriers, and are thought to have an anti-inflammatory effect, thus further influencing the immune system. The exact bacteria involved in these processes are not fully characterized, but some species Lactobacillus and Bifidobacterium have been found to produce neurotransmitters such as acetylcholine and gamma-aminobutyric acid, and Streptococcus, Enterococcus, and Escherichia have been found to produce serotonin, dopamine, and epinephrine (Galland, 2014). These biomarker findings suggest that the beneficial clinical outcomes in the adjuvant probiotic groups with MDD and GAD are a result of the adjuvant probiotic treatment affecting multiple if not all of the pathways by which the microbiome and brain are connected.
As for the observed effect of a decrease in gastrointestinal adverse events in the adjuvant probiotic group of the schizophrenia studies, the mechanism of action is largely unclear. The alleviation of gastrointestinal issues, such as constipation, most likely acted through interactions with the serotonin pathway. As described above, probiotic administration may have resulted in an increase in SCFAs produced by the microbiome, which in turn increases serotonin synthesis through EC cells. Serotonin has been found to activate enteric neural circuitry to initiate peristalsis and reduce constipation (Crowell, 2004). The transient effect of decreasing weight gain for the first 4 weeks could be from a variety of factors. Bifidobacterium administration has been linked to both weight gain and weight loss depending on the strain (Yin et al., 2010). The exact strain used by Yang et al. was not reported (Miyaoka et al., 2018), but increased bacteria with bile salt hydrolase has been found to prevent weight gain through the deconjugation of bile acids (Joyce et al., 2014). This may have been the case for the Yang et al. study, but the general negative impact on energy by olanzapine as well as its own mechanism of action for weight gain may have outweighed the preventative action of the probiotic over time. Although it is thought that probiotics may improve clinical outcomes and symptom severity in populations with schizophrenia through interaction with the immune system (Fond et al., 2020), neither of the studies included a robust collection of immune system related biomarkers, and thus the effect of adjuvant probiotic treatment on the immune system of the patients in these studies is unknown.
Conclusion
Although the studies included in this review were generally found to be of high quality with low risk of bias, there were still some limitations to these studies that impact the generalizability and conclusions that could be drawn from their findings. A major limitation is the small number of studies for each psychiatric illness and the lack of studies investigating other psychiatric illnesses. With only eight studies in total, five of which used a population with MDD, it is difficult to draw strong generalizable conclusions about the effect of adjuvant probiotic treatment on GAD and schizophrenia. Additionally, though the studies included had relatively large sample sizes, further larger scale, double blind, randomized controlled trials are required in the future to make any definitive conclusions. Many of the studies included in this review also did not have comprehensive biomarker collection. To be able to elucidate the mechanisms of action of probiotic supplementation as an adjuvant treatment, as well as to evaluate the colonization of the gut by the probiotics administered and changes in key features of the gut such as intestinal permeability, robust and consistent biomarker collection is necessary. This collection would include biomarkers for the immune system, HPA-axis, serotonin system, and the gut microbiome. Another limitation of the studies was the fact that many used a variety of first-line antidepressant or antipsychotic treatments in combination with the probiotics, which is helpful for evaluating adjunctive probiotic treatment in general but does not give strong insight into the effectiveness of adjunctive probiotic administration with specific antidepressants and antipsychotics. As different psychiatric treatments can have differing effects on the gut microbiome, studies or analyses focusing on one specific intervention could allow for more detail in determining what combination of treatment would be most effective for an individual. These studies are also limited by their length, with the majority being unable to have significant long-term follow-ups to investigate the longevity of the observed effects. Another limitation in this field is the lack of consensus on dosages for probiotics as well as the treatments they are given in combination with. Dose finding studies in the future are needed, in addition to studies investigating the efficacy of adjuvant probiotic treatment at different stages and severity of the illnesses. This is especially relevant for the schizophrenia studies, which included populations with relatively severe clinical symptoms and later stages of the illness. It is possible that in a population with milder symptoms and a more recent onset, adjuvant probiotic therapy could effectively impact clinical outcomes.
Despite these limitations, the findings of these studies suggest the use of adjuvant probiotic treatment with SSRI treatment for MDD and GAD to be superior to SSRI treatment alone. Probiotic adjuvant treatment with antipsychotics could be beneficial for improving the GI issues associated with antipsychotics, but these findings do not suggest that adjuvant probiotic treatment would result in improved clinical outcomes for symptoms of schizophrenia. Though progress in psychiatric research is challenging, these studies have shown that combining probiotic treatment with first line pharmaceutical treatments is promising, and their findings certainly justify continued research in this area. The gut microbiome and the brain are clearly linked, and these studies show that combining treatments that target both areas, respectively, is a viable and efficacious way to combat the symptoms and treat psychiatric illnesses.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
BB and AS completed the initial search of the databases, adhering to the search strategy. EF and one of BB or AS independently assessed the titles and abstracts of records retrieved from a systematic search according to the identified inclusion and exclusion criteria. BB and EF completed the full-text review. AC resolved any disagreements. CS completed quality assessment of eligible articles. All authors contributed to the article and approved the submitted version.
Abbreviations
BDI, Beck Depression Inventory; BMI, body mass index; CFU, colony forming units; DSM, Diagnostic and Statistical Manual of Mental Disorders; EC, enterochromaffin cells; GAD, Generalized Anxiety Disorder; GBA, gut brain axis; GI, gastrointestinal; GLP-1, glucagon-like Protein 1; HAM-A, Hamilton Anxiety Rating Scale; HAM-D, Hamilton Depression Rating Scale 17-item; HPA, hypothalamic pituitary adrenal axis; ICD, International Classification of Diseases; MDD, Major Depressive Disorder; MS, multi-strain probiotic; PANSS, Positive and Negative Syndrome Scale; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PSS-10, Perceived Stress Scale 10; RCT, randomized controlled trial; RoB, risk of bias; SZA, schizoaffective disorder; SCL-90, Symptom Checklist-90; SSRI, selective serotonin reuptake inhibitors; SZ, schizophrenia.
Conflict of interest
RM has received consulting and speaking honoraria from AbbVie, Allergan, Eisai, Janssen, KYE, Lallemand, Lundbeck, Neonmind, Otsuka, and Sunovion, and research grants from CAN-BIND, CIHR, Janssen, Lallemand, Lundbeck, Nubiyota, OBI, and OMHF. AC has received CIHR Doctoral Funding. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Ait Chait Y., Mottawea W., Tompkins T., Hammami R. (2020). Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 10:17878. 10.1038/s41598-020-74934-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association [APA] (2013). Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American psychiatric association. 10.1176/appi.books.9780890425596 [DOI] [Google Scholar]
- Arifdjanova S., Abrurakhmanova Z., Bizulya E., Gumenyuk L., Sorokina L., Gerbali O. (2021). The role of probiotics in combination therapy of depressive disorders. Russ. Open Med. J. 10:e0109. 10.15275/rusomj.2021.0109 [DOI] [Google Scholar]
- Baldwin D., Woods R., Lawson R., Taylor D. (2011). Efficacy of drug treatments for generalised anxiety disorder: Systematic review and meta-analysis. BMJ 342:d1199. 10.1136/bmj.d1199 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. (1996). Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation. 10.1037/t00742-000 [DOI] [Google Scholar]
- Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., et al. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141 599–609, 609e.1–609e.3. 10.1053/j.gastro.2011.04.052 [DOI] [PubMed] [Google Scholar]
- Bermon S., Petriz B., Kajeniene A., Prestes J., Castell L., Franco O. (2015). The microbiota: An exercise immunology perspective. Exerc. Immunol. Rev. 21 70–79. [PubMed] [Google Scholar]
- Ciocan D., Cassard A., Becquemont L., Verstuyft C., Voican C., El Asmar K., et al. (2021). Blood microbiota and metabolomic signature of major depression before and after antidepressant treatment: A prospective case–control study. J. Psychiatry Neurosci. 46 E358–E368. 10.1503/jpn.200159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Kamarck T., Mermelstein R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Crowell M. (2004). Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br. J. Pharmacol. 141 1285–1293. 10.1038/sj.bjp.0705762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J., O’Riordan K., Cowan C., Sandhu K., Bastiaanssen T., Boehme M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99 1877–2013. 10.1152/physrev.00018.2018 [DOI] [PubMed] [Google Scholar]
- Cussotto S., Clarke G., Dinan T., Cryan J. (2019). Psychotropics and the microbiome: A chamber of secrets. Psychopharmacology 236 1411–1432. 10.1007/s00213-019-5185-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M., Dobbelaere M., Sheridan E., Cohen D., Correll C. (2011). Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur. Psychiatry 26 144–158. 10.1016/j.eurpsy.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Derogatis L. R., Savitz K. L. (1999). “The SCL-90-R, brief symptom inventory, and matching clinical rating scales,” in The use of psychological testing for treatment planning and outcomes assessment, ed. Maruish M. E. (Mahwah, NJ: Lawrence Erlbaum Associates Publishers; ), 679–724. [Google Scholar]
- Dickerson F., Stallings C., Origoni A., Katsafanas E., Savage C., Schweinfurth L., et al. (2014). Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning: A randomized, placebo-controlled trial. Prim. Care Companion CNS Disord. 16:26294. 10.4088/PCC.13m01579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandarzadeh S., Effatpanah M., Khosravi-Darani K., Askari R., Hosseini A., Reisian M., et al. (2021). Efficacy of a multispecies probiotic as adjunctive therapy in generalized anxiety disorder: A double blind, randomized, placebo-controlled trial. Nutr. Neurosci. 24 102–108. 10.1080/1028415X.2019.1598669 [DOI] [PubMed] [Google Scholar]
- Evans-Lacko S., Aguilar-Gaxiola S., Al-Hamzawi A., Alonso J., Benjet C., Bruffaerts R., et al. (2018). Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO world mental health (WMH) surveys. Psychol. Med. 48 1560–1571. 10.1017/S0033291717003336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers S., Evans S., Ward K., McInnis M., Ellingrod V. (2017). Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy 37 261–267. 10.1002/phar.1890 [DOI] [PubMed] [Google Scholar]
- Fond G., Lançon C., Korchia T., Auquier P., Boyer L. (2020). The role of inflammation in the treatment of schizophrenia. Front. Psychiatry 11:160. 10.3389/fpsyt.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Manchia M., Panebianco C., Paribello P., Arzedi C., Cossu E., et al. (2020). Exploring the role of gut microbiota in major depressive disorder and in treatment resistance to antidepressants. Biomedicines 8:311. 10.3390/biomedicines8090311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L. (2014). The gut microbiome and the brain. J. Med. Food 17 1261–1272. 10.1089/jmf.2014.7000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelenberg A., Freeman M., Markowitz J., Rosenbaum J., Thase M., Trivedi M., et al. (2010). American psychiatric association practice guidelines for the treatment of patients with major depressive disorder. Am. J. Psychiatry 167 (Suppl. 10), 9–118. [Google Scholar]
- Ghorbani Z., Nazari S., Etesam F., Nourimajd S., Ahmadpanah M., Jahromi S. (2018). The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: A randomized multicenter trial. Arch. Neurosci. 5:e60507. 10.5812/archneurosci.60507 [DOI] [Google Scholar]
- Hamilton M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32 50–55. 10.1111/j.2044-8341.1959.tb00467.x [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M., Greenway F. L. (2016). A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 5 317–320. 10.1016/j.molmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch M., Birnbaum R. (2017). Selective serotonin reuptake inhibitors: Pharmacology, administration, and side effects, eds Roy-Byrne P., Solomon D. (Waltham, MA: UpToDate; ). [Google Scholar]
- Johnson D., Thurairajasingam S., Letchumanan V., Chan K., Lee L. (2021). Exploring the role and potential of probiotics in the field of mental health: Major depressive disorder. Nutrients 13:1728. 10.3390/nu13051728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce S., MacSharry J., Casey P., Kinsella M., Murphy E., Shanahan F., et al. (2014). Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. U.S.A. 111 7421–7426. 10.1073/pnas.1323599111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S., Fiszbein A., Opler L. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 13 261–276. 10.1093/schbul/13.2.261 [DOI] [PubMed] [Google Scholar]
- Kazemi A., Noorbala A., Azam K., Eskandari M., Djafarian K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 38 522–528. 10.1016/j.clnu.2018.04.010 [DOI] [PubMed] [Google Scholar]
- Korbee C., Heemskerk M., Kocev D., van Strijen E., Rabiee O., Franken K., et al. (2018). Combined chemical genetics and data-driven bioinformatics approach identifies receptor tyrosine kinase inhibitors as host-directed antimicrobials. Nat. Commun. 9:358. 10.1038/s41467-017-02777-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Barz M., Anhê F., Varin T., Desjardins Y., Levy E., Roy D., et al. (2015). Probiotics as complementary treatment for metabolic disorders. Diabetes Metab. J. 39 291–303. 10.4093/dmj.2015.39.4.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B. (2010). “The concept of depression as a dysfunction of the immune system,” in Depression: From psychopathology to pharmacotherapy, Vol. 27 ed. Cryan J. F. (Basel: Karger Publishers; ), 53–71. 10.1159/000319504 [DOI] [Google Scholar]
- Li J., Jia H., Cai X., Zhong H., Feng Q., Sunagawa S., et al. (2014). An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 32 834–841. 10.1038/nbt.2942 [DOI] [PubMed] [Google Scholar]
- Liu L., Huh J., Shah K. (2022). Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. EBioMedicine 77:103908. 10.1016/j.ebiom.2022.103908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukić I., Getselter D., Ziv O., Oron O., Reuveni E., Koren O., et al. (2019). Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 9:133. 10.1038/s41398-019-0466-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E., et al. (2018). Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555 623–628. 10.1038/nature25979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A., Sinha C., Kumar Jena A., Ghosh S., Samanta A. (2010). An Investigation on in vitro and in vivo antimicrobial properties of the antidepressant: Amitriptyline hydrochloride. Braz. J. Microbiol. 41 635–645. 10.1590/S1517-83822010000300014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Makino H., Cetinyurek Yavuz A., Ben-Amor K., Roelofs M., Ishikawa E., et al. (2016). Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One 11:e0158498. 10.1371/journal.pone.0158498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern A., Hamlin A., Winter G. (2019). A review of the antimicrobial side of antidepressants and its putative implications on the gut microbiome. Aust. N. Z. J. Psychiatry 53 1151–1166. 10.1177/0004867419877954 [DOI] [PubMed] [Google Scholar]
- Meyyappan A., Forth E., Wallace C., Milev R. (2020). Effect of fecal microbiota transplant on symptoms of psychiatric disorders: A systematic review. BMC Psychiatry 20:299. 10.1186/s12888-020-02654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T., Kanayama M., Wake R., Hashioka S., Hayashida M., Nagahama M., et al. (2018). Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: A prospective open-label trial. Clin. Neuropharmacol. 41 151–155. 10.1097/WNF.0000000000000299 [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151 264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Morais L., Schreiber H., Mazmanian S. (2021). The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19 241–255. 10.1038/s41579-020-00460-0 [DOI] [PubMed] [Google Scholar]
- Morgan A., Crowley J., Nonneman R., Quackenbush C., Miller C., Ryan A., et al. (2014). The antipsychotic olanzapine interacts with the gut microbiome to cause weight gain in mouse. PLoS One 9:e115225. 10.1371/journal.pone.0115225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Bellido J., Munoz-Criado S., Garcıa-Rodrıguez J. (2000). Antimicrobial activity of psychotropic drugs: Selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 14 177–180. 10.1016/S0924-8579(99)00154-5 [DOI] [PubMed] [Google Scholar]
- Neufeld K., Kang N., Bienenstock J., Foster J. (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 23 255–264, e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Nikolova V., Hall M., Hall L., Cleare A., Stone J., Young A. (2021). Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 78 1343–1354. 10.1001/jamapsychiatry.2021.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S., Usall J., Cobo J., Labad X., Kulkarni J. (2012). Gender differences in schizophrenia and first-episode psychosis: A comprehensive literature review. Schizophr. Res. Treatment 2012:916198. 10.1155/2012/916198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odamaki T., Kato K., Sugahara H., Hashikura N., Takahashi S., Xiao J., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 16:90. 10.1186/s12866-016-0708-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K., Cherian J., Gohil K., Atkinson D. (2014). Schizophrenia: Overview and treatment options. Pharm. Ther. 39 638–645. [PMC free article] [PubMed] [Google Scholar]
- Rudzki L., Ostrowska L., Pawlak D., Małus A., Pawlak K., Waszkiewicz N., et al. (2019). Probiotic Lactobacillus plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100 213–222. 10.1016/j.psyneuen.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Sabic D., Sabic A., Bacic-Becirovic A. (2021). Major depressive disorder and difference between genders. Mater. Sociomed. 33 105–108. 10.5455/msm.2021.33.105-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesga-López O., Schneier F., Wang S., Heimberg R., Liu S., Hasin D., et al. (2008). Gender differences in generalized anxiety disorder: Results from the national epidemiologic survey on alcohol and related conditions (NESARC). J. Clin. Psychiatry 69 1606–1616. 10.4088/JCP.v69n1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Long Y., Kang D., Liu C., Xiao J., Wu R., et al. (2021). Effect of Bifidobacterium on olanzapine-induced body weight and appetite changes in patients with psychosis. Psychopharmacology 238 2449–2457. 10.1007/s00213-021-05866-z [DOI] [PubMed] [Google Scholar]
- Yin Y., Yu Q., Fu N., Liu X., Lu F. (2010). Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J. Gastroenterol. 16 3394–3401. 10.3748/wjg.v16.i27.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X., Wang Y., Li X., Jiang J., Kang Y., Pang L., et al. (2021). Gut microbial biomarkers for the treatment response in first-episode, drug-naïve schizophrenia: A 24-week follow-up study. Transl. Psychiatry 11:422. 10.1038/s41398-021-01531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Guo R., Wang W., Ju Y., Wang Q., Ma Q., et al. (2020). Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 25 2905–2918. 10.1038/s41380-019-0475-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.