Abstract
Background
Communication problems in health care may arise as a result of healthcare providers focusing on diseases and their management, rather than people, their lives and their health problems. Patient‐centred approaches to care delivery in the patient encounter are increasingly advocated by consumers and clinicians and incorporated into training for healthcare providers. However, the impact of these interventions directly on clinical encounters and indirectly on patient satisfaction, healthcare behaviour and health status has not been adequately evaluated.
Objectives
To assess the effects of interventions for healthcare providers that aim to promote patient‐centred care (PCC) approaches in clinical consultations.
Search methods
For this update, we searched: MEDLINE (OvidSP), EMBASE (OvidSP), PsycINFO (OvidSP), and CINAHL (EbscoHOST) from January 2000 to June 2010. The earlier version of this review searched MEDLINE (1966 to December 1999), EMBASE (1985 to December 1999), PsycLIT (1987 to December 1999), CINAHL (1982 to December 1999) and HEALTH STAR (1975 to December 1999). We searched the bibliographies of studies assessed for inclusion and contacted study authors to identify other relevant studies. Any study authors who were contacted for further information on their studies were also asked if they were aware of any other published or ongoing studies that would meet our inclusion criteria.
Selection criteria
In the original review, study designs included randomized controlled trials, controlled clinical trials, controlled before and after studies, and interrupted time series studies of interventions for healthcare providers that promote patient‐centred care in clinical consultations. In the present update, we were able to limit the studies to randomized controlled trials, thus limiting the likelihood of sampling error. This is especially important because the providers who volunteer for studies of PCC methods are likely to be different from the general population of providers. Patient‐centred care was defined as a philosophy of care that encourages: (a) shared control of the consultation, decisions about interventions or management of the health problems with the patient, and/or (b) a focus in the consultation on the patient as a whole person who has individual preferences situated within social contexts (in contrast to a focus in the consultation on a body part or disease). Within our definition, shared treatment decision‐making was a sufficient indicator of PCC. The participants were healthcare providers, including those in training.
Data collection and analysis
We classified interventions by whether they focused only on training providers or on training providers and patients, with and without condition‐specific educational materials. We grouped outcome data from the studies to evaluate both direct effects on patient encounters (consultation process variables) and effects on patient outcomes (satisfaction, healthcare behaviour change, health status). We pooled results of RCTs using standardized mean difference (SMD) and relative risks (RR) applying a fixed‐effect model.
Main results
Forty‐three randomized trials met the inclusion criteria, of which 29 are new in this update. In most of the studies, training interventions were directed at primary care physicians (general practitioners, internists, paediatricians or family doctors) or nurses practising in community or hospital outpatient settings. Some studies trained specialists. Patients were predominantly adults with general medical problems, though two studies included children with asthma. Descriptive and pooled analyses showed generally positive effects on consultation processes on a range of measures relating to clarifying patients' concerns and beliefs; communicating about treatment options; levels of empathy; and patients' perception of providers' attentiveness to them and their concerns as well as their diseases. A new finding for this update is that short‐term training (less than 10 hours) is as successful as longer training.
The analyses showed mixed results on satisfaction, behaviour and health status. Studies using complex interventions that focused on providers and patients with condition‐specific materials generally showed benefit in health behaviour and satisfaction, as well as consultation processes, with mixed effects on health status. Pooled analysis of the fewer than half of included studies with adequate data suggests moderate beneficial effects from interventions on the consultation process; and mixed effects on behaviour and patient satisfaction, with small positive effects on health status. Risk of bias varied across studies. Studies that focused only on provider behaviour frequently did not collect data on patient outcomes, limiting the conclusions that can be drawn about the relative effect of intervention focus on providers compared with providers and patients.
Authors' conclusions
Interventions to promote patient‐centred care within clinical consultations are effective across studies in transferring patient‐centred skills to providers. However the effects on patient satisfaction, health behaviour and health status are mixed. There is some indication that complex interventions directed at providers and patients that include condition‐specific educational materials have beneficial effects on health behaviour and health status, outcomes not assessed in studies reviewed previously. The latter conclusion is tentative at this time and requires more data. The heterogeneity of outcomes, and the use of single item consultation and health behaviour measures limit the strength of the conclusions.
Keywords: Humans, Decision Making, Health Behavior, Medical Staff, Medical Staff/education, Medicine, Nursing Staff, Nursing Staff/education, Patient Participation, Patient Satisfaction, Patient‐Centered Care, Patient‐Centered Care/methods, Physician‐Patient Relations, Randomized Controlled Trials as Topic
Plain language summary
Training healthcare providers to be more 'patient‐centred' in clinical consultations
Problems may arise when healthcare providers focus on managing diseases rather than on people and their health problems. Patient‐centred approaches to care delivery in the patient encounter are increasingly advocated by consumers and clinicians and incorporated into training for healthcare providers. We updated a 2001 systematic review of the effects of these training interventions for healthcare providers that aim to promote patient‐centred care in clinical consultations.
We found 29 new randomized trials (up to June 2010), bringing the total of studies included in the review to 43. In most of the studies, training interventions were directed at primary care physicians (general practitioners, internists, paediatricians or family doctors) or nurses practising in community or hospital outpatient settings. Some studies trained specialists. Patients were predominantly adults with general medical problems, though two studies included children with asthma.
These studies showed that training providers to improve their ability to share control with patients about topics and decisions addressed in consultations are largely successful in teaching providers new skills. Short‐term training (less than 10 hours) is as successful in this regard as longer training. Results are mixed about whether patients are more satisfied when providers practice these skills. The impact on general health is also mixed, although the limited data that could be pooled showed small positive effects on health status. Patients' specific health behaviours show improvement in the small number of studies where interventions use provider training combined with condition‐specific educational materials and/or training for patients, such as teaching question‐asking during the consultation or medication‐taking after the consultation. However, the number of studies is too small to determine which elements of these multi‐faceted studies are essential in helping patients change their healthcare behaviours.
Background
Communication problems between healthcare providers and patients are common. Various studies have found that many patients are dissatisfied with the quality of the interaction with their healthcare provider. (Coulter 1998; Ong 1995; Stewart 1995a; Stewart 1995b; Epstein 2011; Mazor 2005; Verghese 2011).Some communication problems have been attributed to the fact that many healthcare providers focus on diseases and their management, rather than on the people, their lives and their health issues.
The concept of 'patient‐centred medicine' was introduced into the medical literature in the mid 1950s by Balint, (Balint 1955; Balint 1956) who contrasted it with 'illness‐centred medicine' (Brown 1999). It has its roots within the paradigm of holism, which suggests that people need to be seen in their biopsychosocial entirety (Henbest 1989), and draws medical attention to patients' individual identities. More recently, calls for increased patient engagement suggest that providers should draw on patients' identities, concerns and preferences in acting on patient‐centeredness in the clinical encounter. The US Institute of Medicine (IOM) defined patient‐centred care as healthcare that establishes a partnership among practitioners, patients and their families to ensure that providers and systems deliver care that is attentive to the needs, values and preferences of patients. (IOM 2001). In their view, this requires mutual, power‐sharing relationships that are collaborative and include the "whole person" orientation.
In this growing literature, the meaning of patient‐centred healthcare continues to include a set of concepts that are compatible. However, different approaches use different sub‐sets of the elements of the approach. This allows "patient‐centred" to be defined somewhat differently across studies. The variability of aims is reflected as well in the heterogeneity of outcomes measured. The term patient‐centred has, at its core, an approach whereby the provider 'tries to enter the patient’s world to see illness through the patient’s eyes' (McWhinney 1989). This means that the provider is guided by the patient’s knowledge, experience (Byrne 1976), needs and preferences (Laine 1996), and comes to understand the patient as a unique human being (Balint 1969). Others (Grol 1990; Lipkin 1984; Winefield 1995) have noted the importance of information‐giving and shared decision‐making in this process. Mead (Mead 2000) has proposed a framework with the following dimensions for studying PCC: the biopsychosocial perspective; the 'patient‐as‐person' ‐ understanding the personal meaning of the illness for each individual patient; sharing power and responsibility; the therapeutic alliance; and the 'doctor‐as‐person' ‐ awareness of the influence of the personal qualities and emotion of the doctor on the doctor‐patient relationship. Shared decision making advocates focus on the need for clinicians to describe options, elicit patient preferences and agree on next steps in the decision‐making process.
Different elements of patient‐centred care (PCC) may be differently constructed and valued by different stakeholders, and for different reasons. Some people regard patient‐centred care as desirable in its own right, while others see it as a means to particular (and varied) ends. Healthcare providers and healthcare consumers may have varied opinions about which components and which outcomes of patient‐centred care are most important. For example, consumers may be more concerned with the extent to which healthcare providers assess consumers' level of knowledge and adjust the consultation accordingly, than with outcomes such as adherence to care plans. The International Alliance of Patients' Organizations, in its declaration on patient‐centred healthcare, includes the involvement of patients in health policy and ready access to information (IAPO 2007).
Patient‐centredness is increasingly being advocated and incorporated into the training of healthcare providers. The growth of interest in training healthcare providers in patient‐centred care has occurred despite a relatively poor empirical understanding of the effects of different interventions to promote it. A growing consensus, however, identifies provider‐patient communication as a key to achieving patient‐centred care. The IOM document specifies as keys that providers use skills and behaviours that promote a relationship in which patients actively participate as partners in healthcare decision making. The US Medical Licensing Examination (USMLE), beginning in 2012, will require that to obtain licensure, US physicians demonstrate in the examination, skills that foster the doctor‐patient relationship; as well as gathering information, providing information, making decisions and supporting emotions (US 2012). The move toward testing patient‐centred care skills in professional education is based on the studies that demonstrate a correlation between effective provider‐patient communication and improved patient health outcomes (Stewart 1995a; Epstein 2007). This review extends that empirical tradition and updates the 2001 Cochrane review, synthesizing the maturing literature to examine rigorously the effects of PCC across studies.
Objectives
To assess the effects of interventions for healthcare providers that aim to promote a patient‐centred approach in clinical consultations. We considered the effects on provider‐patient interactions, healthcare behaviours (including health service utilisation), patients' health and wellbeing, and patients' satisfaction with care.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs), excluding other study designs that were included in the previous version of this review (controlled clinical trials, controlled before and after studies, and interrupted time series). This restriction to RCTs limits the likelihood of sampling error, which is especially important because the providers who volunteer for studies of PCC methods are likely to be different from the general population of providers.
Types of participants
We included studies of all types of healthcare providers, including those training to qualify as healthcare providers.This review focuses primarily on interventions directed at healthcare providers. Some studies, however, combined provider interventions with those given directly to patients. Most assessed some patient outcomes. There were no restrictions on the types of patients for whom outcome data were extracted.
Types of interventions
Any intervention directed at healthcare providers and intended to promote patient‐centred care within clinical consultations was considered.
The update maintains the original definition of patient‐centred care (Lewin 2001), in which shared treatment decision making is a sufficient indicator of patient‐centred care. We defined patient‐centred care as including the following two main features:
healthcare providers share control of consultations, decisions about interventions or the management of the health problems with patients, and/or
healthcare providers focus on the patient as a person, rather than solely on the disease, in consultations.
The review focused on clinical consultations firstly because these are the most usual type of encounters between patients and healthcare providers. Secondly, we wanted to differentiate interventions to promote patient‐centred care in the context of clinical healthcare consultations from related interventions that may be intended to promote patient‐centred approaches in social support or social care. These interventions are likely to be quite different in terms of their target groups; their outcomes; and their policy implications.
An intervention was included if the description of the intervention was adequate to allow review authors to establish that it aimed to increase the patient‐centred behaviours of providers in the clinical consultation. By patient‐centred care we mean behaviours that reflect a philosophy of care that encourages
shared control of the consultation, decisions about interventions or management of the health problems with the patient, and/or
a focus in the consultation on the patient as a whole person who has individual preferences situated within social contexts. This is in contrast to a focus in the consultation on a body part or disease.
In the original review (Lewin 2001), authors assessed the intensity of patient‐centredness and teaching/training tactics for each intervention in the included studies using a three point scale (weak, medium, strong), but the review found this scale to be unreliable. In this update, we used a proxy measure of intervention intensity, namely number of hours, dichotomized as Brief Training (< 10 hours) and Extensive Training (> 10 hours).
Exclusions
We excluded:
studies that considered cultural, disability, sexuality or other sensitivity training only for healthcare providers. Although sensitivity to these issues may be necessary for patient‐centred care, it is not sufficient in itself to constitute patient‐centred care according to our definition.
studies that evaluated training in psychotherapy or counselling for healthcare providers. Although training in psychotherapy and counselling would meet our inclusion criteria, in psychotherapy and counselling (in contrast to most other healthcare situations), communication between healthcare provider and patient is itself the primary treatment. We therefore excluded studies that evaluated training in psychotherapy or counselling unless they specifically indicated that the training aimed to encourage a more patient‐centred approach to psychotherapy or counselling than is usually used.
studies that trained healthcare providers to deliver a specific, secondary intervention initiated by the health provider (e.g. advice on a healthy diet or smoking cessation) in a patient‐centred manner in clinical consultations, regardless of whether the intervention was related to the primary purpose of the consultation as indicated by the patient or their carer. We only classified interventions as patient‐centred if they promoted a patient‐centred approach to care that was integrated with the primary purpose of the consultation rather than being a secondary, 'bolt‐on' component of it initiated by the healthcare provider.
Types of outcome measures
A number of processes and outcomes might be affected by interventions that aim to promote patient‐centred care in the clinical consultation. We extracted all outcomes and grouped them in to the following categories:
| Outcome category | Description |
| A. Consultation processes | Consultation processes, including the extent to which patient‐centred care was judged to be achieved in practice: provider communication skills, consultation process measures |
| B. Satisfaction | Patient satisfaction with care |
| C. Health behaviour | Patient healthcare behaviours, such as concordance with care plans, attendance at follow‐up consultations, and health service utilization |
| D. Health status | Patient health status and well being, including physiological measures (for example of blood pressure); clinical assessments (for example of wound healing); patient self‐reports of symptom resolution or quality of life; and patient self‐esteem |
In this update, the 'satisfaction' category was modified from Lewin 2001 to exclude carers' satisfaction with care, as it was rarely measured, and added heterogeneity to the review's findings (see Potential biases in the review process). We modified the 'health behavior' category to be more consistent with measures of behaviours found in studies in the original review. The previous (Lewin 2001) definition was: "Other healthcare behaviours, including types of care plans agreed; providers' provision of interventions; patients' adoption of lifestyle behaviours; and patients' use of interventions and services".
Exclusions
Studies that did not include any of the outcomes listed above.
Studies which measured only healthcare providers' knowledge, attitudes or intentions, for example by assessing their responses to written vignettes describing patient cases. However, we included studies using simulated patients to assess practice.
Studies so compromised by flaws in their design or execution as to be unlikely to provide reliable data.
Search methods for identification of studies
Electronic searches
We searched:
MEDLINE (OvidSP) (January 2000 to June 17 2010) (Appendix 1),
PsycINFO (OvidSP) (January 2000 to June 2010) (Appendix 2),
CINAHL (EbscoHOST) (January 2000 to June 2010) (Appendix 3), and
EMBASE (OvidSP) (January 2000 to June 2010) (Appendix 4).
For this update, we used the same search strategy as was used in the original review (Lewin 2001). Search strategies were tailored to each database.
Lewin 2001 searched the following databases:
MEDLINE (1966 to December 1999),
HEALTH STAR (1975 to December 1999),
PsycLIT (1987 to December 1999),
CINAHL (1982 to December 1999),
EMBASE (1985 to December 1999).
Searching other resources
We searched the bibliographies of studies assessed for inclusion. Any study authors who were contacted for further information on their studies were also asked if they were aware of any other published or ongoing studies that would meet our inclusion criteria.
Data collection and analysis
Selection of studies
Two authors or acknowledged screening members of the team screened all reports (titles and abstracts) against inclusion criteria (FCD, MHR, CG, JC, SL, GS, AO, RCS, AAO, LF, LBL, KKB). No review authors made selection decisions regarding any of their own studies. We resolved inconsistencies by discussion and consensus. When appropriate, we contacted study authors for further information and clarification. We retrieved in full text all articles that were judged to be potentially relevant from the titles and abstracts. Two review authors then independently assessed these retrieved articles for inclusion. Disagreements were resolved by discussion. In several papers, the description of the intervention was not sufficiently detailed to allow the review authors to judge whether it met the review's inclusion criteria. In these cases, the study authors were contacted and, where possible, we then assessed more detailed descriptions and/or materials.
We excluded studies which did not meet the Criteria for considering studies for this review after full text assessment, as well as studies that were so compromised by flaws in their design or execution as to be unlikely to provide reliable data. We listed these excluded studies, together with the reasons for their exclusion, in the Characteristics of excluded studies table.
Data extraction and management
For included studies, two authors independently extracted full descriptions of the interventions and participants onto a standard outline form (FCD, MHR, CG, SL, JC, GS, AO, and RCS, AAO, LF, LBL, KKB). No authors extracted data from their own studies. Data extraction sheets were checked by the first author for consistency (FCD). Where necessary, other members of the review team were asked to consider and discuss problems. The outcome variables used to measure intervention effects in each category are reported in the Characteristics of included studies table. In many cases, additional outcomes were measured. However, the table indicates 'not applicable' (NA) where none of the study outcomes addressed consultation processes, satisfaction, healthcare behaviour or health status, respectively. Consistent with the original review, two authors (FCD, CGM, GS, SL, JC, RCS, AO, JC, MHR) independently examined each measured outcome and assigned it to the categories described at Types of outcome measures.
We classified interventions by whether they focused only on providers or on providers and patients, with and without condition‐specific educational materials. We grouped outcome data from the studies to evaluate both direct effects on patient encounters (consultation process variables) and effects on patient outcomes (satisfaction, health behaviour change, health status).
Assessment of risk of bias in included studies
Two review authors (FCD, CG, GS) independently assessed the risk of bias of each included study using the criteria listed below taken from the Cochrane Risk of Bias tool in RevMan 5.1:
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding (performance bias and detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
For each criterion, we reported whether it was 'done', 'not done' or 'unclear'. Any discrepancies were resolved by discussion. We report the risk of bias assessment at Risk of bias in included studies, and Figure 1; Figure 2.
1.
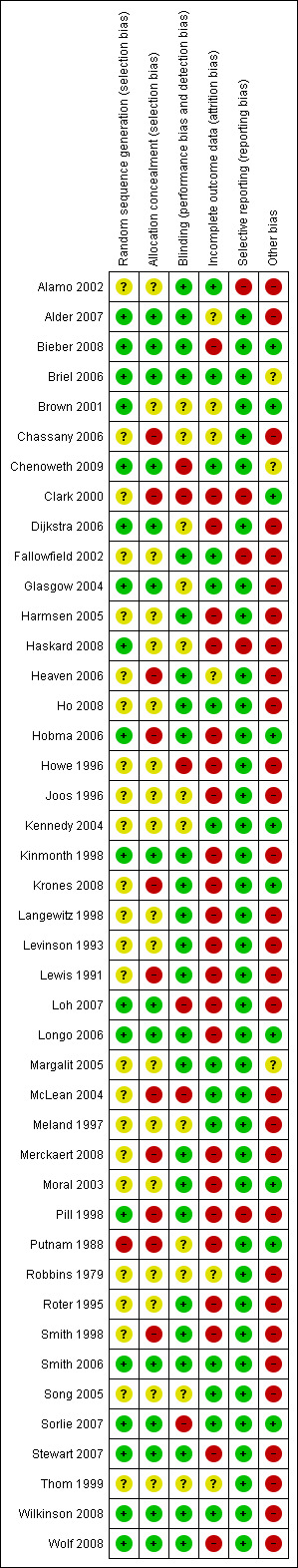
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
For dichotomous outcomes, we recorded the relative risk (RR) and 95% confidence interval (CI). In determining the RR, positive outcomes were defined as events, so RR > 1 indicates a positive effect of the intervention.
For continuous outcomes, we recorded means and standard deviations which were used to compute the standardized mean difference (SMD) and its 95% CI.
Unit of analysis issues
In the pooled analysis, for cluster randomized trials, we reviewed the methods and results reported. If authors used statistical methods that did not account for clustering of observations, we did not meta‐analyse these data. If the authors adjusted for clustering, but did not report the value of the intraclass correlation coefficient (ICC), we contacted the authors. Where reported, we used the ICC value to adjust the sample sizes by dividing the actual sample sizes by the design effect factor (DEFF), which was calculated using ICC as DEFF=1+(M‐1)ICC, where M is the average cluster size (eg, number of patients nested within clinician) (see Notes section of Characteristics of included studies).
Finally, if the studies contained more than two arms, we used pair‐wise comparisons of intervention to control. These steps ensured that comparable estimates of treatment effects were analysed: all from either RCTs with the patient as unit of randomisation and analysis, or from cluster randomized trials adjusted for clustering effect, and all providing between‐arm comparisons for two trial arms.
Dealing with missing data
If the information necessary for meta‐analysis was not reported in the article, we attempted to obtain such information from study authors, including ICC as described above. Further, if an article reported the results of analyses that did not compare two trial arms, then we attempted to re‐analyse the data to derive between‐group comparisons rather than within‐group comparisons. We contacted study authors for any information missing in the article that was needed for re‐analysis, such as the means and standard deviation of the outcomes in each group rather than mean change scores. If we received no response from authors, then we excluded studies with missing data from meta‐analyses (see Table 1).
1. Studies excluded completely from meta‐analysis.
| Study | Type of variable | Reason for Exclusion |
| Dichotomous | ||
| Lewis 1991 | Consultation | Need baseline data and ICCa to adjust for clustering |
| Continuous | ||
| Alamo 2002 | Consultation | Need ICCa to adjust for clustering |
| Bieber 2008 | Consultation Health status Patient satisfaction |
Need ICCa for patients clustered within physicians; Need to confirm with authors if physicians were randomized |
| Brown 2001 | Consultation Health status Health behaviour |
Need ICCa; Need Standard deviations |
| Chassany 2006 | Health status Health behaviour |
Methodological Problem; change scores analyzed instead of post intervention; No ICCa for adjusting for clustering |
| Fallowfield 2002 | Consultation? | Need number of events per doctor or odds for each group reported for table 4 on page 653. Need standard deviations |
| Haskard 2008 | Need standard deviations; Need to reanalyze data because they compared changes within groups | |
| Harmsen 2005 | Need standard deviation for Table 1. All patients. | |
| Heaven 2006 | Methodological Problem: no control; two interventions compared to each other; | |
| Krones 2008 | Consultation Health status Patient satisfaction |
Need standard errors or unadjusted standard deviations for table four; Need ICCa |
| Levinson 1993 | Consultation? | Need short program means for pre intervention and also post intervention separate; Reported as a difference which is unusable; Need Standard deviations pre and post |
| Lewis 1991 | Consultation Health status Patient satisfaction |
Need baseline data and ICCa to adjust for clustering |
| Longo 2006 | Consultation Health status Health behaviour Patient satisfaction |
Need ICCa |
| Margalit 2005 | Methodologically out; No controls; two interventions | |
| Meland 1996 | Patient behaviour? | Need ICCa to adjust for clustering |
| Moral 2003 | Consultation | Need ICCa and standard deviations |
| Pill 1998 | Satisfaction Health status |
Methodological problem: experimental providers were asked to submit a recording which demonstrated the use of the method they had been taught; Need ICCa; Need actual values post with standard deviations ? not change scores |
| Putnam 1988 | Satisfaction Health behaviour Health status |
Need standard deviations; Possible contamination |
| Robbins 1979 | Consultation | Need standard deviations pre and post |
| Smith 1998 | Consultation Satisfaction |
Methodological problem: possible contamination stated by authors; need standard deviations for attitudes and knowledge‐answered by residents; Need satisfaction post means and standard deviations for controls and interventions instead of a difference; |
| Song 2005 | Health status | Methodological problem: possible contamination. Change scores are compared |
| Thom 1999 | Satisfaction Consultation |
Need ICCa |
a) ICC: Intra‐cluster correlation
Assessment of heterogeneity
For groups of outcomes with more than two studies, we assessed heterogeneity using the I2 statistic, and a formal Chi2 test of significance. A value of 50% or higher of the I2 statistic, and P value > 0.1 indicated substantial heterogeneity (Higgins 2003). Where heterogeneity was substantial, an attempt was made to explore the sources of heterogeneity using the a priori generated list of study characteristics (see Data extraction and management above). Characteristics included: academic or non‐academic setting, country, inclusion of an experiential component, number of hours of experiential training, total number of hours of training, individual randomisation or cluster randomisation, usual care control or sham intervention control, whether the outcome analysed was primary or secondary, whether the analysis followed the intention to treat strategy. We conducted subgroup analyses according to the levels of study characteristics. We also investigated methodological problems with the studies as a potential source of heterogeneity.
Data synthesis
Limiting the current update to RCTs allowed us to pool data within categories by conducting meta‐analyses of included studies where similar outcome measures were used within categories. Two authors (SJ, AS) pooled results of RCTs using standardized mean difference (SMD) and relative risks (RR) applying a fixed‐effect model. In each outcome category it was important to keep the direction of the effects the same. For example, higher was worse in some categories, such as depression as an indicator of health status. In other categories, higher was better, such as percentage of patients making an appointment as an indicator of outcomes of consultation processes. The direction of the effects in each category of effects is labelled on the plots (see Data and analyses).
In part due to the inconsistency across categories in the pooled analysis, and because fewer than half of studies could be included in the meta‐analysis, we also conducted a descriptive analysis of the whole set of studies according to the four outcome categories. We report a summary of this analysis at Effects of interventions and a detailed narrative synthesis at Appendix 5. We summarized the results of the descriptive analysis in terms of the number of studies showing a positive effect out of the number of studies that measured that outcome for each of the categories (see Table 2).
2. Impact of Interventions ‐ summary of qualitative analysis.
| Intervention category | Total studies in category |
Studies in which consultation process outcomes favoured intervention / Total studies assessing consultation process (%) |
Studies in which satisfaction outcomes favoured intervention / Total studies reporting satisfaction (%) |
Studies in which patient behaviour outcomes favoured intervention / Total studies reporting patient behaviour (%) |
Studies in which health status outcomes favoured intervention / Total studies reporting health status (%) |
| 1 (PCC training for providers) | 23 | 16/22 (73%) | 6/13 (46%) | 1/4 (25%) | 5/11 (45%) |
| 2 (PCC for providers plus training or materials for patients) | 7 | 5 /6 (83%) | 1/4 (25%) | 0/2 (0%) | 3/4 (75%) |
| 3 (PCC plus condition‐specific training for providers) | 7 | 2/2 (100%) | 2/5 (40%) | 4/6 (67%) | 2/6 (33%) |
| 4 (PCC plus condition‐specific training for providers, plus training for patients) | 6 | 5/5 (100%) | 3/4 (75%) | 3/5 (60%) | 3/6 (50%) |
| Total | 43 | 28/35 (80%) | 12 /26 (46%) | 8/17 (47%) | 12/26 (46%) |
For continuous outcomes, to maintain one direction within each group of outcomes in meta‐analysis (e.g. larger is worse as in anxiety), outcomes with higher scores indicating better outcomes were reverse scored by subtracting the reported means from the scale totals. Where reported, the adjusted estimates and baseline values of the outcomes were recorded.
If reported, one dichotomous and one continuous outcome from each study were included within the four outcome groups: consultation process, satisfaction, health behavior, and health status. If more than one outcome from each group was reported, then the following strategies were implemented to choose one outcome:
primary outcomes were selected over secondary;
total scale scores were selected over sub‐scale scores; and finally
outcomes with median RR or SMD were selected for data synthesis.
Each group of outcomes was also described with the median RR and SMD and the number of studies with significant effects. Because many of the studies were cluster randomized trials, and ICCs were not available in the articles or after contacting the authors, the number of studies in each outcome subgroup was small. This issue and few studies with the number of hours of training reported prevented the implementation of the dose response meta‐regression. Subgroup analyses according to the dichotomous study characteristics shed light on the association between these study characteristics and the magnitude of the intervention effect.
Sensitivity analysis
To determine if the summary effects were dependent on outliers or studies with low methodological quality, we performed the meta‐analyses with and without these studies.
Results
Description of studies
Results of the search
The original review (Lewin 2001) identified 5260 titles and abstracts by electronic searching; 135 of these were judged to potentially meet the entry criteria and the full articles were retrieved for further detailed assessment. The original review included 15 randomized controlled trials (all published before January 2000) and 2 non‐randomized controlled clinical studies.
Our updated searches in June 2010 generated 8469 titles and abstracts, of which 100 were retrieved in full text for further assessment.
Included studies
The original review (Lewin 2001) ostensibly included 15 RCTs and 2 non‐randomized clinical trials. Through discussion with the study author we have since identified that the two papers by Smith et al (Smith 1995; Smith 1998), which were reported as separate trials in the original review, were in fact reports of the same RCT.
We added 29 studies published between January 2000 and May 2010 to the original 14 studies (Lewin 2001), bringing the total number of trials included in this update to 43 (Alamo 2002; Alder 2007; Bieber 2008; Briel 2006; Brown 2001; Chassany 2006; Chenoweth 2009; Clark 2000; Dijkstra 2006; Fallowfield 2002; Glasgow 2004; Harmsen 2005; Haskard 2008; Heaven 2006; Ho 2008; Hobma 2006; Howe 1996; Joos 1996; Kennedy 2004; Kinmonth 1998; Krones 2008; Langewitz 1998; Levinson 1993; Lewis 1991; Loh 2007; Longo 2006; Margalit 2005; McLean 2004; Meland 1997; Merckaert 2008; Moral 2003; Pill 1998; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Smith 2006; Song 2005; Sorlie 2007; Stewart 2007; Thom 1999; Wilkinson 2008; Wolf 2008). These 43 studies were reported in 74 papers. Fewer than half (N = 21) of the 43 studies met the criteria for inclusion in at least one comparison in the meta‐analyses.
Clark 2000 provides additional data for a study included in the original review as Clark 1998.
Interventions and comparisons
The interventions involved training related to a variety of skills, using diverse teaching techniques and lengths of training. Some used placebo controls and some used pure usual care, as noted in the Characteristics of included studies table. Aims of the interventions ranged from improving patient centeredness as a primary goal to improving health behaviour of patients and/or effectiveness of providers in managing medical problems. Studies focused always on providers, by definition. However, the addition in some studies of decision aids and patient education materials (both general and condition‐specific) and patient training suggested that interventions have become more complex, and directed to both the provider and the patient sides of the encounter. While none of the studies included in Lewin 2001 described shared decision making as an aim, four in the current review did aim to improve shared decision making (Bieber 2008; Krones 2008; Loh 2007; Longo 2006).
To capture this emerging diversity in aims of studies, our descriptive results are presented in four intervention categories as follows:
| Intervention category | PCC intervention | Other component | Number of studies |
| 1 | PCC training for providers | None | 23 |
| 2 | PCC training for providers | Training or general educational materials for patients | 7 |
| 3 | PCC training for providers | Condition‐specific training or materials (eg. management of asthma or diabetes mellitus) for providers | 7 |
| 4 | PCC training for providers | Condition‐specific materials or training for both providers and patients | 6 |
Setting
All 43 of the included studies were published in English. Studies were conducted in 13 countries from North America, Europe and Eastern Asia. There were no studies from low and middle income countries.
| Country | Number of studies |
| USA | 16 |
| UK | 10 |
| Germany | 3 |
| Each of Netherlands, Spain, Australia, Switzerland | 2 |
| Each of Canada, France, Holland, Israel, Norway, Taiwan | 1 |
Unit of randomisation
The unit of randomisation was the organisation or practice in 9 studies (Chenoweth 2009; Hobma 2006; Meland 1997; Kinmonth 1998; Pill 1998, Dijkstra 2006; Kennedy 2004, Moral 2003, Krones 2008), the individual healthcare provider in 25 studies (Alder 2007; Alamo 2002; Briel 2006; Chassany 2006; Clark 2000; Fallowfield 2002; Glasgow 2004; Harmsen 2005; Heaven 2006; Ho 2008; Howe 1996; Joos 1996; Langewitz 1998, Levinson 1993; Lewis 1991; Loh 2007; Margalit 2005; Merckaert 2008; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Stewart 2007; Thom 1999; Wilkinson 2008), the patient in 6 studies (Bieber 2008; McLean 2004; Smith 2006; Song 2005; Sorlie 2007; Wolf 2008); and both the provider and patient in 3 studies (Brown 2001; Haskard 2008; Longo 2006).
Trainer characteristics
Training interventions were delivered by healthcare providers and/or research centre staff in 19 studies (Alder 2007; Chenoweth 2009; Chassany 2006; Heaven 2006; Hobma 2006; Howe 1996; Lewis 1991; Krones 2008; Langewitz 1998; Margalit 2005; Pill 1998; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Smith 2006; Sorlie 2007; Stewart 2007; Thom 1999). The remaining 24 studies did not indicate the identity of intervention trainers. Moreover, only ten of the 43 studies reported any kind of training or certification of intervention trainers (Chenoweth 2009; Chassany 2006; Fallowfield 2002; Haskard 2008; Kinmonth 1998; Kennedy 2004; Levinson 1993; Roter 1995; Stewart 2007; Wilkinson 2008).
Consumer involvement
Only three studies (Dijkstra 2006; Kinmonth 1998; Stewart 2007) appeared to have involved consumers in the development of the intervention. None of the 43 studies involved consumers in the actual training of providers.
Patient participants
The 43 included studies evaluating PCC interventions focused on a variety of clinical conditions, although the most common patients were adults with general medical problems. Three studies used simulated patients with a common medical and/or psychosocial problem (Ho 2008; Langewitz 1998; Moral 2003); three others used both real and simulated patients with generalized musculoskeletal pain/fibromyalgia (Alamo 2002), cancer (Wilkinson 2008), and general medical problems (Alder 2007). The remaining 37 studies included only real patients. Two of them included children with asthma (Clark 2000) and a range of different problems (Lewis 1991). The remaining 35 studies enrolled adults with: general medical problems (Briel 2006; Harmsen 2005; Haskard 2008; Heaven 2006; Ho 2008; Hobma 2006; Howe 1996; Joos 1996; Levinson 1993; Longo 2006; Margalit 2005; McLean 2004; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Thom 1999); and more specific medical conditions like diabetes (Dijkstra 2006; Glasgow 2004; Kinmonth 1998; Pill 1998), cancer (Brown 2001; Fallowfield 2002; Merckaert 2008; Stewart 2007), depression (Loh 2007), high coronary heart disease risk (Meland 1997), inflammatory bowel diseases (Kennedy 2004), osteoarthritis (Chassany 2006), fibromyalgia (Bieber 2008), dementia (Chenoweth 2009) and medically‐unexplained symptoms (Smith 2006). Three studies enrolled adult patients undergoing surgery for heart disease (Song 2005; Sorlie 2007) and obesity (Wolf 2008).
Healthcare‐provider participants
In most of the studies, training interventions were directed at primary care physicians (general practitioners, internists, paediatricians or family doctors) or nurses practising in community or hospital outpatient settings. In other studies, specialists were also trained. For example, Alder 2007 trained only obstetricians and gynaecologists. Brown 2001, Fallowfield 2002 and Merckaert 2008 trained medical, radiation, and/or surgical oncologists at specialty cancer centres. Six studies trained nurses and/or nurse practitioners (Heaven 2006; Smith 2006; Song 2005; Sorlie 2007; Wilkinson 2008; Wolf 2008); four studies trained both primary care physicians and nurses (Dijkstra 2006; Kennedy 2004; Kinmonth 1998; Pill 1998), and one study (Chenoweth 2009) trained care givers at residential dementia care sites. The clinical experience of providers varied both within and across studies, ranging from medical students with five years of medical education to providers with more than 20 years of clinical experience. The study sample sizes also varied from 8 to 41 practices in the nine studies randomized by practice, and 4 to 172 providers in the 31 studies that randomized by providers. One study that randomized 30 CME insurance groups did not provide the number of practices and/or providers included (Krones 2008).
The process through which providers were selected to participate in the studies also varied. In two studies (Ho 2008; Smith 1998), participation in the training programmes appeared to have been compulsory (as part of medical school (Ho 2008) or postgraduate (Smith 1998) training); although in Smith 1998, postgraduate trainees were allowed to opt out of the analysis. For the remaining studies, however, participation appeared to be voluntary. Provider recruitment methods were as follows:
| Method of recruiting providers | Study |
| Approached whole practices | Alder 2007; Briel 2006; Dijkstra 2006; Heaven 2006; Hobma 2006; Joos 1996; Kinmonth 1998; Levinson 1993; Lewis 1991; Loh 2007; Longo 2006; Margalit 2005; McLean 2004; Merckaert 2008; Pill 1998; Robbins 1979; Stewart 2007 |
| Approached subsets of providers from specified groups or areas | Clark 2000; Langewitz 1998; Meland 1997; Putnam 1988; Thom 1999; Wilkinson 2008 |
| Approached physicians from the mailing lists of local medical societies | Chassany 2006 |
| Approached physicians from the mailing lists of insurance companies | Glasgow 2004; Krones 2008 |
| Providers were care staff at residential care sites and were selected by managers as competent and interested personnel | Chenoweth 2009 |
| Not specified | Alamo 2002; Bieber 2008; Brown 2001; Fallowfield 2002; Harmsen 2005; Haskard 2008; Howe 1996; Kennedy 2004; Moral 2003; Smith 2006; Song 2005; Sorlie 2007; Wolf 2008). |
In 30 of the 43 studies, the percentage of invited providers who agreed to participate ranged from 5 per cent (Glasgow 2004) to 100 per cent (Alamo 2002). In the other 13 studies this percentage was unclear (Bieber 2008; Brown 2001; Chenoweth 2009; Clark 2000; Dijkstra 2006; Levinson 1993; McLean 2004; Merckaert 2008; Smith 2006; Song 2005; Sorlie 2007; Thom 1999; Wolf 2008).
Outcomes
Most of the included studies evaluated the impact on consultation processes (n = 35, Table 3) and many also evaluated the impact on patient satisfaction (n = 26, Table 4). Patient health behaviours were less frequently assessed (n = 17, Table 5). Patient health status (n = 26, Table 6) was evaluated quite frequently.
3. Category A consultation process outcome measures.
| Study ID | Outcome Category A: Consultation Process | How assessed | Use in analysis |
| Provider consultation communication behaviour | |||
| Alamo 2002 | Provider use of patient‐centred communication behaviours in video recordings of encounters. | Used GATHERES‐CP scoring system | Narrative |
| Alder 2007 | Provider use of patient‐centred communication behaviours in video recordings of encounters, shared decision making | Observation from videotapes as measured by the Maastricht History and Advice Checklist‐Revised (MAAS‐R). | Continuous variable in meta‐analysis |
| Fallowfield 2002 | Provider use of patient‐centred communication behaviours in video recordings of encounters, shared decision making | Medical Interaction Process System (MIPS) | Narrative |
| Haskard 2008 | Physician information giving | Patient report | Narrative |
| Heaven 2006 | Provider use of patient‐centred communication behaviours in audio recordings of encounters | Medical Interview Aural Rating Scale (MIARS) | Narrative |
| Ho 2008 | Provider use of patient‐centred communication behaviours in observed standardized patients | OSCE scores on observed standardized patient encounter | Continuous variable in meta‐analysis |
| Hobma 2006 | Provider use of patient‐centred communication behaviours in video recordings of encounters | MAAS Global Questionnaire for Providers. | Continuous variable in meta‐analysis |
| Howe 1996 | Provider's psychological distress detection rate following training in communication skills among video recordings of own patients | Checklist for video analysis among patients with high scores on General Health Questionnaire (GHQ) | Continuous variable in meta‐analysis |
| Joos 1996 | Provider elicitation of all patient concerns (previously stated on checklist) in audio recordings of encounters | Roter Interactional Analysis System (RIAS) | Continuous variable in meta‐analysis |
| Kinmonth 1998 | Quality of communication with provider | Proportion of patients rating maximum quality | Dichotomous |
| Krones 2008 | Shared decision making, patient perception that doctor knows patient | SDM‐Q, Patient Participation Survey | Narrative |
| Langewitz 1998 | Provider use of patient‐centred communication behaviours in video recordings of encounters with standardized patients | Maastricht History and Advice Checklist‐Revised (MAAS‐R) | Continuous variable in meta‐analysis |
| Levinson 1993 | Provider and patient‐centred communication behaviours |
Change scores: observed from video using RIAS | Narrative |
| Lewis 1991 | Patient‐centered communication style in video recording of actual encounters |
Percentage and number of statements in encounter by Pantell/Stewart coding method | Narrative |
| Loh 2007 | Consultation time; doctor facilitation of patient involvement | Time in min; Participation surveys: PICS, variation of Man‐Song‐Hing scale | Continuous variable in meta‐analysis |
| Longo 2006 | Involving patient in decision making | Observation (Provider score on OPTION instrument for patient agreement and involvement) | Narrative |
| Margalit 2005 | Provider’s biopsychosocial knowledge, intentions, patient‐centred attitudes; Physician detection of patient distress |
Physician self‐report; Patient report (physician detection of patient distress) | Narrative |
| Merckaert 2008 | Patient‐centered communication style in audio recording of simulated and actual encounters |
Audio rating: French translation of “Cancer Research Campaign Workshop Evaluation Manual”; | Continuous variable in meta‐analysis |
| Moral 2003 | Consultation behaviour in standardized patient encounters | Rated by GATHA‐RES (instrument/rating scale designed by authors) | Narrative |
| Pill 1998 | Provider use of patient‐centred communication behaviours in audiotaped encounters. | Investigator developed coding | Narrative |
| Putnam 1988 | Patient and provider use of patient‐centred communication behaviours in audiotaped encounters | Coded verbal response modes (VRMs) | Narrative |
| Robbins 1979 | Provider use of patient‐centred communication behaviours in video taped encounters; empathy scores | Coded responses using Kagan, Brockway, Curkhuff scales; Affect Sensitivity Scale (empathy) | Narrative |
| Roter 1995 | Provider use of emotion‐handling skills in audio recordings of encounters with simulated patients, actual patients | Changes in emotion handling score using study‐specific coding measure | Dichotomous |
| Smith 1998 | Provider use of patient‐centred communication behaviours in audio recorded encounters with real patients, video recorded simulated patients. | Study‐specific rating scales | Narrative |
| Song 2005 | Knowledge of advanced care planning | Patient/surrogate report | Continuous |
| Stewart 2007 | Provider use of patient‐centred communication behaviours in video recordings of encounters. | Score from Patient‐Centred Communication Measure | Continuous variable in meta‐analysis |
| Thom 1999 | Provider's humaneness during visit | Patient perception from score on Physician Humanistic Behaviors Questionnaire | Narrative |
| Wilkinson 2008 | Provider use of patient‐centred communication behaviours in audio recordings of encounters. | Communication Skill Rating Scale coverage score at baseline; skills change score) | Continuous variable in meta‐analysis |
| Wolf 2008 | Reported skills by providers | Structured checklist | Narrative |
| Patient centered actions | |||
| Briel 2006 | Medication prescribed | Provider self‐report | Dichotomous variable in meta‐analysis |
| Clark 2000 | Medication prescribed, treatment/action plan given | Patient/parent report, provider survey | Dichotomous variable in meta‐analysis |
| Dijkstra 2006 | Diabetes‐specific process measures at index visit and 12 months | From medical record | Narrative |
| Glasgow 2004 | Patient‐centred activities completed | Number completed out of a priori list | Continuous variable in meta‐analysis |
| Impact on provider‐patient relationship | |||
| Bieber 2008 | Quality of patient‐physician relationship | By FAPI questionnaire, patient report | Narrative |
| Harmsen 2005 | Mutual understanding | Patient and doctor survey, Mutual Understanding Scale (MUS) | Narrative |
| Other consultation process outcomes | |||
| Brown 2001 | Duration of consultation | Audiotape recording | Narrative |
| Kennedy 2004 | Number of visits to clinic, medical and surgical treatment in hospital | Counts from medical record | Narrative |
| McLean 2004 | Duration of consultation | Timed by the physician | Narrative |
4. Category B satisfaction outcome measures.
| Study ID | Outcome category B: Satisfaction | How assessed | Use in analysis |
| Alamo 2002 | Patient experience of the consultation | Survey at 2 to 3 months | Narrative |
| Alder 2007 | Satisfaction with consultation and relationship | Adapted version of the Kravitz survey | Narrative |
| Bieber 2008 | Patient satisfaction with decision; decisional conflict | Satisfaction with Decision (SWD); Decisional Conflict Scale (DCS) | Narrative |
| Briel 2006 | Patient satisfaction with care received | Score on Langewitz, Patient Satisfaction Survey relative to validation study score | Dichotomous variable in meta‐analysis |
| Clark 2000 | Satisfaction with consultation | Patient report using Likert‐type scale items to assess doctor performance of consultation skills | Narrative |
| Glasgow 2004 | Patient satisfaction with care | Patient satisfaction items of Diabetes Patient Recognition Program (PRP) | Dichotomous variable in meta‐analysis |
| Harmsen 2005 | Satisfaction with consultation | 3‐item survey | Dichotomous variable in meta‐analysis |
| Haskard 2008 | Satisfaction with information, overall care | Physician Information‐giving scale (Heisler), single item: whether recommend doctor to a friend | Narrative |
| Joos 1996 | Patient satisfaction with physician skills | American Board of Internal Medicine Patient Satisfaction Questionnaire | Continuous variable in meta‐analysis |
| Kennedy 2004 | Satisfaction with initial consultation | Consultation satisfaction questionnaire (Baker) | Continuous variable in meta‐analysis |
| Kinmonth 1998 | Patient satisfaction with treatment | Survey dichotomized to high/low | Dichotomous variable in meta‐analysis |
| Krones 2008 | Satisfaction with care process and outcome | Patient Participation Scale (Man‐Son‐Hing) | Narrative |
| Langewitz 1998 | Patient satisfaction; Patients who would recommend doctor to a friend. | Score on the German version of the PSQ by the American Board of Internal Medicine; Proportion recommend | Narrative |
| Lewis 1991 | Child satisfaction with visit; parent satisfaction with visit | Child Satisfaction Questionnaire; Parent Medical Interview Satisfaction Scale | Narrative |
| Loh 2007 | Patient satisfaction with care | German version of CSQ‐8 questionnaire for patients | Continuous variable in meta‐analysis |
| Longo 2006 | Patient satisfaction with communication | COMRADE | Narrative |
| McLean 2004 | Satisfaction with consultation | Consultation Satisfaction Questionnaire (Baker) | Continuous variable in meta‐analysis |
| Merckaert 2008 | Satisfaction with physician communication skills | Perception of the Interview Questionnaire (Devaux) | Narrative |
| Pill 1998 | Satisfaction treatment | SF36 | Narrative |
| Putnam 1988 | Satisfaction with encounter | Medical Interview Satisfaction Scale | Narrative |
| Smith 1998 | Patient satisfaction with medical interview | 29 item locally‐developed scale | Narrative |
| Smith 2006 | Patient satisfaction with provider‐patient relationship | Satisfaction With Provider Patient Relationship Questionnaire (PPR) by Smith | Narrative |
| Stewart 2007 | Patient satisfaction with doctor’s information‐giving and interpersonal skills | Cancer Diagnostic Interview Scale (CDIS) | Continuous variable in meta‐analysis |
| Thom 1999 | Patient's satisfaction with visit | Survey by Davis | Narrative |
| Wilkinson 2008 | Satisfaction with care | 'Patient Satisfaction With Communication' by Ware | Continuous variable in meta‐analysis |
| Wolf 2008 | Satisfaction with care | Baker and Taylor Measurement Scale (BTMS) patient survey | Continuous variable in meta‐analysis |
5. Category C health behaviour outcome measures.
| Study ID | Outcome category C: Health Behavior | How assessed | Use in analysis |
| Alder 2007 | Compliance | Kravitz questionnaire for patients | Narrative |
| Bieber 2008 | Therapeutic modality chosen (medication, exercise, relaxation) | Medical record | Narrative |
| Briel 2006 | Re‐consultation within 14 days | Patient survey at 14 days | Dichotomous variable in meta‐analysis |
| Brown 2001 | Patient consultation behaviours (question asking, information recall) | None | Not included |
| Clark 2000 | Emergency department visits; hospitalizations; school days missed | Parent/patient report | Narrative |
| Glasgow 2004 | Self‐management goal setting | Met NCQA/ADA diabetes Physician Recognition Program (PRP) criteria or not | Dichotomous variable in meta‐analysis |
| Joos 1996 | Medication adherence | Meds score = Number of meds dispensed divided by number prescribed | Continuous variable in meta‐analysis |
| Kennedy 2004 | Making no more than 2 GP visits per year | Medical record | Dichotomous variable in meta‐analysis |
| Kinmonth 1998 | Patients' lifestyle: diet, exercise, smoking | Self‐report | Narrative |
| Krones 2008 | Patient participation in encounter | Patient Participation Scale | Narrative |
| Loh 2007 | Information seeking | PICS‐IS | Continuous variable in meta‐analysis |
| Longo 2006 | Adherence expectation | COMRADE sub scales | Narrative |
| Meland 1997 | Physical activity, smoking | Self‐report | Narrative |
| Pill 1998 | Patient attendance at practice over last 12 months; smoking and alcohol use |
Self‐report | Narrative |
| Putnam 1988 | Medication adherence, Appointment adherence |
Meds = telephone interview Appointment adherence = medical record |
Narrative |
| Roter 1995 | Utilisation of health care by GHQ positive patients | General Healthcare Questionnaire (GHQ) | Narrative |
| Smith 2006 | Antidepressant used to full dose | Medical record review | Dichotomous variable in meta‐analysis |
| Thom 1999 | Continuity with study provider; medication or advice adherence | Medical record review at 6 months | Narrative |
6. Category D health status outcome measures.
| Study ID | Outcome category D: Health status | How assessed | Use in analysis |
| Alamo 2002 | Pain, depression and anxiety | Pain Scale of Nottingham Health Profile; Goldberg Scale of Anxiety, Depression | Narrative |
| Bieber 2008 | Pain, depression; functional capacity; general health status | Pain level (0‐10 VAS) CES‐D; Hannover Functional Quest; SF‐12 | Narrative |
| Briel 2006 | Number of days with restricted activities | Self‐report | Continuous variable in meta‐analysis |
| Brown 2001 | Anxiety | Spielberger | Narrative |
| Chassany 2006 | Pain relief, stiffness, physical functioning/global health; adverse events | WOMAC = physical functioning, stiffness; Adverse events = Lequensne Index | Narrative |
| Chenoweth 2009 | Quality of life in late‐stage dementia | 'Quality of life in late‐stage dementia' survey | Continuous variable in meta‐analysis |
| Dijkstra 2006 | HbA1c level | Medical record review | Continuous variable in meta‐analysis |
| Glasgow 2004 | Quality of life, depression | Patient Health Questionnaire‐depression (PHQ‐9) | Narrative |
| Kennedy 2004 | Number and duration of relapses during the course of the year | HADS –Hospital Anxiety and Depression Scale | Continuous variable in meta‐analysis |
| Kinmonth 1998 | Wellbeing score, quality of life | Not specified | Narrative |
| Krones 2008 | Cardiovascular risk | Mean change on Framingham calibrated for Europeans | Narrative |
| Lewis 1991 | Anxiety (child) | Reported by parent | Narrative |
| Loh 2007 | Depression severity | Brief PHQ‐D patient questionnaire | Dichotomous variable in meta‐analysis |
| Longo 2006 | Anxiety; health status | Anxiety = Spielberger; health status =SF‐12 | Narrative |
| McLean 2004 | Anxiety | Spielberger | Continuous variable in meta‐analysis |
| Meland 1997 | Risk factors for CHD (blood pressure, cholesterol); Combine risk of myocardial infarction compared with a female without risk factors | Mean from record review | Narrative |
| Merckaert 2008 | Change in anxiety | STAI‐S | Continuous variable in meta‐analysis |
| Pill 1998 | Health status Diabetes‐specific measures of well being |
Health status = SF‐36 | Narrative |
| Putnam 1988 | Symptom improvement | Patient questionnaire Index: 3x5 point scales (Mushlin 1978) | Narrative |
| Roter 1995 | Health status | GHQ | Narrative |
| Smith 1998 | Patients' physical and psychosocial well being | Change in health status or not, on GHQ | Dichotomous variable in meta‐analysis |
| Smith 2006 | Mental health | MH scale on SF‐36 survey | Narrative |
| Song 2005 | Anxiety, difficulty making choices | Spielberger STAI | Narrative |
| Sorlie 2007 | Subjective health, overall emotional well‐being | SF‐36 | Continuous variable in meta‐analysis |
| Stewart 2007 | Patients’ psychological distress | Brief Symptom Inventory | Narrative |
| Wilkinson 2008 | Health status | General Health Questionnaire‐12 (GHQ‐12) | Continuous variable in meta‐analysis |
| Wolf 2008 | Bariatric patients post‐op infections, complications | Medical record reviews | Narrative |
Excluded studies
The two non‐randomized clinical trials included in Lewin 2001 were excluded from this update as they no longer met the inclusion criteria (Cope 1986; Roter 1998).
In the Characteristics of excluded studies table we list those studies assessed in full text which were then excluded. The main reasons for exclusion were: ineligible study design, failure to meet patient‐centred criteria, or intervention was not directed at providers.
Risk of bias in included studies
All 43 included studies were at risk of at least one of the potential biases; but none was at high risk for all of them. The details of the risk of bias of included studies are given under each reference in the Characteristics of included studies. See also Figure 1 and Figure 2.
Overall risk of bias was moderate to high, with the highest risks in the elements of random sequence generation and concealment, and blinding of outcome assessors. Conversely, most studies had low risk of reporting bias.
Allocation
Only 18 of 43 studies were judged to have a well‐described randomisation process. Twelve studies explicitly reported concealed and secure allocation (Bieber 2008; Briel 2006; Chenoweth 2009; Dijkstra 2006; Kinmonth 1998; Loh 2007; Longo 2006; Smith 2006; Sorlie 2007; Stewart 2007; Wilkinson 2008; Wolf 2008). Two of these, (Dijkstra 2006; Sorlie 2007) did not describe how the sequence was generated, but allocation was clearly concealed. In the other ten, allocation was made by computer, random number table, or by casting blinded lots. We judged sequence allocation and/or concealment to be adequate in six other studies, even though they were only partially described (Alder 2007; Brown 2001; Glasgow 2004; Haskard 2008; Hobma 2006; Pill 1998). Even after email correspondence with authors, sequence allocation and concealment was judged to be either inadequate or unclear in the remaining 25 studies, raising concerns about selection and confounding biases in these studies (Alamo 2002; Chassany 2006; Clark 2000; Fallowfield 2002; Harmsen 2005; Heaven 2006; Ho 2008; Howe 1996; Joos 1996; Kennedy 2004; Krones 2008; Langewitz 1998; Levinson 1993; Lewis 1991; Margalit 2005; McLean 2004; Meland 1997; Merckaert 2008; Moral 2003; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Song 2005; Thom 1999).
Blinding
Blinding of outcome assessors was reported or clear in the majority (28/43) of studies (Alamo 2002; Alder 2007; Bieber 2008; Briel 2006; Chenoweth 2009; Harmsen 2005; Heaven 2006; Ho 2008; Hobma 2006; Kinmonth 1998; Krones 2008; Langewitz 1998; Levinson 1993; Lewis 1991; Longo 2006; Margalit 2005; Merckaert 2008; Moral 2003; Pill 1998; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Smith 2006; Stewart 2007; Thom 1999 (interviewers, but not chart abstractors); Wilkinson 2008; Wolf 2008). In one study (Fallowfield 2002), outcome assessors for the primary outcome were blinded "as far as possible," but authors did not report blinding for other "subjective and objective" ratings which were planned for future publications. For the remaining 14 studies it was unclear or unlikely that blinding of outcome assessors had been ensured (Brown 2001; Chassany 2006; Clark 2000; Dijkstra 2006; Glasgow 2004; Haskard 2008; Howe 1996; Joos 1996; Kennedy 2004; Loh 2007; McLean 2004; Meland 1997; Song 2005; Sorlie 2007).
Incomplete outcome data
Outcome data were complete in only six studies (Brown 2001; Fallowfield 2002; Ho 2008; Robins 1989; Song 2005; Thom 1999 ). Of the 37 studies with incomplete data, 12 clearly adopted an intention to treat (ITT) approach to statistical analysis (Alamo 2002; Briel 2006; Chenoweth 2009; Glasgow 2004; Joos 1996; Kennedy 2004; Margalit 2005; McLean 2004; Meland 1997; Smith 2006; Sorlie 2007; Wilkinson 2008), raising the possibility of bias in the remaining 25 studies. Five studies stated that ITT analysis was used, but they did not include some participants with missing data in final analyses (Bieber 2008; Chassany 2006; Hobma 2006; Kinmonth 1998; Moral 2003). One study stated that an ITT approach was not used (Roter 1995). In the other 19 studies, it was either unlikely or unclear that this approach had been used (Alder 2007; Clark 2000; Dijkstra 2006; Harmsen 2005; Haskard 2008; Heaven 2006; Krones 2008; Langewitz 1998; Levinson 1993; Lewis 1991; Loh 2007; Longo 2006; McLean 2004; Merckaert 2008; Pill 1998; Putnam 1988; Smith 1998; Stewart 2007; Wolf 2008). Fallowfield 2002Ho 2008Song 2005Brown 2001Robbins 1979Thom 1999 The risk of bias from incomplete outcome data in all studies is illustrated in Figure 1 and Figure 2.
Selective reporting
Only five studies appeared to be at high risk of bias from selective reporting (Alamo 2002; Clark 2000; Fallowfield 2002; Haskard 2008; Pill 1998). All other studies were at low risk for this bias (see Figure 1 and Figure 2).
Other potential sources of bias
Protection against contamination
In studies conducted before1999, attempts to ensure protection from contamination from the intervention to the control group were reported in one study only (Putnam 1988) in which intervention group physicians were asked not to discuss the intervention with control group physicians. After that period, nine more studies attempted to protect against contamination (Brown 2001; Clark 2000; Glasgow 2004; Hobma 2006; Kennedy 2004; Krones 2008; Longo 2006; Moral 2003; Sorlie 2007). However, in the majority of studies, it was unclear or unlikely that protection against contamination was adequate (Alamo 2002; Bieber 2008; Briel 2006;Chenoweth 2009; Dijkstra 2006; Harmsen 2005; Haskard 2008; Heaven 2006; Ho 2008; Howe 1996; Joos 1996; Kinmonth 1998; Langewitz 1998; Levinson 1993; Lewis 1991; Loh 2007; Margalit 2005; McLean 2004; Meland 1997; Merckaert 2008; Pill 1998; Robbins 1979; Roter 1995; Smith 1998; Song 2005; Stewart 2007; Thom 1999; Wilkinson 2008). In five studies (Alder 2007; Chassany 2006; Fallowfield 2002; Smith 2006; Wolf 2008) potential for contamination was high, and no attempts were made to prevent or control it.
Potential for unit of analysis error for some outcomes
Thirty‐two of the studies included in this review (Alamo 2002; Alder 2007; Bieber 2008; Briel 2006; Chassany 2006; Chenoweth 2009; Clark 2000; Dijkstra 2006; Fallowfield 2002; Glasgow 2004; Harmsen 2005; Haskard 2008; Hobma 2006; Joos 1996; Kennedy 2004; Kinmonth 1998; Krones 2008; Levinson 1993; Lewis 1991; Loh 2007; Longo 2006; Margalit 2005; Meland 1997; Merckaert 2008; Moral 2003; Pill 1998; Putnam 1988; Roter 1995; Smith 1998; Stewart 2007; Thom 1999; Wilkinson 2008) randomized health providers or practices/clinics to intervention or control groups and then collected some data respectively at the level of the individual patient or provider. Standard statistical methods that do not account for the cluster effects that may arise in such data will result in the overestimation of the significance of the intervention. Five studies randomized health providers but either did not include patient clusters or did not analyse any patient level data (Heaven 2006; Ho 2008; Howe 1996; Langewitz 1998; Robbins 1979); and the remaining six were randomized by patient (Brown 2001; McLean 2004; Smith 2006; Song 2005; Sorlie 2007; Wolf 2008).
The potential for a unit of analysis error for some outcomes was acknowledged in only 19 of the studies that randomized providers or practices. (Briel 2006; Chassany 2006; Chenoweth 2009; Clark 2000; Dijkstra 2006; Fallowfield 2002; Glasgow 2004; Harmsen 2005; Haskard 2008; Joos 1996; Kennedy 2004; Kinmonth 1998; Krones 2008; Loh 2007; Longo 2006; Roter 1995; Stewart 2007; Thom 1999). However, 28 made adjustments for clustering in the analysis (Alder 2007; Bieber 2008; Briel 2006; Chassany 2006; Chenoweth 2009; Clark 2000; Dijkstra 2006; Fallowfield 2002; Glasgow 2004; Harmsen 2005; Haskard 2008; Howe 1996; Joos 1996; Kennedy 2004; Kinmonth 1998; Krones 2008; Levinson 1993; Lewis 1991; Loh 2007; Longo 2006; Margalit 2005; Merckaert 2008; Moral 2003; Putnam 1988; Roter 1995; Smith 1998; Stewart 2007; Thom 1999; Wilkinson 2008), while four made no such adjustments (Alamo 2002; McLean 2004; Pill 1998; Meland 1997). Although the potential unit of analysis error was not explicitly acknowledged in Hobma 2006, practices were stratified by possible effect modifiers identified by a panel of experts. Clustering is potentially a problem for those studies that randomized providers, due to the clustering effects that cannot be eliminated for patient‐level outcomes. However, when we describe cluster randomized trials in this review, we refer to those that randomized practices.
Baseline measurement
Baseline measures of health provider performance or patient outcomes were conducted in 37 studies and were not collected in six (Alamo 2002; Ho 2008; Lewis 1991; McLean 2004; Roter 1995; Wolf 2008). In 21 studies, no significant differences were found across study groups before the intervention (Alder 2007; Bieber 2008 (except lower depression scores in the non‐randomized comparison group); Briel 2006; Brown 2001; Chassany 2006; Dijkstra 2006; Glasgow 2004; Howe 1996; Joos 1996; Kinmonth 1998; Langewitz 1998; Meland 1997; Pill 1998 (except for two measures); Putnam 1988; Robbins 1979; Smith 1998; Smith 2006; Stewart 2007; Song 2005; Sorlie 2007; Thom 1999). Eight studies reported significant differences between intervention and control groups at baseline and adjustments were made for them (Clark 2000; Fallowfield 2002; Chenoweth 2009; Moral 2003; Harmsen 2005; Heaven 2006; Howe 1996; Wilkinson 2008). Although not tested for significance, baseline differences were accounted for in the main analyses in seven studies (Hobma 2006; Kennedy 2004; Levinson 1993; Loh 2007; Longo 2006; Margalit 2005; Merckaert 2008). The remaining study collected baseline data but did not test for differences at baseline (Haskard 2008).
Effects of interventions
Methodological issues in pooled results
Studies used a variety of measures in all four main outcome categories (consultation process, satisfaction, health behavior, health status). This made pooling results challenging.
Of the 43 included studies, 22 were cluster‐randomized trials. In these studies, for some of the outcomes the unit of randomisation was the same as the unit of analysis, necessitating no adjustment of the sample sizes due to a design effect. Examples of such outcomes include the mean percent of patients whose distress was recognized by a physician, or the mean frequency of using particular types of questions by a physician during patient visits. For other outcomes, the unit of analysis differed from the unit of randomisation, and the intra‐class correlation coefficient (ICC) was needed to adjust the sample sizes for the design effect. Such outcomes included the health status of patients treated by a physician when physicians were randomized to receive the intervention. Notably, only five (Chenoweth 2009; Kennedy 2004; Kinmonth 1998; Krones 2008; Loh 2007) out of the 22 studies had the ICC reported. In four additional studies (Briel 2006; Dijkstra 2006; Glasgow 2004; Harmsen 2005), ICCs were estimated for sample size calculations. Because the ICC was not reported in other relevant articles, and was not provided by authors after email inquiry, we excluded some outcomes from the meta‐analyses. The specific numbers of outcomes from studies included are shown in the summary of analyses of each group of outcomes (see 'Data and analyses'; and Table 2.)
Other methodological problems related to risk for bias were lack of random selection of the patients when physician‐directed programs were evaluated, risk of contamination between study groups, and within group as opposed to between group analyses ('Risk of bias in included studies'; Table 1; Table 7).
7. Studies with some variables excluded from meta‐analysis.
| Study | Type of Variable‐ continuous | Reason for exclusion |
| Glasgow 2004 | Health status | Inconsistency in published report of no significant differences between intervention and control groups for diabetes‐specific quality of life, but with point estimates and standard deviations inconsistent with this finding. |
| Kinmonth 1998 | Consultation Health status Patient satisfaction |
Need standard deviations |
| Langewitz 1998 | Patient satisfaction | Need ICCb |
| McLean 2004 | Consultation | Need standard deviations |
| Merckaert 2008 | Consultation | Methodological problem: patients chosen by physician; did not use some outcomes (selection bias ? health status) |
| Roter 1995 | Consultation | Need ICCb; Need actual scores, standard deviations, pre‐post |
| Smith 2006 | Health status | Need adjusted post scores with standard deviation |
| Stewart 2007 | Consultation Satisfaction Health status |
Need ICCb |
a) PAID‐2 (Problem Areas in Diabetes 2) A questionnaire for diabetes‐specific quality of life.
b) ICC: intra‐cluster correlation
In the Characteristics of included studies table (Notes field) we show both the unadjusted sample sizes, ICC used for calculation, DEFF, and adjusted sample sizes. As seen from the table, the values of ICC differ from study to study. When a value of ICC was not reported for a particular outcome, we used values reported for other outcomes (or their median) if reported in the same paper (for the same study). However, using ICC from another unrelated study was not judged appropriate; it would be unclear which value of ICC to use from a fairly wide range.
We did not undertake sensitivity analysis according to the absence of reported ICCs. Given the number of studies and the amount of missing data, results would be questionable. In summary, we chose a conservative approach. Where any ICC was mentioned in the paper, it was used, and we decided against using some arbitrary values in the absence of any information.
Pooled results by outcome category
Outcome category A: Consultation processes
Summary: Outcomes measured varied across studies (see Table 3), with most measuring some aspect of consultation skills and behaviour (29 studies). Other outcomes included patient‐centred actions in the consultation, impact on the provider‐patient relationship, and impact on the consultation itself (usually duration). The studies in the pooled analysis showed mixed results. Analysis 2.1 Sixteen studies included in the pooled analyses reported a consultation process outcome. The majority of studies measured consultation skills or behaviours In the 4 studies that used dichotomous variables, the pooled analysis showed no effect (RR 0.96, 95%CI 0.82 to 1.13) due largely to the influence of the negative Roter 1995 study, Analysis 1.1, whereas the pooled analysis of the 12 of 16 studies using continuous variables favoured the intervention (SMD 0.70, 95% CI 0.57 to 0.82) Analysis 2.1. This is consistent with the descriptive analysis where three‐quarters of studies assessing consultation processes showed positive results with the remaining quarter indicating no effect or negative results.
2.1. Analysis.

Comparison 2 Continuous Outcomes, Outcome 1 Consultation Process.
1.1. Analysis.

Comparison 1 Dichotomous Outcomes, Outcome 1 Consultation Process.
Heterogeneity was substantial among the four studies using dichotomous variables (I2 = 83%), which was not explained by risk of bias, country of origin, type of centre (academic versus non‐academic), or number of hours of training. Analysis 2.1 There was moderate heterogeneity across the12 studies reporting continuous outcomes (I2 = 58%; Analysis 2.1). Sensitivity analysis revealed that Wilkinson 2008 was the major source of heterogeneity and I2 was reduced to 46% when this study was removed from the analysis. It had a unique a methodological issue whereby of several videotapes of patient visits, intervention nurses selected tapes to be assessed for quality of consultation process. Lack of random selection in the evaluation of the outcomes has a potential for over‐optimistic estimates of the intervention effect. The SMD estimate from this study (1.12) was the largest in this group of outcomes. The other major source of heterogeneity was Loh 2007 (SMD ‐0.18; ie. the point estimate favoured the control group). Exclusion of this study reduced I2 to 49%; and exclusion of both Wilkinson 2008 and Loh 2007 reduced I2 to 30%. None of the other studies contributed significantly to heterogeneity. With Wilkinson 1998 and Loh 2007 excluded, the combined effect of heterogeneity was slightly reduced, but still remained significant. Review of risk of bias (Figure 1), geographic location, or academic centre status did not reveal any consistent patterns of association with heterogeneity.
Consultation process improvement was by far the most consistent finding generated by patient‐centred care training for providers. The exception in the pooled analysis of dichotomous variables, as noted above was due to one large study. It is difficult to identify the difference in approach to training that may account for this large outlier. We also examined the impact of length of training (brief or extensive training). Pooled analysis of both brief training (SMD 0.58, 95% CI 0.28 to 0.89, Analysis 3.1) and extensive training (SMD 0.36, 95% CI 0.01 to 0.71) Analysis 3.2) favoured the interventions. While the brief/extensive training variable was dichotomized at 10 hours of training, the extensive training interventions were all greater than 18 hours.
3.1. Analysis.
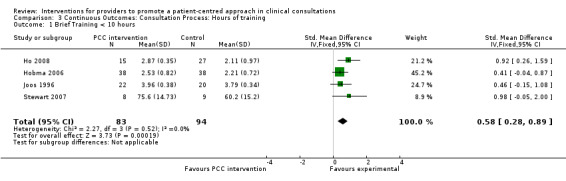
Comparison 3 Continuous Outcomes: Consultation Process: Hours of training, Outcome 1 Brief Training < 10 hours.
3.2. Analysis.

Comparison 3 Continuous Outcomes: Consultation Process: Hours of training, Outcome 2 Extensive training > 18 hours.
Outcome category B: Satisfaction
See Table 4.
Summary: Patient satisfaction favoured PCC interventions across the 11 studies included in the analysis of this outcome, although this outcome was just insignificant in the pooled result of the four studies that used dichotomous measures (RR 0.99, 95% CI 0.93 to 1.06, Analysis 1.2 ).The pooled result of the seven studies that used continuous measures significantly favoured the intervention (SMD 0.35, 95% CI 0.20 to 0.49, Analysis 2.2).
1.2. Analysis.
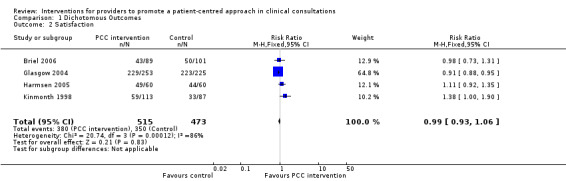
Comparison 1 Dichotomous Outcomes, Outcome 2 Satisfaction.
2.2. Analysis.

Comparison 2 Continuous Outcomes, Outcome 2 Satisfaction.
Again there was substantial heterogeneity for those studies using dichotomous outcomes ( I2 = 86%, Analysis 1.2). In studies with continuous outcomes there was low to moderate heterogeneity ( I2 = 40%) Analysis 2.2. Loh 2007 made had the largest contribution to the overall effect (SMD 0.91, 95% CI 0.44 to 1.38) and contributed all heterogeneity in this group of studies, as reflected in reduction of I2 to 0% when it was excluded. Sensitivity analysis without Loh 2007 reduced the effect (SMD 0.29, 95% CI 0.11 to 0.44). Here also, review of risk of bias (Figure 1), geographic location, or academic centre status did not reveal any consistent patterns of association with heterogeneity.
Outcome category C: Healthcare behaviour
See Table 5.
Summary: The effects of PCC interventions on health behaviour were mixed across the seven studies included in the analysis of this outcome. The pooled results of the four studies using dichotomous measures favoured the intervention (RR 1.28, 95% CI 1.18 to 1.38) Analysis 1.3, while the pooled results from the three studies using continuous measures showed no effect (SMD ‐0.04 (95% CI ‐0.28 to 0.20, Analysis 2.3).
1.3. Analysis.

Comparison 1 Dichotomous Outcomes, Outcome 3 Health Behaviors.
2.3. Analysis.

Comparison 2 Continuous Outcomes, Outcome 3 Health Behaviors.
Heterogeneity was substantial across all studies as reflected by I2 of 94% for those with dichotomous outcome measures and 87% for those with continuous outcome measures.
We used sensitivity analyses of the dichotomous health behaviour outcomes to explore Smith 2006 as a potential source of heterogeneity. However, I2 decreased only to 91% without it. Smith 2006 had the fewest risks of bias (Figure 1) and the total training time for provider (nurses) was 84 hours. This intensive training was followed by twelve 20‐minute sessions of personal contact of providers with patients supplemented with ad‐hoc telephone contacts. In contrast, providers (physicians) were trained for a total of 2 hours in one session in Kennedy 2004; and 3 hours in one session in Glasgow 2004 and 6 hours in Briel. Thus, intensity of training may be an important factor in the effect of intervention on health behaviour outcomes. Two studies with smaller effect sizes (Kennedy 2004; Briel 2006) trained providers only, while the two studies with larger effect sizes involved training of both providers and patients.
Of the three studies with continuous outcomes, Loh 2007 accounted for all the heterogeneity, as demonstrated by I2 = 0% with Loh 2007 excluded. The difference between Loh and the other two studies may be best explained by the types of behavioural outcomes measured. Loh 2007 investigated information seeking among participants (intervention patients were more likely to seek information), while in Joos 1996 and Kennedy 2004, looked at compliance with medication and hospitalisation respectively (control patients achieved higher rates). It could be argued that seeking information is a simpler behaviour to achieve than compliance. Loh 2007 had the best risk of bias profile of the three studies with continuous outcomes (Figure 1). However, it is not clear whether length of training was an important factor here. Two of the three studies had less than 10 hours of training but as the length of training was unclear in Loh 2007, we could not explore the impact of training length further. All three studies involved intervention for both providers and patients. With Loh 2007 excluded from the analysis, the combined effect on continuous health behaviour outcomes Loh 2007favoured the control group Loh 2007 (SMD ‐0.39, 95% CI ‐0.68 to ‐0.09).
Outcome category D: Health status
See Table 6.
Summary: Health status improvements favoured the intervention across the 10 studies included in the analysis of this outcome, including both studies using dichotomous measures and the eight studies using continuous measures.
For dichotomous outcomes, the estimate of the effect was 1.36 (95% CI 1.01 to 1.83, Analysis 1.4) and for continuous outcomes, the estimate of the effect was SMD ‐0.25 (95% CI ‐0.36 to ‐0.15,Analysis 2.4 ).One additional study (Glasgow 2004) reported no significant differences between intervention and control groups for diabetes‐specific quality of life, but reported point estimates and standard deviations inconsistent with this finding. We could not clarify this inconsistency, so this study was not included in Analysis 2.4. We believe this approach was reasonable since this sole aberrant effect was related to a secondary outcome. Heterogeneity was not significant as shown by I2 of 0% for dichotomous and continuous outcomes.
1.4. Analysis.
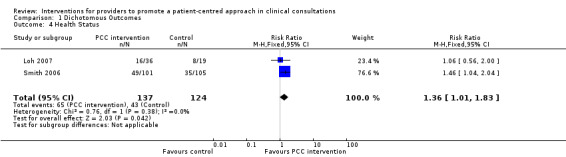
Comparison 1 Dichotomous Outcomes, Outcome 4 Health Status.
2.4. Analysis.
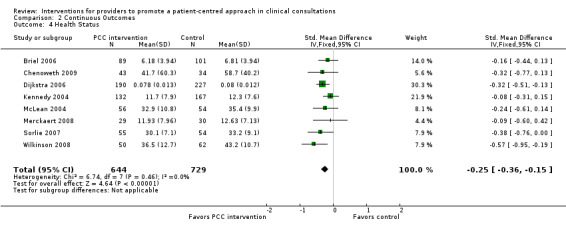
Comparison 2 Continuous Outcomes, Outcome 4 Health Status.
Summary of descriptive results by intervention category
The types of outcomes assessed by each of the included studies varied partly in accordance with the aim and nature of the intervention(s) evaluated and the associated outcome measure. Where large numbers of outcomes were measured in one category, we chose to present key results, either those identified by the study authors or those most consistent with our definition of patient‐centred care. Not all results could be included in the meta‐analysis so a narrative analysis was also conducted. The results of this section are also summarized in Table 2 and reported in detail in Appendix 5.
Outcome category A: Consultation processes
Overall 35 studies assessed consultation processes (see Table 3), 16 of which were included in the meta‐analysis. Results were largely positive across all four categories of interventions (see Types of interventions). Eighty percent of studies assessing a consultation process outcome reported a positive result for the intervention(s).
Outcome category B: Satisfaction
The effect of PCC interventions on patient satisfaction was modest across all four categories of interventions in the 43 studies that assessed this outcome (see Table 4). While the pooled analysis of 11 out of 43 studies shows positive effects for continuous measures and no effect for dichotomous measures, descriptive results are less clear. Where providers only were trained in communication skills, fewer than half (6 of 13 studies or 40%) showed positive effects on satisfaction. Where both providers and patients were trained in communication, only one of the four studies (25%) showed positive results. Where providers were trained in communication skills and provided with condition‐specific content two of five studies (40%) showed positive effects. Where providers and patients were additionally provided with condition‐specific training (CST) materials three out of four studies (75%) showed positive effects on satisfaction. See Table 2.
Outcome category C: Healthcare behaviour
Patient healthcare behavior was the least frequently measured outcome (see Table 5). Seventeen studies measured health behavior, with 8 showing positive results. In the pooled analyses, four studies using dichotomous measures showed a positive effect, while the three using continuous measures did not. In regard to health behaviour change, results varied by the intervention type. In the four studies where providers only were trained in communication, only one (25%) showed a positive effect. Neither of the two studies that trained providers and patients in communication showed a positive effect on behavior. For studies where condition‐specific materials were added, the effects on behaviour were different. In the six studies where providers were trained and provided with condition‐specific content, 4 showed positive effects on patient behaviour. In an additional five studies, both providers and patients were trained in communication and also provided with condition‐specific materials. In these, three studies showed positive effects on patient behaviour. Overall, seven out of the eleven studies (65%) using condition‐specific materials showed an impact on patient behavior. This contrasts with only one of the six studies that did not include condition‐specific materials (17%). See Table 2 for more detail.
Outcome category D: Health status
Health status was measured in 26 studies (see Table 6), with a positive effect shown in 12. The pooled analyses of 10 studies show positive effects for both dichotomous measures and continuous outcome measures, though only 46% of the total group showed positive effects on health status. See Table 2.
Further descriptive detail presented at Appendix 5 shows the heterogeneity, and rich diversity of approaches to teaching providers to improve patient‐centred care skills, with and without added support by training patients and providing condition‐specific information.
Discussion
Summary of main results
The addition of 29 trials in this updated review supports the conclusion of the previous review (Lewin 2001) that interventions for providers to improve patient‐centred care (PCC) skills are largely successful in transferring new skills to providers. This review adds that short‐term training (less than 10 hours) is as successful as longer training. Both were successful in the interval evaluated. This finding held across numerous settings and countries, and with a wide variety of trainers and trainees. PCC skills in the consultation were assessed by video or audiotape in about half of the studies and by patient or physician questionnaires in the rest. Descriptive analysis of simple counts of studies with any positive result suggested that interventions were effective in transferring PCC skills to providers in all the intervention groups. This observation was supported by quantitative analysis of studies that measured continuous outcomes. (Analysis 3.1; Analysis 3.2).
This update strengthens somewhat the case that improved PCC skills results in improved patient satisfaction, in that pooled analysis of continuous measures shows positive results. However, dichotomous measures show no effect, and among all studies, only 12 out of 26 show positive effects on patient satisfaction.
In the earlier review, there were not enough studies to evaluate the effects of the interventions on patient health behaviour or on health status. In this update, there are more data on both. Some studies measured patient behaviours that occurred during the consultation process and others measured behaviours that occurred following the encounter such as healthcare utilization, compliance, and lifestyle changes. In terms of health status, and perhaps health behaviours, there is modest support for the observation that multi‐faceted interventions have an effect that is not found with training for providers only. In only one of the studies that focused only on providers was an impact on health behaviour or health status found. However, in each of the other types of interventions, some effect was seen. In the quantitative analyses, studies that measured health behaviour were mixed, depending on whether continuous or dichotomous outcomes were measured, although studies that measured health status and satisfaction showed consistent benefits. The combined estimate of effect of interventions on patient satisfaction, health behavior and health status were in the low to medium range. This trend is discernable, despite the vast heterogeneity of study designs, and particularly of study outcomes. Studies were undertaken largely in chronic diseases such as diabetes, asthma, depression, and heart disease. Outcome measures included both disease specific and global measures (see Additional tables 4 to 7). It appears that the interventions tend to change just those outcomes they aimed to change, without any positive spill‐over effects to other behaviours such as improving providers’ abilities to listen better or improving relationships with patients. This suggests that the addition of condition‐specific materials to provider training is necessary, but not sufficient to produce desired behavior change. The finding that interventions largely achieved their aims suggests that there are techniques available to improve providers' PCC skills and the addition of condition‐specific materials (though not general education) will improve behaviour change. More research is needed to directly test the effects of interventions aimed at providers only, compared with those aimed at patients and providers, with and without condition‐specific educational materials.
Quality of the evidence
The methodological quality of the studies has improved over time. Increased attention to study quality, and improved reporting of outcomes made meta‐analysis a reasonable undertaking. However, this update identified a number of other methodological weaknesses. First, studies frequently reported only change scores instead of post‐intervention group comparisons and queries to study authors regarding these issues were not answered. Second, many cluster RCTs did not report interclass correlation coefficients. When this occurred, adjustment for clustering was frequently not discussed. No study authors responded to requests for clarification of this issue. This disqualified many studies from meta‐analysis and contributed to possible selection bias in the review. In addition selection bias could not be ruled out in the majority of included studies, as randomisation procedures were frequently not adequately described. Third, contamination remains a significant methodologic problem in those studies that randomized patients or providers and may have mitigated intervention effects. Because the nature of the intervention in patient‐centred skills training can rarely be concealed from patients or providers, contamination is often a problem. While cluster RCTs are a partial solution, concealment is still a problem. As the science of pragmatic trials develops, new strategies for this problem will likely be developed.
Finally, wide variability persists in the measures used to assess the patient centeredness of consultations, patient satisfaction and global health status. A significant contribution that could be made from improved collaboration among researchers in this field would be the wider use of common outcome measures for consultation processes and health status. Disease‐specific measures, of necessity, vary across conditions.
Potential biases in the review process
A strength of the review is the focus on RCTs, which, theoretically, should control for sampling error. However, a limitation of this approach is that it excludes studies with other designs, particularly those undertaken in the context of descriptive healthcare system quality improvement. Further, we identified only published studies, which excludes theses or dissertations. While RCTs are unusual among theses or dissertations, future updates will include this source of scholarship.
Limiting the review to RCTs allowed meta‐analysis. However, the large number of studies that could not be included raises questions about the potential for selection bias in our meta‐analyses. Heterogeneous outcome measures also limits the pooling of results. Funnel plots suggest less potential for publication bias for continuous outcomes (Figure 3; Figure 4; Figure 5; Figure 6) than dichotomous ones (Figure 7; Figure 8; Figure 9; Figure 10).
3.

Funnel plot of comparison: 2 Continuous Outcomes, outcome: 2.1 Consultation Process.
4.
Funnel plot of comparison: 2 Continuous Outcomes, outcome: 2.2 Satisfaction.
5.
Funnel plot of comparison: 2 Continuous Outcomes, outcome: 2.3 Health Behaviors.
6.

Funnel plot of comparison: 2 Continuous Outcomes, outcome: 2.4 Health Status.
7.

Funnel plot of comparison: 1 Dichotomous Outcomes, outcome: 1.1 Consultation Process.
8.
Funnel plot of comparison: 1 Dichotomous Outcomes, outcome: 1.2 Satisfaction.
9.

Funnel plot of comparison: 1 Dichotomous Outcomes, outcome: 1.3 Health Behaviors.
10.

Funnel plot of comparison: 1 Dichotomous Outcomes, outcome: 1.4 Health Status.
We removed carer satisfaction as an outcome in this update with the exception of pediatric studies, which included parents. Satisfaction among adult carers was excluded due to its rarity in the last review and the problem of its contributing added heterogeneity. Excluding carers might be a source of bias, though it would not be likely to change the mixed results found.
The multiplicity of outcome measures within studies, as well as the heterogeneity, were problematic. When studies measured many PCC skills, the result was often that participants scored positively on some and negatively on other skills, leaving an unclear pattern of overall patient‐centeredness. Our descriptive summary (and Table 2) uses the study as the unit of analysis, reporting numbers of positive and negative behaviours by study. This may introduce a positive reporting bias.
Agreements and disagreements with other studies or reviews
Our review agrees with previous reviews that have shown the effectiveness of interventions in transferring PCC skills to providers (Griffin 2004; Lewin 2001; Rao 2007). The update improves previous reports by providing effect sizes. It is the first to provide quantitative summaries of intervention effects, and to show the differential benefits of broad categories of interventions. The small effect sizes suggest that the enthusiasm shown in some reviews (eg. Epstein 2007) is appropriate based on the efficacy of training to change provider PCC skills, but that the effect on patient health should be viewed as a separate and, as yet, unproven assertion. We observed that satisfaction and health behaviour change are positively impacted more frequently in the presence of disease‐specific materials. This is consistent with the systematic review of decision aids by Stacey and colleagues showing positive effects on satisfaction (Stacey 2011).
Authors' conclusions
Implications for practice.
Planners and deliverers of training
This update confirms the findings of the Lewin 2001 review that interventions to promote patient‐centred care (PCC) are effective in transferring PCC skills to healthcare providers and provides new estimates of effect sizes. Overall, there is fairly strong evidence to suggest that most interventions to promote PCC in the clinical consultation lead to significant increases in the patient‐centeredness of consultation processes, as indicated by a range of measures relating to clarifying patients' concerns and beliefs; communicating about treatment options; levels of empathy and patients' perception of providers' attentiveness to them and their concerns as well as their diseases. Is this sufficient to justify the importance that PCC has taken on in training programs in Europe, the UK, and North America? The answer is yes, if PCC is seen as worthy in its own right. The investment in training, and in observational measures of health professional performance are probably justified because interventions to promote PCC within clinical consultations appear to lead to significant increases in the patient‐centeredness of care. The trials included here are dominated by experiments with practicing clinicians and residents/registrars. It may be argued that studies among undergraduate trainees would not be feasible. Designers of undergraduate curricula have gradually adopted PCC approaches, based on the perceived success and importance of this training among practicing clinicians. Once adopted, it would be unethical as well as impractical to deny such training to undergraduate trainees for the sake of a trial. As PCC becomes a mark of quality of care in the United States and other countries, it is anticipated that the field may move from trials of interventions to evidence of competency in patient‐centred skills.
Practice of patient‐centred care
In the original review, we concluded there was some support for PCC Interventions to impact consultation processes and satisfaction, but little data on health behaviours or health status. Health behaviours and health status were more frequently measured in studies in the update. However, they show mixed effects on patient‐specific outcomes. For studies that compared provider training in PCC with no training, the improved patient‐centeredness of the consultation did not lead to changes in patient behaviours or in health status outcomes where those were measured. There is some indication that adding explicit patient and/or provider instruction in disease‐specific management skills to PCC skills training may improve both health behavior and health status. Effect size on health behaviour could not be estimated from included studies. Improving patient health behaviour and outcomes appears to require integrating strategies directed at patients and providers as well as strategies that target specific conditions.
Implications for research.
While the methodological quality of the trials is improving, fewer than half of the studies could be included in the pooled analyses. Interventions generally showed a positive impact on the consultation behaviours taught and assessed, though few studies used the same measure. Measures of patient satisfaction and health status continue to be heterogeneous. In the pooled analyses, continuous measures of both satisfaction and health status show moderate effects, while dichotomous measures show small effects. Patient behaviours and disease‐specific measures will remain idiosyncratic to the clinical conditions of interest, which is a rich mix of mental health, chronic disease, screening and treatment choices. We included health service utilization in the category of health behaviours. This, however, is a growing area of concern in an era of limited healthcare resources and should in the future be looked at separately.
The apparent effectiveness of multifaceted interventions brings with it a challenge to identify ‘active’ elements of effective complex interventions (Craig 2008; Shojania 2005). Adaptation to different health systems with different goals will be enhanced if such key elements can be identified. Head‐to‐head comparisons of different configurations of complex interventions to promote PCC would contribute substantially. Ways of involving healthcare consumers in the design, planning and delivery of interventions to promote PCC need to be actively pursued. In particular, the selection of outcomes should focus on measures of outcomes seen as important by consumers for quality of care, as well as by health policy experts.
All the studies reviewed were completed in high‐income countries. Interventions to promote PCC may have varying acceptability and impact across different health care and cultural settings. In adapting to low‐ and middle‐income countries, it will be important to develop interventions that may involve different components from training to organisational restructuring. In so doing, it should be anticipated that interventions may impact in different ways on consumer and provider satisfaction across different settings. Although patient‐centeredness may be an objective of care in many settings, it is not possible to be confident about the applicability of the reported interventions to low‐ and middle‐income countries. Human resource issues, such as the scarcity of healthcare providers and low motivation to deliver PCC, may limit the feasibility and potential of this approach for improving professional practice and health outcomes.
In thinking broadly about what can be taken from the studies reported here, it is important to take into account the spread of electronic access to information in some areas of the world. It may well be that information resources and training resources can be made available electronically to physicians and community health workers, as opposed to direct person‐to‐person training, as was done in virtually all of the studies reported here. Broad coalitions of national and international organizations may be helpful in developing resources. A clear need is for trials of interventions to promote PCC in low‐ and middle‐income country settings, and in low‐income settings in high‐income countries. In addition, future trials should specifically assess the effects of interventions other than healthcare provider training, such as changes in the organisation of care, in promoting PCC in the clinical consultation. In addition, the included studies in this review did not address care teams, and the most efficient and effective set of provider training, patient training and provision of condition‐specific educational materials.
Implications for health policy
This update strengthens the case for the efficacy of training providers to improve delivery of patient‐centred care to improve patient satisfaction. It suggests, though not as strongly, that the addition of condition‐specific educational materials supports further improvement in patient‐centred care. While further studies are needed, it is reasonable to begin to evaluate effectiveness studies in routine care, and to consider the implications for improved education of providers both in their training, and in post‐graduate training and certification. While measurement heterogeneity limits these conclusions somewhat, a strength of these studies is that they employed direct measures of consultation processes. Direct measures of consultation processes are required to demonstrate success of the interventions. Patient and provider satisfaction measures assess the felt impact of the interventions; such self‐report measures complement observational measures. The impact, particularly of multi‐faceted interventions is promising.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2013 | Amended | Republished with correction to affiliation for Simon Lewin |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 13 June 2012 | New citation required and conclusions have changed | The original review (Lewin 2001) included both randomized controlled trials (RCTs) and quasi‐experimental designs. This update, limited to only RCTs, adds 29 studies (Alamo 2002; Alder 2007; Bieber 2008; Briel 2006; Brown 2001; Chassany 2006; Chenoweth 2009; Dijkstra 2006; Fallowfield 2002; Glasgow 2004; Harmsen 2005; Haskard 2008; Heaven 2006; Ho 2008; Hobma 2006; Kennedy 2004; Krones 2008; Loh 2007; Longo 2006; Margalit 2005; McLean 2004; Merckaert 2008; Moral 2003; Smith 2006; Song 2005; Sorlie 2007; Stewart 2007; Wilkinson 2008; Wolf 2008) and updates one (Clark 2000) of the 14 RCTs in the original review. We excluded the two non‐randomized studies included in the original review. Conclusions changed Authors of the original review concluded there was some support for impact of Interventions to promote patient‐centred care (PCC) within clinical consultations to effect consultation processes and satisfaction, but little data on health behaviours or health status. The update confirms the impact of interventions on consultation processes (qualitative and moderate quantitative effect). We documented moderate qualitative effects on patient satisfaction and small to moderate effects in the quantitative analysis. Health behaviours and health status are more frequently measured in studies in the update. However, they show mixed effects on patient specific outcomes within the study windows reported. Effect size on health behaviour could not be estimated from included studies. Improving patient health behaviour and outcomes appears to require integrating strategies directed at patients and providers as well as strategies that target specific conditions. |
| 17 June 2010 | New search has been performed | We updated searches in June 2010. Methods changes In this update:
|
Acknowledgements
Initial study screening volunteers: In addition to the authors of the study, the following participated in the initial phases of study screening and data abstraction: Professor Linda French, Chair of Family Medicine at the University of Toledo College of Medicine, Toledo, Ohio, USA; Elizabeth Bogdan‐Lovis and Karen Kelly‐Blake of the Michigan State University Center for Ethics, East Lansing, MI, Aileen Antonio‐Santos of Grand Rapids Medical Education Partners, Grand Rapids, Michigan, USA
Peer‐reviewers: The authors thank all peer reviewers for their helpful comments.
Statistical advice: Joanne McKenzie and Jason Wasiak.
The study authors who provided us with additional information regarding study designs and interventions.
Dell Horey, Sophie Hill and Megan Prictor at the editorial base for the Consumer and Communication Review Group.
Funders: Department of Medicine, Michigan State University, USA who provided salary support for Gelareh Sadigh, MD; and World Health Organization (WHO), Switzerland who supplied funding for a research assistant, and for statistical analysis performed at Michigan State University and Norwegian Knowledge Centre for the Health Services. WHO funding also included travel support for Dr. Francesca Dwamena.
We acknowledge the authors of the original Cochrane review (Lewin 2001): Simon Lewin, Zoe Skea, Vikki A. Entwistle, Merrick Zwarenstein and Judy Dick.
Appendices
Appendix 1. MEDLINE search strategy
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 randomized controlled trials.sh.
4 random allocation.sh.
5 double blind method.sh.
6 single‐blind method.sh.
7 1 or 2 or 3 or 4 or 5 or 6
8 (animal not human).sh.
9 7 not 8
10 clinical trial.pt.
11 exp clinical trials/
12 (clin$ adj25 trial$).ti,ab.
13 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
14 placebos.sh.
15 placebo$.ti,ab.
16 random$.ti,ab.
17 research design.sh.
18 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 18 not 8
20 19 not 9
21 comparative study.sh.
22 exp evaluation studies/
23 follow up studies.sh.
24 prospective studies.sh.
25 (control$ or prospectiv$ or volunteer$).ti,ab.
26 21 or 22 or 23 or 24 or 25
27 26 not 8
28 26 not (9 or 20)
29 9 or 20 or 28
30 patient‐centered care/
31 patient‐centered.tw.
32 patient‐centred.tw.
33 person‐centred.tw.
34 person‐centered.tw.
35 patient‐oriented.tw.
36 person‐oriented.tw.
37 patient‐focused.tw.
38 person‐focused.tw.
39 client‐focused.tw.
40 client‐oriented.tw.
41 client‐centred.tw.
42 client‐centered.tw.
43 exp professional‐patient relations/
44 professional‐family relations/
45 patient participation/
46 patient care planning/
47 decision making/
48 exp education, professional/
49 inservice training/
50 (43 or 44) and (45 or 46 or 47 or 48 or 49)
51 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 50
52 29 and 51
Appendix 2. PsycINFO strategy on CSA
1. kw=randomi?ed controlled trial*
2. kw=random*
3. kw=placebo
4. kw=clin* within 25 trial*
5. kw=((singl* or double* or trebl* or tripl*) within 25 (blind* or mask*))
6. kw=pre test pretest or [post test or posttest or control*
7. kw=comparative stud* or evaluation stud* or follow up stud* or prospective stud*
8. DE=prospective studies
9. kw=Prospectiv* or volunteer* or intervention*
10. DE=sampling experimental or experimental design or experimental methods or methodology
11. kw=experiment* or impact or chang* or time series
12. kw=(patient or person or client or consumer) within 1 (centered or centred or focused or focussed or oriented)
13. de=client centered therapy
14. DE=("therapeutic processes" or "psychotherapeutic processes" or "countertransference" or "insight psychotherapeutic process" or "negative therapeutic reaction" or "psychotherapeutic breakthrough" or "psychotherapeutic resistance" or "psychotherapeutic transference" or "therapeutic alliance")
15. DE=client participation or decision making or treatment planning
16. DE=communication skills training
17. DE=clinical methods training or clinical psychology graduate training or clinical psychology internship or community mental health training or mental health inservice training or psychiatric training or psychoanalytic training or psychotherapy training
18. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11
19. 12 or 13
20. 19 and (14 or 15)
21. 20 and (16 or 17)
Appendix 3. CINAHL search strategy on EBSCO platform
S1 randomized controlled trial*
S2 random*
S3 PT clinical trial
S4 (MH "Clinical Trials+")
S5 clin* w25 trial*
S6 singl* w25 blind*
S7 (doubl* 25w blind*) OR (doubl* 25w mask*)
S8 (trebl* 25w blind*) OR (trebl* 25w mask*)
S9 (tripl* 25w blind*) OR (tripl* 25w mask*)
S10 (MH "Placebos")
S11 placebo*
S12 (MH "Study Design+")
S13 S12 or S11 or S10 or S9 or S8 or S7 or S6 or S5 or S4 or S3 or S2 or S1
S14 pre test or pretest or post test or posttest
S15 (MH "Pretest‐Posttest Design+")
S16 (MH "Quasi‐Experimental Studies+")
S17 intervention* or experiment*
S18 impact or change*
S19 evaluat* or effect* or compar*
S20 time series
S21 S20 or S19 or S18 or S17 or S16 or S15 or S14
S22 patient centered or patient centred or person centered or person centred or client centered or client centred or consumer centered or consumer centred
S23 patient focused or patient focused or person focused or person focused or client focused or client focused or consumer focused or consumer focused
S24 patient oriented or person oriented or client oriented or consumer oriented
S25 (MH "Patient Centered Care")
S26 (MH "Patient Centered Care") or (MH "family centered care")
S27 S S26 or S25 or S24 or S23 or S22
S28 (MH "Professional‐Patient Relations+") or (MH "Professional‐Client Relations") or (MH "Professional‐Family Relations")
S29 (MH "Consumer Participation")
S30 (MH "Patient Care Plans+")
S31 (MH "Decision Making+")
S32 (MH "staff development")
S33 (MH education, graduate+)
S34 (MH "education, continuing+")
S35 S34 or S33 or S32 or S31 or S30 or S29
S36 S35 and S28
S37 S36 or S27
S38 S21 or S13
S39 S38 and S37
Appendix 4. EMBASE search strategy
1 randomized controlled trial
2 random$.tw.
3 exp controlled study
4 double blind procedure
5 single blind procedure
6 crossover procedure
7 latin square design
8 multicenter study
9 ((clinical or controlled or comparative or placebo or prospective or random$) adj3 (trial or study)).tw.
10 ((single$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw.
11 (crossover$ or cross‐over$ or (cross adj1 over$)).tw.
12 ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. (36007)
13 or/1‐12
14 evaluation
15 Follow Up
16 Prospective Study
17 (control$ or prospectiv$ or volunteer$).tw.
18 types of study/
19 (intervention$ or experiment$).tw.
20 (time adj series).tw.
21 (pre test or pretest or post test or posttest).tw.
22 (impact or chang$).tw.
23 (evaluat$ or effect? or compar$).tw.
24 case control study/
25 Retrospective Study/
26 cohort analysis/
27 Longitudinal Study/
28 or/14‐27
From April 2006 to May 2010, the following abbreviated filter was used to reduce screening load as we were no longer considering non‐randomized control design
1 randomized controlled trial/ (61682)
2 random$.tw. (126070)
3 exp controlled study/ (1019402)
4 double blind procedure/ (29131)
5 single blind procedure/ (3288)
6 crossover procedure/ (9734)
7 latin square design/ (44)
8 multicenter study/ (21169)
9 ((clinical or controlled or comparative or placebo or prospective or random$) adj3 (trial or study)).tw. (117287)
10 ((single$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. (28148)
11 (crossover$ or cross‐over$ or (cross adj1 over$)).tw. (11860)
12 ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. (36007)
13 or/1‐12 (1123366)
Appendix 5. Detailed narrative review
Intervention category 1: Training for providers only
Twenty‐three RCTs compared training for providers only with a no intervention control group (Alamo 2002; Alder 2007; Chenoweth 2009; Fallowfield 2002; Heaven 2006; Ho 2008; Hobma 2006; Howe 1996; Langewitz 1998; Levinson 1993; Longo 2006; Margalit 2005; McLean 2004; Merckaert 2008; Moral 2003; Putnam 1988; Robbins 1979; Roter 1995; Smith 1998; Stewart 2007; Thom 1999; Wilkinson 2008; Wolf 2008).
Outcome category A: Consultation processes
Twenty‐two studies examined consultation processes. They reported between one and 30 outcomes that assessed a range of provider and patient verbal, humanistic, and/or empathic behaviours in the consultation. The intervention was superior to the control for at least 1 of the outcomes measured in 16 of the 22 studies that measured consultation process (1 did not measure consultation process Chassany 2006). Seven studies assessed providers' videotaped humanistic and empathic behaviours during the consultation process (Alamo 2002; Alder 2007; Fallowfield 2002; Langewitz 1998; Moral 2003; Robbins 1979; Stewart 2007). Among these, the only fully negative study was Alder 2007. Six studies assessed physician communication skills with questionnaires (Hobma 2006; Longo 2006; Margalit 2005; Thom 1999; Wilkinson 2008; Wolf 2008). Among the survey evaluations, the only fully negative study was Wolf 2008. Four studies measured a range of audio‐ taped providers' consultation behaviours (Heaven 2006; Levinson 1993; Putnam 1988; Smith 1998). Of these, the only fully negative study was Levinson 1993. One study used direct observation (Ho 2008) and found the intervention groups had better Objective Structured Clinical Examination scores on assessment of patient perspectives and social factors; basic communication, history taking; and formulation of differential diagnoses.
Rather than assessing communication skills or humanistic behaviours, three studies measured outcomes relating to the providers' ability to detect and/or manage psychological distress in patients (Howe 1996; Merckaert 2008; Roter 1995). In Howe 1996 providers in the intervention group were able to detect a greater amount of psychological distress in patients with emotional problems three months after training. Roter 1995 reported 22 outcomes relating to consultation process, with six to eight outcomes being statistically significant for the intervention groups compared to the control groups. In Merckaert 2008 mean scores of physicians’ ability to detect cancer patient’s distress at baseline and 5 months were not significantly different between intervention and control groups.
The final study (McLean 2004) assessed the effect of intervention on the duration of consultation and found no significant difference between intervention and control groups.
Outcome category B: Satisfaction
Thirteen of the 23 studies reported between 1 and 5 outcomes each on patients' satisfaction with aspects of the provider's manner and/or abilities, or with the visit in general. Results were mixed. Six (Alamo 2002; Langewitz 1998; McLean 2004; Smith 1998; Stewart 2007; Wilkinson 2008) studies reported statistically significant benefit in some aspect of patient satisfaction. Alamo 2002 asked patients to rate how satisfied they were with their ability to discuss pain, how clearly the doctor discussed the cause of pain, and how the doctor listened to their opinions and suggestions concerning management, with the intervention group scoring significantly better than the control group in all three areas. Langewitz 1998 reported that the proportion of patients who stated that they would recommend their doctor to a friend significantly increased in the intervention group following the intervention. McLean 2004 used a validated consultation satisfaction questionnaire with four components: professional care, general satisfaction, depth of relationship, and perceived time. Patients who had acute self‐limiting illnesses, were more satisfied (on one measure only – professional care) when trained GPs were trained (prompted) to ask them about their concerns. Smith 1998 reported five patient satisfaction measures of which two showed statistically significant differences. Immediately after their post intervention medical visit, patients in the intervention group had significantly more confidence in their providers' abilities and were generally more satisfied with their medical visits than patients in the control group, although no significant differences between the groups were reported for patients' satisfaction with opportunities to discuss concerns, provider empathy and comparison of provider with others. In Stewart 2007, the mean score of intervention patients' satisfaction with doctors' information giving and interpersonal skills was positive for the intervention group. Similarly, mean scores for satisfaction with the consultation were positive in Wilkinson 2008.
The seven remaining studies in this category reported no statistically significant differences between the intervention and control groups for either general satisfaction scores or for specific aspects of patient satisfaction with their provider's manner and/or abilities (Alder 2007; Longo 2006; Merckaert 2008; Moral 2003; Putnam 1988; Roter 1995; Thom 1999).
Outcome category C: Health behaviours
Of the four provider training only studies that assessed health behavior (Alder 2007; Longo 2006; Putnam 1988; Thom 1999), the only study that showed significant improvement (Longo 2006) did not measure patient behaviour directly, but rather used a self‐administered questionnaire to assess patients’ confidence in decision‐making and their expectation to adhere to negotiated plans. The other three studies did not show benefit. Putnam 1988 reported on behavioural, medication and appointment adherence, none of which showed any statistically significant changes from before to after the intervention in either the intervention or control group. Thom 1999 measured continuity of patient with provider, self‐reported adherence to advice or treatment, number of referrals, and number of diagnostic tests ordered. None of these showed any significant differences between the two groups approximately six months after post intervention medical visit. Similarly, Roter 1995 found no significant difference between either of two intervention groups (providers trained in problem defining skills only or providers trained in emotion handling skills only) and the control group for the proportion of emotional distressed patients revisiting providers at two weeks, three months and six months after the post intervention medical visit.
Outcome category D: Health status
Eleven studies assessed one outcome each on health status and well being. (Alamo 2002; Chenoweth 2009; Longo 2006; McLean 2004; Merckaert 2008; Putnam 1988; Roter 1995; Smith 1998; Stewart 2007; Wilkinson 2008; Wolf 2008). Five studies (Alamo 2002; Chenoweth 2009; Roter 1995; Stewart 2007; Wilkinson 2008) showed statistically significant, but clinically modest benefits. For example, Alamo 2002 measured intensity of pain, number of tender points, subjective health status, and depression and anxiety scores at 6 months and 12 months found small, but statistically significant differences in favour of the intervention group in number of tender points and anxiety. There were no significant differences in any of the other comparisons. Another study (Roter 1995) measured levels of emotional distress in patients previously identified as having high distress scores. They reported that patients of providers trained in problem defining skills only showed significant reductions in emotional distress at each time point (two weeks, three months, six months from post intervention medical visit) compared with control patients. There was no significant difference in levels of emotional distress for patients of providers trained in emotion handling skills only, compared with control patients. Chenoweth 2009 measured agitation, psychological and psychiatric behaviours, quality of life in end‐stage dementia, incidents, falls and antipsychotic and benzodiazepine drug doses in patients with dementia who received person‐centred care, dementia‐care mapping or no intervention (before intervention, after intervention and at 4 month follow‐up). Agitation significantly decreased in both intervention group at follow‐up, and the rate of falls at 4 month follow‐up significantly decreased in dementia‐care mapping group and significantly increased in person‐centred care group. The other variables were not significantly different within intervention or control groups. Longo 2006; McLean 2004; Merckaert 2008; Putnam 1988; Smith 1998; and Wolf 2008 reported no significant differences between the intervention or control groups for general symptom improvement pre‐ and post‐ intervention.
Intervention category 2. Training for providers plus training for patients
PCC training for providers plus PCC materials and/or training for patients was compared to no intervention in either patient or provider in six studies (Harmsen 2005; Haskard 2008; Joos 1996; Lewis 1991; Song 2005; Sorlie 2007) or to condition‐specific materials for provider and patient in one study (Pill 1998). The details for each of these seven studies are provided in the 'Description of studies' and summarised in the 'Characteristics of included studies' table.
Outcome category A: Consultation processes
Six of the seven studies assessed consultation processes, including provider behaviours and a range of patient behaviours such as initiating discussion on a topic, responses, information recall and participation in healthcare discussions (Harmsen 2005; Haskard 2008; Joos 1996; Lewis 1991; Pill 1998; Song 2005). For this category between three and six outcomes were assessed per study; and five out of six studies had at least one significantly positive outcome in favour of intervention. Harmsen 2005 assessed the effect of an intervention given to both physicians and patients on mutual understanding after one and six months. They documented 11% improvement in mutual understanding (and some improvement in perceived quality of care) at 6 months in consultations with non‐western patients (P = 0.05), but not with western patients. Similarly, Haskard 2008 reported significant benefit of intervention on all three consultation outcomes measured; and Joos 1996 showed statistically significant differences in two out of three outcomes. In Joos 1996 providers in the intervention group elicited patient concerns in a greater proportion of visits than those in the control group; and a higher proportion of patients of intervention providers had favourable perceptions about their medical visit. There was no difference between the intervention and control groups for patients' perceptions of the amount of information given by providers about medications and side effects. Lewis 1991, which focused on communication between providers, children and parents, assessed five consultation process measures, of which two showed statistically significant differences, and Pill 1998 reported a higher percentage of intervention patients who affirmed their current behaviour and initiated discussion of change eight to nine months post intervention. However, they found no significant difference in the percentage of patients who were involved in other aspects of the consultation, such as deciding on topics to discuss and target setting. Song 2005 found no difference in the one consultation outcome measured (patients' knowledge of advanced care planning).
Outcome category B: Satisfaction
Four studies (Harmsen 2005; Joos 1996; Lewis 1991; Pill 1998) measured one to three outcomes describing patient satisfaction. Only one study reported any benefit in favour of the intervention. Lewis 1991 found children in the intervention to be significantly more satisfied with the consultation visit compared with children in the control group, but found no difference in parent's satisfaction. Harmsen 2005 and Joos 1996 reported no significant differences; and Pill 1998 found a statistically significant before and after difference in the control group of patients, but not in the intervention group.
Outcome category C: Health behaviours
Only two studies measured healthcare behaviours, measuring respectively one and two outcomes for this category (Joos 1996; Pill 1998); and neither reported any positive effect of intervention on healthcare behaviour.
Outcome category D: Health status
Four studies (Lewis 1991; Pill 1998; Song 2005; Sorlie 2007) examined aspects of health status and well being, measuring between one and three outcomes for this category. Three of the four studies documented improvement in at least one reported outcome. Intervention patients in Song 2005 reported less difficulty making choices, although patients’ anxiety changes (pre to post) did not significantly differ by group. Similarly, Sorlie 2007 found patients in the intervention group to have significantly less anxiety and better subjective health at discharge, although there was no significant difference between intervention and control patients in changes in depression score. Pill 1998 found no benefit of the intervention on any outcome related to health status. Lewis 1991 measured one outcome ‐ mean levels of anxiety ‐ and showed no statistically significant differences between children in the intervention and control group.
Intervention category 3. Training for providers plus condition‐specific training
PCC training for providers plus condition‐ or behaviour‐specific training or material for providers and/or patients compared with no training intervention was evaluated in three studies (Clark 2000; Kennedy 2004; Smith 2006) and with behaviour‐specific material for providers only in four (Briel 2006; Meland 1997; Bieber 2008; Chassany 2006). Bieber 2008 and Briel 2006 also had a non‐randomized control group with no training intervention, but we did not consider comparisons with this group for this review. The details for each of these studies are provided in the 'Description of studies' and summarised in the 'Characteristics of included studies' table.
Outcome category A: Consultation processes
Two of the seven studies in this intervention category assessed two and eight consultation process outcomes respectively (Bieber 2008; Clark 2000). Both found at least one significant outcome in favour of the intervention. Bieber 2008 used two independent questionnaires to assess the consultation process from the perspectives of both patients and their physicians. Patients’ appraisal of the quality of the consultation process was higher than control patients’ at baseline, 3 months and 1 year. Similarly, providers in the intervention group stated interaction with their patients to be less difficult than did providers in the control group; and the difference between the two groups regarding the difficulty of the provider‐patient interaction remained constant over time. Clark 2000 showed that parents in the intervention group were more likely than parents in the control group to report that the paediatrician was reassuring, encouraging and reassuring two months after the post intervention medical visit. However, no statistically significant differences were found between intervention and control group parents in confidence about managing asthma at home (Clark 2000).
Outcome category B: Satisfaction
Five of the seven studies assessed between one and five outcomes on patient satisfaction (Bieber 2008; Briel 2006; Clark 2000; Kennedy 2004; Smith 2006). Two of them documented at least one positive effect of intervention. Intervention patients in Bieber 2008 reported better satisfaction with their decisions, but there was no difference on their decisional conflict scales. The study did not assess patient satisfaction with their physicians or with their care. Kennedy 2004 and Briel 2006; found no significant differences between trained and untrained providers in patients’ self‐reported satisfaction with initial consultation. Similarly, Clark 2000 found no significant difference between intervention and control in the five outcomes they assessed on patient satisfaction. Conversely, Smith 2006 reported significant improvement in patient satisfaction at 6 months and 12 months in intervention patients compared with control patients, although they did not report the actual satisfaction scores.
Outcome category C: Health behaviours
Six of the seven studies assessed between one and seven outcomes on healthcare behaviours (Bieber 2008; Briel 2006; Clark 2000; Kennedy 2004; Meland 1997; Smith 2006); and four out of these six reported at least one positive outcome of intervention. For example, the intervention group in Bieber 2008 chose a higher mean number of different treatment modalities for their fibromyalgia than control group. Similarly, two of four outcomes in Clark 2000 showed statistically significant differences. According to parent self report, children in the intervention group two months post intervention made significantly fewer non emergency physician office visits and significantly fewer visits following an episode of symptoms, but had no difference in number of emergency department visits or hospitalisations. Intervention patients in Kennedy 2004 made significantly fewer number of outpatient visits for inflammatory bowel disease, although there was no difference in percentage of patients making more than two visits in one year. In addition, significantly higher percentage of intervention patients made their own appointments. Smith 2006 reported higher rates of appropriate antidepressant doses in the intervention group and lower rates of use of controlled substances after 12 months. Meland 1997 and Briel 2006 were the only totally negative study in this group. Meland 1997 found no significant benefits of intervention in the three outcomes they assessed on lifestyle behaviours such as exercise and smoking. Briel 2006 found no significant benefit of intervention in re‐consultation rates within 14 days of study enrolment.
Outcome category D: Health status
Six out of the seven studies reported between one and seven outcomes on health status (Bieber 2008; Briel 2006; Chassany 2006; Kennedy 2004; Meland 1997; Smith 2006); and two of the six reported at least one outcome in favour of the intervention. In Chassany 2006, the intervention improved three different measures of pain even after adjustment for higher use of acetaminophen in the intervention group. The intervention also improved stiffness, physical functioning, and global score; and reduced adverse events. Similarly, the intervention group in Smith 2006 were more likely to have improved SF‐36 mental component summary and physical disability scores after 12 months than control patients. Conversely, Bieber 2008 and Meland 1997 found no significant benefit of the intervention in any of the four outcomes on health status they each reported. The intervention patients in Kennedy 2004 reportedly had a higher number of relapses, but there was no difference in depression, anxiety, or quality of life. There was weak evidence of higher patient enablement in the intervention group in Briel 2006. However, no significant difference was reported for the number of days with restrictions from disease.
Intervention category 4. Training for providers plus training for patients plus condition‐specific training
PCC training for providers, PCC materials for patients plus condition‐ or behaviour‐specific materials for providers and/or patients compared with no training in two studies (Dijkstra 2006; Loh 2007), one CME session on an alternative topic in one study (Krones 2008) and condition‐ or behaviour‐specific materials for providers and/or patients in three studies (Brown 2001; Glasgow 2004; Kinmonth 1998). The details for each of these studies are provided in the 'Description of studies' and summarised in the 'Characteristics of included studies' table.
Outcome category A: Consultation processes
Five of the six studies in this intervention category measured consultation process outcomes, and all of these (Brown 2001; Glasgow 2004; Kinmonth 1998; Krones 2008; Loh 2007) reported at least one consultation process outcome in favour of the intervention. In Brown 2001, the consultation length for providers who were trained to be patient‐centred was shorter than for those who were given prompt sheets without training. Similarly, providers in the intervention group in Glasgow 2004 completed more PCC activities and performed better in three of six condition‐specific process measures. In Kinmonth 1998, patients in the intervention group were more likely to report maximum communication with GPs; but there was no significant difference between the two groups for agreement between patient and provider on main concerns discussed over the year. Similarly, intervention providers had better results in two out of three consultation process outcomes in Krones 2008; and in one of two in Loh 2007.
Outcome category B: Satisfaction
Four of the six studies assessed this outcome (Glasgow 2004; Kinmonth 1998; Krones 2008; Loh 2007); and three of the four reported at least one positive outcome in favour of intervention. Kinmonth 1998 reported two patient satisfaction outcomes of which one showed a statistically significant difference. Patients in the intervention group were more likely than control patients to report high satisfaction with treatment at one year, but there was no difference in satisfaction with style of care. Similarly intervention patients in Krones 2008 and Loh 2007 reported positive effects of intervention in the one outcome they each measured. Glasgow 2004 measured patient satisfaction at baseline and 6 months, but did not report any statistical testing.
Outcome category C: Health behaviours
Five of the six studies assessed between one and three outcomes in this category (Brown 2001; Glasgow 2004; Kinmonth 1998; Krones 2008; Loh 2007); and three of the five reported at least one significant outcome in favour of intervention. For example, Brown 2001 assessed from audiotapes of patient question asking, information needs, and information recall and found that among patients with prompt sheets, patients of trained doctors recalled significantly more information about treatment issues and side effects. Similarly, patients with prompt sheets whose doctors were trained recalled significantly more information in total than those whose doctors were not trained (P = 0.036). There was no difference in frequency of response for the seven items comprising the information needs scale. Patients of untrained providers were significantly more anxious than patients of trained providers. Similarly, Krones 2008 reported less decisional regret in intervention patients; and Loh 2007 found better participation in shared decision making, although they found no difference in adherence to treatment. Alternatively, Kinmonth 1998 found no difference in the three behaviours they assessed, and Glasgow 2004 did not report statistical testing for the two outcomes they assessed.
Outcome category D: Health status
All six studies in this intervention category assessed at least one outcome on health status and well being; the intervention resulted in at least one positive outcome in three of these studies (Brown 2001; Dijkstra 2006; Kinmonth 1998). Brown 2001 used the Spielberg questionnaire to assess anxiety at baseline, immediately after consultations and after 10 days. Patients with prompt sheets alone were significantly more anxious than patients with trained doctors. Similarly, Dijkstra 2006 found better glycated haemoglobin and blood pressure levels in the intervention group, although they found no difference in systolic blood pressure, cholesterol or creatinine levels. In Kinmonth 1998 three of eight outcomes relating to health status and well being showed statistically significant differences, but only one outcome was in favour of intervention. One year after the intervention, overall well being scores were significantly higher in the intervention group. However, average blood triglyceride concentrations and average body mass index were both also higher (i.e. worse) in the intervention group compared with the control group. Furthermore, one year after the intervention, there were no significant differences between the intervention and the control group for mean glycated haemoglobin concentrations; mean total cholesterol; and for mean systolic and diastolic blood pressure. There also were no significant differences between the groups for mean scores on diabetes‐specific quality of life and depressed well being questionnaires, or on various sub‐scales of a generic well being questionnaire (depression, anxiety, and energy subscales). The remaining studies (Glasgow 2004; Krones 2008; Loh 2007) did not find any benefit of intervention on health status. For example, Glasgow 2004 found no difference between the intervention and control groups in either quality of life or depression after 6 months.
Data and analyses
Comparison 1. Dichotomous Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Consultation Process | 4 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.13] |
| 2 Satisfaction | 4 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.93, 1.06] |
| 3 Health Behaviors | 4 | 1097 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.18, 1.38] |
| 4 Health Status | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.01, 1.83] |
Comparison 2. Continuous Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Consultation Process | 12 | 1046 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.70 [0.57, 0.82] |
| 2 Satisfaction | 7 | 813 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.35 [0.20, 0.49] |
| 3 Health Behaviors | 3 | 288 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.28, 0.20] |
| 4 Health Status | 8 | 1373 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.36, ‐0.15] |
Comparison 3. Continuous Outcomes: Consultation Process: Hours of training.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Brief Training < 10 hours | 4 | 177 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.28, 0.89] |
| 2 Extensive training > 18 hours | 3 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.36 [0.01, 0.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alamo 2002.
| Methods |
Randomization procedure: By practice/clinic Informed consent obtained: Yes Protection against contamination: Inadequate. Individual randomisation of providers with some working in the same practice. Some transfer of patients between intervention and control group Outcomes assessors blinded: Adequate: Video tape coder was blinded to provider status. Telephone interviewer was blinded to patient status Intention to treat analysis: Done Potential for unit of analysis error: Primary outcome (patient survey) randomisation by provider practice, but analysis by patient without concern for confounding. The problem was partly acknowledged Comments on study quality: None to add |
|
| Participants |
Profession: Medicine Specialty: General Practice/Family physicians Years experience: Intervention M (SE) 9.7 (1.7) Control 9.2 (1.4) Clinical setting: Health Centers Level of Care: Primary Country: Spain Health problem/Type of Patient: Musculoskeletal chronic pain/ fibromyalgia. Mean age = 44.4 years, 97.3% female, 88.2% married, 54.4% housewives |
|
| Interventions |
Aim of study (hypothesis): To assess whether patient‐centred consultations are more effective than the usual style of consultation used by experienced general practitioners with patients suffering from benign chronic musculoskeletal pain and fibromyalgia. Also, to evaluate the differential characteristics of these two clinical groups of symptoms. Hypothesis: Patient‐centred consultation compared to the conventional treatment would decrease pain by one point on a 10‐point visual analogue scale Content of intervention: Trainers and doctors identified the presentation of the most common problems in primary care, the importance of psychosocial aspects of health and the general objectives of a consultation. Participating doctors practiced communication skills for establishing an effective relationship, obtaining biopsychosocial information, giving information, negotiating, and closing the interview. Role‐play, video examples, and interviews with simulated patients were used. Providers were taught data gathering, relationship building, informing/motivating/shared decision‐making, and behaviours and skills could be replicated Conceptual focus:
(Main features listed in Table 2 of the article) Duration and timing: 18 hours of training. Time spread is unclear Number of providers receiving intervention: 10/20 (start) 10/20 (end) Number of patient receiving intervention: 63/47/(start) 48/33/(end) Fidelity/integrity of intervention: 3 measures explored. (1) Consultation with standardized patient one week after training. (2) Process analysis of audiotapes of consultations. (3) Telephonic questionnaire to patients about experience of their consultation |
|
| Outcomes |
Primary Outcomes: Intensity of Pain (Alamo 2002) & Brief Survey of patient questions (Moral 2001) Consultation process measures: Used GATHERES‐CP scoring system in videotaped consultation s to assess patient‐centeredness; provider able to discuss patient (patient report), observed to clearly discuss cause of pain, listen to and consider options and suggestions. Satisfaction: Patient experience of the consultation. Measured by survey at 2‐3 months. Health behaviours: NA Health status: Pain, depression & anxiety (Pain Scale of Nottingham Health Profile, Goldberg Scale of Anxiety, Depression) |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Doctors were randomly assigned to two groups of 10 doctors" No data was given on sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Randomized by clinic but not stated whether assignment was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Video tape coder was blinded to provider status. Telephone interviewer was blinded to patient status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 29 dropouts, 15 were from experimental and 14 from control, but authors indicated intention to treat analysis was done |
| Selective reporting (reporting bias) | High risk | Sample size calculation was based on hypothesis that intervention compared to control would improve pain by one point on 10 point visual analogue scale. There was no statistical difference in this outcome, but authors reported greater improvement after 1 year in terms of psychological distress and number of tender points |
| Other bias | High risk | There was inadequate protection against contamination and potential for unit of analysis error was not adequately addressed. Moreover, baseline data were not collected |
Alder 2007.
| Methods |
Randomization procedure: Unclear. Groups were randomized and stratified for position and gender. Did not say it was concealed. Per e‐mail correspondence from primary author ‐ randomisation allocation was by statistician using computer generated random order list Informed consent obtained: Yes Protection against contamination: Inadequate. All of the providers in both the control and intervention group worked in the same department in the same hospital during the duration of the study as did the study authors who were 2 of the trainers. Contamination is likely Outcomes assessors blinded: Adequate. Six independent raters, psychology students, were blinded for group and were trained to evaluate videotapes of physician‐patient interactions at T1 and T2. Patients were also blinded Intention to treat analysis: Did not indicate that it was done Potential for unit of analysis error: Yes. It was adjusted for. Randomized by physician, but some outcomes were at level of patient. They used a general linear model for repeated measures with a two‐fold factor group (training and control group) and a two‐fold factor time (pre and post intervention) Comments on study quality: Power analysis was computed to assess the sample size needed for ? level 5% in pre‐intervention differences and 80% in post intervention differences. They needed between 5 and 17 for the different sub scores, planned for 32 and started with 35. 31 were followed up |
|
| Participants |
Profession: Medicine Specialty: Obstetrics & Gynecology Years experience: mean 6.9 years in training group. Mean 4.6 in the control group Clinical setting: University Hospital Obstetrics & Gynecology department Level of Care: Primary Country: Switzerland Health problem/Type of Patient: Both real & simulated patients were used. Health problems were not defined |
|
| Interventions |
Aim of study (hypothesis): To determine whether patient‐physician communication in obstetrics and gynaecology can be improved by a training program and to investigate if physicians with poorer performance before the training show greater improvement in communication skills scores over the course of the study. (605) Content of intervention: The training program consisted of three different parts: workshops, practice seminars and progress assessment meetings
Conceptual Focus:
Number of providers receiving intervention: n = 19/39 start, n = 16/32end Number of patient receiving intervention: T1 real patients = 22, sim = 122, T2 real = 11 sim = 113 Fidelity/integrity of intervention: The training sessions were given by the authors. Video taped sessions were supervised by on of the authors |
|
| Outcomes |
Primary Outcomes: Physician Communication Skills (From analysis of video tapes) Consultation process: Observation from videotapes (patient‐centred communication, establish a therapeutic relationship, understanding the problem, give information and educate, shared decision making) as measured by the MAAS‐R. Satisfaction: Satisfaction with consultation and relationship, as measured by subscales of adapted version of Kravitz survey Health behaviours: Compliance as recorded in the Kravitz questionnaire for patients Health status: NA |
|
| Notes | Linear regression analysis for lower performance at T1 predicting higher improvements in communication skills scores were significant Meta‐analysis: 1) Consultation Process : Continuous (used shared decision making since 5 outcomes in table 2 for continuous; did not take from table three since ICC needed for table 3; table 2 is physician level data‐no ICC needed) Unadjusted sample sizes: intervention: 16 control:16 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Did not give details of sequence generation but author states in e‐mail correspondence that statistician used computer software to generate sequence |
| Allocation concealment (selection bias) | Low risk | Did not specify if it was concealed. Likely, as randomisation was done by statistician |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Six independent raters, psychology students, were blinded for group and were trained to evaluate videotapes of physician‐patient interactions at T1 and T2. Patients were also blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 intervention and 7 controls lost to follow‐up; not clear that intention to treat analysis was used |
| Selective reporting (reporting bias) | Low risk | Sample size calculation was computed to detect pre‐ and post‐ intervention differences in patient satisfaction and communication skills. They found no effects for communication skills or satisfaction scores. They did report some benefit of training in physicians with poor baseline performance, but we did not give credit for this in either the qualitative or quantitative review |
| Other bias | High risk | Contamination was likely, no attempts to control. This may have accounted for the negative findings, as it would lead to underestimation of intervention. Potential for unit of analysis error was addressed |
Bieber 2008.
| Methods |
Randomization procedure: Adequate. At least three groups had similar characteristics and patients were blinded. Groups 1 and 1b were randomized. Central coordination office ensured concealment and did randomisation and allocation of groups using a random number generator. Group 2 was not randomized. Informed consent obtained: Yes Protection against contamination: Not used Outcomes assessors blinded: Adequate. Physicians were blinded to evaluation Intention to treat analysis: Stated as done Potential for unit of analysis error: Yes. Randomized by provider but analysis by patient. Used ANCOVA, ANOVA for adjustment Comments on study quality: We will not consider group 2 data since that group was not randomized. Tested FAPI measure for power, needed sample size of 42 per group for clinically relevant effect. (However, information only group had only 41 where the SDM group had over 42) |
|
| Participants |
Profession: Medicine Specialty: Rheumatology Years experience: Two years working experience Clinical setting: University Health Clinic Level of care: Primary Country: Germany Health problem/Type of patient: Fibromyalgia patients. Over 90% female. Ages 49‐51, 90‐96% female, 51‐73% married, 12‐34% separated |
|
| Interventions |
Aim of study (Hypothesis): To evaluate whether shared decision making training for physicians would improve patient physician interactions (2008), influence therapeutic decisions (2004) and health outcomes of fibromyalgia patients (2006) Content of intervention: In module 1, intervention group providers were taught how to implement a patient computer based information tool about symptoms, diagnosis, pathogenesis, treatment options, and prognosis for fibromyalgia. They were taught about evidence based treatment options for fibromyalgia. In module 2 providers were taught by a shared decision making trained physician how to build a working alliance with patients. They were taught communication skills, patient‐centred communication, interaction skills, handling emotional issues, and practiced in role plays. The randomized control group received training on applying computer based information for patients on fibromyalgia including text and diagrams for patients and evidenced based information about treatment options. There was also a non‐randomized control group which we did not consider for this review and was not included in the 2008 article Conceptual focus:
Duration and timing: 2 modules. (module 1+ 12 lessons in module 2) 1.5 hours long each. Total = 19.5 hours. No data on time spread Number of providers receiving intervention: 4/10 (start) 4/10 (end) Number of patients receiving intervention: 76/149 (start) 44/85 (end) prior to 2008 included group 2 = 48/197 (start) 44/129 (end) Fidelity/integrity of intervention: Consultations were tape recorded and written transcripts were made. SDM patients received consults twice by independent investigators at T2 and T3 |
|
| Outcomes |
Primary outcomes: Patient physician interactions. (Difficult doctor patient relationship questionnaire; DPPRQ questionnaire, physician report) Consultation process: Quality of patient‐physician relationship by FAPI questionnaire, patient report Satisfaction: Patient satisfaction with decision (measured by SWD scale), decisional conflict (measured by DCS scale) Health behaviours: Therapeutic modality chosen (medication, exercise, relaxation) Health status: Pain, depression, functional capacity, general health status |
|
| Notes | References included in review of this article: None except the 3 used for this review listed at the top | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation and allocation of groups using a random number generator |
| Allocation concealment (selection bias) | Low risk | A central coordination office assured that it was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Physicians were blinded to evaluation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of LTFU in patients: 149/164 agreed to participate and were randomized. Only 85 of those randomized received intervention and completed baseline questionnaires. of these complete data at all three points was assessed in only 67 with a 1 year follow‐up rate of 78.8%. Thus overall LTFU was 85/149. These were the patients that were analysed in the study, although authors claimed that analyses were "carried out according to the intention to treat method" |
| Selective reporting (reporting bias) | Low risk | main hypothesis was that SDM persistently improves physician‐patient interaction with FMS patient. Secondary endpoints were measures related to decision‐making process, coping behaviour, and directly health related outcome measures." ANOVA for repeated measures revealed higher interaction quality in SDM group compared with information only group. Patients' appraisal of interaction quality (FAPI scores) went down during 1 year in both groups. However there were no interaction effect between time and group, implying that the difference between the two randomized groups remained constant. Authors also reported appropriate outcomes related to secondary endpoints |
| Other bias | Low risk | No clear evidence of contamination and potential for unit of analysis error addressed; baseline data were collected and accounted for |
Briel 2006.
| Methods |
Randomization procedure: 30 GPs receiving guidelines for the management of acute respiratory tract infections were randomized to receive or not receive training in patient‐centered communication. A further 15 GPs (Group 3), who were not randomized and did not receive the guidelines for acute respiratory tract infections, served as control group. Informed consent obtained: Yes Protection against contamination: It is not stated in the article whether protection against contamination was performed. However, only one physician per practice was allowed to enter the study. Outcomes assessors blinded: trained medical students, blinded to the goal of the trial, conducted follow‐up interviews from the patients by phone. Intention to treat analysis: Stated as done Potential for unit of analysis error: was Present and it was acknowledged by reporting an estimated ICC. Comments on study quality: None |
|
| Participants |
Profession: Medicine Specialty: General medicine, Internal medicine Years experience: years of postgraduate training, median: 9.2; years in private practice, median:14.3 Clinical setting: GPs from two cantons (Basel‐Stadt and Aargau) Level of Care: Primary Country: Switzerland Health problem/Type of Patient: Patients who were visiting as a first consultation for common cold, rhinosinusitis, pharyngitis, exudative tonsillitis, laryngitis, otitis media, bronchitis, exacerbated COPD or influenza. |
|
| Interventions |
Aim of study (hypothesis): to determine whether training physicians in patient‐centered communication would reduce antibiotic prescription rate. Patient outcomes such as days with restricted activities, days off work, re‐consultation rates, patients' satisfaction with received care and their feelings of enablement were additionally investigated. Content of intervention:
Patient‐centered intervention: Physicians attended a six‐hour patient‐centered communication seminar in small groups and received two hours of personal feedback by phone. Conceptual Focus: 1‐ teaching physicians how to understand and modify patients' concepts and beliefs about the use of antibiotics for acute respiratory tract infections. 2‐ teaching physicians to practice elements of active listening, to respond to emotional clues, and to tailor information given to patients. 3‐ introducing physicians to a model by Prochaska, and DiClemente for identifying patients' attitudes and readiness for behavior changes. Number of providers receiving intervention: n = 15/45 (patient centered intervention + condition specific material (Full intervention) ); n= 15/45 (condition specific material (limited intervention)) Number of patient receiving intervention: In full intervention group, 259 patients recruited, 253 patients interviewed at 7 days, 245 patients interview at 14 days. In limited intervention group, 293 patients recruited, 290 patients interviewed at 7 days, 287 patients interview at 14 days. Fidelity/integrity of intervention: Up‐dated guidelines for the management of acute respiratory tract infection were developed by authors and was distributed as a booklet and presented in an interactive two‐hour seminars. Physicians who received the patient‐centered intervention, also attended a six‐hour patient‐centered communication seminar in small groups and received two hours of personal feedback by phone. |
|
| Outcomes |
Primary Outcomes: Prescribed antibiotics reported by pharmacists Consultation process: NA Satisfaction: Satisfaction with care received (Langewitz satisfaction survey relative to validation studies) Health behaviours: Re‐consultation within 14 days Health status: patient enablement, number of days with restricted activities |
|
| Notes | No significant results Meta‐Analysis:
Unadjusted sample sizes: Full intervention: 259/15 (patients/physicians); Limited intervention: 287/15 (patients/physicians) ICC: .097 DEFF: 2.9 Given in article Adjusted sample sizes: Full intervention: n=259/2.9= 89 Limited intervention: n=293/2.9= 101 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The "full intervention" and "limited intervention" groups were recruited randomly. The control group was not randomized. |
| Allocation concealment (selection bias) | Low risk | Allocation to either intervention was concealed using a computer generated list created by an independent institution. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The medical student who were interviewing the patients were blinded to the goal of study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | They have reported the lost to follow‐up and used intention to treat analysis. |
| Selective reporting (reporting bias) | Low risk | All investigated outcomes were reported. |
| Other bias | Unclear risk | It is not stated if protection against contamination was made. However, only one physician per practice was recruited. There was potential for unit of analysis error which was acknowledged by using an estimated ICC. |
Brown 2001.
| Methods |
Randomization procedure: Patients were randomly allocated to one of two groups using a random number table generated by computer. Did not state or describe process for concealment Informed consent obtained: Yes, written consent for participation, including audiotape Protection against contamination: Adequate, whether doctors were able to maintain their standard practice when the randomisation dictated was tested at baseline Outcomes assessor blinded: Unclear. Described how coders were trained but did not indicate that they were blinded to patient assignment. The Cohen's Kappa between re‐ratings by the same rater on content category was 0.945 and between raters, 0.922 respectively Intention to treat analysis: Unclear Potential for unit of analysis error: No Comments on study quality: None |
|
| Participants |
Profession: Medicine Specialty: 5 Medical Oncologists/4 Radiation Oncologists Years experience: No data Clinical setting: University teaching hospital clinic Level of Care: Secondary Country: Australia Health problem/Type of patient: Cancer. Mean age 56.12 years (range 18‐83). 44.3% female, 55.7% male. Education level: 27% completed 10 years, 41.5% completed high school, 9.4% tertiary‐non‐university, 1.9% unknown. Occupation: professionals 38.7%, trades people 12.6%, clerks/sales 22.6%, labourers 15.7%, home duties/students 6.3%, unknown 4.1%. Marital status: single 13.2%, married 62.6%, divorced/separated 12.9%, common law 2.5%, widowed 8.2%, unknown 0.6%. Type of Cancer: breast 19.5%, gastrointestinal 17.3%, lymphoproliferative 13.2%, genitourinary 21%, skin 14.2%, other 14.8%. Disease status: loco‐regional 56.9%, metastasis 35.8%, unknown 7.2%. Estimated prognosis: <1 year 29.8%, 1‐5 years 49.3%, normal 13.9%, unknown 6.9% |
|
| Interventions |
Aim of study (hypothesis): To investigate the effects of a question prompt sheet provided 15‐20 minutes prior to the initial consultation with one of 9 oncologists and active cancer patient question asking, length of the consultation, recall, unmet information needs, anxiety, and satisfaction Content of intervention: Nine doctors were randomly assigned to one of two conditions: A passive doctor group consulted in their standard manner and were not informed of patient assignment. A proactive doctor group was informed when patients had been given a question prompt sheet and actively addressed the prompt sheet following a standardized protocol reviewing each question listed. Proactive doctors were trained in the use of the protocol and given feedback about their performance after 5 consultations. One group of patients were given a question prompt sheet, the second group were not given a prompt sheet. Consultations with patients who did not receive a prompt sheet were to be conducted in the doctors standard manner. Consultation transcripts were analysed and demonstrated that feedback to physicians about their performance improved physician compliance with the protocol Conceptual Focus: Focus on doctor patient relationships and interviewing skills. (1/7) Providers were taught relationship building, informing/motivating/shared decision‐making, behaviours and skills could be replicated but not specifically listed Duration and timing: Unclear Number of providers receiving intervention: No data (9 total allocated to groups) Number of patient receiving intervention: 79 prompt list + trained doctor, 81 no list + trained doctor (158 in control group) Fidelity/integrity of intervention: Yes, by audiotape‐ recording |
|
| Outcomes |
Primary Outcomes: Question asking by patients Consultation process: Consultation length (audiotape recording) Satisfaction: NA Health behaviours: Patient consultation behaviours (question asking, information recall) Health status: Anxiety (Spielberger) |
|
| Notes | References included in review of this article: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated to one of two groups using a random number table generated by computer." |
| Allocation concealment (selection bias) | Unclear risk | Did not state or describe process for concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described how coders were trained but did not indicate that they were blinded to patient assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not state that they used intention to treat analysis, and they did not give any details about loss to follow‐up. They only stated that 318 patients participated in the study |
| Selective reporting (reporting bias) | Low risk | "primary outcome was total question asking" Reported outcome on this but also reported outcomes on anxiety, consultation length and recall. Provided results of all measures in methods section |
| Other bias | Low risk | Controlled and tested for contamination and no potential for unit of analysis error. Provided and accounted for baseline data |
Chassany 2006.
| Methods |
Randomization procedure: Consenting GPs were randomized to CME training group or to a control group Randomization was stratified according to practice location and date of qualification. Method was not given Informed consent obtained: Yes Protection against contamination: contamination was possible, given stratification and no efforts were made to prevent it Outcomes assessors blinded: Not used. Open study design Intention to treat analysis: Done including all patients with at least one assessment after baseline (excluded 9 trained and 15 control patients with no assessments after baseline) Potential for unit of analysis error: Yes. Used ANCOVA with baseline scored as co variable and randomisation group as explicative variable. Some adjustment for effect of different amounts of pain medication used by patients Comments on study quality: Potential for unit of analysis errors acknowledged |
|
| Participants |
Profession: Medicine Specialty: General Practice Years experience: At least 19 years postgraduate experience Clinical setting: Multiple centres. General practices Level of care: Primary Country: France Health problem/Type of patient: Osteoarthritis (lower limb pain and indications for treatment with acetaminophen). Ages 41‐92, 65% female, 83% receiving treatment for at least one additional disease at baseline |
|
| Interventions |
Aim of study (Hypothesis): To evaluate the effects of an interactive training program for general practitioners on pain management in patients with osteoarthritis. The hypothesis was that patients would have greater pain relief when their doctor received the training Content of intervention: The intervention consisted of three parts. The first portion focused on patient physician relationships based on a bio‐psycho‐social model of chronic pain. Participants viewed a video and discussed this topic given trigger questions in small groups of 6 participants. The second portion focused on analysis and evaluation of pain. Participants used tools to evaluate experimental acute pain using themselves as subjects. The third portion was about prescribing and negotiating a therapeutic contract with patients. Participants watched a set of videos and discussed with trigger questions again in small groups. Following the training, they received 8 reminders emphasizing 10 recommendations and national guidelines on chronic pain management. Printed materials for professionals, didactic presentation, illustrative role models (video), and discussion were part of the training. Participants were encouraged to practice between the session and receiving reminders after the session but no practice of skills within the training session. The control group attended the same meeting but was only given information on patient recruitment and consent All patients were prescribed acetaminophen and doctors recruited patients for the study Conceptual focus:
Duration and timing: 1 session lasting 4 hours with 8 reminders sent after the session Number of providers receiving intervention: 84/180 (start) Number of patients receiving intervention: 414/842 (start) 405/818 (end) Fidelity/integrity of intervention: No data |
|
| Outcomes |
Primary outcomes: Pain relief. Consultation Process: NA Satisfaction: NA Health behaviours: NA Health status: Pain Relief, pain, stiffness, physical functioning global health, adverse events (as measured by Lequensne Index, WOMAC Index) |
|
| Notes | References included in review of this article: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation or assignment was not given |
| Allocation concealment (selection bias) | High risk | Did not indicate that randomisation was concealed. Unlikely to have been done given stratification according to practice location and date of qualification |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Outcome assessors including patients who indicated pain were not noted to have been blinded. Unlikely, given that authors report, "patients received written information about the study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing diary data were replaced using last observation carried forward method. ITTA was used after excluded 9/414 trained and 15/428 control patients with no assessments after baseline. LTFU was less than 5% so not likely to matter ("5 and 20 rule"). 32/116 recruited GPs and 20/118 control GPs did not actively participate (i.e., recruit patients) in the study |
| Selective reporting (reporting bias) | Low risk | hypothesis was that pain relief would be greater among patients whose physicians received training. Sample size was calculated to detect a difference of 5mm on VAS scale. Reported findings on primary endpoint as well as a priori secondary endpoints. Even reported portions of patients with adverse events |
| Other bias | High risk | contamination was likely, given stratification according to practice location, but no attempt was made to control for it. However, this would likely lead to underestimate of effect. Potential for unit of analysis error was acknowledged and addressed. Baseline data were collected and accounted for |
Chenoweth 2009.
| Methods |
Randomization procedure: 15 of 30 residential care sites which had similar management structure, staffing, standards and size were randomized to receive one of the three interventions according to complete block design.. Allocation was done by the study statistician, who was unaware of the identity of sites, using SAS program. Three sites which had two separate management and staff were assigned to treatment according to a balanced incomplete block design with the two units at each site treated as separate sites for randomization. Informed consent obtained: Yes Protection against contamination: contamination was possible, although the randomisation was a site level. Outcomes assessors blinded: yes (by means of signed agreement with staff, and managers not to mention the intervention, by ensuring that questionnaires included no intervention, by regularly checking with outcome assessors that they remained unaware of treatment allocation throughout the study. Intention to treat analysis: done, but no further explanation is given Potential for unit of analysis error: was Present and it was acknowledged by adjusting with a within‐site correlation Comments on study quality: Potential for unit of analysis errors acknowledged, the probability of contamination was low. However, the were potential for observer bias, as some of the outcomes were subtle experiences and more difficult to observe and judge. |
|
| Participants |
Profession: residential care sites staff Specialty: Patient care staff in a residential dementia care facility Years experience: not mentioned Clinical setting: residential care site Level of care: Primary Country: Australia Health problem/Type of patient: residents with medical diagnosis of dementia, who were older than 60 years of age, had Australian resident classification scale categories 1‐3 (high dependency), low cognitive function, and need‐driven dementia‐compromised behaviours, and were in permanent placement |
|
| Interventions |
Aim of study (Hypothesis): to investigate the effectiveness of PCC and dementia‐care mapping compared with each other and with conventional dementia care and to examine whether either intervention can decrease need‐driven dementia‐compromised behaviours. Content of intervention: In PCC group, Bradford University's training manual was used as a resource during and after training sessions. Topics covered included understanding that behaviour is a form of communication, recognising that feelings persist despite cognitive impairment, acknowledging feelings during social interactions, and focusing on the unique way that residents express feelings and needs to change usual care. In dementia‐care mapping: the care‐staff were trained by a Bradford‐trained expert, and did mapping to identify factors related to resident well‐being, and report it to nurses to develop individual care plans for residents by considering individual histories, needs, and preferences. Conceptual focus: In PCC group: 1‐how staff actions contribute to behaviours of residents that result from dementia.2‐ emphasising that social interactions, especially those that engage residents on an affective level, help to preserve personhood and build meaningful relationships. In dementia‐care mapping: mapping of composite well being scores for individual residents, associations between care practices and staff‐resident interactions, and well being expressions present in need‐driven dementia‐compromised behaviours. Duration and timing: In PCC group: 2 day training session + support via regular telephone contact In dementia‐care mapping: mapping was performed for 6 hours per day for 2 days for each resident Number of providers receiving intervention: In PCC group: 2 care staff from each of the five sites; in dementia‐care mapping two care staff at each of the five site Number of patients receiving intervention: In person‐centered care group: 98 patients; in dementia‐care mapping group: 109 patients Fidelity/integrity of intervention: In person‐centered care group one of the authors visited each site twice to help staff change practices to include person‐centered care for all 98 residents. In dementia‐care mapping two of the authors also helped the two staffs with dementia‐care mapping after their inter‐rater reliability for scoring had been established. |
|
| Outcomes |
Primary outcomes: need‐driven dementia‐compromised agitation Consultation Process measures: NA Satisfaction: NA Health behaviours: NA Health status: agitation; psychological and psychiatric behaviours; Quality of life in late stage dementia; Incidents and subsequent admission to hospital; Falls; Antipsychotic‐drug doses,Benzodiazepine drug doses (Cohen‐Mansfield agitation inventory;Neuropsychiatric inventory; QUALID; |
|
| Notes |
References included in review of this article: None Meta‐analysis 1) Primary outcome:Health status, Continuous: Agitation Unadjusted sample sizes: intervention: 88 /5 (no/sites); control: 70/5 (no/sites) ICC: .07 given in article DEFF: 1+ICCx(cluster size‐1)=1+.07x (cluster size‐1) DEFF=1+0.07[(88+70)/(10)‐1]=2.036 Adjusted sample sizes: intervention: n=88/2.036=43 control: n=70/2.036= 34 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomisation was performed using complete block design for all except for 3 care sites. These 3 sites which each of them had 2 separate dementia‐care unit and separate management and staff were randomized using balanced incomplete block design |
| Allocation concealment (selection bias) | Low risk | allocation was done by the study statistician who was unaware of the identity of sites, using SAS program. |
| Blinding (performance bias and detection bias) All outcomes | High risk | outcome assessors were each assigned to one intervention group and remained masked to group intervention by means of signed agreement with staff and managers not to mention the intervention, by ensuring that questionnaires included no intervention information, and by regularly checking with the outcome assessors that they remained unaware of treatment allocation throughout the study. However, site staffs which were trained in a particular intervention and were a source of outcome measurement could not be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participation was stable and attrition was low. Intention‐to‐treat analysis was done. |
| Selective reporting (reporting bias) | Low risk | none |
| Other bias | Unclear risk | risk of contamination was present. Potential for unit of analysis error was acknowledged by reporting the within site correlation. |
Clark 2000.
| Methods |
Randomization procedure: Unclear. A convenience sample of 74 physicians were randomly assigned to intervention or control status Informed consent obtained: Yes Protection against contamination: Adequate. Only one doctor per physician group could participate to control for group culture Outcomes assessors blinded: Data collected from parents as well as doctors. Parents and children blinded. Physicians rated their own behaviour and were not blinded Intention to treat analysis: Not done. There were no differences in experimental and control group drop out on demographic variables. However, there were differences in dropout rate related to healthcare utilization and children with more hospital stays and ED visits were more likely to be in the intervention group than the control. This is likely to have led to a conservative estimate of the treatment effect Potential for unit of analysis error: Yes. Acknowledged and adjusted for using generalised estimating equations Comments on study quality: Both physician and patient outcomes were measured but for patients, baseline values were corrected. The problem was acknowledged and to guard against bias, data were collected for parents of patients about physician behaviour as a means of corroborating physician reports. There was a close correlation reported between physician and parent descriptions of behaviour |
|
| Participants |
Profession: Medicine Specialty: Pediatrics primary care Years experience: No data Clinical setting: Private practice Level of care: Primary Country: United States Health problem/Type of patient: Asthma in children. 70% male; 7% < 2yrs, 59% 2‐7yrs, 34% 8‐12yrs; Parents 60% 30‐39yrs; 75% married; ˜90% > high school; 20% 20,000 annual income, 16% < poverty ($15,000/yr); 17% on government assistance for healthcare at baseline; 30% nonwhite (15% Latino/Hispanic, 15% African American) |
|
| Interventions |
Aim of study (Hypothesis): To evaluate the long term impact of an interactive seminar for physicians based on principles of self‐regulation on provider behaviour, children's use of health services for asthma, and parents' views of physician performance. Content of intervention: Physicians were trained to observe, evaluate and react to their own efforts to treat and educate patients. The training used interactive methods focused on helping physicians create conversation with patients to promote the following:
The seminar components included optimal clinical practice based on National Asthma Education and Prevention Program guidelines, patient teaching, and communication. Activities and materials included lectures, videotapes, and case study presentations. They were given printed materials for professionals, didactic presentations about asthma and treatment of asthma, video examples, and patient handouts. Providers were taught data gathering, relationship building, informing, motivating, shared decision making, skills taught could be replicated from the description and references listed. Conceptual focus:
Duration and timing: 2‐3 sessions, 2‐3 hours each for 2‐3 weeks Number of providers receiving intervention: (start) 12/23(end) 12/23 (low income sample) (full group start = 38/74 end = 34/67) Number of patients receiving intervention (start) 17/36 (end) 17/36 (low income sample) (full group start = 336/637 end = 202/369) Fidelity/integrity of intervention: A random sample of patients was selected to assess the effectiveness of the training program |
|
| Outcomes |
Primary outcomes: Patient ED visits & hospitalizations. Physician behavior changes in teaching and communication skills. Parent’s view of paediatrician’s performance. Patient’s use of health care for asthma. (Clark 2000) Consultation process: anti‐inflammatory medication prescribed, treatment/action plan given (patient/parent report, provider survey Satisfaction: Patient report using Likert‐type scale items to assess doctor performance of consultation skills. Health behaviours: Emergency department visits, hospitalizations, school days missed (Parent/patient report) Health status: NA |
|
| Notes |
References included in review of this article: Clark, Gong, Schork, et al. Impact of education for physicians on patient outcomes. Pediatrics. 1998; 101: 831‐6; Brown 2004 included in above review Meta‐analysis: Dichotomous variable=Consultation Process; Patient and provider level data: No ICC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A convenience sample of 74 physicians were randomly assigned to intervention or control status |
| Allocation concealment (selection bias) | High risk | Did not state. Unlikely given description |
| Blinding (performance bias and detection bias) All outcomes | High risk | Data collected from parents as well as doctors. Parents and children blinded. Physicians rated their own behaviour and were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 103/369 patients were lost to follow‐up (i.e. 27.9%) and intention to treat analysis was not done |
| Selective reporting (reporting bias) | High risk | outcomes were split into different papers. Clark assessed physician behaviour changes in teaching communication skills (10 variables), behaviour when prescribing new medicine (5 variables), therapeutic steps (6 variables), and time spent with patient (1 variable); parent's view of the paediatrician's performance (20 variables); and patient's use of healthcare for asthma (four variables). Authors did not report results for all these variables and they did not indicate that they adjusted for multiple comparisons. Reported only final logistic regression models Brown assessed changes in parent's view of physician performance; change in child's health status, and healthcare utilisation and also only presented final logistic regression model for the last 2 categories |
| Other bias | Low risk | Tried to protect against contamination; potential for unit of analysis error was acknowledged and adjusted for. Baseline data was conducted and accounted for, although they were not reported |
Dijkstra 2006.
| Methods |
Randomization procedure: Random allocation done by person outside research group and concealed from investigators. 13 hospitals were stratified on the number of beds and diabetes specialist nurses and randomly assigned to control group (usual care), professional‐directed group or patient‐centered group. Sequence generation was not given Informed consent obtained: Yes (ethics committee approved study) Protection against contamination: Inadequate. Although there was cluster randomization, Diabetes passport (main patient‐centered tool) was promoted in a patient magazine on diabetes Outcomes assessors blinded: Unclear. Primary outcome was determined by diabetes nurse specialists (through chart review) and patient questionnaire. No statement that any were blinded Intention to treat analysis: Not done Potential for unit of analysis error: Yes. Primary outcome (HbA1c) randomisation by practice, but analysis by patient without concern for unit of analysis error. The problem was acknowledged and addressed with a multilevel logistic regression performed stepwise to explain differences in adherence rates Comments on study quality: Multicenter cluster RCT, intracluster correlation coefficient was given. Stratified on the number of beds and diabetes specialist nurses |
|
| Participants |
Profession: Medicine Specialty: Internal medicine physicians (some are part of multi‐professional teams with diabetes specialist nurses, dieticians, and podiatrists) Years experience: Mean years in practice 16.3years. Mean age 47.8 years Clinical setting: Outpatient departments (general and special diabetes clinics) of consenting hospitals. University hospital and hospitals with ongoing intervention studies were excluded Level of care: Primary/secondary Country: The Netherlands Health problem/Type of patient: Type and 2 Diabetes mellitus patients who were visiting their physician for a diabetes check‐up. 47% male, mean age 58 years, Mean HbA1c 7.8 (1.2). No info on education, income, or culture |
|
| Interventions |
Aim of study (hypothesis): To investigate whether a comprehensive strategy involving both patients and professionals with the introduction of a patient centred tool (diabetes passport) as a key component, improves diabetes care Content of intervention: Intervention activities were addressed to both healthcare professionals and to patients in intervention group. Intervention for professionals included first feedback on aggregated patient data, then educational session in which national diabetes opinion leader introduced guidelines on prevention and treatment of diabetes complications as well as diabetes passports and discussed barriers and facilitating factors to implementing the diabetes passports in the clinics. 6 months later, given feedback on clinical performance as well as on the use of the diabetes passport (info collected from patients). Intervention for patients included educational meetings organized in collaboration with the local patient organisations +Diabetes passports (introduced by internists) and leaflets explaining how to use them. Control hospital patients and physicians received no training from researchers and were told to continue usual care. However, national diabetes guidelines were sent to all Dutch hospitals and summary was published in leading Dutch medical journal during the study period. Furthermore, diabetes passport was promoted in patient magazine on diabetes Conceptual Focus: Sharing management of health problem with patient: Passport was developed with patient organization and based on guidelines that aim to educate and record results of medical examinations in order to promote shared disease management; no replicable skills were given (1/7) Duration and timing: data not given Number of providers receiving intervention: 39 Course + feedback (group A), 41 course only (group B), 41 feedback only (group C), 39 control (group D). Hospitals: G1 = 4, G2 = 4, control = 5 (start & end) Number of patient receiving intervention: 150 patients per internist. 1415 patients approached, 1350 given questionnaire. 600 patient in intervention (4 hospitals), 750 in control (5 hospitals). For analysis, n (Intervention) = 351 (58.5%); n (control) = 418 (55.7%); G1 = 248, G2 = 240, control = 276 (start) 77% end Fidelity/integrity of intervention: No data |
|
| Outcomes |
Primary outcomes: HbA1c mean pre‐post Consultation process: Diabetes‐specific process measures at index visit and 12 months from medical record. Satisfaction: NA Health behaviours: NA Health status: HbA1c level (also systolic blood pressure, diastolic blood pressure, cholesterol, creatinine; from medical chart review by local diabetes specialist nurses) |
|
| Notes |
References included in review of this article: 1) Dijkstra RF et al. Diabetic Medicine 2004;21:586‐591. 2) Dijkstra RF et al. 2005;23:164‐70 Meta‐analysis 1) Health Status, Continuous Unadjusted sample sizes: intervention: 351 patients /4 hospitals control: 418 patients /5 hospitals ICC: estimate per article: .01 DEFF: 1+ICCx[(intervention+control/cluster size)‐1] DEFF: 1+.01x[(351+418(/(4+5)‐1]=1.844 Adjusted sample sizes: intervention: 351/1.844 = 190; control: 418/1.844 = 227 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 13 hospitals were stratified on the number of beds and diabetes specialist nurses and randomly assigned to control group (usual care), professional‐directed group or patient‐centered group. Did not indicate how sequence was generated, but likely adequate given that it was done outside research group and concealed |
| Allocation concealment (selection bias) | Low risk | Random allocation done by person outside research group and concealed from investigators |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Primary outcome was determined by diabetes nurse specialists (through chart review) and patient questionnaire. No statement that any were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 1415 patients approached, 1350 were given questionnaire. 600 were allocated to intervention, 750 were allocated to control. Pre‐intervention data was available in 458 (76.3%) intervention and 539 (71.9%) control. Only 351 (58.5%) of intervention and 418 (55.7%) control were analysed. Did not use intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | primary outcome measure was mean HbA1c level drop of 0.5. Secondary outcomes were processes and other outcomes of diabetes. Authors did report results on all variables described in methods |
| Other bias | High risk | Protection against contamination was inadequate. Although there was cluster randomisation, Diabetes passport (main patient‐centered tool) was promoted in a patient magazine on diabetes. However, this would underestimate effects of intervention and study found positive result Potential for unit of analysis error was acknowledged and addressed with a multilevel logistic regression performed stepwise to explain differences in adherence rates. Baseline data were collected and addressed |
Fallowfield 2002.
| Methods |
Randomization procedure: Unclear. Randomly assigned to four groups 160 oncologists, but details not given Informed consent obtained: Yes Protection against contamination: Contamination likely as the 160 oncologists came from 34 centres. There was no mention of protection against contamination. After T2 doctors assigned to feedback or control groups could attend the course and 61 did so. There was no protection for this definite contamination in the follow‐up analysis Outcomes assessors blinded: Probably. "Outcome measures were objective and subjective ratings by researchers, doctors, and patients. Videotapes were reviewed by one of two raters, for whom, as far as possible, time‐point and group allocation were concealed." Intention to treat analysis: Yes for primary outcome. "160 doctors were randomly allocated and completed the study... Applicants on the waiting list replaced five who withdrew or violated protocol... results of MIPS analysis (primary outcome) are for the 160 providers as per randomisation Potential for unit of analysis error: Yes, acknowledged and addressed. "data generated from MIPS analysis are in form of counts of various behaviours... because of correlation between consultations for the same doctor, generalised estimating equations with an exchangeable correlation structure were used for parameter estimation" Comments on study quality: None |
|
| Participants |
Profession: Medicine Specialty: Oncology Years experience: Specialist‐Registrar or above. Years experience, no data Clinical setting: Cancer centres Level of Care: Secondary Country: United Kingdom Health problem/Types of patients: Cancer (confirmed and suspected cases) Female: T1 1= 64% T2 = 58% Male: T1 = 36% T2 = 42%. Age band: 30 or less T1 = 7% T2 = 4%, 31‐50 T1 = 24% T2 = 25%, 51‐70 T1 = 48% T2 = 48%, 70+ T1 = 21% T2 = 23%. Aim of treatment: Curative T1 = 44% T2 = 42%, Palliative T1 = 36% T2 = 39%, Remission T1 = 12% T2 = 9%, Uncertain T1 = 9% T2 = 10%. Cancer: Breast T1 = 29% T2 = 24%, GI/Colorectal T1 = 18% T2 = 22%, Urological T1 = 11% T2 = 10%, Gynecological T1 = 8% T2 = 6%, Hematological T1 = 8% T2 = 7%, Lung T1 = 7% T2 = 7%, Skin/Muscular/Skeletal T1 = 6% T2 = 7%, Other/Unknown/Benign T1 = 6% T2 = 11%, Head/Neck T1 = 5% T2 = 5%, Central Nervous System T1 = 3% T2 = 4% |
|
| Interventions |
Aim of study (hypothesis): To assess the efficacy of written feedback alone, written feedback + training, or training alone on communication skills. It is hypothesized that because of improvement after training interventions, doctors assigned to receive any of the three interventions would show better communication skills on measured variables than doctors who received none of the interventions Content of intervention: The course included 3 days of learner‐centred cognitive, experiential, and behavioral components. Groups of 3‐5 doctors were led by a facilitator with 6 simulated patients skilled in providing constructive feedback. Consultations were filmed in each doctors clinic and reviewed in depth at the start of the course. Doctors identified communication problems and worked on ways of resolving them through role‐play with simulated patients followed by video review and group discussion The written feedback consisted of a comprehensive analysis of doctors communication skills displayed in all videotaped consultations filmed in each doctors clinic, prior to the training, including an explanation of terms used feedback and a glossary of communication skills words and phrases. The feedback included patient satisfaction scores, comments after consultations, congruency of the doctors ratings of patients distress and understanding of information with patients self‐report and included an exit interview with researchers Conceptual Focus:
(2/7) Providers were taught data gathering, relationship building, informing/motivating, and skills that could be replicated that were listed. (Leading questions, focused questions, open questions, empathy, summarizing information, not interrupting, checking understanding and responding appropriately Duration and timing: 3 day course spread over 4 months Number of providers receiving intervention: 39 Course + feedback (group A), 41 course only (group B), 41 feedback only (group C), 39 control (group D) Number of patient receiving intervention: 320 selected for filming Fidelity/integrity of intervention: No data |
|
| Outcomes |
Primary outcomes: The doctor asking leading questions, focused questions, having empathy, summarizing information, interrupting, checking understanding, and responding appropriately Consultation process: Observed (leading questions, focused questions, empathy, summarizes information, interrupts, checks understanding, responds appropriately) as measured by the Medical Interaction Process System/MIPS Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes |
References included in review of this article: Fallowfield, Lipkin, Hall. Teaching senior oncologists communication skills: results from phase 1 of a comprehensive longitudinal program in the UK. J Clin Oncol 1998; 16: 1961‐68 Ford, Hall, Ratcliffe, Fallowfield. The medical interaction process system (MIPS): an instrument for analysing interview of oncologists and patients with cancer. Soc Sci Med 2000; 50: 553‐66 See also: Fallowfield L, Jenkins V, Farewell V, Solis‐Trapala I. Enduring impact of communication skills training: results of a 12‐month follow‐up. British Journal of Cancer (2003) 89, 1445‐9 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random assignment procedures were not described |
| Allocation concealment (selection bias) | Unclear risk | Not stated whether randomisation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Outcome measures were objective and subjective ratings by researchers, doctors, and patients. Videotapes were reviewed by one of two raters, for whom, as far as possible, time‐point and group allocation were concealed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes for primary outcome. "160 doctors were randomly allocated and completed the study... Applicants on the waiting list replaced five who withdrew or violated protocol... results of MIPS analysis (primary outcome) are for the 160 providers as per randomisation |
| Selective reporting (reporting bias) | High risk | Quote "other outcomes will be published elsewhere;" but they were not published during the period of this review |
| Other bias | High risk | There was no protection against likely contamination, but this would underestimate effect of intervention. Potential for unit of analysis error was acknowledged and addressed. Baseline data was collected and addressed, although not reported |
Glasgow 2004.
| Methods |
Randomization procedure: Adequate. Cluster randomized control design. Participating physicians were stratified by size of clinic and setting of practice. Randomized by project statistician. Exact procedure not described Informed consent obtained: Yes Protection against contamination: Contamination was likely as physicians were stratified by size of practice and urban/rural setting in one state. Was not addressed Outcomes assessors blinded: Not stated as used Intention to treat analysis: Done. "Attrition rates were equivalent (5.3% and 4.9%) and minimal across the two conditions at the 6‐month follow‐up and not due to any consistent reasons. Reported analysis on complete cases, but indicated that intention to treat analysis produced identical conclusions Potential for unit of analysis error: Yes, acknowledged and addressed. "To account for clustering of patients within physician, a mixed model was fitted, adjusting for baseline score on the dependent variable with a random physician effect and patient nested within physician." Comments on study quality: None |
|
| Participants |
Profession: Internal Medicine & Nursing Specialty: Family Practice & Internal Medicine Years experience: Treatment group mean 15 years ±6.1. Control group mean 12.8 years ± 8.1 Clinical setting: Urban and rural primary care clinics Level of Care: Primary Country: United States Health problem/Type of patient: Type II diabetes (patient care activities). Mean age: treatment 61 (12.6) control 65 (12.4). Female: treatment 53% control 50.5%. Ethnicity: treatment, white 83.5%, black 1.7%, Hispanic 11.3%, other 3.4% control, white 77.9%, black 2.7%, Hispanic 14.1%, other 5.4%. Education: treatment <high school 13%, high school 27.1%, college 1‐3 years 32%, college grad 27.4% control <high school 14.4%, high school 25.4%, College 1‐3 years 32.8%, college grad 27.4%. Income: <10,000 treatment 12.3% control 10%, 30,000‐49,000 treatment 28%, control 23.9%, >50,000 treatment 33.3% control 32.1% |
|
| Interventions |
Aim of study (hypothesis): To evaluate CD‐ROM assisted diabetes care enhancement program. The hypothesis was that patient and provider communication and quality of care can be improved by the use of interactive technology Content of intervention: Care managers were sent a list of roles & responsibilities and trained to use a patient‐centred self‐management approach in meeting with patients about their diabetes management goals. Each doctors office received a CD‐ROM questionnaire for patients. The questionnaire was focused on diabetes management and development of the patients goals. Patients received a print out of their goals. Reminder calls and faxes were sent to care managers to make follow up calls and schedule participant appointments. In the control doctors offices patients were given a computerized assessment of general health risk behaviours and received a print out of this. Conceptual Focus:
The intervention taught data gathering, informing/motivating/shared decision making, and behaviours and skills that could be replicated. Listed review of medical care needs, self‐care goals that patient identified, and brainstorming strategies to overcome barriers to goals. Duration and timing: 1 session for 3 hours. Other materials were faxed and phoned to providers over 6 months Number of providers receiving intervention: 24/52 (start) 24/52 (end) Number of patient receiving intervention: 469/886 (start) 445/841(end) Fidelity/integrity of intervention: States the intervention was consistently delivered and were measured in implementation section. 99% received computer assessment, 92% discussed with doctor, 99.8% met with care manager, 86.4 received a follow up call from a care manager |
|
| Outcomes |
Primary outcomes: Patient satisfaction by PRP about diabetes health services Consultation process: Labs done, Blood pressure checked, Dilated eye exam done, Foot exam done, Micro albumin checked, Nutrition counselling done, Patient‐centred activities Satisfaction: Patient satisfaction with care as measured by patient satisfaction items of Diabetes patient Recognition Program. Health behaviours: Self‐management goal setting, self‐monitor blood glucose, medical nutrition treatment participation Health status: quality of life, depression (PHQ‐9) |
|
| Notes |
References included in review of this article: Glasgow, Funnell, Bonomi, Davis, Beckham, Wagner. Self‐management aspects of improving chronic illness care breakthrough series implementation with diabetes and heart failure teams. Ann Behav Med 2002; 24: 80‐7 Meta‐analysis: 1) Satisfaction, Dichotomous (patient satisfaction); per article: assuming an ICC as large as .05 Unadjusted sample sizes: intervention: 445 control: 396 ICC: .05 DEFF=1+[(445+396)/(24+28)‐1]0.05=1.759 Adjusted sample sizes: intervention: n=445/1.759== 253 control: n=396/1.759=225 2) Health Behaviors, Dichotomous (self management; goal setting) Unadjusted sample sizes: intervention: 445 control: 396 ICC: .05 DEFF=1+[(445+396)/(24+28)‐1]0.05=1.759 Adjusted sample sizes: intervention: n=445/1.759== 253 control: n=396/1.759=225 3) Consultation process continuous:(lab procedures completed) Unadjusted sample sizes: intervention: 445 control: 396 ICC: .05 DEFF=1+[(445+396)/(24+28)‐1]0.05=1.759 Adjusted sample sizes: intervention: n=445/1.759== 253 control: n=396/1.759=225 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described; but likely adequate, as the project was randomized by project statistician |
| Allocation concealment (selection bias) | Low risk | It is likely since the project statistician randomized groups but concealment is not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated as used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Attrition rates were equivalent (5.3% and 4.9%) and minimal across the two conditions at the 6‐month follow‐up and not due to any consistent reasons. Reported analysis on complete cases, but indicated that intention to treat analysis produced identical conclusions |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure was patient reports of receiving Diabetes Physician Recognition Program (PRP) services. Secondary outcomes were patient satisfaction with 5‐item scale, revised PAID‐2 scale, PHQ‐9 scores. Reported on all these measures in results section |
| Other bias | High risk | Did not protect against contamination, but this would have underestimated effect. Potential for unit of analysis error was acknowledged and adjusted for. Baseline measures were conducted and adjusted for |
Harmsen 2005.
| Methods |
Randomization procedure: Randomisation was not described Informed consent obtained: Insufficient data Protection against contamination: Adequate Outcomes assessors blinded: Adequate. "Interviewers, experts, and research assistants who conducted preliminary data processing were blinded for intervention assignment" Patients were also blinded to group assignment of their providers Intention to treat analysis: Not done Potential for unit of analysis error: Yes, acknowledged and adjusted for. Sample size was calculated "taking the multilevel design into account and assuming an ICC of 0.2" In analysis, "differences between the two patient groups were tested by means of regression analysis with adjustment for baseline fraction, weighing cases (physicians) wit total number of patients seen at baseline plus at measurement concerned" Used multilevel multiple regression techniques Comments on study quality: Underpowered study, no account for multiple testing, different patients were used at each measurement point, the changes reported are likely due to chance with a double intervention. It is not clear how much impact to ascribe to each. Low rates of doctor recruitment preclude generalisability. The study did not address the hypothesis of improving equality of care between western and non‐western patients just reflect on mutual understanding |
|
| Participants |
Profession: Medicine Specialty: General Practice Years experience: Over 5 years in current practice Clinical setting: Primary care practices of all types Level of Care: Primary Country: Holland Health problem/Type of patient: Primary care for a variety of unspecified problems. Median age 30‐49 years. Female 62.8%. Not proficient in Dutch 9.5% |
|
| Interventions |
Aim of study (hypothesis): To evaluate the effects of dual education intervention on intercultural communication given to both doctors and patients on intercultural communication. To reduce differences in mutual understanding, primary outcome, and perception of quality of care in patients with different native origins. The hypothesis was that this will decrease inequalities in care between western and non‐western patients Content of intervention: Training on intercultural communication based on 3‐step method.
Trained doctors in self‐selected strategies to solve gaps in views and culturally determined communication style. Two weeks later in the final session additional problems and advice was discussed. Patients received a co‐intervention of viewing a 12 minute videotape in the waiting room immediately before consultation; available in Dutch, Moroccan‐Arabic, Moroccan‐Berber, and Turkish. The main message of the video was to communicate directly and express misunderstanding or disagreement Conceptual Focus: None checked (0/7). Skills were taught but idiosyncratic and self‐selected Duration and timing: For doctors, 3 sessions over 2.5 days spread over 2 weeks. For patients, one 12 minute session Number of providers receiving intervention: 19/38 (start) 19/36 (end) Number of patient receiving intervention: baseline group 1 = 175, one month group 2 = 161, 6 month group 3 = 151 Fidelity/integrity of intervention: Not given |
|
| Outcomes |
Primary outcomes: Mutual understanding Consultation process: Mutual Understanding (Patient and doctor report) Satisfaction: Satisfaction with consultation by 3 item survey dichotomized to yes/no Health behaviours: NA Health status: NA |
|
| Notes |
References included in review of this article: None Meta‐analysis: Satisfaction, Dichotomous (patient satisfaction; assume ICC of .2 per article) Unadjusted sample sizes: intervention: 151 patients/18 physicians control: 151 patients/17 doctors ICC: .2 DEFF: 1+ICCx(cluster size‐1)=1+0.2[(151+151)/(18+17)‐1]=2.526 Adjusted sample sizes: intervention: n=151/2.526=60 control: n=151/2.526=60 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Did not indicate whether randomisation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Interviewers, experts, and research assistants who conducted preliminary data processing were blinded for intervention assignment" Patients were also blinded to group assignment of their providers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 351/717 (43.9%), 333/848 (39.2%), and 302/842 (35.8%) patients completed interview in first, second and third wave respectively. Did not use intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | Main outcome parameter was mutual understanding. Secondary outcomes were patient satisfaction with consultation and feeling that physician had been considerate. Quality of care was measured with Quote‐Mi. They reported on all measures in results |
| Other bias | High risk | Baseline measurements were collected and adjusted for in multiple regression. Unit of analysis error was acknowledged and adjusted for. Contamination was possible but not addressed ‐ would have underestimated effect |
Haskard 2008.
| Methods |
Randomization procedure: Adequate. Physicians were randomized into one of four groups in a fully crossed 2 x 2 between‐subjects analysis of variance (ANOVA) design assigned by a computer generated random order Informed consent obtained: Yes Protection against contamination: Not Used Outcomes assessors blinded: Unclear. Two groups of raters rated 2000 audio tapes from all three time points and rating were z‐scored within rater to equate individual variability in use of the rating scale but blinding of raters was not mentioned Intention to treat analysis: Not stated as used Potential for unit of analysis error: Yes, acknowledged and adjusted. They randomized by physician and some measures are from patients who were different groups of patients at different times but this was corrected for with ANOVA design Comments on study quality: They used 156 physicians in order to provide adequate power with a robust and generalisable random‐effects model |
|
| Participants |
Profession: Medicine Specialty: Primary care, Obstetrics/genecology, family medicine, internal medicine Years experience: Mean 11.6 years, SD 10.0 years Clinical setting: University medical centres, Department of Veterans Affairs clinic, Staff model HMO Level of Care: Primary Country: United States Health problem/Type of Patient: Counseling about weight loss, exercise, quitting smoking, and alcohol |
|
| Interventions |
Aim of study (hypothesis):
(Expected Outcome): The patient training will show positive effects on physician satisfaction and attitudes, although these effects will be weaker than those of physician training (because patients were not followed over time, and their training was relatively brief). Outcomes will be worse from training only one member of the dyad compared with training both or no training for physician and patient Content of intervention: Part I:First month. 6‐hr interactive workshop on core communication skills in healthcare teaching engaging, empathizing, educating patients about diagnosis, prognosis, and treatment and enlisting patients in mutually agreed upon treatment plans Second month: 6‐hr interactive workshop on patient adherence, enhancing patients’ health lifestyles, reducing health risk behaviours, and building confidence and conviction in patients to make health behavior changes Third month: 6‐hr interactive workshop on sources and nature of interpersonal difficulties between providers and patients, recognizing and assessing tension in relationships, acknowledging problems, discovering meaning, showing compassion, setting boundaries, and helping patients find additional support. Includes key provider‐patient communication competencies detailed in Kalamazoo Consensus statement Part II Coaching sessions (30‐45 min) Review of a routine audio‐taped patient visit, and additional tapes on communicating with terminally ill patients, informed consent, health beliefs, improving adherence, and working with patients with alcohol and nicotine dependence Review of an audio‐taped patient visit involving the issue of patient behavior change and received a copy of Motivational Interviewing (Miller & Rollnick, 1991) Review of a difficult interaction audio‐taped patient visit and receiving a copy of Conversation Repair (Platt, 1995) Patient training 20‐min waiting room pre‐visit intervention involved listening to audio CD with accompanying patient guide book focusing on planning and organizing concerns and questions for physician and encouragement to discuss treatment choices, negotiate best plan, repeat their understanding of the plan, follow‐up of care with their physician, asking questions about medications, tests, procedures, and referral Conceptual Focus: Shared decision making & motivational interviewing.
Number of providers receiving intervention: start group 1 = 31/156, group 1a = 35/156 end group 1 = 27/127, group 1a = 34/127. (Groups 1b & 2 did not receive the provider intervention but group 1b patients received the patient intervention) Number of patient receiving intervention: total number of patients = 2196 no data on patients per group Fidelity/integrity of intervention: Appointments with doctors and patients were audio‐taped |
|
| Outcomes | (They only reported significant results) Primary Outcomes: Patient satisfaction (when physician trained and not trained)Physician satisfaction with patient (when patient trained and not trained); as measured by patient and physician satisfaction surveys Consultation process: Physician information giving, satisfaction with consultation and sensitive communication, whether physician conducted a detailed physical examination. Satisfaction: Rating on Physician Information‐giving scale (Heisler), single item: whether recommend doctor to a friend Health behaviours: NA Health status: NA |
|
| Notes | Also collected Provider Outcomes: 1. Physician Satisfaction with patient. 2. Physician wanted aspects of the physician‐patient relationship to change. (questionnaires & audiotape ratings) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used computer generated random order |
| Allocation concealment (selection bias) | Unclear risk | Article does not state that randomisation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Two groups of raters rated 2000 audio tapes from all three time points and ratings were z‐scored within rater to equate individual variability in use of the rating scale but blinding of raters was not mentioned. Physicians were aware of which patients would receive training |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Had attrition rate of 29/156 (18.6%) of total randomized sample; control, physician only trained, and patient only trained had similar rates; but group with both physicians and patients trained had attrition rate of 29%. Did not use ITT analysis |
| Selective reporting (reporting bias) | High risk | Looked at numerous outcomes and reported only results that were statistically significant. "Bordeline significant items (P < 0.10) were available in an Appendix held by authors. Did not indicate that results had been adjusted for multiple comparisons |
| Other bias | High risk | Unclear whether protected against contamination, but not likely. Acknowledged and adjusted for unit of analysis error with design and in analysis. Collected baseline, but did not provide information on results or indicated that they adjusted for these differences |
Heaven 2006.
| Methods |
Randomization procedure: Unclear, Randomization was conducted following the training workshop and simulated post course assessment to prevent training bias during the workshop. It was stratified to control for those already receiving clinical supervision outside of the study. Blinding and concealment were not mentioned Informed consent obtained: Yes Protection against contamination: Unclear Outcomes assessors blinded: Adequate. "All tapes were rated by one of four raters, who were blind to the training condition and assessment time point." Intention to treat analysis: Not done Potential for unit of analysis error: No Comments on study quality: None |
|
| Participants |
Profession: Nursing Specialty: Nurse specialists in Cancers (68.9%) Nurse specialists in palliative care (1.6%) Years experience: 32.8% less than 1 year, 11.5% one to two years, 24.6% two to five years, 31.1% over 5 years Clinical setting: Hospitals and Primary care Level of care: Primary, secondary, and tertiary care Country: United Kingdom Health problem/Type of patient: Cancer |
|
| Interventions |
Aim of study (Hypothesis): To investigate the potential of clinical supervision enhancing the process of transferring communication skills learned in the training environment to the clinical setting Content of intervention: Nurses in both the intervention and control groups attended a communication skills training workshop for the study. They were learner centred and involved video demonstrations, role‐play and individual feedback on performance. Fifteen‐minute assessment interviews were conducted and audio‐taped using simulated standardized patients before the training and at the completion of the course. Following the course, the intervention group received 4 half days of clinical supervision based on Social Learning Theory. The supervision involved modelling, social influence, attention to physical feelings, review of experiences, focus on challenging expected negative outcomes, supporting positive expectations, boosting self‐confidence, mastery of skills, case review, and providing support. The supervisor also observed a patient visit to allow for feedback on actual performance. Providers were taught data gathering, relationship building, informing and motivating, key interview skills, facilitation of disclosure, and responding to disclosure. The control group did not receive the clinical supervision component Conceptual focus: 5. Interactional skills. 6. Doctor patient relationship/Interviewing skills. (2/7) Duration and timing: 4 half day sessions spread over 1 week with 12 total contact hours Number of providers receiving intervention: 29 (start) 29 (end) Number of patients receiving intervention 469 (start) 449 (end) Fidelity/integrity of intervention: Interviews with standardized patients were audio‐taped |
|
| Outcomes |
Primary outcomes: Changes/Improvement in interviewing skills Consultation process: Key Interviewing skills: skill profile, facilitation of disclosure profile, responding profile, total interview profile (From audiotapes with multilevel analysis of key behaviours). Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes | References included in review of this article: None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Provided no data on sequence generation |
| Allocation concealment (selection bias) | High risk | Not stated. However, it was unlikely given that randomisation was conducted following the training workshop and simulated post course assessment to prevent training bias during the workshop. It was stratified to control for those already receiving clinical supervision outside of the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All tapes were rated by one of four raters, who were blind to the training condition and assessment time point." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not indicate that intention to treat was utilised, however attrition rate was minimal, and not systematic ‐ 4/61 (6.5%) |
| Selective reporting (reporting bias) | Low risk | rated each audio recording using a validated scale and reported them in results |
| Other bias | High risk | Did not attempt to control for contamination, not clear whether this was a potential problem. There was no potential for unit of analysis error. There were differences in baseline demographics and previous communication skills training, 'but this did not impact their baseline scores either with real or simulated patients" |
Ho 2008.
| Methods |
Randomization procedure: Unclear. States they were randomly assigned. It does not specify how Informed consent obtained: Implied. Students were informed. It was approved by an ethics committee Protection against contamination: Inadequate. They did not do baseline measures other than self‐report assessment and all groups were sharing the same internal medicine rotations at the same school Outcomes assessors blinded: Adequate. Standardized patients were not aware which students received the workshop. Students were also blinded to methods used to assess cultural competence and just told that half would get some instruction during the clerkship on cultural competency Intention to treat analysis: Not stated as done Potential for unit of analysis error: No. Randomization was by student, but there was no patient outcome Comments on study quality: Some concern about contamination and small sample size not being generalizable that was partly acknowledged. It is stated that power was calculated and is shown in results somehow despite also saying they were not able to do this |
|
| Participants |
Profession: Medicine Specialty: Medical students Years experience: Mean age: 25. All had prior general communication skills training Clinical setting: Medical school/Internal medicine clerkship Level of Care: Primary Country: Taiwan Health problem/Type of Patient: Unknown problems in standardized patients |
|
| Interventions |
Aim of study (hypothesis): To examine whether a PCC cultural competency curriculum integrated into an Asian medical school clinical clerkship can improve cross‐cultural communication skills Content of intervention: Group 1. The first workshop focused on knowledge and attitudes and included basic concepts such as culture, health disparities, and hidden biases. The second workshop, video clips introduced cross‐cultural communication skills such as eliciting the patients’ perspective and exploring social factors related to illness. Students in the extensive intervention group received an additional 2‐hour workshop which focused on role play using cross‐cultural communication skills. Group 1B. They did not receive role playing workshop time like the extensive intervention group. The first workshop focused on knowledge and attitudes and included basic concepts such as culture, health disparities, and hidden biases. In the second workshop, video clips introduced cross‐cultural communication skills such as eliciting the patients’ perspective and exploring social factors related to illness. Standardized patients received a half‐day training session on how to respond to student questions, mark the checklist, and provide feedback. Measured domains of student behaviours, not specifically cross‐cultural in nature, were basic communication, history‐taking, and differential diagnosis. The simulated patient in the cultural competence case received an additional training hour focusing on eliciting the patient’s perspective and exploring social factors related to illness. This included in patient perspective were eliciting the patient’s explanation of illness, pattern of medication utilization, concerns about treatment, and utilization of alternative treatments. Social factors rated were eliciting the patient’s sources of social support, impact of illness on work, affordability of medication, prescription literacy, and access to clinics. Conceptual Focus: 4) a focus in the consultation on the patient as a whole person who has individual preferences situated within social contexts. 5) interactional skills 6) Doctor patient relationship/Interviewing skills 7) Bio‐psycho‐social model. (4/7) Number of providers receiving intervention: start 1.15/57 1b.15/5 end 1.15/57 1b. 15/57 Number of patient receiving intervention: at least 3 per student (unknown number total). Fidelity/integrity of intervention: None |
|
| Outcomes |
Primary Outcomes: Post workshop test Consultation process: Patient perceptions; observed skills (social factors, basic communication skills, history taking, differential diagnosis, patient perspectives) Satisfaction: NA Health behaviours: NA Patient health status: NA |
|
| Notes |
Meta‐analysis: 1) Consultation Process : Continuous No ICC needed. Physician level data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States they were randomly assigned. Does not specify methods used |
| Allocation concealment (selection bias) | Unclear risk | Did not indicate whether concealed or not |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Standardized patients were not aware which students received the workshop. Students were also blinded to methods used to assess cultural competence and just told that half would get some instruction during the clerkship on cultural competency |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no attrition. All randomized students completed the study |
| Selective reporting (reporting bias) | Low risk | Reported on outcomes described in methods |
| Other bias | High risk | Contamination was likely, no attempts to control but this would underestimate treatment effects. There was no potential for unit of analysis error. They did not do baseline measures other than self‐report assessment |
Hobma 2006.
| Methods |
Randomization procedure: Practices were stratified by region and experience with modern educational methods. "Balanced randomisation of practices was done." Used 'allocate random data' option in SPSS. Likely not concealed because done by principal researcher and project's research assistant Informed consent obtained: Yes Protection against contamination: Yes. GPs participated individually, but randomisation was done at practice level so practice either had all intervention or all control group physicians, stratified by region and experience. Done to avoid contamination Outcomes assessors blinded: "For the first observation, blinding was not feasible, because limited time was scheduled between assessment ad feedback in the intervention group. For the second observation (2‐6 months after intervention, observers were blinded to whether provider was intervention or control." Intention to treat analysis: "Analyses were done as 'intention to treat' analyses if data were available: all participants, whether they participated in educational activities on doctor‐patient communication or not, were included in analyses." Potential for unit of analysis error: Yes. Randomized at the practice level and collected data at provider level. UoA error not explicitly acknowledged, but "practices were stratified by region and experience in working with modern educational methods; these were identified in an expert panel prior to the study as possible effect modifiers." Comments on study quality: Used linear regression, using post score as dependent variable and pre‐score and treatment as independent variables |
|
| Participants |
Profession: Medicine Specialty: General Practice Years experience: No data Clinical setting: Primary Care Level of care: Primary Care Country: The Netherlands Health problem/Type of patient: Primary Care patients |
|
| Interventions |
Aim of study (Hypothesis): To examine the effectiveness of a learner‐centred approach that focuses on actual needs to improve general practitioners communication with patients. The hypothesis was that a learner‐centred approach which included individual performance assessment followed by small group meetings tailored to doctors individual needs may be more successful in improving doctor patient communication than traditional CME approaches Content of intervention: Doctors were led in small groups by regional general practitioners specializing in doctor‐patient communication who had received 8 hours of training. The intervention group participated in discussion groups and received written materials about doctor‐patient communication. They were taught relationship building and informing/motivating doctor patient communication skills. The control group was offered written information on doctor‐ patient communication and self‐assessment questionnaires Conceptual focus: 6. Doctor patient relationship/interviewing skills. (1/7) and 8. Which ever topics doctors needed to improve Duration and timing: Three or Four two hour long sessions over a seven month time period. (6 to 8 direct contact hours) Number of providers receiving intervention: 44/86(start) 33/56(end) Number of patients receiving intervention: No data Fidelity/integrity of intervention: No data |
|
| Outcomes |
Primary outcomes: Doctor/patient communication in consultation process (MAAS‐Global total written questionnaires for providers.) Consultation process: Assessed consultation encounter skills (introduction, request for help, physical exam, diagnosis, management, evaluation of consultation, exploration, emotions, Information giving, summarization, structuring, empathy) as measured by the MAAS Global Questionnaire for Providers. Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes |
Meta‐analysis: 1) Consultation Process : Continuous Some clustering (1‐2 Physicians/practice); Physician level data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Practices were stratified by region and experience with modern educational methods. "Balanced randomisation of practices was done." Used 'allocate random data' option in SPSS |
| Allocation concealment (selection bias) | High risk | Likely not concealed because done by principal researcher and project's research assistant |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "For the first observation, blinding was not feasible, because limited time was scheduled between assessment ad feedback in the intervention group. For the second observation (2‐6 months after intervention, observers were blinded to whether provider was intervention or control." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "used intention to treat analyses if data were available" but attrition rates (i.e., no video observations) at times 1 and 2 for intervention groups were 5/49 (10.2%) and 11/49 (22.4%) respectively; and for controls were respectively 9/41 (17.6%) and 13/51 (25.5%) |
| Selective reporting (reporting bias) | Low risk | Reported 12/13 items on MAAS scale. "Follow‐up consultation" item probably not applicable in the first visit |
| Other bias | Low risk | Protected against contamination. Potential for unit of analysis error was not explicitly acknowledged; however, practices were stratified by region and experience in working with modern educational methods, which were determine a priori by an expert panel as possible effect modifiers. Did not provide statistical testing on baseline differences, but results given as mean pre‐post difference. In linear regression, used post score as dependent variable and pre‐score (baseline) and treatment as independent variables |
Howe 1996.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Unclear Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: Yes |
|
| Participants |
Speciality: General practitioners Clinical setting: General practices, UK Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of intervention: Training was in the form of a self‐directed educational package. It aimed to help providers improve their detection of psychological distress in patients (through a process of reflexive learning). It included:
Duration and timing: This work was undertaken in the providers own time, and within three months of doing the exercise, the data collection procedure was repeated to see if their performance as detectors of distress had altered Numbers of providers receiving intervention: 10 Numbers of patients followed up in IG: not stated (2764 patients overall) Review authors' score for intensity of the patient centeredness of the intervention: 4/10 Review authors' score for intensity of the teaching strategies used: 1/10 Control group received no training Numbers of providers in CG: 9 Numbers of patients followed up in CG: not stated (2764 patients overall) |
|
| Outcomes |
Consultation process: Provider's psychological detection rate Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes |
Measures used: For provider's psychological detection rate: Type: GP rating scale of psychological distress Index: 6 point scale (Crossley 1992; Goldberg 1988) indicating the degree of psychological disturbance present in patient Meta‐analysis: 1) Consultation Process : Continuous No clustering; no ICC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not describe sequence generation and acquisition |
| Allocation concealment (selection bias) | Unclear risk | Did not describe randomisation procedure |
| Blinding (performance bias and detection bias) All outcomes | High risk | Did not indicate whether or not outcome assessors were blinded; but clearly, providers (who rated patients' psychological distress) were not blinded to their experimental status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate moderate: 2/20 (10%) providers randomized; Did not use intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | Reported results of outcome measures described in methods |
| Other bias | High risk | Probable contamination was not addressed, but likely would have underestimated the positive benefit found. Minimal potential for unit of analysis error as both randomisation and analysis were at the provider level (outcome was detection rate of general practitioner). Baseline data was collected and adjusted for with Wilcoxin rank sum test to compare before and after data for each group |
Joos 1996.
| Methods |
Study design: RCT Informed consent obtained: yes Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Unclear Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: Yes, but adjustments made |
|
| Participants |
Speciality: General physicians and internal medicine residents Clinical setting: General medicine outpatient clinics, USA Types of patients: Adults taking oral medication for at least one chronic condition |
|
| Interventions |
Content of intervention: The intervention was designed to enhance providers' ability to elicit, identify and respond effectively to patient requests. Teaching methods included readings, lecture, discussion, review of videotapes, and role‐playing. Outlines and two or three focused readings were prepared for each session. The first session included:
The 16 item Patient Requests for Services questionnaire/clinical tool was designed to enhance information transfer between patient and provider. Patients filled this out prior to a clinic visit and it was attached to the front of their medical chart. Providers were encouraged to review it before seeing the patient. The types of services patients could request on the form included information about their disease conditions and treatment; counselling regarding habit and behaviour change; discussions of their concerns with the provider; assistance with emotional and social problems; and tests and referral to specialists. The second session reviewed providers experience with using the tool during the previous week and focused on how to help patients follow recommendations. The third session was devoted to practice and feedback of skills using simulated patients who role‐played four different scenarios. Duration and timing: 3 x 90 minute sessions at two week intervals Numbers of providers receiving intervention: 22 Numbers of patients followed up in IG: 185 Review authors' score for intensity of the patient‐centeredness of the intervention: 9/10 Review authors' score for intensity of the teaching strategies used: 3/10 Control group received training in medical decision making Numbers of providers in CG: 20 Numbers of patients followed up in CG: 163 |
|
| Outcomes |
Consultation process: Provider use of clinical tool in visit; frequency with which provider elicited all of a patient's concerns; patient's perceptions of amount of information they received about their disease conditions and medications Satisfaction: Patient's satisfaction with physician skills Health behaviours: Medication adherence Health status: NA |
|
| Notes |
Measures used: For provider use of clinical tool and frequency with which all patient's concerns were elicited: Type: Analysis of audiotapes (Roter coding system, Roter 1977) For patient satisfaction with care: Type: American Board of Internal Medicine Patient Satisfaction Questionnaire Index: 26 items (Carter 1989) where 1 = poor; 5 = excellent For patient's perceptions of amount of information they received about their disease conditions and medications: Type: Patient questionnaire Index: 5 point scale (no reference given) where 1 = nothing at all; 5 = all there is to know. For medication compliance and appointment keeping: Type: pharmacy records; appointment files. Meta‐analysis: 1) Consultation Process : Continuous Physician the unit of analysis 2) Satisfaction with Care, Continuous Physician the unit of analysis; no ICC needed 3) Healthcare Behaviors, Continuous Physician the unit of analysis: no ICC needed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not report on how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Did not describe randomisation process |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not indicate whether or not outcome assessors were blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was minimal for 1/43 (2.3%) for physicians; but it was high for patients:101/409 (24.7%) of all patients, 42/209 (20.1%) of intervention and 48/191(25.1%) of control patients who completed baseline data collection had some missing data. ITT analysis |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | High risk | Contamination was likely as study conducted in one university‐based VA hospital; and it was not addressed. This would have led to underestimate of effect of intervention on transfer of communication skill. It also may have led to Type 2 error with conclusions of negative effects on patient outcomes. Potential for unit of analysis error was acknowledged and addressed: " The GEE approach is an extension of logistic regression that adjusts for the effects of clustering and permits the use of covariates. However, because it tends to be unreliable in samples in which there are fewer than 40 clusters per treatment group, we chose to use the general linear model for the special case of zero‐one dependent variable elicitation of all patient concerns, which also allowed us to account for clustering and include a covariate" Baseline data collected and adjusted for in final regression model |
Kennedy 2004.
| Methods |
Randomization procedure: Did not describe how sequence was generated or whether it was concealed Informed consent: Insufficient data Protection against contamination: Used cluster randomisation to protect against contamination Outcomes assessors blinded: Did not indicate whether or not outcome assessors were blinded Intention to treat analysis: Was done. Additional statistical methods were used to address missing exit questionnaires Potential for unit of analysis error: Possible. The problem is acknowledged and adjusted for at baseline with ICC. In analyses, they used procedures in STATA that were based on theoretical assumptions specific to clustered survey data Comments on study quality: Cluster randomized trial but no differences in patient population |
|
| Participants |
Profession: Medicine, Nursing, Secretary Specialty: Gastroenterology team Years experience: No data Clinical setting: Follow up outpatient clinics, National Health Service, Inpatient, General practice Level of Care: Primary & Secondary Country: England Health problem/Type of patient: Ulcerative colitis or Crohns disease. Mean age 45.5, Female 57.6%, currently working 57.4%, retired 21.1%, married or living with a partner 62.1%, education past age 16 43.6%, ulcerative colitis 63.5%, pattern of relapse followed by periods of no symptoms 52.4%, in active phase of disease 25%, no current disease problem 75%, mean duration of illness just under 9.1 years with range of 0‐53 years |
|
| Interventions |
Aim of study (hypothesis): To determine if a whole systems approach to self‐management using a guidebook developed with patients combined with physicians trained in PCC care improves clinical outcomes and leads to cost effective use of National Health Services Content of intervention: The training included description of the background to the research and successful pilot project. Training components included description of patient‐centred skills, demonstration video, role play, video feedback training, and discussion. The two major component of the patient‐centred consultation were addressing the impact of the disease on the patient and establishing with the patient what treatment works. The participants were instructed in open ended questions, picking up cues from patients, clarification, summarizing, checking whether information was understood, and a collaborative approach to treatment. Skills were demonstrated using a video of a model consultation that included how to use a guidebook for self‐management, making a written management plan, and enabling self‐referral to the clinic. A role play was recorded and discussed and participants practiced skills in role plays. Consumer involvement was used in producing the handbook Conceptual Focus:
Contained Guidebook for patients about managing open access. Patients control appointment making Duration and timing: One session for 2 hours Number of providers receiving intervention: 9 sites (24 team members) Number of patient receiving intervention: 403/710 (start) 190/320 (end) Fidelity/integrity of intervention: No data |
|
| Outcomes |
Primary outcomes: Quality of life Consultation process: Number of visits to clinic, medical and surgical treatment in hospital. Satisfaction: Satisfaction with inpatient and outpatient care received (Consultation Satisfaction Questionnaire by Baker) Health behaviours: Making no more than 2 GP visits per year Health status: Number and duration of relapses during the course of the year, details of symptoms, quality of life (Patient diaries, Inflammatory bowel Disease Quality of Life scale) |
|
| Notes |
References included in review of this article:
Meta‐analysis: 1) Health Behaviors, Dichotomous (ICC was estimated from the median ICCs: .030, .033, .047, .054, .109 Thus .047 is the median) Patients making no more than 2 visits to GP during trial year (yes/no) Unadjusted sample sizes: intervention: 232 control: 288 ICC: .047 DEFF: 1+ICCx(cluster size‐1)=1+0.047[(232+288)/(9+10)‐1]=2.239 Adjusted sample sizes: intervention: n=232/2.239=104 control: n=288/2.239=129 2) Satisfaction with Care, Continuous (satisfaction with initial consult) Unadjusted sample sizes: intervention: 260 control: 358 ICC: .047 DEFF: 1+ICCx(cluster size‐1)=1+0.047[(260+358)/(19)‐1]=2.482 Adjusted sample sizes: intervention: 260/2.482=105 control: n=358/2.482=144 3) Healthcare Behaviors, Continuous (number of kept appointments during trial) Used ICC of .109 for number of hospital appointments Unadjusted sample sizes: intervention: 274 control: 364 ICC: .109 DEFF: 1+ICCx(cluster size‐1)=4.551 DEFF: 1+.109x[(274+364)/19)‐1]=4.551 Adjusted sample sizes: intervention: n=274/4.551=60 control: 364/4.551=80 4) Health Status, Continuous (ICC provided as .030 for HADS –Hospital Anxiety and Depression Scale) Unadjusted sample sizes: intervention: 242 control: 306 ICC: .030 DEFF: 1+ICCx(cluster size‐1)=1.835 DEFF: 1+.030x[(242+306)/19)‐1]=1.835 Adjusted sample sizes: intervention: 242/1.835=132 control: n=306/1.835=167 Patient randomized; patient data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not describe sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Did not indicate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not indicate whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate in intervention was 36/279 (12.9%) and in control was 95/403 (23.6%). However, ITT analysis was done. "To adjust for missing exit questionnaires, logistic regression was used to estimate th probability of questionnaire return on the basis of hospital and patient characteristics. The inverse of these probabilities was assigned to individual cases as weights in main analysis. Where data were skewed, bootstrapping was used to confirm statistical significance" |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | Low risk | Used cluster randomisation to protect against contamination. Potential unit of analysis error was acknowledged and addressed by computing ICCs. In analyses, they used procedures in STATA that were based on theoretical assumptions specific to clustered survey data |
Kinmonth 1998.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Blind and secure Protection against contamination: Unclear Outcome assessors blind?: Yes Intention to treat analysis: Done Potential for unit of analysis error for some outcomes?: Yes, but adjustments made |
|
| Participants |
Speciality: General practitioners and practice nurses Clinical setting: General practices, UK Types of patients: Adults with type 2 diabetes |
|
| Interventions |
Content of intervention:
Practices were encouraged to base care on British Diabetic Association Guidelines. Practices were given BDA materials for the practice and for the patients as suggested resources
At least one GP and one practice nurse from each practice attended a half day training session. During the training session they reviewed the evidence for the patient‐centred approach and were encouraged to consider both patient and provider agendas. They were given booklets for patients that encouraged patients to prepare for their consultation and the consideration of both patient and provider agendas
Practice nurses attended a further full day of skills training to practice skills learned. These included: eliciting and listening well to the patient agenda; learning to negotiate behaviour change; using a framework for the consultation and behavioural change materials. Practice nurses attended two follow‐up half days at six‐monthly intervals for group support concerning the patient‐centred approach and to review recruitment to the trial with the research team. Duration and timing: For GPs = 1 x 0.5 days For nurses = 3 x 0.5 days (2 were optional support sessions); 1x 1.0 days. Timespread from 1st to last session = 12 months Numbers of providers receiving intervention: 23 GPs; 32 practice nurses (21 practices) Numbers of patients followed up in IG: 142 Review authors' score for intensity of the patient‐centred ness of the intervention: For GPs = 3/10, for nurses = 5/10 Review authors' score for intensity of the teaching strategies used: For GPs = 1/10, for nurses = 7/10 Control group received condition specific material for providers and patients Numbers of providers in CG: 23 practice nurses (20 practices) Numbers of patients followed up in CG: 108 |
|
| Outcomes |
Consultation process: Agreement between patient and provider on main concerns over the previous year; patient ratings of communication with doctors and nurses Satisfaction: Patient satisfaction with treatment and style of care Health behaviours: Patients' lifestyle: diet, exercise, smoking Health status: Well being score, quality of life |
|
| Notes |
Measures used: For agreement between patient and provider on main concerns over the previous year: Type: patient questionnaire Index: 4 point scale (no reference given) For patient ratings of communication with doctors and nurses: Type: patient questionnaire Index: 6 point scale (no reference given) where 6 = patient always able to tell practitioner very personal things, ask the practitioner about troubling things, and get the practitioner to understand his or her point of view. For patient satisfaction with treatment and style of care: Type: patient questionnaire Index: 36 point scale (Bradley 1994) For patients' lifestyle: Type: patient questionnaire Index: Not stated (Godin 1985; Murphy 1992; Roe 1994). Smoking was confirmed by clinical measure For all clinical health status and well being outcomes: Type: various clinical measures For perceived control of diabetes Type: patient questionnaire Index: 30 point scale (Bradley 1994) For functional and psychological status: Type: Quality of life and well being patient questionnaire Index: Various different scales including depression, anxiety, energy and positive well being sub scales (Bradley 1994) Meta‐analysis: 1) Consultation Process, Dichotomous: Maximum score for communication with GP Unadjusted sample sizes: intervention: 140 /20(no/practices); control: 107/19 (no/practices) ICC: .046 DEFF: 1+ICCx(cluster size‐1)=1+.046x (cluster size‐1) DEFF=1+0.046[(140+107)/(20+19)‐1]=1.2453 Adjusted sample sizes: intervention: n=140/1.2453=112 control: n=107/1.2453= 86 2) Satisfaction, Dichotomous: High Satisfaction with treatment Unadjusted sample sizes: intervention: 140/20 (No/practices) control: 108/20 (no/practices) ICC: .046 DEFF: 1+ICCx(cluster size‐1)=1+0.046[(140+108)(20+20)‐1]=1.239 Adjusted sample sizes: intervention: n=140/1.239=113 control: n=108/1.239=87 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "initial stratified random allocation of practices by computer" |
| Allocation concealment (selection bias) | Low risk | Did not state whether allocation was concealed, but likely from the description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Practice teams agreed to randomisation to "different approaches to early diabetes care." Assessment of patients was by research nurse who was unaware of the groups. The trial was conducted within a wider study of the incidence and presentation of type 2 diabetes." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was minimal for practices 1/22 (4.5%) intervention and 1/21 (4.8%) control; but 57/199 (28.6%) intervention patients and 53/161 (32.9%) control patients had some missing data after 1 year. Analysis was by intention to treat (but tables included only those with complete data) |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured. |
| Other bias | High risk | Contamination was possible given stratified (by district and practice size) randomisation, but likely would have led to underestimation of positive treatment effects reported Unit of analysis error was addressed: "Patients' results were corrected for clustering at practice level (STATACORP 1997) and adjusted for stratifiers". Intraclass coefficients (ICC) were calculated for HbA1c and BMI. Baseline data was collected and there were no significant important differences in practices, practitioners and patients |
Krones 2008.
| Methods |
Randomization procedure: Randomized by pre‐existing CME groups and stratified by rural or urban location of member practices. Patients were nested within practice. No concealment Informed consent obtained: Yes Protection against contamination: They excluded group with prior training with the same materials and other cardiovascular risk calculators. They did not protect against contamination from within group practices Outcomes assessors blinded: Adequate. Patients were the outcomes assessors and were unaware of their physicians group allocation Intention to treat analysis: Not stated as done Potential for unit of analysis error: Randomization by CME group/doctor and analysis was by patient. This was considered in analysis for sample size needed to have adequate power. Showing they would need at least 786 patients. They had over 900 patients. They defined patient as unit of analysis for sample size calculation, but they did not explicitly acknowledge Comments on study quality: They met the calculations they for adequate power for their study to be generalisable |
|
| Participants |
Profession: Medicine Specialty: Family Practice Years experience: No data on years of experience Clinical setting: Health Centers Level of Care: Primary Country: Germany Health problem/Type of Patient: Cardiovascular disease (Risk) Consecutive patients with cholesterol measured within 4 weeks |
|
| Interventions |
Aim of study (hypothesis): To determine the effect of promoting the effective communication of absolute cardiovascular disease (CVD) risk and shared decision making through disseminating a simple decision aid for use in family practice consultations Content of intervention: Participants in the intervention arm attended 2 CME sessions lasting 2 hours each. They discussed epidemiology and background of global CVD risk Calculation, the ethics of shared decision making, emphasized practical communication strategies and materials to be applied during consultation. A script‐like decision aid was practiced through role playing. Physicians received feedback from peers in their groups They were taught how to calculate and demonstrate the effect of several preventive measures. They were provided printed materials for patients with individual prognosis through a marked smiley face rating Conceptual focus: Shared Decision Making to:
Number of providers receiving intervention: start 80 end (follow up) 44 Number of patient receiving intervention: start 82 end (follow up) 47 Fidelity/integrity of intervention: None listed |
|
| Outcomes |
Primary Outcomes: Patient satisfaction, Patient participation (patient report questionnaire Patient Participation Scale & SDM‐Q Scale Short Form.) Consultation process: Shared decision making steps reported by patient, patient perceived that doctor knows patient, patient knowledge of condition learned in encounter. Satisfaction: Satisfaction with process and result of care (Measured by Patient Participation Scale by Man‐Son‐Hing) Health behaviours: Patient participation in the Encounter (Patient Participation Scale) Health status: Mean change of cardiovascular risk on Framingham calibrated for Europeans. |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by pre‐existing CME groups and stratified by rural or urban location of member practices. Patients were nested within practice. Did not describe sequence generation |
| Allocation concealment (selection bias) | High risk | No concealment was stated, and is unlikely in this pragmatic cluster randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients were the outcomes assessors and were unaware of their physicians group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates of practices and patients in randomized practices: 40/80 (50%) and 90/550 (16.4%) respectively in intervention; and in controls 41/82 (50%) and 116/582 (19.9%) respectively. ITT was not done. Missing values were simply excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | Low risk | Protected against contamination with cluster randomisation. Unit of analysis was acknowledged and addressed with random effects models in Stata and mixed models in SPSS to allow for clustering by practice and CME groups. Also estimated ICC. Baseline data was obtained. Intervention group had higher number of practices with >1500 patients, but is was adjusted for in analysis |
Langewitz 1998.
| Methods |
Study design: RCT Informed consent: Implied for residents (3/44 refused); no data on patients Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind? Yes Intention to treat analysis: Not done (no loss to follow up) Potential for unit of analysis error for some outcomes?: No |
|
| Participants |
Speciality: Paediatric doctors Clinical setting: Primary care practices, USA Types of patients: Paediatric asthma patients and parents |
|
| Interventions |
Content of intervention: The four teaching objectives of the intervention were; to help the patient clarify his/her concerns; to find relevant information; to offer a negotiation process and; to invite patient participation in decision making. Techniques of active listening were taught and providers were encouraged to use PCC communication techniques. NB: Providers were instructed to clarify time limits and to announce explicitly a change in the topic and the structure of the communication, e.g., by announcing a shift from a patient‐centred phase to a provider centred part. The intervention consisted of three elements:
All participants performed two videotaped interviews with simulated patients three weeks before the intervention started and ten months later performed two videotaped interviews with simulated patients. Duration and timing: 22.5 hrs of specific communication training over a six month period. Numbers of providers receiving intervention: 20 Numbers of patients followed up in IG: 4 (actors) Review authors' score for intensity of the patient centeredness of the intervention: 9/10 Review authors' score for intensity of the teaching strategies used: 10/10 Control group received no training Numbers of providers in CG: 23 Numbers of patients followed up in CG: 4 (same 4 actors as in IG) |
|
| Outcomes |
Consultation process: Sum score of provider use of behaviours relating to helping the patient clarify his/her concerns; finding relevant information,inviting patient participation in decision making; offering a negotiation process; provider overall performance throughout the entire consultation. Satisfaction: patient satisfaction scores; proportion of patients who would recommend doctor to a friend. Health behaviours: NA Health status and well being: NA |
|
| Notes |
Measures used: For all consultation/practice process outcomes: Type: The Maastricht History and Advice Checklist‐Revised rating scale (van Thiel 1991) Index: MAAS‐R contains two types of scores; global scores ranging from 0 (does not occur), 1 (bad performance) to 5 (very good performance) that rate either specific behaviours or the quality of e.g., data gathering, and checklists where the occurrence of a certain behaviour or the mention of specific information is marked. For patient satisfaction with care: Type: patient questionnaire (issued by the American Board of Internal Medicine) Index: 14 item (Matthews 1989) and also contains two dichotomous (yes/no) variables. Meta‐analysis 1) Consultation process, Continuous Consultation at physician level; patent satisfaction at patient level No ICC needed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not describe sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Did not describe randomisation process |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "raters were blind as to group assignment of residents" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was minimal 1/43 (2.3%), but intention to treat analysis was not done |
| Selective reporting (reporting bias) | Low risk | Reported on all measured outcomes |
| Other bias | High risk | No attempts were made to control contamination, would underestimate effects of intervention Minimal potential for unit of analysis error Baseline characteristics data were collected, no significant differences were found |
Levinson 1993.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Yes Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: yes, but adjustments made |
|
| Participants |
Speciality: Family practitioners and general internists Clinical setting: Primary care practices, USA Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of intervention Providers attended a workshop which reviewed a wide range of communication skills that are consistent with a 'patient‐centred' style of interviewing. The programme includes didactic presentations and case‐based discussions focusing on four fundamental skills in the medical interview: engaging patient participation, communicating empathy, educating patients, and enlisting patients in healthcare discussions. Behaviours taught included: eliciting the patient's concerns; more use of open‐ended questions/less closed‐ended questions; more giving of information about medical illness and therapy; more psychosocial discussion; more asking the patient's opinion; more listening and less talking; summarizing what the patient says; allowing the patient to tell a story without interrupting. All providers in this group had give routine medical visits audio‐taped prior to the workshop and five visits audio‐taped after the workshop. Duration and timing: 1x 4 1/2 hour workshop Numbers of providers receiving intervention: 16 Numbers of patients followed up in the IG: 75 Review authors' score for intensity of the patient centeredness of the intervention:9/10 Review authors' score for intensity of the teaching strategies used: 2/10 Control group received no training Numbers of providers in CG: 15 Numbers of patients followed up in CG: 75 |
|
| Outcomes |
Consultation process: Change scores in provider and patient‐centred communication behaviours (positive talk, biomedical information giving, closed‐ended questions, open‐ended questions, psychosocial talk)
Provider and patients' negative and positive emotions during visits. Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes | Measures used: For all consultation/practice process outcomes, analysis of audio tapes of encounters was used. Method: Roter Interactional Analysis System (Roter 1991).This system codes each phrase or complete thought in the visit, by either patient or provider, into one of 34 mutually exclusive and exhaustive content categories. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not describe how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Did not describe randomisation procedure |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Audiotapes of the medical visit were content‐coded by blinded judges using the Roter Interactional Analysis System (RIAS) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | attrition rate was 3/53 (5.7%) of randomized physicians. There was incomplete data on 61/473 (12.9%) patients. Did not indicate intention to treat done or address missing values in any other way |
| Selective reporting (reporting bias) | Low risk | Reported on all outcome measures |
| Other bias | High risk | Contamination was possible and not addressed, but would lead to underestimation of positive effect found. Potential for unit of analysis error in some outcomes was addressed: "t‐tests comparing the pre‐post change scores of physicians on the four physician and two patient categories... A second analysis was performed using ANCOVA ... controlling for pretest scores as covariate to take into account any baseline differences between the groups in the dependent measure." Collected baseline data, did not report significant testing, but adjusted for differences in ANCOVA; pre‐post |
Lewis 1991.
| Methods |
Study design: RCT Informed consent: Implied. Stated that 14% of residents refused; 20% of patients refused. Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Yes Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: Yes, but adjustments made |
|
| Participants |
Speciality: Paediatric residents and fellows Clinical setting: General paediatric practices, USA Types of patients: Children (accompanied by their patients) consulting with various problems |
|
| Interventions |
Content of intervention: The intervention targeted all three participants in the medical interview (provider, parent and child). Each participant viewed a videotape. The three tapes shared four main aims:
Child videotape ‐ 10 minutes/viewed immediately prior to visit. Featured a young boy demonstrating how to communicate effectively during a medical visit. Encouraged children to see themselves as active, thoughtful participants in their own health care and modelled communication and assertiveness skills. After viewing, the children received workbooks to note down questions for providers and any information discussed. Children formulated a question they wanted to ask the provider and practiced telling it to the researcher. Parent videotape ‐ 10 minutes/viewed just prior to visit. Presented vignettes of medical visits which demonstrated effective communication skills for parents. Presented evidence re. importance of provider‐patient communication and child involvement in health care, as well as factors that affect children's understanding of medical information. Provider videotape ‐ 15 minutes/viewed as part of a 1 hour training session (in which they also received research articles on health consequences of effective communication, examples of appropriate interviewing techniques for children, and an acronym designed to remind them of critical interviewing skills). Presented research evidence relating to children's understanding of health‐related information and the consequences of effective communication . Vignettes were also used to demonstrate a number of provider communication skills, including participating with the parent and child in agenda setting, and facilitating their expression of concerns. After each visit with study patient, providers filled out a self‐assessment form designed to help them reflect on their performance. At three months, eight months, and 15 months after intervention providers received a written reminder of the intervention, data on the reported implementation of each goal and a self‐assessment form Duration and timing: providers received 1x 1hr training session Numbers of providers receiving intervention: 20 Numbers of patients followed up in IG: 81 Review authors' score for intensity of the patient centeredness of the intervention: 7/10 Review authors' score for intensity of the teaching strategies used: 6/10 Control group (providers, children and parents) received non patient‐centred educational videotape intervention (providers on assessment of febrile infants; children and parents on bicycle safety). Numbers of providers in CG: 14 Numbers of patients followed up in CG: 60 |
|
| Outcomes |
Consultation process: percentage of provider recommendations addressed to child or child and parent; number of child substantive initiations and responses; total number of statements; percentage of provider recommendations recalled by child; percentage of medication recommendations recalled by child. Satisfaction: child satisfaction with visit (Child Satisfaction Questionnaire); parent satisfaction with visit (Parent Medical Interview Satisfaction Scale). Health behaviours: NA Health status: child's anxiety as reported by parent. |
|
| Notes | Measures used: For percentage of provider recommendations addressed to child or child and parent; number of child substantive initiations and responses; and total number of statements: Type: Analysis of consultation videotapes Index: A coding system (Stewart 1981) was used to code the content, direction, origin, and type (initiation, response, interruption) of each statement during the medical visit. For percentage of provider recommendations recalled by child; percentage of medication recommendations recalled by child: Type: three open ended questions For child satisfaction with visit: Type: shortened version of Child Satisfaction Questionnaire Index: 4 point scale where 4 = high satisfaction (Rifkin 1988) For parent satisfaction: Type: Parent Medical Interview Satisfaction Scale Index: 5 point scale where 5 = high satisfaction (Lewis 1986) For child anxiety: Type: child questionnaire Index: 2 point scale where 2 = high anxiety (Venham 1979) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "pediatric residents were paired and one member was randomly assigned to the experimental group" |
| Allocation concealment (selection bias) | High risk | Did not state whether or not randomisation was concealed, but unlikely given description of randomisation process |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Videotapes were coded by a research assistant blind to the study hypotheses and design" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was 22/56 (39.3%) of physician randomized and they did not do intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | High risk | Contamination was possible but not addressed. Would have underestimated positive effects found, but may have led to Type 2 error for some outcomes (satisfaction) potential for unit of analysis error (patient clusters) was addressed; "data were analysed using mixed‐effects analysis of variance (BMDP 3V microcomputer statistical program). Parent gender and experimental status were include as fixed effects, and physician was included as a random effect." Baseline data on demographics collected (no significant differences) but not on outcomes, did not have pre‐post |
Loh 2007.
| Methods | ||
| Participants |
Profession: Medicine Specialty: General Practice Years experience: The average years professional experience was 13.0 ‐ 7.0 years (control group 10.6 +/‐ 7.4 years, intervention group 14.3 +/‐ 6.7 years). Clinical setting: University clinic GP practice Level of Care: Primary Country: Germany Health problem/Type of Patient: Newly diagnosed Depression |
|
| Interventions |
Aim of study (hypothesis): The aim of this study was to assess, if patient participation in decision‐making with a shared decision‐making intervention leads to improved treatment adherence, satisfaction. Improved patient involvement in treatment decision‐making would lead to higher likelihood of adherence, satisfaction, and improved clinical outcomes. Content of intervention: Physician training with lectures, question/discussion, practice role‐playing, video examples of SDM, a decision board for use during the consultation, printed patient information about depression and encouraging shared decision making. Conceptual Focus:
Number of providers receiving intervention: start 20/30 end 15/23 Number of patient receiving intervention: start 263/405 end 191/287 Fidelity/integrity of intervention: Attendance was recorded. 85% (17) attended the first event. 75% (15) attended the following two events. 95% (19) attended the last event. Eleven (55%) attended all five events and nine (45%) attended at least three training sessions. |
|
| Outcomes |
Primary Outcomes: Doctor facilitation & Patient participation by PICS scale doctor facilitation scale & Man‐Son‐Hing patient participation scale. Consultation process: Consultation time measured by physicians; doctor facilitation accomplished (patient report, provider report) Satisfaction: Patient satisfaction with care (German version of CSQ‐8 questionnaire for patients) Health behaviours: Treatment adherence, information seeking, participation in care process Health status: Depression severity (Brief PHQ‐D patient questionnaire) |
|
| Notes |
Meta‐analysis: 1) Health Status, Dichotomous (clinical outcomes –Depressive Symptom Severity Reduction %; (Brief PHQ‐D) Unadjusted sample sizes: intervention: 128 patients/15 physicians control: 66 patients/8 physicians ICC: .345 as given DEFF: 1+ICC)x(cluster size‐1)=1+0.345[(128+66)/(15+8)‐1]=3.565 Adjusted sample sizes: intervention: n=128/3.565=36 control: n=66/3.565=19 2) Consultation process, Continuous. Consultation time in minutes Unadjusted sample sizes: intervention: 128 patients/15 physicians control: 66 patients/8 physicians ICC: .563 as given DEFF: 1+ICC)x(cluster size‐1) DEFF: 1+.563x[(128+66)/(15+8)‐1]=5.186 Adjusted sample sizes: intervention: n=128/5.186=25 control: n=66/5.186=13 3) Satisfaction with Care, Continuous. Patient Satisfaction (ZUF‐8) Unadjusted sample sizes: intervention: 128 patients/15 physicians control: 66 patients/8 physicians ICC: .174 DEFF: 1+ICC)x(cluster size‐1) DEFF: 1+.174x[(128+66)/(15+8)‐1]=2.294 Adjusted sample sizes: intervention: n=128/2.294=56 control: n=66/2.294=29 4) Healthcare Behaviors, Continuous. Information Seeking (PICS‐IS) Unadjusted sample sizes: intervention: 128 patients/15 physicians control: 66 patients/8 physicians ICC: .110 as given DEFF: 1+ICC)x(cluster size‐1) DEFF: 1+.110x[(128+66)/(15+8)‐1]=1.818 Adjusted sample sizes: intervention: n=128/= 70 control: n=66/=36 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Two thirds of were randomly assigned to the intervention group by drawing blinded lots under supervision of the principal investigator and two researchers. The remaining third comprised the control group". The unequal distribution in the intervention and control groups was by design due to the possible higher drop out rate for the intervention group because of effort required |
| Allocation concealment (selection bias) | Low risk | By virtue of the lots being blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated, but unlikely given that outcome assessors were physicians (who could not be blinded) and patients who were not noted to be blind to their doctors' training status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not address high attrition rate ‐ 5/20 (25%) physicians and 72/263 (27.3%) patients in intervention; and 2/10 (20%) and 46/142 932.4) respectively in controls. Did not say they used intention to treat analysis and pre‐ and post numbers in Table 2 suggests they didn't |
| Selective reporting (reporting bias) | Low risk | Described scales in methods and reported results on all outcomes measured |
| Other bias | High risk | Did not protect against contamination of doctors, but this would underestimate effect of intervention, and study showed positive effect. Potential unit of analysis error by virtue of clustering of patients under physicians was acknowledged and addressed via ANCOVA with adjustment for clustering effect. they calculated variance inflation factor from the intracluster correlation coefficient (ICC). baseline differences were not significant tested, but was addressed by comparing pre‐post differences |
Longo 2006.
| Methods |
Randomization procedure: Randomization conducted by statistician using random number generator and implement while concealed. Randomized by provider with patient clusters Informed consent obtained: Yes Protection against contamination: Adequate. Only one practitioner per practice was included. Three practitioners were excluded from potential pool because of prior exposure to the training content during developmental work conducted earlier in other areas Outcomes assessors blinded: Adequate Intention to treat analysis: Done Potential for unit of analysis error: Yes. This problem is acknowledged and accounted for ‐ addressed with a three‐level statistical model with rater at level 1, consultation at level 2 and provider at level 3. For patient outcomes, calculated ICC and used for sample size calculation Comments on study quality: None |
|
| Participants |
Profession: Medicine Specialty: General practice Years experience: Recent grads with 1 to 10 years experience Clinical setting: Unknown Level of Care: Primary Country: United Kingdom Health problem/Type of patient: Atrial fibrillation, prostatism, menorrhagia, menopausal symptoms. Urban and Rural, mean age: Prostatic symptoms 63, A. Fib 65, Menorrhagia 45, HRT = 56 |
|
| Interventions |
Intervention Aim of study (hypothesis): To operationalize risk communication and standard medicine as specific and comparable interventions. To determine if each intervention was successful and if successful what order to use them with patients. In addition there was a study in parallel to the RCT to identify patient preferences for shared decision making relative to other attributes of a consultation and whether their preferences changed as a result of experiencing a shared decision making consultation Content of intervention: Providers were randomized to attend two different workshops with two sessions in each workshop using presentations, discussion, written handouts, and participation in consultation with standardized patients using a previously piloted skill development process. The first workshop session knowledge was taught and skills were demonstrated. In the second session intervention skills were practiced with standardized patients. The intervention group was taught shared decision making and the other group was taught risk communication in their workshops Conceptual Focus: 5. Interactional skills. (1/7) Duration and timing: Two session, duration and time spread not given. Number of providers receiving intervention: 20 (start) 20 (end) Number of patient receiving intervention: 747 (start) 715 exit Q, 655 at 1 month, 618 at 6 months Fidelity/integrity of intervention: Not stated |
|
| Outcomes |
Primary outcomes: Risk communication & patient confidence in decision as measured by COMRADE Consultation process: Observation of involving patient in decision making (Provider score on OPTION instrument for patient agreement and involvement) Satisfaction: Patient satisfaction with communication as measured by COMRADE. Health behaviours: Adherence expectation (COMRADE sub scales) Health status: Anxiety (Spielberger short form), health status (SF‐12) |
|
| Notes | ES moderate Risk Communication preceding shared decision making added 7.7, P < 0.001 preceding Risk Communication NS | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation and allocations by random number generation by trial statistician" |
| Allocation concealment (selection bias) | Low risk | "randomisation was concealed from those implementing interventions or assessments" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | audiotape raters were blind to study group allocation of providers or patients; both providers and patients were blinded to the decision‐making or risk communication focus of the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | attrition rate for docs was low at 1/21 (4.7%); but after 1 month (when study outcomes were assessed) was 427/1082 (39.4%) of patients invited and 92/747 (12.3%) of patients who attended. Intention to treat analysis was not done in this study with crossover design. Authors attempted to analyse possible bias from non‐response and noted that "there was no difference statistically for age and condition type between 655 responders and 92 non‐responders after 1 month." Comment: this does not adequately address incomplete data. |
| Selective reporting (reporting bias) | Low risk | process outcomes were reported in Elwyn et al., patient outcomes were reported in Edwards et al, and Longo et al. |
| Other bias | Low risk | attempted to protect against contamination; unit of analysis error was addressed with a three‐level statistical model with rater at level 1, consultation at level 2 and provider at level 3. For patient outcomes, calculated ICC and used for sample size calculation. Baseline data were collected and analyses compared baseline with intervention phases. |
Margalit 2005.
| Methods |
Randomization procedure: 44 GPs who participated in a course were randomly allocated into 2 groups one with didactic method and second with interactive method. Did not state how sequence was generated or allocated or whether it was concealed. Informed consent obtained: Yes for doctors, No data for patients. Protection against contamination: contamination was likely as included providers were from one district to participate in a CME course. No attempt was made to prevent contamination Outcomes assessors blinded: Adequate. Scores on knowledge and intentions were scored by two physicians who were blinded. Intention to treat analysis: Done Potential for unit of analysis error: Yes. This was not acknowledged, but adjusted for, using mixed model with individual physician as random effect and time (before/after) and teaching method as the two fixed‐effects. Comments on study quality: Possible selection bias with physicians choosing their patients for video taping their own interviews may be biased. Results for groups separated were not significant much of the time but combined groups all showed a significant difference. Questions about the power of this study |
|
| Participants |
Profession: Medicine Specialty: General Practice Years experience: No data Clinical setting: Outpatient health centres. University study Level of Care: Primary Country: Israel Health problem/Type of patient: No data |
|
| Interventions |
Aim of study (hypothesis): To teach bio‐psycho‐social approach to practicing providers. The hypothesis was that the interactive teaching approach would improve knowledge, intentions, patient‐centred attitudes, and professional self‐esteem more than the didactic education only Content of intervention: Providers were given CME training aimed at promoting BPS approach using lectures, interactive teaching and role playing geared for better learning in adults. Role plays included common types of patient and family encounters. Small group discussions had 3‐5 members. The didactic section included lectures, reading assignments, and tests. The trainers were board certified family physicians experienced in the Short family therapy in ambulatory medicine (SFAT‐AM) teaching method. The control group received the same lectures, readings, and tests without any of the interactive components Conceptual Focus:
Duration and timing: 12 sessions for 4‐6 hours per session over a 12 week time spread Number of providers receiving intervention: 22/44 Number of patient receiving intervention: No data Fidelity/integrity of intervention: No data |
|
| Outcomes |
Primary outcomes: Providers bio‐psycho‐social knowledge Consultation process measures: Provider’s biopsychosocial knowledge, intentions, patient‐centred attitudes; professional self‐esteem (Physician self‐report); physician detection of patient distress as measured by patient report of physician understanding of their disease. Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes | References included in review of this article: Eshet 1993 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated into 2 groups: one with didactic method and second with interactive method. Did not state how sequence was generated or allocated or whether it was concealed |
| Allocation concealment (selection bias) | Unclear risk | Did not state whether allocation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Scores on knowledge and intentions were scored by two physicians who were blinded. Did not state whether "attitudes" assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All collected measures were reported |
| Other bias | Unclear risk | baseline data collected, accounted for with before and after analysis. Potential for unit of analysis error was not acknowledged explicitly, but adjusted for, using mixed model with individual physician as random effect and time (before/after) and teaching method as the two fixed‐effects |
McLean 2004.
| Methods |
Randomization procedure: "If patients were eligible, top sheet from randomly arranged pile was turned over. If underside said 'control', consultation proceeded as normal if it said 'intervention', it included prompts to facilitate elicitation of concerns." Authors did not describe how sequence of randomisation was generated (i.e., how the 'random pile' was arranged; or who arranged it) Informed consent obtained: Yes Protection against contamination: Not used Outcomes assessors blinded: "open randomized controlled trial" Intention to treat analysis: Unclear. Potential for unit of analysis error: Not likely. This problem was not addressed or adjusted for Comments on study quality: Potential for measurement bias, not clear how control interview differed from intervention ones and were given by the same interviewers who were not blinded |
|
| Participants |
Profession: Medicine Specialty: General Practice Years experience: Not listed Clinical setting: Semi‐rural general practices Level of Care: Primary Country: United Kingdom Health problem/Type of patient: Minor self‐limiting illness: Cough, upper respiratory tract infection, virus, ear infection, other acute self‐limiting illness |
|
| Interventions |
Aim of study (hypothesis): “To measure the costs and benefits of using a prompt to elicit patient concerns when they consult for minor illness” Content of intervention: Two trainers taught all 5 providers the inclusion strategy, how to randomize patients, the intervention interview following prompts, and time recording. The intervention interview consisted of three specific prompts asking patients to explain their concerns. “ May I ask if you have any concerns about this”(illness/pain) you have come about today?” followed by “ Anything in particular about the??” and if still not giving information to ask, “ What is it about the ? that concerns you?” The same providers delivered the intervention interviews and the control interviews in which the prompts were not to be used Conceptual Focus:
Duration and timing: No data on provider training time, 110 total patient interviews occurred about 10 minutes long Number of providers receiving intervention: 5/5 Number of patient receiving intervention: 56/110 (54 control) Fidelity/integrity of intervention: Interviews were audio taped or videotaped and presented to a blinded independent assessor to determine if they were assessed correctly |
|
| Outcomes |
Primary outcomes: Duration of consultation Consultation process: Duration of consultation (Timed by the physician) Satisfaction: Satisfaction with consultation (Consultation Satisfaction Questionnaire (CSQ) by Baker Health behaviour: NA Health status: Anxiety (Spielberger) |
|
| Notes |
Meta‐analysis: 1) Satisfaction with Care, Continuous Patient randomized ; patient data; no ICC 2) Health Status, Continuous |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "If patients were eligible, top sheet from randomly arranged pile was turned over. If underside said 'control', consultation proceeded as normal if it said 'intervention', it included prompts to facilitate elicitation of concerns." Authors did not describe how sequence of randomisation was generated (i.e., how the 'random pile' was arranged or who arranged it) |
| Allocation concealment (selection bias) | High risk | Didn't state whether or not randomisation sequence was concealed, but unlikely given then they just had pile of papers rather than e.g., sequentially numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Didn't say whether or not outcome assessors were blinded, but unlikely, given that authors described trial as "an open randomized controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported an attrition rate of 0 (i.e., 56/56 patients in intervention and 54/54 patients in control group were analysed); and they stated that "missing values in incomplete questionnaires were replaced by series mean of relevant variable." Although not stated, analysis was effectively by intention to treat |
| Selective reporting (reporting bias) | Low risk | Results of all outcome measures were reported |
| Other bias | High risk | Contamination was possible and not addressed, but would likely lead to underestimation of the reported positive result. Potential for unit of analysis error was likely not an issue as randomisation was at patient level. Baseline data was not collected or adjusted for |
Meland 1997.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Unclear Intention to treat analysis: Done Potential for unit of analysis error for some outcomes?: Yes. Not acknowledged or adjusted for |
|
| Participants |
Speciality: General practitioners Clinical setting: General practices, Norway Types of patients: Adult males with coronary heart disease risk |
|
| Interventions |
Content of intervention: The educational session aimed at encouraging and sustaining the patient's presently performed health promotion efforts, and to counsel on behaviour change after each patient had chosen their task from a menu containing options on the following lifestyle changes: cholesterol reduction; weight reduction; salt reduced diets; leisure time exercise; smoking cessation; and stress management. Providers were instructed to restrict themselves to an advisory function and to respect patient choice. They were encouraged to ask patients about what specific behaviours they would adopt in order to achieve their chosen goals and to make written contracts with their patients Patients were given self‐help material based on cognitive behaviour change principles. They were offered a stress‐coping audiotape containing general relaxation and self cognitive instructions. Duration and Timing: 1x 2 hour educational session supported by a video tape demonstration. Numbers of providers receiving intervention:11 Numbers of patients followed up in IG: 58 Review authors' scores for intensity of the patient‐centredness of the intervention: 0/10 Review authors' scores for intensity of the teaching strategies: 0/10 Control group received behaviour specific material (didactic brochures, aimed at cholesterol reduction, weight reduction, salt reduced diets, leisure time exercise, and smoking cessation). Numbers of providers in CG: 11 Numbers of patients followed up in CG: 52 |
|
| Outcomes |
Consultation process: NA Satisfaction: NA Health behaviours: Physical activity, smoking Health status: Risk factors for CHD ( blood pressure, cholesterol) Combine risk of myocardial infarction compared with a female without risk factors by medical record review |
|
| Notes | Measures used: For measure of physical activity: Type: Patient questionnaire Index: one question with 7 point scale (Blair 1985) and two questions from the Nord Trondelag health survey (Maere 1991) For smoking behaviour; and all other health status and well being outcomes except mean log infarction score: Type: various clinical measures Mean log infarction scores were based on Norwegian epidemiological data (Bjartveit 1987) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The centres were randomized to either PCSD care or CC." Didn't describe how sequence was generated or allocated |
| Allocation concealment (selection bias) | Unclear risk | Did not describe randomisation process |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not indicate whether outcome assessors were blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | attrition rate was moderate: 17/127 (13.4%); 11/69(15.9%) in intervention and 6/58 (10.3% control). "In eight cases 6 months' missing measurements were replaced by the preceding measures. Single missing values at the 12‐month visit were not replaced." Intention to treat analysis was used: "In the intention to treat analysis, patients avoiding the inclusion visit and all dropouts were analysed with their last measurement entered." |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | High risk | Contamination was not addressed Potential for unit of analysis error was neither acknowledged nor addressed Baseline data were collected on patients ‐ "two groups were comparable at start of study" but no significant testing given. Did not adjust for baseline differences |
Merckaert 2008.
| Methods |
Randomization procedure: Providers were randomly assigned after basic training, but didn't describe how sequence was generated or whether it was concealed Informed consent obtained: Yes Protection against contamination: Not used Outcomes assessors blinded: Adequate. Trained audio tape raters were blind to trained or untrained status of the physicians and of the assessment time. Not stated for patient interviews (i.e. Patient perception and satisfaction) Intention to treat analysis: Not done Potential for unit of analysis error: Yes (2005). This problem were not acknowledged explicitly, but adjusted for Comments on study quality: Randomization by provider and analysis by patient and chosen by their doctors for the rated interviews. Possible confounders ignored. Looking at the data there appears to be lower ratings in the control physicians detection of patients distress and baseline rating of the intervention group than the actual patient ratings of distress. This would seem that the intervention may have made some difference despite the measures taken for this study |
|
| Participants |
Profession: Medicine Specialty: Oncology Years experience: Physician specialists in surgical or medical oncology Intervention group: mean age: 41 (6.6), female 48%, medical practice 16.5 mean years (6.5), oncology practice 13.5 mean years (6.8), mean number of cancer patients seen per week 28 (24) Control group: mean age: 44 (7.7), female 42%, medical practice 18.2 mean years (7.3), oncology practice 15.0 (8.0) mean years, mean number of cancer patients seen per week 25 (19) Clinical setting: Cancer care treatment centres Level of Care: Secondary and Tertiary Country: Belgium Health problem/Type of patient: Cancer 2 simulated patients and 1 actual patient per provider |
|
| Interventions |
Aim of study (hypothesis): To assess the improvement in physicians communication skills resulting from participation in consolidation workshops after attending a basic training program. The hypothesis was communication skill improvements acquired during consolidation workshops would be reflected in patients perceptions of and satisfaction with their physicians performance, as recorded in actual patient interviews. (2005) Consolidation workshops would be needed to improve physicians detection of patients distress. (2008) patients and relatives distress and reduce patient and relatives anxiety. To investigate the impact of communication variables on changing patients anxiety & distress Content of intervention: All providers in both the intervention and control group attended the basic training workshop. The basic training workshop included a 2‐hour plenary on theoretical information, 2 handbooks, and 2 lectures. All providers were trained in a 2 day and 13 hour long sessions. The information included functions of PPR in cancer care, handling patient distress, role playing based on clinical problems with immediate feedback. Discussion topics included breaking bad news, coping with patients uncertainties and distress, detecting psychopathologic reactions, and interacting when patients relatives are present. The intervention group only attended 6 consolidation workshops after the basic training that included role‐plays with feedback and discussion of clinical problems brought by the participants aimed at evaluating and implementing newly acquired skills to the workplace over a 5 month time period Conceptual Focus:
Duration and timing: Basic training: 3 sessions: Two 8‐hour day sessions and one 3‐hour evening session. Consolidation workshops: Six 3‐hour evening sessions. (14 sessions, 38 hours, over 5 months.) Number of providers receiving intervention: 6447 (start) 27/56 (end) Number of patient receiving intervention: Total number of simulated patients was not indicated. For actual patients, different patients were used for each assessment period. (2008) start 71/58 end (2 different groups used) Fidelity/integrity of intervention: Interviews were video taped. Interviews that were measured were rated by trained psychologists who were tested for concordance rates and were supervised weekly to check rating accuracy. Computer program processed for inconsistencies |
|
| Outcomes |
Primary outcomes: Patient satisfaction with their physician’s communication skills (2003). (2005) Patients distress. (2008) patient and relatives distress & patient and relatives anxiety Consultation process: Assessed skills(Simulated patient): Increased open and open directive questions; Increased utterances alerting patients to reality. (Actual patient) Increase in acknowledgements; Increase in empathic statements; Increase in educated guesses; Increase in negotiations. (Patient reports): Higher scores concerning physician’s understanding of their disease (Taped interview rater/CRCWEM). (Observation): Physician’s detection of patient’s distress (2 person interview)., Physician’s detection of patient’s distress (3 person interview), Physician’s detection of relative’s distress (Differences between ratings. Physician report (VAS), patient report (HADS), relatives report (HADS), Satisfaction: Satisfaction with physician communication skills as measured by the Perception of the Interview Questionnaire by Devaux. Health behaviours: NA Health status: Change in patient anxiety (STAI‐S) |
|
| Notes |
Meta‐analysis 1) Consultation process continuous 1 pt/physician; no ICC needed 2) Health Status, Continuous 1 patient/1 physician: no ICC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Providers were randomly assigned after basic training, but didn't describe how sequence was generated or whether it was concealed |
| Allocation concealment (selection bias) | High risk | Did not indicate whether randomisation was concealed. Special selection bias ‐ patients who assessed health status were chosen by physician |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Trained audio tape raters were blind to trained or untrained status of the physicians and of the assessment time. Not stated for patient interviews (i.e. Patient perception and satisfaction) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Had attrition rates of 14/72(19.4%) of total sample; 7/30(23.3%) of control and 7/35(20%) of intervention. Did not use ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | High risk | Contamination was possible and not addressed, but likely to have led to underestimate of positive effect. Potential for unit of analysis error was not explicitly acknowledged but addressed with mixed effects modelling with control for group and time. Baseline data were measured and accounted with pre‐post comparison |
Moral 2003.
| Methods |
Randomization procedure: Randomized by training unit. Did not describe sequence generation or concealment Informed consent obtained: Not stated for standardized patients, Yes for doctors Protection against contamination: Adequate, used cluster randomisation by practice and groups were distanced geographically Outcomes assessor blinded: Adequate. Raters of GATHA‐RES were blinded to group assignment of residents Intention to treat analysis: Stated they used an intention to treat model but excluded 28 residents (11 intervention, 17 control) from the analysis Potential for unit of analysis error: Yes. Randomized by practice but analysed at the individual resident level. Did not acknowledge exclusion but adjusted for previous training and training unit using ANCOVA for repeated measures. (44.6% of tutors in intervention has prior training in interviewing compared to 26% in the control group P < 0.0001. 21% of the intervention group had experience teaching interactive clinical interviewing compared to the control having only 3% P < 0.0001) Comments on study quality: |
|
| Participants |
Profession: Medicine Specialty: Family Practice Years experience: Third Year Residents 65.5%, 75% had Ph.D. research, 19% had previous primary care experience Clinical setting: Family practice training units Level of Care: Primary Country: Spain Health problem/Type of patient: Case A: Biomedical problem with no difficult psychosocial problem. Case B: Organic problem (headache or low back pain) + psychosocial problem. Case C: Organic Problem (arthritis or abdominal pain) with fears of under diagnosed serious organic disease + intense emotion who requests referral |
|
| Interventions |
Aim of study (hypothesis): To evaluate the effectiveness of a clinical interviewing training program for third year practice trainees and to determine which factors influence residents’ training in clinical communication Content of intervention: The intervention was delivered to small groups of five to seven trainees. The course was based on student’s experience. Providers were taught data gathering, relationship building, informing, motivating, and shared decision‐making. They were given printed materials, didactic presentations, illustrative role models, had time for discussion, opportunity to practice within sessions, and encouragement to practice between sessions. No patient handouts were provided. The training could be replicated Conceptual Focus:
Duration and timing: 14 sessions with 14‐28 direct contact hours given in 2 hour sessions over the course of 6‐8 months Number of providers receiving intervention: 105 residents Number of patient receiving intervention: 6 standardized patient encounters per residents: 3 before and 3 after Fidelity/integrity of intervention: Communication skills of residents were evaluated with GATHA‐RES rating scale. (rates general data gathering, interviewer communication, and interviewing skills |
|
| Outcomes |
Consultation process: Consultation Behavior (as rated by GATHA‐RES, an instrument/rating scale designed by authors) Satisfaction: NA Health behaviours: NA Health status: NA |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used cluster randomisation, did not describe sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Did not state whether or not it was concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Raters of GATHA‐RES were blinded to experimental condition and before/after status of residents |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Had moderate attrition rate of 11/105 (10.5%) in intervention and 17/88 (19.3%) in control. "Used intention to treat after exclusion of those lost to follow up." |
| Selective reporting (reporting bias) | Low risk | Reported results on validated measure described in methods |
| Other bias | Low risk | Attempted to protect against contamination by randomising by groups that were distanced geographically. Potential for unit of analysis error was not explicitly acknowledged but used as covariates training centre and previous clinical training in ANCOVA for repeated measures. Measured and adjusted for baseline differences |
Pill 1998.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if Blind/ secure Protection against contamination: Unclear Outcome assessors blind?: Yes Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: Yes. Not acknowledged or adjusted for |
|
| Participants |
Speciality: General practitioners and nurses Clinical setting: Primary care practices, UK Types of patients: Adults with type 2 diabetes |
|
| Interventions |
Content of intervention: Training sessions comprising discussion, demonstration of the technology and often role play. Continuing contact with the practices was achieved by bimonthly newsletters, personal contacts with the research nurse, two group meetings held seven months apart and by being invited to make an audio recording of one or more clinical consultations in which the method was being used. The intervention adopted many of the principles of motivational interviewing. It aimed to encourage the provider to negotiate individual care plans that built on the patient's perceptions of their disease and their readiness to change their lifestyles. The core message was that the patient should be allowed to air their personal concerns about their condition, to select which particular topic they felt most relevant for discussion and, if appropriate, to set a specific target for themselves. A visual agenda setting chart and three other visual aids were encouraged to be used with patients (a readiness‐to‐change ruler; a diary and a balance chart to weigh up the pros and cons of a given change). Duration and timing: At least two training sessions (1.5 hour sessions) Numbers of providers receiving intervention: 15 Numbers of patients followed up in IG: 77 Review authors' score for intensity of the patient centeredness of the intervention: 5/10 Review authors' score for intensity of the teaching strategies used: 7/10 Control group were provided with the standard BDA leaflets to use with their patients Numbers of providers in CG: 14 Numbers of patients followed up in CG: 95 |
|
| Outcomes |
Consultation process: Provider use of patient‐centred communication behaviours (sharing dialogue, sharing decision making) Satisfaction: Satisfaction with recent consultations and treatment received Health behaviours: Patient attendance at practice over last 12 months; smoking and alcohol use Health status: health status and diabetes‐specific measures of well being; numbers of complications; body mass index; weight; diastolic and systolic blood pressure; glyco‐Hb readings |
|
| Notes |
Measures used:
For all consultation/practice process outcomes:
Type: Analysis of audiotapes (no reference given)
For patient attendance at practice:
Type: patient questionnaire (no reference given)
For satisfaction with treatment: SF36 For smoking and alcohol use; complications; body mass index; weight; blood pressure; and glyco‐Hb readings: Type: various clinical measures For health status and diabetes‐specific measures of well being and patient satisfaction with recent consultations and treatment received: Type: SF‐36 questionnaire and 7 new scales (specifically designed for the intervention, Hackett 1996) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Unit of randomisation was the practice... Recruitment was carried sequentially over 6 months by the GP member of the intervention team, the order of approach being determined by a random order list... After recruitment, each practice was allocated by block randomisation independently to each arm of the trial" |
| Allocation concealment (selection bias) | High risk | Not stated, but not likely, given description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All tapes were numbered, transcribed and coded blind by the evaluation team... patient data were collected in the subject's home by a psychologist who was blind to their experimental status" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate: (18/95)19% of intervention patients and 7/95 (7%) of control patients did not complete follow‐up data collection. Did not use intention to treat analysis |
| Selective reporting (reporting bias) | High risk | Multiple comparisons, reported significant levels only on some outcomes, did not adjust for multiple comparisons |
| Other bias | High risk | Possibility of contamination was not addressed, may have led to Type 2 error in important outcomes Potential for unit of analysis error was not acknowledged or addressed Baseline data were collected. Differences were accounted for with pre‐post comparisons |
Putnam 1988.
| Methods |
Study design: RCT Informed consent: Implied. Residents all agreed; Patients 480/906 agreed Allocation procedure: Unclear if blind/secure Protection against contamination: Yes Outcome assessors blind?: Yes Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: Yes, but adjustments made |
|
| Participants |
Speciality: Internal medicine residents Clinical setting: Medical walk‐in clinic, USA Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of intervention: Group sessions followed by individual sessions where the trainer reviewed audiotapes of the provider's recent encounter with a patient (focusing on the provider's listening skills and provider's explanations of the patient's illness or its treatment). In group sessions, active listening and giving thorough information about illness and treatment was stressed and techniques discussed included: using respectful silence, verbal encouragements, occasional reflections; avoidance of asking too many questions (especially closed) during first five minutes of interviews; avoidance of using evaluative words to acknowledge patient communication; the importance of spending time giving patients information; and the importance of giving information in non‐technical terms. Providers participated in an active listening exercise during this group session. Each provider was given a short manual that described and gave examples of patient exposition and provider explanation Duration and timing: One or two group sessions followed by five or six individual sessions. Total average training time = 3.7 hours, of which 2.3 hours was spent in individual sessions Numbers of providers receiving intervention: 11 Numbers of patients followed up in IG: 156 Review authors' score for intensity of the patient centeredness of the intervention: 4/10 Review authors' score for intensity of the teaching strategies: 5/10 Control group received no training Numbers of providers in CG: 8 Numbers of patients followed up in CG: 112 |
|
| Outcomes | Consultation process: Provider and patient use of included patient‐centred communication behaviours. Satisfaction: Satisfaction with encounter Health behaviours: medication, appointment adherence Health status: Symptom improvement | |
| Notes |
Measures used: For all consultation/practice process outcomes: Type: Analysis of audiotapes Index: Coded using a general‐purpose conceptually‐based taxonomy of verbal response modes (Stiles 1978) For patient satisfaction (affective and cognitive): Type: Medical Interview Satisfaction Scale (MISS) Index: 28 item with 7 point scale ranging from 'very strongly agree' to 'very strongly disagree' (Wolf 1978; Wolf 1980; Wolf 1981) For behavioural and medication adherence: Type: Structured telephone interviews For appointment adherence: Type: Outpatient appointment books checked For symptom improvement: Type: patient questionnaire Index: 3x5 point scales (Mushlin 1978) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The months of July, September, and November were arbitrarily selected for training... During the months of August, October, and December residents received no training" |
| Allocation concealment (selection bias) | High risk | Randomisation procedure described as above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Residents and patients were blinded to the hypothesis of study, but did not indicate whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates for patients was 212/480 (44%) and intention to treat analysis was not done |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | Low risk | protected against contamination: "residents in the experimental group were told not to say anything about their training, to avoid contaminating the interviews of residents in the control groups" Potential for unit of analysis error was addressed: "This was thought to be the most conservative way to measure the differences between groups, for it controlled for the effect of the uneven number of interviews per residents. Analysis of covariance was performed with initial interviewing behaviour as covariate baseline characteristics were collected; there were no differences between the groups |
Robbins 1979.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Yes Intention to treat analysis: Done Potential for unit of analysis error for some outcomes?: No |
|
| Participants |
Speciality: Internal medicine residents Clinical setting: Hospital department of Internal Medicine, USA Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of intervention: Training involved critically reviewing videotapes of consultations and discussion of the following issues:
For the remainder of the two month rotation, providers reviewed one or two of their own patient interviews per week with a trained faculty member. There were also weekly meetings during which the learned skills were practiced and defined. Upon completion of the programme, the provider should be able to perform a medical interview that effectively demonstrates the use of facilitating responses, attention to psychosocial aspects of illness, and expression of empathy. Duration and timing: 8 x 1‐2 hours training (over a two month period) Numbers of providers receiving intervention: 26 Numbers of patients followed up in IG: Not stated (no patient numbers given) Review authors' score for intensity of the patient‐centredness of the intervention: 2/10 Review authors' score for intensity of the teaching strategies: 9/10 Control group received no training Numbers of providers in CG: 25 Numbers of patients followed up in CG: Not stated (no patient numbers given) |
|
| Outcomes | Consultation process: Provider empathy score, total affective response score, sharing dialogue with patients. Satisfaction: NA Health behaviours: NA Health status: NA | |
| Notes |
Measures used: For rating the level of empathy: Type: analysis of videotapes Index: The Carkhuff rating scale was used (Truax 1967) For various provider interview behaviours: Type Analysis of videotapes Index: The Kagan rating scale was used to rate specific interview skills taught (Kagan 1975) and the Brockway scale was used to rate use of appropriate medical interviewing skills (Brockway 1978) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "subjects within each postgraduate year were randomly assigned to the experimental or control group." Did not describe how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Did not indicate whether or not randomisation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not indicate whether or not outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not give data on attrition; and did not indicate whether or not ITT analysis was performed |
| Selective reporting (reporting bias) | Low risk | Reported on all outcomes measured |
| Other bias | High risk | did not attempt to protect against contamination, would have underestimated positive effects of intervention Minimal potential for unit of analysis error as unit of analysis was same as level of randomisation No significant baseline differences |
Roter 1995.
| Methods |
Study design: RCT Informed consent obtained: Patients: yes; physicians: implied. Authors state that 16% of physicians contacted agreed to participate Allocation procedure: Unclear if blind/secure Protection against contamination: Not done Outcome assessors blind?: Yes Intention to treat analysis: Not done Potential for unit of analysis error for some outcomes?: Yes, but adjustments made |
|
| Participants |
Speciality: Primary care physicians Clinical setting: Internal medicine and family practice, USA Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of Intervention: Emotion‐Handling skills intervention group (EH) The sessions involved lectures, discussion and role play. The first two hours of the first session consisted of:
The last two hours of the first session and all of the second session consisted of small group work focusing on practice of targeted skills (with preceptor and a simulated patient). Skills were displayed on a flip chart. Homework assignment given between the two sessions. Providers given a portable tape‐recorder to tape themselves practicing skills on one or two patients. Tapes brought to second session for discussion. Emotion‐Handling Skills included:
Identical in all aspects to group 1a, except in skills trained. The sessions involved lectures, discussion and role play. The first two hours of the first session consisted of:
The last two hours of the first session and all of the second session consisted of small group work focusing on practice of targeted skills (with preceptor and a simulated patient). Skills were displayed on a flip chart. Homework assignment given between the two sessions. Providers were given a portable tape‐recorder to tape themselves practicing skills on one or two patients. Tapes brought to second session for discussion. Problem‐defining skills included:
Duration and timing: Both intervention groups attended 2 x 4 hour sessions given one week apart in the evening Numbers of providers receiving intervention: EH = 22; PD = 23 Numbers of patients followed up in IG: Not stated (311 patients overall) Review authors' score for intensity of the patient‐centredness of the intervention: EH = 3/10; PD = 8/10 Review authors' score for intensity of the teaching strategies used: EH = 6/10; PD = 6/10 Control group received no training Numbers of providers in CG: 24 Numbers of patients followed up in CG: Not stated (311 patients overall) |
|
| Outcomes |
Consultation process: Changes in patient as person score, sharing dialogue, sharing decision‐making, provider use of various patient‐centred communication skills (provider recognition of emotional problems/distress; provider management of emotional problems; clinical proficiency in identifying distress) Satisfaction: NA Health behaviours: Utilisation of health care by GHQ positive patients Health status: health status as measured by GHQ among those who were health status positive at baseline |
|
| Notes |
Measures used:
For provider use of communication skills:
Type: analysis of audiotapes
Index: a study‐specific method of coding was designed (no reference given)
For provider recognition of emotional problems/distress and provider management of emotional problems:
Type: provider self report
Index: no reference given
For clinical proficiency in identifying distress:
Type: analysis of audiotapes and consultation letters
For healthcare utilisation:
Type: telephone interview, where patients were asked about number of visits to provider
For GHQ status of patients who were GHQ positive at baseline:
Type: Patient General Health Questionnaire
Index: 28 items (Goldberg 1988) Meta‐analysis: Consultation Process, Dichotomous, not adjusted for clustering; measure at the level of provider; no ICC needed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eighty physicians were randomly assigned, but did not describe process of sequence generation and allocation |
| Allocation concealment (selection bias) | Unclear risk | Did not indicate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All GHQ‐positive patients were contacted by telephone 2 weeks, 3 months, and 6 months after their audiotaped visit by an interviewer blinded to the patient's experimental status." Audiotape coders were also blinded to the training status of participants." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | attrition rate for physicians was 19/88 (21.6%); intention to treat analysis was not done |
| Selective reporting (reporting bias) | Low risk | Reported on measured outcomes |
| Other bias | High risk | Did not attempt to protect against contamination; would have underestimated positive effects of intervention Potential for unit of analysis error was addressed with a "nested analysis of variance design in which patients were nested within physicians and physicians were nested within the three study groups Baseline characteristics were measured, no difference for physicians. "Differences in patients were noted and adjusted for with covariance analyses when related to the dependent variable." |
Smith 1998.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Yes Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes?: Yes, but adjustments made |
|
| Participants |
Speciality: Medical/family residents in postgraduate year 1 Clinical setting: Primary care outpatient clinics, USA Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of intervention: The training was experiential and skills oriented and was guided by competency‐based objectives that were both learner and teacher centred. The focus of the training was efficient data gathering, emotion handling, patient education, and the management of psychosocial and psychiatric problems in primary care settings Four interviewing models were used to enhance learning:
Non‐interviewing training objectives included helping providers develop self‐awareness of potentially harmful personal reactions During training, a brief discussion of each interviewing model (or other objective) was followed by demonstration of and repeated practice with the model through role playing Providers were given a syllabus of required readings and other materials Duration and timing: 12 x seminar sessions and 20 x supervisory sessions. Training took place in a four week full time teaching (residency) block Numbers of providers receiving intervention: 31 Numbers of patients followed up in IG: Not stated Review authors' score for intensity of the patient‐centeredness of the intervention: 2/10 Review authors' score for intensity of the teaching strategies used: 10/10 Control group received no training Numbers of providers in CG: 32 Numerus of patients followed up in CG: Not stated |
|
| Outcomes | Consultation process: Provider use of data gathering skills with actual patient; provider use of various data gathering skills with simulated patient; provider use of informing and motivating skills with simulated patient, sharing dialogue. Satisfaction: Patient satisfaction with medical interview. Health behaviours: NA Health status: Patients' physical and psychosocial well being | |
| Notes |
Measures used: For all consultation/practice process measures: Type: Analysis of audio and videotapes of consultations Measure: No reference given For patient satisfaction: Type: Patient questionnaire Index: 29 item, 5 point scale (Smith 1995a) For patients' physical and psychosocial well being: Type: General Health Questionnaire and Functional Health Questionnaire. For health status=Change in health status or not on GHQ. Index: Not stated (Goldberg 1979; Greenfield 1985) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Residents were assigned to receive training either during the first 6 months of post graduate year 1 (training groups) or later in postgraduate year 1 after they served as controls (control group)." Did not indicate how sequence was generated |
| Allocation concealment (selection bias) | High risk | "An effort to assign equal numbers of men and women to the training and control groups was limited by scheduling constraints." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Raters were blinded to group assignments and data collection points" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition in 2/65 (3.1%) residents trained refused to participate in evaluation. However, "when a measure was incomplete for a resident, that measure for that resident was omitted and the data were analysed with fewer participants." E.g., In table1, attrition rate was 8/65(12.3%) Intention to treat analysis was not done |
| Selective reporting (reporting bias) | Low risk | Reported on all measured outcomes |
| Other bias | High risk | Did not attempt to protect against contamination, would have underestimated reported positive effects of intervention Potential for unit of analysis error was addressed: "The influence of the training program was assessed by analyses of covariance; a pre‐training measure served as a covariate." Baseline characteristics were obtained, most analyses were adjusted for pre‐test scores |
Smith 2006.
| Methods |
Randomization procedure: Adequate. Statistician was blinded to the identity of participants and allocated them to intervention or control group using a computerized random number generator Informed consent obtained: Yes Protection against contamination: Contamination was likely as all providers (intervention and control) were at the same institution. No attempt was made to control for it Outcomes assessors blinded: Adequate. Interviewers were blinded Intention to treat analysis: Done and stated Potential for unit of analysis error: Was not an issue, Randomisation was at patient level and there was no data collected at provider level Comments on study quality: None |
|
| Participants |
Profession: Nursing Specialty: Nurse Practitioners (Primary care providers) Years experience: No experience in mental health as primary care physicians or case managers Clinical setting: HMO Level of Care: Primary Country: United States Health problem/Type of patient: Medically unexplained symptoms. Average age: 47.7 years. Female: 79.1%. Married: 69‐75%. < 16 years of education: 72‐81% |
|
| Interventions |
Aim of study (hypothesis): Comprehensive primary care intervention for patients with medically unexplained symptoms would lead to improved mental health 12 months after baseline Content of intervention: Nurse practitioners were taught a 5‐step PCC method to establish a positive patient provider relationship and communicate effectively and a 3‐step PCC method to inform and motivate patients about treatment was used. Training covered evidence‐based patient‐centred interviewing, cognitive behavioral treatment approaches, treatment of medically unexplained symptoms, commonly occurring psychiatric problems in primary care, diagnosis and treatment of commonly seen health issues in primary care, and in the use of antidepressant medications Conceptual Focus:
Duration and timing: 10 weeks of training in 4 hour sessions for a total of 84 hours of training Number of providers receiving intervention: 4/25 (start) 4/25 (end) Number of patient receiving intervention: 101/206 (start) 98/200 (end) Fidelity/integrity of intervention: Audiotapes were made of a sample of encounters between nurses and patients. Nurses documented each patient encounter and wrote a qualitative summary of each case |
|
| Outcomes |
Primary outcomes: An increase of 4 or more points on the mental component summary (MCS) of the Short Form‐36 Consultation process: NA Satisfaction: Satisfaction with relationship measured by Satisfaction with Patient‐Provider Relationship Questionnaire (Smith) Health behaviours: Antidepressant used to full dose, decrease in controlled substances use (Nursing documentation forms and chart reviews) Health status: Mental health as measured by the Short Form‐36 survey |
|
| Notes | Use of antidepressants to full dose was strongly associated with improved MCS scores at 12 months P = 0.12 References included in review of this article: Lyles 2003 Meta‐analysis: Healthcare Behaviors, Dichotomous. No ICC needed. Health Status, Dichotomous. No ICC needed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The statistician randomized participants, was blinded, and used a random number generator to assign groups |
| Allocation concealment (selection bias) | Low risk | Not stated, but likely as blinded statistician generated numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interviewers were blinded, although it was not possible to blind patients, NPS, or usual care physicians |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate was minimal 3/101 (3.0) in intervention and 3/105 (2.9) in control, and investigators used ITT analysis |
| Selective reporting (reporting bias) | Low risk | Used multiple scales. Only reported final model, but gave good description of how candidate variables were selected |
| Other bias | High risk | Contamination was likely and no attempt was made to control, but would likely have led to underestimation of the positive result Potential for unit of analysis was not an issue as both randomisation and analysis was at patient level. No data was collected at provider level Baseline data for patients was collected and accounted for |
Song 2005.
| Methods |
Randomization procedure: Did not describe process of randomisation Informed consent obtained: Yes Protection against contamination: Not used Outcomes assessors blinded: Not used Intention to treat analysis: Done but not stated (no lost to follow ups) Potential for unit of analysis error: No Comments on study quality: None |
|
| Participants |
Profession: Nursing Specialty: trained nurse facilitator Years experience: Unknown Clinical setting: Cardiothoracic surgery clinic Level of Care: Tertiary Country: United States Health problem/Type of patient: Cardiac surgical patients (education on advanced care planning and end of life issues). Mean age: treatment group 69.81(8.57) control group 68 (7.99). Female: treatment group 50% control group 44%. Married: treatment group 88% control 75%. All were white. Catholic: treatment group 44% control group 19%. Protestant: treatment group 50% control group 69%. Education: > High school: treatment group 94% control group 94%. Income: 11,000‐25 treatment group 44% control group 44%. >25‐50,000 treatment group 50% control group 31%. Past advanced directive completed: treatment group 56% control group 38% |
|
| Interventions |
Aim of study (hypothesis): To evaluate short term effects of patient‐centred advanced care planning compared to usual care. The hypothesis was that patient‐centred advanced care planning would improve congruence between patient and surrogate understanding of preferences, reduce anxiety, difficulty making choices, and improve knowledge of advanced care planning Content of intervention: The intervention included patients and their surrogates. The nurse facilitator led the group in a 5 stage interview based on a representational approach. The five stages were:
Conceptual focus:
Duration and timing: Unknown for the nurse facilitator, for patients and surrogates: One session 20‐45 minutes long Number of providers receiving intervention: Unknown Number of patient receiving intervention: 16/32 (start & end) Fidelity/integrity of intervention: None |
|
| Outcomes |
Primary outcomes: Congruence between patient and surrogate Consultation process: Knowledge of advanced care planning (patient/surrogate report) Satisfaction: NA Health behaviours: NA Health status: Anxiety measured by Spielberger SAI |
|
| Notes |
Meta‐analysis 1) Consultation process continuous One clinic: no ICC needed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not describe procedure for randomisation |
| Allocation concealment (selection bias) | Unclear risk | Did not provide any data on this |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not provide data on this |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported an attrition rate of 0. Outcome measures were complete in 32/32 randomized |
| Selective reporting (reporting bias) | Low risk | Reported on all measures in methods |
| Other bias | High risk | Contamination was likely, not addressed. Likely to have underestimated positive effects, but may have led to Type 2 error in one of the outcomes (anxiety). Unit of analysis error was not issue as randomized at patient level and data collected at patient level. Baseline was measured on 2 of the outcomes (anxiety and congruence) and were noted to be similar in the groups. However, did not measure baseline difficulty in making choices ‐ one of the primary outcomes |
Sorlie 2007.
| Methods |
Randomization procedure: Adequate. Physician allocation was blinded and the identity of treating physicians was concealed. Treating physicians were blinded. Randomized by patient Informed consent obtained: Yes Protection against contamination: Attempted to control. Nurses did not communicate and physicians were blinded to group assignment Outcomes assessors blinded: The data entry person was blinded. Surveys were completed by patients who were not blinded Intention to treat analysis: Done Potential for unit of analysis error: No. Randomized by patient and outcomes by patients Comments on study quality: No provider outcomes or process measures listed |
|
| Participants |
Profession: Nursing Specialty: Hospital nurses Years experience: Not described Clinical setting: Hospital Level of Care: Tertiary Country: Norway Health problem/Type of patient: CABG Surgery patients |
|
| Interventions |
Aim of study (hypothesis): To determine whether this intervention would have an effect on emotional well‐being at the time of hospital discharge and which psychological outcome variables were affected Content of intervention: A manual defining the desirable attitudes and behaviours included in the information procedure and corresponding rating form was used. To secure adherence to the manual, all information sessions were audio‐taped and every two weeks a selection of these was rated independently by the nurses and the project leader. The ratings were compared and a consensus of ratings was arrived at. The approach was discussed and a consensus as how to approach individual patients was made. The training for nurses included printed materials for professionals, didactic presentation, discussion, printed handouts for patients, and encouragement to practice skills between sessions The patient intervention consisted of a 12‐minute video for patients made for the study and was viewed by patients at home prior to admission and again during the first information session at admission. They received two 40‐minute sessions about PCC information with trained nurses. The first session was at admission and the second was at hospital discharge. The control group received 40‐minute admission and discharge information sessions. All patients were given the same pre‐op checklist, behavioral instructions, and post‐op information on prevention life style changes. These sessions did not emphasize establishing trusting relationships, provide support, or information tailored to specific patient needs as the intervention groups materials did Conceptual focus: Sharing the management of health problems with the patient. A focus in the consultation on the patient as a whole person who has individual preferences situated within social contexts. Interactional skills. Doctor patient relationship/Interviewing skills. Duration and timing: The training was spread over 3 months. No data on number of hours or sessions for the provider training Number of providers receiving intervention: Unclear Number of patient receiving intervention: 55/109 patients at start, all were followed up Fidelity/integrity of intervention: Yes. Audio‐taping of intervention delivery reviewed every 2 weeks for 3 months and a training manual was used |
|
| Outcomes |
Primary outcomes: Patient health status Consultation process: NA (Data not provided) Report that intervention nurse skills were evaluated and remediated over 3 months. Satisfaction: NA Health behaviours: NA Health status: subjective health, overall emotional well‐being (SF36) |
|
| Notes |
Meta‐analysis: 1) Health Status, Continuous No ICC needed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Didn't say how sequence was generated, but likely adequate because physician who performed allocation sequence was blind to all patient data and not involved in the treatment of participating patients |
| Allocation concealment (selection bias) | Low risk | Concealed with opaque, sealed and sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Treating physicians and data entry clerk, were blinded to assignment, but outcome assessors (patients) and their information providing nurses were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate after 2 years was 19/55 (34.5%) intervention and 20/54 (37%) control; but intention to treat analyses was done with total n at discharge (primary outcome). "All patients in the study sample were analysed at all time points ("last observation carried forward analysis")... also checked differences between the groups when the only patients for whom data at the different time points were included ("random effects models")." |
| Selective reporting (reporting bias) | Low risk | Reported results for all study outcomes measured |
| Other bias | Low risk | Attempted to control for contamination: "Trained nurses did not provide information to patients in the control group. The intervention was not discussed with the nurses that provided the control group information"; Minimal potential for unit of analysis error as both randomisation and analysis were at patient level; Quote: " Collected baseline data and indicated that "no significant differences between the groups in patient reported outcome measures at baseline" Unit of analysis was not an issue. Collected baseline data and noted there were no differences |
Stewart 2007.
| Methods |
Randomization procedure: Randomization was done by the project coordinator. Physicians were recruited in blocks by specialty category and city. After the whole block of physicians had been recruited, the physicians were allocated using a random number table Informed consent obtained: Yes Protection against contamination: Inadequate. The family physicians did not work in the same practice, the surgeons, surgical residents and oncologists did, opening the door to possible contamination Outcomes assessors blinded: Adequate. Audio‐tape coder and real patients were masked to doctor’s allocation Intention to treat analysis: Not stated as done Potential for unit of analysis error: Yes, acknowledged and adjusted. Randomized by physicians and assessed patient They adjusted for this using SAS “procedure mixed” also to increase precision. They used ANCOVA to test for differences between the two groups on communication measures controlling for baseline scores of the doctors Comments on study quality: They considered power calculations to estimate sample size and needed 51 patients per group to permit analysis for clustering of patients within one doctor. They had 51 patients per group |
|
| Participants |
Profession: Medicine Specialty: Oncologists, General Surgeons, Family physicians Years experience: Intervention Control Year of graduation, No. (%) Before 1986 13 (52) 12 (46) 1986 or after 12 (48) 14 (53) Total 25 (100) 26 (100) Clinical setting: University health centres Level of Care: Primary Country: Canada Health problem/Type of Patient: Breast Cancer Patient Characteristics were similar in the 2 groups with respect to marital status (intervention, 52% married vs control, 48%), mean age (58.4 intervention vs 59.5 control, years), mean scores on preference for information (7.6 intervention vs 6.9 control), and involvement in decisions (2.7 intervention vs 2.6 control). Differences were observed with respect to education (intervention group 54% with high school or less vs 46% in the control group) and mean number of medical conditions (1.1 intervention vs 1.4 control). |
|
| Interventions |
Aim of study (hypothesis): The intervention would change verbal communication of surgeons, oncologists, and family physicians, and would influence breast cancer patients’ perceptions of the patient‐physician interaction and their own health. (388) Breast cancer patients of the oncologists and surgeons would have higher scores on perceptions of patient‐centred communication, be more satisfied with the physician’s information‐giving and interpersonal skills, experience less psychological distress, and feel better after the visit with the doctor (388) Content of intervention:
The intervention group attended 6 hours with all of the components. The control attended 2 hours and received just the literature about the benefits of patient physician communication and video demonstrations with discussion only. They did not practice skills or receive personal feedback on their skills Conceptual Focus: 2) sharing decisions about interventions. 3) sharing the management of the health problems with the patient. 4) a focus in the consultation on the patient as a whole person who has individual preferences situated within social contexts. 5) interactional skills 6) Doctor patient relationship/Interviewing skills (5/7) Number of providers receiving intervention: start 25/51 end 25/51 Number of patient receiving intervention: 230 at baseline. Start & end 51/102 Fidelity/integrity of intervention: Each physician saw 4 cases in videotaped sessions that were rated by two well trained raters |
|
| Outcomes |
Primary Outcomes: Patient Centered Communication Measure (with sub scales) Consultation process: Observed skills from videotapes (exploration of illness experience, validation of patients’ illness experience, offering support/Sharing information, physician description and patient response, creating an experience of control/ mutual discussion of management plan, mastering the whole person experience/ exploration of whole person issues, validation of whole person issues) Satisfaction: Patient satisfaction with doctor’s information‐giving and interpersonal skills as measured by Cancer Diagnostic Interview Scale (CDIS) Health behaviours: NA Health status: Patients’ psychological distress, patient perception of well‐being |
|
| Notes |
Brown 2001 may be a portion of this larger study with just the 9 oncologists. It is not clear whether the 9 oncologists in the Brown trial were the same group Meta‐analysis: 1) Consultation process, continuous: Total score table 3 2) Satisfaction, continuous: Table 4 No ICC needed for this data from table three and table five because adjusted for clustering 3) Anti‐depressant use to full dose, dichotomous |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by project coordinator using random number table |
| Allocation concealment (selection bias) | Low risk | Did not state whether or not concealed, but likely concealed given that randomisation occurred "after the whole block of physicians had been recruited |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Trained audiotape raters and real patients were blinded, even though simulated patients and physicians were not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was almost no missing data for the primary outcome (1 control patient did not answer one of the questions on the questionnaire): "All 51 providers who were randomized completed both the intervention and doctor measures. However, patient response rate was only 44.3% (46.4% in intervention; and 42.5% in control). Intention to treat was not done |
| Selective reporting (reporting bias) | Low risk | Reported on all primary and secondary measures |
| Other bias | High risk | Contamination was likely and not controlled for. This may have led to Type 2 error in some of the reported results. Potential unit of analysis error was acknowledged and addressed both in sample size calculations and in analysis that "adjusted for clustering effects within doctors using SAS procedure mixed." Baseline data was collected authors 'controlled for preprogram communication scores," but not for other differences (education and mean number of medical conditions ‐ control group was more educated and had slightly higher number of medical conditions (1.4 vs. 1.1)) |
Thom 1999.
| Methods |
Study design: RCT Informed consent: Insufficient data Allocation procedure: Unclear if blind/secure Protection against contamination: Unclear Outcome assessors blind?: Unclear Intention to treat analysis: Unclear Potential for unit of analysis error for some outcomes: Yes, but acknowledged and adjusted for |
|
| Participants |
Speciality: Community‐ based family physicians Clinical setting: Community‐ based family practices, USA Types of patients: Adults consulting with various problems |
|
| Interventions |
Content of intervention Workshop designed to teach skills that build and maintain patient‐provider trust. It addressed:
Specific behaviours related to developing trust were targeted:
Problem‐based learning techniques were used. The workshop included brief didactic presentations, group discussion, viewing of videotaped encounters with patients, and role‐playing. Duration and timing: Seven hours (one day workshop) Numbers of providers receiving intervention:10 Numbers of patients followed up in IG: Not stated (343 patients overall) Review authors' score for intensity of the patient‐centredness of the intervention: 5/10 Review authors' score for intensity of the patient‐centredness of the teaching strategies used: 5/10 Control group received no training Numbers of providers in CG: 10 Numbers of patients followed up in CG: Not stated (343 patients overall) |
|
| Outcomes |
Consultation process: Provider's humaneness during visit, mean number of diagnostic tests, referrals Satisfaction: Patient's satisfaction with visit; patient's trust in the provider Health behaviours: Continuity with study provider; medication or advice adherence, Health status: NA |
|
| Notes | Measures used: For provider's humaneness during visit: Type:Patient questionnaire (Physician Humanistic Behaviours Questionnaire) Index:19 items (Weaver 1993) For patient satisfaction with visit: Type: patient questionnaire Index: not stated (Davis 1991) For patient trust in the provider: Type: patient questionnaire (Trust in the Physician scale) Index: not stated (Anderson 1990) For continuity with study provider and adherence to advice or prescribed medication: Type: Patient questionnaire Index: 2 questions (no reference given) For numbers of referrals made and number of diagnostic tests ordered: Type: Data from patients' charts | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Ten of the physicians were then randomized to receive the intervention." Did not describe how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Did not indicate whether or not randomisation was concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not indicate whether or not outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not provide data on attrition or missing data and did not indicate that intention to treat analysis was done |
| Selective reporting (reporting bias) | Low risk | Reported on all measured outcomes |
| Other bias | High risk | No attempt made to avoid contamination, could have led to Type 2 error Potential for unit of analysis was acknowledged and adjusted for: "The effect of the intervention on outcomes was tested using analysis of variance techniques to adjust for the non‐independence of observations from patients seen by the same physician Baseline characteristics were similar between the two groups |
Wilkinson 2008.
| Methods |
Randomization procedure: Randomization was based on a random number sequence, using a computer randomized number generator, and stratified for the 10 course locations? Random allocation was performed by the statistician before the commencement of the study and placed in a sealed envelope, which was kept securely by the administrator within the central research department. On receipt of completed tapes 1 and 2, the nurses were randomized in the order that the tapes arrived at the researcher’s office. The research coordinator contacted the administrator by telephone to find out allocation Informed consent obtained: Yes Protection against contamination: contamination was possible but not addressed Outcomes assessors blinded: The independent rater was blinded to which group tapes were from and whether it was the first, second or third taping. Intention to treat analysis: Primary analysis was on an intention to treat basis Potential for unit of analysis error: Some measures were analysed by patient and simulated patients with randomisation by provider. However, the main outcomes are provider outcomes and ANCOVA for intervention groups before scores were fitted as covariates Comments on study quality: Power was calculated with 90% power at the 5% significance level with 80 nurses per group. They had 85 & 87 in the groups. Raters kappa statistics for inter‐rater reliability were assessed for each item rated |
|
| Participants |
Profession: Nursing Specialty: Registered Nurses in cancer and palliative care Years experience: (RN at least 1 year). Mean time since qualified: Intervention 18.6 (9.4) Control 18.3 (10.6) Clinical setting: Hospice, Community nursing service, other Level of Care: Secondary Country: UK (10 geographic locations) Health problem/Type of Patient: Cancer Care both real and simulated patients |
|
| Interventions |
Aim of study (hypothesis): To evaluate the effectiveness of the 3‐day Wilkinson communication skills course in ability to change nurses’ communication skills Following a 3‐day communication skills course, nurses’ communication skills would improve compared with nurses who did not take the course. Nurses attending the course would have a greater level of confidence in communicating with patients. Patients assessed by nurses following a course would have lower levels of emotional distress, anxiety, and higher levels of satisfaction compared with those treated by nurses not attending the course Content of intervention: The course included didactic teaching or communication and evidence base for communication skills training. Discussion of positive and negative communication behaviours. Learning strategies for handling difficult situations. Discussion of the emotional impact of communication Interactive demonstration of the communication skills model. Role plays with actors to practice skills with feedback from participants and facilitators. Audio‐tapes of nurse‐patient interviews with feedback led by the facilitator. Consolidation materials were provided including CD‐ROM, handouts, reading list, and references Conceptual Focus:
Number of providers receiving intervention: start 85/172 end 84/170 Number of patient receiving intervention: 321 tapes were completed and rated, 112 real patients. Intervention: 12 missing tape #3, 3 not usable = 50 patients. Control: 62 patients (4 missing tape #3) Fidelity/integrity of intervention: Audio‐taped interviews during the training sessions |
|
| Outcomes |
Primary Outcomes: 1. Communication Skills Consultation process: Observed from audio tapes (coverage score at baseline; skills change score) Satisfaction: Patient survey with care as measured by Patient Satisfaction With Communication survey by J. Ware Health behaviours: NA Health status: health status as measured General Health Questionnaire‐12 (GHQ‐12). |
|
| Notes | Provider outcomes were also measured. Confidence with patients score 1. Baseline 2. Follow‐up 16 wks 3. Change from t1 to t2 Meta‐analysis 1) Consultation process continuous 1 patient/physician: no ICC needed 2) Satisfaction with Care, Continuous 1 patient/1 physician: no ICC 3) Health Status, Continuous 1 patient/1 physician: no ICC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was based on a random number sequence, using a computer randomized number generator |
| Allocation concealment (selection bias) | Low risk | Random allocation was performed by the statistician before the commencement of the study and placed in a sealed envelope, which was kept securely by the administrator within the central research department |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The independent rater was blinded to which group tapes were from and whether it was the first, second or third taping |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Had minimal attrition rate: 1/85 (1.2) in intervention and 1/87 (1.1 in control). Intention to treat analysis was done with n = 85 and 87. 11% 0f nurses group had missing data. Those with missing data had lower baseline score but were otherwise similar to the res. However, because they were mostly in the control group effect would be to underestimate the effect of the positive intervention |
| Selective reporting (reporting bias) | Low risk | They reported on all outcomes measured |
| Other bias | High risk | Contamination was possible, but not addressed. Would have underestimated positive effects. Potential for unit of analysis error was addressed with ANCOVA in which intervention group scores were fitted as covariates. Baseline differences in primary outcome were adjusted for with pre‐post analysis |
Wolf 2008.
| Methods |
Randomization procedure: Randomized by individual patient. Used sealed opaque sequentially numbered envelopes, a blocked procedure to assure equal entry into both groups. Participants were blinded to assignment Informed consent obtained: Yes & was approved by IRB Protection against contamination: Inadequate: The intervention and control groups worked on the same unit in the same hospital. They were assigned to separate hallways. The use of one unit was done to prevent confounding variables such as having different unit leaders, different care protocols, and ancillary personnel Outcomes assessors blinded: Study participants were not informed of their assignment and patients did the outcome measures Intention to treat analysis: Not used Potential for unit of analysis error: No. Randomized by patient, analysed patient level data Comments on study quality: Power was calculated (pos hoc) and showed an estimated sample size needed of 1,500 per group to attain power of 0.80 with P = 0.05. This study only had 58 per group |
|
| Participants |
Profession: Nursing Specialty: Bariatric nursing Years experience: Intervention group mean years experience 8.0 (7.6). Control group mean years experience 9.07 (10.03). Total number of years nurses cared for bariatric patients ranged from 1 to 4 years Clinical setting: University hospital Level of Care: Tertiary Country: United States Health problem/Type of patient: Bariatric surgery patients |
|
| Interventions |
Aim of study (hypothesis): To determine whether using PCC affects patient satisfaction, perceptions of care, and quality outcomes Content of intervention: Providers were introduced to patient‐centred communication concepts and communication skills. Skills focused on establishing mutual understanding with patients, understanding frustration and anger, understanding guilt and anxiety, communication to facilitate behavior change, motivational interviewing, and skills for exploring patients’ thoughts, expectations and feelings. They were educated about the purpose of the study, used a scripted preadmission call for making individual care plans. The training included a didactic presentation, role plays, discussion, chances to practice within sessions, encouragement to practice between sessions, and audio visual aids used in teaching. Intervention group patients were given a pre‐admission call where nurses explored the patient’s current thoughts, expectations, and feelings to establish mutual understanding that included patients concerns, expectations and learning needs into an individualized plan of care. They were asked to identify a care partner of their choice to include in their care plans. They had daily collaboration to review or alter their plan of care. The control group did not receive a pre‐admission call. Both patient groups received a post‐discharge call to review their transition home and address questions. Conceptual Focus:
Duration and timing: 2 sessions, 5 hours each (total 10 hours) time spread not listed Number of providers receiving intervention: 11/26 start and end Number of patient receiving intervention: start 129 patients total. 58/116 followed up Fidelity/integrity of intervention: Each interview guide was signed and dated by the team member interviewing the patients. A structured checklist of patient intervention was used and monitored biweekly by the research team |
|
| Outcomes |
Primary outcomes: Quality of care and satisfaction with care Consultation process: Reported skills by standardized patient (seeing patient, responding, watching over, explaining Satisfaction: Satisfaction with care as measured by Baker and Taylor Measurement Scale (BTMS) patient survey Health behaviours: NA Health status: Bariatric patients post‐op infections, falls, length of stay, complications total, renal failure, atrial fibrillation, gastric bleed, adhesions, pain consult (from post discharge chart reviews.) |
|
| Notes |
Meta‐analysis: 1) Satisfaction with Care, Continuous Patient unit of analysis, No ICC needed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Didn't indicate sequence generation, but likely adequate given that they were concealed |
| Allocation concealment (selection bias) | Low risk | "patients were randomized using sealed opaque sequentially numbered envelopes, a blocked procedure to assure equal entry into both groups." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Study participants were not informed of their assignment." Study participants assessed primary outcomes with questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate was moderate : "13/129 (10.1%) randomized patients were lost." Intention to treat analysis was not done. Quote: "Data were screened for accuracy and missing data points. All assumptions of planned analysis were met." |
| Selective reporting (reporting bias) | Low risk | All measured outcomes were reported |
| Other bias | High risk | Authors admit contamination may have led to Type 2 error: "The decision to use one unit was made to ensure that patients were managed by the same surgeon and experienced the same surgical routine. However, diffusion of the intervention could have occurred as a result of all patients (control and experimental) being admitted to the same unit, despite steps taken to minimize this potential." Minimal potential for unit of analysis error as both randomisation and analyses were at patient level. Did not collect or account for baseline data on primary outcomes |
ANCOVA: Analysis of covariance
ANOVA: analysis of variance
CES‐D: Center for Epidemiological Studies Depression (Scale)
CG: control group
CME: Continuing Medical Education
COPD: Chronic obstructive Pulmonary Disease
CSQ‐8: Client Satisfaction Questionnaire (8‐item)
CVD: Cardiovascular disease
DCS: Decisional Conflict Scale
DDP‐RQ: Difficult Doctor Patient Relationship Questionnaire
ED: Emergency Department
GATHA‐RES: An instrument to assess the clinical interviews of residents in family medicine
GP: General practitioner
HbA1c: glycated haemoglobin
ICC: intra‐cluster correlation
IG: Intervention group
ITTA: Intention to treat analysis
LTFU: lost‐to‐follow‐up
MAAS‐R: The revised Maastricht History‐Taking and Advice Checklist
MIPS ‐ Medical Interaction Process System
mo.: Month
PCC: Patient‐centred care
PHQ‐D: Physician health Questionnaire depression scale
PICS‐DF: A scale on doctor facilitation
PICS‐IS: A scale on patient information seeking
QUALID: Quality of Life in Late‐Stage Dementia
RCT: Randomized controlled trial
RR: Relative risk
SAS: Statistical Analysis Software
SDM: Shared decision‐making
SE: standard error
SF‐36: Medical Outcomes Study 36‐item Short Form
SPID: Sum of Pain Intensity Difference (scale)
SWD: Satisfaction with Decision (Scale)
VAS: Visual analogue scale
WOMAC: Western Ontario McMaster Osteoarthritis Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alroy 1984 | Intervention did not meet patient centred criteria |
| Baile 1997 | Ineligible study design. No relevant outcomes assessed |
| Baile 1999 | Intervention did not meet patient centred criteria. Ineligible study design. No relevant outcomes assessed |
| Beckman 1990 | Intervention did not meet patient centred criteria. Ineligible study design |
| Bensing 1985 | Ineligible study design |
| Berg 1983 | Intervention did not meet patient centred criteria. Ineligible study design. No relevant outcomes assessed |
| Blaasvaer 1998 | Intervention not directed at healthcare providers |
| Bohme 1998 | Patients are receiving psychotherapeutic treatment |
| Boscart 2009 | Not a randomised controlled trial |
| Breunlin 1990 | No relevant outcomes assessed |
| Calhoun 1985 | Intervention did not meet patient centred criteria. Ineligible study design |
| Caris‐Verhallen 2000 | Not focused on clinical consultation |
| Cope 1986 | Ineligible study design. Not parallel study. Controlled before and after design |
| Covinsky 1998 | Intervention not directed at healthcare providers |
| Cox 1981 | Ineligible study design. Intervention not directed at healthcare providers |
| Cummings 1989 | Intervention did not meet patient centred criteria |
| Dick 1997 | Intervention not directed at health care providers |
| Dougherty 1998 | Intervention did not meet patient centred criteria |
| Douglas 1996 | Intervention not directed at health care providers |
| Edberg 1996 | Intervention did not meet patient centred criteria |
| Eijkman 1977 | No numerical data available (contacted authors) |
| Ericson 1997 | Ineligible study design |
| Evans 1987 | Intervention did not meet patient centred criteria |
| Evans 1991 | Intervention did not meet patient centred criteria |
| Evans 1992 | Intervention did not meet patient centred criteria |
| Evans 1993 | Ineligible study design |
| Fallowfield 1998 | Intervention did not meet patient centred criteria. Ineligible study design. No relevant outcomes assessed |
| Family Heart Study | Intervention did not meet patient centred criteria |
| Farhall 1998 | Ineligible study design |
| Farsad 1978 | Intervention did not meet patient centred criteria |
| Fine 1977 | No relevant outcomes assessed |
| Finnema 2000 | Not focused on clinical consultation |
| Foley 1997 | Intervention did not meet patient centred criteria |
| Fox 1997 | Intervention not directed at health care providers. |
| Goldberg 1980 | Intervention did not meet patient centred criteria. |
| Greenberg 1999 | Intervention did not meet patient centred criteria. Ineligible study design. |
| Greenfield 1988 | Intervention not directed at health care providers. |
| Guillory‐Dunbar 1994 | Intervention did not meet patient centred criteria. Ineligible study design. No relevant outcomes assessed. |
| Haisch 1996 | Intervention not directed at health care providers. |
| Handmaker 1999 | Intervention not directed at health care providers. |
| Hebert 1992 | Follow up data to Ockene 1991. |
| Hunsdon 1984 | Ineligible study design. No relevant oucomes assessed. |
| Inui 1976 | Intervention did not meet patient centred criteria. |
| Jacob 1988 | No relevant outcomes assessed (contacted authors). |
| Johnson 1996 | Intervention did not meet patient centred criteria. |
| Kauss 1980 | Ineligible study design. |
| Kihlgren 1990 | Intervention was outside clinical consultation. |
| Kihlgren 1992 | Intervention was outside clinical consultation. |
| Kihlgren 1993 | Intervention was outside clinical consultation. |
| Kosower 1996 | Ineligible study design. |
| Kramer 1987 | Intervention did not meet patient centred criteria. |
| Ladyshewsky 1997 | Intervention did not meet patient centred criteria. Ineligible study design. |
| Landefeld 1995 | Intervention did not meet patient centred criteria. Intervention not directed at health care providers. |
| Llewellyn‐Jones 1999 | Intervention did not meet patient centred criteria. |
| Maguire 1977 | Intervention did not meet patient centred criteria. |
| Maguire 1986 | Intervention did not meet patient centred criteria. |
| Maiman 1988 | Intervention did not meet patient centred criteria. |
| Maisiak 1996 | Intervention not directed at health providers. Intervention was outwith clinical consultation. |
| Martin 1998 | Intervention not directed at health care providers. |
| Mayer 1998 | Ineligible study design. |
| McCourt 1998 | Intervention did not meet patient centred criteria. Intervention not directed at health care providers. |
| McManus 1993 | Ineligible study design. |
| Meland 1996 | Intervention did not meet patient centred criteria. |
| Miller 1993 | Not directed at health care providers (All health care providers received patient centred care intervention). |
| Morgan 1996 | Ineligible study design. |
| Myers 1991 | Ineligible study design. No relevent outcomes assessed. |
| Nathan 1991 | Ineligible study design. |
| Novack 1992 | Ineligible study design. |
| Ockene 1988 | Ineligible study design. |
| Ockene 1991 | Not directed at health care providers (All health care providers received patient centred care intervention). Authors contacted. |
| Ockene 1994 | Not directed at health care providers (All health care providers received patient centred care intervention). |
| Ockene 1995 | For outcome of interest, ineligible study design. |
| Ockene 1997 | For outcome of interest, ineligible study design. |
| Ockene 1999 | Ineligible intervention (secondary, additional health‐provider initiated component of the consultation). |
| Ockene 1999b | Ineligible intervention (secondary, additional health‐provider initiated component of the consultation). |
| Ogden 1997 | No relevant outcomes assessed. |
| Olson 1987 | Intervention did not meet patient centred criteria. Ineligible study design. |
| OXCHECK study group | Intervention not directed at health care providers. |
| Perkonigg 1995 | No relevant outcomes assessed (contacted authors). |
| Phillips 1997 | Ineligible study design. |
| Poole 1979 | Intervention did not meet patient centred criteria. |
| Quirk 1993 | Not directed at health care providers (All health care providers received patient centred care intervention). |
| Rabinowitz 1994 | Intervention did not meet patient centred criteria. Ineligible study design. No relevant outcomes assessed. |
| Razavi 1988 | Does not meet our criteria for patient centred care intervention. No relevant outcomes assessed. |
| Robins 1989 | No relevant outcomes assessed. |
| Roche 1996 | Intervention did not meet patient centred criteria. |
| Rollnick 1997 | Ineligible study design. |
| Roter 1990 | Intervention did not meet patient centred criteria. |
| Roter 1998 | Ineligible study design. Not parallel study. Controlled before and after design. |
| Saltmarche 1998 | Ineligible study design. |
| Sanson‐Fisher 1978 | Intervention did not meet patient centred criteria. |
| Scheidt 1986 | Intervention did not meet patient centred criteria. |
| Schubert 1989 | Intervention was outwith the clinical consultation. |
| Seim 1995 | Ineligible study design. |
| Sidorov 1997 | Ineligible study design. Intervention did not meet patient centred criteria. |
| Simek‐Downing 1985 | Intervention did not meet patient centred criteria. Ineligible study design. |
| Simkin‐Silverman1997 | Intervention did not meet patient centred criteria. |
| Smith 1991 | No relevant outcomes assessed. |
| Snoek 1986 | Intervention did not meet patient centred criteria. Ineligible study design. |
| Stein 1999 | Ineligible study design. |
| Steyn 1997 | Ineligible study design. |
| Stillman 1977 | Intervention did not meet patient centred criteria. |
| Szekely 1986 | Ineligible study design. Intervention did not meet patient centred criteria. |
| Ter Horst 1980 | Ineligible study design. |
| Ter Horst 1984 | Intervention did not meet patient centred criteria. No relevant outcomes assessed. |
| Teusch 1997 | Intervention did not meet patient centred criteria. Intervention not directed at health care providers. |
| Thompson 1982 | Ineligible study design. |
| Thompson 1990 | Intervention not directed at health care providers. |
| Utting 2000 | Ineligible study design. |
| Vaidya 1999 | Intervention did not meet patient centred criteria. Ineligible study design. |
| Vail 1996 | Ineligible study design. No relevant outcomes assessed. |
| Verhaak 1988 | Ineligible study design. |
| Ward 1975 | Ineligible study design. |
| Ward 1996 | Intervention did not meet patient centred criteria. |
| White 1999 | Ineligible study design. |
| Wilkinson 1998 | Ineligible study design. |
| Willetts 1997 | Ineligible study design. |
| Wist 1993 | Intervention did not meet patient centred criteria. Ineligible study design. No relevant outcomes assessed. |
Differences between protocol and review
This study excluded non‐randomized studies because of the increased availability of better quality studies. Given our decision to only include RCTs, we adapted earlier search strategies to look only for RCTs. We used accepted RCT filters developed by Consumers and Communication Group and/or Cochrane for the different databases as these filters were designed to pick up these types of studies.
In the original review, we assessed the intensity of patient‐centredness and teaching/training tactics for each intervention in the included studies using a three point scale (weak, medium, strong). In the update, the three point scale was found to be unreliable. A post‐hoc measure of intensity in number of hours was used, dichotomized as Brief Training (< 10 hours) and Extensive Training (≥ 10 hours).
In the update, the 'satisfaction' category was modified to exclude carers' satisfaction with care, as it was rarely measured, and added heterogeneity to the review's findings. The 'health behavior' category was modified to be more consistent with measures of behaviours found in studies. The previous (Lewin 2001) definition was: "Other healthcare behaviours, including types of care plans agreed; providers' provision of interventions; patients' adoption of lifestyle behaviours; and patients' use of interventions and services".
This update includes an assessment of risk of bias for each study using the Cochrane criteria for judging risk of bias.
Contributions of authors
JC conducted the literature search. FCD, MHR, CG, SL, GS, LF, AO, RCS, JC, EBL, AAS applied the inclusion criteria, assessed the quality, extracted the data for the included studies and conducted the descriptive analysis. AS, SJ, and FCD conducted the meta‐analyses. FCD drafted and revised the manuscript for this update with input from the other review authors.
Sources of support
Internal sources
-
Department of Medicine, Michigan State University, USA.
Salary support for Gelareh Sadigh, MD
External sources
-
World Health Organization, Switzerland.
Supplied funding for a research assistant, and for statistical analysis performed at Michigan State University and Norwegian Knowledge Centre for the Health Services. Funding also included travel support for Dr. Francesca Dwamena.
Declarations of interest
Simon Lewin is an editor for the Cochrane Consumers and Communication Review Group. He did not have any influence over the Review Group's editorial process or decision to publish this review.
Edited (no change to conclusions)
References
References to studies included in this review
Alamo 2002 {published data only}
- Alamo MM, Moral RR, Torres LAP. Evaluation of a patient‐centred approach in generalized musculoskeletal chronic pain/fibromyalgia patients in primary care. Patient Education and Counseling 2002;48:23‐31. [DOI] [PubMed] [Google Scholar]
- Moral RR, Alamo MM, Jurado MA, Torres LP. Effectiveness of a learner‐centred training programme for primary care physicians in using a patient‐centred consultation style. Family Practice 2001 Feb;18(1):60‐3. [DOI] [PubMed] [Google Scholar]
Alder 2007 {published data only}
- Alder J, Christen R, Zemp E, Bitzer J. Communication skills training in obstetrics and gynaecology: whom should we train? A randomised controlled trial. Archives of Gynecology and Obstetrics 2007 Dec;276(6):605‐12. [DOI] [PubMed] [Google Scholar]
Bieber 2008 {published data only}
- Bieber C, Mueller KG, Blumenstiel K, Schneider A, Richter A, Wilke S, et al. Long‐term effects of a shared decision‐making intervention on physician‐patient interaction and outcome in fibromyalgia ‐ a qualitative and quantitative 1 year follow‐up of a randomised controlled trial. Patient Education and Counseling 2006;63(3):357‐66. [DOI] [PubMed] [Google Scholar]
- Bieber C, Müller KG, Blumenstiel K, Hochlehnert A, Wilke S, Hartmann M, et al. A shared decision‐making communication training program for physicians treating fibromyalgia patients: Effects of a randomised controlled trial. Journal of Psychosomatic Research 2008;64(1):13‐20. [DOI] [PubMed] [Google Scholar]
- Bieber C, Müller KG, Blumenstiel K, Schuller‐Roma B, Richter A, Hochlehnert A, et al. Shared decision making (SDM) with chronic pain patients. The patient as a partner in the medical decision making process. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2004;47(10):985‐91. [DOI] [PubMed] [Google Scholar]
Briel 2006 {published data only}
- Briel M, Langewitz W, Tschudi P, Young J, Hugenschmidt C, Bucher HC. Communication training and antibiotic use in acute respiratory tract infections. A cluster randomised controlled trial in general practice. Swiss Medical Weekly 2006;136:241‐7. [DOI] [PubMed] [Google Scholar]
Brown 2001 {published data only}
- Brown RF, Butow P, Dunn SM, Tattersall MHN. Promoting patient participation and shortening cancer consultations: a randomised trial. British Journal of Cancer 2001;85(9):1273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chassany 2006 {published data only}
- Chassany O, Boureau F, Liard F, Bertin P, Serrie A, Ferran P, et al. Effects of training on general practitioners' management of pain in osteoarthritis: A randomised multicenter study. Journal of Rheumatology 2006;33(9):1827‐34. [PubMed] [Google Scholar]
Chenoweth 2009 {published data only}
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein‐Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person‐centred care, dementia‐care mapping, and usual care in dementia: a cluster‐randomised trial. Lancet Neurology 2009;8(4):317‐25. [DOI] [PubMed] [Google Scholar]
Clark 2000 {published data only}
- Brown R, Bratton SL, Cabana MD, Kaciroti N, Clark NM. Physician asthma education program improves outcomes for children of low‐income families. Chest 2004;126(2):369‐74. [DOI] [PubMed] [Google Scholar]
- Clark NM, Gong M, Schork MA, Evans D, Roloff D, Hurwitz M, et al. Impact of education for physicians on patient outcomes. Pediatrics 1998;101(5):831‐6. [DOI] [PubMed] [Google Scholar]
- Clark NM, Gong M, Schork MA, Evans D, Roloff D, Hurwitz M, et al. Long‐term effects of asthma education on patient satisfaction and use of health services. European Respiratory Journal 2000;16:15‐21. [DOI] [PubMed] [Google Scholar]
- Clark NM, Notherwehr F, Gong M, Evans D, Maiman LA, Hurwitz ME, et al. Physician‐patient partnership in managing chronic illness. Academic Medicine 1995;70:957‐9. [DOI] [PubMed] [Google Scholar]
Dijkstra 2006 {published data only}
- Dijkstra RF, Braspenning JCC, Huijsmans Z, Akkermans RP, Ballegooie E, Have P, et al. Introduction of diabetes passports involving both patient and professionals to improve hospital outpatient diabetes care. Diabetes Research and Clinical Practice 2005;68(2):126‐34. [DOI: 10.1016/j.diabres.2004.09.020] [DOI] [PubMed] [Google Scholar]
- Dijkstra RF, Niessen LW, Braspenning JCC, Adang E, Grol R. Patient centred and professional‐directed implementation strategies for diabetes guidelines: a cluster‐randomized trial‐based cost‐effectiveness analysis. Diabetic Medicine 2006;23(2):164‐70. [DOI] [PubMed] [Google Scholar]
Fallowfield 2002 {published data only}
- Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled trial. Lancet 2002;359(9307):650‐6. [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Jenkins V, Farewell V, Solis‐Trapala I. Enduring impact of communication skills training: Results of a 12‐month follow‐up. British Journal of Cancer 2003;89(8):1445‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield LJ, Lipkin M, Hall A. Teaching senior oncologists communication skills: results from phase 1 of a comprehensive longitudinal program in the UK. Journal of Clinical Oncology 1998;16:1961‐8. [DOI] [PubMed] [Google Scholar]
- Ford S, Hall A, Ratcliffe D, Fallowfield L. The Medical Interaction Process System (MIPS): an instrument for analysing interviews of oncologists and patients with cancer. Social Science Medicine 2000;50:553‐66. [DOI] [PubMed] [Google Scholar]
Glasgow 2004 {published data only}
- Glasgow RE, Funnell MM, Bonomi AE, Davis C, Beckham V, Wagner EH. Self‐management aspects of improving chronic illness care breakthrough series implementation with diabetes and heart failure teams. Annals of Behavioral Medicine 2002;24:80‐7. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, et al. A practical randomised trial to improve diabetes care. Journal of General Internal Medicine 2004;19(12):1167‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harmsen 2005 {published data only}
- Harmsen H, Bernsen R, Meeuwesen L, Thomas S, Dorrenboom G, Pinto D, et al. The effect of educational intervention on intercultural communication: results of a randomised controlled trial. British Journal of General Practice 2005;55(514):343‐50. [PMC free article] [PubMed] [Google Scholar]
Haskard 2008 {published data only}
- Haskard KB, Williams SL, DiMatteo MR, Rosenthal R, White MK, Goldstein MG. Physician and patient communication training in primary care: effects on participation and satisfaction. Health Psychology 2008;27(5):513‐22. [DOI] [PubMed] [Google Scholar]
Heaven 2006 {published data only}
- Heaven C, Clegg J, Maguire P. Transfer of communication skills training from workshop to workplace: the impact of clinical supervision. Patient Education and Counseling 2006;60:313‐25. [DOI] [PubMed] [Google Scholar]
Ho 2008 {published data only}
- Ho MJ, Yao G, Lee KL, Beach MC, Green AR. Cross‐cultural medical education: can patient‐centred cultural competency training be effective in non‐Western countries?. Medical Teacher 2008;30(7):719‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hobma 2006 {published data only}
- Hobma S, Ram P, Muijtjens A, Vleuten C, Grol R. Effective improvement of doctor‐patient communication: a randomised controlled trial. British Journal of General Practice 2006;56(529):580‐6. [PMC free article] [PubMed] [Google Scholar]
Howe 1996 {published data only}
- Howe A. Detecting psychological distress: Can general practitioners improve their own performance?. British Journal of General Practice 1996;46:407‐10. [PMC free article] [PubMed] [Google Scholar]
Joos 1996 {published data only}
- Joos SK, Hickam DH, Gordon GH, Baker LH. Effects of a physician communication intervention on patient care outcomes. Journal of General Internal Medicine 1996;11:147‐55. [DOI] [PubMed] [Google Scholar]
Kennedy 2004 {published data only}
- Kennedy A, Robinson A, Hann M, Thompson D, Wilkin D, North‐West Region Gastrointestinal Research Group. A cluster‐randomised controlled trial of a patient‐centred guidebook for patients with ulcerative colitis: Effect on knowledge, anxiety and quality of life. Health & Social Care in the Community 2003;11(1):64‐72. [DOI] [PubMed] [Google Scholar]
- Kennedy AP, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al. A randomised controlled trial to assess the effectiveness and cost of a patient orientated self management approach to chronic inflammatory bowel disease. Gut 2004;53(11):1639‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Kennedy A, Nelson E, Robinson A. Uncovering the limits of patient‐centeredness: Implementing a self‐management trial for chronic illness. Qualitative Health Research 2005;15(2):224‐39. [DOI] [PubMed] [Google Scholar]
Kinmonth 1998 {published data only}
- Kinmonth AL, Spiegal N, Woodcock A. Developing a training programme in patient‐centred consulting for evaluation in a randomised controlled trial; diabetes care from diagnosis in British primary care. Patient Education and Counseling 1996;29:75‐86. [DOI] [PubMed] [Google Scholar]
- Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current well being and future disease risk. BMJ 1998;317:1202‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock AJ, Kinmonth AL, Campbell MJ, Griffin SJ, Spiegal NM. Diabetes care from diagnosis: effects of training in patient‐centred care on beliefs, attitudes and behaviour of primary care professionals. Patient Education and Counseling 1999;37:65‐79. [DOI] [PubMed] [Google Scholar]
Krones 2008 {published data only}
- Krones T, Keller H, Sönnichsen A, Sadowski EM, Baum E, Wegscheider K, et al. Absolute cardiovascular disease risk and shared decision making in primary care: A randomised controlled trial. Annals of Family Medicine 2008;6(3):218‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langewitz 1998 {published data only}
- Langewitz WA, Eich P, Kiss A, Wossmer B. Improving communication skills: a randomised controlled behaviorally oriented intervention study for residents in internal medicine. Psychosomatic Medicine 1998;60:268‐76. [DOI] [PubMed] [Google Scholar]
Levinson 1993 {published data only}
- Levinson W, Roter D. The effects of two continuing medical education programs on communication skills of practicing primary care physicians. Journal of General Internal Medicine 1993;8:318‐24. [DOI] [PubMed] [Google Scholar]
Lewis 1991 {published data only}
- Lewis CC, Pantell RH, Sharp L. Increasing patient knowledge, satisfaction, and involvement: randomized trial of a communication intervention. Pediatrics 1991;88(2):351‐8. [PubMed] [Google Scholar]
Loh 2007 {published data only}
- Ende J, Kazis L, Ash A, Moskowitz MA. Measuring patients' desire for autonomy: Decision making and information‐seeking preferences among medical patients. Journal of General Internal Medicine 1989;4:23‐30. [DOI] [PubMed] [Google Scholar]
- Lerman C, Brody D, Caputo C, et al. Patients' perceived involvement in care scale: Relationship to attitudes about illness and medical care. Journal of General Internal Medicine 1990;5:29‐33. [DOI] [PubMed] [Google Scholar]
- Loh A, Kremer N, Giersdorf N, Jahn H, Hänselmann S, Bermejo I, Härter M. Information and participation interests of patients with depression in clinical decision making in primary care [Informations‐ und Partizipationsinteressen depressiver Patienten bei der medizinischen Entscheidungsfindung in der hausarztlichen Versorgung]. Zeitschrift für ärztliche Fortbildung und Qualität im Gesundheitswesen [German Journal for Quality in Healthcare] 2004;98(2):101‐7. [PubMed] [Google Scholar]
- Loh A, Simon D, Wills CE, Kriston L, Niebling W, Härter M. The effects of a shared decision‐making intervention in primary care of depression: a cluster‐randomised controlled trial. Patient Education and Counseling 2007;67(3):324‐32. [DOI] [PubMed] [Google Scholar]
Longo 2006 {published data only}
- Edwards A, Elwyn G, Hood K, Atwell C, Robling M, Houston H, et al. Patient‐based outcome results from a cluster randomised trial of shared decision making skill development and use of risk communication aids in general practice. Family Practice 2004;21(4):347‐54. [DOI] [PubMed] [Google Scholar]
- Elwyn G, Edwards A, Hood K, Robling M, Atwell C, Russell I, et al. Achieving involvement: process outcomes from a cluster randomised trial of shared decision making skill development and use of risk communication aids in general practice. Family Practice 2004;21(4):337‐46. [DOI] [PubMed] [Google Scholar]
- Longo MF, Cohen DR, Hood K, Edwards A, Robling M, Elwyn G, et al. Involving patients in primary care consultations: assessing preferences using discrete choice experiments. British Journal of General Practice 2006;56(522):35‐42. [PMC free article] [PubMed] [Google Scholar]
Margalit 2005 {published data only}
- Eshet I, Margalit APA, Amagor G. Short Family Therapy in Ambulatory Medicine (SFAT‐AM): Treatment approach in 10‐15 minute encounters. Family Practice 1993;10:178‐87. [DOI] [PubMed] [Google Scholar]
- Margalit AP, Glick SM, Benbassat J, Cohen A, Katz M. Promoting a biopsychosocial orientation in family practice: effect of two teaching programs on the knowledge and attitudes of practising primary care physicians. Medical Teacher 2005;27(7):613‐18. [DOI] [PubMed] [Google Scholar]
McLean 2004 {published data only}
- McLean M, Armstrong D. Eliciting patients' concerns: a randomised controlled trial of different approaches by the doctor. British Journal of General Practice 2004;54(506):663‐6. [PMC free article] [PubMed] [Google Scholar]
Meland 1997 {published data only}
- Meland E, Laerum E, Ulvik RJ. Effectiveness of two preventive interventions for coronary heart disease in primary care. Scandinavian Journal of Primary Health Care 1997;15(1):57‐64. [DOI] [PubMed] [Google Scholar]
Merckaert 2008 {published data only}
- Delvaux N, Razavi D, Marchal S, Bredart A, Farvacques C, Slachmuylder JL. Effects of a 105 hours psychological training program on attitudes, communication skills and occupational stress in oncology: a randomised study. British Journal of Cancer 2004;90(1):106‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard A, Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, et al. Factors that influence cancer patients' and relatives' anxiety following a three‐person medical consultation: impact of a communication skills training program for physicians. Psycho‐oncology 2008;17(5):488‐96. [DOI] [PubMed] [Google Scholar]
- Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, Etienne AM, et al. Factors influencing physicians' detection of cancer patients' and relatives' distress: can a communication skills training program improve physicians' detection? . Psycho‐oncology 2008;17(3):260‐9. [DOI] [PubMed] [Google Scholar]
- Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, Etienne AM, et al. Factors that influence physicians' detection of distress in patients with cancer ‐ Can a communication skills training program improve physicians' detection?. Cancer 2005;104(2):411‐21. [DOI] [PubMed] [Google Scholar]
- Razavi D, Merckaert I, Marchal S, Libert Y, Conradt S, Boniver J, et al. How to optimise physicians' communication skills in cancer care: Results of a randomised study assessing the usefulness of post training consolidation workshops. Journal of Clinical Oncology 2003;21(16):3141‐9. [DOI] [PubMed] [Google Scholar]
Moral 2003 {published data only}
- Moral RR, Salvador JJR, Torres LP, Castillejo JAP. Effectiveness of a clinical interviewing training program for family practice residents: a randomised controlled trial. Family Medicine 2003;35(7):489‐95. [PubMed] [Google Scholar]
Pill 1998 {published data only}
- Pill R, Stott NCH, Rollnick SR, Rees M. A randomized controlled trial of an intervention designed to improve the care given in general practice to type II diabetic patients: Patient outcomes and professional ability to change behaviour. Family Practice 1998;15(3):229‐35. [DOI] [PubMed] [Google Scholar]
- Stott NCH, Rees M, Rollnick S, Pill RM, Hackett P. Professional responses to innovation in clinical method: Diabetes care and negotiating skills. Patient Education and Counseling 1996;29:67‐73. [DOI] [PubMed] [Google Scholar]
- Stott NCH, Rollnick S, Rees MR, Pill RM. Innovation in clinical method: diabetes care and negotiating skills. Family Practice 1995;12(4):413‐8. [DOI] [PubMed] [Google Scholar]
Putnam 1988 {published data only}
- Putnam SM, Stiles WB, Jacob MC, James SA. Teaching the medical interview: an intervention study. Journal of General Internal Medicine 1988;3:38‐47. [DOI] [PubMed] [Google Scholar]
Robbins 1979 {published data only}
- Robbins AS, Kauss DR, Heinrich R, Abrass I, Dreyer J, Clyman B. Interpersonal skills training: evaluation in an internal medicine residency. Journal of Medical Education 1979;54:885‐94. [PubMed] [Google Scholar]
Roter 1995 {published data only}
- Roter DL, Hall JA, Kern DE, Barker R, Cole KA, Roca RP. Improving physicians' interviewing skills and reducing patients' emotional distress. Archives of Internal Medicine 1995;155:1877‐84. [PubMed] [Google Scholar]
Smith 1998 {published data only}
- Smith RC, Lyles JS, Mettler J, Stoffelmayr BE, Vanegeren LF, Marshall AA, et al. The effectiveness of intensive training for residents in interviewing: A randomized controlled study. Annals of Internal Medicine 1998;128(2):118‐26. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lyles JS, Mettler JA, Marshall AA, Egeren LF, Stoffelmayr BE, et al. A strategy for improving patient satisfaction by the intensive training of residents in psychosocial medicine: a controlled, randomized study. Academic Medicine 1995;70(8):729‐32. [DOI] [PubMed] [Google Scholar]
- Smith RC, Mettler JA, Stoffelmayr BE, Lyles JS, Marshall AA, Egeren LF, et al. Improving residents' confidence in using psychosocial skills. Journal of General Internal Medicine 1995;10:315‐20. [DOI] [PubMed] [Google Scholar]
Smith 2006 {published data only}
- Lyles JS, Hodges A, Collins C, Lein C, Given CW, Given B, et al. Using nurse practitioners to implement an intervention in primary care for high‐utilizing patients with medically unexplained symptoms. General Hospital Psychiatry 2003;25(2):63‐73. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lyles JS, Gardiner J, Sirbu C, Hodges A, Collins C, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomised controlled trial. Journal of General Internal Medicine 2006;21(7):671‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2005 {published data only}
- Song MK, Kirchhoff KT, Douglas J, Ward S, Hammes B. A randomised, controlled trial to improve advance care planning among patients undergoing cardiac surgery. Medical Care 2005;43(10):1049‐53. [DOI] [PubMed] [Google Scholar]
Sorlie 2007 {published data only}
- Sorlie T, Busund R, Sexton J, Sorlie D. Video information combined with individualized information sessions: effects upon emotional well being following coronary bypass surgery. A randomised trial. Patient Education and Counseling 2007;65(2):180‐8. [DOI] [PubMed] [Google Scholar]
Stewart 2007 {published data only}
- Stewart M, Brown JB, Hammerton J, Donner A, Gavin A, Holliday RL, et al. Improving communication between doctors and breast cancer patients. Annals of Family Medicine 2007;5(5):387‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thom 1999 {published data only}
- Thom DH. Training physicians to increase patient trust. Journal of Evaluation in Clinical Practice 2000;6(3):245‐53. [DOI] [PubMed] [Google Scholar]
- Thom DH, Bloch DA, Segal ES. An intervention to increase patients' trust in their physicians. Academic Medicine 1999;74(2):195‐8. [DOI] [PubMed] [Google Scholar]
Wilkinson 2008 {published data only}
- Wilkinson S, Perry R, Blanchard K, Linsell L. Effectiveness of a three‐day communication skills course in changing nurses' communication skills with cancer/palliative care patients: a randomised controlled trial. Palliatative Medicine 2008;22(4):365‐75. [DOI] [PubMed] [Google Scholar]
Wolf 2008 {published data only}
- Wolf D, Lehman L, Quinlin R, Rosenzweig M, Friede S, Xullo T, et al. Can nurses impact patient outcomes using a patient‐centred care model?. Journal of Nursing Administration 2008;38(12):532‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alroy 1984 {published data only}
- Alroy G, Ber R, Kramer D. An evaluation of the short‐term effects of an interpersonal skills course. Medical Education 1984;18:85‐9. [DOI] [PubMed] [Google Scholar]
Baile 1997 {published data only}
- Baile WF, Lenzi R, Kudelka AP, Maguire P, Novack D, Goldstein M, et al. Improving physician‐patient communication in cancer care. Journal of Cancer Education 1997;12(3):166‐73. [DOI] [PubMed] [Google Scholar]
Baile 1999 {published data only}
- Baile WF, Kudelka AP, Beale EA, Glober GA, Myers EG, Greisinger AJ, et al. Communication skills training in oncology. American Cancer Society 1999;86(5):887‐97. [PubMed] [Google Scholar]
Beckman 1990 {published data only}
- Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first‐year trainees. Journal of General Internal Medicine 1990;5:42‐5. [DOI] [PubMed] [Google Scholar]
Bensing 1985 {published data only}
- Bensing JM, Sluijs EM. Evaluation of an interview training course for general practitioners. Social Science and Medicine 1985;20(7):737‐44. [DOI] [PubMed] [Google Scholar]
Berg 1983 {published data only}
- Berg RA, Rimsza ME, Eisenberg N, Ganelin RS. Evaluation of a successful biosocial rotation. American Journal of Diseases of Children 1983;137:1066‐8. [DOI] [PubMed] [Google Scholar]
Blaasvaer 1998 {published data only}
- Blaasvaer S, Laerum E. A patient‐centred approach to health care: the effects on health‐related behaviour, consultation satisfaction and psychological well‐being [Effekter av helsepedagogisk tilnaerming pa helseatferd, konsultasjonstilfredshet og psykisk velvaere]. Tidsskr Nor Loegeforen nr 1998;118(3):370‐6. [PubMed] [Google Scholar]
Bohme 1998 {published data only}
- Bohme H, Finke J, Teusch L. Effects of inpatient client‐centred psychotherapy in various illness: 1‐year follow‐up [Effekte stationarer gesprachspsychotherapie bei verschiedenen krankheitsbildern: 1‐jahres‐katamnese]. Psychotherapie, Psychosomatic Medizinche Psychologie 1998;48(1):20‐9. [PubMed] [Google Scholar]
Boscart 2009 {published data only}
- Boscart VM. A communication intervention for nursing staff in chronic care. Journal of Advanced Nursing 2009;65(9):1823‐32. [DOI] [PubMed] [Google Scholar]
Breunlin 1990 {published data only}
- Breunlin DC, Richman JS, Lattimer A. An evaluation strategy for behavioural pediatrics training. Family Systems Medicine 1990;8(1):48‐56. [Google Scholar]
Calhoun 1985 {published data only}
- Calhoun JG, Woolliscroft JO, Beauchamp C. Changing orientations of medical students to patients' care: biomedical versus biopsychosocial models. Psychological Reports 1985;56:91‐4. [DOI] [PubMed] [Google Scholar]
Caris‐Verhallen 2000 {published data only}
- Caris‐Verhallen WMCM, Kerkstra A, Bensing JM, Grypdonck MHF. Effects of video interaction analysis training on nurse‐patient communication in the care of the elderly. Patient Education and Counseling 2000;39:91‐103. [DOI] [PubMed] [Google Scholar]
Cope 1986 {published data only}
- Cope DW, Linn LS, Leake BD, Barrett PA. Modification of residents' behavior by preceptor feedback of patient satisfaction. Journal of General Internal Medicine 1986;1:394‐8. [DOI] [PubMed] [Google Scholar]
Covinsky 1998 {published data only}
- Covinsky KE, Palmer RM, Kresevic DM, Kahana E, Counsell SR, Fortinsky RH, et al. Improving functional outcomes in older patients: Lessons from an acute care for elders unit. Joint Commission Journal on Quality Improvement 1998;24(2):64‐76. [DOI] [PubMed] [Google Scholar]
Cox 1981 {published data only}
- Cox A, Holbrook D, Rutter M. Psychiatric interviewing techniques VI. Experimental study: Eliciting feelings. British Journal of Psychiatry 1981;139:144‐52. [DOI] [PubMed] [Google Scholar]
Cummings 1989 {published data only}
- Cummings SR, Richard RJ, Duncan CL, Hansen B, Martin RV, Gerbert B, et al. Training physicians about smoking cessation: A controlled trial in private practices. Journal of General Internal Medicine 1989;4:482‐9. [DOI] [PubMed] [Google Scholar]
Dick 1997 {published data only}
- Dick J, Lombard C. Shared vision ‐ a health education project designed to enhance adherence to anti‐tuberculosis treatment. The International Journal of Tuberculosis and Lung Disease 1997;1(2):181‐6. [PubMed] [Google Scholar]
Dougherty 1998 {published data only}
- Dougherty MC, Dwyer JW, Pendergast JF, Tomlinson BU, Boyington AR, Vogel WB, et al. Community‐based nursing: continence care for older rural women. Nursing Outlook 1998;46(5):233‐44. [DOI] [PubMed] [Google Scholar]
Douglas 1996 {published data only}
- Douglas S, Daly BJ, Rudy EB, Sereika SM, Menzel L, Song R, et al. Survical experience of chronically critically ill patients. Nursing Research 1996;45(2):73‐7. [DOI] [PubMed] [Google Scholar]
Edberg 1996 {published data only}
- Edberg AK, Hallberg IR, Gustafson L. Effects of clinical supervision on nurse‐patient cooperation quality. Clinical Nursing Research 1996;5(2):127‐49. [DOI] [PubMed] [Google Scholar]
Eijkman 1977 {published data only}
- Eijkman MAJ, Karsdorp NE, Boeke B, Karsdorp‐Bimmerman EHLM. Experiences with a training course in patient counselling. Journal of Dental Education 1977;41(10):623‐5. [PubMed] [Google Scholar]
Ericson 1997 {published data only}
- Ericson D, Christersson C, Manogue M, Rohlin M. Clinical guidelines and self‐assessment in dental education. European Journal of Dental Education 1997;1:123‐8. [DOI] [PubMed] [Google Scholar]
Evans 1987 {published data only}
- Evans BJ, Kiellerup FD, Stanley RO, Burrows GD, Sweet B. A communication skills programme for increasing patients' satisfaction with general practice consultations. British Journal of Medical Psychology 1987;60:373‐8. [DOI] [PubMed] [Google Scholar]
Evans 1991 {published data only}
- Evans BJ, Stanley RO, Mestrovic R, Rose L. Effects of communication skills training on students' diagnostic efficiency. Medical Education 1991;25:517‐26. [DOI] [PubMed] [Google Scholar]
Evans 1992 {published data only}
- Evans BJ, Stanley RO, Burrows GD. Communication skills training and patients' satisfaction. Health Communication 1992;4(2):155‐70. [Google Scholar]
- Evans BJ, Stanley RO, Burrows GD, Sweet B. Lectures and skills workshops as teaching formats in a history‐taking skills course for medical students. Medical Education 1989;23:364‐70. [DOI] [PubMed] [Google Scholar]
Evans 1993 {published data only}
- Evans BJ, Sweet B, Coman GJ. Behavioural assessment of the effectiveness of a communication programme for medical students. Medical Education 1993;27:344‐50. [DOI] [PubMed] [Google Scholar]
Fallowfield 1998 {published data only}
- Fallowfield L, Lipkin M, Hall A. Teaching senior oncologists communication skills: results from phase I of a comprehensive longitudinal program in the United Kingdom. Journal of Clinical Oncology 1998;16(5):1961‐8. [DOI] [PubMed] [Google Scholar]
Family Heart Study {published data only}
- Family Heart Study Group. Randomised controlled trial evaluating cardiovascular screening and intervention in general practice: principal results of British family heart study. British Medical Journal 1994;308:313‐20. [PMC free article] [PubMed] [Google Scholar]
Farhall 1998 {published data only}
- Farhall J, Webster B, Hocking B, Leggatt M, Riess C, Young J. Training to enhance partnerships between mental health professionals and family caregivers: A comparative study. Psychiatric Services 1998;49(11):1488‐90. [DOI] [PubMed] [Google Scholar]
Farsad 1978 {published data only}
- Farsad P, Galliguez P, Chamberlin R, Roghmann KJ. Teaching interviewing skills to paediatric house officers. Pediatrics 1978;61(3):384‐8. [DOI] [PubMed] [Google Scholar]
Fine 1977 {published data only}
- Fine, VK, Therrien ME. Empathy in the doctor‐patient relationship: skill training for medical students. Journal of Medical Education 1977;52:752‐7. [DOI] [PubMed] [Google Scholar]
Finnema 2000 {published data only}
- Finnema EJ, Droes RM, Ettema TP, Ooms ME, Ader HJ, Ribbe MW, et al. The effect of emotion‐oriented care on nursing home residents with dementia and their nursing assistants: a randomised clinical trial. In: Finnema E editor(s). Emotion‐oriented care in dementia: a psychosocial approach. Amsterdam: Vrije Universiteit, 2000:135‐63. [Google Scholar]
Foley 1997 {published data only}
- Foley ME, Nespoli G, Conde E. Using standardized patients and standardized physicians to improve patient‐care quality: results of a pilot study. The Journal of Continuing Education in Nursing 1997;28(5):198‐204. [DOI] [PubMed] [Google Scholar]
Fox 1997 {published data only}
- Fox PJ, Breuer W, Wright JA. Effects of a health promotion program on sustaining health behaviours in older adults. American Journal of Preventive Medicine 1997;13(4):257‐64. [PubMed] [Google Scholar]
Goldberg 1980 {published data only}
- Goldberg DP, Steele JJ, Smith C, Spivey L. Training family doctors to recognise psychiatric illness with increased accuracy. The Lancet 1980;2(8193):521‐3. [DOI] [PubMed] [Google Scholar]
Greenberg 1999 {published data only}
- Greenberg LW, Ochsenschlagert D, O'Donnell R, Mastruserio J, Cohen GJ. Communicating bad news: a paediatric department's evaluation of a simulated intervention. Pediatrics 1999;103(6):1210‐7. [DOI] [PubMed] [Google Scholar]
Greenfield 1988 {published data only}
- Greenfield S, Kaplan SH, Ware JE, Yano EM, Frank HJL. Patients' participation in medical care: effects on blood sugar control and quality of life in diabetes. Journal of General Internal Medicine 1988;3:448‐57. [DOI] [PubMed] [Google Scholar]
Guillory‐Dunbar 1994 {published data only}
- Guillory‐Dunbar BM. Effects of staff‐patient relations training on employees and their feelings about their work. Health Care Supervisor 1994;13(1):26‐30. [PubMed] [Google Scholar]
Haisch 1996 {published data only}
- Haisch J, Braun S, Bohm BO, Stock D. Effects of patient education in type II diabetic patients after clinic admission. Results of a 3 month catamnesis after new patient‐centred education [Schulungseffekte bei typ‐II‐diabetikern nach einem klinikaufenthalt]. Psychotherapie, Psychosomatic, Medizinis Che Psychologie 1996;46(11):400‐4. [PubMed] [Google Scholar]
Handmaker 1999 {published data only}
- Handmaker NS, Miller WR, Manicke M. Findings of a pilot study of motivational interviewing with pregnant drinkers. Journal of Studies on Alcohol 1999;60(2):285‐7. [DOI] [PubMed] [Google Scholar]
Hebert 1992 {published data only}
- Hebert JR, Kristeller J, Ockene JK, Landon J, Luippold R, Goldberg RJ, et al. Patient characteristics and the effect of three physician‐delivered smoking interventions. Preventive Medicine 1992;21:557‐73. [DOI] [PubMed] [Google Scholar]
Hunsdon 1984 {published data only}
- Hunsdon S, Clarke SS. The impact of illness on patients and families: social workers teach medical students. Social Work in Health Care 1984;10(2):41‐52. [DOI] [PubMed] [Google Scholar]
Inui 1976 {published data only}
- Inui TS, Yourtee EL, Williamson JW. Improved outcomes in hypertension after physician tutorials: A controlled trial. Annals of Internal Medicine 1976;84:646‐51. [DOI] [PubMed] [Google Scholar]
Jacob 1988 {published data only}
- Jacob MR. Putting research into practice: the impact of interpersonal skills training on responses to patients' emotional concerns by nursing staff in a general hospital. The Florida Nurse 1988;36(9):18. [PubMed] [Google Scholar]
- Jacob MR. The impact of interpersonal skills training on responses to patients' emotional concerns by nursing staff in a general hospital. D Ed dissertation, University of Georgia 1987. [PubMed]
Johnson 1996 {published data only}
- Johnson JA, Kopp KC. Effectiveness of standardized patient instruction. Journal of Dental Education 1996;60(3):262‐6. [PubMed] [Google Scholar]
Kauss 1980 {published data only}
- Kauss DR, Robbins AS, Abrass I, Bakaitis RF, Anderson LA. The long‐term effectiveness of interpersonal skills training in medical schools. Journal of Medical Education 1980;55:595‐601. [DOI] [PubMed] [Google Scholar]
Kihlgren 1990 {published data only}
- Kihlgren M, Hallgren A, Norberg A, Brane G, Karlsson I. Effects of the training of integrity‐promoting care on the interaction at a long‐term ward. Scandinavian Journal of Caring Sciences 1990;4(1):21‐8. [DOI] [PubMed] [Google Scholar]
Kihlgren 1992 {published data only}
- Kihlgren M, Lindsten IG, Norberg A, Karlsson I. The content of the oral daily reports at a long‐term ward before and after staff training in integrity promoting care. Scandinavian Journal of Caring Sciences 1992;6(2):105‐12. [DOI] [PubMed] [Google Scholar]
Kihlgren 1993 {published data only}
- Kihlgren M, Kuremyr D, Norberg A, Brane G, Karlson I, Engstrom B, et al. Nurse‐patient interaction after training in integrity promoting care at a long‐term ward: analysis of video‐recorded morning care sessions. International Journal of Nursing Studies 1993;30(1):1‐13. [DOI] [PubMed] [Google Scholar]
Kosower 1996 {published data only}
- Kosower E, Inkelis S, Berman N, Seidel J. Evaluating telephone T.A.L.K. Journal of Biocommunication 1996;23(1):27‐31. [PubMed] [Google Scholar]
Kramer 1987 {published data only}
- Kramer D, Ber R, Moore M. Impact of workshop on students' and physicians' rejecting behaviours in patient interviews. Journal of Medical Education 1987;62:904‐10. [DOI] [PubMed] [Google Scholar]
Ladyshewsky 1997 {published data only}
- Ladyshewsky R, Gotjamanos E. Communication skill development in health professional education: the use of standardised patients in combination with a peer assessment strategy. Journal of Allied Health 1997;26(4):177‐86. [PubMed] [Google Scholar]
Landefeld 1995 {published data only}
- Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomised trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. NEJM 1995;332(20):1338‐44. [DOI] [PubMed] [Google Scholar]
Llewellyn‐Jones 1999 {published data only}
- Llewellyn‐Jones RH, Baikie KA, Smithers H, Cohen J, Snowdon J, Tennant CC. Multifaceted shared care intervention for late life depression in residential care: Randomised controlled trial. BMJ 1999;319:676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 1977 {published data only}
- Maguire GP, Clarke D, Jolley B. An experimental comparison of three courses in history‐taking skills for medical students. Medical Education 1977;11:175‐82. [DOI] [PubMed] [Google Scholar]
Maguire 1986 {published data only}
- Maguire P, Fairbairn S, Fletcher C. Consultation skills of young doctors: I ‐ Benefits of feedback training in interviewing as students persist. British Medical Journal 1986;292:1573‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maiman 1988 {published data only}
- Maiman LA, Becker MH, Liptak GS, Nazarian LF, Rounds KA. Improving paediatrician's compliance‐enhancing practices. American Journal of Diseases of Children 1988;142:773‐9. [DOI] [PubMed] [Google Scholar]
Maisiak 1996 {published data only}
- Maisiak R, Austin JS, West SG, Heck L. The effect of person‐centred counselling on the psychological status of persons with systemic lupus erythematosus or rheumatoid arthritis. Arthritis Care and Research 1996;9(1):60‐6. [DOI] [PubMed] [Google Scholar]
Martin 1998 {published data only}
- Martin DP, Diehr P, Conrad DA, Davis JH, Leickly R, Perrin EB. Randomized trial of a patient‐centred hospital unit. Patient Education and Counseling 1998;34:125‐33. [DOI] [PubMed] [Google Scholar]
Mayer 1998 {published data only}
- Mayer TA, Cates RJ, Mastorovich MJ, Royalty DL. Emergency department patient satisfaction: customer service training improves patient satisfaction and ratings of physician and nurse skill. Journal of Healthcare Management 1998;43(5):427‐41. [PubMed] [Google Scholar]
McCourt 1998 {published data only}
- McCourt C, Page L, Hewison J, Vail A. Evaluation of one‐to‐one midwifery: Women's responses to care. Birth 1998;25(2):73‐80. [DOI] [PubMed] [Google Scholar]
McManus 1993 {published data only}
- McManus IC, Vincent CA, Thom S, Kidd J. Teaching communication skills to clinical students. BMJ 1993;306:1322‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meland 1996 {published data only}
- Meland E, Laerum E, Maeland JG. Life style intervention in general practice: effects on psychological well‐being and patient satisfaction. Quality of Life Research 1996;5:348‐54. [DOI] [PubMed] [Google Scholar]
Miller 1993 {published data only}
- Miller WR, Benefield RG, Tonigan JS. Enhancing motivation for change in problem drinking: a controlled comparison of two therapist styles. Journal of Consulting and Clinical Psychology 1993;61(3):455‐61. [DOI] [PubMed] [Google Scholar]
Morgan 1996 {published data only}
- Morgan ER, Winter RJ. Teaching communication skills. Archives of Pediatric and Adolescent Medicine 1996;150:638‐42. [DOI] [PubMed] [Google Scholar]
Myers 1991 {published data only}
- Myers RE, Stephens SA, Boyce AA, Hermann J. Educating allied health professionals to provide care for cancer patients and their families. Journal of Health & Social Policy 1991;3(2):49‐69. [DOI] [PubMed] [Google Scholar]
Nathan 1991 {published data only}
- Nathan RG, Hohmann LK, Nusbaum HJ. Initial evaluation of a hidden agenda ‐ method of teaching the interview. Family Medicine 1991;23(4):285‐6. [PubMed] [Google Scholar]
Novack 1992 {published data only}
- Novack DH, Dube C, Goldstein MG. Teaching medical interviewing: a basic course on interviewing and the physician‐patient relationship. Archives of Internal Medicine 1992;152:1814‐20. [DOI] [PubMed] [Google Scholar]
Ockene 1988 {published data only}
- Ockene JK, Quirk ME, Goldberg RJ, Kristeller JL, Donnelly G, Kalan KL, et al. A residents' training program for the development of smoking intervention skills. Archives of Internal Medicine 1988;148:1039‐45. [PubMed] [Google Scholar]
Ockene 1991 {published data only}
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomised clinical trial. Journal of General Internal Medicine 1991;6:1‐8. [DOI] [PubMed] [Google Scholar]
Ockene 1994 {published data only}
- Ockene JK, Adams A, Pbert L, Luippold R, Herbert JR, Quirk M, et al. The physician‐delivered smoking intervention project: factors that determine how much the physician intervenes with smokers. Journal of General Internal Medicine 1994;9:379‐84. [DOI] [PubMed] [Google Scholar]
Ockene 1995 {published data only}
- Ockene JK, Ockene IS, Quirk ME, Hebert JR, Saperia GM, Luippold RS, et al. Physician training for patient‐centred nutrition counselling in a lipid intervention trial. Preventive Medicine 1995;24:563‐70. [DOI] [PubMed] [Google Scholar]
Ockene 1997 {published data only}
- Ockene JK, Wheeler EV, Adams A, Hurley TG, Hebert J. Provider training for patient‐centred alcohol counselling in a primary care setting. Archives of Internal Medicine 1997;157(20):2334‐41. [PubMed] [Google Scholar]
Ockene 1999 {published data only}
- Ockene IS, Hebert JR, Ockene JK, Merriam PA, Hurley TG, Saperia GM. Effect of training and a structured office practice on physician‐delivered nutrition counselling: the Worchester‐area trial for counselling in hyperlipidaemia (WATCH). American Journal of Preventive Medicine 1996;12(4):252‐8. [PubMed] [Google Scholar]
- Ockene IS, Hebert JR, Ockene JK, Saperia GM, Stanek Ed, Nicolosi R, et al. Effect of physician‐delivered nutrition counselling training and an office‐support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidaemic population. Archives of Internal Medicine 1999;159:725‐31. [DOI] [PubMed] [Google Scholar]
Ockene 1999b {published data only}
- Ockene JK, Adams A, Hurley TG, Wheeler EV, Hebert JR. Brief physician‐ and nurse practitioner‐delivered counselling for high‐risk drinkers. Archives of Internal Medicine 1999b;159(Oct 11):2198‐205. [DOI] [PubMed] [Google Scholar]
Ogden 1997 {published data only}
- Ogden J, Hoppe R. The relative effectiveness of two styles of educational package to change practice nurses' management of obesity. International Journal of Obesity 1997;21:963‐71. [DOI] [PubMed] [Google Scholar]
Olson 1987 {published data only}
- Olson JK, Iwasiw CL. Effects of a training model on active listening skills of post‐RN students. Journal of Nursing Education 1987;26(3):104‐7. [DOI] [PubMed] [Google Scholar]
OXCHECK study group {published data only}
- Muir J, Mant D, Jones L, Yudkin P. Effectiveness of health checks conducted by nurses in primary care: results of the OXCHECK study after one year. British Medical Journal 1994;308:308‐12. [Google Scholar]
Perkonigg 1995 {published data only}
- Perkonigg A, Wittchen HU, Winkler S. The "patient anxiety seminar" as a graduate education program ‐ effectiveness and use in general practice [Das fortbildungsprogramm "patientenseminar angst" ‐ wirksamkeit and einsatz in der praxis]. Zeitschrift fur Arztliche Fortbildung (Jena) 1995;89(4):370‐7. [PubMed] [Google Scholar]
Phillips 1997 {published data only}
- Phillips RM, Baldwin BA. Teaching psychosocial care to long‐term care nursing assistants. Journal of Continuing Education in Nursing 1997;28(3):130‐4. [DOI] [PubMed] [Google Scholar]
Poole 1979 {published data only}
- Poole AD, Sanson‐Fisher RW. Understanding the patient: a neglected aspect of medical education. Social Science & Medicine ‐ Medical Psychology & Medical Sociology 1979;13A(1):37‐43. [DOI] [PubMed] [Google Scholar]
- Poole DA, Sanson‐Fisher RW. Long‐term effects of empathy training on the interview skills of medical students. Patient Counselling and Health Education 1980;Third Quarter:125‐7. [DOI] [PubMed] [Google Scholar]
Quirk 1993 {published data only}
- Quirk ME, Godkin MA, Schwenzfeier E. Evaluation of two AIDS prevention interventions for inner‐city adolescent and young adult women. American Journal of Preventive Medicine 1993;9(1):21‐6. [PubMed] [Google Scholar]
Rabinowitz 1994 {published data only}
- Rabinowitz S, Kushnir T, Ribak J. Developing psychosocial mindedness and sensitivity to mental health issues among primary care nurses using the balint group method. Israel Journal of Psychiatry and Related Sciences 1994;31(4):280‐6. [PubMed] [Google Scholar]
Razavi 1988 {published data only}
- Razavi D, Delvaux N, Farvacques C, Robaye E. Immediate effectiveness of brief psychological training for health professionals dealing with terminally ill cancer patients: a controlled study. Social Science and Medicine 1988;27(4):369‐75. [DOI] [PubMed] [Google Scholar]
Robins 1989 {published data only}
- Robins LS, Wolf FM. The effect of training on medical students' responses to geriatric patient concerns: results of a linguistic analysis. The Gerontologist 1989;29(3):341‐4. [DOI] [PubMed] [Google Scholar]
Roche 1996 {published data only}
- Roche AM, Eccleston P, Sanson‐Fisher R. Teaching smoking cessation skills to senior medical students: a block‐randomised controlled trial of four different approaches. Preventive Medicine 1996;25:251‐8. [DOI] [PubMed] [Google Scholar]
Rollnick 1997 {published data only}
- Rollnick S, Butler CC, Stott N. Helping smokers make decisions: the enhancement of brief intervention for general medical practice. Patient Education and Counseling 1997;31:191‐203. [DOI] [PubMed] [Google Scholar]
Roter 1990 {published data only}
- Roter DL, Cole KA, Kern DE, Barker LR, Grayson M. An evaluation of residency training in interviewing skills and the psychosocial domain of medical practice. Journal of General Internal Medicine 1990;5:347‐54. [DOI] [PubMed] [Google Scholar]
Roter 1998 {published data only}
- Roter D, Rosenbaum J, Negri B, Renaud D, DiPrete‐Brown L, Hernandez O. The effects of a continuing medical education programme in interpersonal communication skills on doctor practice and patient satisfaction in Trinidad and Tobago. Medical Education 1998;32:181‐9. [DOI] [PubMed] [Google Scholar]
Saltmarche 1998 {published data only}
- Saltmarche A, Kolodny V, Mitchell GJ. An educational approach for patient‐focused care. Journal of Nursing Staff Development 1998;14(2):81‐6. [PubMed] [Google Scholar]
Sanson‐Fisher 1978 {published data only}
- Sanson‐Fisher RW, Poole AD. Training medical students to empathize: an experimental study. Medical Journal of Australia 1978;1:473‐6. [PubMed] [Google Scholar]
Scheidt 1986 {published data only}
- Scheidt PC, Lagoritz S, Ebbeling WL, Figelman AR, Moessner HF, Singer JE. Evaluation of system providing feedback to students on videotaped patient encounters. Journal of Medical Education 1986;61:585‐90. [DOI] [PubMed] [Google Scholar]
Schubert 1989 {published data only}
- Schubert DSP, Billowitz A, Gabinet L, Friedson W. Effect of liaison psychiatry on attitudes toward psychiatry, rate of consultation, and psychosocial documentation. General Hospital Psychiatry 1989;11:77‐87. [DOI] [PubMed] [Google Scholar]
Seim 1995 {published data only}
- Seim HC, Verhoye JR. Comparison of training techniques using a patient‐centred approach to smoking cessation. Medical Education 1995;29:139‐43. [DOI] [PubMed] [Google Scholar]
Sidorov 1997 {published data only}
- Sidorov J, Christianson M, Girolami S, Wydra C. A successful tobacco cessation program led by primary care nurses in a managed care setting. The American Journal of Managed Care 1997;3(3):207‐14. [PubMed] [Google Scholar]
Simek‐Downing 1985 {published data only}
- Simek‐Downing L, Quirk M. Videotape analyses of medical students' interviewing skills. Family Medicine 1985;17(2):57‐60. [PubMed] [Google Scholar]
Simkin‐Silverman1997 {published data only}
- Simkin‐Silverman LR, Wing RR. Management of obesity in primary care. Obesity Research 1997;5(6):603‐12. [DOI] [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith RC, Osborn G, Hoppe RB, Lyles JS, Egeren L, Henry R, et al. Efficacy of a one‐month training block in psychosocial medicine for residents: a controlled study. Journal of General Internal Medicine 1991;6:535‐43. [DOI] [PubMed] [Google Scholar]
Snoek 1986 {published data only}
- Snoek FJ, Verrips GHW. Learning cooperation. An evaluation of a training programme in co‐operative skills for dental students [Evaluatie van een training in samenwerkingsvaardigheden ten behoeve van doctoraalstudenten tandheelkunde]. Ned Tijdschr Tandheelkd 1986;93(3):120‐3. [PubMed] [Google Scholar]
Stein 1999 {published data only}
- Stein TS, Kwan J. Thriving in a busy practice: physician‐patient communication training. Effective Clinical Practice 1999;2(2):63‐70. [PubMed] [Google Scholar]
Steyn 1997 {published data only}
- Steyn M, Merwe N, Dick J, Borcherds R, Wilding RJC. Communication with TB patients; a neglected dimension of effective treatment?. Curationis 1997;20(1):53‐6. [PubMed] [Google Scholar]
Stillman 1977 {published data only}
- Stillman PL, Sabers DL, Redfield BM. Use of trained mothers to teach interviewing skills to first‐year medical students: A follow‐up study. Pediatrics 1977;60(2):165‐9. [PubMed] [Google Scholar]
Szekely 1986 {published data only}
- Szekely B, Botwin D, Eidelman BH, Becker M, Elman N, Schemm R. Nonpharmacological treatment of menstrual headache: relaxation‐biofeedback behavior therapy and person‐centred insight therapy. Headache 1986;26(2):86‐92. [DOI] [PubMed] [Google Scholar]
Ter Horst 1980 {published data only}
- Ter Horst G, Bekker M, Moltzer G. Communication skills training by means of videotaped interaction of dental students with their patients. Development, realisation and evaluation of a trial educational method [Training in communicatieve vaardigheden met behulp van video‐opnamen vanpatientenbehandelingen]. Ned Tijdschr Tandheelkd 1980;87(December):480‐4. [PubMed] [Google Scholar]
Ter Horst 1984 {published data only}
- Ter Horst G, Leeds JG, Hoogstraten J. Effectiveness of communication skills training for dental students. Psychological Reports 1984;55:7‐11. [DOI] [PubMed] [Google Scholar]
Teusch 1997 {published data only}
- Teusch L, Bohme H, Gastpar M. The benefit of an insight‐oriented and experiential approach on panic and agoraphobia symptoms. Psychotherapy and Psychosomatics 1997;66:293‐301. [DOI] [PubMed] [Google Scholar]
Thompson 1982 {published data only}
- Thompson TL, Stoudemire A, Mitchell WD. Effects of a psychiatric liaison program on internists' ability to assess psychosocial problems. International Journal of Psychiatry in Medicine 1982;12(2):153‐60. [DOI] [PubMed] [Google Scholar]
Thompson 1990 {published data only}
- Thompson SC, Nanni C, Schwankovsky L. Patient‐oriented interventions to improve communication in a medical office visit. Health Psychology 1990;9(4):390‐404. [DOI] [PubMed] [Google Scholar]
Utting 2000 {published data only}
- Utting MR, Campbell F, Rayner C, Whitehouse CR, Dornan TL. Consultation skills of medical students before and after changes in curriculum. Journal of the Royal Society of Medicine 2000;93:247‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaidya 1999 {published data only}
- Vaidya VU, Greenberg LW, Patel KM, Strauss LH, Pollack MM. Teaching physicians how to break bad news. Archives of Pediatric and Adolescence Medicine 1999;153:419‐22. [DOI] [PubMed] [Google Scholar]
Vail 1996 {published data only}
- Vail R, Mahon‐Salazar C, Morrison A, Kalet A. Patients as teachers: an integrated approach to teaching medical students about the ambulatory care of HIV infected patients. Patient Education and Counseling 1996;27:95‐101. [DOI] [PubMed] [Google Scholar]
Verhaak 1988 {published data only}
- Verhaak PFM. Detection of psychologic complaints by general practitioners. Medical Care 1988;26(10):1009‐20. [DOI] [PubMed] [Google Scholar]
Ward 1975 {published data only}
- Ward NG, Stein L. Reducing emotional distance: a new method to teach interviewing skills. Journal of Medical Education 1975;50:605‐14. [PubMed] [Google Scholar]
Ward 1996 {published data only}
- Ward J, Sanson‐Fisher R. Does a 3‐day workshop for family medicine trainees improve preventive care? A randomised control trial. Preventive Medicine 1996;25:741‐7. [DOI] [PubMed] [Google Scholar]
White 1999 {published data only}
- White MK, Malik T. Teaching clinician‐patient communication in the treatment of breast diseases. Journal of Women's Health 1999;8(1):39‐44. [DOI] [PubMed] [Google Scholar]
Wilkinson 1998 {published data only}
- Wilkinson S, Roberts A, Aldridge J. Nurse‐patient communication in palliative care: an evaluation of a communication skills programme. Palliative Medicine 1998;12:13‐22. [DOI] [PubMed] [Google Scholar]
Willetts 1997 {published data only}
- Willets LE, Leff J. Expressed emotion and schizophrenia: the efficacy of a staff training programme. Journal of Advanced Nursing 1997;26:1125‐33. [DOI] [PubMed] [Google Scholar]
Wist 1993 {published data only}
- Wist E. Teaching communication with cancer patients and terminally ill patients to medical students. Journal of Cancer Education 1993;8(2):119‐22. [DOI] [PubMed] [Google Scholar]
Additional references
Anderson 1990
- Anderson LA, Dedrick RF. Development of the trust in physician scale: a measure to assess interpersonal trust in patient‐physician relationships. Psychological Reports 1990;67:1091‐100. [DOI] [PubMed] [Google Scholar]
Balint 1955
- Balint M. The doctor, his patient and the illness. Lancet 1955;1:318. [DOI] [PubMed] [Google Scholar]
Balint 1956
- Balint M. The doctor, his patient and the illness. London: Pitman, 1956. [DOI] [PubMed] [Google Scholar]
Balint 1969
- Balint M, Ball DH, Hare ML. Training medical students in patient‐centered medicine. Comprehensive Psychiatry 1969;10(4):249‐58. [DOI] [PubMed] [Google Scholar]
Bjartveit 1987
- Bjartveit K. Handbook for the cardiovascular disease survey. Vol. 160, National Health Screening Service, 1987. [Google Scholar]
Blair 1985
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven‐day recall in a community survey and controlled experiments. American Journal of Epidemiology 1985;122:794‐804. [DOI] [PubMed] [Google Scholar]
Bradley 1994
- Bradley C, ed. Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Management. Chur, Switzerland: Harwood Academic, 1994. [Google Scholar]
Brockway 1978
- Brockway BS. Evaluating physician competency: what difference does it make?. Evaluation and Program Planning 1978;1:211‐20. [DOI] [PubMed] [Google Scholar]
Brown 1999
- Brown SJ. Patient‐centred communication. Annual Review of Nursing Research 1999;17:85‐104. [PubMed] [Google Scholar]
Byrne 1976
- Byrne P, Long B. Doctors Talking to Patients. London: HMSO, 1976. [Google Scholar]
Carter 1989
- Carter WB, Inui TS. How patients judge the humanistic skills of their physicians. Philadelphia: American Board of Internal Medicine Final Report on the Patient Satisfaction Questionnaire 1989.
Coulter 1998
- Coulter A, Entwistle V, Gilbert D. Informing Patients: An Assessment of the Quality of Patient Information Materials. London: Kings Fund, 1998. [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337(a1655):979‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crossley 1992
- Crossley D, Myers P, Wilkinson G. Assessment of psychological care in general practice. BMJ 1992;305:1333‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1991
- Davis AR, Ware JE. GHAA's consumer satisfaction survey. Group Health Association of America. Washington, DC 1991.
Epstein 2007
- Epstein AM, Street RL Jr. Patient‐Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. Bethesda MD: National Cancer Institute, NIH, 2007. [Google Scholar]
Epstein 2011
- Epstein RM, Street RL Jr. The values and value of patient‐centered care. Annals of Family Medicine 2011;9(2):100‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Godin 1985
- Godin G, Shephard RJ. A simple method to assess exercise behaviour in the community. Canadian Journal of Applied Sport Sciences 1985;10:141‐6. [PubMed] [Google Scholar]
Goldberg 1979
- Goldberg DP, Hillier VF. A scaled version of the General Health Questionnaire. Psychological Medicine 1979;9:139‐45. [DOI] [PubMed] [Google Scholar]
Goldberg 1988
- Goldberg D, Williams P. A user's guide to the General Health Questionnaire. Windsor: NFER Nelson, 1988. [Google Scholar]
Greenfield 1985
- Greenfield S, Kaplan S, Ware JE. Expanding patient involvement in care. Effects on patient outcomes. Annals of Internal Medicine 1985;102:520‐8. [DOI] [PubMed] [Google Scholar]
Griffin 2004
- Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, Grant J, Stewart M. Effect on health‐related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Annals of Family Medicine 2004;2(6):595‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grol 1990
- Grol R, Whitfield M, Maeseneer J, Mokkink H. Attitudes to risk taking in medical decision making among British, Dutch and Belgian general practitioners. British Journal of General Practice 1990;40(333):134‐6. [PMC free article] [PubMed] [Google Scholar]
Hackett 1996
- Hackett PMW, Pill RM, Stott NCH. Tests for diabetes treatment satisfaction tested. Diabetic Medicine 1996;13:685‐6. [DOI] [PubMed] [Google Scholar]
Henbest 1989
- Henbest RJ, Stewart MA. Patient‐centredness in the consultation: a method for measurement. Family Practice 1989;6(4):249‐53. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
IAPO 2007
- International Alliance of Patients' Organizations (IAPO). What is Patient‐Centered Healthcare? A Review of Definitions and Principles. 2nd Edition. London: IAPO, 2007. [Google Scholar]
IOM 2001
- IOM. Crossing the Quality Chasm. A New Health System for the 21st Century. Washington DC: National Academy Press, 2001. [PubMed] [Google Scholar]
Kagan 1975
- Kagan N. Influencing human interaction: eleven years with IPR. Canadian Counsellor 1975;9:74‐97. [Google Scholar]
Kiresuk 1986
- Kiresuk R, Sherman RE. Goal attainment scaling: A general method for evaluating comprehensive community mental health programs. Community Mental Health Journal 1986;44:443‐53. [DOI] [PubMed] [Google Scholar]
Laine 1996
- Laine C, Davidoff F. Patient‐centered medicine: a professional evolution. JAMA 1996;275:152‐6. [PubMed] [Google Scholar]
Lewis 1986
- Lewis C, Scott DE, Pantell RH, et al. Parent satisfaction with children's medical care: development, field test and validation of a questionnaire. Medical Care 1986;24:209‐15. [DOI] [PubMed] [Google Scholar]
Lipkin 1984
- Lipkin M Jr, Quill TE, Napodano RJ. The medical interview: a core curriculum for residencies in internal medicine. Annals of Internal Medicine 1984;100(2):277‐84. [DOI] [PubMed] [Google Scholar]
Maere 1991
- Maere A, Bjorndal A, Holmen J, Midthjell K, Kjaersgaard P. Physical activity among adults in a Norwegian county. Tidsskr Nor Laegeforen 1991;111:3695‐9. [PubMed] [Google Scholar]
Matthews 1989
- Matthews DA, Feinstein AR. A new instrument for patients' ratings of physician performance in the hospital setting. Journal of General Internal Medicine 1989;4:14‐22. [DOI] [PubMed] [Google Scholar]
Mazor 2005
- Mazor KM, Ockene JK, Rogers HJ, Carlin MM, Quirk ME. The relationship between checklist scores on a communication OSCE and analogue patients' perceptions of communication. Advances in Health Sciences Education: Theory and Practice 2005;10(1):37‐51. [DOI] [PubMed] [Google Scholar]
McWhinney 1989
- McWhinney I. The need for a transformed clinical method. In: Stewart M, Roter D editor(s). Communicating with Medical Patients. London: Sage, 1989. [Google Scholar]
Mead 2000
- Mead N, Bower P. Patient‐centredness: a conceptual framework and review of the empirical literature. Social Science and Medicine 2000;51(7):1087‐110. [DOI] [PubMed] [Google Scholar]
Moral 2001
- Moral RR, Alamo MM, Jurado MA, Torres LP. Effectiveness of a learner‐centred training programme for primary care physicians in using a patient‐centred consultation style. Family Practice 2001;18(1):60‐3. [DOI] [PubMed] [Google Scholar]
Murphy 1992
- Murphy E. Lay health concepts and response to medical advice about lifestyle modification: the case of people with a diagnosis of non insulin dependent diabetes. [Dissertation], Southhampton: University of Southampton 1992.
Ong 1995
- Ong LML, Haes JCJM, Hoos AM, Lammes FB. Doctor‐patient communication: a review of the literature. Social Science and Medicine 1995;40(7):903‐18. [DOI] [PubMed] [Google Scholar]
Rao 2007
- Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make a difference in conversations between physicians and patients: a systematic review of the evidence. Medical Care 2007;45(4):340‐9. [DOI] [PubMed] [Google Scholar]
Rifkin 1988
- Rifkin L, Wolf M, Lewis C, Pantell R. Children's perceptions of physicians and medical care: Two measures. Journal of Pediatric Psychology 1988;13:247‐54. [DOI: 10.1093/jpepsy/13.2.247] [DOI] [PubMed] [Google Scholar]
Roe 1994
- Roe L, Strong C, Whiteside C, Neil A, Mant D. Dietary interventions in primary care: validity of the DINE method for dietary assessment. Family Practice 1994;11:375‐81. [DOI] [PubMed] [Google Scholar]
Roter 1977
- Roter DL. Patient participation in the patient‐provider interaction: the effects of patient question asking on the quality of interaction, satisfaction and compliance. Health Education Monographs 1977;5:281‐315. [DOI] [PubMed] [Google Scholar]
Roter 1991
- Roter DL. The Roter Interactional Analysis System (RIAS) Coding Manual. Baltimore MD: School of Hygiene and Public Health, Johns Hopkins University, 1991. [Google Scholar]
Shojania 2005
- Shojania KG, Grimshaw JM. Evidence‐based quality improvement: the state of the science. Health Affairs 2005;24(1):138‐49. [DOI] [PubMed] [Google Scholar]
Stacey 2011
- Stacey D, Bennett CL, Barry MJ, Col NF, Eden KB, Holmes‐Rovner M, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD001431.pub3] [DOI] [PubMed] [Google Scholar]
Stewart 1981
- Stewart TJ, Pantell RH, Dias JK, Wells PA, Ross AW. Children as patients: a communications process study in family practice. Journal of Family Practice 1981;13(6):827‐35. [PubMed] [Google Scholar]
Stewart 1995a
- Stewart M. Effective physician‐patient communication and health outcomes: a review. Canadian Medical Association Journal 1995;152(9):1423‐33. [PMC free article] [PubMed] [Google Scholar]
Stewart 1995b
- Stewart M, Brown JB, Weston WW, McWhinney IR, McWilliam CL, Freeman TR. Patient‐Centred Medicine: Transforming the Clinical Method. Thousand Oaks, California: Sage, 1995. [Google Scholar]
Stiles 1978
- Stiles WB. Manual for a taxonomy of verbal response modes. Institute for Research in Social Science: University of North California at Chapel Hill, Chapel Hill, 1978. [Google Scholar]
Truax 1967
- Truax CB, Carkhuff RR. Toward Effective Counseling and Psychotherapy. Chicago: Aldine Press, 1967. [Google Scholar]
US 2012
- US Medical Licensing Examination (USMLE). 2012 Step 2 CS Content Description and information for examinees testing for USMLE. Philadelphia, PA: USMLE, 2012. [Google Scholar]
van Thiel 1991
- Thiel J, Kraan HF, Vleuten CP. Reliability and feasibility in measuring medical interviewing skills: the revised Maastricht History‐Taking and Advice Checklist (MAAS‐R). Medical Education 1991;25:224‐9. [DOI] [PubMed] [Google Scholar]
Venham 1979
- Venham LL, Gaulin‐Kremer E. A self‐report measure of situational anxiety for young children. Pediatric Dentistry 1979;2:91‐6. [PubMed] [Google Scholar]
Verghese 2011
- Verghese A, Brady E, Kapur CC, Horwitz RI. The bedside evaluation: ritual and reason. Annals of Internal Medicine 2011;155(8):550‐3. [DOI] [PubMed] [Google Scholar]
Weaver 1993
- Weaver MJ, Ow CL, Walker DJ, Degenhardt EF. A questionnaire for patients' evaluations of their physicians' humanistic behaviours. Journal of General Internal Medicine 1993;8:135‐9. [DOI] [PubMed] [Google Scholar]
Winefield 1995
- Winefield HR, Murrell TG, Clifford JV, Farmer EA. The usefulness of distinguishing different types of general practice consultation, or are needed skills always the same?. Family Practice 1995;12(4):402‐7. [DOI] [PubMed] [Google Scholar]
Wolf 1978
- Wolf MH, Putnam SM, James SA, Stiles WB. The medical interview satisfaction scale: development of a scale to measure patient perceptions of physician behaviour. Journal of Behavioural Medicine 1978;1:391‐401. [DOI] [PubMed] [Google Scholar]
Wolf 1980
- Wolf MH. Patient beliefs, personality traits, health perceptions, and response biases are predictors of medical interview satisfaction. PhD dissertation, University of North Carolina at Chapel Hill 1980.
Wolf 1981
- Wolf MH, Stiles WB. [Further development of the medical interview satisfaction scale]. Paper presented at the American Psychological Association Convention. Los Angeles, California, August 1981.
References to other published versions of this review
Lewin 2001
- Lewin SA, Skea ZC, Entwistle V, Zwarenstein M, Dick J. Interventions for providers to promote a patient‐centered approach in clinical consultations. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003267] [DOI] [PubMed] [Google Scholar]


