Abstract
Pulmonary arterial hypertension (PAH) is a rare disease that can be caused by (likely) pathogenic germline genomic variants. In addition to the most prevalent disease gene, BMPR2 (bone morphogenetic protein receptor 2), several genes, some belonging to distinct functional classes, are also now known to predispose to the development of PAH. As a consequence, specialist and non-specialist clinicians and healthcare professionals are increasingly faced with a range of questions regarding the need for, approaches to and benefits/risks of genetic testing for PAH patients and/or related family members. We provide a consensus-based approach to recommendations for genetic counselling and assessment of current best practice for disease gene testing. We provide a framework and the type of information to be provided to patients and relatives through the process of genetic counselling, and describe the presently known disease causal genes to be analysed. Benefits of including molecular genetic testing within the management protocol of patients with PAH include the identification of individuals misclassified by other diagnostic approaches, the optimisation of phenotypic characterisation for aggregation of outcome data, including in clinical trials, and importantly through cascade screening, the detection of healthy causal variant carriers, to whom regular assessment should be offered.
Short abstract
Idiopathic, anorexigen-induced, congenital heart disease-associated and heritable PAH, and pulmonary veno-occlusive disease patients should be offered genetic counselling and testing with a gene panel including all disease genes for the condition https://bit.ly/3ga3HEc
Pulmonary arterial hypertension
Pulmonary arterial hypertension (PAH) is a rare and severe disorder characterised by the obliteration of remodelled pulmonary microvessels resulting in increased pulmonary vascular resistance (PVR), progressively elevated pulmonary arterial pressure (PAP), right ventricle hypertrophy and failure as well as death if untreated [1]. PAH has been traditionally defined haemodynamically by mean PAP (mPAP) ≥25 mmHg, pulmonary arterial wedge pressure ≤15 mmHg and PVR >3 WU [2]. The cut-off of 25 mmHg exceeds the upper limit of a normal mPAP, i.e. 20 mmHg, which was suggested as a new cut-off value for diagnosing pulmonary hypertension during the Sixth World Symposium on Pulmonary Hypertension in 2018 [3] and was recently accepted as a new haemodynamic cut-off in addition to PVR >2 WU [4]. Patients with PAH present with non-specific symptoms (fatigue, dyspnoea on exertion, chest pain and (pre)syncope) that often lead to a delay in seeking medical advice by the patient and referral delay by the physician often resulting in the appropriate diagnosis at already advanced stages of the disease, leading to a worse prognosis. The mean delay between onset of symptoms and diagnosis of 2.8 years could be shortened by a greater awareness of this disease among clinicians [5]. Available vasodilator drugs targeting nitric oxide, endothelin and prostacyclin pathways fail to cure the disease. New agents are being tested in clinical trials addressing underlying molecular mechanisms to meet the urgent need for new therapeutic targets [6, 7]. This set of new drugs underscores the importance of a thorough molecular characterisation of our patients to understand if subsets of patients respond or do not respond to particular treatments.
The first description of PAH as a distinct entity was made by Ernst von Romberg, a German physician who described the pulmonary vascular disease as “pulmonary vascular sclerosis” on autopsy in 1891 [8]. Since the mid-1900s, when right heart catheterisation (RHC) became possible, idiopathic PAH (IPAH, called primary pulmonary hypertension (PPH) at that time) was recognised. PAH has an incidence between 2.5 and 7.5 cases per million per year and a prevalence ranging from 15 to 50 per million, according to the French [5] and Scottish registries [9]. In the US REVEAL registry and the US PAH Biobank, patients with PAH associated with other conditions (APAH) comprised the largest subpopulation (50.7% and 48.2%, respectively), followed closely by IPAH [10]. Connective tissue disease accounted for half of the APAH cases [11].
A possible genetic origin was described in 1954 by Dresdale et al. [12], who observed familial cases. In the 1980s, an autosomal dominant mode of inheritance was described in 14 families with PPH/PAH [13]. In the late 1990s and in 2000, the PPH1 locus was mapped to chromosome 2q31–32 [14, 15], later in 2000 to 2q33 [16], and in 2000, heterozygous pathogenic germline variants in the bone morphogenetic protein receptor type 2 (BMPR2) gene were found to be responsible for the majority of familial PAH (FPAH) cases [17, 18]. This finding opened a new era, highlighting the genetic causes of PAH, leading to the identification of several other genes associated with PAH. This process resulted in the refinement of the clinical classification of PAH, with heritable PAH (HPAH) categorised as a distinct subcategory of Group 1 (PAH) during the Fourth World Symposium on Pulmonary Hypertension in 2008 [19]. In the most recent classification of pulmonary hypertension, this distinction remains, including subtypes for IPAH (Group 1.1) and HPAH (Group 1.2) (table 1) [3, 4]. About 3% of all PAH patients are characterised as HPAH and around 40% as IPAH [5, 11].
TABLE 1.
2022 classification of pulmonary hypertension [4]
| 1 PAH |
| 1.1 Idiopathic PAH |
| 1.1.1 Non-responders at vasoreactivity testing |
| 1.1.2 Acute responders at vasoreactivity testing |
| 1.2 Heritable |
| 1.3 Associated with drugs and toxins |
| 1.4 Associated with: |
| 1.4.1 Connective tissue disease |
| 1.4.2 HIV infection |
| 1.4.3 Portal hypertension |
| 1.4.4 Congenital heart disease |
| 1.4.5 Schistosomiasis |
| 1.5 PAH with features of venous/capillary (PVOD/PCH) involvement |
| 1.6 Persistent pulmonary hypertension of the newborn syndrome |
| 2 Pulmonary hypertension associated with left heart disease |
| 2.1 Pulmonary hypertension due to heart failure |
| 2.1.1 With preserved ejection fraction |
| 2.1.2 With reduced or mildly reduced ejection fraction |
| 2.2 Valvular heart disease |
| 2.3 Congenital/acquired cardiovascular conditions leading to post-capillary pulmonary hypertension |
| 3 Pulmonary hypertension associated with lung diseases and/or hypoxia |
| 3.1 Obstructive lung disease or emphysema |
| 3.2 Restrictive lung disease |
| 3.3 Lung disease with mixed restrictive/obstructive pattern |
| 3.4 Hypoventilation syndromes |
| 3.5 Hypoxia without lung disease (e.g. high altitude) |
| 3.6 Developmental lung disorders |
| 4 Pulmonary hypertension associated with pulmonary artery obstructions |
| 4.1 Chronic thromboembolic pulmonary hypertension |
| 4.2 Other pulmonary artery obstructions |
| 5 Pulmonary hypertension with unclear and/or multifactorial mechanisms |
| 5.1 Haematological disorders |
| 5.2 Systemic disorders |
| 5.3 Metabolic disorders |
| 5.4 Chronic renal failure with or without haemodialysis |
| 5.5 Pulmonary tumour thrombotic microangiopathy |
| 5.6 Fibrosing mediastinitis |
PAH: pulmonary arterial hypertension; PVOD: pulmonary veno-occlusive disease; PCH: pulmonary capillary haemangiomatosis.
Data from several PAH registries show a consistent female predominance; on average 70–80% of patients are female, with variation according to the subgroup, e.g. up to 90% are female in connective tissue disease APAH (Group 1.4.1) [20, 21]. While variable across registries, female predominance may be less apparent or even absent in elderly patients in particular with a smoking history [22–24] and in pre-pubertal children [25]. In younger adult patients, the disease prevalence is also less biased, with twice as many females compared with males [26].
Genetics of PAH
The term HPAH includes familial cases of PAH and sporadic cases when there is an underlying (likely) pathogenic variant in a predisposing gene. In about 70–87% of FPAH and 12–20% of IPAH patients a genetic cause can be identified in the currently known PAH genes [10, 27, 28]. HPAH is most often caused by heterozygous pathogenic variants in the BMPR2 gene, encoding a member of the bone morphogenetic protein receptor family of transmembrane serine/threonine kinases (BMPR2). Pathogenic variants in this gene predispose to a narrowing of the small pulmonary arteries by driving cell proliferation and preventing apoptosis. Vascular remodelling results in increased proliferation of pulmonary arterial smooth muscle cells and fibroblasts. This can lead to the formation of neointimal lesions and complex plexiform lesions where nests of proliferating endothelial cells are also seen. More than 800 different, independent pathogenic BMPR2 variants have been identified to date [29, 30]. In addition, by 2018 a total of 17 PAH genes had been acknowledged at the Sixth World Symposium on Pulmonary Hypertension [31]. Many of these genes have been shown to belong to or to be associated with the BMPR2/transforming growth factor (TGF)-β pathway.
The main pattern of inheritance in PAH is autosomal dominant with incomplete penetrance. Thus, one inherited or newly arisen (de novo) variant can be sufficient to lead to disease development. However, not all heterozygous individuals develop PAH. For BMPR2 variants the penetrance has been estimated to be around 30%, with 42% of heterozygous women and 14% of heterozygous men developing PAH [32]. Hence, for BMPR2 the penetrance is also sexually dimorphic, with the penetrance for PAH in female BMPR2 variant carriers being at least twice that of male variant carriers [30]. Thus, the disease may “skip” a generation and manifest again in the following generation [33].
Of note, a few PAH genes can also be autosomal recessively inherited or act semidominantly. Patients with biallelic variants have been shown to present with more severe and earlier onset clinical phenotypes [27, 34, 35].
In contrast to classical PAH, the heritable form of pulmonary veno-occlusive disease (PVOD) and/or pulmonary capillary haemangiomatosis (PCH) is characterised by autosomal recessive inheritance due to biallelic pathogenic variants in the eukaryotic translation initiation factor 2α kinase 4 gene (EIF2AK4) [36–39]. While PVOD patients usually present with reduced diffusing capacity of the lung for carbon monoxide (DLCO) and abnormalities in computed tomography (CT), heritable PVOD patients are in addition characterised by an earlier age of onset than PVOD patients without biallelic EIF2AK4 variants [39, 40].
Genetic testing in PAH with and without associated conditions
Genetic testing may help clinicians to better characterise the phenotype of PAH patients and identify potentially misclassified patients, facilitating appropriate management. Considering that individuals with BMPR2 pathogenic variants, on average, develop PAH at a younger age, present with a more compromised haemodynamic profile and carry a higher risk of death or lung transplantation [41], a genetic diagnosis may have significant consequences for clinical management and therapeutic strategies. For example, more frequent monitoring may be recommended, to allow for early therapy escalation and/or starting with combination therapy. Furthermore, genetic testing can be helpful for risk stratification of family members and it may allow for reproductive options such as pre-implantation genetic testing.
PVOD/PCH can be difficult to diagnose, and is characterised by radiological abnormalities, low DLCO and poor response to PAH therapies [42]. PVOD/PCH has likely been underdiagnosed as distinguishing features may not be apparent in early stages of disease. Biallelic EIF2AK4 pathogenic variants represent at least 25% of all PVOD/PCH cases [43]. Genetic testing may identify biallelic EIF2AK4 variants in patients misclassified as having IPAH [39, 44]. As PVOD/PCH patients have a poor prognosis and can develop pulmonary oedema with PAH therapies, genetic testing could identify these misclassified patients, allowing appropriate management and early referral for lung transplantation as rapid disease progression in EIF2AK4 patients has been reported [39, 45]. PVOD/PCH has also been described more frequently in consanguineous families [36, 38].
The development of PAH in patients with hereditary haemorrhagic telangiectasia (HHT) (Osler–Weber–Rendu disease) led to the identification of other PAH-predisposing genes: activin A receptor type II-like kinase 1 (ACVRL1), endoglin (ENG) and mothers against decapentaplegic homologue 4 (SMAD4). These genes also belong to the BMP/TGF-β family. The cardinal features of HHT are mucocutaneous telangiectasias, recurrent epistaxis, macroscopic arteriovenous malformations and, in rare cases, PAH. HHT is autosomal dominantly transmitted with onset around puberty and essentially complete penetrance of HHT by the age of 60 years, although there is a much lower penetrance for PAH. Only very few HHT predisposing variant carriers with PAH older than 60 years have been described without any signs for HHT [10]. In contrast, PAH associated with an ACVRL1 mutation is characterised by a young median age of onset of 20 years and thus PAH may be the first obvious sign of subsequently developing HHT in these young patients [46]. Indeed, genetic testing can identify these patients and facilitate the recognition of HHT complications (arteriovenous malformations) in the patients and their relatives.
Several predisposing PAH genes are also associated with lung development abnormalities in children and adults, such as T-box protein 4 (TBX4). This gene has been previously associated with small patella syndrome (OMIM: 601719) with or without PAH, and is inherited in an autosomal dominant fashion with incomplete penetrance and variable expressivity [47, 48]. An enrichment of pathogenic TBX4 variants has been observed in paediatric PAH [49, 50]. Genetic diagnosis can assist in assessment of associated hip and knee issues. Similarly, protein-truncating variants in the gene encoding vascular endothelial growth factor receptor 2 (KDR) were identified in patients with interstitial lung disease in addition to PAH [48, 50–52]. In these conditions, PAH may be associated with low DLCO (KDR) or bronchial abnormalities (TBX4). Genetic testing may help to differentiate these patients from Group 3 pulmonary hypertension due to chronic lung diseases in particular if low DLCO is present together with a past smoking history [24, 53].
In patients with congenital heart disease APAH (Group 1.4.4), particularly in children with this condition, pathogenic variants in the transcription factor SRY-box transcription factor 17 (SOX17) have been identified in 3–7% of patients [54–56]. The majority of these patients presented with simple heart defects such as arterial septal defects, patent ductus arteriosus, patent foramen ovale and ventricular septal defects [54, 56]. In addition, chest CT abnormalities such as dilated, tortuous pulmonary vessels and ground-glass opacities and haemoptysis were described in a subset of patients [56]. While pathogenic SOX17 variants are more common in congenital heart disease APAH they have also been described in FPAH and IPAH (table 2) [10, 27, 28, 56]. The same SOX17 variant was even identified in PAH patients from the same family with and without associated congenital heart disease [57]. Similarly, pathogenic variants in congenital heart disease APAH patients were not only identified in SOX17 but also in other PAH genes such as TBX4 and BMPR2 [10].
TABLE 2.
Number of reported disease-causing (pathogenic or likely pathogenic) variants across genes and pulmonary hypertension groups
| Gene | Group 1 PAH | Group 2 | Group 3 | Group 4 | Group 5 | ||||||||||
| IPAH | HPAH | DPAH | CTD APAH | HIV APAH | POPH | CHD APAH | Sch. APAH |
PVOD/
PCH |
PPHN | CTEPH | Other | ||||
| ACVRL1 | 20–25 | 5–10 | <5 | <5 | <5 | ||||||||||
| ATP13A3 | 20–25 | <5 | <5 | <5 | <5 | ||||||||||
| BMPR2 | >650 | >350 | <5 | <5 | <5 | 10–15 | <5 | <5 | <5 | ||||||
| CAV1 | 5–10 | 5–10 | <5 | <5 | <5 | ||||||||||
| EIF2AK4 | 10–15 | 5–10 | <5 | 40–50 | |||||||||||
| ENG | 5–10 | <5 | <5 | <5 | <5 | ||||||||||
| GDF2 | 50–60 | <5 | <5 | <5 | |||||||||||
| KCNK3 | 15–20 | 5–10 | <5 | ||||||||||||
| KDR | 5–10 | <5 | <5 | ||||||||||||
| SMAD9 | 15–20 | <5 | <5 | <5 | 5–10 | ||||||||||
| SOX17 | 30–40 | <5 | <5 | <5 | 10–15 | ||||||||||
| TBX4 | 70–80 | 5–10 | <5 | <5 | 10–15 | 10–15 | |||||||||
PAH: pulmonary arterial hypertension; I/H/D: idiopathic/heritable/associated with drugs and toxins; APAH: associated PAH; CTD: connective tissue disease; POPH: portopulmonary hypertension; CHD: congenital heart disease; Sch.: schistosomiasis; PVOD: pulmonary veno-occlusive disease; PCH: pulmonary capillary haemangiomatosis; PPHN: persistent pulmonary hypertension of the newborn; CTEPH: chronic thromboembolic pulmonary hypertension. Data compiled from [10, 27, 30, 50–52, 54, 56, 57, 80, 81, 98–106].
Little evidence is available for patients with PAH associated with drugs and toxins (Group 1.3). Current evidence shows that only patients with previous anorexigen intake may harbour pathogenic variants and should, therefore, undergo genetic counselling and testing [58, 59]. For other specific drug or toxin exposures only few cases with pathogenic variants were reported [60, 61]. In most other patients no predisposing genetic variant was identified in addition to the drug or toxin exposure. For example, in a subset of more than 20 patients who ingested colza oil and subsequently developed PAH, no pathogenic variant could be identified (J.A. Tenorio-Castaño, personal communication). Similarly, in a subset of 13 PVOD patients with previous intermediate or high trichlorethylene exposure, no pathogenic variant could be identified, whereas five out of 12 PVOD patients with absent or trivial exposure to the same substance were carriers of biallelic EIF2AK4 variants [62].
Our recommendation is that genetic testing should be performed at least in patients with a family history of PAH, patients with IPAH, patients with anorexigen-induced PAH and patients with congenital heart disease APAH. Patients with suspected or confirmed PVOD/PCH and other developmental lung disorders should also be offered genetic counselling and testing. Cascade genetic testing should be offered to relatives of index cases with PAH with identified pathogenic/likely pathogenic variants according to the guidelines of the American College of Medical Genetics and Genomics (ACMG) [63].
Currently there is insufficient evidence to recommend genetic testing for pulmonary hypertension patients in Groups 2–5 (table 2). Only if there is familial aggregation of pulmonary hypertension or any of the aforementioned differential diagnoses is being considered should genetic testing be offered.
Genetic testing and clinical screening for relatives of PAH patients
Regular screening and follow-up of at-risk family members of HPAH patients is particularly important to diagnose HPAH at an early stage, initiate early treatment and hopefully improve prognosis. Asymptomatic individuals at genetic risk should have regular clinical assessments. Screening assessments intervals may vary between 6 months to 3 years, based on gene, assessment results, trajectory, gender and family history. As soon as any symptoms such as breathlessness appear, a full clinical work-up should be immediately conducted. Therefore, family members should be informed to be alert for these symptoms.
Screening should ideally be performed annually and include medical history, New York Heart Association/World Health Organization dyspnoea class, physical examination, ECG, pulmonary function testing (PFT), N-terminal pro-brain natriuretic peptide (NT-proBNP)/BNP and echocardiography [2]. In addition, echocardiography during exercise and cardiopulmonary exercise testing (CPET) can provide valuable additional information on PAP increase during exercise [15]. Subtle clinical changes may only be obvious during exercise in an otherwise healthy individual [4]. An increase of systolic PAP >40 mmHg during mild exercise has been demonstrated in 30% of family members of HPAH patients and in only 10% of controls [64]. This hypertensive pulmonary response could indicate early vascular changes as only family members with this response and a pathogenic BMPR2 variant subsequently developed manifest PAH [65]. Screening intervals of asymptomatic variant carriers with a hypertensive exercise response may therefore be even shorter than for individuals without a hypertensive exercise response.
An absolute minimum screening should include NT-proBNP/BNP levels, which may be checked by the local general practitioner, and anamnesis via phone by the treating pulmonary hypertension expert, albeit at shorter intervals such as every 6 months. While this more frequent minimum screening is less burdensome and costly for the family member, it cannot substitute for a full clinical screening with the full set of the aforementioned assessments, which should then be conducted at less frequent time intervals.
Regular screening can help to identify family members at risk who can be offered diagnostic RHC [66]. Therefore, informing the patient of the familial implications and providing genetic counselling to interested family members is a medico-legal responsibility once a (likely) pathogenic variant has been identified in a PAH proband. A starting point is the family history and pedigree. With consent of the PAH patient, first-degree family members should be offered genetic counselling and testing. During genetic counselling disadvantages of knowing about a potential genetic predisposition also have to be addressed (see the later section on genetic counselling). By identifying those variant carriers with PAH in a given family further insight may be gained regarding possible second hits of a genetic, epigenetic or environmental nature, providing clues about disease penetrance [67].
A recent study (DELPHI-2) performed annual screening in asymptomatic BMPR2 mutation carriers including clinical assessment, ECG, PFT, DLCO measurement, 6-min walk test, CPET, chest radiography, echocardiography and NT-proBNP level [68]. In addition, an optional RHC at rest and exercise was performed at baseline. 55 subjects (median age 37 years) were included. At baseline, no PAH was suspected based on echocardiography and NT-proBNP levels. All subjects accepted the optional RHC at inclusion, which identified two mild PAH cases (3.6%) and 12 subjects with exercise pulmonary hypertension (21.8%). At long-term follow-up (>5 years), three additional cases were diagnosed who were still in functional class I–II. In these patients, echocardiography and NT-proBNP were useful in detecting mildly symptomatic PAH with more severe haemodynamic impairment. In clinical practice, screening is often extended to include mildly symptomatic patients and these data suggest that echocardiography remains a useful screening tool in this setting. Also in DELPHI-2, patients with exercise pulmonary hypertension at baseline seemed to be at higher risk for developing PAH than patients with normal haemodynamics at rest and exercise [68]. Finally, a PAH incidence in BMPR2 mutation carriers of 2.3% per year (0.99% per year in men and 3.5% per year in women) was calculated for the first time. All PAH cases remained at low-risk status on oral therapy at last follow-up, illustrating the benefit of an early detection. International multicentre studies are needed to define the best multimodal screening programmes and follow-up intervals to allow early detection and effective treatment of PAH, and to accurately estimate the numbers of at-risk family members who develop disease across the lifespan and by sex.
Which PAH genes should be included in genetic testing?
In addition to BMPR2 as the gene responsible for the largest proportion of HPAH, pathogenic variants in its co-receptors activin receptor-like kinase 1 (ACVRL1) and endoglin (ENG) are found predominantly in patients with HHT, of whom around 2% also develop PAH [69, 70]. Moreover, heterozygous or biallelic pathogenic variants in a ligand of BMPR2, i.e. bone morphogenetic protein 9 (GDF2) [35], and the BMPR2 downstream pathway gene SMAD9 [71] are less common genetic causes of PAH.
Apart from BMPR2 pathway genes, disease-causing variants in genes strongly associated with PAH have also been identified in genes that encode a plasma membrane protein (caveolin-1 (CAV1)), a potassium channel protein (KCNK3) and an ion channel protein (ATP13A3) [28, 72, 73]. For KCNK3, ATP13A3 and GDF2, not only monoallelic but also biallelic variants have been described with a more severe presentation of the respective patients [27, 34, 35, 74].
The transcription factor genes SOX17 and TBX4 have been implicated in PAH often manifesting already in childhood [28, 47, 49]. As detailed earlier, EIF2AK4 should also be analysed to help to clarify a diagnosis of PVOD/PCH particularly in patients with CT abnormalities and low DLCO. Another subset of PAH patients with low DLCO and parenchymal lung abnormalities may carry a pathogenic variant of the KDR gene, a result consistent with the findings obtained by Bayesian inference on the DLCO phenotype [51, 52].
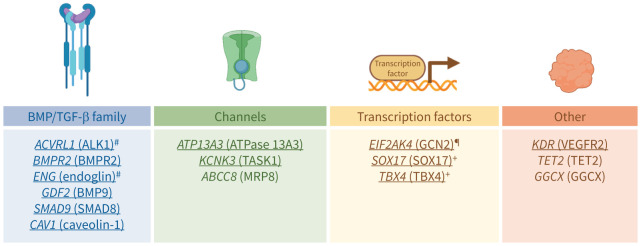
The ClinGen Pulmonary Hypertension Gene Curation Expert Panel has identified the aforementioned genes (ACVRL1, ATP13A3, BMPR2, CAV1, EIF2AK4, ENG, GDF2, KCNK3, KDR, SMAD9, SOX17 and TBX4) as having a “strong” or “definitive” gene–disease relationship (personal communication; www.clinicalgenome.org). Three genes were classified as having a “moderate” gene–disease relationship, including the only recently identified potassium channel gene ABCC8 [75], the vitamin K pathway gene GGCX [10] and a gene involved in DNA methylation regulation by catalysing the conversion of methylcytosine to 5-hydroxymethylcytosine TET2 [76]. Finally, six additional genes involved in PAH have been recently reported and so far identified in only a few PAH patients. Therefore, they have limited evidence for a gene–disease relationship. These genes include the water channel aquaporin-1 gene AQP1 [28], the transcription factor gene KLF2 [77], the BMPR2 ligand gene BMP10 [78], the kallikrein-1 gene KLK1 [10], the extracellular matrix gene fibulin 2 gene FBLN2 and the platelet-derived growth factor (PDGF)-D gene PDGFD [79]. Thus, since the Sixth World Symposium on Pulmonary Hypertension, the list of PAH genes has grown steadily. The genes with a strong recommendation for inclusion on the PAH panel are underlined in figure 1.
FIGURE 1.
Main genes recommended to be included in genetic testing of pulmonary arterial hypertension (PAH) patients and their relatives. The underlined genes are the genes which should definitely be included in the PAH gene panel. The other genes may be included but results should be interpreted with caution as they await further supporting evidence. #: genes associated with hereditary haemorrhagic telangiectasia (Osler–Weber–Rendu disease); ¶: gene associated with pulmonary veno-occlusive disease/pulmonary capillary haemangiomatosis; +: genes associated with lung development abnormalities. BMP: bone morphogenetic protein; TGF: transforming growth factor. Protein symbols/names/aliases are given in brackets.
How should testing be performed?
The clinical features of the patient and family history may guide the genetic testing chosen. Depending on the resources available, genetic testing should start with an affected individual and include BMPR2 at a minimum. A PAH gene panel sequencing approach should be employed that includes BMPR2 as well as at least all “strong” evidence PAH genes as defined by the ClinGen Pulmonary Hypertension Gene Curation Expert Panel using next-generation sequencing as a “gold standard”. This approach replaces the previous stepwise method of using sequential Sanger sequencing of selected genes due to lower costs and greater efficiency of panel testing with next-generation sequencing [80]. The gene list should be revised as additional genes are identified. PAH patients who had received testing of only a limited number of genes in the past should be offered a new round of sequencing [81]. In addition, testing should include methods for large genomic rearrangements screening, including copy number variations. Methods such as multiplex ligation-dependent probe amplification, quantitative PCR, digital droplet PCR and microarrays (mainly comparative genomic hybridisation arrays and single nucleotide polymorphism arrays) are the “gold standard” techniques. Alternatively, sequence-based methods such as whole-exome sequencing (WES) and gene panel sequencing can also be used to quantify read counts to assess intragenic or whole-gene deletions or duplications, particularly regarding BMPR2, ACVRL1, ENG and TBX4, since these occur frequently in these genes.
For children, familial cases or PAH patients with a congenital anomaly regardless of age without an identified pathogenic variant after gene panel testing including all disease genes for the condition, WES or whole-genome sequencing (WGS) can be useful and should include parents to better assess de novo variants in offspring employing a trio-WES or trio-WGS strategy. Once a (likely) pathogenic variant is identified in the family, targeted testing can be performed for the familial variant in symptomatic or asymptomatic family members. Variants should be classified according to ACMG guidelines [63] and submitted to the ClinVar database (www.ncbi.nlm.nih.gov/clinvar) by the diagnostic laboratory.
How are the genetic test results derived and how is the report interpreted?
After the blood/saliva/buccal swab sample of the proband together with all required signed consent forms for genetic testing arrive in the genetic diagnostic laboratory, genomic DNA is extracted. Sequence data are generated for the genes requested. The sequences are aligned and compared with databases of known benign variants. Rare variants and copy number variations are classified using defined criteria, such as those outlined by the ACMG guidelines [63]. The criteria assess different aspects of the variant and consider: 1) variant frequency in the general population (i.e. the gnomAD genome aggregation database; https://gnomad.broadinstitute.org) and in the affected patient population; 2) predicted impact of a nucleotide change leading to an amino acid substitution, premature stop codon, alternate splicing leading to skipping of an exon or inclusion of an intron, deletion or insertions of amino acids; 3) location of the variant within important protein domains; 4) published functional studies for the given variant; 5) co-segregation of the variant with the disease in the family; 6) de novo appearance of the variant in the proband; 7) pathogenicity prediction according to in silico bioinformatic tools; and 8) pattern of inheritance of the variants and disease mode of inheritance.
According to these criteria each variant is classified as benign (class I), likely benign (class II), variant of uncertain significance (class III), likely pathogenic (class IV) or pathogenic (class V) [82]. Class IV and V variants are considered to be likely causative for the disease, while class I and II variants are considered not to be disease causing. Variants with either insufficient or conflicting evidence are classified as variants of uncertain significance.
For example, variants present in more than 1% of the general population are considered benign for PAH (class I), while a rare variant (less than 0.001%) that has never been described in a healthy individual but has been observed in other PAH patients and was functionally shown to reduce or abolish protein function would be classified as a pathogenic variant (class V). In between (likely) benign and (likely) pathogenic variants are those variants of uncertain significance (class III). For these variants not enough evidence is available to upgrade or downgrade them to give them a clear benign or pathogenic character. Such variants should be re-evaluated at regular intervals, e.g. every 3 years, to consider newly published data such as functional evidence or additional cases in patients or controls. In addition, co-segregation with the disease in further affected family members can add helpful information. Association of a class III variant with a pathogenic variant on the other allele of a gene whose biallelic loss of function is lethal is of great help. Also, segregation of the variant in the parents can inform about its inherited or de novo nature. Pulmonary hypertension experts may monitor the ClinVar public database for further information of a specific variant or contact laboratories to request a re-evaluation if it is not done automatically. The large majority of these variants will eventually be downgraded to likely benign variants as they are discovered in further healthy control probands. Variants of uncertain significance are not to be acted on clinically and pre-implantation diagnostics for these variants should not be performed.
To correctly classify such variants and interpret the results appropriately, laboratories performing genetic testing should ideally be certified by an official agency and participate in regular quality control for the genomic studies by external agencies (e.g. the European Molecular Quality Network (EMQN); www.emqn.org) to adhere to all regulations, and use established and accepted methods for sample handling, sequencing, data analyses and reporting. Depending on the country and the regulatory agency, the final genetic diagnostic report is approved and signed by at least one professionally trained human geneticist.
Genetic counselling
Genetic testing for symptomatic individuals should be performed in PAH centres of excellence. The multidisciplinary team should include clinicians with expertise in genetics, including cardiologists, pulmonologists, geneticists and genetic counsellors. Policies and regulations about who can order genetic testing differ by country. Pre-symptomatic genetic testing of asymptomatic individuals should include a genetic professional consultation to explore understanding of the genetic test, implications of a positive or negative result, risk of disease based upon age/gender/genetic status and actions to be taken if genetic results are positive (surveillance and possible reproductive decisions). Pre-symptomatic genetic testing in minors/adolescents should be carefully considered to preserve the right to an open future for the child since there is not a proven method of disease prevention and because penetrance is incomplete. A healthy child with a pathogenic variant may unconsciously be treated differently by his/her parents compared with siblings without such predisposition. This should be carefully discussed during genetic counselling, in particular considering the incomplete penetrance of PAH-associated variants. Thus, the most immediate benefits for the child at hereditary risk would be to obviate the need for clinical assessments and the relief of anxiety in the case of a negative test result. Not all countries allow genetic testing of minors for genetic conditions associated with PAH. When appropriate, based upon the maturity of the adolescent, the adolescent and the parents should all be included in the discussion to ensure the opinions of the adolescent are considered. If a genetic test identified a familial variant, pre-implantation diagnostics may be discussed. While pregnancy itself is to be avoided in female PAH patients [2], it is being debated as a trigger for disease onset in asymptomatic variant carriers [83, 84]. Because there is no proven way to prevent or cure PAH, genetic counselling is important to explore whether the individual is psychologically prepared to determine his/her genetic status and the availability of emotional support. Although many countries have laws protecting against genetic discrimination, these laws do not necessarily cover life insurance, private health insurances, disability insurance or long-term care and therefore exploring insurance policies prior to genetic testing is a consideration. Any positive result revealing a genetic predisposition could possibly lead to a rejection of a new insurance policy or a higher premium.
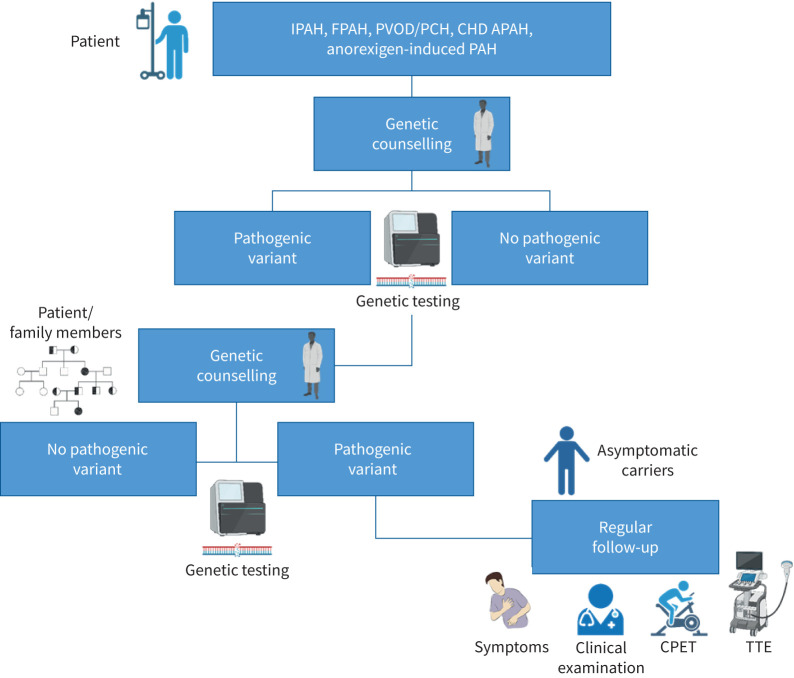
Upon return of the results, many individuals who test positive are concerned because of the poorer prognosis associated with some PAH genes (i.e. BMPR2) and/or worries/guilt about having passed it onto their children. Even those who test negative may need time to adjust to “survivor guilt”. Those who test negative for a pathogenic variant in the family do not have to be concerned about genetic testing for any of their descendants since the pathogenic variant cannot be transmitted from someone who does not carry the variant. In contrast, variant carriers have a 50% chance of passing the variant to their offspring. Specific genetic counselling should be given for recessive autosomal transmission of EIF2AK4-linked PVOD/PCH. 25% of the offspring will be carriers of biallelic pathogenic variants when both parents carry a single heterozygous pathogenic variant. Moreover, the risk of consanguineous unions should be explained to families with pathogenic EIF2AK4 variants. Carrying a pathogenic variant of EIF2AK4 on a single allele is not considered sufficient to lead to disease onset. Disseminating information about genetic test results to family members can be challenging due to the complex nature of the information, guilt and family dynamics [85]. Genetic professionals can act as a critical liaison for families to educate and counsel family members once a (likely) pathogenic variant has been identified. The path of genetic counselling and testing is illustrated in figure 2.
FIGURE 2.
Genetic counselling path for pulmonary arterial hypertension (PAH) patients and their relatives. Patients should receive genetic counselling before and after genetic testing. If a (likely) pathogenic variant is identified, family testing should be encouraged. If asymptomatic carriers are identified they should undergo regular clinical follow-up. IPAH: idiopathic PAH; FPAH: familial PAH; PVOD: pulmonary veno-occlusive disease; PCH: pulmonary capillary haemangiomatosis; CHD APAH: congenital heart disease-associated PAH; CPET: cardiopulmonary exercise testing; TTE: transthoracic echocardiography.
For some patients and relatives, access to testing can be difficult. In some countries, insurance may not cover genetic counselling and testing despite the benefits for the proband. This situation can be even worse in minority or underprivileged communities. Thus, registration of the patient in a PAH expert centre may help to overcome these obstacles.
Areas of uncertainty
While PAH is a disease with a mainly autosomal dominant inheritance pattern, a few patients have been described in whom more than one pathogenic variant in different genes was present and might contribute to disease manifestation. For example, these so-called oligogenic cases have been described in a family with a BMPR2 frameshift variant and an EIF2AK4 exon-skipping variant [86]. An earlier study had reported a severe case of PAH in a child with a BMPR2 and a KCNA5 variant [87]. Thus, in rare cases two variants may increase the severity of the disease or lead to an earlier age of onset of PAH. However, the contribution of this potential oligogenic inheritance remains to be established in PAH.
It is unknown whether the substantial variation usually observed among PAH patients in response to available treatments might be determined, at least in part, by genetic predisposition. More than a decade ago it was already shown that BMPR2 variant carriers rarely respond acutely to vasodilators and are hence less likely to benefit from calcium channel blocker therapy [88, 89]. Relatively few studies focused on gene variants that may interact with drug responses to PAH therapies. Benza et al. [90] demonstrated that variants in endothelin metabolism may predict outcomes in PAH patients treated with endothelin receptor antagonists. Two recent case series explored the hypothesis that BMPR2 pathogenic variants may be associated with a reduced haemodynamic response to inhaled or parenteral prostanoids [91, 92]. The conclusions were different, but it is interesting to notice that the different results might be related to the aggressiveness of the therapeutic approach. These efforts might ultimately provide a step towards the application of precision medicine in PAH. In this regard, it will be interesting to determine whether patients with a genetic risk show a differential response to novel emerging drugs which aim to reduce cellular proliferation and vascular remodelling through the BMP pathway (e.g. sotatercept [6]) or the PDGF receptor pathway (e.g. the inhaled tyrosine kinase inhibitor seralutinib [7]).
Patients with persistent pulmonary hypertension of the newborn (Group 1.6 [4]) often present with complex comorbidities or disorders and may harbour genetic variants in developmental genes [55]. The exact influence of the genes on the phenotype of the patients, apart from the acknowledged PAH genes such as TBX4 and BMPR2, remains to be established [93, 94].
Patients with congenital heart disease APAH may present with a diverse range of cardiac phenotypes. How much the specific cardiac defects contribute to PAH or whether there are pleiotropic effects of some genes on cardiac and pulmonary vascular development remains to be determined. Among patients with congenital heart defects, those patients with a defect repaired early and APAH may be more likely to harbour a pathogenic variant.
PVOD/PCH is defined by clear clinical characteristics in CT, reduced DLCO and in hereditary forms by biallelic EIF2AK4 pathogenic variants. EIF2AK4 variants have also been reported in a limited number of PAH patients which may have been unrecognised PVOD patients [39]. Interestingly, protein expression of EIF2AK4 was similarly reduced in BMPR2 heterozygotes and sporadic PVOD patients without variants in EIF2AK4 [40]. The presence of an additional EIF2AK4 variant in BMPR2 heterozygotes explaining the disease penetrance in a HPAH family could also point towards an autosomal dominant contribution of EIF2AK4 mutations in some cases [86]. Thus, despite being characterised as two different forms of PAH, one might speculate that PVOD and PAH are a spectrum of the same disease. An 8-year follow-up of a sibling with two sisters with PVOD illustrated pulmonary arterial remodelling as a first sign of a lung disease with mild dyspnoea without elevated PAP, while after 8 years lung transplantation revealed pronounced venous remodelling and capillary proliferation [95]. This hypothesis is also supported by a PVOD-like phenotype, which has been described in BMPR2 mutation carriers [96, 97] and which has even received an OMIM entry (265450) prior to the detection of EIF2AK4 as a genetic cause for PVOD. Accordingly, a new terminology has been adopted for the classification of PVOD/PCH patients in the 2022 pulmonary hypertension guidelines for this subgroup of PAH (Group 1.5): “PAH with features of venous/capillary (PVOD/PCH) involvement” [4].
The strength of the International Consortium for Genetic Studies in PAH (PAH-ICON; https://pahicon.com) is the connection of worldwide experts, large and small centres, together achieving larger numbers of patients to address questions like oligogenic inheritance and genetic contributions to persistent pulmonary hypertension of the newborn, and characterise patients’ phenotypes and rare genotypes in detail. Data collected and analysed by PAH-ICON will also provide clues to therapeutic responses of novel molecular agents addressing the underlying pathophysiological imbalances.
Summary of recommendations
At least probands diagnosed with IPAH, FPAH, PVOD/PCH and anorexigen-induced and congenital heart disease APAH should be offered genetic counselling and genetic testing.
A PAH gene panel sequencing approach should be employed that includes at least all “strong” evidence PAH genes (ACVRL1, ATP13A3, BMPR2, CAV1, EIF2AK4, ENG, GDF2, KCNK3, KDR, SMAD9, SOX17 and TBX4) using next-generation sequencing. The gene list should be revised as additional genes are identified and new supporting evidence for previously described possible disease genes becomes available.
The testing method should be complemented by methods to quantify read counts to assess intragenic or whole-gene deletions or duplications, particularly for BMPR2, ACVRL1, ENG and TBX4.
Symptomatic individuals with FPAH, children with normal genetic results on a PAH gene panel and patients with a congenital anomaly regardless of age should consider exome/genome sequencing including multiple affected family members in FPAH and both parents in children to increase the chance of identifying the disease-causing variant.
Cascade genetic testing should be offered in consultation with genetic professionals in families with an identified PAH-associated pathogenic/likely pathogenic variant.
Asymptomatic genetically at-risk individuals should be followed regularly and should be aware of PAH-associated symptoms to enable early diagnosis of PAH and early initiation of treatment if evidence of PAH emerges.
Variants of uncertain significance should be re-evaluated at regular intervals to take into account newly published data. Pulmonary hypertension experts may monitor the ClinVar database for further information of a specific variant or contact laboratories to request a re-evaluation.
Shareable PDF
Footnotes
PAH-ICON members: Micheala A. Aldred (Indiana University School of Medicine, Indianapolis, IN, USA), Stephen L. Archer (Department of Medicine, Queen's University, Kingston, ON, Canada), Eric D. Austin (Department of Pediatrics, Division of Allergy, Immunology and Pulmonary Medicine, Vanderbilt University, Nashville, TN, USA), Roberto Badagliacca (Pulmonary Hypertension Unit, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome, Italy), Srimmitha Balanchandar (School of Medicine, Indiana University, Indianapolis, IN, USA), Joan-Albert Barberà (Department of Pulmonary Medicine, Hospital Clinic-IDIBAPS, University of Barcelona, Spain; Biomedical Research Networking Center on Respiratory Diseases (CIBERES), Spain), Raymond L. Benza (The Cardiovascular Institute, Allegheny General Hospital, Pittsburgh, PA, USA), Rolf M. F. Berger (Pediatric and Congenital Cardiology, Center for Congenital Heart Diseases, Beatrix Children's Hospital, University Medical Center Groningen, The Netherlands), Harm Jan Bogaard (Department of Lung Disease, Amsterdam UMC (location VUmc), Amsterdam, The Netherlands), Sébastien Bonnet (Pulmonary Hypertension Research Group, Centre de Recherche de l'Institut de Cardiologie et de Pneumologie de Quebec, Quebec City, QC, Canada; Department of Medicine, Université Laval, Quebec City, QC, Canada), Karin A. Boomars (Department of Pulmonary Medicine, Erasmus MC, University Medical Center Rotterdam, The Netherlands), Olivier Boucherat (Department of Medicine, Université Laval, Quebec City, QC, Canada; Pulmonary Hypertension and Vascular Biology Research Group, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Department of Medicine, Université Laval, Quebec City, QC, Canada), Murali M. Chakinala (Division of Pulmonary and Critical Care Medicine, Department of Medicine, Washington University School of Medicine, St Louis, MO, USA), Robin Condliffe (Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, UK; Royal Hallamshire Hospital, Sheffield, UK), Rachel Lynn Damico (Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA), Marion Delcroix (Department of Pneumology, University Hospital Leuven, Leuven, Belgium), Ankit A. Desai (Indiana University, Indianapolis, IN, USA), Anna Doboszynska (Department of Pulmonology, Faculty of Medicine, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland), Dennis Dooijes (Department of Genetics, Utrecht University Medical Center, Utrecht, The Netherlands), C. Greg Elliott (Pulmonary Division, Intermountain Medical Center, Murray, UT, USA), Melanie Eyries (Département de Génétique, AP-HP, Hôpital Pitié-Salpêtrière, Paris, France; INSERM UMRS 1166, Sorbonne Université and Institute for Cardiometabolism and Nutrition (ICAN), Paris, France), Maria Pilar Escribano Subías (Department of Cardiology, Hospital Universitario 12 de Octubre, Madrid, Spain; Ciber-CV, Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares, Madrid, Spain; Centro de Referencia Nacional de Hipertensión Pulmonar Compleja and ERN-Lung-Pulmonary Hypertension Referal Center, Madrid, Spain; Instituto de Investigación Sanitaria del Hospital Universitario 12 de Octubre (Imas12), Red SAMID, Madrid, Spain), Henning Gall (Justus-Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI) and Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany), Beatriz García-Aranda (Department of Cardiology, Hospital Universitario 12 de Octubre, Madrid, Spain), Stefano Ghio (Division of Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy), Hossein Ardeschir Ghofrani (Justus-Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI) and Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany; Department of Medicine, Imperial College London, London, UK), Rizwan Hamid (Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN, USA), Paul M. Hassoun (Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA), Anna R. Hemnes (Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA), Katrin Hinderhofer (Laboratory of Molecular Genetic Diagnostics, Institute of Human Genetics, Heidelberg University, Heidelberg, Germany), Arjan C. Houweling (Department of Clinical Genetics, Amsterdam UMC (location VUmc), Amsterdam, The Netherlands), Luke S. Howard (National Heart and Lung Institute, Imperial College London, London, UK), Marc Humbert (Faculté de Médecine, Université Paris-Sud and Université Paris-Saclay, Le Kremlin-Bicêtre, France; INSERM UMR_S 999, Le Plessis-Robinson, France; AP-HP, Service de Pneumologie, Centre de Référence de l'Hypertension Pulmonaire Sévère, Département Hospitalo-Universitaire (DHU) Thorax Innovation (TORINO), Hôpital de Bicêtre, Le Kremlin-Bicêtre, France), David G. Kiely (Sheffield Pulmonary Vascular Disease Unit, Royal Hallamshire Hospital, Sheffield, UK), Gabor Kovacs (Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria; Medical University of Graz, Graz, Austria), David Langleben (Center for Pulmonary Vascular Disease, Cardiology Division, Jewish General Hospital and McGill University, Montreal, QC, Canada), Pablo Lapunzina (Institute of Medical and Molecular Genetics (INGEMM)-IdiPAZ, Hospital Universitario La Paz-UAM, Madrid, Spain; CIBER Enfermedades Respiratorias, Centro de Investigación Biomédica en Red de Enfermedades Raras, ISCIII, Madrid, Spain; ITHACA, European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability, Hospital Universitario La Paz, Madrid, Spain), Allan Lawrie (National Heart and Lung Institute, Imperial College London, London, UK), Jim E. Loyd (Vanderbilt University Medical Center, Nashville, TN, USA), Rajiv D. Machado (Molecular and Clinical Sciences Research Institute, St George's University of London, London, UK), Giovanna Manzi (Department of Clinical, Anesthesiological and Cardiovascular Sciences, I School of Medicine, Sapienza University of Rome, Policlinico Umberto I, Rome, Italy), Jennifer M. Martin (Department of Medicine, University of Cambridge, Cambridge, UK), Evangelos D. Michelakis (Department of Medicine, Alberta Cardiovascular and Stroke Research Centre, University of Alberta, Edmonton, AB, Canada), Shahin Moledina (Great Ormond Street Hospital, London, UK), John H. Newman (Division of Allergy, Pulmonary and Critical Care Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA), William C. Nichols (Division of Human Genetics, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA), Nuria Ochoa Parra (Pulmonary Hypertension Unit, Department of Cardiology, Hospital Universitario Doce de Octubre, Madrid, Spain; Centro de Investigación Biomédica en Red en Enfermedades Cardiovasculares, Instituto de Salud Carlos III (CIBERCV), Madrid, Spain), Andrea Olschewski (Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria), Horst Olschewski (Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria; Medical University of Graz, Graz, Austria), Dviya Pandya (Department of Medicine, University of Cambridge, Cambridge, UK), Silvia Papa (Pulmonary Hypertension Unit, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Italy), Mike W. Pauciulo (Division of Human Genetics, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, University of Cincinnati College of Medicine, Cincinnati, OH, USA), Roxane Paulin (Pulmonary Hypertension Research Group, Centre de Recherche de l'Institut de Cardiologie et de Pneumologie de Quebec, Quebec City, QC, Canada), Roberto Poscia (Pulmonary Hypertension Unit, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome, Italy), Martina Prapa (Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK; Royal Papworth Hospital NHS Foundation Trust, Cambridge, UK), Steeve Provencher (Department of Medicine, Université Laval, Quebec City, QC, Canada; Pulmonary Hypertension and Vascular Biology Research Group, Institut Universitaire de Cardiologie et de Pneumologie de Québec, Department of Medicine, Université Laval, Quebec City, QC, Canada), Marlene Rabinovitch (Cardiovascular Institute, Department of Pediatrics, Stanford University School of Medicine, Stanford, CA, USA; Division of Pulmonary and Critical Care Medicine, Department of Medicine, Stanford University School of Medicine/VA Palo Alto, Palo Alto, CA, USA; The Vera Moulton Wall Center for Pulmonary Vascular Disease, Stanford, CA, USA), Laura Scelsi (Division of Cardiology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy), Werner Seeger (Justus-Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI) and Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany), Memoona Shaukat (Laboratory of Molecular Genetic Diagnostics, Institute of Human Genetics, Heidelberg University, Heidelberg, Germany; Center for Pulmonary Hypertension, Thoraxklinik Heidelberg gGmbH at Heidelberg University Hospital, Heidelberg, Germany and Translational Lung Research Center Heidelberg (TLRC), German Center for Lung Research (DZL), Heidelberg, Germany), Natascha Sommer (Justus-Liebig University, Excellence Cluster Cardio-Pulmonary Institute (CPI) and Universities of Giessen and Marburg Lung Center (UGMLC), Member of the German Center for Lung Research (DZL), Giessen, Germany), Laura Southgate (Molecular and Clinical Sciences Research Institute, St George's University of London, London, UK; Department of Medical and Molecular Genetics, King's College London, London, UK), Duncan J. Stewart (Ottawa Hospital Research Institute, Sinclair Centre for Regenerative Medicine and the University of Ottawa, ON, Canada), Andrew Sweatt (Department of Pulmonary, Allergy and Critical Care Medicine, Stanford University, Stanford, CA, USA), Emilia M. Swietlik (Department of Medicine, University of Cambridge, UK), Hemant K. Tiwari (Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA), Roberto Torre (Pulmonary Hypertension Unit, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Rome, Italy), Carmen Treacy (Department of Medicine, University of Cambridge, Cambridge Biomedical Campus, Cambridge, UK), Olga Tura-Ceide (Department of Pulmonary Medicine, Hospital Clínic-IDIBAPS, University of Barcelona, Spain; Biomedical Research Networking center on Respiratory diseases (CIBERES), Madrid, Spain; Department of Pulmonary Medicine, Dr Josep Trueta University Hospital de Girona, Santa Caterina Hospital de Salt and the Girona Biomedical Research Institute (IDIBGI), Girona, Spain), Carmine Dario Vizza (Pulmonary Hypertension Unit, Department of Cardiovascular and Respiratory Sciences, Sapienza University of Rome, Italy), Anton Vonk Noordegraaf (Department of Pulmonology, Amsterdam Cardiovascular Sciences, Amsterdam UMC (location VUmc), Amsterdam, The Netherlands), Carrie Welch (Department of Pediatrics, Columbia University Medical Center, New York, NY, USA), Martin R. Wilkins (National Heart and Lung Institute, Imperial College London, London, UK), Roham T. Zamanian (Department of Medicine, Stanford University Medical Center, Stanford, CA, USA) and Dmitry Zateyshchikov (Federal Scientific Clinical Centre of Federal Medical and Biological Agency, Genetic Laboratory, Moscow, Russia).
Conflict of interest: C.A. Eichstaedt reports lecture fees from MSD, outside the submitted work; and has a patent “Gene panel specific for pulmonary hypertension and its uses” (European Patent ID: EP3507380) issued. C. Belge reports consulting fees, participation on advisory boards and lecture honoraria from Janssen and MSD/Bayer; travel support from MSD/Bayer, outside the submitted work. W.K. Chung reports scientific advisory board participation with Regeneron Genetics Center, outside the submitted work. E. Grünig reports grants from Actelion, Bayer, GSK, United Therapeutics, Novartis, Bellerophon, OMT, Pfizer and REATA; lecture fees and consultancy fees from Actelion, Bayer/MSD and GSK; travel support from Janssen; advisory board participation with MSD and Ferrer, outside the submitted work; has a patent “Gene panel specific for pulmonary hypertension and its uses” (European Patent ID: EP3507380) issued; and has also served in leadership roles for ADue and pH e.V., outside the submitted work. D. Montani reports grants from Acceleron, Janssen and Merck; consulting fees from Acceleron; lecture honoraria from Bayer, Janssen and Merck, outside the submitted work. R.C. Trembath reports lecture fees from Clinical Cases, outside the submitted work. N.W. Morrell reports employment and stock/stock options from Centessa Pharmaceuticals. All other authors have nothing to disclose.
Support statement: J.A. Tenorio-Castaño is supported by the Instituto de Salud Carlos III, grant numbers FISPI21/01593 and FISPI21/01690, the annual call of the Foundation Against PAH (FCHP; www.fchp.es) and FEDER annual grant (https://enfermedades-raras.org/index.php). N.W. Morrell is supported by the British Heart Foundation (BHF). Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351: 1425–1436. doi: 10.1056/NEJMra040291 [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. doi: 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, McLaughlin V, Gibbs JSR, et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med 2021; 384: 1204–1215. doi: 10.1056/NEJMoa2024277 [DOI] [PubMed] [Google Scholar]
- 7.Frantz RP, Benza RL, Channick RN, et al. TORREY, a phase 2 study to evaluate the efficacy and safety of inhaled seralutinib for the treatment of pulmonary arterial hypertension. Pulm Circ 2021; 11: 20458940211057071. doi: 10.1177/20458940211057071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Romberg E. Über Sklerose der Lungenarterie. [On sclerosis of pulmonary arteries.] Dtsch Archiv Klin Med 1891; 48: 197–206. [Google Scholar]
- 9.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. doi: 10.1183/09031936.00078411 [DOI] [PubMed] [Google Scholar]
- 10.Zhu N, Pauciulo MW, Welch CL, et al. Novel risk genes and mechanisms implicated by exome sequencing of 2572 individuals with pulmonary arterial hypertension. Genome Med 2019; 11: 69. doi: 10.1186/s13073-019-0685-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGoon MD, Miller DP. REVEAL: a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev 2012; 21: 8–18. doi: 10.1183/09059180.00008211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dresdale DT, Michtom RJ, Schultz M. Recent studies in primary pulmonary hypertension, including pharmacodynamic observations on pulmonary vascular resistance. Bull NY Acad Med 1954; 30: 195–207. [PMC free article] [PubMed] [Google Scholar]
- 13.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis 1984; 129: 194–197. doi: 10.1164/arrd.1984.129.1.194 [DOI] [PubMed] [Google Scholar]
- 14.Nichols WC, Koller DL, Slovis B, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet 1997; 15: 277–280. doi: 10.1038/ng0397-277 [DOI] [PubMed] [Google Scholar]
- 15.Grünig E, Janssen B, Mereles D, et al. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation 2000; 102: 1145–1150. doi: 10.1161/01.CIR.102.10.1145 [DOI] [PubMed] [Google Scholar]
- 16.Deng Z, Haghighi F, Helleby L, et al. Fine mapping of PPH1, a gene for familial primary pulmonary hypertension, to a 3-cM region on chromosome 2q33. Am J Respir Crit Care Med 2000; 161: 1055–1059. doi: 10.1164/ajrccm.161.3.9906051 [DOI] [PubMed] [Google Scholar]
- 17.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 2000; 26: 81–84. doi: 10.1038/79226 [DOI] [PubMed] [Google Scholar]
- 18.Deng Z, Morse JH, Slager SL, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000; 67: 737–744. doi: 10.1086/303059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. doi: 10.1093/eurheartj/ehp297 [DOI] [PubMed] [Google Scholar]
- 20.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013; 62: D51–D59. doi: 10.1016/j.jacc.2013.10.023 [DOI] [PubMed] [Google Scholar]
- 21.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL registry. Chest 2010; 137: 376–387. doi: 10.1378/chest.09-1140 [DOI] [PubMed] [Google Scholar]
- 22.Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol 2013; 62: D1–D3. doi: 10.1016/j.jacc.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 23.Hoeper MM, Gibbs JSR. The changing landscape of pulmonary arterial hypertension and implications for patient care. Eur Respir Rev 2014; 23: 450–457. doi: 10.1183/09059180.00007814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoeper MM, Pausch C, Grünig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant 2020; 39: 1435–1444. doi: 10.1016/j.healun.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 25.Berger RM, Beghetti M, Humpl T, et al. Clinical features of paediatric pulmonary hypertension: a registry study. Lancet 2012; 379: 537–546. doi: 10.1016/S0140-6736(11)61621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. doi: 10.1016/j.ijcard.2012.10.026 [DOI] [PubMed] [Google Scholar]
- 27.Eichstaedt CA, Sassmannshausen Z, Shaukat M, et al. Gene panel diagnostics reveals new pathogenic variants in pulmonary arterial hypertension. Respir Res 2022; 23: 74. doi: 10.1186/s12931-022-01987-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gräf S, Haimel M, Bleda M, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat Commun 2018; 9: 1416. doi: 10.1038/s41467-018-03672-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado RD, Southgate L, Eichstaedt CA, et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat 2015; 36: 1113–1127. doi: 10.1002/humu.22904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Southgate L, Machado RD, Gräf S, et al. Molecular genetic framework underlying pulmonary arterial hypertension. Nat Rev Cardiol 2020; 17: 85–95. doi: 10.1038/s41569-019-0242-x [DOI] [PubMed] [Google Scholar]
- 31.Morrell NW, Aldred MA, Chung WK, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801899. doi: 10.1183/13993003.01899-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin EK, Newman JH, Austin ED, et al. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 892–896. doi: 10.1164/rccm.201205-0886OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfarr N, Fischer C, Ehlken N, et al. Hemodynamic and genetic analysis in children with idiopathic, heritable, and congenital heart disease associated pulmonary arterial hypertension. Respir Res 2013; 14: 3. doi: 10.1186/1465-9921-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navas Tejedor P, Tenorio Castano J, Palomino Doza J, et al. An homozygous mutation in KCNK3 is associated with an aggressive form of hereditary pulmonary arterial hypertension. Clin Genet 2017; 91: 453–457. doi: 10.1111/cge.12869 [DOI] [PubMed] [Google Scholar]
- 35.Wang G, Fan R, Ji R, et al. Novel homozygous BMP9 nonsense mutation causes pulmonary arterial hypertension: a case report. BMC Pulm Med 2016; 16: 17. doi: 10.1186/s12890-016-0183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014; 46: 65–69. doi: 10.1038/ng.2844 [DOI] [PubMed] [Google Scholar]
- 37.Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014; 145: 231–236. doi: 10.1378/chest.13-2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenorio J, Navas P, Barrios E, et al. A founder EIF2AK4 mutation causes an aggressive form of pulmonary arterial hypertension in Iberian Gypsies. Clin Genet 2014; 88: 579–583. doi: 10.1111/cge.12549 [DOI] [PubMed] [Google Scholar]
- 39.Hadinnapola C, Bleda M, Haimel M, et al. Phenotypic characterisation of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation 2017; 136: 2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nossent EJ, Antigny F, Montani D, et al. Pulmonary vascular remodeling patterns and expression of general control nonderepressible 2 (GCN2) in pulmonary veno-occlusive disease. J Heart Lung Transplant 2018; 37: 647–655. doi: 10.1016/j.healun.2017.09.022 [DOI] [PubMed] [Google Scholar]
- 41.Evans JD, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 2016; 4: 129–137. doi: 10.1016/S2213-2600(15)00544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montani D, Lau EM, Dorfmüller P, et al. Pulmonary veno-occlusive disease. Eur Respir J 2016; 47: 1518–1534. doi: 10.1183/13993003.00026-2016 [DOI] [PubMed] [Google Scholar]
- 43.Montani D, Girerd B, Jais X, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med 2017; 5: 125–134. doi: 10.1016/S2213-2600(16)30438-6 [DOI] [PubMed] [Google Scholar]
- 44.Best DH, Sumner KL, Smith BP, et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest 2017; 151: 821–828. doi: 10.1016/j.chest.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 45.Zeng X, Chen F, Rathinasabapathy A, et al. Rapid disease progress in a PVOD patient carrying a novel EIF2AK4 mutation: a case report. BMC Pulm Med 2020; 20: 186. doi: 10.1186/s12890-020-01186-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girerd B, Montani D, Coulet F, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med 2010; 181: 851–861. doi: 10.1164/rccm.200908-1284OC [DOI] [PubMed] [Google Scholar]
- 47.Kerstjens-Frederikse WS, Bongers EM, Roofthooft MT, et al. TBX4 mutations (small patella syndrome) are associated with childhood-onset pulmonary arterial hypertension. J Med Genet 2013; 50: 500–506. doi: 10.1136/jmedgenet-2012-101152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thore P, Girerd B, Jais X, et al. Phenotype and outcome of pulmonary arterial hypertension patients carrying a TBX4 mutation. Eur Respir J 2020; 55: 1902340. doi: 10.1183/13993003.02340-2019 [DOI] [PubMed] [Google Scholar]
- 49.Zhu N, Gonzaga-Jauregui C, Welch CL, et al. Exome sequencing in children with pulmonary arterial hypertension demonstrates differences compared with adults. Circ Genom Precis Med 2018; 11: e001887. doi: 10.1161/CIRCGEN.117.001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galambos C, Mullen MP, Shieh JT, et al. Phenotype characterisation of TBX4 mutation and deletion carriers with neonatal and paediatric pulmonary hypertension. Eur Respir J 2019; 54: 1801965. doi: 10.1183/13993003.01965-2018 [DOI] [PubMed] [Google Scholar]
- 51.Eyries M, Montani D, Girerd B, et al. Familial pulmonary arterial hypertension by KDR heterozygous loss of function. Eur Respir J 2020; 55: 1902165. doi: 10.1183/13993003.02165-2019 [DOI] [PubMed] [Google Scholar]
- 52.Swietlik EM, Greene D, Zhu N, et al. Bayesian inference associates rare KDR variants with specific phenotypes in pulmonary arterial hypertension. Circ Genom Precis Med 2021; 14: e003155. doi: 10.1161/CIRCGEN.120.003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoeper MM, Dwivedi K, Pausch C, et al. Phenotyping of idiopathic pulmonary arterial hypertension: a registry analysis. Lancet Respir Med 2022; 10: 937–948. doi: 10.1016/S2213-2600(22)00097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu N, Welch CL, Wang J, et al. Rare variants in SOX17 are associated with pulmonary arterial hypertension with congenital heart disease. Genome Med 2018; 10: 56. doi: 10.1186/s13073-018-0566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch CL, Chung WK. Genetics and genomics of pediatric pulmonary arterial hypertension. Genes 2020; 11: 1213. doi: 10.3390/genes11101213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montani D, Lechartier B, Girerd B, et al. An emerging phenotype of pulmonary arterial hypertension patients carrying SOX17 variants. Eur Respir J 2022: 60: 2200656. doi: 10.1183/13993003.00656-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang TM, Wang SS, Xu YJ, et al. SOX17 loss-of-function mutation underlying familial pulmonary arterial hypertension. Int Heart J 2021; 62: 566–574. doi: 10.1536/ihj.20-711 [DOI] [PubMed] [Google Scholar]
- 58.Humbert M, Deng Z, Simonneau G, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J 2002; 20: 518–523. doi: 10.1183/09031936.02.01762002 [DOI] [PubMed] [Google Scholar]
- 59.Souza R, Humbert M, Sztrymf B, et al. Pulmonary arterial hypertension associated with fenfluramine exposure: report of 109 cases. Eur Respir J 2008; 31: 343–348. doi: 10.1183/09031936.00104807 [DOI] [PubMed] [Google Scholar]
- 60.Lerche M, Eichstaedt CA, Hinderhofer K, et al. Mutually reinforcing effects of genetic variants and interferon-beta 1a therapy for pulmonary arterial hypertension development in multiple sclerosis patients. Pulm Circ 2019; 9: 2045894019872192. doi: 10.1177/2045894019872192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perrin S, Montani D, O'Connell C, et al. Nasal decongestant exposure in patients with pulmonary arterial hypertension: a pilot study. Eur Respir J 2015; 46: 1211–1214. doi: 10.1183/13993003.00051-2015 [DOI] [PubMed] [Google Scholar]
- 62.Montani D, Lau EM, Descatha A, et al. Occupational exposure to organic solvents: a risk factor for pulmonary veno-occlusive disease. Eur Respir J 2015; 46: 1721–1731. doi: 10.1183/13993003.00814-2015 [DOI] [PubMed] [Google Scholar]
- 63.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grünig E, Weissmann S, Ehlken N, et al. Stress Doppler echocardiography in relatives of patients with idiopathic and familial pulmonary arterial hypertension: results of a multicenter European analysis of pulmonary artery pressure response to exercise and hypoxia. Circulation 2009; 119: 1747–1757. doi: 10.1161/CIRCULATIONAHA.108.800938 [DOI] [PubMed] [Google Scholar]
- 65.Hinderhofer K, Fischer C, Pfarr N, et al. Identification of a new intronic BMPR2-mutation and early diagnosis of heritable pulmonary arterial hypertension in a large family with mean clinical follow-up of 12 years. PLoS One 2014; 9: e91374. doi: 10.1371/journal.pone.0091374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coghlan JG, Denton CP, Grünig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340–1349. doi: 10.1136/annrheumdis-2013-203301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang P, Yu PB. In search of the second hit in pulmonary arterial hypertension. Circ Res 2019; 124: 6–8. doi: 10.1161/CIRCRESAHA.118.314270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montani D, Girerd B, Jais X, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J 2021; 58: 2004229. doi: 10.1183/13993003.04229-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lyle MA, Fenstad ER, McGoon MD, et al. Pulmonary hypertension in hereditary hemorrhagic telangiectasia. Chest 2016; 149: 362–371. doi: 10.1378/chest.15-0535 [DOI] [PubMed] [Google Scholar]
- 70.Margelidon-Cozzolino V, Cottin V, Dupuis-Girod S, et al. Pulmonary hypertension in hereditary haemorrhagic telangiectasia is associated with multiple clinical conditions. ERJ Open Res 2021; 7: 00078-2020. doi: 10.1183/23120541.00078-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasim MT, Ogo T, Ahmed M, et al. Molecular genetic characterization of SMAD signaling molecules in pulmonary arterial hypertension. Hum Mutat 2011; 32: 1385–1389. doi: 10.1002/humu.21605 [DOI] [PubMed] [Google Scholar]
- 72.Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med 2013; 369: 351–361. doi: 10.1056/NEJMoa1211097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Austin ED, Ma L, LeDuc C, et al. Whole exome sequencing to identify a novel gene (caveolin-1) associated with human pulmonary arterial hypertension. Circ Cardiovasc Genet 2012; 5: 336–343. doi: 10.1161/CIRCGENETICS.111.961888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machado R, Welch CL, Haimel M, et al. Biallelic variants of ATP13A3 cause dose-dependent childhood-onset pulmonary arterial hypertension characterised by extreme morbidity and mortality. J Med Genet 2022; 59: 906–911. doi: 10.1136/jmedgenet-2021-107831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bohnen MS, Ma L, Zhu N, et al. Loss-of-function ABCC8 mutations in pulmonary arterial hypertension. Circ Genom Precis Med 2018; 11: e002087. doi: 10.1161/CIRCGEN.118.002087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Potus F, Pauciulo MW, Cook EK, et al. Novel mutations and decreased expression of the epigenetic regulator TET2 in pulmonary arterial hypertension. Circulation 2020; 141: 1986–2000. doi: 10.1161/CIRCULATIONAHA.119.044320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eichstaedt CA, Song J, Rodríguez Viales R, et al. First identification of Krüppel-like factor 2 mutation in heritable pulmonary arterial hypertension. Clin Sci 2017; 131: 689–698. doi: 10.1042/CS20160930 [DOI] [PubMed] [Google Scholar]
- 78.Eyries M, Montani D, Nadaud S, et al. Widening the landscape of heritable pulmonary hypertension mutations in paediatric and adult cases. Eur Respir J 2019; 53: 1801371. doi: 10.1183/13993003.01371-2018 [DOI] [PubMed] [Google Scholar]
- 79.Zhu N, Swietlik EM, Welch CL, et al. Rare variant analysis of 4241 pulmonary arterial hypertension cases from an international consortium implicates FBLN2, PDGFD, and rare de novo variants in PAH. Genome Med 2021; 13: 80. doi: 10.1186/s13073-021-00891-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song J, Eichstaedt CA, Rodríguez Viales R, et al. Identification of genetic defects in pulmonary arterial hypertension by a new gene panel diagnostic tool. Clin Sci 2016; 130: 2043–2052. doi: 10.1042/CS20160531 [DOI] [PubMed] [Google Scholar]
- 81.van den Heuvel LM, Jansen SMA, Alsters SIM, et al. Genetic evaluation in a cohort of 126 Dutch pulmonary arterial hypertension patients. Genes 2020; 11: 1191. doi: 10.3390/genes11101191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plon SE, Eccles DM, Easton D, et al. Sequence variant classification and reporting: recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum Mutat 2008; 29: 1282–1291. doi: 10.1002/humu.20880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Limoges M, Langleben D, Fox BD, et al. Pregnancy as a possible trigger for heritable pulmonary arterial hypertension. Pulm Circ 2016; 6: 381–383. doi: 10.1086/686993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jaliawala HA, Parmar M, Summers K, et al. A second hit? Pulmonary arterial hypertension, BMPR2 mutation, and exposure to prescription amphetamines. Pulm Circ 2022; 12: e12053. doi: 10.1002/pul2.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Girerd B, Montani D, Jais X, et al. Genetic counselling in a national referral centre for pulmonary hypertension. Eur Respir J 2016; 47: 541–552. doi: 10.1183/13993003.00717-2015 [DOI] [PubMed] [Google Scholar]
- 86.Eichstaedt CA, Song J, Benjamin N, et al. EIF2AK4 mutation as “second hit” in hereditary pulmonary arterial hypertension. Respir Res 2016; 17: 141. doi: 10.1186/s12931-016-0457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang G, Knight L, Ji R, et al. Early onset severe pulmonary arterial hypertension with ‘two-hit’ digenic mutations in both BMPR2 and KCNA5 genes. Int J Cardiol 2014; 177: e167–e169. doi: 10.1016/j.ijcard.2014.08.124 [DOI] [PubMed] [Google Scholar]
- 88.Rosenzweig EB, Morse JH, Knowles JA, et al. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant 2008; 27: 668–674. doi: 10.1016/j.healun.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 89.Elliott CG, Glissmeyer EW, Havlena GT, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation 2006; 113: 2509–2515. doi: 10.1161/CIRCULATIONAHA.105.601930 [DOI] [PubMed] [Google Scholar]
- 90.Benza RL, Gomberg-Maitland M, Demarco T, et al. Endothelin-1 pathway polymorphisms affect outcome in pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 1345–1354. doi: 10.1164/rccm.201501-0196OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Scelsi L, Greco A, Acquaro M, et al. BMPR2 mutations and response to inhaled or parenteral prostanoids: a case series. Pulm Circ 2021; 11: 20458940211037275. doi: 10.1177/20458940211037275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boucly A, Savale L, Jaïs X, et al. Sequential combination therapy with parenteral prostacyclin in BMPR2 mutations carriers. Pulm Circ 2022; 12: e12023. doi: 10.1002/pul2.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taha F, Southgate L. Molecular genetics of pulmonary hypertension in children. Curr Opin Genet Dev 2022; 75: 101936. doi: 10.1016/j.gde.2022.101936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haarman MG, Kerstjens-Frederikse WS, Vissia-Kazemier TR, et al. The genetic epidemiology of pediatric pulmonary arterial hypertension. J Pediatr 2020; 225: 65–73. doi: 10.1016/j.jpeds.2020.05.051 [DOI] [PubMed] [Google Scholar]
- 95.Montani D, Dorfmüller P, Girerd B, et al. Natural history over 8 years of pulmonary vascular disease in a patient carrying bi-allelic EIF2AK4 mutations. Am J Respir Crit Care Med 2018; 198: 537–541. doi: 10.1164/rccm.201802-0317LE [DOI] [PubMed] [Google Scholar]
- 96.Oriaku I, LeSieur MN, Nichols WC, et al. A novel BMPR2 mutation with widely disparate heritable pulmonary arterial hypertension clinical phenotype. Pulm Circ 2020; 10: 2045894020931315. doi: 10.1177/2045894020931315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Montani D, Achouh L, Dorfmüller P, et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine 2008; 87: 220–233. doi: 10.1097/MD.0b013e31818193bb [DOI] [PubMed] [Google Scholar]
- 98.Tenorio Castaño JA, Hernandez-Gonzalez I, Gallego N, et al. Customized massive parallel sequencing panel for diagnosis of pulmonary arterial hypertension. Genes 2020; 11: 1158. doi: 10.3390/genes11101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsoi SM, Jones K, Colglazier E, et al. Persistence of persistent pulmonary hypertension of the newborn: a case of de novo TBX4 variant. Pulm Circ 2022; 12: e12108. doi: 10.1002/pul2.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang XJ, Lian TY, Jiang X, et al. Germline BMP9 mutation causes idiopathic pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801609. doi: 10.1183/13993003.01609-2018 [DOI] [PubMed] [Google Scholar]
- 101.Baloira A, Bastos M, Pousada G, et al. Pulmonary arterial hypertension associated with hereditary spherocytosis and splenectomy in a patient with a mutation in the BMPR2 gene. Clin Case Rep 2016; 4: 752–755. doi: 10.1002/ccr3.610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baloira Villar A, Pousada Fernández G, Núñez Fernández M, et al. Clinical and molecular study of 4 cases of pulmonary hypertension associated with sarcoidosis. Arch Bronconeumol 2015; 51: e19–e21. doi: 10.1016/j.arbres.2014.03.022 [DOI] [PubMed] [Google Scholar]
- 103.Barozzi C, Galletti M, Tomasi L, et al. A combined targeted and whole exome sequencing approach identified novel candidate genes involved in heritable pulmonary arterial hypertension. Sci Rep 2019; 9: 1425. doi: 10.1038/s41598-018-37277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Braam EAJE, Quanjel MJR, Van Haren-Willems JHGM, et al. Extensive pulmonary sarcoid reaction in a patient with BMPR-2 associated idiopathic pulmonary arterial hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2016; 33: 182–185. [PubMed] [Google Scholar]
- 105.Eichstaedt CA, Verweyen J, Halank M, et al. Myeloproliferative diseases as possible risk factor for development of chronic thromboembolic pulmonary hypertension – a genetic study. Int J Mol Sci 2020; 21: 3339. doi: 10.3390/ijms21093339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feng Y-X, Liu D, Sun M-L, et al. BMPR2 germline mutation in chronic thromboembolic pulmonary hypertension. Lung 2014; 192: 625–627. doi: 10.1007/s00408-014-9580-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01471-2022.Shareable (482.6KB, pdf)