Summary
Regenerative agriculture (RA) is gaining traction globally as an approach for meeting growing food demands while avoiding, or even remediating, the detrimental environmental consequences associated with conventional farming. Momentum is building for science to provide evidence for, or against, the putative ecosystem benefits of RA practices relative to conventional farming. In this perspective article, we advance the argument that consideration of the soil microbiome in RA research is crucial for disentangling the varied and complex relationships RA practices have with the biotic and abiotic environment, outline the expected changes in soil microbiomes under RA, and make recommendations for designing research that will answer the outstanding questions on the soil microbiome under RA. Ultimately, deeper insights into the role of microbial communities in RA soils will allow the development of biologically relevant monitoring tools which will support land managers in addressing the key environmental issues associated with agriculture.
Subject areas: Soil science, Applied microbiology, Microbiome, Agricultural science, Agricultural soil science
Graphical abstract
Soil science; Applied microbiology; Microbiome; Agricultural science; Agricultural soil science
Introduction
One of the greatest challenges that humanity is currently facing is meeting growing food demands while reducing environmental damage from agriculture. To guarantee food security into the future, it is estimated that food production needs to roughly double.1 Most of the current demand is met by conventional agriculture. Conventional agriculture contributes heavily to the deterioration of our soil and water environments, the accumulation of greenhouse gasses, and biodiversity losses, in part due to high stock densities, heavy pesticide and fertilizer use, monocultures, and soil tilling.2,3,4 Drastic changes are required, not only to address the extent of soil degradation and related productivity and biodiversity loss but also to ensure food security into the future. Regenerative agriculture (RA) is touted as a “back to basics” solution to improve soil quality and biodiversity, while maintaining or improving productivity and profitability.5
While RA lacks an accepted definition, it is commonly described as a set of on-farm practices that generally differ from conventional farming in terms of the types and intensities of human disturbances and/or inputs within the farm system6 (Table 1). These practices vary, but most often include a lack of tillage (mechanical disturbance), excluding synthetic fertilizer and pesticide use, inclusion of increased plant diversity, and the integration and altered management of crops and livestock.5,6 Limited evidence shows that RA can improve soil nutrient content and physical structure,7,8,9 while increasing profit and reducing pests.5 Perhaps most importantly, RA is purported to increase atmospheric carbon sequestration and is, therefore, touted as a strategy to help address global climate change issues.10 While the RA “movement” is being increasingly adopted by farmers at the grassroots level, comprehensive scientific research on the ecosystem-level changes induced by RA, relative to conventional farming systems, has only recently gained momentum; thus, questions remain about the extent to which regenerative approaches are able to achieve better environmental outcomes, such as soil quality improvements and climate change mitigation, compared to conventional practices.
Table 1.
Descriptions of practices commonly associated with regenerative agriculture, the rationale for their implementation, and examples of research relevant to each
| Practice | Description | Rationale for implementation | Examples |
|---|---|---|---|
| Reduced tillage | Soil disturbances are minimized by adopting a no-till or conservation-tillage approach, the latter meaning plant residues are maintained on at least 30% of the soil surface. Direct-drill cropping and pasture-sowing methods are adopted. | Can decrease energy consumption and reduce CO2 emissions while potentially increasing carbon sequestration (but see Cai et al.11). Reduces soil erosion, improves soil fertility, and increases biodiversity and water retention | Holland,12; Blanco-Canqui and Ruis,13 |
| Cover crops and crop rotation | Using close-growing crops to cover the soil between normal crop production or between trees/vines in orchards and vineyards. Production crops alternate sequentially on the same land. | Improves soil nutrients, while reducing erosion and weed growth. Legume cover crops can increase soil N content and reduce the need for fertilizers. Crop rotation improves production and disrupts insect/pest reproduction cycles | Blanco-Canqui and Ruis,13; Adetunji et al.14; Shah et al.15 |
| High-diversity pasture | Replacing traditional low-diversity pastures (usually made up of one or two species) with diverse mixes, often selected specifically to suit the farm purpose. | Better pasture utilization, growing season extension, increased nutrition for grazing animals, increased milk production, and improved animal welfare. Thought to increase overall biodiversity and improve ecosystem functioning. | Pembleton et al.16; Distel et al.17 |
| No synthetic fertilizers or pesticides | Reduce or eliminate the need for synthetic fertilizers, instead using things like organic compost and bio-supplements (e.g., compost, seaweed extracts, fish hydrolysates, and vermicast). Avoidance of pesticide use. | Avoids the negative environmental impacts of synthetic chemicals, including increased greenhouse gas emissions and eutrophication of aquatic ecosystems. | Paungfoo-Lonhienne et al.18 |
| Grazing management | Rotating livestock across smaller paddocks or delineating grazing areas for short periods and only returning to a previously grazed paddock when pasture has recovered and regrown. | Manipulating the amount of time that grass spends in the active growth phase by managing the duration and timing of grazing is thought to enhance soil carbon sequestration. Furthermore, preventing overgrazing means rootstocks are less impacted, allowing for quicker recovery. | Teague and Barnes,19 |
Because soil microbial communities are crucial for maintaining soil quality and are a fundamental component of the soil ecosystem,20,21 the soil microbiome is likely to serve as the belowground “engine” for delivering many of the key benefits of RA practices. A difference in microbial community composition in RA sites compared to conventional systems has been confirmed,22 and the activity of microbes under RA may increase.9 Microbial biomass is also known to increase in RA sites compared to conventional systems.8 This is not surprising, given that there is clear evidence that aboveground activity impacts both the bacterial23,24 and fungal25 belowground ecosystem components. However, the limited scientific research on RA that has been conducted to date has largely overlooked relationships between RA practices and soil microbial communities. We need to investigate how both the composition and functional profiles of the microbiome change. For example, genes that can be used to define substrate utilization and the capacity for nutrient cycling, toxin or heavy metal degradation, and microbial stress responses can be quantified.
The importance of scientific research on soil microbiomes under RA
Soil biota, including the microbiome, is increasingly recognized as the crucial foundation of ecosystem resilience and functioning.26,27 For example, microbes enhance soil fertility through their mediation of the biogeochemical cycles28 and elevated carbon storage in soils with higher plant diversity has been shown to be directly mediated by soil microbial processes.29 Microbial activity, for example, from fungi in the rhizosphere, can impact the hydrophobicity of soil30 and therefore has consequences for soil hydraulic function that need to be considered.31 Thus, soil microbial communities are a vital component of the success, or otherwise, of RA practices.
Understanding the role of the soil microbiome in RA outcomes could provide opportunities to increase the efficiency and/or efficacy of RA. This could be achieved by indirectly manipulating the soil microbiome32 and altering management practices33 or more directly by modifying soil microbial communities, for example, by introducing beneficial microorganisms.34 The latter is likely to be challenging to implement, although there are examples of commercially available microbial products to enhance soil fertility or act as biocontrol agents.34 Though perhaps more in line with RA principles would be the application of compost and compost teas which contain diverse and abundant microbial organisms.35 Indirect methods will only be feasible if we can improve our understanding of the relationship between RA practices and soil microbial communities. Knowing what aspects of microbial activity are key elements to desired soil condition outcomes will create opportunities for optimizing the soil microbiome to get the greatest possible agroecosystem benefit and to maximize production and profit potentials.
While there are several examples of verification schemes, such as the “Certified Regenerative” by A Greener World and “Ecological Outcomes Verification” by the Savory Institute, the lack of a clear definition for RA and the practices to be deployed means these certification programmes are faced with challenges. As a solution, outcomes, rather than or as well as practices, could be monitored.6 For this purpose, these outcomes should not be based solely on changes in abiotic variables such as soil nutrients, pollutants, and soil structure, as has often been the case for soil monitoring under agriculture.36 Biological indicators make up less than 20% of indicators currently in use, and despite the increased appreciation for the importance of biology in soil health, their use has not increased.37 Where microbial measures are used, these often consist of monitoring richness, biomass, or ratios of fungi to bacteria, and the science behind the measures is commonly insufficient meaning interpretation for management and policy purposes is difficult.38 That is not to say that microbial data cannot be useful, rather the type of data that are used and the scientific understanding behind those is crucial. Approaches utilizing molecular methods (e.g., DNA-based detection of microbes) are likely to offer the best opportunities for incorporating microbial insights into soil health for monitoring and management.38 Frameworks monitoring the progress of RA need to ensure that the biotic component of the soil is also assessed. The first step in creating microbiological indicators of RA’s impact on the soil ecosystem is understanding the relationships between RA practices and the soil microbiome.
Possible impacts of regenerative agricultural practices on the soil microbiome
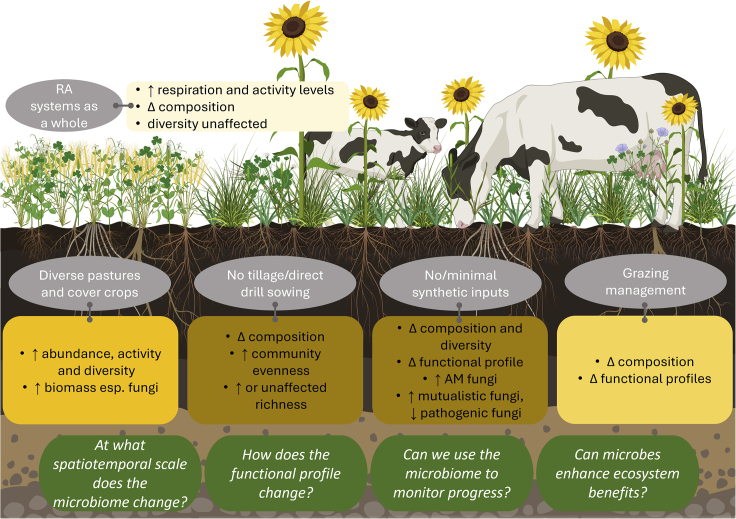
Understanding RA’s impact on soil microbial communities is particularly challenging as RA is guided by principles rather than dictated by strict protocols, resulting in a suite of practices that fall under the umbrella of regenerative farming. Nonetheless, there are commonalities in how RA is applied, even if the specific nature of those practices varies among farms and farming systems. Research assessing the effects of specific farm management practices and their impact on the soil microbiome also provides insight into how particular principles of RA might impact the soil microbial communities (Figure 1); these practices, and what is known about their effects on soil microbial communities, are outlined below.
Figure 1.
A summary of the changes in soil microbial communities known to occur under regenerative agriculture (RA) practices, compared to conventional agriculture
Gray boxes state the RA practice, and the connected yellow boxes contain the microbiome changes associated with that practice reported by previous research; darker shades of yellow represent increasing supporting literature for those possible impacts on the soil microbiome. Reported differences between RA and conventional agriculture were increased (↑), decreased (↓), non-direction changes (Δ), or no changes. For details on specific compositional or functional profile changes, see section 3 and references therein. Green boxes highlight key knowledge gaps. This figure was created with BioRender.com.
Minimize physical soil disturbance by using no-till approaches
Closely associated with RA is the absence of, or reduction in, tillage to minimize soil disturbances.6 Tillage is used for seedbed preparation, soil aeration, turning over cover crops, and incorporating fertilizer into the root zone,39 but it can have negative impacts on soil properties, for example, by breaking up the soil structure, accelerating erosion, and increasing surface runoff.13 The ecological impacts of tillage, in terms of the biological organisms in the soil, have been investigated and described for many decades, with clear differences observed between tilled and untilled soils.40 Microbial biomass and enzyme activities increase in the absence of tillage.41 Furthermore, when comparing soil microbiomes between conventionally tilled and reduced-tillage or no-tillage sites, research suggests that, while there is no observable difference in microbial community richness, the taxonomic composition does change.42,43 However, increases in microbial richness have also been observed in the absence of tillage.44,45 Whether or not differences are observed in microbial communities could be due to variation in underlying soil environmental conditions, highlighting the importance of research across different ecosystems.
The impact of tillage on the soil microbiome can directly result from physical disturbance, and fungal mycelia are particularly sensitive to physical disturbances due to their filamentous nature.46 However, differences in fungal communities observed between till and no-till sites can also be linked to differences in soil organic carbon, rather than the disturbance per se.47 Similarly, since tillage results in a more homogeneous soil environment, this reduction in the diversity of microhabitats can result in a less-diverse bacterial community that is dominated by a much smaller number of phyla.44
In all the above studies, the comparison between tillage and non-tillage was conducted in conventional systems. It could therefore be argued that the microbiomes of these agricultural soils are still strongly selected for by a relatively uniform abiotic environment, for example, through low-diversity rotational crops creating a homogeneous organic substrate. Indeed, even no-till sites contain soil microbial communities that are more similar to those in agricultural soils than those in natural grasslands, showing that physical disturbance is not the only aspect of agriculture that impacts soil microbiomes.43 RA practices go far beyond simply “no-tillage”; in combination, they may have as yet unobserved consequences for the soil microbiome.
Increasing aboveground biodiversity
The goal of RA to increase aboveground biodiversity by having diverse crop rotations, growing cover crops, and encouraging diverse pastures has the potential to increase net primary productivity and belowground carbon sequestration48,49; this is one of the core principles of RA. A strong relationship exists between plant diversity and soil microbial communities on a global scale; plant beta-diversity in grasslands is significantly positively correlated with bacterial and fungal beta-diversity, even when accounting for environmental variation.50 The use of cover crops and diverse production crops can also increase the abundance, activity, and diversity of soil microbes, although crop management practices and climate impact the observed effects.51,52 Interestingly, fungal communities might vary more with cover crop species composition than bacteria, which respond more to local soil conditions,53 but the implications of this for environmental effects in agricultural systems are unknown.
It has been suggested that soil microbes can promote plant diversity indirectly by affecting soil variables, such as nutrient availability,54 so understanding the soil microbiome under RA practices could be important for ensuring environmental and production benefits of planting diverse pastures, as well as the maintenance of that diversity. Mycorrhizal diversity and plant diversity are especially closely interlinked, with diversity in one enhancing diversity in the other.55 However, the nature of these interaction webs is not entirely understood. More diverse plant systems have increased root biomass and a corresponding increase in root exudates; this has been shown to result in an increase in both bacterial and fungal biomass and a shift in the fungal to bacterial biomass ratio.56 Ratios of fungal and bacterial biomass can give important insights into the functioning of soil ecosystems, whereby bacterial-dominated microbial communities have been linked to losses in nitrogen and lower rates of carbon sequestration when compared to soils that are fungal dominated.57,58,59 Increasing plant diversity in pastures could result in increased fungal biomass, which could therefore help RA practices achieve carbon sequestration goals. However, understanding the dynamics of this increase and how variation in plant diversity interacts with other RA practices is crucial.
Managing stock densities and grazing rotations
Managing and optimizing the duration and timing of grazing are thought to be one strategy by which soil carbon sequestration can be achieved because aboveground biomass and belowground carbon flux are optimal when grass spends more time in the active growth phase.49 While further research is required to understand exactly how efficient this is for increasing soil organic matter and carbon stock and how best to implement it, altering grazing patterns is likely to impact the soil microbial communities. Overall, there is clear evidence that grazed pastures contain different microbial communities and that this effect can “spill over” into neighboring environments.60 Fungal community composition differs between cattle-disturbed soils compared to non-disturbed soils, and richness and fungal biomass are higher in grazed soils.61 Some of this increased richness is likely due to the introduction of cattle-associated fungi. However, indirect effects on soil chemical profiles caused by excrement and urine deposits and changes in plant root turnover associated with the trampling and grazing activities can explain a great deal of soil microbial community variation.61 Grazing has been shown to affect soil bacterial communities more than fungal communities, with increased bacterial diversity and decreased biomass observed.62 Bacterial communities also show changes in functional profiles in response to grazing, including increases in nitrogen mineralization, nitrification genes, virulence, stress, and antibiotic resistance genes, while denitrification, nitrogen reduction, and carbon fixation are decreased.63 Even mild grazing by lambs has been shown to result in functional changes in bacterial communities, especially in relation to nitrification.64 Rotational grazing can reduce the abundance of antimicrobial resistance genes, the presence of which is of environmental concern.65 Such research shows that grazing activity correlates with changes in soil microbial communities; however, these studies largely compare grazed to un-grazed soils. While there is evidence that the response of the soil microbial community is correlated with the time spent under grazing66 and grazing intensity,67 there is a lack of research into how altered grazing patterns will impact the soil microbial communities. Furthermore, the type of animal being used for grazing is likely to be diverse under RA; deposition of cattle manure versus poultry litter changed the abundance of antimicrobial resistance genes mentioned above, supporting the differing impact different grazing animals will have.65 The full effect of this management practice in the context of application within RA is therefore unclear.
Moving away from synthetic fertilizer use
To meet the specific nutrient requirements for high-yield crop plant production, synthetic chemical fertilizers have long been applied to agricultural soils. However, their use has harmful environmental impacts, including increased greenhouse gas emissions and eutrophication of aquatic ecosystems.68 In line with the general philosophy of RA, a primary aim is often to reduce or eliminate the use of synthetic fertilizers. Synthetic nitrogen fertilizers have been reported to result in both a decrease69 and contradictorily an increase70 of arbuscular mycorrhizal (AM) fungi diversity, suggesting changes could be context specific or determined through interactive effects. Long-term nitrogen fertilization experiments have reported that bacterial diversity is not affected, though the community composition does change along with differences in the functional profiles, including shifts in the catabolic capabilities, and increases in the relative abundance of genes associated with nucleic acid replication, electron transport, and protein metabolism.71 Shifts in community composition are also observed for AM fungal communities even after the short-term application of chemical fertilizers.70 Nitrogen and phosphorus additions have been shown to increase the relative abundance of fungal pathogens, while decreasing the abundance of mutualists,72 highlighting the potentially detrimental ecosystem shifts caused by synthetic fertilizer use. However, fungal saprotrophs, which play crucial roles in soil carbon flux and storage, are not impacted by nutrient amendments.72 Ultimately, given that synthetic fertilizers appear to affect at least microbial community composition and function, and potentially diversity and richness, phasing out their use in RA soils is likely to significantly change the observed microbial communities; however, the specific effects of this on microbial community functions relevant for RA outcomes are unknown.
Some changes in microbial community composition and function due to synthetic fertilizer use are likely to be caused by the indirect effects of fertilizers on vegetation and soil physiochemistry. Indeed, when assessing changes in fungal communities after nutrient inputs across different continents, it appears the changes are largely indirect and the result of nutrient-induced shifts in plant communities.72 This could explain why the exact nature of such changes varies among studies. Compared to synthetic fertilizers, organic amendments are higher in carbon content. This is thought to increase the inoculation capacity of AM fungi on plant roots as it can promote mycelial proliferation.73 Indeed, synthetic fertilizers are known to decrease AM fungal spore density and root colonization rates,74 suggesting that regenerative practices, which avoid such fertilizer application, might encourage more AM fungi-plant associations than conventional agriculture, allowing plants better access to nutrients already present in the soil via symbiotic partners. This would, in turn, reduce or remove the need for fertilizer use. However, whether or not AM fungi have a role in increasing production in an agricultural setting in the absence of nutrient supplementation remains contested.75 Further work is required to understand the impact of RA practices, in unison, on AM fungi and crop production.
Finally, synthetic inputs are not limited to fertilizers. The application of synthetic pesticides to reduce disease and thereby increase crop production can have far-reaching impacts from toxicity to non-target organisms, soil contamination, and impacting human health through food contamination.76 While the relationship between microbial communities and pesticide use has been less studied than for fertilizer, it is clear that there are observable impacts on the microbial communities including increases in microbial biomass carbon and microbial enzyme activity.77,78 Understanding how the elimination of all synthetic inputs changes the microbial communities is crucial to understanding the overall changes associated with RA-managed soils.
How should microbial investigations be conducted to evaluate RA?
The above-mentioned principles, which underpin RA and their expected impacts on soil microbial communities, highlight that adopting regenerative practices is likely to alter the soil microbiome significantly. However, studying these practices in isolation does not provide an accurate representation of the variation in microbial communities that we expect to find in regenerative soils. The outcomes of many of the management decisions themselves depend on the combinations of practices adopted and their interactions with local environmental conditions. To truly understand the ecosystem changes that will occur with the adoption of RA, we need to take a holistic approach by investigating RA systems rather than individual RA practices.
Given the wide variety of existing RA practices, microbial community structures, and geographical and habitat-specific circumstances, there is likely to be a wide variation in soil environments between RA sites, as well as a development of the soils over time under RA. This, in turn, would result in high spatiotemporal variation in soil microbiomes, when comparing different RA sites or tracking particular sites through time, which needs to be accounted for in research designs. It is crucial that comparative studies adopt large sample sizes so that patterns can be observed through the noise, while incorporating “conventional” sites which are paired with or closely matched to the RA sites. Furthermore, we know that previous land uses leave a “legacy” effect on the soil microbial communities, including important functions such as the cycling of nitrogen.79 Microbiomes under RA will therefore be structured not only by present practices but also by past land-use management, and changes are likely to be gradual. For some of the individual practices we described in the above section, we have some understanding of how microbial communities respond through time. For example, bacterial and non-AM fungal biomass “recovers” to pre-till levels within three years of a single tillage event, while AM fungal biomass does not.80 But for most studies cited above, results are based on a single time point and do not cover an adequate temporal change to assess long-term implications. Adding temporal components to research is crucial to understand the rates of change and the cumulative effects of time spent under RA. Alternatively, this can be achieved by using a space-for-time approach where sites that have been under RA management for various amounts of time are included. A combination of approaches, studying the soil microbiome at a range of spatial and temporal scales, will be required to enhance our understanding of the complexities of these phenomena under natural conditions.
Unlike much of the previous work described above, future research should focus not just on compositional changes but should assess the functional contribution of the soil microbiome. Microbial communities can show functional variance even if their compositions are similar,81 while compositionally different microbial communities can have the same functional potential due to functional redundancy.82 Despite most of the studies having focused only on compositional changes, the functional profiles of the soil microbiome will vary in RA sites compared to conventional farming. As discussed, the practices that make up RA will change the soil environment, and soils with different physicochemical properties are known to house microbial communities with different functional profiles.83 Given the importance of microbial functions to the soil environment, understanding how these functions change is critical for appreciating the overall impact of RA practices on the soil ecosystem.
Finally, soil microbial communities are most commonly investigated in only the top 10–15 cm of soil. However, the microbiomes in surface soils differ from those found in deeper soils.84 Particularly important is that the functions also change with depth, including functions likely to be of particular interest for RA, such as nutrient metabolism genes, because deeper soils contain fewer genes for the metabolism of nitrogen and more genes for phosphorous metabolism than surface soils.85 Given that RA practices are likely to impact more than just surface soils, for example, by encouraging deeper rootstocks, investigating the microbial community changes deeper in the soil profile will enhance our overall understanding of the impacts of regenerative practices on soil condition.
Conclusion
RA systems are complex and can be context specific; understanding these systems requires multidisciplinary investigations at a range of spatiotemporal scales and across geographic areas and farming systems. Soil microbial communities should not be overlooked in this research as we add more scientific knowledge to the field. Understanding the role of soil microbial communities in RA will not only allow the development of biologically relevant monitoring tools but potentially could also help us understand how microbial communities can be manipulated or supplemented to better support the goals of RA. Implementation of such practices supports meeting increasing global food demands while reducing the environmental burden of agricultural production.
Acknowledgments
SMH is supported by the Royal Society of New Zealand through a Rutherford Foundation Postdoctoral Fellowship.
Author contributions
Writing - original draft, SMH; visualization, SMH; writing – reviewing and editing, GL, BSC, and HLB.
Declaration of interests
The authors declare no competing interests.
References
- 1.Foley J.A., Ramankutty N., Brauman K.A., Cassidy E.S., Gerber J.S., Johnston M., Mueller N.D., O’Connell C., Ray D.K., West P.C., et al. Solutions for a cultivated planet. Nature. 2011;478:337–342. doi: 10.1038/nature10452. [DOI] [PubMed] [Google Scholar]
- 2.Bruinsma J. 2003. World Agriculture: Towards 2015/2030 : An FAO Perspective (Earthscan) [Google Scholar]
- 3.Kamilaris A., Assumpcio A., Blasi A.B., Torrellas M., Prenafeta-Boldú F.X. In: From Science to Society Progress in IS. Otjacques B., Hitzelberger P., Naumann S., Wohlgemuth V., editors. Springer International Publishing; 2018. Estimating the environmental impact of agriculture by means of geospatial and big data analysis: the case of catalonia; pp. 39–48. [DOI] [Google Scholar]
- 4.Emmerson M., Morales M.B., Oñate J.J., Batáry P., Berendse F., Liira J., Aavik T., Guerrero I., Bommarco R., Eggers S., et al. In: Advances in Ecological Research Large-Scale Ecology: Model Systems to Global Perspectives. Dumbrell A.J., Kordas R.L., Woodward G., editors. Academic Press; 2016. Chapter two - how agricultural intensification affects biodiversity and ecosystem services; pp. 43–97. [DOI] [Google Scholar]
- 5.LaCanne C.E., Lundgren J.G. Regenerative agriculture: merging farming and natural resource conservation profitably. PeerJ. 2018;6:e4428. doi: 10.7717/peerj.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton P., Civita N., Frankel-Goldwater L., Bartel K., Johns C. What is regenerative agriculture? A review of scholar and practitioner definitions based on processes and outcomes. Front. Sustain. Food Syst. 2020;4:577723. [Google Scholar]
- 7.Xu N., Bhadha J.H., Rabbany A., Swanson S. Soil health assessment of two regenerative farming practices on sandy soils. Sustain. Agric. Res. 2019;8:61–71. doi: 10.22004/ag.econ.301910. [DOI] [Google Scholar]
- 8.Fenster T.L.D., Oikawa P.Y., Lundgren J.G. Regenerative almond production systems improve soil health, biodiversity, and profit. Front. Sustain. Food Syst. 2021;5 doi: 10.3389/fsufs.2021.664359. [DOI] [Google Scholar]
- 9.Luján Soto R., Martínez-Mena M., Cuéllar Padilla M., de Vente J. Restoring soil quality of woody agroecosystems in Mediterranean drylands through regenerative agriculture. Agric. Ecosyst. Environ. 2021;306:107191. doi: 10.1016/j.agee.2020.107191. [DOI] [Google Scholar]
- 10.Kenne G.J., Kloot R.W. The carbon sequestration potential of regenerative farming practices in South Carolina, USA. Am. J. Clim. Change. 2019;08:157–172. doi: 10.4236/ajcc.2019.82009. [DOI] [Google Scholar]
- 11.Cai A., Han T., Ren T., Sanderman J., Rui Y., Wang B., Smith P., Xu M., Li Y. Declines in soil carbon storage under no tillage can be alleviated in the long run. Geoderma. 2022;425:116028. doi: 10.1016/j.geoderma.2022.116028. [DOI] [Google Scholar]
- 12.Holland J.M. The environmental consequences of adopting conservation tillage in Europe: reviewing the evidence. Agric. Ecosyst. Environ. 2004;103:1–25. doi: 10.1016/j.agee.2003.12.018. [DOI] [Google Scholar]
- 13.Blanco-Canqui H., Ruis S.J. No-tillage and soil physical environment. Geoderma. 2018;326:164–200. doi: 10.1016/j.geoderma.2018.03.011. [DOI] [Google Scholar]
- 14.Adetunji A.T., Ncube B., Mulidzi R., Lewu F.B. Management impact and benefit of cover crops on soil quality: a review. Soil Tillage Res. 2020;204:104717. doi: 10.1016/j.still.2020.104717. [DOI] [Google Scholar]
- 15.Shah K.K., Modi B., Pandey H.P., Subedi A., Aryal G., Pandey M., Shrestha J. Diversified crop rotation: an approach for sustainable agriculture production. Adv. Agric. 2021;2021:e8924087. doi: 10.1155/2021/8924087. [DOI] [Google Scholar]
- 16.Pembleton K.G., Tozer K.N., Edwards G.R., Jacobs J.L., Turner L.R., Pembleton K.G., Tozer K.N., Edwards G.R., Jacobs J.L., Turner L.R. Simple versus diverse pastures: opportunities and challenges in dairy systems. Anim. Prod. Sci. 2015;55:893–901. doi: 10.1071/AN14816. [DOI] [Google Scholar]
- 17.Distel R.A., Arroquy J.I., Lagrange S., Villalba J.J. Designing diverse agricultural pastures for improving ruminant production systems. Front. Sustain. Food Syst. 2020;4:596869. [Google Scholar]
- 18.Paungfoo-Lonhienne C., Visser J., Lonhienne T.G.A., Schmidt S. Past, present and future of organic nutrients. Plant Soil. 2012;359:1–18. doi: 10.1007/s11104-012-1357-6. [DOI] [Google Scholar]
- 19.Teague R., Barnes M. Grazing management that regenerates ecosystem function and grazingland livelihoods. Afr. J. Range Forage Sci. 2017;34:77–86. doi: 10.2989/10220119.2017.1334706. [DOI] [Google Scholar]
- 20.Lehman R.M., Acosta-Martinez V., Buyer J.S., Cambardella C.A., Collins H.P., Ducey T.F., Halvorson J.J., Jin V.L., Johnson J.M.F., Kremer R.J., et al. Soil biology for resilient, healthy soil. J. Soil Water Conserv. 2015;70:12A–18A. doi: 10.2489/jswc.70.1.12A. [DOI] [Google Scholar]
- 21.Frąc M., Hannula S.E., Bełka M., Jędryczka M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018;9:707. doi: 10.3389/fmicb.2018.00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz de Otálora X., Epelde L., Arranz J., Garbisu C., Ruiz R., Mandaluniz N. Regenerative rotational grazing management of dairy sheep increases springtime grass production and topsoil carbon storage. Ecol. Indicat. 2021;125:107484. doi: 10.1016/j.ecolind.2021.107484. [DOI] [Google Scholar]
- 23.Mendes L.W., de Lima Brossi M.J., Kuramae E.E., Tsai S.M. Land-use system shapes soil bacterial communities in Southeastern Amazon region. Appl. Soil Ecol. 2015;95:151–160. doi: 10.1016/j.apsoil.2015.06.005. [DOI] [Google Scholar]
- 24.Hermans S.M., Buckley H.L., Case B.S., Curran-Cournane F., Taylor M., Lear G. Bacteria as emerging indicators of soil condition. Appl. Environ. Microbiol. 2017;83:e02826-16. doi: 10.1128/AEM.02826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee S., Walder F., Büchi L., Meyer M., Held A.Y., Gattinger A., Keller T., Charles R., van der Heijden M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019;13:1722–1736. doi: 10.1038/s41396-019-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dini-Andreote F., van Elsas J.D. Modern Soil Microbiology. CRC Press; 2019. The soil microbiome—an overview. [Google Scholar]
- 27.Jansson J.K., Hofmockel K.S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020;18:35–46. doi: 10.1038/s41579-019-0265-7. [DOI] [PubMed] [Google Scholar]
- 28.Basu S., Kumar G., Chhabra S., Prasad R. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Verma J.P., Macdonald C.A., Gupta V.K., Podile A.R., editors. Elsevier; 2021. Chapter 13 - role of soil microbes in biogeochemical cycle for enhancing soil fertility; pp. 149–157. [DOI] [Google Scholar]
- 29.Lange M., Eisenhauer N., Sierra C.A., Bessler H., Engels C., Griffiths R.I., Mellado-Vázquez P.G., Malik A.A., Roy J., Scheu S., et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015;6:6707. doi: 10.1038/ncomms7707. [DOI] [PubMed] [Google Scholar]
- 30.Hallett P.D. A brief overview of the causes, impacts and amelioration of soil water repellency – a review. Soil Water Res. 2008;3:S21–S29. doi: 10.17221/1198-swr. [DOI] [Google Scholar]
- 31.Robinson D.A., Hopmans J.W., Filipovic V., van der Ploeg M., Lebron I., Jones S.B., Reinsch S., Jarvis N., Tuller M. Global environmental changes impact soil hydraulic functions through biophysical feedbacks. Glob. Chang. Biol. 2019;25:1895–1904. doi: 10.1111/gcb.14626. [DOI] [PubMed] [Google Scholar]
- 32.Hu H.-W., He J.-Z., Hu H.-W., He J.-Z. Manipulating the soil microbiome for improved nitrogen management. Microbiol. Aust. 2018;39:24–27. doi: 10.1071/MA18007. [DOI] [Google Scholar]
- 33.Hartman K., van der Heijden M.G.A., Wittwer R.A., Banerjee S., Walser J.-C., Schlaeppi K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome. 2018;6:14. doi: 10.1186/s40168-017-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parnell J.J., Berka R., Young H.A., Sturino J.M., Kang Y., Barnhart D.M., DiLeo M.V. From the lab to the farm: an industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 2016;7:1110. doi: 10.3389/fpls.2016.01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naidu Y., Meon S., Kadir J., Siddiqui Y. Microbial starter for the enhancement of biological activity of compost tea. Int. J. Agric. Biol. 2010;12:51–56. [Google Scholar]
- 36.Winder J. Alberta Environmentally Sustainable Agriculture (AESA) Soil Quality Monitoring Program. Alberta Agriculture, Food and Rural development, Conservation Branch; 2003. Soil quality monitoring programs: a literature review. [Google Scholar]
- 37.Lehmann J., Bossio D.A., Kögel-Knabner I., Rillig M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020;1:544–553. doi: 10.1038/s43017-020-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fierer N., Wood S.A., Bueno de Mesquita C.P. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 2021;153:108111. doi: 10.1016/j.soilbio.2020.108111. [DOI] [Google Scholar]
- 39.El Titi A. 1st Edition. CRC Press; 2002. Soil Tillage in Agroecosystems. [Google Scholar]
- 40.Hendrix P.F., Parmelee R.W., Crossley D.A., Coleman D.C., Odum E.P., Groffman P.M. Detritus food webs in conventional and No-tillage agroecosystems. Bioscience. 1986;36:374–380. doi: 10.2307/1310259. [DOI] [Google Scholar]
- 41.Zuber S.M., Villamil M.B. Meta-analysis approach to assess effect of tillage on microbial biomass and enzyme activities. Soil Biol. Biochem. 2016;97:176–187. doi: 10.1016/j.soilbio.2016.03.011. [DOI] [Google Scholar]
- 42.de Graaff M.-A., Hornslein N., Throop H.L., Kardol P., van Diepen L.T.A. In: Advances in Agronomy. Sparks D.L., editor. Academic Press; 2019. Chapter One - effects of agricultural intensification on soil biodiversity and implications for ecosystem functioning: a meta-analysis; pp. 1–44. [DOI] [Google Scholar]
- 43.Frøslev T.G., Nielsen I.B., Santos S.S., Barnes C.J., Bruun H.H., Ejrnæs R. The biodiversity effect of reduced tillage on soil microbiota. Ambio. 2022;51:1022–1033. doi: 10.1007/s13280-021-01611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta A., Dick W.A. Bacterial community diversity in soil under two tillage practices as determined by pyrosequencing. Microb. Ecol. 2015;70:853–859. doi: 10.1007/s00248-015-0609-4. [DOI] [PubMed] [Google Scholar]
- 45.Legrand F., Picot A., Cobo-Díaz J.F., Carof M., Chen W., Le Floch G. Effect of tillage and static abiotic soil properties on microbial diversity. Appl. Soil Ecol. 2018;132:135–145. doi: 10.1016/j.apsoil.2018.08.016. [DOI] [Google Scholar]
- 46.Klein D.A., Paschke M.W. A soil microbial community structural-functional index: the microscopy-based total/active/active fungal/bacterial (TA/AFB) biovolumes ratio. Appl. Soil Ecol. 2000;14:257–268. doi: 10.1016/S0929-1393(00)00061-5. [DOI] [Google Scholar]
- 47.Gao M., Li H., Li M. Effect of No tillage system on soil fungal community structure of cropland in mollisol: a case study. Front. Microbiol. 2022;13:847691. doi: 10.3389/fmicb.2022.847691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McClelland S.C., Paustian K., Schipanski M.E. Management of cover crops in temperate climates influences soil organic carbon stocks: a meta-analysis. Ecol. Appl. 2021;31:e02278. doi: 10.1002/eap.2278. [DOI] [PubMed] [Google Scholar]
- 49.Prescott C.E., Rui Y., Cotrufo M.F., Grayston S.J. Managing plant surplus carbon to generate soil organic matter in regenerative agriculture. J. Soil Water Conserv. 2021;76:99A–104A. doi: 10.2489/jswc.2021.0920A. [DOI] [Google Scholar]
- 50.Prober S.M., Leff J.W., Bates S.T., Borer E.T., Firn J., Harpole W.S., Lind E.M., Seabloom E.W., Adler P.B., Bakker J.D., et al. Plant diversity predicts beta but not alpha diversity of soil microbes across grasslands worldwide. Ecol. Lett. 2015;18:85–95. doi: 10.1111/ele.12381. [DOI] [PubMed] [Google Scholar]
- 51.King A.E., Hofmockel K.S. Diversified cropping systems support greater microbial cycling and retention of carbon and nitrogen. Agric. Ecosyst. Environ. 2017;240:66–76. doi: 10.1016/j.agee.2017.01.040. [DOI] [Google Scholar]
- 52.Kim N., Zabaloy M.C., Guan K., Villamil M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020;142:107701. doi: 10.1016/j.soilbio.2019.107701. [DOI] [Google Scholar]
- 53.Castle S.C., Samac D.A., Gutknecht J.L., Sadowsky M.J., Rosen C.J., Schlatter D., Kinkel L.L. Impacts of cover crops and nitrogen fertilization on agricultural soil fungal and bacterial communities. Plant Soil. 2021;466:139–150. doi: 10.1007/s11104-021-04976-z. [DOI] [Google Scholar]
- 54.Van Der Heijden M.G.A., Bardgett R.D., Van Straalen N.M. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 55.Tedersoo L., Bahram M., Zobel M. How mycorrhizal associations drive plant population and community biology. Science. 2020;367:eaba1223. doi: 10.1126/science.aba1223. [DOI] [PubMed] [Google Scholar]
- 56.Eisenhauer N., Lanoue A., Strecker T., Scheu S., Steinauer K., Thakur M.P., Mommer L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. 2017;7:44641. doi: 10.1038/srep44641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Six J., Frey S.D., Thiet R.K., Batten K.M. Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci. Soc. Am. J. 2006;70:555–569. doi: 10.2136/sssaj2004.0347. [DOI] [Google Scholar]
- 58.de Vries F.T., van Groenigen J.W., Hoffland E., Bloem J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol. Biochem. 2011;43:997–1005. doi: 10.1016/j.soilbio.2011.01.016. [DOI] [Google Scholar]
- 59.de Vries F.T., Thébault E., Liiri M., Birkhofer K., Tsiafouli M.A., Bjørnlund L., Bracht Jørgensen H., Brady M.V., Christensen S., de Ruiter P.C., et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl. Acad. Sci. USA. 2013;110:14296–14301. doi: 10.1073/pnas.1305198110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu J., Buckley H.L., Curry L., Stevenson B.A., Schipper L.A., Lear G. Livestock exclusion reduces the spillover effects of pastoral agriculture on soil bacterial communities in adjacent forest fragments. Environ. Microbiol. 2021;23:2919–2936. doi: 10.1111/1462-2920.15473. [DOI] [PubMed] [Google Scholar]
- 61.Jirout J., Šimek M., Elhottová D. Inputs of nitrogen and organic matter govern the composition of fungal communities in soil disturbed by overwintering cattle. Soil Biol. Biochem. 2011;43:647–656. doi: 10.1016/j.soilbio.2010.12.001. [DOI] [Google Scholar]
- 62.Wu Y., Chen D., Delgado-Baquerizo M., Liu S., Wang B., Wu J., Hu S., Bai Y. Long-term regional evidence of the effects of livestock grazing on soil microbial community structure and functions in surface and deep soil layers. Soil Biol. Biochem. 2022;168:108629. doi: 10.1016/j.soilbio.2022.108629. [DOI] [Google Scholar]
- 63.Yang Y., Wu L., Lin Q., Yuan M., Xu D., Yu H., Hu Y., Duan J., Li X., He Z., et al. Responses of the functional structure of soil microbial community to livestock grazing in the Tibetan alpine grassland. Glob. Chang. Biol. 2013;19:637–648. doi: 10.1111/gcb.12065. [DOI] [PubMed] [Google Scholar]
- 64.Ma X., Zhang Q., Zheng M., Gao Y., Yuan T., Hale L., Van Nostrand J.D., Zhou J., Wan S., Yang Y. Microbial functional traits are sensitive indicators of mild disturbance by lamb grazing. ISME J. 2019;13:1370–1373. doi: 10.1038/s41396-019-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y., Ashworth A.J., DeBruyn J.M., Durso L.M., Savin M., Cook K., Moore P.A., Jr., Owens P.R. Antimicrobial resistant gene prevalence in soils due to animal manure deposition and long-term pasture management. PeerJ. 2020;8:e10258. doi: 10.7717/peerj.10258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heyde M.v.d., Bennett J.A., Pither J., Hart M. Longterm effects of grazing on arbuscular mycorrhizal fungi. Agric. Ecosyst. Environ. 2017;243:27–33. doi: 10.1016/j.agee.2017.04.003. [DOI] [Google Scholar]
- 67.Zhao F., Ren C., Shelton S., Wang Z., Pang G., Chen J., Wang J. Grazing intensity influence soil microbial communities and their implications for soil respiration. Agric. Ecosyst. Environ. 2017;249:50–56. doi: 10.1016/j.agee.2017.08.007. [DOI] [Google Scholar]
- 68.Zhang X., Fang Q., Zhang T., Ma W., Velthof G.L., Hou Y., Oenema O., Zhang F. Benefits and trade-offs of replacing synthetic fertilizers by animal manures in crop production in China: a meta-analysis. Glob. Chang. Biol. 2020;26:888–900. doi: 10.1111/gcb.14826. [DOI] [PubMed] [Google Scholar]
- 69.Lin X., Feng Y., Zhang H., Chen R., Wang J., Zhang J., Chu H. Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in North China revealed by 454 pyrosequencing. Environ. Sci. Technol. 2012;46:5764–5771. doi: 10.1021/es3001695. [DOI] [PubMed] [Google Scholar]
- 70.Xiang X., Gibbons S.M., He J.-S., Wang C., He D., Li Q., Ni Y., Chu H. Rapid response of arbuscular mycorrhizal fungal communities to short-term fertilization in an alpine grassland on the Qinghai-Tibet Plateau. PeerJ. 2016;4:e2226. doi: 10.7717/peerj.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fierer N., Lauber C.L., Ramirez K.S., Zaneveld J., Bradford M.A., Knight R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012;6:1007–1017. doi: 10.1038/ismej.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lekberg Y., Arnillas C.A., Borer E.T., Bullington L.S., Fierer N., Kennedy P.G., Leff J.W., Luis A.D., Seabloom E.W., Henning J.A. Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nat. Commun. 2021;12:3484. doi: 10.1038/s41467-021-23605-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J., Li J., Yang Y., Wang Y., Zhang Y., Wang P. Effects of conventional and organic agriculture on soil arbuscular mycorrhizal fungal community in low-quality farmland. Front. Microbiol. 2022;13:914627. doi: 10.3389/fmicb.2022.914627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin C., Wang Y., Liu M., Li Q., Xiao W., Song X. Effects of nitrogen deposition and phosphorus addition on arbuscular mycorrhizal fungi of Chinese fir (Cunninghamia lanceolata) Sci. Rep. 2020;10:12260. doi: 10.1038/s41598-020-69213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryan M.H., Graham J.H. Is there a role for arbuscular mycorrhizal fungi in production agriculture? Plant Soil. 2002;244:263–271. doi: 10.1023/A:1020207631893. [DOI] [Google Scholar]
- 76.Tudi M., Daniel Ruan H., Wang L., Lyu J., Sadler R., Connell D., Chu C., Phung D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health. 2021;18:1112. doi: 10.3390/ijerph18031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arora S., Sahni D. Pesticides effect on soil microbial ecology and enzyme activity- an overview. J. Appl. Nat. Sci. 2016;8:1126–1132. doi: 10.31018/jans.v8i2.929. [DOI] [Google Scholar]
- 78.Wołejko E., Jabłońska-Trypuć A., Wydro U., Butarewicz A., Łozowicka B. Soil biological activity as an indicator of soil pollution with pesticides – a review. Appl. Soil Ecol. 2020;147:103356. doi: 10.1016/j.apsoil.2019.09.006. [DOI] [Google Scholar]
- 79.Hermans S.M., Taylor M., Grelet G., Curran-Cournane F., Buckley H.L., Handley K.M., Lear G. From pine to pasture: land use history has long-term impacts on soil bacterial community composition and functional potential. FEMS Microbiol. Ecol. 2020;96:fiaa041. doi: 10.1093/femsec/fiaa041. [DOI] [PubMed] [Google Scholar]
- 80.Wortmann C.S., Quincke J.A., Drijber R.A., Mamo M., Franti T. Soil microbial community change and recovery after one-time tillage of continuous No-till. Agron. J. 2008;100:1681–1686. doi: 10.2134/agronj2007.0317. [DOI] [Google Scholar]
- 81.Castañeda L.E., Barbosa O. Metagenomic analysis exploring taxonomic and functional diversity of soil microbial communities in Chilean vineyards and surrounding native forests. PeerJ. 2017;5:e3098. doi: 10.7717/peerj.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Louca S., Polz M.F., Mazel F., Albright M.B.N., Huber J.A., O’Connor M.I., Ackermann M., Hahn A.S., Srivastava D.S., Crowe S.A., et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018;2:936–943. doi: 10.1038/s41559-018-0519-1. [DOI] [PubMed] [Google Scholar]
- 83.Bahram M., Hildebrand F., Forslund S.K., Anderson J.L., Soudzilovskaia N.A., Bodegom P.M., Bengtsson-Palme J., Anslan S., Coelho L.P., Harend H., et al. Structure and function of the global topsoil microbiome. Nature. 2018;560:233–237. doi: 10.1038/s41586-018-0386-6. [DOI] [PubMed] [Google Scholar]
- 84.Eilers K.G., Debenport S., Anderson S., Fierer N. Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol. Biochem. 2012;50:58–65. doi: 10.1016/j.soilbio.2012.03.011. [DOI] [Google Scholar]
- 85.Rchiad Z., Dai M., Hamel C., Bainard L.D., Cade-Menun B.J., Terrat Y., St-Arnaud M., Hijri M. Soil depth significantly shifted microbial community structures and functions in a semiarid prairie agroecosystem. Front. Microbiol. 2022;13:815890. doi: 10.3389/fmicb.2022.815890. [DOI] [PMC free article] [PubMed] [Google Scholar]