Summary
Phosphoenolpyruvate carboxykinase (PCK) plays a critical role in cytosolic gluconeogenesis, and defects in PCK1 cause a fasting-aggravated metabolic disease with hypoglycemia and lactic acidosis. However, there are two genes encoding PCK, and the role of the mitochondrial resident PCK (encoded by PCK2) is unclear, since gluconeogenesis is cytosolic. We identified three patients in two families with biallelic variants in PCK2. One has compound heterozygous variants (p.Ser23Ter/p.Pro170Leu), and the other two (siblings) have homozygous p.Arg193Ter variation. All three patients have weakness and abnormal gait, an absence of PCK2 protein, and profound reduction in PCK2 activity in fibroblasts, but no obvious metabolic phenotype. Nerve conduction studies showed reduced conduction velocities with temporal dispersion and conduction block compatible with a demyelinating peripheral neuropathy. To validate the association between PCK2 variants and clinical disease, we generated a mouse knockout model of PCK2 deficiency. The animals present abnormal nerve conduction studies and peripheral nerve pathology, corroborating the human phenotype. In total, we conclude that biallelic variants in PCK2 cause a neurogenetic disorder featuring abnormal gait and peripheral neuropathy.
Keywords: PCK2, peripheral neuropathy, mitochondria, neurogenetic
We have identified biallelic pathogenic variants in PCK2 in three individuals with a neurogenetic condition that features abnormal gait and evidence of peripheral neuropathy. We provide supportive data from patient fibroblasts of mutation impact on PCK2 expression and activity, and from a Pck2 knockout mouse model that corroborates the peripheral neuropathy phenotype.
Introduction
Phosphoenolpyruvate carboxykinase (PCK) plays a critical role in intermediary metabolism, converting the tricarboxylic acid cycle intermediate oxaloacetate into the gluconeogenic precursor phosphoenolpyruvate. There are two human genes encoding PCK. Cytosolic phosphoenolpyruvate carboxykinase (PCK1) encodes a protein with clear roles in hepatic gluconeogenesis, and loss-of-function variants cause a syndrome (MIM 261680) dominated by hypoglycemia during fasting.1 Mitochondrial phosphoenolpyruvate carboxykinase (PCK2) encodes a protein with 70% identity to PCK1 that is targeted to the mitochondrial matrix and expressed primarily in pancreas, kidney, liver, and fibroblasts.2
The cellular role of PCK2 is unclear, as gluconeogenesis is cytosolic. The mitochondrial PCK2 has kinetic properties similar to those of the cytosolic form and can substitute for PCK1 when targeted to the cytosol.3 One hypothesis specific to its function in the mitochondrial matrix is that PCK2 is required for the consumption of mitochondrial guanosine triphosphate (GTP) and the regulation of pancreatic insulin secretion.4 However, an animal model deficient in Pck2, while having impaired glucose-stimulated insulin secretion, was non-diabetic.5
While inherited defects in mitochondrial PCK2 were previously claimed in the medical literature (MIM 261650),6,7 none of the patients described were known or found to have pathogenic variants in PCK2, making it unlikely that the disorder was correctly described.8 In this study, we identify three individuals with biallelic PCK2 variants and a phenotype consistent with an inherited neurogenetic condition featuring abnormal gait and peripheral neuropathy.
Results
Case presentations
Using exome sequencing, we identified three patients in two families with a common phenotype and likely pathogenic variants in PCK2 (Figure 1A). Patient 1 came to medical attention as a 3-year-old girl with ataxia and weakness, which was exacerbated by illness. Specifically, she experienced several episodes of severe gait instability resulting in transient loss of ambulation and necessitating hospitalization. Between episodes she was found to have mild, persistent gait ataxia and mild distal > proximal weakness.
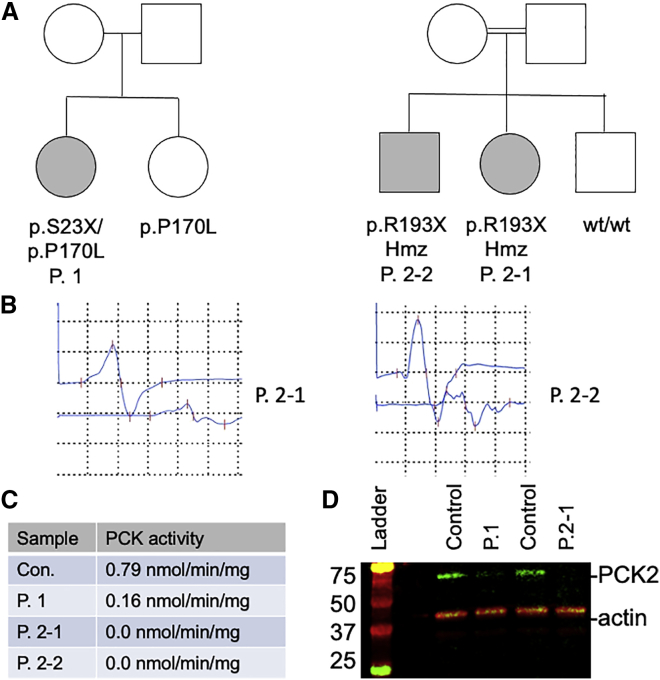
Figure 1.
PCK2 variants are associated with peripheral neuropathy
(A) Pedigrees from two families with biallelic variants in PCK2.
(B) Nerve conduction studies (NCS) from right tibial nerve. In patient P.2-1, NCS showed low proximal and distal compound muscle action potential (CMAP) amplitudes (2.5 mV and 0.79 mV, respectively) with reduced conduction velocity (31.1 m/s) and temporal dispersion. In patient P.2-2, NCS showed low proximal and distal CMAP amplitudes (0.89 mV and 0.25 mV, respectively) with reduced conduction velocity (32.3 m/s) and temporal dispersion.
(C) Reduction in PCK2 activity in fibroblast lysates from patients harboring biallelic PCK2 variants (Con., unrelated normal control).
(D) Western blot of fibroblast lysates from patients P.1 and P.2-1 and from two unrelated controls. Actin was provided as a loading control.
Owing to the episodic nature of the presentation, particularly given exacerbation with illness, an inborn error of metabolism was suspected and investigated. Mild elevations in lactate were occasionally noted (2.4–3.5 mM during her initial hospitalization) but metabolic studies were otherwise unremarkable, and a 24-h fasting study provoked a ketotic response (β-hydroxybutyrate 0.8 mM at 24 h).
She was lost to follow-up for many years until she presented again in adulthood. She continues to experience gait ataxia but has maintained independent ambulation. No additional diagnostic data were able to be obtained; of note, nerve conduction studies have never been performed.
Whole-exome sequencing identified p.Ser23Ter (c.68C>G [NM004563.4]) and p.Pro170Leu (c.509C>T) variants in PCK2 and no other potentially causative variants. An unaffected sibling carried the p.Pro170Leu variant only. The allele frequency of p.Ser23Ter is 1.87 × 10−3 and p.Pro170Leu is 4.78 × 10−5 in a database of large-scale sequencing projects (gnomAD v2.1.1, May 1, 2021 release).9 The prevalence of p.Ser23Ter is unexpectedly high for a loss-of-function allele. Of note, a single p.Ser23Ter homozygous individual is found enumerated in GnomAD.
A second family was identified after referral of a 12-year old girl (patient 2-1) for abnormal gait. Her gait changes were progressive, with symptom onset at age 10 years and with increasing clumsiness and falls over an approximate span of 2 years. Family history revealed that her older brother (patient 2-2) was similarly affected and that the parents are first cousins. Physical examination of the proband was notable for distal muscle atrophy and bilateral pes cavus, distal muscle weakness, and absent reflexes in the knees and ankles. Sensory exam was abnormal in the lower extremities to all modalities. Of note, both the proband and her brother had evidence of asymmetry in terms of muscle atrophy and weakness. Unlike with individual 1, there was no clear demonstration of fluctuation in symptomatology or worsening with illness in either sibling.
Nerve conduction studies, performed on both siblings, showed a sensory-motor demyelinating peripheral neuropathy with evidence of temporal dispersion and conduction block (Figure 1B and Table S1). As with the physical exam, there were asymmetrical changes in nerve conduction. Metabolic studies, including lactate, pyruvate, amino acids, and urine organic acids, were unremarkable. The proband was initially diagnosed, based on the nerve conduction studies, with chronic inflammatory demyelinating polyneuropathy and treated with intravenous immunoglobulin. After failure of response to therapy, genetic testing was pursued. PMP22 deletion/duplication testing was normal, and a Charcot-Marie-Tooth disease multigene panel was unremarkable. Whole-exome sequencing identified homozygous p.Arg193Ter variants in PCK2 in both siblings (c.577C>T [NM004563.4]), which had a population allele frequency of 8.14 × 10−5. A third sibling in the family had normal gait and did not carry the variant. Of note, no other disease-associated pathogenic variants were identified in either sibling.
Validation studies: Patient fibroblasts show reduced PCK2 levels and activity
Fibroblast samples were obtained from all patients. Since PCK1 is not expressed in fibroblasts,2 enzymatic studies of PCK activity in these cells reflects PCK2 alone. There was a partial loss of fibroblast PCK2 activity in fibroblasts from patient 1, and a complete loss of activity in cells from patients 2-1 and 2-2 (Figure 1C). Western blotting of fibroblast lysates confirmed a partial loss of detectable PCK2 in patient 1 and a complete loss of protein in patient 2-1 (Figure 1D). Oximetry studies of intact fibroblasts showed no significant difference in basal or maximal oxygen consumption between control cells and cells from patient 2-1 (Figure S1), consistent with a lack of defect in mitochondrial oxidative phosphorylation.
Characterization of a Pck2 knockout mouse line
To further investigate the connection between the phenotype and PCK2 variants, we created and evaluated a mouse model of Pck2 deficiency (Pck2_em1_del or Pck2 KO). This mouse line, generated on a C57BL/6NCrl background, harbors a homozygous 252 bp deletion in Pck2, resulting in a p.Ser142Hisfs∗21 nonsense mutation (see supplemental material and methods for details of the generation of this strain). Homozygous animals were viable with no overt phenotype but were obtained at slightly below expected frequency (22%; 53/240), with a mild bias toward male survival (30% of males homozygous, 15% of females homozygous).
Murine embryonic fibroblast (MEF) lines were created from wild-type, heterozygous, and homozygous Pck2 KO animals. Western blotting of the cell lines showed an absence of protein in the Pck2 KO animals and a partial loss of protein in the heterozygotes (Figure 2A). Studies of PCK activity in lysates showed a corresponding loss of activity in Pck2 KO MEFs compared with wild-type MEFs (wild type = 0.57 ± 0.04 nmol/min/mg protein, Pck2 KO = 0.0 nmol/min/mg protein).
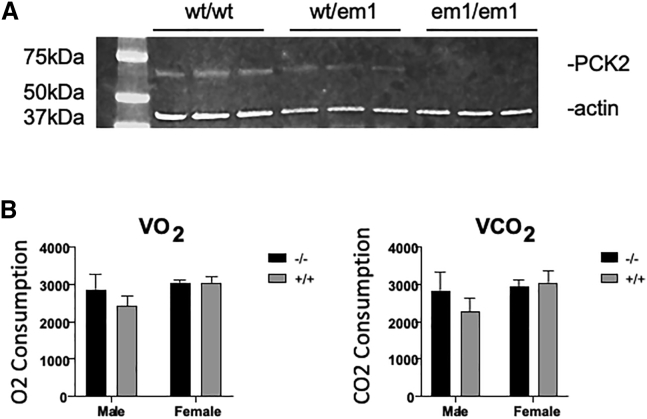
Figure 2.
Characterization of mouse embryonic fibroblasts from Pck2 knockout mice
(A) Western blotting of wild-type (wt), heterozygous (wt/em1), and Pck2 knockout (em1/em1) animals. Actin was used as a control. Protein extracts were derived from mouse embryonic fibroblasts for the indicated genotypes.
(B) Metabolic studies of Pck2 knockout compared with wild-type mice (n = 8 animals studied).
VO2 p = 0.20 and VCO2 p = 0.017 by two-way ANOVA without Bonferroni correction.
We performed a battery of phenotypic analyses on the Pck2 KO animals, comparing them with wild-type littermates, with independent evaluation of both male and female animals because of the observed difference in viability. A small difference was seen in metabolic studies in the males, with increased volume of oxygen consumed (VO2) and volume of CO2 produced (VCO2) in the KO animals that was no longer significant after correction for multiple testing (Figure 2B). Treadmill testing was used to evaluate the animal’s gait. Minor differences were noted in gait, primarily in female animals, but these again were not significant after correction for multiple testing.
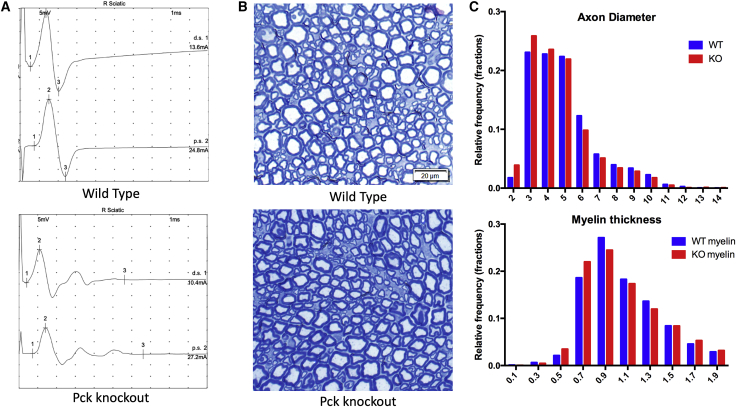
We then focused additional investigations on the peripheral nervous system. Pck2 KO animals and wild-type littermates were evaluated by nerve conduction studies, where we observed in KO mice an asymmetric reduction of the conduction velocities (Figure 3A), mainly in males, associated with variable penetrance. Some of the KO mice also showed mild temporal dispersion, a finding not seen in any wild-type mice. Considering 40 m/s as the lower limit of normal for sural nerve conduction velocity, 6 of 15 Pck2 KO mice had reduced values (i.e., <40 m/s) while only 1 of 15 wild-type littermates showed a reduction (Table S2). Lastly, we examined for peripheral nerve pathology using both light and electron microscopy. No obvious structural abnormalities were consistently observed. Quantitative analysis of sciatic nerves, however, revealed variability in both axon and myelin sheath diameter (Figure 3B), with significant shifts to disproportionate amounts of small or large fibers (Figures 3C and S2). In total, these findings support the presence in KO mice of a demyelinating peripheral neuropathy of variable penetrance.
Figure 3.
Peripheral nervous system changes in Pck2 knockout mice
(A) Sciatic nerve conduction studies of Pck2 knockout mice reveal reduced conduction velocities and temporal dispersion (Table S2) (n = 15 per genotype). Shown are representative tracings from a wild-type littermate and a Pck2 knockout mouse.
(B) Representative semi-thin sections of sciatic nerve. No overt abnormalities were seen, although there was the appearance of an increased proportion of small fibers.
(C) Quantification of axon diameter and myelin thickness as represented by frequency distribution plots. Difference in distribution between genotypes was p = 0.0001 (Wilcoxon rank test, n = 8 per genotype with >50 fibers measured per nerve/animal).
Discussion
In summary, our studies demonstrate that the loss of PCK2 causes a neurogenetic disorder featuring abnormal gait with evidence of demyelinating peripheral neuropathy. This phenotype stands in contrast to the decompensating metabolic condition that has been associated with the loss of PCK1. The difference in phenotype is likely related to the targeting of PCK to the mitochondrial matrix and its expression in a different set of tissues. There is no evidence of any diabetic phenotype in the patients or animal model, although there were small changes in mitochondrial coupling in the male Pck2 KO animals.
Previous studies of PCK2 posited a role in pancreatic insulin release. However, none of our patients are diabetic, and patient 1 had an unremarkable glucose tolerance test as a child. Although PCK2 is apparently highly expressed in liver and pancreas,2 we observed no deficits in our patients or in the KO mice corresponding to hepatic or endocrine or exocrine pancreatic function. We conclude that a loss of mitochondrial PCK activity is not diabetogenic.
The clinical phenotype, at least in family 2 and also supported by the mouse studies, is in keeping with a recessive form of Charcot-Marie-Tooth disease (CMT type 4) or hereditary motor and sensory neuropathy. A key unanswered question of this study concerns the mechanism through which the loss of mitochondrial PCK activity causes peripheral neuropathy. Previous studies have implicated PCK2 in the maintenance of neuronal progenitor state in cell culture,10 and loss of this function may explain the histopathological finding of excessive variability of axon diameter. However, it is unclear how this finding relates to the demyelination changes observed in our patients or to the temporal dispersion of nerve conduction signals seen in both patients and mice. From a pathomechanistic prospective, PCK2 deficiency fits with the broader subgroup of peripheral neuropathies due to abnormalities in mitochondrial structure and/or function, a group that includes mutations in MFN2 (CMT2A), SURF1 (CMT4K), and PDK3 (CMTX6).
Of note, neither isoform of PCK is highly expressed in the central nervous system, although PCK2 is detectable in many brain regions where PCK1 expression is absent. A study of dogs with paroxysmal dyskinesia has recently implicated a heterozygous variant in the GTP-binding domain of PCK2 as causative.11 One possible avenue for future studies is investigating whether the clearance of GTP in the mitochondrial matrix by PCK may be important for the maintenance of neuronal health.
The phenotype described in our patients is relatively mild in that all three individuals have maintained the ability to ambulate independently. Given the relatively high allele frequency of loss-of-function variants in PCK2, particularly the p.Ser23Ter allele, combined with mild phenotypic presentations, we posit that deficiency in PCK2 may be a fairly common genetic cause of peripheral neuropathy that is significantly underdiagnosed. As the sequencing of patients with ataxia, gait disturbance, and/or abnormal nerve conduction studies becomes more common, we suspect that PCK2 variants will be increasingly recognized as a cause of human neurological disease.
Acknowledgments
We thank the patients and families for agreeing to participate in this study. This study was funded in part through a Rare Disease Models and Mechanisms grant from the Canadian Institutes of Health Research (N.S., M.S.). Additional support was provided by the Mogford Campbell Family endowed chair fund (J.J.D.). We thank the Toronto Center for Phenogenomics for assistance with the Pck2 knockout mouse model.
Declaration of interests
N.S. is an employee of Synlogic, and K.A. is a part-time employee of Deep Genomics.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2023.100182.
Contributor Information
Neal Sondheimer, Email: neal.sondheimer@sickkids.ca.
James J. Dowling., Email: james.dowling@sickkids.ca.
Supplemental information
References
- 1.Vieira P., Cameron J., Rahikkala E., Keski-Filppula R., Zhang L.H., Santra S., Matthews A., Myllynen P., Nuutinen M., Moilanen J.S., et al. Novel homozygous PCK1 mutation causing cytosolic phosphoenolpyruvate carboxykinase deficiency presenting as childhood hypoglycemia, an abnormal pattern of urine metabolites and liver dysfunction. Mol. Genet. Metabol. 2017;120:337–341. doi: 10.1016/j.ymgme.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Modaressi S., Brechtel K., Christ B., Jungermann K. Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression. Biochem. J. 1998;333:359–366. doi: 10.1042/bj3330359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escós M., Latorre P., Hidalgo J., Hurtado-Guerrero R., Carrodeguas J.A., López-Buesa P. Kinetic and functional properties of human mitochondrial phosphoenolpyruvate carboxykinase. Biochem. Biophys. Rep. 2016;7:124–129. doi: 10.1016/j.bbrep.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stark R., Pasquel F., Turcu A., Pongratz R.L., Roden M., Cline G.W., Shulman G.I., Kibbey R.G. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J. Biol. Chem. 2009;284:26578–26590. doi: 10.1074/jbc.M109.011775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abulizi A., Cardone R.L., Stark R., Lewandowski S.L., Zhao X., Hillion J., Ma L., Sehgal R., Alves T.C., Thomas C., et al. Multi-Tissue acceleration of the mitochondrial phosphoenolpyruvate cycle improves whole-body metabolic health. Cell Metabol. 2020;32:751–766.e11. doi: 10.1016/j.cmet.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson B.H., Taylor J., Sherwood W.G. The genetic heterogeneity of lactic acidosis: occurrence of recognizable inborn errors of metabolism in pediatric population with lactic acidosis. Pediatr. Res. 1980;14:956–962. doi: 10.1203/00006450-198008000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Clayton P.T., Hyland K., Brand M., Leonard J.V. Mitochondrial phosphoenolpyruvate carboxykinase deficiency. Eur. J. Pediatr. 1986;145:46–50. doi: 10.1007/BF00441851. [DOI] [PubMed] [Google Scholar]
- 8.Bodnar A.G., Cooper J.M., Holt I.J., Leonard J.V., Schapira A.H. Nuclear complementation restores mtDNA levels in cultured cells from a patient with mtDNA depletion. Am. J. Hum. Genet. 1993;53:663–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Álvarez Z., Hyroššová P., Perales J.C., Alcántara S. Neuronal progenitor maintenance requires lactate metabolism and PEPCK-M-directed cataplerosis. Cerebr. Cortex. 2016;26:1046–1058. doi: 10.1093/cercor/bhu281. [DOI] [PubMed] [Google Scholar]
- 11.Nessler J., Hug P., Mandigers P.J.J., Leegwater P.A.J., Jagannathan V., Das A.M., Rosati M., Matiasek K., Sewell A.C., Kornberg M., et al. Mitochondrial PCK2 missense variant in shetland sheepdogs with paroxysmal exercise-induced dyskinesia (PED) Genes. 2020;11:774–815. doi: 10.3390/genes11070774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.