Abstract
Hemoplasmas can cause severe hemolytic anemia in humans. To explore the genetic diversity and the potential transmission routes of hemoplasmas among bat population, bats and bat-ectoparasites including bat-flies, bat-mites, and bat-ticks were collected in Eastern and Central China from 2015 to 2021, and tested with PCR for hemoplasmas 16S rRNA gene. Based on 16S rRNA PCR, 18.0% (103/572) adult bats were positive for hemoplasmas, but none of 11 fetuses from hemoplasmas-positive pregnant bats was positive for hemoplasmas. These results indicated that adult bats had a high prevalence of hemoplasma, but vertical transmission of hemoplasmas did not occurr in the bats. Based on the 16S rRNA gene PCR, the minimum infection rate of bat-ectoparasite for hemoplasmas was 4.0% (27/676), suggesting that bat-ectoparasite also had a high prevalence for hemoplasmas. Phylogenetic analysis revealed that bat hemoplasmas from this study clustered into 4 genotypes (I-IV). Genotype I clustered together with hemoplasmas identified in bats from America. Genotype II shared high similarity with a human-pathogenic hemoplasma Candidatus Mycoplasma haemohominis. Genotype III and IV were unique, representing 2 new hemoplasma genotypes. Only genotype I was identified in both bats and all bat-ectoparasites including bat-flies, bat-mites, and bat-ticks. In conclusion, bats and bat-ectoparasites from China harbored abundant genetically diverse hemoplasmas including potential human-pathogenic hemoplasmas, indicating bats and bat-ectoparasites may play important roles in the maintenance and transmission of hemoplasmas in the natural foci.
Keywords: Bat, Ectoparasites, Hemoplasmas, Hemotropic mycoplasmas, Genetic diversity
Highlights
-
•
Bats from China carry a diversity of novel hemoplasmas with a high prevalence, including potential human-pathogens.
-
•
Bat-ectoparasites including fly, mite, and tick also carry hemoplasmas and they may be the vectors of hemoplasmas in bats.
1. Introduction
Bats belong to the order Chiroptera, which is the second largest order of mammals next to Rodentia, and are geographically widespread, especially abundant in the tropical and subtropical regions. As the only mammal that develop the true capability of powered flight, bats can travel a long distance, and have a wide range of activities. Bats are important sources of emerging infectious diseases, and they are considered as natural reservoirs of several viruses that cause severe diseases in humans, such as Hendra virus, Nipah virus, Marburg virus, MERS coronavirus, SARS coronavirus [1,2]. During the past two decades, a large number of novel viruses have been identified in bats worldwide [3], while bacterial pathogens in bats remain much more neglected [4].
Hemoplasmas, also known as hemotropic mycoplasmas, are epierythrocytic parasites within the order Mycoplasmatales, which were formerly known as Haemobartonella and Eperythrozoon. They are unculturable, cell wall-less bacteria that parasitize on the surface of erythrocytes of diverse mammals [5]. Hemoplasmas infections in humans and animals vary from asymptomatic to severe hemolytic anemia [6]. A diversity of hemoplasmas was identified in a wide range of wild and domestic animals [[7], [8], [9], [10]]. Several hemoplasmas were reported to cause human infection, including Candidatus Mycoplasma haematoparvum from dog, M. ovis from sheep, M. haemofelis from cat, and M. suis from pig [[11], [12], [13], [14]] (Fig. 1).
Fig. 1.
Map showing current knowledge on human infection with epierythrocytic mycoplasmas and bat-related hemoplasmas. Countries or regions that reported human cases were marked with the pentagram (★). Countries or regions that reported hemoplasmas in bats and bat-ectoparasites were highlighted and marked with icons (bat , bat-fly
, bat-fly , bat-tick
, bat-tick ).
).
A diversity of novel hemoplasmas was identified in bats from several countries, mostly in the Americas (the United States, Chile, Belize, Brazil, Peru, and Costa Rica), also in Australia, New Caledonia, Nigeria, Germany, and Spain [[15], [16], [17], [18], [19], [20], [21], [22], [23]] (Fig. 1). However, there is a lack of understanding on the genetic diversity of hemoplasmas in bats from Asia. Cases of human infection of Candidatus Mycoplasma haemohominis, which usually leads to severe febrile splenomegaly and autoimmune hemolytic anemia, even spleen rupture with fatal outcomes, have been reported world widely including the United Kingdom, Japan, New Caledonia, and Australia [16,[24], [25], [26]] in recent years (Fig. 1). Thus, Ca. M. haemohominis is likely an emerging disease in humans, and that evidence in New Caledonia [16] suggests zoonotic potential from bats. Currently, it is unknown how hemoplasmas are maintained and transmitted among bats. Although researchers suggested that hemoplasmas in bats appear to be transmitted through direct contact and arthropods [6], more evidence is in need to clarify the transmission mode of hemoplasmas among bat population.
In this study, the prevalence and genetic diversity of hemoplasmas in bats and bat-ectoparasites from Eastern and Central China was investigated. Besides, the potential roles of vector-borne transmission and vertical transmission of hemoplasmas among bats were evaluated.
2. Materials and methods
2.1. Ethics statement
The bat capture for microbiological studies was approved by the Ethics Committee of the Medical School, Wuhan University (WHU2020-YF0023).
2.2. Sampling and species identification of bats and bats ectoparasites
Bats and bat ectoparasites were sampled during an ongoing program of identifying pathogens in bats. Three rural areas were selected for sample collection sites including 1 site from a prefectural city (Linyi city, 117°51′58.60“ E / 35°42’10.58” N) in Shandong Province in Eastern China, 2 areas from 2 prefectural cities (Xianning city, 114°18′50.63“ E / 29°47’5.83” N, and Jingzhou city 111°51′3.25“ E / 30°7’44.85” N) in Hubei Province in Central China. Mist nets were settled near the entrance of karst caves before sunset. Once captured, bats were sacrificed by inhaling ethyl ether, and transported to the laboratories on ice immediately. Information about bat species, sex, and reproductive status was recorded. Bat-ectoparasites were collected from the wing and fur of bats using forceps. Bat blood and organ samples were collected and stored at −80 °C before analysis. Bats and ectoparasites were identified by taxonomic morphological keys [[27], [28], [29], [30]], and 3–5 samples of each species were subjected to PCR assay targeting mitochondrial cytochrome B (cytB) gene, mitochondrial cytochrome oxidase subunit I (COI) or 16S rRNA gene to confirm the accuracy of the classification [[31], [32], [33]] (Table S1). For bat samples lack of morphological information, species were identified by amplifying and sequencing cytB gene as described above.
2.3. Molecular detection of hemoplasmas in bats and bat-ectoparasites
DNA was extracted from bat blood or liver samples, bat-flies, bat-mites, and bat-ticks using Trelief Animal Genomic DNA Kit (Tsingke, Beijing, China). All the DNA samples were stored at −20 °C before analysis.
For hemoplasma detection, a conventional PCR targeting a 600 bp fragment of the 16S rRNA was performed as previously described [34] (Table S1) for initial screening. PCR was carried out under the following conditions: an initial denaturing cycle at 95 °C for 5min followed by 35 cycles at 95 °C for 30 s, 65 °C for 30 s, and 72 °C for 60 s, and a final extension cycle at 72 °C for 10min. Negative controls were set in each PCR assay using nuclease-free water instead of DNA as PCR template. To avoid cross-contamination, no positive control was included. PCR products were analyzed by electrophoresis on 1.2% agarose gel stained with Goldview™ Nucleic Acid Gel Stain (10,000×) (GL biotech, Shanghai, China). Positive samples were additionally subjected to PCR assays targeting a longer fragment of the 16S rRNA gene, and a 300 bp fragment of the 23S rRNA gene of hemoplasmas (Table S1). Agarose gel bands with expected size were excised, and extracted with SanPrep Column DNA Gel Extraction Kit (Sangon Biotech, Shanghai, China). Purified PCR products were directly sequenced, or inserted into T-Vector pMD19 (Simple) (Takara, Shiga, Japan) for TA cloning. At least three positive colonies of each sample were sequenced with universal primers M13–47 and M13–48.
2.4. Phylogenetic analysis
Chromatograms were checked with Chromas 2.5.1 (Technelysium, Tewantin, QLD, Australia) to ensure the accuracy of sequencing. Qualified sequences were then compared to the existing sequences deposited in GenBank with Basic Local Alignment Search Tool (BLAST) on National Center for Biotechnology Information (NCBI). Sequences from this study and reference sequences downloaded from the GenBank database were imported into MEGA-X (https://www.megasoftware.net), and aligned with Cluster W. Best-fit evolutionary model was chosen following Bayesian Information Criterion (BIC) to build phylogenetic trees, and Mycoplasma pneumoniae was used as outgroup. A haplotype data file of 16S rRNA was generated in DnaSP 5 (http://www.ub.edu/dnasp/) using aligned sequences, and a nucleotide sequence type (ntST) network was then conducted in PopArt (http://popart.otago.ac.nz/index.shtml) with Median Joining network method [35].
2.5. Statistical analyses
The positive rate of hemoplasmas by sample type was analyzed using Chi-square test. Information of sex, and reproductive status (pregnant or not) was only collected in samples in 2021, and details of ectoparasite index was not recorded. Hence, in order to evaluate the prevalence of hemoplasmas by these factors, Chi-square test or Fisher's exact test was performed using available data in 2021 with SPSS 21.0 (https://www.ibm.com/analytics/spss-statistics-software), and differences were statistically significant if P values were <0.05.
3. Results
3.1. Sampling and species identification of bats and bat-ectoparasites
In total, 608 bats including 572 adults, and 36 fetuses were collected from rural areas in three prefectural cities (Linyi, Xianning, Jingzhou) in Shandong, and Hubei provinces from 2015 to 2021. The bats were classified into 12 species of 3 families, including Miniopterus schreibersii in the family of Miniopteridae, Hypsugo alaschanicus, Eptesicus serotinus, Myotis adversus, Myotis altarium, Myotis davidii, Myotis fimbriatus, Myotis laniger, Myotis pequinius, and Myotis ricketti in the family of Vespertilionidae, Rhinolophus pusillus, and Rhinolophus ferrumequinum in the family of Rhinolophidae (Table 1).
Table 1.
Sampling information and prevalence of hemoplasmas in bats from Shandong and Hubei provinces, China from 2015 to 2021.
| Sampling time | Sampling area | Host animal | Sample type | No. of samples | No. of positive samples (%) |
|---|---|---|---|---|---|
| March – October 2015 | Mengyin District, Linyi, Shandong | Rhinolophidae | |||
| R. ferrumequinum | blood | 2 | 0 | ||
| Vespertilionidae | |||||
| E. serotinus | blood | 23 | 1 (4.3) | ||
| M. ricketti | 5 | 1 (20.0) | |||
| M. fimbriatus | 11 | 5 (45.5) | |||
| M. penquinius | 48 | 0 | |||
| Subtotal | 89 | 7 (7.9) | |||
| May 2018 | Xianan District, Xianning, Hubei | Miniopteridae | |||
| M. schreibersii | liver | 8 | 0 | ||
| Vespertilionidae | |||||
| H. alaschanicus | liver | 4 | 0 | ||
| E. serotinus | 2 | 0 | |||
| M. adversus | 28 | 0 | |||
| M. altarium | 2 | 0 | |||
| M. davidii | 36 | 1 (2.7) | |||
| M. laniger | 2 | 0 | |||
| M. fimbriatus | 3 | 0 | |||
| M. pequinius | 53 | 0 | |||
| M. ricketti | 2 | 0 | |||
| Subtotal | 140 | 1 (0.7%) | |||
| September 2019 | Songzi District, Jingzhou, Hubei | Miniopteridae | |||
| M. schreibersii | liver | 3 | 0 | ||
| Vespertilionidae | |||||
| M. davidii | liver | 13 | 7 (53.8) | ||
| Subtotal | 16 | 7 (43.8) | |||
| July 2020 | Tongshan County, Xianning, Hubei | Vespertilionidae | |||
| M. adversus | liver | 24 | 1 (4.2) | ||
| M. laniger | 1 | 0 | |||
| M. davidii | 87 | 1 (1.1) | |||
| blood | 15 | 0 | |||
| Rhinolophidae | |||||
| R. pusillus | liver | 56 | 4 (7.1) | ||
| blood | 18 | 2 (11.1) | |||
| Subtotal | 201 | 8 (4.0) | |||
| May 2021 | Tongshan County, Xianning, Hubei | Miniopteridae | |||
| M. schreibersii | blood | 4 (1) | 4 (80) | ||
| liver | 2 (2) | 0 | |||
| Vespertilionidae | |||||
| M. adversus | blood | 47 (1) | 40 (85.1) | ||
| M. davidii | blood | 48 (7) | 34 (70.8) | ||
| liver | 25 (25) | 2 (8) | |||
| Subtotal | 126 (36) | 80 (64.0) | |||
| Total | 572(36) | 103 (18.0) | |||
Note: Subscripts in the parentheses represented the number of pregnant bats.
Abbreviations: R. ferrumequinum, Rhinolophus ferrumequinum; E. serotinus, Eptesicus serotinus; M. ricketti, Myotis ricketti; M. fimbriatus, Myotis fimbriatus; M. penquinius, Myotis pequinius; M. schreibersii, Miniopterus schreibersii; H. alaschanicus, Hypsugo alaschanicus; M. adversus, Myotis adversus; M. altarium, Myotis altarium; M. davidii, Myotis davidii; M. laniger, Myotis laniger; R. pusillus, Rhinolophus pusillus.
A total of 676 bat-ectoparasites including bat-flies, bat-mites, and bat-ticks were retrieved from bats collected in Tongshan, Xianning City, Hubei Province from 2020 to 2021. Based on the taxonomic morphological keys, bat-flies were classified into two species: the larger bat-flies (Penicillidia monoceros), and the smaller bat-flies (Nycteribia sp.). Bat-mites were classified into Spinturnix sp. and Eyndhovenia sp., and all bat-ticks were identified as Ixodes simplex. The larger bat-flies and adult bat-ticks were individually processed, while the smaller bat-flies, bat-mites, and bat-tick nymphs were pooled for DNA extraction (2–20 individuals per pool). Species identification of bat-ectoparasites collected in 2020 were described previously [36]. Together, 300 DNA samples were obtained from bat-ectoparasites (Table 2).
Table 2.
Sampling information and prevalence of hemoplasmas in bat ectoparasites (bat-flies, bat-mites, and bat-ticks) from Hubei Province, China from 2020 to 2021.
| Sampling time | Sampling area | Ectoparasites | No. of samples | No. of DNA samples | No. of positive samples | Minimum infection rate (%) |
|---|---|---|---|---|---|---|
| July 2020 | Tongshan County, Xianning City, Hubei Province | Nycteribiidae | ||||
| P. monoceros | 119 | 119 | 11 | 9.2 | ||
| Spinturnicidae | ||||||
| Spinturnix sp. | 200 | 11‡ | 1 | 0.5 | ||
| Eyndhovenia sp. | ||||||
| Subtotal | 319 | 130 | 12 | 3.8 | ||
| May 2021 | Tongshan County, Xianning City, Hubei Province | Nycteribiidae | ||||
| P. monoceros | 88 | 88 | 7 | 8.0 | ||
| Nycteribia sp. | 40 | 13‡ | 1 | 2.5 | ||
| Spinturnicidae | ||||||
| Spinturnix sp. | 140 | 22‡ | 4 | 2.9 | ||
| Eyndhovenia sp. | ||||||
| Ixodidae | ||||||
| I. simplex | 26 | 26 | 2 | 7.7 | ||
| 63 | 21‡ | 1 | 1.6 | |||
| Subtotal | 357 | 170 | 15 | 4.2 | ||
| Total | 676 | 300 | 27 | 4.0 | ||
Abbreviations: P. monoceros, Penicillidia monoceros; I. simplex, Ixodes simplex.
Samples in pools.
Representative sequences of bat species (cytB) and bat-ectoparasites (16S rRNA, COI) were deposited in the GenBank with accession numbers KX655809-KX655810, KX655817, KX655826, KX655831, KX655837, KX655840, MH888177-MH888183, MH888178, MW085077-MW085079, OP779865-OP779887 (bat), OP758801-OP758805 (bat-fly), MZ483874- MZ483876, MZ238086- MZ238089 (bat-mite), OP750378-OP750380 (bat-tick).
3.2. Prevalence of hemoplasmas in bats and bat-ectoparasites
The 16S rRNA PCR showed that 18.0% (103/572) bats were hemoplasma positive. Seven out of 12 bat species were positive for hemoplasmas, including M. schreibersii in the family of Miniopteridae, E. serotinus, M. adversus, M. davidii, M. fimbriatus, and M. ricketti in the family of Vespertilionidae, and R. pusilus in the family of Rhinolophidae (Table 1). For bats sampled during the breeding season, 31.4% (11/36) pregnant bats were hemoplasma-positive, but their fetuses were all negative to hemoplasmas. No statistical difference was observed between the positive rate among bat sexes (P = 0.39 > 0.05), or female bats with different reproductive status (P = 1.00 > 0.05).
We obtained blood, liver, and spleen from each bat collected from Tongshan, Hubei Province in 2021. Thus, we determined the hemoplasma positive rate in different bat sample types. The 16S rRNA PCR showed that the positive rate in blood, liver, and spleen were 78.8% (78/99), 21.7% (20/92), and 2.3% (2/88), respectively. The hemoplasma infection rate is significantly high in blood sample than in livers and spleen tissues (χ2 = 130.5, P < 0.001, Table S2).
The hemoplasma positive rate in bat-ectoparasites collected from Tongshan, Xianning, Hubei was 4.0% (27/676). For bat-flies, the hemoplasma positive rate in the larger bat-flies (P. monoceros) collected in 2020 and 2021 were 9.2% (11/119) and 8.0% (7/88), respectively; and for the smaller bat-flies (Nycteribia sp.) collected in 2021, the minimum infection rate (MIR) was 2.5% (1/40). The hemoplasma MIR for bat-mites (Spinturnix sp., and Eyndhovenia sp.) collected in 2020 and 2021 were 0.5% (1/200) and 2.9% (4/140), respectively. For bat-ticks (I. simplex) collected in 2021, the hemoplasma positive rate was 7.7% (2/26) for adult ticks, and 1.6% (1/63) for nymph ticks, respectively (Table 2). Statistical difference in hemoplasma positive rate was observed between larger bat-flies and bat-mites in 2020 (χ2 = 13.4, P < 0.001).
3.3. Phylogenetic analysis
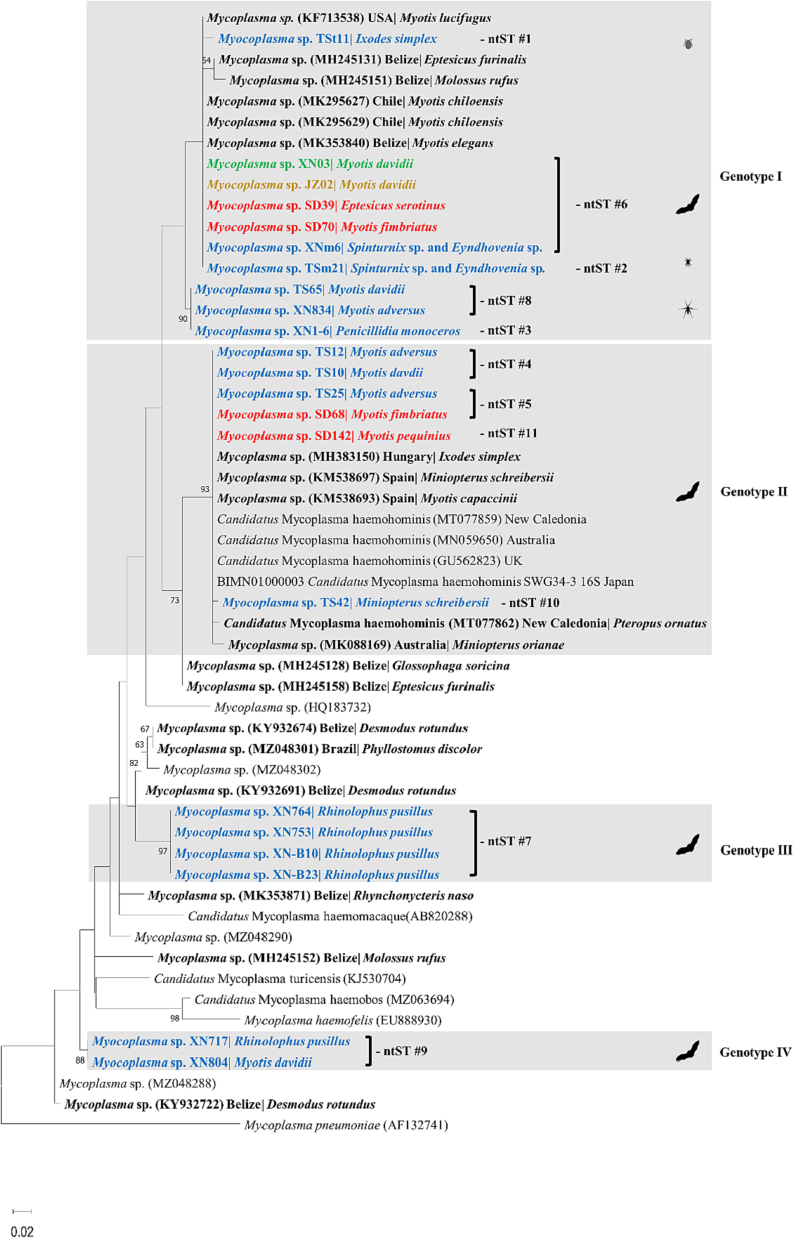
Of the 130 hemoplasma positive samples, 16S rRNA gene sequences were obtained from 85 samples with intense PCR bands. Based on the phylogenetic analysis of the 16S rRNA gene, hemoplasmas identified in bats and their ectoparasites were divided into four genotypes (I-IV), among them 2 hemoplasma strains might represent novel genotypes of hemoplasmas (III-IV) (Fig. 3). Eleven ntSTs of hemoplasmas were characterized in this study, with 5 in genotype I (ntST 1, 2, 3, 6, 8), 4 in genotype II (ntST 4, 5, 10, 11), 1 in genotype III (ntST 7), and 1 in genotype IV (ntST 9) (Table 3, Fig. 2, Fig. 3).
Fig. 3.
Nucleotide sequence type (ntST) network with hemoplasma 16S rRNA sequences (approximately 535 bp). Each pie chart represented a different ntST, and pie size was scaled by the ntST frequency, while different pie color corresponding to different sample species. Genotypes defined by phylogenetic analysis were indicated in red circles. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Genotypes, ntST, BLASTn results of bat-borne hemoplasmas based on 16S rRNA gene and their corresponding sample information of bats and ectoparasites.
| Genotype | No. of sequenced samples | ntST | GenBank Accession number | Accession number of the closest match | Nucleotide identity (%) | Species | No. of sequences | Sampling site | Sampling year |
|---|---|---|---|---|---|---|---|---|---|
| I | 53 | 1 | ON426383 | MK295627 | 99.1 | I. simplex | 1 | Xianning | 2021 |
| M. davidii | 1 | Xianning | 2021 | ||||||
| 2 | ON426384 | 99.3 | Spinturnix sp., Eyndhovenia sp. | 1‡ | Xianning | 2021 | |||
| 3 | ON426385 | 98.3 | P. monoceros | 1 | Xianning | 2020 | |||
| M. davidii | 2 | Xianning | 2021 | ||||||
| 6 | ON426388 | MK295627 | 99.6 | M. adversus | 13 | Xianning | 2021 | ||
| M. davidii | 1 | Xianning | 2018 | ||||||
| 4 | Jingzhou | 2019 | |||||||
| 9 | Xianning | 2021 | |||||||
| M. fimbriatus | 1 | Linyi | 2015 | ||||||
| E. serotinus | 1 | Linyi | 2015 | ||||||
| P. monoceros | 1 | Xianning | 2020 | ||||||
| 3 | Xianning | 2021 | |||||||
| Spinturnix sp., Eyndhovenia sp. | 1‡ | Xianning | 2021 | ||||||
| Spinturnix sp., Eyndhovenia sp. | 1‡ | Xianning | 2020 | ||||||
| I. simplex | 1‡ | Xianning | 2021 | ||||||
| 8 | ON426390 | MK295627 | 98.5 | M. davidii | 6 | Xianning | 2021 | ||
| M. adversus | 1 | Xianning | 2020 | ||||||
| 4 | Xianning | 2021 | |||||||
| II | 26 | 4 | ON426386 | KM538693 | 99.8 | M. davidii | 11 | Xianning | 2021 |
| M. adversus | 8 | Xianning | 2021 | ||||||
| 5 | ON426387 | KM538693 | 99.6 | M. adversus | 1 | Xianning | 2021 | ||
| M. fimbriatus | 3 | Linyi | 2015 | ||||||
| 10 | ON426392 | KM538698 | 99.6 | M. schreibersii | 2 | Xianning | 2021 | ||
| 11 | ON426393 | KM538693 | 99.8 | M. pequinius | 1 | Linyi | 2015 | ||
| III | 4 | 7 | ON426395 | KY932691 | 95.0 | R. pusillus | 4 | Xianning | 2020 |
| ON426396 | Xianning | 2020 | |||||||
| ON426397 | Xianning | 2020 | |||||||
| ON426389 | Xianning | 2020 | |||||||
| IV | 2 | 9 | ON426391 | KY932722 | 95.5 | R. pusillus | 1 | Xianning | 2020 |
| ON426394 | M. davidii | 1 | Xianning | 2020 | |||||
| Total | 85 | 85 |
Note: ‡ Samples in pools.
Fig. 2.
Phylogenetic analysis of hemoplasmas based on 16S rRNA gene (approximately 562 bp). The phylogenetic tree was constructed using the Maximum-likelihood method, Kimura 2-parameter model with Gamma distribution (K2 + G) and 1000 bootstrap analysis in MEGA-X. Sequences from this study were color-coded and sequences obtained from bats and bat-ectoparasites were shown in bold. Genotypes detected in this study were highlighted in grey boxes.
The first clade including 53 sequences obtained in this study and was assigned as genotype I, which clustered together with hemoplasmas detected in Myotis bats from the Americas (Chile, Belize, and the United States). This genotype was detected in bats (E. serotinus, M. adversus, M. davidii, and M. fimbriatus), bat-flies (P. monoceros), bat-mites (Spinturnix sp., and Eyndhovenia sp.), and bat-ticks (I. simplex), and was identified in all sampling sites (Fig. 2). Hemoplasmas identified in 26 bats positioned in the second clade, namely genotype II, clustering within the human-pathogenic Ca. M. haemohominis group. This genotype was detected in M. fimbriatus and M. pequinius from Linyi, Shandong Province in 2015, and M. adversus, M. davidii, and M. schreibersii from Tongshan, Hubei Province in 2021 (Fig. 2). Four sequences exclusively from Rhinolophus bats formed a monophyletic clade, and were assigned as genotype III. The final clade comprising two sequences from Rhinolophus pusillus and Myotis davidii, positioned distant from other hemoplasma sequences (Fig. 2).
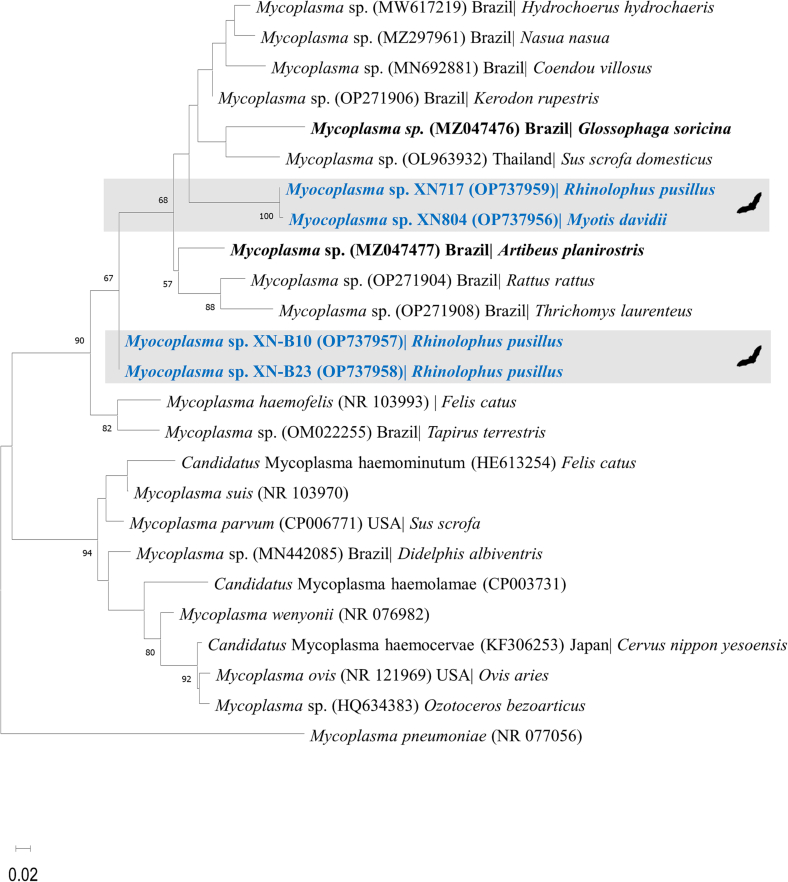
The two novel genotypes (III and IV) were further characterized with 23S rRNA gene phylogentic analysis. Phylogenetic tree of the 23S rRNA gene sequence (280 bp) showed that hemoplasmas were divided into 2 major clades (Fig. 4). The first clade, including two sequences from R. pusillus and M. davidii, positioned next to a group including a Glossophaga soricina bat (accession number MZ047476) in Brazil and other mammals. The second clade comprising 2 sequences from R. pusillus, clustered separately from other sequences within the Hemofelis group.
Fig. 4.
Phylogenetic analysis of hemoplasmas based on 23S rRNA gene (approximately 270 bp). The phylogenetic tree was constructed using the Maximum-likelihood method, Kimura 2-parameter model with Gamma distribution (K2 + G) and 1000 bootstrap analysis in MEGA-X. Sequences obtained from bats were shown in bold.
The hemoplasma DNA sequences obtained in this study were deposited in the GenBank with accession numbers ON426383–ON426397 and OP737956–OP737959.
4. Discussion
Our results demonstrated that bats from China carried abundant genetic diverse hemoplasmas. Hemoplasmas were identified in bats M. fimbriatus, M. penquinius, and E. serotinus in the family of Vespertilionidae, and R. pusillus in the family of Rhinolophidae for the first time, expanding our knowledge on the host range and geographic distribution of bat-related hemoplasmas.
4.1. Prevalence of hemoplasmas in bats
The overall prevalence of hemoplasmas in bats in this study is very high (18.0%, 103/572) but it still lower than those reports from Americas and Europe, which reported the hemoplasma infection rate in bats reaching to nearly 50–100% (43.0% (58/135) in Brazil [18], 51.0% (239/469) in Belize [15], 96.8% (30/31) in Spain [17]. The difference of hemoplasma PCR positive rate in this study and previous studies may be caused by different infection rate of hemoplasmas due to different locations and species of bats and genetic diversity of hemoplasmas affecting PCR sensitivity. Another possible reason is that tissue tropism of hemoplasmas. We have demonstrated the PCR positive rate of hemoplasmas was significantly high in blood samples than in liver and spleen tissues. This is a retrospective study and most samples of bats used were liver tissues. Therefore, our study may underestimate the hemoplasma prevalence in bats.
The prevalence of hemoplasmas was remarkably high in bats collected from Jingzhou, in 2019 (43.8% using liver) and Xianning, Hubei Province in 2021 (63.0% using blood and liver). A recent laboratory experiment revealed that Mycoplasma haemomuris-like bacterium infection in rodents reached peak infection level after 25–45 days post infection and stabilized in low bacterial loads for the rodents' lifetime [37]. Bats in the present study might share the same pattern of infection: bats with high frequency of infection, might be during an acute outbreak with high hemoplasma concentrations, while for bats in stable chronic stage of hemoplasmas, they may have a low bacterial load and leading to low PCR positive rate. This phenomenon was also observed in bats and other mammals in Brazil [18,38].
4.2. Genetic diversity and host specificity of hemoplasmas in bats
Bats and bat-ectoparasites harbored abundant genetic diverse hemoplasmas based on 16S rRNA gene. Genotype I, previously reported in bats from North and South America, was the major hemoplasmas identified in this study (Table 3). Genotype II, which positioned closely with human-pathogenic Ca. M. haemohominis clade, was identified in bats collected from the two provinces in China. Drs. Drancourt M. and Raoult D. proposed a sequence-based identification of a new bacterium that two bacteria are considered as two different species if their 16S rRNA genes share <97.0% similarity [39]. According to this criterion, genotype III and IV sharing 95.5% and 95.0% nucleotide similarity with other hemoplasma sequences in the GenBank, indicated they are novel genotypes. Phylogenetic analysis of 23S rRNA further characterized their novelty. Besides, Genotype III was exclusively identified in R. pusilus bats, while genotypes I and II were mainly found in bats belong to the genera of Myotis and Miniopterus. Host specificity was also observed in bats from Belize and Brazil, suggesting that hemoplasma evolution might be linked to bat speciation [15,18]. Inter-species transmission of hemoplasmas could happen among bats. A study in Nigeria found that one genotype was associated with both Eidolon and Rhinolophus bats [40]. Here, genotype IV was identified in both Myotis and Rhinolophus bats. Future studies are needed to elucidate the evolution of hemoplasmas in bats.
4.3. Public health significance of bat hemoplasmas
Ca. M. haemohominis infection in humans usually led to serious outcomes. The first human case of Ca. M. haemohominis was reported in 2011 in the UK [26]. Subsequently, human cases were also reported in New Caledonia, Australia, and Japan [16,24,25] (Fig. 2). It is reported that this pathogen has also been identified in bats in the family of Miniopteridae from Spain, Pteropondidae in New Caledonia, Pteropondidae and Molossidae in Nigeria [16,17,40]. Especially in New Caledonia, researchers found that Ca. M. haemohominis strains identified in patients, local flying foxes, and parasitic bat-flies were highly similar [16], indicating zoonotic potential from bats, and that bats might be natural hosts of Ca. M. haemohominis. We herein identified bats in the family of Vespertilionidae and Miniopteridae carried hemoplasmas which closely related to the human-pathogenic Ca. M haemohominis in China, and local physicians and officials should be alert to the potential emergence of human infections.
4.4. Transmission routes of hemoplasmas in bats
The transmission mechanism of hemoplasmas among animals remained unclarified, and direct contact, arthropod borne, airborne, and vertical transmission were postulated [6]. Bats are infested by a variety of ectoparasites, such as bat-flies, bat-mites, and bat-ticks. As a blood-borne pathogen, blood-sucking arthropods might play an important role in the transmission of hemoplasmas among bats. Previous studies reported the identification of hemoplasmas in bat-flies [16,18], and bat-ticks [41]. In this study hemoplasmas were identified in bat-flies (Nycteribiidae: P. monoceros, and Nycteribia sp.), bat-mites (Spinturnicidae: Spinturnix sp., and Eyndhovenia sp.), and bat-ticks (Ixodidae: I. simplex). These results indicated that bat-ectoparasites may play a role in transmission of hemoplasmas to bats, and bat-ectoparasites together with their hosts may be important in the maintenance and transmission of hemoplasmas in natural foci. The limitation of our study is that we could not demonstrate whether the hemoplasmas were the same between the host and their ectoparasites because we did not register the host-ectoparasite relationships. The role of ectoparasites as the vector and reservoir of hemoplasmas need to be confirmed by transmission experiments of hemoplasmas in laboratory animals.
Previous studies revealed that tick and flea might play a less important role in the transmission of hemoplasmas in rodents, dogs, and grey foxes [37,42,43]. An experiment found that none of the recipient rodents became infected after infested by fleas removed from hemoplasma-positive rodent donors, suggesting that flea-borne transmission might not play an important role in the transmission of hemoplasmas in rodents [37]. No strong association was found between hemoplasma infection status in dogs and the presence of parasitic ticks [42], or between hemoplasma prevalence in grey foxes and fleas [43]. A study in Brazil found that parasitic ticks and fleas from hemoplasma infected wild animals were negative [44].
Erythrocytic pathogens might be transmitted vertically from mother to baby during pregnancy and delivery. Previous study found that the prevalence and bacterial loads of M. suis in newborn but pre-suckling piglets were higher than sows, indicating the existence of vertical transmission [45]. Vertical transmission of hemoplasmas was also reported in dogs, cria, and cattle [[46], [47], [48]]. However, a laboratory infection experiment did not observe the evidence of vertical transmission in rodents [37]. In this study, fetuses of 11 hemoplasma-positive pregnant bats were negative for hemoplasmas, indicating that transplacental vertical transmission of hemoplasmas was unlikely to occur in bats. However, our result might be biased by the small sample size.
In conclusion, bats and bat-ectoparasites from China harbored abundant genetic diverse hemoplasmas including potential human-pathogenic hemoplasmas, indicating bats and bat-ectoparasites may play important roles in the maintenance and transmission of hemoplasmas in the natural foci.
Authors' contributions
HJH, XJY, and RW designed the experiments. RW conducted the experiments with the help of ZML, QMP, and XLG. RW performed the result analysis. XX, CMZ, HJH, RW, ZML, and QMP carried out the field work. RW wrote the original manuscript. HJH and XJY revised the manuscript. All the authors read and approved the final manuscript.
Fundings
This work was supported by the National Nature Science Funds of China (grant number: 81971939), the China Postdoctoral Science Foundation Funded Project (grant number: 2019M662720), and the Innovation Research of Hubei Postdoctoral Science and Technology.
Declaration of Competing Interest
The authors declare no conflict of interest.
Contributor Information
Rui Wang, Email: rhea_wang@whu.edu.cn.
Xiao-Lan Gu, Email: guxiaolan@whu.edu.cn.
Chuan-Min Zhou, Email: 00033465@whu.edu.cn.
Hui-Ju Han, Email: 17731811382@163.com.
Xue-Jie Yu, Email: yuxuejie@whu.edu.cn.
Appendix A. Appendices
The following are the supplementary data related to this article.
Primers used for species identification of bats and bat-ectoparasites and molecular detection of hemoplasmas.
Comparison of the prevalence of hemoplasmas in blood, liver, and spleen samples of bats from Tongshan, Hubei Province, China, 2021.
Data availability
Data will be made available on request.
References
- 1.Olival K.J., Hosseini P.R., Zambrana-Torrelio C., Ross N., Bogich T.L., Daszak P. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546(7660) doi: 10.1038/nature22975. 646–50. Epub 2017/06/22. PubMed PMID: 28636590; PubMed Central PMCID: PMCPMC5570460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han H.J., Wen H.L., Zhou C.M., Chen F.F., Luo L.M., Liu J.W., et al. Bats as reservoirs of severe emerging infectious diseases. Virus Res. 2015;205:1–6. doi: 10.1016/j.virusres.2015.05.006. Epub 2015/05/23. PubMed PMID: 25997928; PubMed Central PMCID: PMCPMC7132474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson C.J., Gibb R.J., Albery G.F., Brierley L., Connor R.P., Dallas T.A., et al. The Global Virome in One Network (VIRION): an atlas of vertebrate-virus associations. mBio. 2022;13(2) doi: 10.1128/mbio.02985-21. e0298521. Epub 2022/03/02. PubMed PMID: 35229639; PubMed Central PMCID: PMCPMC8941870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muhldorfer K. Bats and bacterial pathogens: a review. Zoonoses Public Health. 2013;60(1):93–103. doi: 10.1111/j.1863-2378.2012.01536.x. 22862791 Epub 2012/08/07. [DOI] [PubMed] [Google Scholar]
- 5.Messick J.B. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet. Clin. Pathol. 2004;33(1):2–13. doi: 10.1111/j.1939-165x.2004.tb00342.x. 15048620 Epub 2004/03/30. [DOI] [PubMed] [Google Scholar]
- 6.Millan J., Di Cataldo S., Volokhov D.V., Becker D.J. Worldwide occurrence of haemoplasmas in wildlife: insights into the patterns of infection, transmission, pathology and zoonotic potential. Transbound. Emerg. Dis. 2021;68(6):3236–3256. doi: 10.1111/tbed.13932. 33210822 Epub 2020/11/20. [DOI] [PubMed] [Google Scholar]
- 7.Byamukama B., Tumwebaze M.A., Tayebwa D.S., Byaruhanga J., Angwe M.K., Li J., et al. First molecular detection and characterization of hemotropic Mycoplasma species in Cattle and Goats from Uganda. Animals (Basel) 2020;10(9) doi: 10.3390/ani10091624. Epub 2020/09/16. PubMed PMID: 32927890; PubMed Central PMCID: PMCPMC7552329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes A.J., Elshafie N.O., Kmetiuk L.B., Ullmann L.S., Brandao A.P.D., Haisi A., et al. Hemotropic mycoplasmas (hemoplasmas) in wild boars, hunting dogs, and hunters from two Brazilian regions. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14038. Epub 2021/02/20. PubMed PMID: 33605554. [DOI] [PubMed] [Google Scholar]
- 9.Lashnits E., Neupane P., Bradley J.M., Richardson T., Thomas R., Linder K.E., et al. Molecular prevalence of Bartonella, Babesia, and hemotropic Mycoplasma species in dogs with hemangiosarcoma from across the United States. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227234. e0227234. Epub 2020/01/11. PubMed PMID: 31923195; PubMed Central PMCID: PMCPMC6953799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massini P.F., Drozino R.N., Otomura F.H., Mongruel A.C.B., Valente J.D.M., Toledo M.J.O., et al. Detection of Hemotropic Mycoplasma sp. in white-eared opossums (Didelphis albiventris) from southern Brazil. Rev. Bras. Parasitol. Vet. 2019;28(4):797–801. doi: 10.1590/s1984-29612019058. Epub 2019/08/08. PubMed PMID: 31390439. [DOI] [PubMed] [Google Scholar]
- 11.dos Santos A.P., dos Santos R.P., Biondo A.W., Dora J.M., Goldani L.Z., de Oliveira S.T., et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg. Infect. Dis. 2008;14(12) doi: 10.3201/eid1412.080964. 1922–4. Epub 2008/12/03. PubMed PMID: 19046522; PubMed Central PMCID: PMCPMC2634649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maggi R.G., Compton S.M., Trull C.L., Mascarelli P.E., Mozayeni B.R., Breitschwerdt E.B. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J. Clin. Microbiol. 2013;51(10) doi: 10.1128/JCM.01125-13. 3237–41. Epub 2013/07/19. PubMed PMID: 23863574; PubMed Central PMCID: PMCPMC3811635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sykes J.E., Lindsay L.L., Maggi R.G., Breitschwerdt E.B. Human coinfection with Bartonella henselae and two hemotropic mycoplasma variants resembling Mycoplasma ovis. J. Clin. Microbiol. 2010;48(10) doi: 10.1128/JCM.01029-10. 3782–5. Epub 2010/08/13. PubMed PMID: 20702675; PubMed Central PMCID: PMCPMC2953074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan C.L., Liang A.B., Yao C.B., Yang Z.B., Zhu J.G., Cui L., et al. Prevalence of Mycoplasma suis (Eperythrozoon suis) infection in swine and swine-farm workers in Shanghai, China. Am. J. Vet. Res. 2009;70(7):890–894. doi: 10.2460/ajvr.70.7.890. Epub 2009/07/02. PubMed PMID: 19566474. [DOI] [PubMed] [Google Scholar]
- 15.Becker D.J., Speer K.A., Brown A.M., Fenton M.B., Washburne A.D., Altizer S., et al. Ecological and evolutionary drivers of haemoplasma infection and bacterial genotype sharing in a Neotropical bat community. Mol. Ecol. 2020;29(8) doi: 10.1111/mec.15422. 1534–49. Epub 2020/04/04. PubMed PMID: 32243630; PubMed Central PMCID: PMCPMC8299350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Descloux E., Mediannikov O., Gourinat A.C., Colot J., Chauvet M., Mermoud I., et al. Flying fox hemolytic fever, description of a new zoonosis caused by Candidatus Mycoplasma haemohominis. Clin. Infect. Dis. 2021;73(7) doi: 10.1093/cid/ciaa1648. e1445-e53. Epub 2020/10/30. PubMed PMID: 33119064. [DOI] [PubMed] [Google Scholar]
- 17.Millan J., Lopez-Roig M., Delicado V., Serra-Cobo J., Esperon F. Widespread infection with hemotropic mycoplasmas in bats in Spain, including a hemoplasma closely related to “Candidatus Mycoplasma hemohominis”. Comp. Immunol. Microbiol. Infect. Dis. 2015;39:9–12. doi: 10.1016/j.cimid.2015.01.002. Epub 2015/02/07. PubMed PMID: 25655409. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda P., Torres J.M., Lourenco E.C., Albery G.F., Herrera H.M., de Oliveira C.E., et al. Molecular detection and genotype diversity of hemoplasmas in non-hematophagous bats and associated ectoparasites sampled in peri-urban areas from Brazil. Acta Trop. 2022;225 doi: 10.1016/j.actatropica.2021.106203. 106203. Epub 2021/10/25. PubMed PMID: 34688630. [DOI] [PubMed] [Google Scholar]
- 19.Fritschi J., Marti H., Seth-Smith H.M.B., Aeby S., Greub G., Meli M.L., et al. Prevalence and phylogeny of Chlamydiae and hemotropic mycoplasma species in captive and free-living bats. BMC Microbiol. 2020;20(1):182. doi: 10.1186/s12866-020-01872-x. Epub 2020/06/28. PubMed PMID: 32590949; PubMed Central PMCID: PMCPMC7318495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holz P.H., Lumsden L.F., Legione A.R., Hufschmid J. Polychromophilus melanipherus and haemoplasma infections not associated with clinical signs in southern bent-winged bats (Miniopterus orianae bassanii) and eastern bent-winged bats (Miniopterus orianae oceanensis) Int. J. Parasitol. Parasites Wildl. 2019;8 doi: 10.1016/j.ijppaw.2018.11.008. 10–8. Epub 2019/01/09. PubMed PMID: 30619705; PubMed Central PMCID: PMCPMC6287050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascarelli P.E., Keel M.K., Yabsley M., Last L.A., Breitschwerdt E.B., Maggi R.G. Hemotropic mycoplasmas in little brown bats (Myotis lucifugus) Parasit. Vectors. 2014;7:117. doi: 10.1186/1756-3305-7-117. Epub 2014/03/25. PubMed PMID: 24655520; PubMed Central PMCID: PMCPMC3994326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacristan I., Acuna F., Aguilar E., Garcia S., Lopez M.J., Cevidanes A., et al. Assessing cross-species transmission of hemoplasmas at the wild-domestic felid interface in Chile using genetic and landscape variables analysis. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-53184-4. 16816. Epub 2019/11/16. PubMed PMID: 31727935; PubMed Central PMCID: PMCPMC6856521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Volokhov D.V., Becker D.J., Bergner L.M., Camus M.S., Orton R.J., Chizhikov V.E., et al. Novel hemotropic mycoplasmas are widespread and genetically diverse in vampire bats. Epidemiol. Infect. 2017;145(15) doi: 10.1017/S095026881700231X. 3154–67. Epub 2017/10/25. PubMed PMID: 29061202; PubMed Central PMCID: PMCPMC6538534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcorn K., Gerrard J., Cochrane T., Graham R., Jennison A., Irwin P.J., et al. First report of Candidatus Mycoplasma haemohominis infection in Australia causing persistent fever in an animal Carer. Clin. Infect. Dis. 2021;72(4):634–640. doi: 10.1093/cid/ciaa089. 32006025 Epub 2020/02/02. [DOI] [PubMed] [Google Scholar]
- 25.Hattori N., Kuroda M., Katano H., Takuma T., Ito T., Arai N., et al. Candidatus Mycoplasma haemohominis in Human, Japan. Emerg. Infect. Dis. 2020;26(1) doi: 10.3201/eid2601.190983. 11–9. Epub 2019/12/20. PubMed PMID: 31855136; PubMed Central PMCID: PMCPMC6924906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steer J.A., Tasker S., Barker E.N., Jensen J., Mitchell J., Stocki T., et al. A novel hemotropic mycoplasma (Hemoplasma) in a patient with hemolytic anemia and pyrexia. Clin Infect Dis. 2011;53(11) doi: 10.1093/cid/cir666. E147-E51. Epub 2011/10/25. PubMed PMID: WOS:000297279900003; PubMed Central PMCID: PMCPMC3205199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones J.K., Jr., Nowak R.M., Paradiso J.L. Walker’s Mammals of the World. The Johns Hopkins Univ. Press, Baltimore, 4th ed., l:i–xlvi + 1–568 + xlvii–xli and 2:i–x + 569–1362 + xi–xxv, illustrated, 1983. J. Mammal. 1984;65(1):171. doi: 10.2307/1381225%J. [DOI] [Google Scholar]
- 28.Dick C.W., Patterson B.D. In: Micromammals and Macroparasites: From Evolutionary Ecology to Management. Morand S., Krasnov B.R., Poulin R., editors. Springer Japan; Tokyo: 2006. Bat flies: Obligate ectoparasites of bats; pp. 179–194. [Google Scholar]
- 29.Estrada-Peña A., Mihalca A.D., Petney T.N., editors. Ticks of Europe and North Africa. Springer International Publishing; 2017. [Google Scholar]
- 30.Rudnick A., editor. A Revision of the Mites of the Family of SPINTURNICIDAE (Acarina) 1960. [Google Scholar]
- 31.Ishii A., Ueno K., Orba Y., Sasaki M., Moonga L., Hang’ombe B.M., et al. A nairovirus isolated from African bats causes haemorrhagic gastroenteritis and severe hepatic disease in mice. Nat. Commun. 2014:5. doi: 10.1038/ncomms6651. PubMed PMID: WOS:000347227700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3(5):294–299. Epub 1994/10/01. PubMed PMID: 7881515. [PubMed] [Google Scholar]
- 33.Bruyndonckx N., Dubey S., Ruedi M., Christe P. Molecular cophylogenetic relationships between European bats and their ectoparasitic mites (Acari, Spinturnicidae) Mol. Phylogenet. Evol. 2009;51(2):227–237. doi: 10.1016/j.ympev.2009.02.005. 19236931 Epub 2009/02/25. [DOI] [PubMed] [Google Scholar]
- 34.Varanat M., Maggi R.G., Linder K.E., Breitschwerdt E.B. Molecular prevalence of Bartonella, Babesia, and hemotropic Mycoplasma sp. in dogs with splenic disease. J. Vet. Intern. Med. 2011;25(6):1284–1291. doi: 10.1111/j.1939-1676.2011.00811.x. 22092618 Epub 2011/11/19. [DOI] [PubMed] [Google Scholar]
- 35.Bandelt H.J., Forster P., Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999;16(1):37–48. doi: 10.1093/oxfordjournals.molbev.a026036. 10331250 Epub 1999/05/20. [DOI] [PubMed] [Google Scholar]
- 36.Han H.J., Li Z.M., Li X., Liu J.X., Peng Q.M., Wang R., et al. Bats and their ectoparasites (Nycteribiidae and Spinturnicidae) carry diverse novel Bartonella genotypes, China. Transbound. Emerg. Dis. 2021 doi: 10.1111/tbed.14357. Epub 2021/10/26. PubMed PMID: 34695291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen C., Shemesh M., Garrido M., Messika I., Einav M., Khokhlova I., et al. Haemoplasmas in wild rodents: routes of transmission and infection dynamics. Mol. Ecol. 2018;27(18):3714–3726. doi: 10.1111/mec.14826. 30074652 Epub 2018/08/04. [DOI] [PubMed] [Google Scholar]
- 38.de Oliveira L.B., Calchi A.C., Vultão J.G., Yogui D.R., Kluyber D., Alves M.H., et al. Molecular investigation of haemotropic mycoplasmas and Coxiella burnetii in free-living Xenarthra mammals from Brazil, with evidence of new haemoplasma species. Transbound. Emerg. Dis. 2022 doi: 10.1111/tbed.14523. Epub 2022/03/18. PubMed PMID: 35298081. [DOI] [PubMed] [Google Scholar]
- 39.Drancourt M., Raoult D. Sequence-based identification of new bacteria: a proposition for creation of an orphan bacterium repository. J. Clin. Microbiol. 2005;43(9) doi: 10.1128/JCM.43.9.4311-4315.2005. 4311–5. Epub 2005/09/08. PubMed PMID: 16145070; PubMed Central PMCID: PMCPMC1234117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Cataldo S., Kamani J., Cevidanes A., Msheliza E.G., Millan J. Hemotropic mycoplasmas in bats captured near human settlements in Nigeria. Comp. Immunol. Microbiol. Infect. Dis. 2020;70 doi: 10.1016/j.cimid.2020.101448. 32109761 101448. Epub 2020/02/29. [DOI] [PubMed] [Google Scholar]
- 41.Hornok S., Szoke K., Meli M.L., Sandor A.D., Gorfol T., Estok P., et al. Molecular detection of vector-borne bacteria in bat ticks (Acari: Ixodidae, Argasidae) from eight countries of the Old and New Worlds. Parasit. Vectors. 2019;12(1):50. doi: 10.1186/s13071-019-3303-4. Epub 2019/01/24. PubMed PMID: 30670048; PubMed Central PMCID: PMCPMC6343265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barker E.N., Tasker S., Day M.J., Warman S.M., Woolley K., Birtles R., et al. Development and use of real-time PCR to detect and quantify Mycoplasma haemocanis and “Candidatus Mycoplasma haematoparvum” in dogs. Vet. Microbiol. 2010;140(1–2) doi: 10.1016/j.vetmic.2009.07.006. 167–70. Epub 2009/08/04. PubMed PMID: 19646827; PubMed Central PMCID: PMCPMC2805721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Millán J., Travaini A., Cevidanes A., Sacristán I., Rodríguez A. Assessing the natural circulation of canine vector-borne pathogens in foxes, ticks and fleas in protected areas of Argentine Patagonia with negligible dog participation. Int. J. Parasitol. Parasites Wildl. 2019;8:63–70. doi: 10.1016/j.ijppaw.2018.11.007. Epub 2019/01/10. PubMed PMID: 30622893; PubMed Central PMCID: PMCPMC6319024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Sousa K.C.M., Herrera H.M., Secato C.T., Oliveira A.D.V., Santos F.M., Rocha F.L., et al. Occurrence and molecular characterization of hemoplasmas in domestic dogs and wild mammals in a Brazilian wetland. Acta Trop. 2017;171:172–181. doi: 10.1016/j.actatropica.2017.03.030. 28366511 Epub 2017/04/04. [DOI] [PubMed] [Google Scholar]
- 45.Stadler J., Willi S., Ritzmann M., Eddicks M., Ade J., Hoelzle K., et al. Detection of Mycoplasma suis in pre-suckling piglets indicates a vertical transmission. BMC Vet. Res. 2019;15(1):252. doi: 10.1186/s12917-019-2001-y. Epub 2019/07/22. PubMed PMID: 31324179; PubMed Central PMCID: PMCPMC6642596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Almy F.S., Ladd S.M., Sponenberg D.P., Crisman M.V., Messick J.B. Mycoplasma haemolamae infection in a 4-day-old cria: support for in utero transmission by use of a polymerase chain reaction assay. Can. Vet. J. 2006;47(3) 229–33. Epub 2006/04/12. PubMed PMID: 16604978; PubMed Central PMCID: PMCPMC1371050. [PMC free article] [PubMed] [Google Scholar]
- 47.Lashnits E., Grant S., Thomas B., Qurollo B., Breitschwerdt E.B. Evidence for vertical transmission of Mycoplasma haemocanis, but not Ehrlichia ewingii, in a dog. J. Vet. Intern. Med. 2019;33(4) doi: 10.1111/jvim.15517. 1747–52. Epub 2019/05/28. PubMed PMID: 31127669; PubMed Central PMCID: PMCPMC6639480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaoka F., Suzuki J., Hirata T., Ichijo T., Furuhama K., Harasawa R., et al. Vertical transmission of Mycoplasma wenyonii in cattle, supported by analysis of the ribonuclease P RNA gene - short communication. Acta Vet. Hung. 2015;63(3):271–274. doi: 10.1556/004.2015.025. 26551417 Epub 2015/11/10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for species identification of bats and bat-ectoparasites and molecular detection of hemoplasmas.
Comparison of the prevalence of hemoplasmas in blood, liver, and spleen samples of bats from Tongshan, Hubei Province, China, 2021.
Data Availability Statement
Data will be made available on request.