Abstract
Introduction
Human immunodeficiency virus (HIV/AIDS) infected patients have a higher risk of opportunistic infections (OIs) depending on their immunological status, especially CD4 + cell count. Toxoplasma gondii, hepatitis C virus (HCV), and hepatitis B virus (HBV) are important OIs among Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) patients. However, little is known about co‐infection of these pathogens among HIV‐infected individuals and their correlation with the patient's CD4 + cell count. Hence, this study aimed to investigate the serological and molecular status of T. gondii infection among HIV‐infected individuals who had co‐infection with HBV and HCV infections.
Methods
A total of 100 HIV/AIDS patients in two cities in the southwest of Iran was tested for T. gondii Immunoglobulin G (IgG) and Immunoglobulin M (IgM) antibodies as well as DNA detection by polymerase chain reaction (PCR) targeting the RE gene. HBV and HCV were detected by hepatitis B surface antigen (HBsAg) test, hepatitis C antibody (HCV Ab) test, and Real‐Time PCR. The number of CD4 + cell counts was determined by Flow cytometry.
Results
Anti‐T. gondii IgG was positive in 22% of the patients, but anti‐T. gondii IgM and PCR were negative in all samples. HBV and HCV were positive in 8% and 33% of the patients, respectively. Co‐infections were as followed: HIV + HCV (16%), HIV + HCV + T. gondii (11%), HIV + T. gondii (5%), HIV + HBV (1%), HIV + HBV + T. gondii (1%), HIV + HBV + HCV (1%), and HIV + HBV + HCV + T. gondii (5%). A significant decline in CD4 + cell counts was found in such co‐infection groups (HIV + T. gondii, HIV + HCV + T. gondii, and HIV + HBV + HCV + T. gondii) compared with the HIV mono‐infection group.
Conclusions
Our study showed that co‐infections of T. gondii, HCV, and HBV were common among HIV‐infected patients and co‐infections had a negative correlation with CD4 + cell counts of the patients.
Keywords: CD4, hepatitis B, hepatitis C, HIV/AIDS, Iran, T cell, toxoplasmosis
HIV/AIDS infected patients have a higher risk of opportunistic infections (OIs) depending on their immunological status, especially CD4 cell count. Toxoplasma gondii, hepatitis C virus (HCV), and hepatitis B virus (HBV) are important OIs among HIV/AIDS patients. The prevalence of HCV, HBV, and anti‐Toxoplasma IgG antibody were 33%, 8%, and 22%, respectively. Anti‐T. gondii IgM and PCR was negative in all samples. A significant decline in CD4 cell counts was found in such co‐infection groups (HIV + T. gondii, HIV + HCV + T. gondii, and HIV + HBV + HCV + T. gondii) compared with the HIV mono‐infection group. This study showed that co‐infections of T. gondii, HCV, and HBV had a negative correlation with CD4 counts of the patients.
1. INTRODUCTION
The human immunodeficiency virus (HIV) is one of the major causes of morbidity and mortality around the world. According to the estimation, about 37.7 million people lived with HIV at the end of 2020. Accordingly, in 2020, 680,000 people died from HIV‐related causes and 1.5 million people acquired HIV. 1 People living with HIV (PLWH) have a higher risk of opportunistic infections (OIs) dependent on their immunological status, stage of HIV, or adherence to antiretroviral therapy (ART). 2 , 3 Furthermore, CD4 T lymphocyte number has a critical role in disease progression and response to ART in PLWH, 4 , 5 , 6 , 7 while an increased incidence and number of OIs have been documented with diminishing CD4 + cell count. 2 , 3 As such, the clinical courses of OIs (e.g., toxoplasmosis and Cytomegalovirus infections) were correlated with a decline in the CD4 + cell count. 8 , 9
Toxoplasmosis, caused by the protozoan parasite Toxoplasma gondii, is an important OIs in HIV/AIDS patients. 3 , 10 The primary infection occurs through ingestion of contaminated water or food with oocysts shed by cats or by eating raw or undercooked meat containing tissue cysts of T. gondii. 11 , 12 Congenital transmission, blood transfusion, and organ transplantation are other important routes of T. gondii infection. 11 , 12 Toxoplasmosis is usually self‐limiting and asymptomatic in immunocompetent individuals, 12 However, in immunocompromised patients, the infection could be life‐threatening (e.g., HIV/AIDS patients, organ transplant recipients, and patients with cancer) infection with fatal outcome. 13 , 14 , 15 , 16 , 17 In HIV/AIDS patients, reactivation of latent toxoplasmosis may cause severe infections, which results in disseminated infection or encephalitis. 13 , 18 , 19 According to a meta‐analysis, the pooled worldwide prevalence of T. gondii‐HIV co‐infection was estimated 35.8% (95% confidence interval [CI]: 30.8–40.7), while the prevalence in low‐income, middle‐income, and high‐income countries were 54.7% (95% CI: 35·8–73.5), 34.2% (95% CI: 27.4–40.9), and 26.3% (95% CI: 20.4–32.2), respectively. 10 On the other hand, the number of CD4 + count (<200/mm3) is a significant risk factor for toxoplasmosis in PLWH. 20 As such, the results of a recent meta‐analysis revealed that the risk of cerebral toxoplasmosis increases 27.94 times in PLWH with CD4 + T cells <100/mm3. 21
Hepatitis B virus (HBV) and hepatitis C virus (HCV) are a major public health concern, especially among HIV infected patients. 22 , 23 As HIV, HBV, and HCV share similar transmission routes (e.g., sexual route, drug injection, and needle stick injury), co‐infection with these viruses is more common than in the general population. 23 , 24 Chronic hepatitis B (CHB) infection is a major cause of cirrhosis and hepatocellular carcinoma (HCC). 22 According to a systematic review and meta‐analysis, the global prevalence of HBV among HIV individuals was 7.6% (5.6%–12.1%) or 2.7 (2.0–4.2) million co‐infections. Accordingly, the odds of HBV infection were 1.4 times higher among HIV‐positive compared to HIV‐negative individuals. 22 HCV is a common opportunistic pathogen among HIV‐infected individuals, estimating a third of HIV‐infected individuals have HCV co‐infection in Europe and the United States of America (USA). 25 HCV infection is a leading cause of chronic liver disease as well as liver failure and liver transplantation around the world. 23 , 24 In 2015, the worldwide prevalence of HIV‐HCV co‐infection was estimated 2,278,400 (1,271,300–4,417,000) cases, and the odds of HCV infection were six times higher in HIV‐infected individuals (5.8, 95% CI: 4.5–7.4) than their HIV‐negative counterparts. 26 Furthermore, co‐infection of HIV with HCV and HBV accelerated liver injury 23 , 24 , 27 and increased the risk of kidney disease. 28 , 29 , 30 An inverse correlation between CD4 + cell count and persistent HBV viremia (CD4 < 200 mm3) 31 and an increased risk of mortality (CD4 < 500/mm3) 32 has been reported among PLWH coinfected with HBV. Progression of liver fibrosis was also correlated with declining CD4 + T cells among PLWH coinfected with HCV. 33
Co‐infections can synergically augment the severity of some infectious diseases. 34 , 35 , 36 For instance, virus‐virus co‐infection can heighten virus replication and persistence, altered immunological responses, and disease intensity. 37 HIV‐HCV co‐infections promotes hepatocellular injury and boosts certain inflammatory cytokines. 38 Our previous studies showed that maternal ToRCH (toxoplasmosis, rubella, CMV, and HSV) co‐infections were an increased risk of abortion among pregnant women than single infection. 39 Accumulating evidence has shown that co‐infections have more severe consequences than the single infections. 35
A number of studies reported a higher prevalence of toxoplasmosis in HIV‐infected individuals compared to healthy individuals. 10 , 16 However, little is known about the prevalence of triple co‐infection of T. gondii, HBV, and HCV and their correlation with CD4 + T cell count among PLWH. Hence, our study aimed to estimate the prevalence rates of T. gondii, HBV, and HCV infections among HIV‐infected individuals and their correlation with CD4 cell count.
2. MATERIALS AND METHODS
2.1. Study population and sampling
The present study was conducted among 100 confirmed cases of HIV‐infected individuals who had medical records in the health centers of Jahrom and Fasa cities (Fars Province, south of Iran) during 2020–2021. These cities have a hot semi‐arid climate, and each of them has more than 100,000 population. This study protocol was approved by the Ethical Committee of Jahrom University of Medical Sciences (IR.JUMS.REC.1398.065). The patient's information was extracted from their medical records. We obtained about five milliliters of venous blood samples from each patient, after centrifugation, the serum samples were separated for serologic evaluation, and the buffy coat samples were used for DNA extraction and molecular detection.
2.2. CD4 cell counts, HBV, and HCV status
Information about CD4 + T cell counts (determined by Flow cytometry), HBV, and HCV status were obtained from the patient's medical records. Accordingly, HBV and HCV were previously detected by hepatitis B surface antigen (HBs Ag) test, hepatitis C antibody (HCV Ab) test, and Real‐Time polymerase chain reaction (RT‐PCR).
2.3. Anti‐T. gondii antibody serologic test
Anti‐Toxoplasma IgM and IgG antibodies were detected by a commercial enzyme‐linked immunosorbent assay (ELISA) kit (Pishtaz Teb) according to the manufacturer's procedure. The cut‐off values at the upper and lower limit of 11 IU/mL were considered as positive and negative results, respectively.
2.4. Molecular detection of T. gondii
DNA was extracted from buffy coat samples using a commercial solution (DNG Plus) according to the manufacturer's protocol. The buffy coat samples were used for DNA extraction using the phenol–chloroform–isoamyl alcohol method, as described in a previous study. 40 PCR was performed using a set of highly sensitive and specific primers for T. gondii (the RE gene) that amplified a region of 529 base pair (bp) fragments. 41 The PCR primers 41 and cycling conditions were described in previous reports (Tables S1 and S2). 42 DNA of the RH strain of T. gondii was used as positive control and double distilled water was used as negative control. For each PCR reaction, a negative and positive control was included. PCR products were electrophoresed in agarose gel (stained with safe stain, Sinaclon, Iran) and visualized under UV transilluminator.
2.5. Statistical analysis
Correlations of CD4 + T cell counts with infection status were analyzed by statistics as a powerful statistical software SPSS (ver. 20) using Chi‐square test. The data are presented here as mean ± standard deviation (SD) of three independent experiments.
3. RESULTS
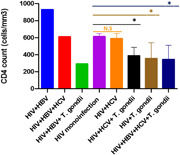
The mean ages of the patients were 43.79 years (±10.47 standard deviation (SD)) ranging from 20 to 63 years old. From the 100 patients, 52% and 48% were males and females, respectively (Table 1). HBV and HCV were positive in 8% and 33% of the HIV‐positive patients, respectively (Table 2). Anti‐T. gondii IgG was positive in 22% of the patients, but anti‐T. gondii IgM was negative in all samples. Moreover, T. gondii DNA was negative in all HIV‐positive patients (Table 2). Out of 100 HIV‐positive patients, 60% did not have co‐infection with HBV, HCV, or T. gondii, while the rest of the patients (40%) had co‐infections, including HIV + HCV (16%), HIV + T. gondii (5%), HIV + HBV (1%), HIV + HCV + T. gondii (11%), HIV + HBV + T. gondii (1%), HIV + HBV + HCV (1%), and HIV + HBV + HCV + T. gondii (5%) (Figure 1 and Table S3). There was a significant decline in CD4 + T cell counts in such co‐infection groups (HIV + T. gondii, HIV + HCV + T. gondii, and HIV + HBV + HCV + T. gondii) compared with the HIV mono‐infection group (Figure 2 and Table S3).
Table 1.
Sex and age of HIV‐infected individuals.
| Cities | Age, year (mean ± SD) | Sex | |
|---|---|---|---|
| Male | Female | ||
| Jahrom (N = 50) | 43 ± 9.89 | 29 (58%) | 21 (42%) |
| Fasa (N = 50) | 44.58 ± 11.07 | 23 (46%) | 27 (54%) |
| Total (N = 100) | 43.79 ± 10.47 | 52 (52%) | 48 (48%) |
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
Table 2.
Infection status among HIV‐infected individuals.
| Type of co‐infections | Jahrom | Fasa | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (N = 29) | Female (N = 21) | Total (N = 50) | Male (N = 23) | Female (N = 27) | Total (N = 50) | Male (N = 50) | Female (N = 50) | Total (N = 100) | |
| HBV | 2 (6.8%) | 0 | 2 (4%) | 4 (17.4%) | 2 (7.4%) | 6 (12%) | 6 (12%) | 2 (4%) | 8 (8%) |
| HCV | 7 (24.1%) | 9 (42.8%) | 16 (32%) | 7 (30.4%) | 10 (37%) | 17 (34%) | 14 (28%) | 19 (38%) | 33 (33%) |
| Toxoplasma gondii | 6 (20.7%) | 2 (9.5%) | 8 (16%) | 5 (21.7%) | 9 (33.3%) | 14 (28%) | 11 (22%) | 11 (22%) | 22 (22%) |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Figure 1.
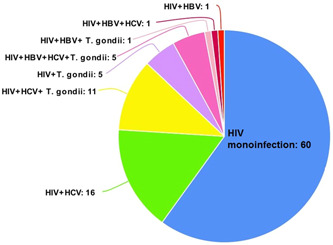
Pattern of co‐infection among HIV‐infected individuals. HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
Figure 2.
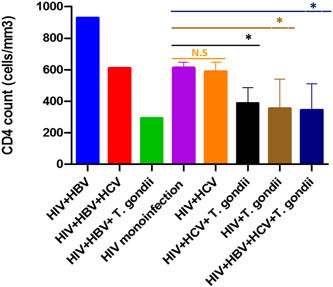
Correlation of CD4 cell counts with co‐infection status. Co‐infections were compared with the HIV‐monoinfection group (* p < .0001). We were unable to compare CD4 cell counts in HIV + HBV, HIV + HBV + HCV, and HIV + HBV + T. gondii groups because there was one patient in each of these groups. HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
4. DISCUSSION
In the current study, we performed sero‐molecular tests to screen T. gondii infection among PLWH. Overall, anti‐T. gondii IgG was positive in 22% of the patients, but anti‐T. gondii IgM and PCR was negative in all samples. Our findings revealed that out of 100 HIV‐positive patients, 60% did not have co‐infection with HBV, HCV, or T. gondii, while 40% of the patients had co‐infections. Of them, the prevalence of HCV, HBV, and T. gondii infection were 33%, 8%, and 22% among the HIV‐positive patients, respectively. Moreover, a significantly declined level of CD4 + T cell count was observed among T. gondii seropositive patients compared to the seronegative group. Generally, T. gondii IgG seropositivity without IgM and PCR positive results indicate latent infection. It should be noted that reactivation of latent toxoplasmosis is the major cause of toxoplasmic encephalitis (TE) in HIV/AIDS patients. 18
Regarding T. gondii/HIV co‐infection in Iran, a previous study in Jahrom (the same region of this study) revealed that 21.1% of HIV‐infected individuals had anti‐T. gondii IgG antibody. Indeed, T. gondii seropositive patients had significantly lower levels of CD4 + T cell count than seronegative patients. 43 Another report in western Iran revealed that anti‐T. gondii IgG and IgM seropositivity among 40.8% and 2.6% of HIV‐positive patients, respectively, although, no statistically significant correlation was found between toxoplasmosis and CD4 + T cell count. 44 In the north of Iran, Rahimi et al. 45 found a high seroprevalence rate of T. gondii IgG (96.3%) among HIV‐infected individuals, while IgM was negative in all of them. A recent study in the southwest of Iran demonstrated that 9.3%, 7.8%, and 9.3% of HIV‐positive patients had anti‐T. gondii IgG, IgM, and T. gondii DNA. 46 A serologic study among HIV‐positive patients in Tehran (the capital of Iran) revealed that the prevalence rates of anti‐T. gondii IgG and IgM were 49.75% and 1%, respectively. It is worth noting a significant association was found between the rate of toxoplasma encephalitis and CD4 + T count (p < .001). 47 A study among 208 HIV/AIDS patients In Shiraz, southern Iran, revealed that 18.2% of the patients had T. gondii seropositive, while TE was recorded in 89.6% and 10.4% of Toxoplasma seropositive and seronegative patients, respectively. 48 The difference in seroprevalence rate of T. gondii among HIV‐infected individuals in Iran may be due to various factors, such as living in areas with humid climate, contact with cats and soil, consumption of raw/undercooked meat, 49 , 50 consumption of unwashed/raw vegetables. 51 All of these factors could increase the exposure of human to the parasite. For example, the north of Iran (which has the highest seroprevalence rates of toxoplasmosis 46 ) has a humid climate, which provides a suitable condition for parasite oocyst survival in the soil and environment. 52 In this condition, percentages of latent infection among meat producing animals are more common than regions with low humid climate, leading to increased rates of meat‐born toxoplasmosis. 50 , 53 , 54
We found that 40% of the patients had co‐infection (Figure 1 and Table S3). Interestingly, we found that the levels of CD4 + T cell counts were significantly declined in HIV‐positive patients who had co‐infections, including (HIV + T. gondii, HIV + HCV + T. gondii, and HIV + HBV + HCV + T. gondii compared with HIV mono‐infection group. A number of studies have been reported an increased prevalence rate of HCV 26 and HBV 22 among PLWH. Furthermore, studies suggest that co‐infection of HIV with HCV and HBV accelerates liver and kidney disease. 27 , 29 , 30 On the other hand, there are evidence for an association between T. gondii infection and chronic liver disease (CLD) 55 , 56 as well as nonalcoholic fatty liver disease (NAFLD). 57 A study in Burkina Faso demonstrated significantly higher prevalence rate of T. gondii/HBV, T. gondii/HCV, and HCV/HBV co‐infections among HIV‐positive pregnant women compared to HIV‐negative counterparts. 58
A number of studies demonstrated that co‐infection can worsen the severity of infectious diseases. 34 , 35 , 59 Virus‐virus co‐infection can enhance virus replication and persistence, dampen immunological response and increase disease intensity. 37 HIV‐HCV co‐infection increased HCV RNA levels, promoted hepatocellular injury, inflammation, and fibrosis, and accelerates progression to cirrhosis and end‐stage liver disease. 38 , 60 Respiratory Syncytial Virus (RSV) exacerbated severity of influenza A virus disease in mouse models. 61 ToRCH (toxoplasmosis, rubella, CMV, and HSV) co‐infections increased the risk of abortion than single infection among pregnant women. 39 HIV and T. gondii co‐infection could disturb the immune regulatory mechanisms. 62 El‐Sayed et al. 56 found that a significantly increased parasitemia among CLD patients compared with the control group (30% vs. 6%; p < .001). Additionally, T. gondii/HBV and T. gondii/HCV co‐infection was 33.3% and 31.4%, respectively, alongside with a significant association between HCV viral load and T. gondii parasitemia. T. gondii positive CLD patients had a significant increase of liver enzymes than T. gondii negative patients. 56 Accumulating evidence suggests that co‐infections have more severe sequels than the single infections. 35
New evidence revealed that T. gondii can be sexually transmitted from male to female in humans 63 , 64 , 65 as well as in animal models (e.g., Rats, 66 dogs, 67 sheep, 68 and goats 69 ). This is an important point because sexual transmission is one of the main routes of HIV, HBV, and HCV transmission. 23 , 24 Therefore, co‐infection of T. gondii with these viruses should be more considered for prophylaxis, screening, and management of the infection.
5. STUDY LIMITATIONS AND SUGGESTIONS
Our study has some limitations, including 1) limited sample size; 2) lack of clinical data of the patients; 3) lack of laboratory parameters of the patients. Based on these limitations, a larger investigation with a higher number of patients could be recommended in future investigations. Moreover, it should be recommended to obtain clinical data and laboratory findings of PLWH coinfected with toxoplasmosis, HBV, and HCV.
6. CONCLUSIONS
Our findings showed a high prevalence rate of co‐infection and their negative impact on CD4 + T cell counts of HIV‐infected patients. Routine screening of T. gondii as well as HCV and HBV should be recommended in HIV‐infected individuals.
AUTHOR CONTRIBUTIONS
Ahmadreza Bazmjoo: Conceptualization; formal analysis; validation. Mohammad Aref Bagherzadeh: Formal analysis; methodology. Rahim Raoofi: Supervision. Ali Taghipour: Data curation; formal analysis; validation; visualization. Samaneh Mazaherifar: Methodology. Hojatallah Sotoodeh: Methodology. Zahra Ostadi: Methodology. Enayat Shadmand: Methodology. Mirza Ali Mofazzal Jahromi: Methodology; validation. Amir Abdoli: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; writing—original draft; writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
ETHICS STATEMENT
This study was approved by the research and ethics committee of the Jahrom University of Medical Sciences, Jahrom, Iran (the ethics code: IR.JUMS.REC.1398.065).
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors acknowledge the Jahrom University of Medical Sciences, Jahrom, Iran, for supporting this study. The authors also would like to thank the Clinical Research Development Unit of Peymanieh Educational and Research and Therapeutic Center of the Jahrom University of Medical Sciences for supporting this work. This study was supported by the Jahrom University of Medical Sciences, Jahrom, Iran.
Bazmjoo A, Bagherzadeh MA, Raoofi R, et al. Toxoplasma gondii, HBV, and HCV co‐infection and their correlation with CD4 cells among Iranian HIV‐positive patients. Immun Inflamm Dis. 2023;11:e794. 10.1002/iid3.794
DATA AVAILABILITY STATEMENT
All data are available from the corresponding author upon reasonable request.
REFERENCES
- 1. HIV/AIDS . 2021. https://www.who.int/news-room/fact-sheets/detail/hiv-aids#:%7E:text=Transmission,child%20during%20pregnancy%20and%20delivery
- 2. Shaw J, Matin N. Opportunistic infections in HIV. Medicine. 2022;50(5):294‐297. [Google Scholar]
- 3. Dufaur L, Matin N. Important opportunistic infections in HIV. Medicine. 2018;46(6):352‐355. [Google Scholar]
- 4. Ford N, Chiller T. CD4 cell count: a critical tool in the human immunodeficiency virus response. Clin Infect Dis. 2022;74(8):1360‐1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Phillips AN, Staszewski S, Weber R, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286(20):2560‐2567. [DOI] [PubMed] [Google Scholar]
- 6. Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple‐drug therapy. JAMA. 2001;286(20):2568‐2577. [DOI] [PubMed] [Google Scholar]
- 7. Ford N, Meintjes G, Pozniak A, et al. The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis. 2015;15(2):241‐247. [DOI] [PubMed] [Google Scholar]
- 8. Šimeková K, Nováková E, Rosoľanka R, Masná J, Antolová D. Clinical course of opportunistic infections—toxoplasmosis and cytomegalovirus infection in HIV‐Infected patients in Slovakia. Pathogens. 2019;8(4):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmes CB, Wood R, Badri M, et al. CD4 Decline and Incidence of Opportunistic Infections in Cape Town South Africa: implications for Prophylaxis and Treatment. J Acquir Immune Defic Syndr. 2006;42(4):464‐469. [DOI] [PubMed] [Google Scholar]
- 10. Wang Z‐D, Wang S‐C, Liu H‐H, et al. Prevalence and burden of Toxoplasma gondii infection in HIV‐infected people: a systematic review and meta‐analysis. Lancet HIV. 2017;4(4):e177‐e188. [DOI] [PubMed] [Google Scholar]
- 11. Montoya J, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965‐1976. [DOI] [PubMed] [Google Scholar]
- 12. Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012;7(1):1‐17. [PMC free article] [PubMed] [Google Scholar]
- 13. Abdoli A, Barati M, Dalimi A, Pirestani M, Hoseini Shokouh SJ. Toxoplasmosis among patients with immunocompromising conditions: a snapshot. J Arch Military Med. 2016;4(4):e41832. [Google Scholar]
- 14. Abdoli A. Neglected risk factors for HIV and Toxoplasma gondii co‐infection. Lancet HIV. 2017;4(4):e152. [DOI] [PubMed] [Google Scholar]
- 15. Rasti S, Hassanzadeh M, Soliemani A, et al. Serological and molecular survey of toxoplasmosis in renal transplant recipients and hemodialysis patients in Kashan and Qom regions, central Iran. Ren Fail. 2016;38(6):970‐973. [DOI] [PubMed] [Google Scholar]
- 16. Wang Z‐D, Liu H‐H, Ma Z‐X, et al. Toxoplasma gondii Infection in Immunocompromised Patients: a Systematic Review and Meta‐Analysis. Front Microbiol. 2017;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Israelski DM, Remington JS. Toxoplasmosis in patients with cancer. Clin Infect Dis. 1993;17(suppl_2):S423‐S435. [DOI] [PubMed] [Google Scholar]
- 18. Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15(2):211‐222. [DOI] [PubMed] [Google Scholar]
- 19. Kodym P, MalÝ M, Beran O, et al. Incidence, immunological and clinical characteristics of reactivation of latent Toxoplasma gondii infection in HIV‐infected patients. Epidemiol Infect. 2015;143(3):600‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rostami A, Riahi SM, Abdollahzadeh Sagha S, et al. Seroprevalence estimates of latent and acute toxoplasma infections in HIV+ people—call for action in underprivileged communities. Microorganisms. 2021;9(10):2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dwinata IM, Widyadharma IPE, Dewi PR, Tedyanto EH. Risk factors of cerebral toxoplasmosis in HIV patients: a systematic review. Rom J Neurol. 2021;20(3):305‐310. [Google Scholar]
- 22. Platt L, French CE, McGowan CR, et al. Prevalence and burden of HBV co‐infection among people living with HIV: a global systematic review and meta‐analysis. J Viral Hepatitis. 2020;27(3):294‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sohrab SS, Suhail M, Ali A, Qadri I, Harakeh S, Azhar EI. Consequence of HIV and HCV co‐infection on host immune response, persistence and current treatment options. Virus Dis. 2018;29(1):19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abutaleb A, Sherman KE. A changing paradigm: management and treatment of the HCV/HIV‐co‐infected patient. Hepatol Int. 2018;12(6):500‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rockstroh JK, Spengler U. HIV and hepatitis C virus co‐infection. Lancet Infect Dis. 2004;4(7):437‐444. [DOI] [PubMed] [Google Scholar]
- 26. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co‐infection in people living with HIV: a global systematic review and meta‐analysis. Lancet Infect Dis. 2016;16(7):797‐808. [DOI] [PubMed] [Google Scholar]
- 27. Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV‐hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017;31(15):2035‐2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fabrizi F, Dixit V, Martin P, Messa P. Hepatitis C virus increases the risk of kidney disease among HIV‐positive patients: systematic review and meta‐analysis. J Med Virol. 2016;88(3):487‐497. [DOI] [PubMed] [Google Scholar]
- 29. Fabrizi F, Donato FM, Messa P. Association between hepatitis C virus and chronic kidney disease: a systematic review and Meta‐Analysis. Annals of Hepatology. 2018;17(3):364‐391. [DOI] [PubMed] [Google Scholar]
- 30. Mweemba A, Zanolini A, Mulenga L, et al. Chronic hepatitis B virus coinfection is associated with renal impairment among Zambian HIV‐infected adults. Clin Infect Dis. 2014;59(12):1757‐1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Msomi N, Parboosing R, Wilkinson E, et al. Persistent hepatitis B viraemia with polymerase mutations among HIV/HBV co‐infected patients on HBV‐active ART in KwaZulu‐Natal, South Africa. Viruses. 2022;14(4):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kouamé GM, Gabillard D, Moh R, et al. Higher risk of mortality in HIV‐HBV co‐infected patients from Sub‐Saharan Africa is observed at lower CD4+ cell counts. Antivir Ther. 2021;26(1‐2):25‐33. [DOI] [PubMed] [Google Scholar]
- 33. Puoti M, Bonacini M, Spinetti A, et al. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001;183(1):134‐137. [DOI] [PubMed] [Google Scholar]
- 34. Abdoli A, Taghipour A, Pirestani M, et al. Infections, inflammation, and risk of neuropsychiatric disorders: the neglected role of “co‐infection”. Heliyon. 2020;6(12):e05645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hunter P. Co‐infection: when whole can be greater than the sum: the complex reaction to co‐infection of different pathogens can generate variable symptoms. EMBO Rep. 2018;19(8):e46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abdoli A, Falahi S, Kenarkoohi A. COVID‐19‐associated opportunistic infections: a snapshot on the current reports. Clin Exp Med. 2022;22(3):327‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumar N, Sharma S, Barua S, Tripathi BN, Rouse BT. Virological and immunological outcomes of coinfections. Clin Microbiol Rev. 2018;31(4):e00111‐e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shmagel K, Saidakova E, Shmagel N, et al. Systemic inflammation and liver damage in HIV/hepatitis C virus coinfection. HIV Med. 2016;17(8):581‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rasti S, Ghasemi FS, Abdoli A, Piroozmand A, Mousavi SGA, Fakhrie‐Kashan Z. ToRCH “co‐infections” are associated with increased risk of abortion in pregnant women. Congenit Anom (Kyoto). 2016;56(2):73‐78. [DOI] [PubMed] [Google Scholar]
- 40. Abdoli A, Barati M, Pirestani M, Dalimi A. Screening of toxoplasmosis in cancer patients: a concern. Trop Doct. 2019;49(1):31‐34. [DOI] [PubMed] [Google Scholar]
- 41. Homan WL, Vercammen M, De Braekeleer J, Verschueren H. Identification of a 200‐ to 300‐fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int J Parasitol. 2000;30(1):69‐75. [DOI] [PubMed] [Google Scholar]
- 42. Abdoli A, Dalimi A, Soltanghoraee H, Ghaffarifar F. Molecular detection of Toxoplasma gondii in house sparrow (Passer domesticus) by LAMP and PCR methods in Tehran, Iran. J Parasitic Dis. 2016;40(4):1317‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rezanezhad H, Sayadi F, Shadmand E, et al. Seroprevalence of Toxoplasma gondii among HIV patients in Jahrom, Southern Iran. Korean J Parasitol. 2017;55(1):99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nazari N, Bozorgomid A, Janbakhsh A, Bashiri F. Toxoplasma gondii and human immunodeficiency virus co‐infection in Western Iran: A cross sectional study. Asian Pac J Trop Med. 2018;11(1):58‐62. [Google Scholar]
- 45. Rahimi MT, Mahdavi SA, Javadian B, et al. High seroprevalence of Toxoplasma gondii antibody in HIV/AIDS individuals from north of Iran. Iran J Parasitol. 2015;10(4):584‐589. [PMC free article] [PubMed] [Google Scholar]
- 46. Nikbakht G, Behrouzi M, Mousavizadeh A, et al. Seroprevalence of Toxoplasma gondii infection among HIV‐positive patients in Southwest Iran and associated risk factors: a case‐control study. Trans R Soc Trop Med Hyg. 2022;116(10):930‐934. 10.1093/trstmh/trac016 [DOI] [PubMed] [Google Scholar]
- 47. Minoo M, Farhad M, Sara J, et al. Seroprevalence of toxoplasmosis in HIV+/AIDS patients in Iran. Acta Med Iranica. 1970;49(4):213‐218. [PubMed] [Google Scholar]
- 48. Davarpanah MA, Mehrbani D, Neyrami R, Ghahramanpouri M, Darvishi M. Toxoplasmosis in HIV/AIDS patients in Shiraz, Southern Iran. Iran Red Crescent Med J. 2007;9(1):22‐27. [Google Scholar]
- 49. Khademi SZ, Ghaffarifar F, Dalimi A, Davoodian P, Abdoli A. Prevalence and risk factors of Toxoplasma gondii infection among pregnant women in Hormozgan Province, South of Iran. Iran J Parasitol. 2019;14(1):167. [PMC free article] [PubMed] [Google Scholar]
- 50. Khademi SZ, Ghaffarifar F, Dalimi A, Dayer MS, Abdoli A. Toxoplasma gondii in slaughtered sheep in high‐ and low‐humidity regions in the South of Iran: molecular prevalence and genotype identification. Vet Med Int. 2021;2021:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Badri M, Olfatifar M, Karim MR, et al. Global prevalence of intestinal protozoan contamination in vegetables and fruits: a systematic review and meta‐analysis. Food Control. 2022;133:108656. [Google Scholar]
- 52. Maleki B, Ahmadi N, Olfatifar M, et al. Toxoplasma oocysts in the soil of public places worldwide: a systematic review and meta‐analysis. Trans R Soc Trop Med Hyg. 2021;115(5):471‐481. [DOI] [PubMed] [Google Scholar]
- 53. Rasti S, Marandi N, Abdoli A, Delavari M, Mousavi SGA. Serological and molecular detection of toxoplasma gondii in sheep and goats in Kashan, Central Iran. Journal of Food Safety. 2018;38(2):e12425. [Google Scholar]
- 54. Almeria S, Dubey JP. Foodborne transmission of Toxoplasma gondii infection in the last decade. an overview. Res Vet Sci. 2020;135:371‐385. [DOI] [PubMed] [Google Scholar]
- 55. Tian AL, Li GX, Elsheikha HM, et al. Seroepidemiology of Toxoplasma gondii infection in patients with liver disease in eastern China. Epidemiol Infect. 2017;145(11):2296‐2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. El‐Sayed NM, Ramadan ME, Ramadan ME. Toxoplasma gondii infection and chronic liver. Diseases: Evidence of an Association. Trop Med Infect Dis. 2016;1(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Huang J, Zhang H, Liu S, et al. Is Toxoplasma gondii infection correlated with nonalcoholic fatty liver disease?‐ a population‐based study. BMC Infect Dis. 2018;18(1):629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Simpore J, Savadogo A, Ilboudo D, et al. Toxoplasma gondii, HCV, and HBV seroprevalence and co‐infection among HIV‐positive and ‐negative pregnant women in Burkina Faso. J Med Virol. 2006;78(6):730‐733. [DOI] [PubMed] [Google Scholar]
- 59. Mofazzal Jahromi MA, Sefidfard M, Taghipour A, et al. Latent infections, coronavirus disease 2019 and psychiatric disorders: the friend of my enemy. Clin Translat Discov. 2022;2(4):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blackard JT, Sherman KE. HCV/ HIV co‐infection: time to re‐evaluate the role of HIV in the liver? J Viral Hepatitis. 2008;15(5):323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. George JA, AlShamsi SH, Alhammadi MH, Alsuwaidi AR. Exacerbation of influenza A virus disease severity by respiratory syncytial virus co‐infection in a mouse model. Viruses. 2021;13(8):1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li JR, Gong RY, Li YP, Bai Y, You F, Deng S. Research on HIV/Toxoplasma gondii co‐infection and cytokine levels among intravenous drug users. Parasite Immunol. 2010;32(2):161‐164. [DOI] [PubMed] [Google Scholar]
- 63. Flegr J, Klapilová K, Kaňková S. Toxoplasmosis can be a sexually transmitted infection with serious clinical consequences. Med Hypotheses. 2014;83(3):286‐289. [DOI] [PubMed] [Google Scholar]
- 64. Hlaváčová J, Flegr J, Řežábek K, Calda P, Kaňková Š. Male‐to‐Female presumed transmission of toxoplasmosis between sexual partners. Am J Epidemiol. 2021;190(3):386‐392. [DOI] [PubMed] [Google Scholar]
- 65. Tong WH, Hlaváčová J, Abdulai‐Saiku S, Kaňková Š, Flegr J, Vyas A. Presence of Toxoplasma gondii tissue cysts in human semen: toxoplasmosis as a potential sexually transmissible infection. J Infect. 2023;86(1):60‐65. [DOI] [PubMed] [Google Scholar]
- 66. Dass SAH, Vasudevan A, Dutta D, Soh LJT, Sapolsky RM, Vyas A. Protozoan parasite Toxoplasma gondii manipulates mate choice in rats by enhancing attractiveness of males. PLoS One. 2011;6(11):e27229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Arantes TP, Lopes WDZ, Ferreira RM, et al. Toxoplasma gondii: evidence for the transmission by semen in dogs. Exp Parasitol. 2009;123(2):190‐194. [DOI] [PubMed] [Google Scholar]
- 68. Lopes WDZ, Rodriguez JD, Souza FA, et al. Sexual transmission of Toxoplasma gondii in sheep. Vet Parasitol. 2013;195(1):47‐56. [DOI] [PubMed] [Google Scholar]
- 69. Santana LF, Rossi GAM, Gaspar RC, Pinto VMR, Oliveira GP, Costa AJ. Evidence of sexual transmission of Toxoplasma gondii in goats. Small Ruminant Res. 2013;115(1):130‐133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All data are available from the corresponding author upon reasonable request.


