Abstract
The side effects and drug resistance during chemotherapy seriously affect the outcome of and may lead to the failure of chemotherapy for patients with hepatoma. The aim of the present study was to investigate the association between the expression of ATP-binding cassette transporter G2 (ABCG2) in hepatoma cells and the drug resistance of hepatoma. An MTT assay was used to determine the half-maximal inhibitory concentration (IC50) of Adriamycin (ADM) in hepatoma HepG2 cells after treatment with ADM for 24 h. An ADM-resistant hepatoma cell subline, HepG2/ADM, was generated from the HepG2 hepatoma cell line through a stepwise selection with ADM doses from 0.01 to 0.1 µg/ml. The HepG2/ABCG2 cell line, an ABCG2-overexpressing hepatoma cell line, was established by transfecting the ABCG2 gene into HepG2 cells. The MTT assay was then used to detect the IC50 of ADM in HepG2/ADM and HepG2/ABCG2 cells after treatment with ADM for 24 h and the resistance index was calculated. The apoptosis, cell cycle and ABCG2 protein expression levels in HepG2/ADM, HepG2/ABCG2 cells, HepG2/PCDNA3.1 and their parental HepG2 cells were detected by flow cytometry. In addition, flow cytometry was used to detect the efflux effect of HepG2/ADM and HepG2/ABCG2 cells after ADM treatment. ABCG2 mRNA expression in cells was detected by reverse transcription-quantitative PCR. After 3 months of ADM treatment, HepG2/ADM cells grew stably in the cell culture medium containing 0.1 µg/ml ADM and the cells were named HepG2/ADM cells. ABCG2 was overexpressed in HepG2/ABCG2 cells. The IC50 of ADM in HepG2, HepG2/PCDNA3.1, HepG2/ADM and HepG2/ABCG2 cells was 0.72±0.03, 0.74±0.01, 11.17±0.59 and 12.75±0.47 µg/ml, respectively. The cell apoptotic rate of HepG2/ADM and HepG2/ABCG2 cells was not significantly different compared with that of HepG2 and HepG2/PCDNA3.1 cells (P>0.05), but the G0/G1 phase population of the cell cycle decreased and the proliferation index increased significantly (P<0.05). The expression levels of ABCG2 gene and protein in HepG2/ADM and HepG2/ABCG2 cells were significantly higher than those in HepG2 and HepG2/PCDNA3.1 cells (P<0.01), but there was no significant difference between HepG2 and HepG2/PCDNA3.1 cells (P>0.05). The ADM efflux effect of HepG2/ADM and HepG2/ABCG2 cells was significantly higher than that of parental HepG2 and HepG2/PCDNA3.1 cells (P<0.05). Therefore, the present study demonstrated that ABCG2 expression is highly increased in drug-resistant hepatoma cells and that high expression of ABCG2 is involved in the drug resistance of hepatoma by reducing the intracellular drug concentration.
Keywords: hepatoma, drug resistance, flow cytometry, ATP-binding cassette transporter G2, gene transfection
Introduction
Hepatoma is a common malignant digestive system tumor with high morbidity (854,000) and mortality (810,000) (2015 global incidence and deaths for hepatoma) (1), and seriously threatens the health and lives of individuals. The treatment of hepatoma includes surgery, interventional therapy and radiotherapy (2).
Chemotherapy is the main treatment for patients with hepatoma who have lost the opportunity for surgical treatment. The side effects and drug resistance during chemotherapy seriously affect the outcome of and may lead to the failure of chemotherapy. The aim of the present study was to explore the mechanism of drug resistance in hepatoma and targeted interventions that may effectively improve the outcome of chemotherapy.
It has been found that the adenosine triphosphate (ATP) binding transporter family has an important role in the development of multidrug resistance by reducing the concentration of chemotherapeutic drugs in tumor cells (3-5), among which ATP binding cassette (ABC)B1 and ABCG2 are most closely associated with multidrug resistance (6-9).
ABCG2 is involved in the formation of multidrug resistance in numerous types of tumors and is the main marker of side population cells (10), which are involved in the formation of cancer stem cells (11). Chen (12) reported that ABCG2 protein is a marker of glioma stem cells and side population cells, and found that ABCG2 is related to drug resistance in glioma. Wang et al (13) reported that ABCG2 is a marker of breast cancer stem cells and that ABCG2 is related to the chemoresistance, tumor recurrence, metastasis and invasion of breast cancer. However, to the best of our knowledge, the association between ABCG2 and the multidrug resistance of hepatoma has rarely been reported. In the present study, drug-resistant cells of hepatoma were established and the expression of ABCG2 in such drug-resistant cells was detected. The association between the multidrug resistance of ABCG2 and hepatoma was then discussed.
ABCG2 may cause a decrease of the effective drug concentration in tumor cells by the efflux of chemotherapeutic drugs, which leads to drug resistance (13). Therefore, the aim of the present study was to discuss the mechanism of ADM resistance and provide an ADM-resistant cell model for the clinical study of ADM resistance in hepatoma cells. To this end, drug-resistant hepatoma cells were established and the expression of ABCG2 in these drug-resistant cells was detected. Flow cytometry (FCM) was used to detect the concentration of Adriamycin (ADM) in HepG2/ADM (drug-resistant hepatoma cells), HepG2/ABCG2, HepG2/PCDNA3.1 and their parental cells (HepG2) and to analyze the efflux function of HepG2/ADM and HepG2/ABCG2 cells. The association between the multidrug resistance of ABCG2 and hepatoma was then discussed.
Materials and methods
Chemicals and reagents
ABCG2 antibody (cat. no. sc-18841) was purchased from Santa Cruz Biotechnology, Inc. IgG-FITC antibody (cat. no. 115-095-003) was purchased from Jackson ImmunoResearch Laboratories, Inc. Propidium iodide/RNase (cat. no. 550825) and Annexin V-FITC/propidium iodide (cat. no. 556547) were purchased from Becton, Dickinson and Company. ADM (cat. no. H33021980) was purchased from Hanhui Pharmaceuticals Co., Ltd.
Establishment of the ADM-resistant hepatoma cell line
The HepG2 cell line (cat. no. CL-0103) was obtained from Procell Life Science & Technology Co., Ltd., and was authenticated by STR profiling and analyzed for mycoplasma contamination. The HepG2 cell line was cultured in MEM containing 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 1% penicillin and streptomycin. Cells were sustained in an incubator at 37˚C with 5% CO2. An ADM-resistant hepatoma cell line was established from the HepG2 cells by continuous exposure to increasing concentrations of ADM, from 0.01 to 0.1 µg/ml for 3 months. One of the surviving clones was isolated and designated as HepG2/ADM cells.
ABCG2 gene transfection
pcDNA3.1-ABCG2 plasmid [sequences: CDS of ABCG2 (BC021281) gene (494…2,461)] containing ABCG2 cDNA and empty pcDNA3.1 plasmid were purchased from Wuhan Genesil Biotechnology Co., Ltd. Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was used as the transfection reagent, according to the manufacturer's instructions, and stably transfected cell clones were selected using 600 mg/l G418 (without penicillin and streptomycin) subsequent to transfection for 72 h. The transfection reagents used were as follows. i) Reagent 1: 5 µl of Lipofectamine® 2000 was mixed with 245 µl of MEM culture medium (FBS- and antibiotic-free), and then placed at room temperature for 5 min; ii) reagent 2: 4 µl of pcDNA3.1-ABCG2 or pcDNA3.1 plasmid (containing 2 µg plasmid) was mixed with 246 µl of MEM culture medium (FBS- and antibiotic-free) and then placed at room temperature for 5 min. Reagents 1 and 2 were mixed gently, placed at room temperature for 20 min and then added into the wells. The HepG2 cells transfected with pcDNA3.1-ABCG2 or pcDNA3.1 and selected with 600 mg/l G418 for 10 days were termed HepG2/ABCG2 or HepG2/pcDNA3.1 cells, respectively. ABCG2 mRNA and protein expression levels were monitored to ascertain the efficacy and specificity of the transfection using reverse transcription-quantitative PCR and FCM, respectively.
Cytotoxicity assay
The ADM anticancer drug sensitivity of the HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cells was determined using the MTT assay. This method is based on the capacity of viable cells to metabolize a yellow tetrazolium salt, MTT, using mitochondrial succinate dehydrogenase, into purple formazan crystals when dissolved in acidified propan-2-ol; the resulting purple solution is then spectrophotometrically measured at 490 nm (14). The cells were seeded into 96-well culture plates at a density of 1x104 cells/ml. The serial concentrations of ADM (0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10 and 50 µg/ml) were added in a final volume of 200 µl/well. Normal saline (vehicle; containing 0 µg/ml ADM) was used for the control group. Following drug treatment in an incubator at 37˚C with 5% CO2 for 24 h, the medium was replaced with an equal volume of complete culture medium containing 0.5 mg/ml MTT and incubated at 37˚C with 5% CO2 for 4 h. The medium was removed, 180 µl DMSO was added to each well and plates were continuously shaken for 10 min at room temperature. The cytotoxic effects of the drugs were determined using the optical density (OD) values from a microplate reader at an absorption wavelength of 490 nm (OD490). The inhibitory rate (IR) was calculated using the following formula in order to calculate the half-maximal inhibitory concentration (IC50) of the cells: IR (%)=(1-OD490 treated cells/OD490 control cells) x100%. The resistance index was determined as the IC50 of the resistant cells (HepG2/ADM or HepG2/ABCG2 cells)/IC50 of the parental cells (HepG2 cells). Each experiment was performed in triplicate wells.
Detection of ABCG2 mRNA expression in cells
Total RNA of cells was extracted by RNA isolater (Vazyme Biotech) according to the manufacturer's instructions. Following extraction of total RNA from HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cells, total RNA was reversely transcribed into cDNA using the HiScript II 1st Strand cDNA Synthesis Kit (Vazyme Biotech) according to the manufacturer's instructions, and PCR amplification was then performed using the cDNA as a template. The thermocycling conditions were as follows: 95˚C for 5 min (pre-denaturation), followed by 40 cycles of 95˚C for 15 sec, 58˚C for 30 sec and 72˚C for 25 sec, and a final cycle of 95˚C for 15 sec, 58˚C for 1 min and 95˚C for 30 sec. SYBR-Green I was used as the fluorescent dye. Real-time PCR was performed using AceQ qPCR SYBR Green Master Mix kit (Vazyme Biotech) according to the manufacturer's instructions. Human GAPDH was used as the internal reference for standardization. The primers used were as follows: ABCG2 forward, 5'-GGTCAGAGTGTGGTTTCTGTAGCA-3' and reverse, 5'-GTGAGAGATCGATGCCCTGCTTTA-3'; and GAPDH forward, 5'-ACCACAGTCCATGCCATCAC-3' and reverse, 5'-TCCACCACCCTGTTGCTGTA-3'. The relative expression level of ABCG2 mRNA was calculated using the 2-ΔΔCq method (ΔCq=CqABCG2-CqGAPDH) (15). Each experiment was performed three independent replicates.
Assessment of cell apoptosis and cell cycle distribution using FCM
HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 and HepG2 cells were collected during the logarithmic growth phase. A total of 1 ml of the single-cell suspension (containing 1x106 cells) was prepared, washed with cold PBS once and suspended in 100 µl of 1X binding buffer. Subsequently, 10 µl Annexin V-FITC was added and the mixture was placed on ice for 15 min in the dark. Next, 380 µl of 1X binding buffer and 10 µl propidium iodide were added and the cell mixture was incubated on ice for 15 min in the dark, washed with cold PBS once and suspended with 1 ml PBS. The apoptosis of the cells was measured using an FC500 flow cytometer (Beckman Coulter, Inc.). EXPO32 ADC software (version 1.2; Beckman Coulter, Inc.) was used to analyze the immunofluorescence data and evaluate the apoptotic rate. Each experiment was performed as three independent replicates.
A total of 1 ml of the single-cell suspension (containing 1x106 cells) was prepared, washed with cold PBS twice and fixed with 70% ethanol at 4˚C for 6 h. Following this, the single-cell suspension was washed with PBS twice. The cells were then suspended in 100 µl PBS and 1 ml propidium iodide was added to the suspension for staining at 4˚C for 30 min. Subsequently, the cells were detected using the FC500 flow cytometer. MultiCycle AV software (version 275; Phoenix Flow Systems, Inc.) was used to analyze the cell cycle. The proliferative index (PI) was calculated according to the following formula: PI=(S+G2/M)/(G0/1+S+G2/M) x100%. Each experiment was performed as three independent replicates.
Assessment of ABCG2 protein expression in cells using FCM
HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 and HepG2 cells were collected during the logarithmic growth phase. A total of 1 ml of the single-cell suspension (containing 1x106 cells) was first prepared, then washed with cold PBS once and resuspended in 100 µl PBS. A total of 10 µl of ABCG2 antibody (dilution, 1:100; cat. no. sc-18841; Santa Cruz Biotechnology, Inc.) was added to each sample. The suspension was then incubated for 30 min at room temperature, washed with cold PBS once and resuspended in 100 µl of PBS. IgG-FITC secondary antibodies (dilution, 1:100; cat. no. 115-095-003; Jackson ImmunoResearch Laboratories, Inc.) were added to each sample. The suspension was then incubated for 30 min in the dark, washed with cold PBS once and resuspended in 1 ml PBS. The stained cells were analyzed using the FC500 flow cytometer and the data were analyzed using EXPO32 ADC software, with the mean fluorescence intensity representing the expression of ABCG2 protein. Each experiment was performed three independent replicates.
ADM efflux by HepG2/ADM and HepG2/ABCG2 cells
The cellular accumulation and efflux of ADM were analyzed by flow cytometry. HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 and HepG2 cells (4x105 cells) were incubated with 0.1 µg/ml ADM at 37˚C for 2 h and washed twice with ice-cold PBS. The cells were resuspended in ADM-free complete culture medium and incubated in an incubator at 37˚C with 5% CO2 for 1 h. The cells were washed with ice-cold PBS and the ADM (the ADM has an intrinsic fluorescence peak at 595 nm) (16) retained in the cells was detected using the FC500 flow cytometer and the data were analyzed using EXPO32 ADC software, with the mean fluorescence intensity representing the ADM content. Each experiment was performed three independent replicates.
Statistical analysis
All data are presented as the mean ± the SD (n=3) and were statistically analyzed using one-way ANOVA or two-way ANOVA (MTT assay: Two independent variables-ADM concentration and group) followed by Tukey's or Bonferroni's test (SPSS software version 21; IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Drug resistance of HepG2/ADM and HepG2/ABCG2 cells to ADM
Following anticancer drug (ADM) treatment for 24 h, the cell growth inhibitory rate was detected by an MTT assay. As presented in Fig. 1, compared with the control group (0 µg/ml ADM), the HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cell survival decreased in a dose-dependent manner after treatment with concentrations of ADM ranging from 0.001 to 50 µg/ml. The cell growth inhibitory rate in HepG2/ADM and HepG2/ABCG2 cells was significantly lower than that in HepG2 and HepG2/pcDNA3.1 cells (P<0.01). The IC50 was calculated through the MTT assay. The IC50 of HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 and HepG2 cells was 11.17±0.59, 12.75±0.47, 0.74±0.01 and 0.72±0.03 µg/ml, respectively. The resistance index of HepG2/ADM and HepG2/ABCG2 cells to ADM was 15.51 and 17.71, respectively. HepG2/ADM and HepG2/ABCG2 cells were resistant to ADM.
Figure 1.
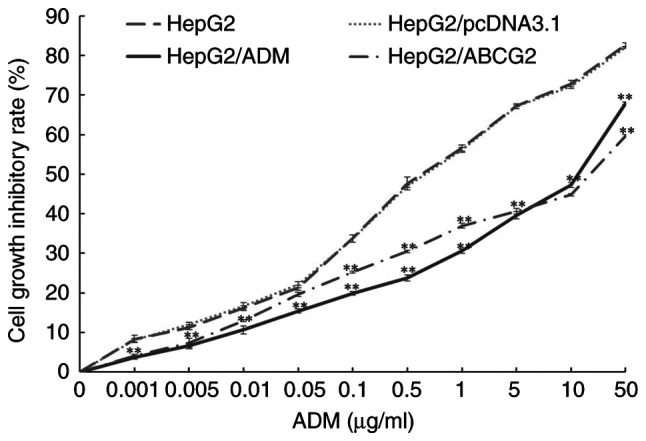
Cell growth inhibitory rate of cells following treatment with various concentrations of ADM. HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cells were treated with various concentrations of ADM for 24 h and the cell growth inhibitory rate was detected using the MTT assay. HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cell survival decreased in a dose-dependent manner following treatment with different concentrations of ADM, ranging from 0.001 to 50 µg/ml. The cell growth inhibitory rate on HepG2/ADM and HepG2/ABCG2 cells was significantly lower than that in HepG2 and HepG2/pcDNA3.1 cells. Values are expressed as the mean +/- standard deviation (n=3). **P<0.01 vs. HepG2 and HepG2/pcDNA3.1 cells. ADM, Adriamycin; ABCG2, adenosine triphosphate-binding cassette transporter G2.
Apoptotic rate of cells
The results of the FCM analysis indicated no significant difference in the apoptotic rate of HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 cells and their parental HepG2 cells (P>0.05; Fig. 2).
Figure 2.
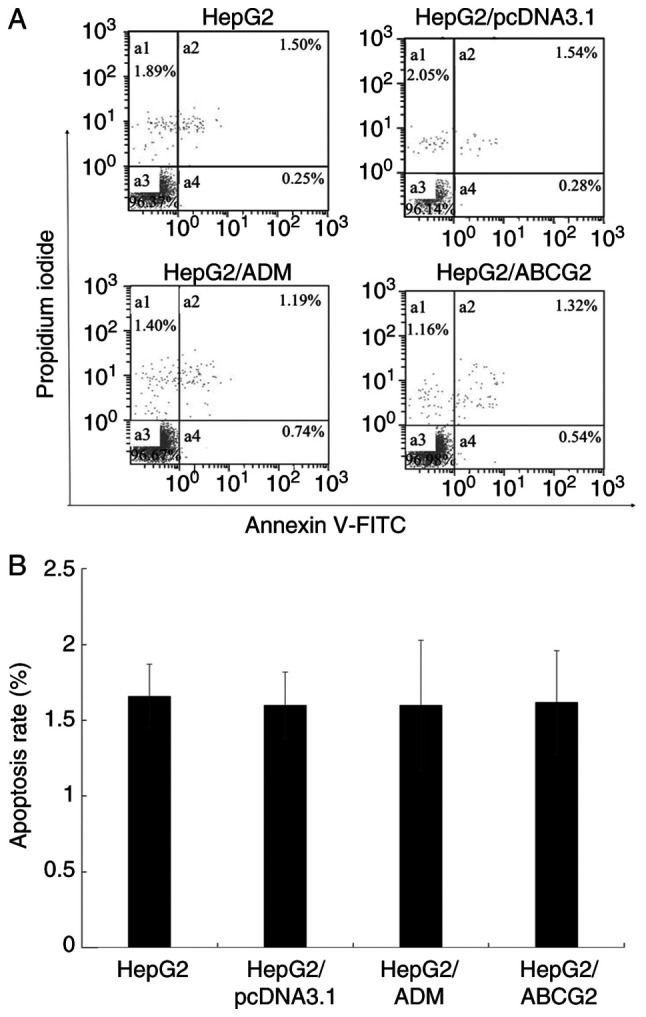
Cell apoptotic rate detected by flow cytometry. (A) The cell apoptotic rate of HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cells was detected by flow cytometry. (B) The flow cytometry results indicated that there was no significant difference between the apoptotic rate of HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 cells and their parental HepG2 cells. Values are expressed as the mean +/- standard deviation (n=3). ADM, Adriamycin; ABCG2, adenosine triphosphate-binding cassette transporter G2.
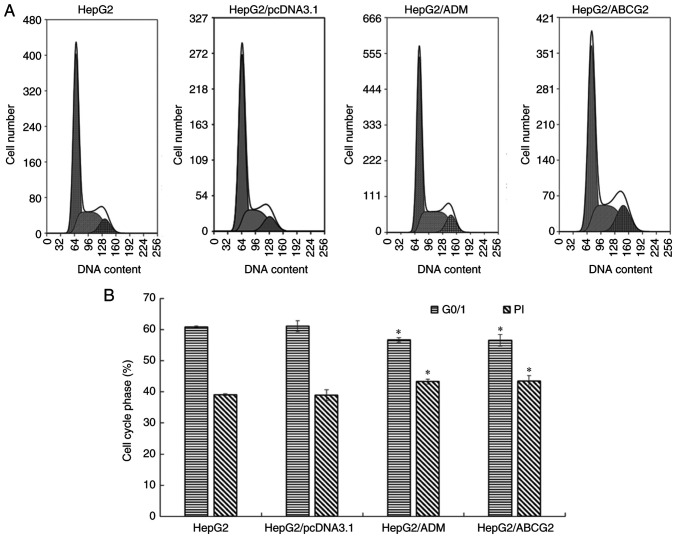
Evaluation of the cell cycle using FCM
The results of the FCM analysis (Fig. 3) indicated that the G0/G1 phase population of HepG2/ADM and HepG2/ABCG2 cells decreased significantly compared with that of their parental HepG2 cells and HepG2/pcDNA3.1 cells (P<0.05). The PI of HepG2/ADM and HepG2/ABCG2 cells increased significantly compared with their parental HepG2 cells and HepG2/pcDNA3.1 cells (P<0.05). Compared with HepG2/ABCG2 cells, HepG2/ADM exhibited no significant difference in G0/G1 phase and PI (P>0.05); compared with HepG2 cells, HepG2/pcDNA3.1 had no significant difference in G0/G1 phase and PI (P>0.05).
Figure 3.
Cell cycle detected by flow cytometry. (A) The cell cycle distribution was detected by flow cytometry. (B) The results indicated that the G0/G1 phase population of HepG2/ADM and HepG2/ABCG2 cells was significantly lower, while the proliferation index was significantly higher, compared with their parental HepG2 cells and HepG2/pcDNA3.1 cells. Values are expressed as the mean +/- standard deviation (n=3). *P<0.05 vs. HepG2 and HepG2/pcDNA3.1 cells; ADM, Adriamycin; ABCG2, adenosine triphosphate-binding cassette transporter G2; PI, proliferative index.
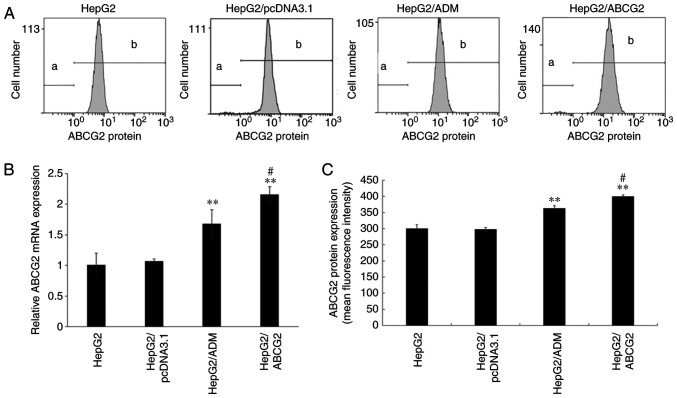
Expression of ABCG2 gene and protein in drug-resistant hepatoma cells and their parental cells
The results indicated that the gene and protein expression levels of ABCG2 in the HepG2/ADM and HepG2/ABCG2 cells were significantly higher than those in HepG2 and HepG2/pcDNA3.1 cells (P<0.01), but there was no significant difference between HepG2/pcDNA3.1 and HepG2 cells (P>0.05). Compared with HepG2/ADM cells, the expression levels of ABCG2 gene and protein in HepG2/ABCG2 cells increased significantly (P<0.05), as indicated in Fig. 4.
Figure 4.
ABCG2 gene and protein expression in cells detected by RT-qPCR and flow cytometry. (A) The expression level of ABCG2 protein in cells was detected by flow cytometry. (B) The expression level of ABCG2 mRNA in cells was detected by RT-qPCR. The expression level of ABCG2 mRNA in HepG2/ADM and HepG2/ABCG2 cells was significantly higher than that in HepG2 and HepG2/pcDNA3.1 cells (P<0.01), and the expression level of ABCG2 mRNA in HepG2/ABCG2 cells was significantly higher than that in HepG2/ADM cells (P<0.05), but there was no significant difference between the HepG2/pcDNA3.1 and HepG2 cells. (C) The expression level of ABCG2 protein in HepG2/ADM and HepG2/ABCG2 cells was significantly higher than that in HepG2 and HepG2/pcDNA3.1 cells (P<0.01), and the expression level of ABCG2 protein in HepG2/ABCG2 cells was significantly higher than that in HepG2/ADM cells (P<0.05), but there was no significant difference between the HepG2/pcDNA3.1 and HepG2 cells. Values are expressed as the mean +/- standard deviation (n=3). **P<0.01 vs. HepG2 and HepG2/pcDNA3.1 cells; #P<0.05 vs. HepG2/ADM cells. a and b stand for ‘gates’ of flow cytometry. ADM, Adriamycin; ABCG2, adenosine triphosphate-binding cassette transporter G2; RT-qPCR, reverse transcription-quantitative PCR.
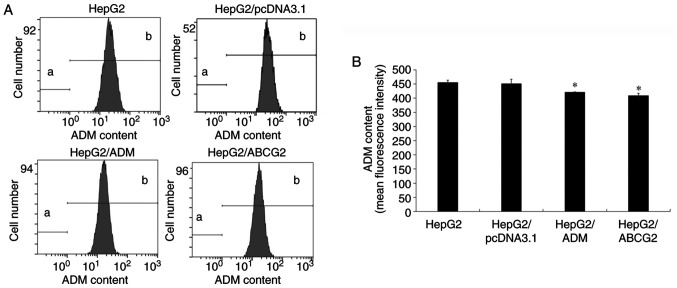
ADM efflux effect of HepG2/ADM and HepG2/ABCG2 cells assayed by FCM
An ADM efflux effect assay was used to investigate how the HepG2/ADM and HepG2/ABCG2 cells resisted the anticancer agent using FCM (Fig. 5). The cells were incubated at 37˚C with 0.1 µg/ml ADM for 2 h and then without ADM for 1 h. The level of ADM decreased in HepG2/ADM and HepG2/ABCG2 cells compared with HepG2 and HepG2/pcDNA3.1 cells (P<0.05). There was no significant difference between HepG2/pcDNA3.1 and HepG2 cells (P>0.05). Thus, the results showed that the ADM efflux effect of the HepG2/ADM and HepG2/ABCG2 cells was more pronounced than that of the HepG2 and HepG2/pcDNA3.1 cells.
Figure 5.
ADM efflux effect of HepG2/ADM and HepG2/ABCG2 cells assayed by flow cytometry. (A) The HepG2, HepG2/pcDNA3.1, HepG2/ADM and HepG2/ABCG2 cells were incubated at 37˚C with 0.1 µg/ml ADM for 2 h, and then without ADM for 1 h. The ADM fluorescence intensity, which represents the ADM content in the cells, was detected by flow cytometry. (B) The ADM fluorescence intensity in the HepG2/ADM and HepG2/ABCG2 cells was lower than that in the HepG2 and HepG2/pcDNA3.1 cells, indicating that the ADM efflux effect of HepG2/ADM and HepG2/ABCG2 cells was more efficient than that of the HepG2 and HepG2/pcDNA3.1 cells. There was no significant difference between the HepG2/pcDNA3.1 and HepG2 cells. Values are expressed as the mean +/- standard deviation (n=3). *P<0.05 vs. HepG2 and HepG2/pcDNA3.1 cells. a and b stand for ‘gates’ of flow cytometry. ADM, Adriamycin; ABCG2, adenosine triphosphate-binding cassette transporter G2.
Discussion
Hepatoma is a common malignant tumor of the digestive system, and is characterized by high morbidity and mortality (1,2). Chemotherapy is one of the conventional treatments for hepatoma, particularly for patients unable to undergo surgery at an advanced stage. The factors that affect the efficacy of chemotherapy include the side effects of the chemotherapeutic drugs and the multidrug resistance of tumor cells to chemotherapeutic drugs (17). Exploring the mechanism and finding the target of multidrug resistance is important for reversing multidrug resistance in the clinic.
The most widely studied and clinically significant mechanisms of multidrug resistance in tumor cells include the following: i) The membrane transporter-mediated drug efflux pump mechanism (18), consisting of drug delivery through transmembrane transporters to reduce the level of antitumor drugs in multidrug-resistant cells; ii) the enzyme-mediated mechanism, which consists of the activation of cell oxidation and the glutathione-related detoxification enzyme system, to inactivate drugs or accelerate the excretion of drugs directly (19); and iii) the regulating mechanism through apoptotic genes that mediate the increase of the expression of anti-apoptotic factors, such as Bcl-2 expression, and the decrease of the gene and protein expression of apoptotic factors, so that multidrug-resistant cells prevent apoptosis induced by antitumor drugs (20,21).
The drug efflux pump mechanism mediated by membrane transporters is one of the most widely studied multidrug resistance mechanisms (11,22,23). Cell transmembrane transporters may pump intracellular antitumor drugs out of cells and reduce the concentration of chemotherapeutic drugs in cells, thus producing multidrug resistance to tumors. Several studies have suggested that the majority of transmembrane transporters belong to the ABC transporter family (3,24-27). To date, >100 types have been found, of which ≥48 types are in humans. The most widely studied ABC proteins are ABCB1 and ABCG2 (6,7,28-30). ABCG2 is associated with multidrug resistance in a variety of tumor cells (31-38), and is involved in forming side population cells and tumor stem cells (10,39,40). However, studies on the relationship between ABCG2 and multidrug resistance of hepatoma cells are rare (41). The present study established drug-resistant hepatoma cells by increasing the drug concentration in cell culture and gene transfection. The role of ABCG2 in the formation of multidrug resistance in hepatoma cells was then discussed.
The IC50 value of ADM in HepG2 cells was calculated using the MTT assay. The ADM concentration in the culture of drug-resistant hepatoma cells was set up according to the IC50 value of ADM in HepG2 cells. The ADM concentration in the culture was increased from 0.01 to 0.1 µg/ml. Eventually, HepG2 cells grew steadily in the culture containing 0.1 µg/ml ADM and these cells were designated HepG2/ADM cells. The morphology of HepG2/ADM cells was more irregular than that of the others and the cell volume was increased (data not shown). The resistance index of HepG2/ADM cells to ADM was 15.51. The expression levels of ABCG2 protein and gene in HepG2/ADM cells were significantly higher than those in parental HepG2 cells. In order to verify that ABCG2 is involved in the drug resistance of hepatoma cells, HepG2/ABCG2 cells overexpressing the ABCG2 gene were established by gene transfection. The results indicated that HepG2/ABCG2 cells were also resistant to ADM and the drug resistance index was 17.71. These results suggested that ABCG2 is involved in the drug resistance of hepatoma cells (HepG2/ADM and HepG2/ABCG2).
The apoptosis and cell cycle of HepG2/ADM, HepG2/ABCG2, HepG2/pcDNA3.1 and HepG2 cells were detected by FCM. The results indicated no difference in the apoptotic rate of HepG2/ADM and HepG2/ABCG2 cells compared with HepG2 and HepG2/pcDNA3.1 cells, but the G0/G1 phase decreased and the PI increased significantly. The proliferation of HepG2/ADM and HepG2/ABCG2 cells was higher than that of their parental cells.
ABCG2 is a member of the ABC superfamily. The multidrug resistance mechanism of the ABC superfamily involves drug efflux (42-45), which decreases the intracellular drug concentration. The ADM efflux effect of HepG2/ADM and HepG2/ABCG2 cells was significantly higher than that of HepG2 and HepG2/pcDNA3.1 cells, which suggested that the drug resistance of hepatoma cells is related to the efflux of chemotherapeutics regulated by ABCG2. These results are consistent with the mechanism of drug resistance induced by ABCG2 in the literature (42-45).
In conclusion, in the present study, it was demonstrated that HepG2/ADM and HepG2/ABCG2 cells were resistant to ADM. The mechanism of drug resistance was related to high expression of ABCG2 in cells and the resulting increase of ADM efflux, which led to a decreased concentration of ADM in the cells, resulting in drug resistance. However, assessing the drug resistance of only one chemotherapeutic agent (ADM) was a limitation of the present study. The molecular mechanisms underlying the drug resistance of hepatoma cells are complex, necessitating further study in the future.
Acknowledgements
The present study is based on a previously published meeting abstract presented at the 17th International Congress of Immunology (October 19-23, 2019, Beijing, China) and published as an abstract entitled ‘Study on the drug resistance of HepG2 cells induced by adenosine triphosphate (ATP)-binding cassette transporter G2’ in the European Journal of Immunology, vol. 49, Supplement S3, p3073.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YL wrote the manuscript. YL, BD, JL, XL, CH, JW and LL performed the experiments. BD and JL conducted the statistical analysis. LL designed the study and revised the manuscript. All authors have read and approved the final manuscript. YL and LL confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al. Global, regional, and national cancer incidence, motality, years of life lost, yeas lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. Global Burden of Disease Cancer Collaboration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ju DY, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20:793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas C, Tampé R. Structural and mechanistic principles of ABC transporters. Ann Rev Biochem. 2020;89:605–636. doi: 10.1146/annurev-biochem-011520-105201. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Qin Z, Cai S, Yu L, Hu H, Zeng S. The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin Drug Metab Toxicol. 2021;17:291–306. doi: 10.1080/17425255.2021.1887139. [DOI] [PubMed] [Google Scholar]
- 6.Hegedus C, Telbisz A, Hegedus T, Sarkadi B, Ozvegy-Laczka C. Lipid regulation of the ABCB1 and ABCG2 multidrug transporters. Adv Cancer Res. 2015;125:97–137. doi: 10.1016/bs.acr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 7.To KK, Poon DC, Wei Y, Wang F, Lin G, Fu LW. Vatalanib sensitizes ABCB1 and ABCG2-overexpressing multidrug resistant colon cancer cells to chemotherapy under hypoxia. Biochem Pharmacol. 2015;97:27–37. doi: 10.1016/j.bcp.2015.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Limniatis G, Georges E. Down-regulation of ABCB1 by collateral sensitivity drugs reverses multidrug resistance and up-regulates enolase I. J Biochem. 2022;172:37–48. doi: 10.1093/jb/mvac032. [DOI] [PubMed] [Google Scholar]
- 9.Kukal S, Guin D, Rawat C, Bora S, Mishra MK, Sharma P, Paul PR, Kanojia N, Grewal GK, Kukreti S, et al. Multidrug efflux transporter ABCG2: expression and regulation. Cell Mol Life Sci. 2021;78:6887–6939. doi: 10.1007/s00018-021-03901-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie ZY, Lv K, Xiong Y, Guo WH. ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from colon cancer. Oncol Res Treat. 2014;37:666–668. doi: 10.1159/000368842. [DOI] [PubMed] [Google Scholar]
- 11.Kozovska Z, Gabrisova V, Kucerova L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed Pharmacother. 2014;68:911–916. doi: 10.1016/j.biopha.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Chen D. Tumor formation and drug resistance properties of human glioblastoma side population cells. Mol Med Rep. 2015;11:4309–4314. doi: 10.3892/mmr.2015.3279. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Wang Y, Zhong J. Side population cells and drug resistance in breast cancer. Mol Med Rep. 2015;11:4297–4302. doi: 10.3892/mmr.2015.3291. [DOI] [PubMed] [Google Scholar]
- 14.Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018 doi: 10.1101/pdb.prot095505. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Motlagh NS, Parvin P, Ghasemi F, Atyabi F . Fluorescence properties of several chemotherapy drugs: Doxorubicin, paclitaxel and bleomycin. Biomed Opt Express. 2016;25:2400–2406. doi: 10.1364/BOE.7.002400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Q, Yang Z, Nie Y, Shi Y, Fan D. Multi-drug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett. 2014;347:159–166. doi: 10.1016/j.canlet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Lu JF, Pokharel D, Bebawy M. MRP1 and its role in anticancer drug resistance. Drug Metab Rev. 2015;47:406–419. doi: 10.3109/03602532.2015.1105253. [DOI] [PubMed] [Google Scholar]
- 19.Masanek U, Stammler G, Volm M. Modulation of multidrug resistance in human ovarian cancer cell lines by inhibition of P-glycoprotein 170 and PKC isoenzymes with antisense oligonucleotides. J Exp Ther Oncol. 2002;2:37–41. doi: 10.1046/j.1359-4117.2002.01004.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YF, Li XH, Shi YQ, Wu YY, Li N, He Q, Ji Q, Wang RQ, Yang SM, Fang DC. CIAPIN1 confers multidrug resistance through up-regulation of MDR-1 and Bcl-L in LoVo/Adr cells and is independent of p53. Oncol Rep. 2011;25:1091–1098. doi: 10.3892/or.2011.1148. [DOI] [PubMed] [Google Scholar]
- 21.Lo YL, Wang W, Ho CT. 7,3',4'-Trihydroxyisoflavone modulates multidrug resistance transporters and induces apoptosis via production of reactive oxygen species. Toxicology. 2012;302:221–232. doi: 10.1016/j.tox.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Kunická T, Souček P. Importance of ABCC1 for cancer therapy and prognosis. Drug Metab Rev. 2014;46:325–342. doi: 10.3109/03602532.2014.901348. [DOI] [PubMed] [Google Scholar]
- 23.Misra R, Das M, Sahoo BS, Sahoo SK. Reversal of multidrug resistance in vitro by co-delivery of MDR1 targeting siRNA and doxorubicin using a novel cationic poly(lactide-co-glycolide) nanoformulation. Int J Pharm. 2014;475:372–384. doi: 10.1016/j.ijpharm.2014.08.056. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh MJ, Chen MK, Yu YY, Sheu GT, Chiou HL. Psoralen reverses docetaxel-induced multidrug resistance in A549/D16 human lung cancer cells lines. Phytomedicine. 2014;21:970–977. doi: 10.1016/j.phymed.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Wu CP, Hsiao SH, Murakami M, Lu YJ, Li YQ, Huang YH, Hung TH, Ambudkar SV, Wu YS. Alpha-mangostin reverses multidrug resistance by attenuating the function of the multidrug resistance-linked ABCG2 transporter. Mol Pharm. 2017;14:2805–2814. doi: 10.1021/acs.molpharmaceut.7b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murakami M, Ohnuma S, Fukuda M, Chufan EE, Kudoh K, Kanehara K, Sugisawa N, Ishida M, Naitoh T, Shibata H, et al. Synthetic analogs of curcumin modulate the function of multidrug resistance-linked ATP-binding cassette transporter ABCG2. Drug Metab Dispos. 2017;45:1166–1177. doi: 10.1124/dmd.117.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan YF, Zhang W, Zeng L, Lei ZN, Cai CY, Gupta P, Yang DH, Cui Q, Qin ZD, Chen ZS, Trombetta LD. Dacomitinib antagonizes multidrug resistance (MDR) in cancer cells by inhibiting the efflux activity of ABCB1 and ABCG2 transporters. Cancer Lett. 2018;421:186–198. doi: 10.1016/j.canlet.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Bieber MM, Teng NN. Hedgehog signaling regulates drug sensitivity by targeting ABC transporters ABCB1 and ABCG2 in epithelial ovarian cancer. Mol Carcinog. 2014;53:625–634. doi: 10.1002/mc.22015. [DOI] [PubMed] [Google Scholar]
- 29.Sodani K, Tiwari AK, Singh S, Patel A, Xiao ZJ, Chen JJ, Sun YL, Talele TT, Chen ZS. GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2- and ABCB1-mediated drug resistance. Biochem Pharmacol. 2012;83:1613–1622. doi: 10.1016/j.bcp.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepper ER, Nooter K, Verweij J, Acharya MR, Figg WD, Sparreboom A. Mechanisms of resistance to anticancer drugs: The role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics. 2005;6:115–138. doi: 10.1517/14622416.6.2.115. [DOI] [PubMed] [Google Scholar]
- 31.Sodani K, Patel A, Anreddy N, Singh S, Yang DH, Kathawala RJ, Kumar P, Talele TT, Chen ZS. Telatinib reverses chemotherapeutic multidrug resistance mediated by ABCG2 efflux transporter in vitro and in vivo. Biochem Pharmacol. 2014;89:52–61. doi: 10.1016/j.bcp.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenfeldt MT, Bell LA, Long JS, O'Prey J, Nixon C, Roberts F, Dufès C, Ryan KM. E2F1 drives chemotherapeutic drug resistance via ABCG2. Oncogene. 2014;33:4164–4172. doi: 10.1038/onc.2013.470. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi T, Ross DD. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin J Cancer. 2012;31:73–99. doi: 10.5732/cjc.011.10320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yousaf M, Ali M. Modulation of ABCG2 surface expression by Rab5 and Rab21 to overcome multidrug resistance in cancer cells. Xenobiotica. 2020;50:988–996. doi: 10.1080/00498254.2020.1716107. [DOI] [PubMed] [Google Scholar]
- 35.Kokubo S, Ohnuma S, Murakami M, Kikuchi H, Funayama S, Suzuki H, Kajiwara T, Yamamura A, Karasawa H, Sugisawa N, et al. A phenylfurocoumarin derivative reverses ABCG2-mediated multidrug resistance in vitro and in vivo. Int J Mol Sci. 2021;22(12502) doi: 10.3390/ijms222212502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mioč M, Telbisz Á, Radman K, Bertoša B, Šumanovac T, Sarkadi B, Kralj M. Interaction of crown ethers with the ABCG2 transporter and their implication for multidrug resistance reversal. Histochem Cell Biol. 2022;158:261–277. doi: 10.1007/s00418-022-02106-z. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y, Guo Z, Fan C, Chen J, Xu S, Liu Z. Rationally screened and designed ABCG2-binding aptamers for targeting cancer stem cells and reversing multidrug resistance. Anal Chem. 2022;94:7375–7382. doi: 10.1021/acs.analchem.2c00863. [DOI] [PubMed] [Google Scholar]
- 38.Zattoni IF, Delabio LC, Dutra JP, Kita DH, Scheiffer G, Hembecker M, Pereira GDS, Moure VR, Valdameri G. Targeting breast cancer resistance protein (BCRP/ABCG2): Functional inhibitors and expression modulators. Eur J Med Chem. 2022;237(114346) doi: 10.1016/j.ejmech.2022.114346. [DOI] [PubMed] [Google Scholar]
- 39.Zhang QH, Dou HT, Xu P, Zhuang SC, Liu PS. Tumor recurrence and drug resistance properties of side population cells in high grade ovary cancer. Drug Res (Stuttg) 2015;65:153–157. doi: 10.1055/s-0034-1375609. [DOI] [PubMed] [Google Scholar]
- 40.Britton KM, Eyre R, Harvey IJ, Stemke-Hale K, Browell D, Lennard TWJ, Meeson AP . Breast cancer, side population cells and ABCG2 expression. Cancer Lett. 2012;323:97–105. doi: 10.1016/j.canlet.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tandia M, Mhiri A, Paule B, Saffroy R, Cailliez V, Noé G, Farinotti R, Bonhomme-Faivre L. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P-glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): Monocentric study. Cancer Chemother Pharmacol. 2017;79:759–766. doi: 10.1007/s00280-017-3268-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Zhang YK, Wang YJ, Kathawala RJ, Patel A, Zhu H, Sodani K, Talele TT, Ambudkar SV, Chen ZS, Fu LW. WHI-P154 enhances the chemotherapeutic effect of anticancer agents in ABCG2-overexpressing cells. Cancer Sci. 2014;105:1071–1078. doi: 10.1111/cas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang XK, To KK, Huang LY, Xu JH, Yang K, Wang F, Huang ZC, Ye S, Fu LW. Afatinib circumvents multidrug resistance via dually inhibiting ATP binding cassette subfamily G member 2 in vitro and in vivo. Oncotarget. 2014;5:11971–11985. doi: 10.18632/oncotarget.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai CL, Liang YJ, Wang YS, Tiwari AK, Yan YY, Wang F, Chen ZS, Tong XZ, Fu LW. Sensitization of ABCG2-overexpressing cells to conventional chemotherapeutic agent by sunitinib was associated with inhibiting the function of ABCG2. Cancer Lett. 2009;279:74–83. doi: 10.1016/j.canlet.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Jing W, Zhou M, Chen R, Ye X, Li W, Su X, Luo J, Wang Z, Peng S. In vitro and ex vivo anti-tumor effect and mechanism of Tucatinib in leukemia stem cells and ABCG2-overexpressing leukemia cells. Oncol Rep. 2021;45:1142–1152. doi: 10.3892/or.2020.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.