Abstract
During development, resident stem cell populations contribute to the growth and maturation of tissue and organs. In skeletal muscle, muscle stem cells, or satellite cells, are responsible for the maturation of postnatal myofibers. However, the role satellite cells play in later stages of postnatal growth, and thus, when they enter a mature quiescent state is controversial. Here we discuss the current literature regarding the role satellite cells play in all stages of postnatal growth, from birth to puberty onset to young adulthood. We additionally highlight the implications of satellite cell loss or dysfunction during developmental stages, both in the context of experimental paradigms and disease settings.
Keywords: skeletal muscle, satellite cells, development, regeneration, aging, cancer, pediatric
Introduction
Resident stem cell populations provide host organ systems with the ability to regenerate and repair damaged tissue. Skeletal muscle is endowed with its own resident muscle stem cell, the satellite cell (SC). First discovered by Mauro in 1961, these cells are named for their unique position, found in between the overlaying basal lamina and myofiber itself [1]. In adulthood, SCs (identified by expression of Pax7) reside in a quiescent state, waiting to partake in programs of adaptation, repair, or regeneration [2]. The adult SC pool is rather heterogenous in nature, with a multitude of factors governing their quiescent state. This review will not focus on adult SCs, but a recent encompassing review has touched upon these subjects in detail [3]. In a paradigm of skeletal muscle injury, SCs will activate and give rise to progenitors that fuse to repair degenerated myofibers. The progression of SC-derived progenitors towards terminal myogenic commitment and fusion competency is regulated by a coordinated expression of myogenic regulatory factors [4]. Numerous groups have demonstrated the indispensable role of SCs in skeletal muscle regeneration [5–7]. Additionally, SCs are activated in other processes, including neuromuscular junction (NMJ) maintenance/regeneration and exercise mediated skeletal muscle adaptation [8–12]. Thus, SCs remain a target of intense investigation for paradigms designed to restore or accelerate the recovery of injured, diseased, and/or aged skeletal muscle.
The embryonic origin of skeletal muscle has been covered in great depth [13, 14]. In brief, trunk and limb skeletal muscles are derived from the somites during embryonic development. Initially, Pax3/Pax7-expressing myogenic progenitors contribute to primary myogenesis. As development proceeds, Pax7-expressing myogenic progenitors continue to contribute to late embryonic and postnatal skeletal muscle growth [15]. With time, some Pax7+ cells will escape terminal commitment and form the pool of muscle stem cells found interspersed along the length of individual adult myofibers [16–18]. Myofibers, the contractile unit of skeletal muscle, are unique due to their multinucleated nature. An individual myofiber has hundreds to thousands of myonuclei running along its length. It has long been established that SCs are the principal source of myonuclei during development [19]. Each myonuclei is derived from a SC that divided, gave rise to a myogenic progenitor, and that progenitor fused to the developing myofiber.
Until recently, the role of SCs in postnatal muscle growth and the timing of their entry into quiescence was controversial, leaving the molecular mechanisms understudied. In this review, we discuss the most recent advances describing SC-derived myonuclear contribution to postnatal development. We will focus on the critical, yet underappreciated, developmental paradigm; the transition from prepubertal growth to puberty, and onwards to young adulthood. This review will describe the physiological relevance and underlying mechanisms regulating SC fate and function during all stages of postnatal development. Additionally, we highlight recent studies regarding the establishment of the quiescent adult SC pool and the developmental events regulating this process. Lastly, we conclude by investigating the implications of SC dysfunction and loss during development, both in the context of genetic models and disease.
Postnatal skeletal muscle growth
Understanding the role of SCs during juvenile growth is important for not only basic developmental science, but potentially revealing a role for SC dysfunction in juvenile skeletal muscle disorders. In humans, it has been suggested that myonuclear number increases with age during the first 18 years of life [20]. Additionally, a recent study has demonstrated that in terms of myofiber size and myonuclear number, human myofibers scale similarly to that of mice [21]. This observation, as well as the murine condensed lifecycle, make it a suitable model to understand the paradigms of juvenile muscle development.
During the first three weeks of murine growth, body mass increases rapidly (nearly 8-fold) [22]. At 3–4 weeks of age (P21-P28), mice are typically weaned from their mother. The period of growth between weaning and puberty onset, is known as prepuberty [23]. In the developmental timeline of the mouse, it has been reported that genetic background of the animal can influence the timing of puberty onset [24, 25]. Additionally, and similar to humans, pubertal onset in female mice can occur earlier than that of males. The opening of the vagina, an external marker of puberty onset, has been reported to occur at ~P29 [26]. On average though, mice attain puberty at about 6 weeks (P42) [23, 27, 28]. Lastly, sexual maturity and young adulthood is reached by about 8–12 weeks of age, with the average being 10 weeks [23]. These time points, as well as a comparison relative to humans, has been highlighted in Figure 1.
Figure 1.

Illustration comparing the developmental life cycle of mice and humans. Information is adapted from Dutta and Sengupta, 2016 [23].
During postnatal development, the increase in skeletal muscle size is primarily facilitated by increasing individual myofiber cross-sectional area (CSA) and length. Extensor digitorum longus (EDL) myofibers increase in CSA by nearly 5-fold (from ~100 to 550 μm2) from P1 to P21 [22, 29]. This rapid growth continues during prepuberty, with EDL and soleus (SOL) myofibers nearly tripling in CSA between P21 and P42 [30]. By P42 the distribution of EDL and SOL CSA is similar to that of a 12-week young adult, indicating that developmental increases in CSA slow beyond this time point [30]. During these periods of rapid myofiber growth, the total number of myofibers does not change much. This is established rather early in development, with no changes being recorded post P7 in the EDL [29]. In order to facilitate the rapid growth of myofibers, SCs are actively dividing and giving rise to progenitors to fuse to the growing myofibers. This addition of individual myonuclei is termed myonuclear accretion. It has long been formally demonstrated that the SCs are the principal source of myonuclei during development [19]. However, in the murine model, there has been debate as to when developmental myonuclear accretion finally ceases.
Until recent studies proving otherwise, it was accepted that SC-derived myonuclear contribution ceases by P21 in the developing mouse [29, 31, 32]. Furthermore, at P21 all SCs were deemed mitotically quiescent, with the remaining myofiber hypertrophy independent of myonuclear accretion [29, 31, 33]. These notions were backed up by myonuclear counts (a readout of SC activity) in EDL myofibers, in which there was no significant difference when comparing P21 vs P56 ages (245 vs 256 myonuclei/myofiber respectively) [29]. However, recently numerous groups have identified residual SC activity extending into the prepubertal developmental period (Figure 2).
Figure 2.
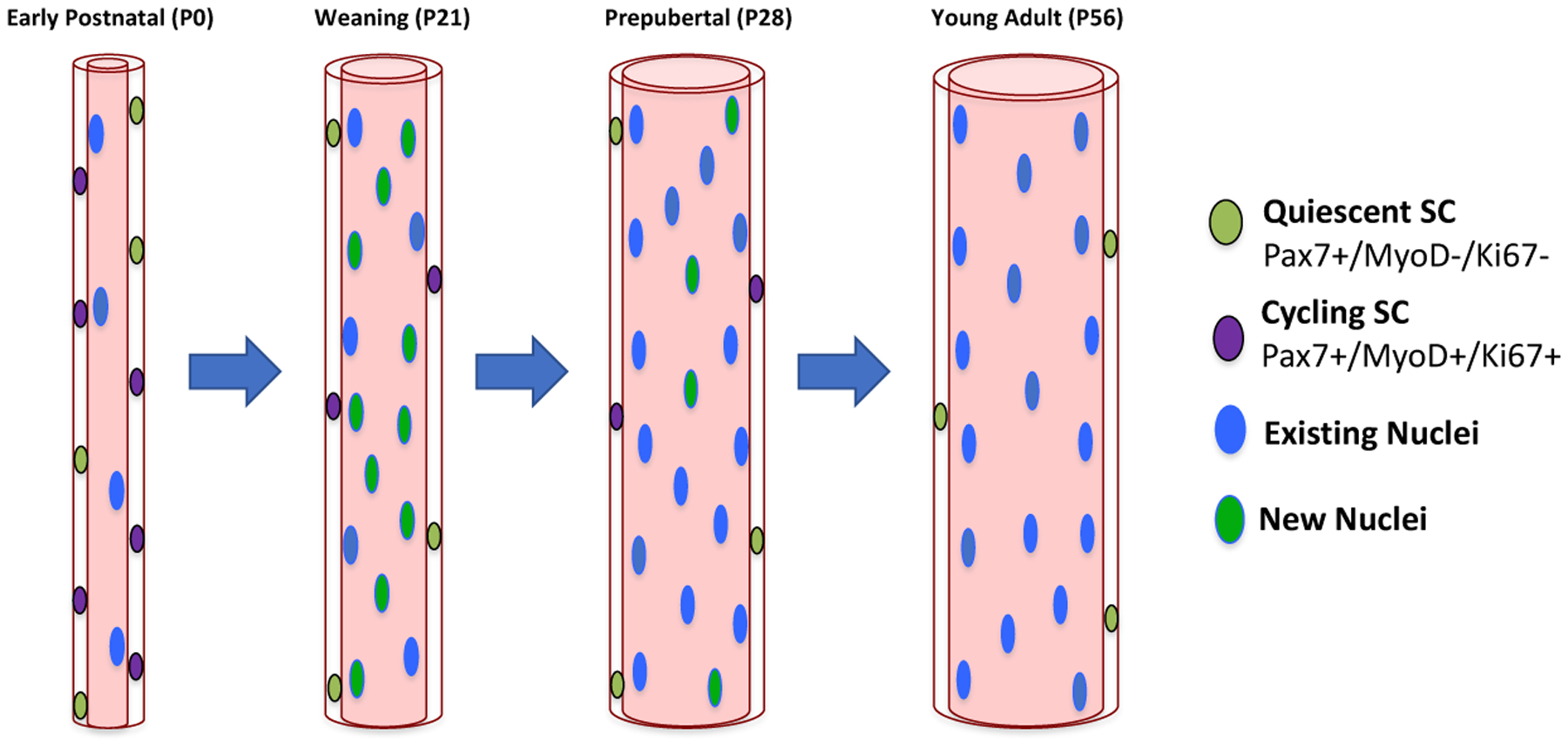
Representation of murine myofiber growth during postnatal stages. The majority of myonuclear accretion occurs prior to P21 [29]. However, during prepuberty, residual addition of myonuclei occurs to support myofiber growth [30, 34, 35, 39, 45]. As development proceeds, the cycling SC pool is reduced in number until the adult quiescent pool size is established at P56 [30, 45].
Contrary to White et al, other groups have observed an increase in myonuclear number post P21. In the EDL muscle of young mice, Huh et al observed a 35% increase in myonuclear number between P21 and P35 (203 vs 273 myonuclei/myofiber) [34]. A recent study by Cramer et al observed similar increases in prepubertal EDL myonuclear number [35]. This study assessed EDL myonuclear number at P14, P21, P28, P35, P42, and P150. Performing counts on a myonuclei per millimeter (MN/mm) basis, they observed from P14-P28 the EDL myonuclear number remained at a steady state of ~55 MN/mm. However, a significant boost was observed from P28-P35 to ~60 MN/mm. If an average P28 EDL myofiber is ~7 millimeters in length [22], this averages out to an additional 35 myonuclei per EDL myofiber between P28 and P35. In our own developmental study, we performed myonuclear counts on P21, P28, and P42 EDLs. Consistent with Cramer et al, we observed a significant increase from 61 MN/mm at P28 to 67 MN/mm at P42 in the EDL [30]. We additionally conducted counts in the slow-contracting soleus (SOL) muscle. The SOL muscle has nearly double the myonuclei of the fast-contracting EDL muscle, perhaps necessary for its role as a load-bearing muscle and in ambulation. We observed SOL MN/mm to increase from ~110 to 135 from P28 to P42 [30]. Lastly, other groups have assessed myonuclear number at a young developmental age and later on in life as adults, finding a significant increase post weaning age [36–38].
Lineage tracing assays during development have been used to understand SC dynamics and activity. Pawlikowski et al utilized a TdTomato reporter during developmental stages to label SCs and assess contribution to myofibers [39]. This study observed that the EDL muscle reached a steady state of fusion at ~8 weeks of age. Interestingly, the slow-contracting SOL muscle had extended fusion out until ~12 weeks of age. In another lineage tracing study, we utilized a Pax7CreERT2/+;Rosa26nTnG/+ (P7nTnG) mouse to assess myonuclear contribution during prepubertal ages [30]. Upon tamoxifen injection this P7nTnG mouse enables GFP expression in SCs and derived myonuclei, allowing for assessment of SC activity during a period time. Consistent with Pawlikowski et al, we observed substantial GFP+ myonuclei during prepubertal growth, with a drop-off to a minimal amount at about ~6–8 weeks in the EDL. The SOL, consistent with the aforementioned study, had prolonged contribution out to 12 weeks of age. In accordance with the aforementioned increases in prepubertal myonuclear number, these lineage tracing studies demonstrate relatively robust SC activity up to puberty or even into young adulthood, depending on the muscle examined.
With the evidence of residual SC activity during prepubertal development, the question arises, when do SCs truly establish quiescence? Additionally, what is mediating the transition from a cycling developmental paradigm to that apparent in the quiescent adult muscle? In the following section, we review current literature investigating SC dynamics that govern the establishment of quiescence. Additionally, we discuss whole muscle adaptations that could influence these changes.
Postnatal SC fate and the establishment of quiescence
During postnatal growth, not all SCs divide and contribute myonuclei at the same rate. Described by Schultz in 1996, the SC pool in the postnatal rat is comprised of both a fast (~80%) and slow (~20%) dividing population [40]. The slow dividing population, which spent more time in G0 between divisions, was postulated to serve as a reserve source of stem cells. This notion is consistent with cell heterogeneity observed in other stem cell systems [41]. Techniques used to track cell division kinetics suggested that the stem cells with the fewest amount of divisions (label-retaining cells- LRCs) serve as a source for pool maintenance via self-renewal [42]. By dividing infrequently, they could be protecting the pool from unnecessary risks to their genome integrity. In SCs, it was demonstrated that LRCs possess self-renewal capabilities that are progressively lost with aging [43]. Further investigating the differences between non-label retaining cells (nLRCs) and LRCs, Chakkalakal et al observed that these populations are formed at birth and persist throughout postnatal development [44]. Consistent with the reports of SC activity post P21, Chakkalalakal et al observed ~20–25% of LRCs and nLRCs were positive for markers of proliferation (Ki67+ and MyoD+) at P21. By P50, the aforementioned markers of SC proliferation became negligible as the SC subpopulations began to resemble an adultlike quiescent state.
Following up on SC dynamics during postnatal growth, a recent study thoroughly characterized SC myogenic progression during juvenile stages [45]. This study, by Gattazzo et al, assessed markers of myogenic fate and proliferation at the following time points: early postnatal (P0–P10), juvenile (P15), weaning (P21), prepubertal (P28), and adult (P49-P56). Utilizing flow cytometry, they observed the cycling (Ki67+) SC pool at birth was ~65%, followed by a drop at P6 to ~39%. At the weaning and prepubertal timepoints, ~25% of SCs were still Ki67+. They further confirmed this data by assessing cycling (Pax7+, Ki67+) and non-cycling markers (Pax7+, Ki67-) within the fast-contracting Tibialis Anterior (TA) muscle. Consistent with the flow cytometry data, cycling SCs became progressively less prevalent following birth (~49% at P10). However, at the prepubertal P28 ~29% of TA SCs were still actively cycling. By P56, cycling SCs were completely absent. In line with these notions, CD34 expression (a marker of quiescence) begins to appear in SCs at P21, but expression continues to increase beyond that age as the remaining SC pool reaches full quiescence [46]. Gattazzo et al also observed an ~33% reduction in TA SC number between P28 and P56, consistent with previous reports in the EDL and SOL [30]. Thus based on SC number and markers of cell cycling, the quiescent SC pool is established at ~P56, well beyond the previously described P21 time point [29, 31, 32]. Gattazzo et al elegantly demonstrated that postnatal growth is accompanied by waves of differentiation and commitment. However, the question remains as to what is governing the establishment of the definitive quiescent SC pool.
The onset of puberty is characterized by the induction of sex hormones, testosterone in males and estradiol in females [47]. It has been revealed that these sex hormones play a role in establishing the pool of quiescent SCs [48]. In this study, Kim et al demonstrated that sex hormones (both estrogens and androgens) can induce Notch signaling in juvenile cycling SCs, driving them to a quiescent state (Figure 3). Specifically, dihydrotestosterone (DHT) was injected into 10-day old juvenile mice, causing a significant reduction in Pax7+, Ki67+ cells. Injection of DHT caused an upregulation of the Notch signaling pathway within SCs, which was also increased naturally near puberty onset. The importance of Notch signaling in maintaining quiescence has also been demonstrated throughout adulthood [49, 50]. Lastly, Kim et al established that the loss of sex hormones (either through a surgical orchiectomy or pharmacological antagonist) resulted in a delayed entry into quiescence outwards to 8 weeks of age. Consistent with these observations, another study demonstrated that the castration of adult mice increased BrDU+ and Ki67+ SCs, effectively disrupting quiescence [51]. Additionally, estradiol is important for the lifelong maintenance of female SCs, with an ovariectomy resulting in decreased SC number and impaired self-renewal and differentiation deficiencies [52]. Taken together, these studies all demonstrate the importance of sex hormones and Notch signaling in not only establishing SC quiescence, but also in maintaining it.
Figure 3.

Illustration depicting the potential developmental signals governing the progression of SCs to quiescence. The induction of sex hormones has been shown to induce Mib1 expression in myofibers, leading to elevated Notch signaling in cycling SCs [48]. Additionally, whole muscle RNA Sequencing has suggested alterations in ECM composition around the time of puberty onset [30]. However, the effect of the development and maturation of the ECM on SC fate has yet to be examined.
Although the induction of sex hormones certainly plays a paramount role in eliciting SC quiescence, it is possible that other developmental paradigms may alter SC fate decisions during this time. One of these could be the maturation of the extracellular matrix (ECM) during postnatal growth. The skeletal muscle ECM is a complex hierarchy, which is comprised of 3 connective tissue layers: the epimysium (fascia), perimysium, and endomysium. All three layers function to facilitate contraction and the transmission of force from tendon to myofiber. Additionally, the ECM, which is composed of collagens, laminins, fibronectin, and protoeogylcans, exists within the SC microenvironment or niche [53]. Thus, alterations in ECM content have the ability to influence how SCs respond to stimuli and regulate their quiescent state [54–56].
Contrary to the plethora of literature in adult, not much has been uncovered as to how the ECM matures during postnatal growth. Our group has observed, using whole muscle RNA Sequencing, that the ECM undergoes rapid changes in makeup during prepubertal development [30]. Between four and six weeks of age, there is a stark down regulation of ECM components, at the mRNA level. What is mediating this rapid change in ECM content needs to be further defined, as well as the implications of these changes on SC fate during prepubertal growth (Figure 3). A recent study utilized single cell RNA-Seq to characterize myonuclear heterogeneity during multiple time points throughout the life of the mouse [57]. This study was also able to capture other resident muscle populations. Comparing the number of FAPS (fibro-adipogenic progenitors) at the juvenile P21 versus adult 5-month time points, there are less FAPS (as a % of nuclei) at the adult stage. With FAPS being one potential source of ECM proteins, it is conceivable they are gradually lost during development as the muscle matures (similar to the loss of SC number during late postnatal growth) [58]. This progressive loss may regulate the development of the ECM during postnatal growth. However, at this time, these are just observations and a rigorous characterization, during all stages of postnatal development, is needed.
Taken together, recent evidence points to the murine quiescent SC pool being established at ~8 weeks of age [45]. More complex than initially postulated, waves of SC commitment and differentiation punctuate postnatal development. This progression is accompanied by the gradual pruning of SC pool size, as it moves towards quiescence. Additionally, in this section, we highlighted developmental paradigms and molecular changes that are involved in mediating this progression to quiescence. However, what are the consequences of actually losing SCs and their derived myonuclei during postnatal growth? In the following section, we highlight recent findings utilizing genetic mouse models to manipulate myonuclear accretion during normal developmental muscle growth.
Genetic disruption of SC-derived myonuclear contribution during postnatal growth
Genetic mouse models of SC depletion have been used under a number of different contexts. A common model is the Pax7CreER; Rosa26DTA (P7DTA) mouse, in which injection of tamoxifen results in SC depletion in an inducible manner. This model was employed to demonstrate the absolute requirement of SCs in muscle regeneration [5, 6]. In the adult animal, there has been mixed results regarding the effect of SC depletion on muscle fiber size in mechanical overload and homeostatic aging experiments [9, 59–62]. In a sedentary adult, it seems as though SC depletion will result in myofiber atrophy in some, but not all, muscles. However, these deficits, when observed, manifest over a relatively protracted period of time. In this section, we review the most recent studies employing genetic models to deplete SCs and/or disrupt myonuclear accretion during postnatal development.
To understand the consequences of SC loss during postnatal growth, our group (using the P7DTA model) depleted SCs at prepubertal 4 weeks of age and assessed the consequences at 8 weeks of age [30]. In this short period of time, we observed a significant reduction in myonuclear number and myofiber CSA in EDL and SOL myofibers. These deficits in myofiber size translated to a reduction in EDL and SOL absolute force generation capacity, when assessed with ex vivo muscle physiology analysis. Recently, we followed up these observations in a long-term aging paradigm, in which SCs were depleted at 4 weeks of age and animals were sacrificed at 16–18 months of age [63]. Consistent with our previous results, SC-depleted animals (~65% reduction in SC number) had a lifelong reduction in myofiber CSA and myonuclear number in EDL and SOL muscles, highlighting the crucial role for SCs during this short prepubertal developmental period. On average, myofiber CSA and myonuclear number were reduced by ~20% compared to control animals. These experiments, both in the short-term and long-term, demonstrate the importance of residual prepubertal myonuclear accretion to the maturation and development of myofiber size and integrity.
Due to the necessity of SCs for postnatal muscle development, Murach et al probed if overload-induced hypertrophy was also dependent upon the developmental stage of the mouse [64]. Utilizing a P7DTA mouse, SCs were ablated at a young adult stage (8 weeks of age) or mature (16 weeks of age) stage, followed by synergistic ablation surgery. Through the excision of the gastrocnemius and SOL muscles, this surgery overloads the plantaris musclecausing the myofibers to adapt and hypertrophy. Murach et al observed that in the young SC depleted animals, there was a failure of the overloaded plantaris muscles to hypertrophy. Contrary to this, there was a significant increase in plantaris CSA in the SC depleted mature overload conditions. The group concluded that maturational period of the mouse is a key determinant in the ability of the muscle to adapt to a hypertrophic stimulus. It is possible that at the time of depletion, the plantaris did not attain its entire complement of developmental myonuclei, limiting its ability to adapt to an overload stimulus. Indeed, examination of another load-bearing posterior muscle (the SOL) has revealed significant and prolonged myonuclear accretion (post 8 weeks of age) [30, 38, 39].
Recently, two concurrent studies investigated the consequences of diminished myonuclear accretion during neonatal and postnatal development [21, 35]. These studies made use of an SC-specific inducible Myomaker (Mymk) knockout mouse (Mymk-KO). Myomaker is a muscle-specific seven-transmembrane protein that controls myoblast fusion ability [65]. It was previously demonstrated, in adults SCs, that the knockout of Mymk resulted in fusion incompetence in regenerative and functional overload scenarios [66, 67]. Thus, by utilizing this mouse model, one could manipulate myonuclear accretion during developmental stages.
Utilizing the Mymk-KO mouse, Cramer et al deleted Mymk at a number of different early and postnatal ages. Blocking myonuclear fusion at P0 (referred to as Δ1), P6 (Δ2), and P13 (Δ3) resulted in a reduction in EDL myonuclear number by 75, 55, and 25% respectively (four weeks post tamoxifen). Focusing on the Δ1 condition, the 75% reduction in myonuclear number was not sufficient to establish a normal level of developmental muscle function. This resulted in an upregulation of apoptotic and oxidative stress genes in the whole muscle, and lethality of Δ1 animals starting at ~200 days of age. Interestingly, the maladaptive phenotype was not observed in Δ2 animals, as the level of myonuclear accretion had been sufficient to allow for adaptation and overall survival.
Both Cramer et al and Hansson et al, further delved into the adaptations that enabled Δ2 animals to survive with such drastic reductions in myonuclear number [21, 35]. In concurrent data, the groups identified that the significant 55% reduction in EDL myonuclear number was apparent by P14 (one week after Mymk knockout) and sustained outwards to P150. Interestingly, there was no difference in CSA between WT and Mymk-KO myofibers from P14-P28, but at P35 CSA diverged with EDL Mymk-KO myofibers being smaller outwards to P150. Notably, this divergence occurred at a time when there is an uptick of myonuclei per unit length, associated with a final wave of SC activity prior to the establishment of the definitive adult quiescent SC pool[30, 35, 45, 48, 63]. Upon assessing mRNA concentration (normalized to the number of individual myonuclei) for Δ1 and Δ2 animals, it was revealed that there was a 5.3-fold and 3.5-fold increase in the output of mRNA per myonucleus respectively. Due to the lethality of the D1 animals, it was hypothesized that this sheer increase in mRNA output was not sustainable for survival. In contrast, the D2 animals had potentially reached a minimum number of myonuclei that enabled adaptation and survival, albeit with reduced myofiber CSA and peak force generation capacity into adulthood.
Each of the aforementioned studies demonstrates the importance of SC-dependent myonuclear contribution to the development and maturation of skeletal muscle. The overall findings and timelines of these studies have been outlined in Figure 4. Importantly, the consequences of SC disruption during development are readily apparent, and have lifelong consequences. In contrast, SC depletion in sedentary adult mice leads to myofiber atrophy in some, but not all, muscle and this manifests over a relatively lengthy timeframe [9, 61, 62]. It should be noted that these juvenile depletion experiments were all performed on sedentary mice. It would be interesting to note if the deficits in myofiber size, myonuclear number, and force generation capacity translate to exercise intolerance. Indeed, it has recently been demonstrated that SC depletion in adult animals blunts not only their capacity to exercise, but adaptative abilities to respond to exercise [11, 12]. Given the critical role of SCs during early postnatal and prepubertal muscle maturation, we can speculate that SC depletion at the juvenile stage would result in impaired muscle adaptation to exercise. To conclude, SC activity is crucial for proper muscle development and maturation, setting the required foundation for functional adult skeletal muscles.
Figure 4.

Schematic representation of studies that have depleted SCs/genetically disrupted myonuclear contribution during postnatal growth. The major findings of these individual experiments have been listed in the included table. Due to consistency of myonuclear counts being performed on a myonuclei per millimeter basis (MN/mm), the percent decrease has been listed for the respective muscle. However, assessment of CSA was performed both in crosssection and on fixed myofibers, so only general trends have been listed.
Stressors and disorders during muscle development
Due to the dynamic nature of SCs during postnatal growth, they may be especially susceptible to insults during this time. Additionally, interfering with the developmental role of SCs could have lifelong consequences. Indeed, a recent paper demonstrated that Pax7 loss of function mutations resulted in myopathies in five juvenile patients [68]. These myopathies were characterized by an exhaustion of the SC pool, leading to diminished myonuclear accretion and thus decreased muscle growth. In this section, we overview the impact of cancer therapies on the cycling juvenile SC pool and further discuss the deleterious long-term consequences. Additionally, we highlight a role for SC dysfunction in one of the most common childhood motor disorders, cerebral palsy.
Cancer therapies
Radiation and chemotherapeutic agents are the most commonly used therapies to combat cancer by targeting rapidly dividing cells, a hallmark of cancer cells. Although effective in cancer mitigation, these therapies can have undesirable off-target cytotoxic effects that can negatively impact the long-term wellbeing of patients. This is especially true in children, as the damage to actively dividing progenitors and stem cells involved in tissue growth and development can have lifelong consequences. Indeed, the long-term follow-up of pediatric cancer survivors has revealed premature aging phenotypes in numerous organs [69–73]. In regards to skeletal muscle, survivors can demonstrate sarcopenic qualities such as muscle weakness and loss of size [71, 74, 75]. Therefore, understanding the mechanisms governing the muscle maturation process prior to adulthood, and how cancer therapies alter these mechanisms is crucial to prevent developmental aberrations. To this end, there is a concerted effort to better understand the effects of cancer therapies on developing organ systems, with the hope of enabling survivors to lead longer and healthier lives [73].
Ionizing radiation induces double-stranded DNA breaks that, when severe enough, result in cellular apoptosis or mitotic catastrophe [76]. While the post-mitotic muscle fiber is relatively radioresistant, the effect of irradiation on SCs can be dependent upon dose and age of the animal. A previous study by Pagel and Partidge, examined the effect of a single 18 Gy high dose of X-ray radiation on the hindlimb of juvenile 16 day-old and mature 15 week-old adult mice [77]. When mice were irradiated at 16 days of age and examined 58 days later, there was a significant reduction in irradiated muscle weight and CSA (myonuclear counts were not performed). In the adult animals, when examined across the same time frame, these phenotypes were not observed.
Our group has recently published two studies investigating the consequences of cancer therapies on juvenile muscle maturation and development [63, 78]. In the first study, Paris et al utilized a syngeneic rhabdomyosarcoma (RMS) tumor model [78]. Originating from immature skeletal muscle, RMS is the most common childhood sarcoma [79]. In the experimental paradigm, RMS cells were inoculated into mouse lower limbs at 3.5 weeks of age. The animals were then irradiated with a fractionated 8.2 Gy 3X (M,W,F) treatment at 4 weeks of age, with this completely eliminating ~70% of the RMS tumors. Upon assessing EDL and SOL myofiber CSA and myonuclear number from tumor-free animals, Paris et al observed a significant reduction in both parameters four weeks post-irradiation. These deficits were further magnified when radiotherapy was combined with the chemotherapeutic agent Vincristine. The combination of radiotherapy and Vincristine also exacerbated deficits in balance/motor coordination.
In our concurrent study, we further investigated the impact of the relevant tumor-mitigating 8.2 Gy 3X regimen on prepubertal muscle maturation [63]. Assessing SC number ~2–4 hours after the completion of our fractionated regimen, there was already a significant reduction in SC number in both EDL and SOL numbers. This immediate radiation-induced loss of SC number was specific to the prepubertal period, as adult animals were more radioresistant to SC loss. This deficit in SC number was sustained throughout the life of the animal, as the irradiated TA had ~68% less SCs 14 months post-irradiation. Consistent with Pagel and Pattridge, irradiation during prepuberty resulted in a loss of muscle mass in TA, EDL, and SOL muscles in the long-term. Additionally, aged irradiated EDL and SOL myofibers were ~70–80% the CSA of control myofibers, while having ~20% less myonuclei. Interestingly, these deficits were replicated in our lifelong P7DTA model of prepubertal SC depletion. Thus, loss of myonuclear accretion during postnatal developmental growth is a principal driver of the radiation-induced lifelong deficits in myofiber size and myonuclear number.
These aforementioned studies highlight the susceptibility of juvenile skeletal muscle to long-term damage by cancer therapies and radiation exposure. Future studies will need to focus on how SCs can be protected during the treatments or potentially how to galvanize the remaining irradiated SCs. However, clinically relevant ways to protect from radiation-induced cell loss have proven to be elusive. Despite many years of research, the free radical scavenger, Amifostine, is only one of two drugs used clinically as a radioprotectant, albeit in a very limited fashion [80, 81]. Thus, attempting to stimulate the remaining irradiated SC pool may prove to be a more effective option. One way of doing this is by better understanding what makes some SCs more “radio-resistant” than others. A recent study identified a population of adult SCs (enriched in Pax3 expression) that were able to withstand radiation-induced stressors, through the regulation of reactive oxygen species [82]. However, it is unclear if this same population exists in the context of juvenile irradiation. Exercise may be another potential avenue to stimulate remaining SCs. Indeed, a recent study, performed in adult mice, reported improvements in irradiated SC number following bouts of weekly exercise [83].
Cerebral Palsy (CP)
Cerebral palsy, the most common developmental motor disorder, affects 2–4 children per 1000 every year [84, 85]. This disorder is derived from a brain injury, which occurs prenatally in 70–80% of cases [86]. Children with CP will present with muscle weakness, reduction in longitudinal muscle growth, contractures, and limitations in joint range of movement [87–89]. Additionally, myofiber CSA is markedly decreased in children with CP [90, 91]. In order to combat these symptoms, rehabilitative approaches are utilized in attempts to strengthen and stretch the afflicted muscle during development. However, the biological basis of some of these phenotypes continues to elude the medical community.
To understand the mechanisms behind CP’s impaired muscle growth and muscular contractions, Dr. Richard Lieber’s group has implicated SC dysfunction in these processes. In two separate studies, one utilizing flow cytometry and the other immunohistochemistry, this group demonstrated that muscle biopsies from CP patients have ~60–70% less SCs [92, 93]. Thus, they hypothesized that CP contractures may arise from a reduced ability to add sarcomeres (the basic force-generating unit), due to a reduction in the SC pool. This hypothesis was tested recently using a genetic mouse model of SC depletion (P7DTA) in an experimental condition meant to simulate the contractures observed in CP [87]. In this condition, Dayanidhi et al casted a limb of P7DTA (SC depleted) and control (CTL) animals for two weeks in a plantarflexion position, chronically shortening the SOL muscle and resulting in ~20% reduction in SOL serial sarcomeres. After 30 days, the casts were removed and animals were allowed to regain ankle range of motion for 30 days. Consistent with the initial hypothesis, P7DTA animals were unable to regain range of motion in the casted limb compared to the CTL group. This deficit was due to an inability of the casted SOL muscle to add sarcomeres, due to the loss of SCs. Together these studies implicate a role of SC dysfunction or loss in CP, especially in the formation of muscle contractures.
Conclusion
In this review, we discussed recent literature relevant to postnatal muscle development, specifically the role SCs play in this process. We highlighted the continued activity of murine SCs post weaning age (P21), contrary to prior literature [29, 31–33]. It is important to recognize that there is still residual SC activity extending into the prepuberal period [30, 34, 35, 39, 45]. Additionally, interfering with SC dynamics during this time can obstruct the normal developmental paradigm, resulting in lifelong consequences in skeletal muscle size and function [21, 35, 63].
In terms of future directions, the role of the muscle-nerve connection in regulating postnatal SC fate is an interesting paradigm. Studies in the 1980s by Dr. Gerta Vrbová’s group, demonstrated that denervation of young rat muscle resulted in a loss of muscle function and size outwards to a year post-operation [94, 95]. Contrary to this, the denervated adult muscle was able to bounce back to essentially normal. However, the effect of juvenile denervation on SC fate and myonuclear accretion was not, and has yet to be assessed. Due to the juvenile muscle disorders that arise from neuromuscular insults, better understanding how denervation affects SC dynamics and thus muscle growth can lead to promising therapeutics [96, 97]. In conclusion, by better understanding all facets of postnatal muscle maturation, we can harness this information to help children suffering from muscle disorders to lead fuller and longer lives.
Acknowledgments
Thank you to lab members: Dr. Roméo Blanc and Jake Kallenbach for their insightful comments and proofreading; Dr. Nicole Paris for figure generation and layout. Graphical abstract was created with BioRender.com
Funding
This work was supported by URMC Wilmot Cancer Institute pilot funding and NIH grants R01AG051456 and R01CA220467 to J.V.C and F31 training grant F31AR076175 to J.F.B.
Abbreviations:
- CSA
Cross-sectional area
- EDL
Extensor Digitorum Longus
- ECM
Extracellular matrix
- LRC
Label-retaining cell
- MN/mm
Myonuclei per millimeter
- nLRC
Non-label-retaining cell
- SC
satellite cell
- SOL
Soleus
- TA
Tibialis Anterior
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mauro A (1961) Satellite cell of skeletal muscle fibers, J Biophys Biochem Cytol. 9, 493–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P & Rudnicki MA (2000) Pax7 Is Required for the Specification of Myogenic Satellite Cells, Cell. 102, 777–786. [DOI] [PubMed] [Google Scholar]
- 3.Ancel S, Stuelsatz P & Feige JN (2021) Muscle Stem Cell Quiescence: Controlling Stemness by Staying Asleep, Trends Cell Biol. [DOI] [PubMed] [Google Scholar]
- 4.Yin H, Price F & Rudnicki MA (2013) Satellite cells and the muscle stem cell niche, Physiol Rev. 93, 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA & Kardon G (2011) Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration, Development. 138, 3625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lepper C, Partridge TA & Fan CM (2011) An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration, Development. 138, 3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S & Galy A (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration, Development. 138, 3647–56. [DOI] [PubMed] [Google Scholar]
- 8.Liu W, Wei-LaPierre L, Klose A, Dirksen RT & Chakkalakal JV (2015) Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions, Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Klose A, Forman S, Paris ND, Wei-LaPierre L, Cortes-Lopez M, Tan A, Flaherty M, Miura P, Dirksen RT & Chakkalakal JV (2017) Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration, Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goh Q, Song T, Petrany MJ, Cramer AA, Sun C, Sadayappan S, Lee SJ & Millay DP (2019) Myonuclear accretion is a determinant of exercise-induced remodeling in skeletal muscle, Elife. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englund DA, Murach KA, Dungan CM, Figueiredo VC, Vechetti IJ Jr., Dupont-Versteegden EE, McCarthy JJ & Peterson CA (2020) Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity, Am J Physiol Cell Physiol. 318, C1178–C1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Englund DA, Figueiredo VC, Dungan CM, Murach KA, Peck BD, Petrosino JM, Brightwell CR, Dupont AM, Neal AC, Fry CS, Accornero F, McCarthy JJ & Peterson CA (2021) Satellite Cell Depletion Disrupts Transcriptional Coordination and Muscle Adaptation to Exercise, Function. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chal J & Pourquie O (2017) Making muscle: skeletal myogenesis in vivo and in vitro, Development. 144, 2104–2122. [DOI] [PubMed] [Google Scholar]
- 14.Buckingham M & Rigby PW (2014) Gene regulatory networks and transcriptional mechanisms that control myogenesis, Dev Cell. 28, 225–38. [DOI] [PubMed] [Google Scholar]
- 15.Hutcheson DA, Zhao J, Merrell A, Haldar M & Kardon G (2009) Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin, Genes Dev. 23, 997–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Relaix F, Rocancourt D, Mansouri A & Buckingham M (2005) A Pax3/Pax7-dependent population of skeletal muscle progenitor cells, Nature. 435, 948–53. [DOI] [PubMed] [Google Scholar]
- 17.Kassar-Duchossoy L, Giacone E, Gayraud-Morel B, Jory A, Gomes D & Tajbakhsh S (2005) Pax3/Pax7 mark a novel population of primitive myogenic cells during development, Genes Dev. 19, 1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gros J, Manceau M, Thome V & Marcelle C (2005) A common somitic origin for embryonic muscle progenitors and satellite cells, Nature. 435, 954–8. [DOI] [PubMed] [Google Scholar]
- 19.Moss FP & Leblond CP (1970) Satellite cells as the source of myonuclei in growing rats, Anat Rec, 421–435. [DOI] [PubMed] [Google Scholar]
- 20.Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F & van Loon LJ (2014) Satellite cells in human skeletal muscle; from birth to old age, Age (Dordr). 36, 545–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansson K-A, Eftestøl E, Bruusgaard JC, Juvkam I, Cramer AW, Malthe-Sørenssen A, Millay DP & Gundersen K (2020) Myonuclear content regulates cell size with similar scaling properties in mice and humans, Nature Communications. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gokhin DS, Ward SR, Bremner SN & Lieber RL (2008) Quantitative analysis of neonatal skeletal muscle functional improvement in the mouse, J Exp Biol. 211, 837–43. [DOI] [PubMed] [Google Scholar]
- 23.Dutta S & Sengupta P (2016) Men and mice: Relating their ages, Life Sci. 152, 244–8. [DOI] [PubMed] [Google Scholar]
- 24.Nelson JF, Karelus K, Felicio LS & Johnson TE (1990) Genetic Influences on the Timing of Puberty in Mice, Biology of Reproduction. 42, 649–655. [DOI] [PubMed] [Google Scholar]
- 25.Pinter O, Beda Z, Csaba Z & Gerendai I (2007) Difference in the onset of puberty in selected inbred mouse strains Endocrine Abstracts. 14, 617. [Google Scholar]
- 26.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U & Levine JE (2010) Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons, Proc Natl Acad Sci U S A. 107, 22693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagenauer MH, Perryman JI, Lee TM & Carskadon MA (2009) Adolescent changes in the homeostatic and circadian regulation of sleep, Dev Neurosci. 31, 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kercmar J, Tobet SA & Majdic G (2014) Social isolation during puberty affects female sexual behavior in mice, Front Behav Neurosci. 8, 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White RB, Bierinx AS, Gnocchi VF & Zammit PS (2010) Dynamics of muscle fibre growth during postnatal mouse development, BMC Dev Biol. 10, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachman JF, Klose A, Liu W, Paris ND, Blanc RS, Schmalz M, Knapp E & Chakkalakal JV (2018) Prepubertal skeletal muscle growth requires Pax7-expressing satellite cell-derived myonuclear contribution, Development. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lepper C, Conway SJ & Fan C-M (2009) Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements, Nature. 460, 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stantzou A, Schirwis E, Swist S, Alonso-Martin S, Polydorou I, Zarrouki F, Mouisel E, Beley C, Julien A, Le Grand F, Garcia L, Colnot C, Birchmeier C, Braun T, Schuelke M, Relaix F & Amthor H (2017) BMP signaling regulates satellite cell dependent postnatal muscle growth, Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Relaix F & Zammit PS (2012) Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage, Development. 139, 2845–56. [DOI] [PubMed] [Google Scholar]
- 34.Huh MS, Young KG, Yan K, Price-O’Dea T & Picketts DJ (2017) Recovery from impaired muscle growth arises from prolonged postnatal accretion of myonuclei in Atrx mutant mice, PLoS One. 12, e0186989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cramer AAW, Prasad V, Eftestøl E, Song T, Hansson K-A, Dugdale HF, Sadayappan S, Ochala J, Gundersen K & Millay DP (2020) Nuclear numbers in syncytial muscle fibers promote size but limit the development of larger myonuclear domains, Nature Communications. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardasis CA & Cooper GW (1975) An analysis of nuclear numbers in individual muscles fibers during differentiation and growth: A satellite cell-muscle fiber growth unit, J Exp Zoology. 191, 347–358. [DOI] [PubMed] [Google Scholar]
- 37.Neal A, Boldrin L & Morgan JE (2012) The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration, PLoS One. 7, e37950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruusgaard JC, Liestol K & Gundersen K (2006) Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice, J Appl Physiol (1985). 100, 2024–30. [DOI] [PubMed] [Google Scholar]
- 39.Pawlikowski B, Pulliam C, Betta ND, Kardon G & Olwin BB (2015) Pervasive satellite cell contribution to uninjured adult muscle fibers, Skelet Muscle. 5, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz E (1996) Satellite Cell Proliferative Compartments in Growing Skeletal Muscles, Developmental Biology. 175, 84–94. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs E (2009) The tortoise and the hair: slow-cycling cells in the stem cell race, Cell. 137, 811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ono Y, Masuda S, Nam HS, Benezra R, Miyagoe-Suzuki Y & Takeda S (2012) Slowdividing satellite cells retain long-term self-renewal ability in adult muscle, J Cell Sci. 125, 1309–17. [DOI] [PubMed] [Google Scholar]
- 43.Chakkalakal JV, Jones KM, Basson MA & Brack AS (2012) The aged niche disrupts muscle stem cell quiescence, Nature. 490, 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakkalakal JV, Christensen J, Xiang W, Tierney MT, Boscolo FS, Sacco A & Brack AS (2014) Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state, Development. 141, 1649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gattazzo F, Laurent B, Relaix F, Rouard H & Didier N (2020) Distinct Phases of Postnatal Skeletal Muscle Growth Govern the Progressive Establishment of Muscle Stem Cell Quiescence, Stem Cell Reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garcia-Prat L, Perdiguero E, Alonso-Martin S, Dell’Orso S, Ravichandran S, Brooks SR, Juan AH, Campanario S, Jiang K, Hong X, Ortet L, Ruiz-Bonilla V, Flandez M, Moiseeva V, Rebollo E, Jardi M, Sun HW, Musaro A, Sandri M, Del Sol A, Sartorelli V & Munoz-Canoves P (2020) FoxO maintains a genuine muscle stem-cell quiescent state until geriatric age, Nat Cell Biol. 22, 1307–1318. [DOI] [PubMed] [Google Scholar]
- 47.Ober C, Loisel DA & Gilad Y (2008) Sex-specific genetic architecture of human disease, Nat Rev Genet. 9, 911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Han GC, Seo JY, Park I, Park W, Jeong HW, Lee SH, Bae SH, Seong J, Yum MK, Hann SH, Kwon YG, Seo D, Choi MH & Kong YY (2016) Sex hormones establish a reserve pool of adult muscle stem cells, Nat Cell Biol. 18, 930–40. [DOI] [PubMed] [Google Scholar]
- 49.Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM & Rando TA (2012) Notch signaling is necessary to maintain quiescence in adult muscle stem cells, Stem Cells. 30, 232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V & Tajbakhsh S (2012) A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state, Stem Cells. 30, 243–52. [DOI] [PubMed] [Google Scholar]
- 51.Klose A, Liu W, Paris ND, Forman S, Krolewski JJ, Nastiuk KL & Chakkalakal JV (2018) Castration induces satellite cell activation that contributes to skeletal muscle maintenance, Journal of Cachexia, Sarcopenia and Muscle – Rapid Communications. 1. [PMC free article] [PubMed] [Google Scholar]
- 52.Collins BC, Arpke RW, Larson AA, Baumann CW, Xie N, Cabelka CA, Nash NL, Juppi HK, Laakkonen EK, Sipila S, Kovanen V, Spangenburg EE, Kyba M & Lowe DA (2019) Estrogen Regulates the Satellite Cell Compartment in Females, Cell Rep. 28, 368–381 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grzelkowska-Kowalczyk K (2016) The Importance of Extracellular Matrix in Skeletal Muscle Development and Function in Composition and Function of the Extracellular Matrix in the Human Body. [Google Scholar]
- 54.Fry CS, Kirby TJ, Kosmac K, McCarthy JJ & Peterson CA (2017) Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy, Cell Stem Cell. 20, 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA & Bonaldo P (2013) Collagen VI regulates satellite cell self-renewal and muscle regeneration, Nat Commun. 4, 1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas K, Engler AJ & Meyer GA (2015) Extracellular matrix regulation in the muscle satellite cell niche, Connect Tissue Res. 56, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petrany MJ, Swoboda CO, Sun C, Chetal K, Chen X, Weirauch MT, Salomonis N & Millay DP (2020) Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers, Nat Commun. 11, 6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Biferali B, Proietti D, Mozzetta C & Madaro L (2019) Fibro-Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network, Front Physiol. 10, 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE & Peterson CA (2011) Effective fiber hypertrophy in satellite cell-depleted skeletal muscle, Development. 138, 3657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Egner IM, Bruusgaard JC & Gundersen K (2016) Satellite cell depletion prevents fiber hypertrophy in skeletal muscle, Development. 143, 2898–906. [DOI] [PubMed] [Google Scholar]
- 61.Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ & Peterson CA (2015) Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia, Nat Med. 21, 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M & Kardon G (2015) Muscle stem cells contribute to myofibres in sedentary adult mice, Nat Commun. 6, 7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bachman JF, Blanc RS, Paris ND, Kallenbach JG, Johnston CJ, Hernady E, Williams JP & Chakkalakal JV (2020) Radiation-Induced Damage to Prepubertal Pax7+ Skeletal Muscle Stem Cells Drives Lifelong Deficits in Myofiber Size and Nuclear Number, iScience. 23, 101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murach KA, White SH, Wen Y, Ho A, Dupont-Versteegden EE, McCarthy JJ & Peterson CA (2017) Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice, Skelet Muscle. 7, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Millay DP, O’Rourke JR, Sutherland LB, Bezprozvannaya S, Shelton JM, Bassel-Duby R & Olson EN (2013) Myomaker is a membrane activator of myoblast fusion and muscle formation, Nature. 499, 301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Millay DP, Sutherland LB, Bassel-Duby R & Olson EN (2014) Myomaker is essential for muscle regeneration, Genes Dev. 28, 1641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goh Q & Millay DP (2017) Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy, Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feichtinger RG, Mucha BE, Hengel H, Orfi Z, Makowski C, Dort J, D’Anjou G, Nguyen TTM, Buchert R, Juenger H, Freisinger P, Baumeister S, Schoser B, Ahting U, Keimer R, Nguyen CE, Fabre P, Gauthier J, Miguet M, Lopes F, AlHakeem A, AlHashem A, Tabarki B, Kandaswamy KK, Bauer P, Steinbacher P, Prokisch H, Sturm M, Strom TM, Ellezam B, Mayr JA, Schols L, Michaud JL, Campeau PM, Haack TB & Dumont NA (2019) Biallelic variants in the transcription factor PAX7 are a new genetic cause of myopathy, Genet Med. 21, 2521–2531. [DOI] [PubMed] [Google Scholar]
- 69.Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T & Kirkland JL (2015) Frailty in childhood cancer survivors, Cancer. 121, 1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ness KK, DeLany JP, Kaste SC, Mulrooney DA, Pui CH, Chemaitilly W, Karlage RE, Lanctot JQ, Howell CR, Lu L, Srivastava DK, Robison LL & Hudson MM (2015) Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia, Blood. 125, 3411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ness KK, Mertens AC, Hudson MM, Wall MM, Leisenring WM, Oeffinger KC, Sklar CA, Robison LL & Gurney JG (2005) Limitations on physical performance and daily activities among long-term survivors of childhood cancer, Ann Intern Med. 143, 639–47. [DOI] [PubMed] [Google Scholar]
- 72.Ness KK, Plana JC, Joshi VM, Luepker RV, Durand JB, Green DM, Partin RE, Santucci AK, Howell RM, Srivastava DK, Hudson MM, Robison LL & Armstrong GT (2020) Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer Journal of Clinical Oncology. 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guida JL, Ahles TA, Belsky D, Campisi J, Cohen HJ, DeGregori J, Fuldner R, Ferrucci L, Gallicchio L, Gavrilov L, Gavrilova N, Green PA, Jhappan C, Kohanski R, Krull K, Mandelblatt J, Ness KK, O’Mara A, Price N, Schrack J, Studenski S, Theou O, Tracy RP & Hurria A (2019) Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors, J Natl Cancer Inst. 111, 1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rayar M, Webber CE, Nayiager T, Sala A & Barr RD (2013) Sarcopenia in children with acute lymphoblastic leukemia, J Pediatr Hematol Oncol. 35, 98–102. [DOI] [PubMed] [Google Scholar]
- 75.Paulino AC (2004) Late effects of radiotherapy for pediatric extremity sarcomas, Int J Radiat Oncol Biol Phys. 60, 265–74. [DOI] [PubMed] [Google Scholar]
- 76.Eriksson D & Stigbrand T (2010) Radiation-induced cell death mechanisms, Tumour Biol. 31, 363–72. [DOI] [PubMed] [Google Scholar]
- 77.Pagel CNP, T. A. (1998) Covert persistence of mdx mouse myoptahy is revealed by acute and chronic effects of irradiation, Journal of the Nuerological Sciences. 164, 103–116. [DOI] [PubMed] [Google Scholar]
- 78.Paris ND, Kallenbach JG, Bachman JF, Blanc RS, Johnston CJ, Hernady E, Williams JP & Chakkalakal JV (2020) Chemoradiation impairs myofiber hypertrophic growth in a pediatric tumor model, Sci Rep. 10, 19501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paulino AC & Okcu MF (2008) Rhabdomyosarcoma, Curr Probl Cancer. 32, 7–34. [DOI] [PubMed] [Google Scholar]
- 80.Williams JP, Calvi L, Chakkalakal JV, Finkelstein JN, O’Banion MK & Puzas E (2016) Addressing the Symptoms or Fixing the Problem? Developing Countermeasures against Normal Tissue Radiation Injury, Radiat Res. 186, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kouvaris JR, Kouloulias VE & Vlahos LJ (2007) Amifostine: the first selective-target and broad-spectrum radioprotector, Oncologist. 12, 738–47. [DOI] [PubMed] [Google Scholar]
- 82.Scaramozza A, Park D, Kollu S, Beerman I, Sun X, Rossi DJ, Lin CP, Scadden DT, Crist C & Brack AS (2019) Lineage Tracing Reveals a Subset of Reserve Muscle Stem Cells Capable of Clonal Expansion under Stress, Cell Stem Cell. 24, 944–957 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.D’Souza D, Roubos S, Larkin J, Lloyd J, Emmons R, Chen H & De Lisio M (2019) The Late Effects of Radiation Therapy on Skeletal Muscle Morphology and Progenitor Cell Content are Influenced by Diet-Induced Obesity and Exercise Training in Male Mice, Sci Rep. 9, 6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dayanidhi S & Lieber RL (2014) Skeletal muscle satellite cells: mediators of muscle growth during development and implications for developmental disorders, Muscle Nerve. 50, 723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR & R. Z (2007) How common are the “common” neurologic disorders?, Neurology. 68, 326–337. [DOI] [PubMed] [Google Scholar]
- 86.Krigger KW (2006) Cerebral Palsy: An Overview, American Family Physician. 73, 91–100. [PubMed] [Google Scholar]
- 87.Dayanidhi S, Kinney MC, Dykstra PB & Lieber RL (2020) Does a Reduced Number of Muscle Stem Cells Impair the Addition of Sarcomeres and Recovery from a Skeletal Muscle Contracture? A Transgenic Mouse Model, Clin Orthop Relat Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mathewson MA, Chambers HG, Girard PJ, Tenenhaus M, Schwartz AK & Lieber RL (2014) Stiff muscle fibers in calf muscles of patients with cerebral palsy lead to high passive muscle stiffness, J Orthop Res. 32, 1667–74. [DOI] [PubMed] [Google Scholar]
- 89.Smith LR, Lee KS, Ward SR, Chambers HG & Lieber RL (2011) Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length, J Physiol. 589, 2625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barber LA, Read F, Lovatt Stern J, Lichtwark G & Boyd RN (2016) Medial gastrocnemius muscle volume in ambulant children with unilateral and bilateral cerebral palsy aged 2 to 9 years, Dev Med Child Neurol. 58, 1146–1152. [DOI] [PubMed] [Google Scholar]
- 91.Barber L, Hastings-Ison T, Baker R, Barrett R & Lichtwark G (2011) Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy, Dev Med Child Neurol. 53, 543–8. [DOI] [PubMed] [Google Scholar]
- 92.Smith LR, Chambers HG & Lieber RL (2013) Reduced satellite cell population may lead to contractures in children with cerebral palsy, Dev Med Child Neurol. 55, 264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dayanidhi S, Dykstra PB, Lyubasyuk V, McKay BR, Chambers HG & Lieber RL (2015) Reduced satellite cell number in situ in muscular contractures from children with cerebral palsy, J Orthop Res. 33, 1039–45. [DOI] [PubMed] [Google Scholar]
- 94.Lowrie MB, Krishnan S & Vrbova G (1982) Recovery of slow and fast muscles following nerve injury during early post-natal development in the rat, The Journal of Physiology. 331, 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lowrie MB & Vrbova G (1984) Different pattern of recovery of fast and slow muscles following nerve injury in the rat, The Journal of Physiology. 349, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nikolaou S, Cramer AA, Hu L, Goh Q, Millay DP & Cornwall R (2019) Proteasome inhibition preserves longitudinal growth of denervated muscle and prevents neonatal neuromuscular contractures, JCI Insight. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim JK, Jha NN, Feng Z, Faleiro MR, Chiriboga CA, Wei-Lapierre L, Dirksen RT, Ko CP & Monani UR (2020) Muscle-specific SMN reduction reveals motor neuronindependent disease in spinal muscular atrophy models, J Clin Invest. 130, 1271–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]


