Abstract
Semaphorins were originally identified as neuronal guidance molecules mediating their attractive or repulsive signals by forming complexes with plexin and neuropilin receptors. Subsequent research has identified functions for semaphorin signaling in many organs and tissues outside of the nervous system. Vital roles for semaphorin signaling in vascular patterning and cardiac morphogenesis have been demonstrated, and impaired semaphorin signaling has been associated with various human cardiovascular disorders including persistent truncus arteriosus, sinus bradycardia and anomalous pulmonary venous connections. Here, we review the functions of semaphorins and their receptors in cardiovascular development and disease and highlight important recent discoveries in the field.
Keywords: cardiac development, vasculature development, semaphorin, plexin, neuropilin
Introduction
Semaphorins are a large family of secreted or membrane associated glycoproteins that are highly conserved both structurally and functionally throughout evolution from viruses to mammalians. Semaphorins and neuropilin receptors were initially identified as axon guidance molecules in the developing nervous system (He and Tessier-Lavigne, 1997; Luo et al., 1993). Subsequently, semaphorins have been shown to function in a wide range of developmental, physiological and pathological processes outside of the central nervous system including lymphocyte activation, neural crest cell migration, vascular endothelial cell motility, bone growth, tumor angiogenesis and progression, lung branching morphogenesis and cardiovascular development (Cora et al., 2014; Kruger et al., 2005; Neufeld and Kessler, 2008; Neufeld et al., 2012; Roth et al., 2009).
Semaphorin signals act by juxtacrine, paracrine and autocrine mechanisms to modulate cellular behavior and are categorized into eight classes according to their structural features and species of origin (Kruger et al., 2005). Class 1 and 2 semaphorins are found in invertebrates, classes 3–7 are found in vertebrates and class 8 semaphorins are encoded by viruses (Figure 1). Though semaphorins differ in their amino acid sequence and overall structural characteristics, all members of the family contain a conserved ~500 amino acid “sema” domain located near the amino terminus of the mature protein. The sema domain is essential for receptor binding specificity and signaling (He and Tessier-Lavigne, 1997; Koppel et al., 1997). The sema domains of several different semaphorins were recently characterized by mutagenesis, X-ray crystallography and three-dimensional modeling, which reveals that the sema domain is a variant of the seven blade β-propeller fold with overall structural similarities to the β-propeller repeats of α-integrins (Siebold and Jones, 2013).
Figure 1. Structure of semaphorins and their receptors.
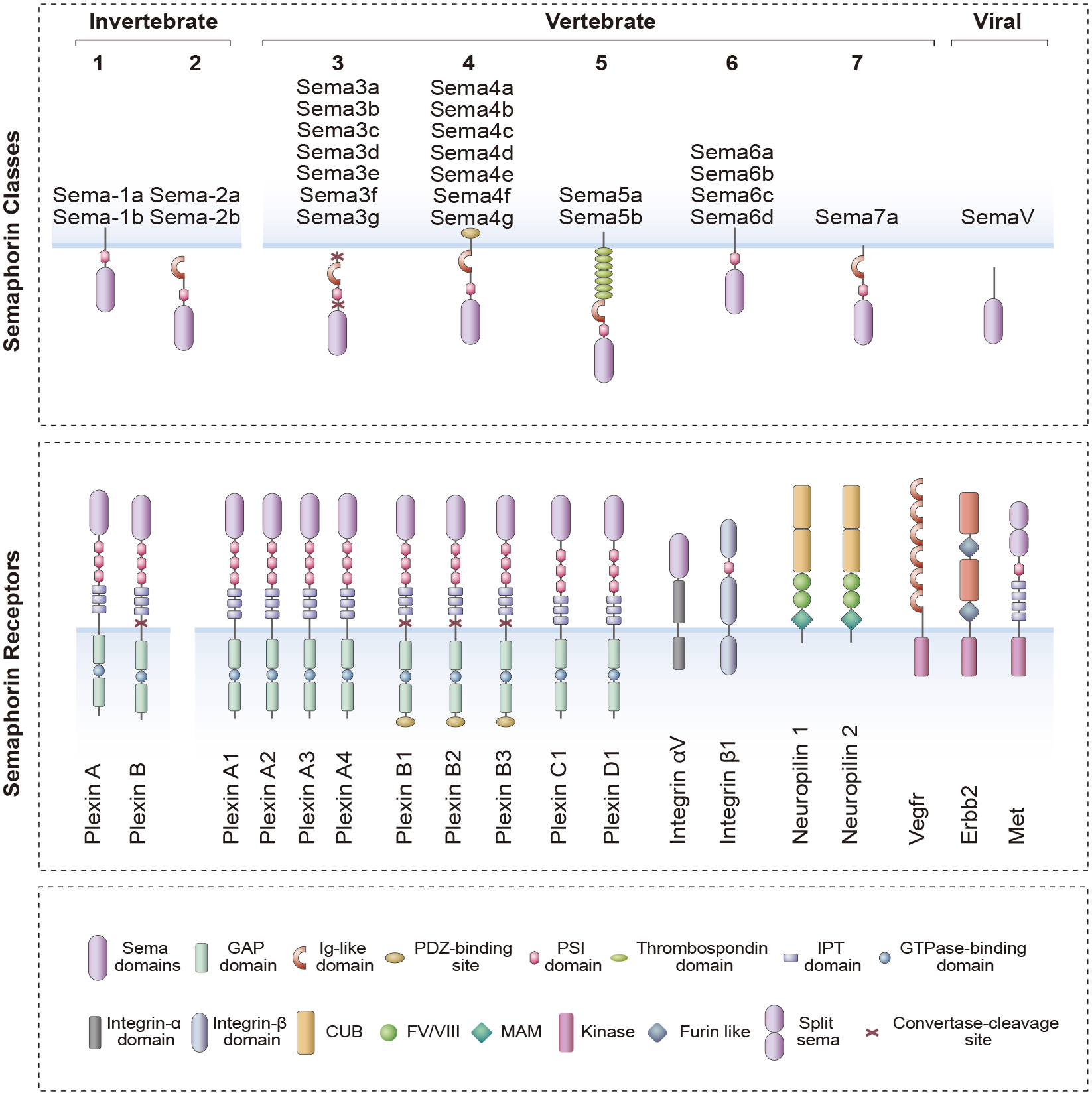
Semaphorins are categorized into eight classes based on their structural features and species origin. Semaphorins are defined by the presence of a conserved “sema” domain near the N-terminus of the protein. Class 1 and 2 semaphorins are found in invertebrates, Class 3–7 in vertebrates and SemaV in certain viruses. Semaphorins are secreted (Sema2a-2b, Sema3a-3g and SemaV), membrane-bound (Sema-1a,1b, Sema4a-4g, Sema5a-5b and Sema6a-6d) or GPI-anchored proteins (Sema7a). Two distinct transmembrane receptor families have been identified as semaphorin receptors, plexins and neuropilins. Shown here are the eleven members of the plexin family, including the invertebrate plexins (Plexin A and Plexin B), and the two members of the neuropilin family. Generally, membrane-bound semaphorins (class 4–7) directly bind to plexins. However, most secreted semaphorins (class 3) require neuropilins (Nrp1 and Nrp2) as binding co-receptors to enable binding to plexins. Neuropilins provide binding specificity for the class 3 semaphorins, whereas plexins, which also contain a sema domain, serve as signaling partners. An exception to this rule is Sema3e; a secreted semaphorin that can directly binds to plexinD1. Sema7a, a GPI-anchored semaphorin, signals through integrin receptors. Vegfr2 can form a receptor complex with plexin A1 that is required for Sema6d-mediated signaling in cardiac development. Class 4 and 5 semaphorins are associated with plexin B1–3 receptors, and the receptor tyrosine kinases Met and Erbb2 can function as coreceptors with plexin Bs for certain class 4 semaphorins. Protein domains are indicated: GAP, GTPase-activating protein; Ig-like, immunoglobulin like; PSI, plexin semaphorin integrin; IPT, Ig-like plexin transcription factors; CUB, complement C1r/C1s, Uegf, Bmp1; FV/VIII, coagulation factor V/VIII homology like; MAM, meprin like.
The different classes of semaphorins are characterized by class specific structural motifs. For example, classes 2–4 and 7 contain a single copy of an immunoglobulin-like domain, while class 5 semaphorins contain seven copies of a thrombospondin domain. All vertebrate and invertebrate semaphorins contain a PSI (plexin-semaphorin-integrin) domain that is required for homodimerization (Klostermann et al., 1998). Another important feature that differs between different semaphorin classes is the presence of membrane anchoring sequences. Classes 2 and 3 semaphorins are produced as secreted proteins, whereas classes 1, 4, 5 and 6 are intrinsic membrane proteins and class 7 semaphorins are membrane anchored proteins, which can be further processed into soluble proteins by proteolytic cleavage (Figure 1) (Siebold and Jones, 2013).
Two families of semaphorin receptors have been identified: plexins and neuropilins (Figure 1 and Tables 1,2). The plexins are a conserved family of large proteins (~200 kDa), initially identified as cell surface molecules mediating cell adhesion (Ohta et al., 1995). Nine members have been identified in vertebrates that are divided into four classes: class A (plexin A1, A2, A3, A4), class B (plexin B1, B2, B3), class C (plexin C1) and class D (plexin D1) (Tamagnone et al., 1999). Unlike semaphorins, plexin architecture is conserved throughout the family. The extracellular domain of plexins contains one sema domain followed by three PSI and three IPT (Ig-like, plexin and transcription factor) repeats (Kruger et al., 2005). The sema domain acts as an autoinhibitory element modulating plexin activation in addition to its role in binding ligand (Figure 1). For example, plexin A1 (Plxna1) mutants lacking the sema domain are constitutively active suggesting that the sema domain is necessary to maintain Plxna1 in its basal inactive state (Takahashi and Strittmatter, 2001). The IPT domain plays an important role in protein-protein interaction (Watarai et al., 2008).
Table 1.
Semaphorin ligand mutants with cardiovascular defects
| Mutation(s) | Receptors or Ligands | Stage of lethality | Cardiovascular phenotypes and functions | Refs. |
|---|---|---|---|---|
| Semaphorins | ||||
| Sema3a −/− | Plexin A1-A4, D1, Nrp1-2, Vegfr2 | Postnatal | Atrial defects, sinus bradycardia, lymphatic valve defects, vascular patterning defects. Sema3a inhibits angiogenesis. | (Bouvree et al., 2012; Chen et al., 2013; Ieda et al., 2007; Jurisic et al., 2012) |
| Sema3b −/− | Plexin A1-A4, Nrp1-2, Vegfr2 | Viable | No obvious cardiac defects. Sema3b inhibits angiogenesis. | (Falk et al., 2005) |
| Sema3c −/− | Plexin A2, D1, Nrp1-2 | Postnatal (varies with strain) | PTA, VSD, aortic arch defects. Sema3c promotes angiogenesis. | (Feiner et al., 2001) |
| Sema3d −/− | Nrp1 | Viable | Anomalous pulmonary venous connection, ASD. Sema3d repels pulmonary vein endothelial cells. | (Degenhardt et al., 2013a; Katz et al., 2012) |
| Sema3e −/− | Plexin As (neuropilin dependent), Plexin D1 | Viable | Vascular patterning defects. Sema3e restricts blood vessel growth to the intersomitic boundaries. Inhibits angiogenesis. A 61-kDa Sema3e isoform promotes endothelial cell migration. | (Casazza et al., 2010; Gu et al., 2005) |
| Sema3f −/− | Plexin A3, A4, Nrp1-2 | Viable | No obvious phenotype. Sema3f inhibits angiogenesis and lymphangiogenesis. | (Sahay et al., 2003) |
| Sema3g −/− | Nrp1-2 | Viable | Vascular remodeling defects. Sema3g promote angiogenesis. | (Kutschera et al., 2011) |
| Sema4a −/− | Plexin B1-3, Plexin D1, Met, Erbb2 | Viable | Enhanced angiogenesis in response to Vegf or inflammatory stimuli. Sema4a inhibits angiogenesis. | (Kumanogoh et al., 2005; Toyofuku et al., 2007) |
| Sema4d −/− | Plexin B1-2, C1, Met, Erbb2 | Viable | Delayed atherosclerotic plaque formation due to impaired neovascularization. Sema4d promotes angiogenesis. | (Yukawa et al., 2010; Zhu et al., 2009) |
| Sema5a −/− | Plexin A3, B3, Nrp2, Met, Erbb2 | Viable or embryonic | Defective cranial blood vessel remodeling. Sema5a promotes angiogenesis. | (Fiore et al., 2005; Matsuoka et al., 2011) |
| Sema6a −/− | Plexin A1, A2, A4, Vegfr2 | Viable | Abnormal retinal vascular development. Sema6a promotes angiogenesis. A soluble form of Sema6a inhibits angiogenesis. | (Segarra et al., 2012) |
| Sema6d −/− | Plexin A1 | Viable | No obvious phenotype in mice. Sema6d modulates compact layer expansion and trabeculation in chick. | (Takamatsu et al., 2010; Toyofuku et al., 2004a; Toyofuku et al., 2004 b) |
VSD, ventricular septal defect; PTA, persistent truncus arteriosus; ASD, atrial septal defect.
Table 2.
Semaphorin receptor mutants with cardiovascular defects
| Mutation(s) | Receptors or Ligands | Stage of lethality | Cardiovascular phenotypes and functions | Refs. |
|---|---|---|---|---|
| Plexins | ||||
| Plxna1 −/− | Sema3a, Sema5a or 5b, Sema6c or 6d | Viable | No obvious cardiac defects. Lymphatic valves are abnormal. | (Bouvree et al., 2012; Takegahara et al., 2006) |
| Plxna2 −/− | Sema6a or 6b | Viable | PTA and lack of aortic and pulmonary channel septation with incomplete penetrance. Double knockouts lacking Plxna2 and Plxna4 exhibit cardiovascular defects with higher penetrance. | (Suto et al., 2007; Toyofuku et al., 2008) |
| Plxna4 −/− | Sema6a or 6b | Viable | Double knockouts lacking Plxna2 and Plxna4 exhibit cardiovascular defects with higher penetrance. | (Toyofuku et al., 2008) |
| Plxnd1−/− and Plxnd1ecKO | Sema3a, 3c (neuropilin dependent) Sema3e, 4a (neuropilin-independent) | Postnatal | PTA, VSD, atrial defects, coronary artery and aortic arch defects. | (Gitler et al., 2004; Zhang et al., 2009) |
| Neuropilins | ||||
|
Nrp1
−/−
Nrp1 ecKO Nrp1 −Sema |
Coreceptor for class 3 semaphorins and others | Embryonic E10.5-12.5 Perinatal Postnatal |
Various cardiac and vascular defects including PTA and aortic arch defects. PTA, BAE, AOC, VSD. BAE, VSD, lymphatic vessels and valve defects. |
(Gu et al., 2003; Kawasaki et al., 1999) |
| Nrp2 −/− | Coreceptor for class 3 semaphorins and others | Viable | Lymphatic vessel and capillary formation defect. | (Giger et al., 2000; Yuan et al., 2002) |
| Nrp1−/−; Nrp2−/− | Coreceptor for class 3 semaphorins and others | Embryonic (~E8.0) | Vascular anomalies in both embryos and placenta. | (Takashima et al., 2002) |
| Nrp1Sema-; Nrp2−/− | Coreceptor for class 3 semaphorins and others | Not reported | PTA, BAE, AOC and VSD, no vascular defects reported. | (Gu et al., 2003) |
BAE, Bilateral atrial enlargement; AOC, anomalous origin of the coronary arteries; VSD, ventricular septal defect; PTA, persistent truncus arteriosus.
The intracellular domains of plexins contain two segments that show sequence similarity to Ras-family-specific guanosine triphosphatase (GTPase)-activating proteins (RasGAP). These segments fold to form an intact GAP domain highly similar to the GAP domain of p120 RasGAP (Siebold and Jones, 2013). The GAP domains are separated by a linker region that includes a Rho GTPase–binding domain (RBD). Three Rho family GTPases, Rac1, Rnd1, and RhoD have been shown to bind the RBD of plexin receptors (Tong et al., 2007). Mutation of the two conserved arginine residues that correspond to the invariant catalytic residues of Ras GAPs abolish plexin-mediated cellular responses (Rohm et al., 2000), indicating that the GAP domains are essential for plexin signaling. Accumulating evidence suggests that activation of the GAP activity of plexins requires two simultaneous events: binding of a semaphorin ligand to the extracellular domain and binding of a Rho GTPase to the RBD (Wang et al., 2012). Recently, crystal structures of the RBDs of various plexin receptors demonstrated that this region has a ubiquitin-like fold, which is also present in RasGAPs (Tong and Buck, 2005). Although plexins share structural homology with tyrosine kinase receptors such as c-Met, they do not exhibit tyrosine kinase activity, and details of downstream signaling events remain to be fully elucidated.
Most membrane bound semaphorins bind directly to the plexin receptors. However this is generally not the case with (class 3) semaphorins, with the exception of semaphorin 3e (Sema3e), which require an initial interaction with a neuropilin co-receptor (Nrp1/Nrp2) to transduce their signal to plexin receptors (Gu et al., 2003). Neuropilins are promiscuous co-receptors regarding both ligands and receptor partners that participate in a broad range of signaling pathways (Gu et al., 2003; Hillman et al., 2011; Muders, 2011; Neufeld et al., 2002; Rizzolio and Tamagnone, 2011). Importantly with regard to the cardiovascular system, neuropilins can partner with the vascular endothelial growth factor (Vegf) receptor to mediate angiogenic Vegf signaling to endothelial cells.
Below, we highlight the recent advances in the field of semaphorin-plexin signaling in cardiovascular development and contributions to congenital cardiac diseases. Cardiovascular abnormalities associated with defective semaphorin signaling are summarized in Tables 1 and 2.
Cardiovascular development: an overview
The heart is the first functional organ to form during embryogenesis. Heart development (Figure 2) begins with the migration of mesodermal precursors away from the primitive streak to form a bow-shaped structure called the cardiac crescent. This region can be divided into the first heart field, which gives rise to the linear heart tube, and the second heart field, which gives rise to portions of the atria, the outflow tract and the right ventricle (Epstein, 2010). Initially, the heart consists of two layers; an inner endocardium and an outer myocardium. Subsequently, the myocardial layer expands and proepicardial progenitor cells migrate over the surface of the heart, contribute fibroblasts that invade the myocardial layer, and form the outermost epicardial layer. Complex asymmetric morphogenetic movements coupled with uneven growth rates contribute to looping and chamber formation. A series of septation events results in a fully functional four-chambered heart integrated with systemic and pulmonary circulations (Epstein, 2010). This process is facilitated by neural crest cells, which migrate from the dorsal neural tube and invade the cardiac outflow tract, contributing to the smooth muscle of the great vessels and which are required for innervation of the heart.
Figure 2. Cardiac development.
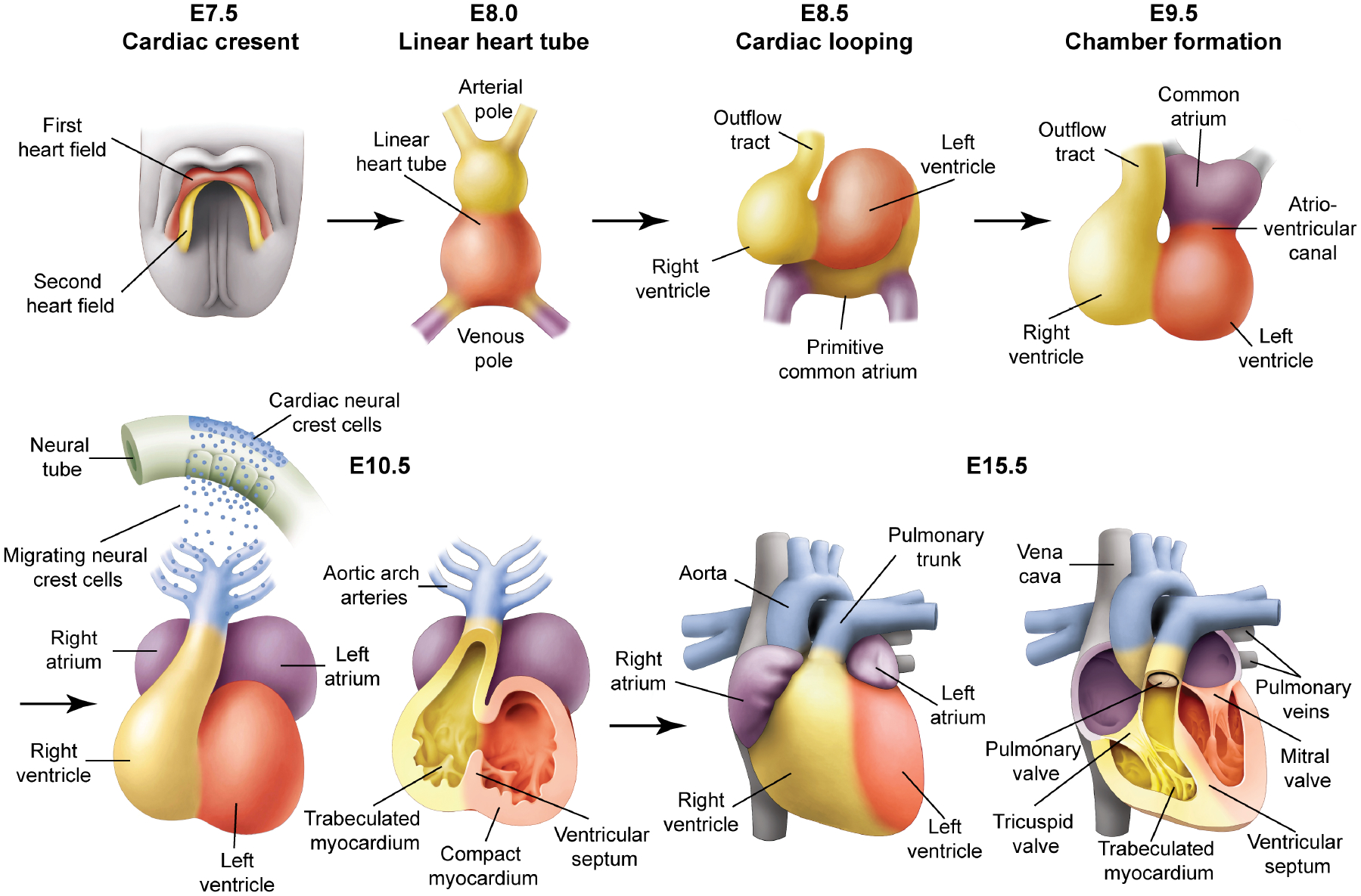
The heart originates from mesodermal cells in the primitive streak. During gastrulation, cardiac progenitors migrate to the splanchnic mesoderm to form the cardiac crescent. At E7.5 in the mouse, the cardiac crescent can be divided into two heart field lineages based on differential gene expression and their respective contribution to heart, a first heart field (red) and a second heart field (yellow), which is located posteriorly and medially to the first heart field. At E8.0, the linear heart tube is present. At E8.5, the looping is associated with uneven growth of cardiac chambers. The outflow tract is at the arterial pole and the inflow tract and primitive atria are at the venous pole. By E9.5, the common atrium has moved superior to the ventricles and is separated by a distinct atrio-ventricular canal. By E10.5, cardiac neural crest cells from the dorsal neural tube migrate via the pharyngeal arches to the cardiac outflow tract. Further cardiac development involves a series of septation events and myocardial trabeculation that result in a mature four-chambered heart integrated with the circulatory system depicted at E15.5.
As the heart is forming, endothelial progenitor cells coalesce to form a primitive vascular plexis and connect with one another to form tubes (vasculogenesis) (Figure 3). The growing vessels branch, expand and migrate (angiogenesis) in stereotypical patterns under the influence of extrinsic growth factors such as Vegf and various guidance molecules including semaphorins. Arterial and venous endothelium adopt unique identities and gene expression characteristics. The lymphatic vasculature emerges from the venous vessels including the cardinal vein at around E10.5 in the mouse, and mature into a characteristically patterned vascular system that drains extracellular fluid and cellular constituents from organs and peripheral tissues, returning lymph to the venous systemic system (Tammela and Alitalo, 2010).
Figure 3. Schematic overview of mammalian vascular development.
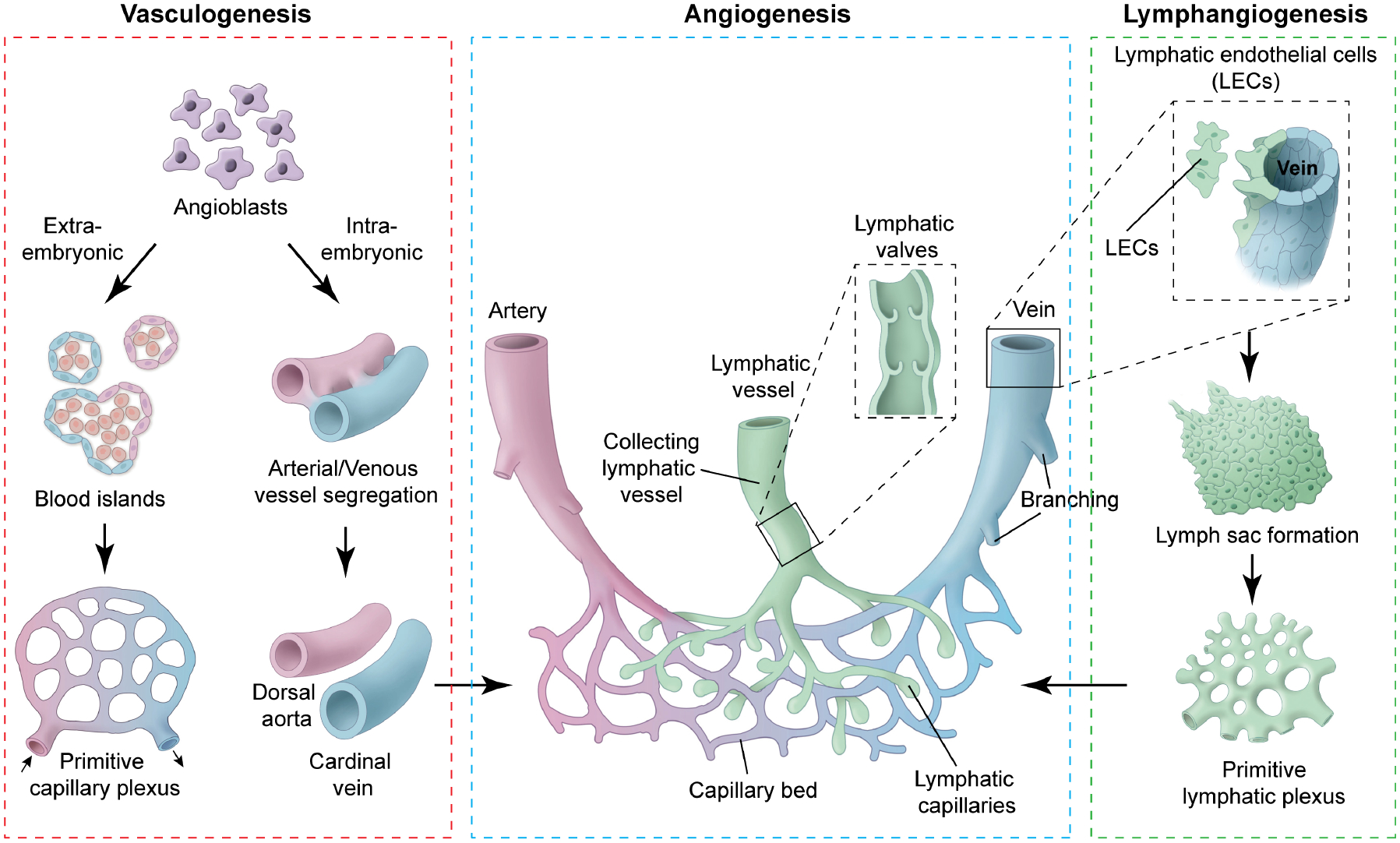
Vascular endothelial progenitor cells (angioblasts) are derived from mesodermal cells during gastrulation. These mesodermal cells have potential to give rise to both blood and endothelium. However, their fate is restricted once they have emigrated into extra-embryonic tissue (extra embryonic ectoderm, yolk sac and allantois) and intra-embryonic tissues (embryonic ectoderm). In extra-embryonic tissue, these angioblasts aggregate to form blood islands. Fusion of blood islands leads to the formation of a honeycomb-shaped primitive capillary plexus. However, in intra-embryonic tissue, angioblasts aggregate and directly form the dorsal aorta and cardinal vein, without a vascular plexus intermediate. The primitive vascular plexus along with dorsal aorta and cardinal vein undergo remodeling to form a mature circulatory system. Lymphatic endothelial cells (LECs) are specified in embryonic cardinal veins at defined locations. Under the influence of Vegfc, produced by the mesenchymal cells surrounding the cardinal veins, LEC precursors lose their polarity, delaminate from the veins, migrate and aggregate to form the primary lymph sacs. Furthermore, sprouting, remodeling and expansion of the lymph sacs and primitive lymphatic plexus lead to the formation of mature peripheral lymphatic vasculature. There are two types of lymphatic vessels: collecting lymphatic vessels and lymphatic capillaries. Lymphatic capillaries are thin-walled, blind-ended vessels that absorb interstitial fluid and transport it to the collecting lymphatic vessels. Collecting lymphatic vessels are surrounded by smooth muscle cells and have bicuspid valve to facilitate unidirectional lymphatic flow.
Over the past several years, a growing body of literature implicates semaphorin signaling in many aspects of cardiovascular development (Tables 1 and 2), and a growing number of human disorders are being associated with genetic mutation or variation in semaphorin signaling pathway genes.
Myocardial compaction and trabeculation
An essential process in cardiac development is chamber maturation and defects with this can lead to a variety of congenital abnormalities including non‐compaction, systolic dysfunction, diastolic dysfunction, and arrhythmia (Teekakirikul et al., 2013). During chamber development the myocardium consists of two layers; an outer compact and an inner trabeculated layer. The trabeculated myocardium is covered by the endocardium and the interaction between these two layers is essential for cardiomyocyte proliferation and differentiation. Left ventricular non-compaction (LVNC) is a well-studied cardiac maturation disorder characterized by extensively trabeculated myocardium of the left ventricle and a thin compact zone (Oechslin and Jenni, 2011). LVNC can lead to heart failure, stroke, ventricular arrhythmias and/or sudden cardiac death (Bhatia et al., 2011; Ichida et al., 1999; Paterick and Tajik, 2012).
Toyofuku et al demonstrated that the Sema6d-Plxna1 interaction is required for cardiac chamber development (Toyofuku et al., 2004a; Toyofuku et al., 2004b). Sema6d is expressed in compact and trabeculated myocardial cells; however, its receptor Plxna1 is expressed by trabeculated myocardial and endocardial cells (Toyofuku et al., 2004a; Toyofuku et al., 2004b). Knockdown of Sema6d, Plxna1 or both in chick embryos results in a non-compaction phenotype characterized by a thin compact myocardium (Toyofuku et al., 2004a; Toyofuku et al., 2004b). Further experiments suggest that membrane bound Sema6d can function as both a ligand and a receptor for Plxna1 to modulate compact layer expansion and trabeculation (Toyofuku et al., 2004b). Surprisingly, Sema6d knockout mice are viable and show no obvious cardiac defects (Takamatsu et al., 2010). Additional roles for semaphorins in myocardial maturation are likely. For example, Sema3a mutant mice display abnormal right ventricular and atrial myocardium (Behar et al., 1996).
Neural crest and cardiac outflow tract development
Many signaling pathways have been implicated in regulating specification, induction, migration, proliferation, differentiation and survival of cardiac neural crest cells (High and Epstein, 2008; Stoller and Epstein, 2005). Disruption of cardiac neural crest during development leads to specific forms of congenital heart disease including interruption of the aortic arch, persistent truncus arteriosus (PTA) and double outlet right ventricle (DORV) (Stoller and Epstein, 2005). Various studies have demonstrated that semaphorin-plexin signaling can regulate the migration of cardiac neural crest cells to the forming heart.
Sema3c is expressed in the OFT myocardium and branchial arch mesenchyme throughout the septation and remodeling process (Feiner et al., 2001). Genetic deletion of Sema3c causes postnatal lethality due to PTA, ventricular septal defects and interruption of the aortic arch. Molecular analysis revels abnormal migration of cardiac neural crest cells in Sema3c−/− mouse embryos associated with anomalous expression of Foxc1, Ednra and Plxna2 by neural crest (Feiner et al., 2001). The OFT and aortic arch defects in Sema3c mutants are similar to neural crest-ablated chick embryos suggesting that Sema3c expression is essential for OFT development and aortic arch patterning. Recent work in chick embryos suggests that cardiac neural crest cell migration is determined by both repulsive and attractive cues provided by semaphorins. Sema3c can provide attractive cues while membrane bound Sema6a and Sema6b can provide repulsive cues to migrating neural crest (Toyofuku et al., 2008). However, Sema3c is also expressed by migrating cardiac neural crest and may signal to surrounding endothelium expressing Plxnd1 and Nrp1/2 resulting in reciprocal signaling cascades that ultimately affect neural crest patterning and differentiation. Interestingly, Sema3c expression by cardiac neural crest is Gata6 dependent and Gata6 mutations in mice and humans are associated with congenital heart disease including PTA (Kodo et al., 2009; Lepore et al., 2006).
A host of additional studies using genetically modified mice further implicate semaphorin signaling in cardiac neural crest and outflow tract development (Brown et al., 2001; Gitler et al., 2004; Gu et al., 2003; Toyofuku et al., 2008; Zhang et al., 2009). Plxna2, which may act as a receptor for Sema6a/b (Toyofuku et al., 2008) is expressed by migrating cardiac neural crest (Brown et al., 2001) and loss of expression in mice results in impaired migration and cardiovascular abnormalities including PTA and lack of aortic and pulmonary artery septation (Toyofuku et al., 2008). Loss of Plxna2 leads to the upregulation of plexin A4 (Plxna4) in the OFT suggesting a compensatory response (Toyofuku et al., 2008), and double mutants for Plxna2 and Plxna4 exhibit OFT defects with higher penetrance than Plxna2−/− mice (Toyofuku et al., 2008). Recently Li et al demonstrated that neural crest specific Plxna2 gene activation is Brg1 (Brahma-related gene 1) dependent. Brg1, an ATPase subunit of the Brg1/BAF chromatin-remodeling complex, interacts at the Plxna2 promoter with chromodomain-helicase-DNA-binding protein 7 (Chd7), which is encoded by a gene mutated in humans with CHARGE syndrome characterized by OFT defects. Neural crest specific Brg1 mutants also display defects in OFT formation (Li et al., 2013).
Plxnd1 is expressed by endothelial cells during cardiovascular development (Gitler et al., 2004; Zhang et al., 2009). Plxnd1 null pups and endothelial-specific Plxnd1 knockouts die shortly after birth due with PTA, defective aortic arch arteries and ventricular septal defects (Gitler et al., 2004; Zhang et al., 2009). Mutations of PLXND1 have been associated with isolated cases of truncus arteriosus in humans (Ta-Shma et al., 2013). Recent work by Worzfeld et al. demonstrates that transgenic mice harboring mutations in the GAP domain of Plxnd1 recapitulate the phenotype of Plxnd1 null mice suggesting that the GAP domain is essential for Plxnd1 signaling during cardiac development (Worzfeld et al., 2014). Plxnd1 may interact with Nrp1 to transduce a Sema3c signal (Toyofuku et al., 2008) but the spectrum of Plxnd1 ligands has yet to be fully defined, and the mechanism by which loss of Plxnd1 signaling in endothelium results in OFT defects remains obscure. Given the known roles of Notch signaling in OFT formation (High and Epstein, 2008), it is attractive to hypothesize that loss of Plxnd1 results in abnormal Notch signaling from endothelium to surrounding mesenchyme, including neural crest, but this hypothesis awaits experimental validation.
Cardiac defects similar to Sema3c, Plxna2 and Plxnd1 mutants have been described in Nrp1 knockout (Kawasaki et al., 1999) and endothelial-specific Nrp1 knockout mice (Gu et al., 2003). However, PTA is not observed in mice expressing a form of neuropilin-1 unable to bind class 3 semaphorins (Nrp1sema−) suggesting that sema-independent Nrp1 signaling in endothelial cells is essential for OFT septation (Gu et al., 2003). Interestingly, Nrp1sema− mice on a Nrp2 null background do display PTA, suggesting functional redundancy. Since Nrp1 can bind Vegf in addition to semaphorins, it is possible that Vegf-dependent Nrp1 signaling modulates OFT development accounting for these findings. However, Gu et al. recently reported the phenotype of a Nrp1 mutant mouse in which Nrp1 was mutated to specifically abolish Vegf binding. The resulting Nrp1Vegf− mice survive to adulthood and do not display OFT defects (Gelfand et al., 2014). The relative influence of Vegf versus semaphorin signaling via neuropilins remains to be fully elucidated.
Epicardial development
The epicardial layer of the heart is derived from a transient structure called the proepicardial organ (PEO) that arises from the mesothelium of the septum transversum (Gittenberger-de Groot et al., 1998; Manner et al., 2001; Mikawa and Fischman, 1992; Mikawa and Gourdie, 1996). During embryonic development, the epicardium provides progenitor cells that give rise to various cardiac lineages including fibroblasts, endothelial and smooth muscle, with important contributions to the coronary vasculature (Limana et al., 2011; Singh and Epstein, 2012; Singh et al., 2011). The epicardium also plays an important role in cardiac injury and repair. Embryonic gene programs in the epicardium are activated after myocardial injury and factors are released from the activated epicardium that affect cardiac repair and regeneration, partly by modulating angiogenesis (Zhou et al., 2011).
The proepicardial contribution to cardiac lineages has been controversial, with conflicting findings in chick and mouse models. The PEO is a heterogeneous tissue comprised of genetically distinct sub-populations of progenitor cells with distinct fates (Degenhardt et al., 2013b; Katz et al., 2012). One population, capable of contributing to coronary endothelium, is characterized by expression of Sema3d (Chen et al., in press; Katz et al., 2012). Additionally, Sema3d expressing proepicardial cells also contribute to the endothelial lining of the sinus venosus, a tissue previously reported to contribute to coronary endothelial cells (Katz et al., 2012; Red-Horse et al., 2010). Although not yet directly demonstrated, it is likely that epicardial-derived semaphorins function to pattern coronary vasculature and innervation during development. These functions could also contribute to epicardial signals that modulate the response to injury in the adult.
Pulmonary vein patterning
The pulmonary veins function to return oxygenated blood from the lungs to the left atrium. Inappropriate patterning of the pulmonary veins during development can result in abnormal connections to the right atrium, or to one of the venous structures that connects to the right atrium such as the coronary sinus or vena cavae. This type of anomalous pulmonary venous connection (APVC) can cause hypoxia and volume overload to the right side of the heart, requiring surgical intervention if severe. Little is known about the developmental signals that pattern the pulmonary veins or about the genetic or environmental causes of APVC in humans.
Sema3d null mice exhibit APVC and have provided novel insights into the developmental pathophysiology of pulmonary venous malformations (Degenhardt et al., 2013a). As the pulmonary veins form during mid gestation, Sema3d is expressed in the mesocardial reflections adjacent to the maturing vascular plexus. Sema3d is thought to act as a repulsive guidance molecule to constrain and direct the developing pulmonary venous endothelial cells towards the left atrium. This is supported by the ability of Sema3d to repel endothelial cells in vitro, and the abnormal invasion of endothelial cells into the region where Sema3d is normally expressed in the Sema3d nulls (Aghajanian et al., 2014; Degenhardt et al., 2013a). Sema3d is able to bind to the endothelial cells of the nascent pulmonary vein, which express Nrp1, and Nrp1 is required for Sema3d-mediated endothelial repulsion (Aghajanian et al., 2014; Degenhardt et al., 2013a). (Although Nrp1 has been suggested to be arterial specific (Herzog et al., 2001), more recent studies indicate that it is also expressed by some venous and lymphatic endothelium (Degenhardt et al., 2013a)).
Mutations or genetic variants of SEMA3D may account for some cases of APVC in humans. In one case, a patient with APVC was identified with a very rare variant (not found in several thousand controls) resulting in a phenylalanine-to-leucine (F602L) substitution (Degenhardt et al., 2013a). The mutation results in a significantly reduced ability to bind Nrp1 and to repel endothelial cells in vitro. In the future, it will be of interest to investigate the potential contributions of genetic variants in both SEMA3D and the genes encoding other members of the Sema3d signaling cascade in APVC.
Autonomic innervation of the heart
Cardiac tissues are innervated by sympathetic and parasympathetic nerve fibers that provide critical connections for regulation of heart rate, contractility and blood pressure in response to changes in physiologic demand. The cardiac sympathetic nerves, which are located subepicardially, stimulate heart rate, while parasympathetic nerves are generally subendocardial and function to inhibit heart rate (Zipes, 2008). The ventricular sympathetic innervation is characterized by a gradient from base to apex (Crick et al., 1999; Crick et al., 1994; Pierpont et al., 1985). Specialized conduction tissues such as the sinoatrial node, atrioventricular node and His bundles are highly innervated compared to the myocardium. Abnormal innervation can be associated with lethal arrhythmias in diseased hearts (Cao et al., 2000a; Cao et al., 2000b; Opthof et al., 1991).
Sema3a is initially expressed in the subendocardial cells of the trabeculated myocardium of both ventricles. Later, it is restricted to Purkinje fibers (specialized conduction cells) along the ventricular wall. Through embryonic development, Sema3a expression is consistent with a role in patterning the sympathetic innervation in the heart (Ieda et al., 2007). Although the majority of Sema3a knockout mice die within a week of birth, some survive past weaning (Behar et al., 1996). In these animals, sympathetic cardiac innervation is mis-patterned and results in a slow heart rate (sinus bradycardia) (Ieda et al., 2007). Cardiac-specific overexpression of Sema3a significantly reduces sympathetic innervation suggesting a repulsive and inhibitory role for Sema3a in neuronal growth and patterning. These Sema3a transgenic animals display spontaneous premature ventricular contractions and an increased susceptibility to ventricular arrhythmias (Ieda et al., 2007). Recently, Chen et al, reported that overexpression of Sema3a in border zone myocardium surrounding a recent myocardial infarction can reduce hyperinnervation that normally accompanies the response to injury, consequently reducing susceptibility to ventricular tachycardia (Chen et al., 2013).
Recently, a genetic variant in the human SEMA3A gene was associated with unexplained cardiac arrest and ventricular arrhythmia (Nakano et al., 2013). A nonsynonymous polymorphism (I334V, rs138694505A>G) was identified in the 10th exon of the SEMA3A gene and this variant was significantly more common in a cohort of 83 Japanese patients diagnosed with unexplained cardiac arrest than in 2958 healthy controls. Similar to Sema3a knockout mice, patients with this I334V variant have abnormal patterning of sympathetic nerves and sinus bradycardia. The I334V variant protein has reduced activity in a repulsion assay (Nakano et al., 2013).
Vascular patterning
PlexinD1
Semaphorin signaling contributes to growth and patterning of the entire vascular system, and the complexity and redundancy by which these signals interact with each other and with other angiogenic pathways to influence normal and pathologic blood vessels is becoming increasingly apparent. Perhaps the most visually dramatic and convincing evidence for the ability of secreted class 3 semaphorins to guide growing endothelial sprouts derives from work in zebrafish, where the emerging intrasomitic blood vessels can be visualized in real time. Knockdown of plxnd1 expressed by endothelial cells, or of sema3a secreted by adjacent somitic mesoderm, produces abnormally patterned vessels with too many angiogenic sprouts due to loss of a repellent guidance signal (Torres-Vazquez et al., 2004).
Plxnd1 can probably respond to multiple class 3 semaphorins. It can bind Sema3e even in the absence of a neuropilin co-receptor, and mouse knockouts of both Plxnd1 and Sema3e display intersomitic vascular patterning defects (Gitler et al., 2004; Gu et al., 2005). Sema3e-Plxnd1 signaling is also required for proper dorsal aortae patterning in the early embryo (Meadows et al., 2013). Plxnd1 can repress angiogenesis by antagonizing the proangiogenic activity of Vegf (Zygmunt et al., 2011), and Sema3e-Plxnd1 signaling affects retinal angiogenic cell fate decisions by regulating cell responsiveness to Vegf and Notch in tip and stalk cells (Kim et al., 2011).
However, Plxnd1 mutants have a more severe cardiovascular phenotype that that of Sema3e mutants, suggesting that Plxnd1 in endothelial cells may transduce signals from other semaphorins. In support of this possibility, Plxnd1 in complex with Nrp1 or Nrp2 is capable of binding to Sema3a, Sema3c and Sema4a in vitro (Gitler et al., 2004; Toyofuku et al., 2007). Plxnd1-deficient endothelial cells fail to respond to Sema3a in Boyden chamber and aortic ring assays (Zhang et al., 2009).
Semaphorins and the vasculature
Multiple class 3 semaphorins can modulate endothelial behavior. Sema3a signals through Nrp1 on endothelial cells to inhibit proliferation, survival, migration, adhesion and capillary sprouting in aortic ring assays and can modulate retinal neovascularization after injury as well as vascular permeability (Acevedo et al., 2008; Joyal et al., 2011; Miao et al., 1999). However, whether knockout of Sema3a causes abnormalities of murine vascular development remains controversial (Serini et al., 2003; Vieira et al., 2007). Sema3b repels endothelial cells by binding to Nrp1, therefore functioning as an angiogenesis inhibitor (Varshavsky et al., 2008). Sema3c induces endothelial cell proliferation, adhesion and migration (Banu et al., 2006). Sema3d inhibits endothelial tube formation, adhesion, and motility through Nrp1 and PI3K/Akt dependent pathways (Aghajanian et al., 2014). Sema3f inhibits adhesion of endothelial cells to fibronectin and FGF-induced human umbilical vein endothelial cell proliferation and survival (Kessler et al., 2004; Serini et al., 2003). Sema3g acts as a positive regulator of angiogenesis by stimulating endothelial cells and activating smooth muscle cells (Kutschera et al., 2011).
Membrane-bound semaphorins possess both pro- and anti-angiogenic properties during development and in pathological conditions. For example, Sema4a binds to Plxnd1 and inhibits developmental angiogenesis (Toyofuku et al., 2007), and Sema4d signals via Plxnb1 on endothelial cells to regulate motility (Yukawa et al., 2010). Sema5a signals through the receptor Plxnb3 (Artigiani et al., 2004) and mice lacking Sema5a show defective remodeling of cranial blood vessels. Branching of cranial cardinal veins is reduced in Sema5a mutants compared to controls (Fiore et al., 2005). Sema6a binds to Plxna2 and Plxna4 receptors (Suto et al., 2007) and regulates vascular development and adult angiogenesis (Segarra et al., 2012). Interestingly, a soluble form of Sema6a can inhibit angiogenesis (Dhanabal et al., 2005) and cleaved forms of other semaphorins have been described with altered angiogenic functions (Basile et al., 2007; Casazza et al., 2010; Guo et al., 2013; Varshavsky et al., 2008).
The role played by neuropilin receptors in the vasculature is complicated by the fact that they can participate directly in Vegf signaling by heterodimerizing with Vegfr2 (Kdr) (Kawasaki et al., 1999; Lee et al., 2002; Soker et al., 1998). Nrp1 knockout embryos display a wide range of vascular defects, and overexpression of Nrp1 leads to excessive formation of blood vessels and capillaries (Kitsukawa et al., 1995). Nrp1 is strongly expressed on the newly formed vasculature during wound healing and blocking of Nrp1 signaling reduces vascular density within the wound (Matthies et al., 2002). Nrp2 deficient mice are viable (Chen et al., 2000; Giger et al., 2000) but Vegf-induced retinal neovascularization is significantly compromised in Nrp2−/− mice (Shen et al., 2004). Double Nrp1/Nrp2 knockout mice die as early as E8.5 and display a more severe vasculature phenotype than single knockouts suggesting partial functional redundancy (Takashima et al., 2002).
Lymphangiogenesis and tumor angiogenesis
Semaphorins, plexins and neuropilins have all been implicated in tumor angiogenesis, a topic that has been the subject of several excellent recent reviews (Ch’ng and Kumanogoh, 2010; Neufeld and Kessler, 2008; Neufeld et al., 2012; Sakurai et al., 2012). Semaphorin ligands expressed by tumor cells and by tumor associated immune cells can stimulate pro-angiogenic pathways in endothelial cells, and the possibility of developing anti-angiogenic therapies for cancer by targeting these pathways is being actively explored.
Sema3a and its receptors Nrp1, Nrp2 and Plxna1 are expressed in lymphatic vessels and participate in both lymphatic valve formation and assembly of lymphatic vessels (Bouvree et al., 2012; Jurisic et al., 2012). Sema3a, Plxna1 and Nrp1sema−/− knockout mice have small and poorly formed lymphatic valves (Bouvree et al., 2012). Nrp2 knockout mice have malformed lymphatic vessels and capillaries, but no lymphatic valve defects (Yuan et al., 2002). Sema3a, and probably other membrane bound and secreted semaphorins, can participate in both lymphatic patterning and valve formation. Defective lymphatic patterning and homeostasis can result in significant morbidity related to lymphedema and other complications, suggesting that a better understanding of the role of semaphorins in lymphangiogenesis will be important for targeting and minimizing side effects of potential therapies that target semaphorin, plexin and neuropilin pathways.
Concluding remarks
Since the discovery of semaphorins as axon-guidance molecules, semaphorin signaling has emerged as an important pathway in nearly every organ and tissue. The cardiovascular system is no exception, and a wealth of data underscores the critical role that semaphorins play during cardiovascular development and in various disease processes. The complex code that dictates which semaphorin ligands will interact with which receptors to produce various outputs is only beginning to emerge, and the complexity increases when we consider combinations of heterodimeric receptors and interplay with related signaling pathways. Abnormalities in semaphorin signaling components account for developmental patterning defects and congenital heart disease, and further elucidation of the mechanisms by which semaphorins affect the vasculature offer opportunities for new pro- and anti-angiogenic therapies for cancer, tissue ischemia, and other disorders.
Acknowledgements:
We would like to thank Lili Guo for helping us with the artwork. We apologize to colleagues whose work was not discussed and cited in this review due to limitations in space and scope. MKS is supported by funds from DUKE-NUS Graduate Medical School Singapore and Goh foundation (DUKE-NUS/GRSF/2014/0002R). JAE is supported by RO1 HL118768, the Spain Fund and the Cotswold Foundation.
References:
- Acevedo LM, Barillas S, Weis SM, Gothert JR, and Cheresh DA (2008). Semaphorin 3A suppresses VEGF-mediated angiogenesis yet acts as a vascular permeability factor. Blood 111, 2674–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian H, Choi C, Ho VC, Gupta M, Singh MK, and Epstein JA (2014). Semaphorin 3d and Semaphorin 3e Direct Endothelial Motility through Distinct Molecular Signaling Pathways. J Biol Chem 289, 17971–17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, and Tamagnone L (2004). Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep 5, 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu N, Teichman J, Dunlap-Brown M, Villegas G, and Tufro A (2006). Semaphorin 3C regulates endothelial cell function by increasing integrin activity. Faseb J 20, 2150–2152. [DOI] [PubMed] [Google Scholar]
- Basile JR, Holmbeck K, Bugge TH, and Gutkind JS (2007). MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem 282, 6899–6905. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, and Fishman MC (1996). Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature 383, 525–528. [DOI] [PubMed] [Google Scholar]
- Bhatia NL, Tajik AJ, Wilansky S, Steidley DE, and Mookadam F (2011). Isolated noncompaction of the left ventricular myocardium in adults: a systematic overview. J Card Fail 17, 771–778. [DOI] [PubMed] [Google Scholar]
- Bouvree K, Brunet I, Del Toro R, Gordon E, Prahst C, Cristofaro B, Mathivet T, Xu Y, Soueid J, Fortuna V, et al. (2012). Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ Res 111, 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CB, Feiner L, Lu MM, Li J, Ma X, Webber AL, Jia L, Raper JA, and Epstein JA (2001). PlexinA2 and semaphorin signaling during cardiac neural crest development. Development 128, 3071–3080. [DOI] [PubMed] [Google Scholar]
- Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, et al. (2000a). Nerve sprouting and sudden cardiac death. Circ Res 86, 816–821. [DOI] [PubMed] [Google Scholar]
- Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, et al. (2000b). Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101, 1960–1969. [DOI] [PubMed] [Google Scholar]
- Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, et al. (2010). Sema3E-Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest 120, 2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng ES, and Kumanogoh A (2010). Roles of Sema4D and Plexin-B1 in tumor progression. Molecular cancer 9, 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Bagri A, Zupicich JA, Zou Y, Stoeckli E, Pleasure SJ, Lowenstein DH, Skarnes WC, Chedotal A, and Tessier-Lavigne M (2000). Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron 25, 43–56. [DOI] [PubMed] [Google Scholar]
- Chen HI, Sharma B, Akerberg B, Nurmi HJ, Kivela R, Saharinen P, Aghajanian H, McKay AS, Bogard P, Chang AH, et al. (in press). The sinus venosus contributes to coronary vasculature through VEGF-C-stimulated angiogenesis. Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Li YG, Jiao KL, Zhang PP, Sun Y, Zhang LP, Fong XF, Li W, and Yu Y (2013). Overexpression of Sema3a in myocardial infarction border zone decreases vulnerability of ventricular tachycardia post-myocardial infarction in rats. J Cell Mol Med 17, 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cora D, Astanina E, Giraudo E, and Bussolino F (2014). Semaphorins in cardiovascular medicine. Trends in molecular medicine 20, 589–598. [DOI] [PubMed] [Google Scholar]
- Crick SJ, Sheppard MN, Ho SY, and Anderson RH (1999). Localisation and quantitation of autonomic innervation in the porcine heart I: conduction system. J Anat 195 (Pt 3), 341–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick SJ, Wharton J, Sheppard MN, Royston D, Yacoub MH, Anderson RH, and Polak JM (1994). Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation 89, 1697–1708. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Singh MK, Aghajanian H, Massera D, Wang Q, Li J, Li L, Choi C, Yzaguirre AD, Francey LJ, et al. (2013a). Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nat Med 19, 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Singh MK, and Epstein JA (2013b). New approaches under development: cardiovascular embryology applied to heart disease. J Clin Invest 123, 71–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanabal M, Wu F, Alvarez E, McQueeney KD, Jeffers M, MacDougall J, Boldog FL, Hackett C, Shenoy S, Khramtsov N, et al. (2005). Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biol Ther 4, 659–668. [DOI] [PubMed] [Google Scholar]
- Epstein JA (2010). Franklin H. Epstein Lecture. Cardiac development and implications for heart disease. N Engl J Med 363, 1638–1647. [DOI] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, et al. (2005). Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 48, 63–75. [DOI] [PubMed] [Google Scholar]
- Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, and Raper JA (2001). Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development 128, 3061–3070. [DOI] [PubMed] [Google Scholar]
- Fiore R, Rahim B, Christoffels VM, Moorman AF, and Puschel AW (2005). Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol Cell Biol 25, 2310–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand MV, Hagan N, Tata A, Oh WJ, Lacoste B, Kang KT, Kopycinska J, Bischoff J, Wang JH, and Gu C (2014). Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, et al. (2000). Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron 25, 29–41. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, and Epstein JA (2004). PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell 7, 107–116. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Vrancken Peeters MP, Mentink MM, Gourdie RG, and Poelmann RE (1998). Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res 82, 1043–1052. [DOI] [PubMed] [Google Scholar]
- Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, and Ginty DD (2003). Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell 5, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, and Ginty DD (2005). Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science 307, 265–268. [DOI] [PubMed] [Google Scholar]
- Guo HF, Li X, Parker MW, Waltenberger J, Becker PM, and Vander Kooi CW (2013). Mechanistic basis for the potent anti-angiogenic activity of semaphorin 3F. Biochemistry 52, 7551–7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, and Tessier-Lavigne M (1997). Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell 90, 739–751. [DOI] [PubMed] [Google Scholar]
- Herzog Y, Kalcheim C, Kahane N, Reshef R, and Neufeld G (2001). Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev 109, 115–119. [DOI] [PubMed] [Google Scholar]
- High FA, and Epstein JA (2008). The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9, 49–61. [DOI] [PubMed] [Google Scholar]
- Hillman RT, Feng BY, Ni J, Woo WM, Milenkovic L, Hayden Gephart MG, Teruel MN, Oro AE, Chen JK, and Scott MP (2011). Neuropilins are positive regulators of Hedgehog signal transduction. Genes Dev 25, 2333–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida F, Hamamichi Y, Miyawaki T, Ono Y, Kamiya T, Akagi T, Hamada H, Hirose O, Isobe T, Yamada K, et al. (1999). Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol 34, 233–240. [DOI] [PubMed] [Google Scholar]
- Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, et al. (2007). Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med 13, 604–612. [DOI] [PubMed] [Google Scholar]
- Joyal JS, Sitaras N, Binet F, Rivera JC, Stahl A, Zaniolo K, Shao Z, Polosa A, Zhu T, Hamel D, et al. (2011). Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A. Blood 117, 6024–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV, and Detmar M (2012). An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res 111, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz TC, Singh MK, Degenhardt K, Rivera-Feliciano J, Johnson RL, Epstein JA, and Tabin CJ (2012). Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev Cell 22, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, and Fujisawa H (1999). A requirement for neuropilin-1 in embryonic vessel formation. Development 126, 4895–4902. [DOI] [PubMed] [Google Scholar]
- Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, and Neufeld G (2004). Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res 64, 1008–1015. [DOI] [PubMed] [Google Scholar]
- Kim J, Oh WJ, Gaiano N, Yoshida Y, and Gu C (2011). Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev 25, 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsukawa T, Shimono A, Kawakami A, Kondoh H, and Fujisawa H (1995). Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development 121, 4309–4318. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lohrum M, Adams RH, and Puschel AW (1998). The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J Biol Chem 273, 7326–7331. [DOI] [PubMed] [Google Scholar]
- Kodo K, Nishizawa T, Furutani M, Arai S, Yamamura E, Joo K, Takahashi T, Matsuoka R, and Yamagishi H (2009). GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc Natl Acad Sci U S A 106, 13933–13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel AM, Feiner L, Kobayashi H, and Raper JA (1997). A 70 amino acid region within the semaphorin domain activates specific cellular response of semaphorin family members. Neuron 19, 531–537. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, and Guan KL (2005). Semaphorins command cells to move. Nat Rev Mol Cell Biol 6, 789–800. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, et al. (2005). Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 22, 305–316. [DOI] [PubMed] [Google Scholar]
- Kutschera S, Weber H, Weick A, De Smet F, Genove G, Takemoto M, Prahst C, Riedel M, Mikelis C, Baulande S, et al. (2011). Differential endothelial transcriptomics identifies semaphorin 3G as a vascular class 3 semaphorin. Arterioscler Thromb Vasc Biol 31, 151–159. [DOI] [PubMed] [Google Scholar]
- Lee P, Goishi K, Davidson AJ, Mannix R, Zon L, and Klagsbrun M (2002). Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc Natl Acad Sci U S A 99, 10470–10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore JJ, Mericko PA, Cheng L, Lu MM, Morrisey EE, and Parmacek MS (2006). GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J Clin Invest 116, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Xiong Y, Shang C, Twu KY, Hang CT, Yang J, Han P, Lin CY, Lin CJ, Tsai FC, et al. (2013). Brg1 governs distinct pathways to direct multiple aspects of mammalian neural crest cell development. Proc Natl Acad Sci U S A 110, 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limana F, Capogrossi MC, and Germani A (2011). The epicardium in cardiac repair: from the stem cell view. Pharmacol Ther 129, 82–96. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, and Raper JA (1993). Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 75, 217–227. [DOI] [PubMed] [Google Scholar]
- Manner J, Perez-Pomares JM, Macias D, and Munoz-Chapuli R (2001). The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs 169, 89–103. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, Kumar SR, Suto F, Chedotal A, Peachey NS, et al. (2011). Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron 71, 460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies AM, Low QE, Lingen MW, and DiPietro LA (2002). Neuropilin-1 participates in wound angiogenesis. Am J Pathol 160, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SM, Ratliff LA, Singh MK, Epstein JA, and Cleaver O (2013). Resolution of defective dorsal aortae patterning in Sema3E-deficient mice occurs via angiogenic remodeling. Dev Dyn 242, 580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, and Klagsbrun M (1999). Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol 146, 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, and Fischman DA (1992). Retroviral analysis of cardiac morphogenesis: discontinuous formation of coronary vessels. Proc Natl Acad Sci U S A 89, 9504–9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, and Gourdie RG (1996). Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol 174, 221–232. [DOI] [PubMed] [Google Scholar]
- Muders MH (2011). Neuropilin and neuropilin associated molecules as new molecular targets in pancreatic adenocarcinoma. Anticancer Agents Med Chem 11, 442–447. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Chayama K, Ochi H, Toshishige M, Hayashida Y, Miki D, Hayes CN, Suzuki H, Tokuyama T, Oda N, et al. (2013). A nonsynonymous polymorphism in semaphorin 3A as a risk factor for human unexplained cardiac arrest with documented ventricular fibrillation. PLoS Genet 9, e1003364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, and Kessler O (2008). The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer 8, 632–645. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Kessler O, and Herzog Y (2002). The interaction of Neuropilin-1 and Neuropilin-2 with tyrosine-kinase receptors for VEGF. Adv Exp Med Biol 515, 81–90. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Sabag AD, Rabinovicz N, and Kessler O (2012). Semaphorins in angiogenesis and tumor progression. Cold Spring Harb Perspect Med 2, a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin E, and Jenni R (2011). Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur Heart J 32, 1446–1456. [DOI] [PubMed] [Google Scholar]
- Ohta K, Mizutani A, Kawakami A, Murakami Y, Kasuya Y, Takagi S, Tanaka H, and Fujisawa H (1995). Plexin: a novel neuronal cell surface molecule that mediates cell adhesion via a homophilic binding mechanism in the presence of calcium ions. Neuron 14, 1189–1199. [DOI] [PubMed] [Google Scholar]
- Opthof T, Misier AR, Coronel R, Vermeulen JT, Verberne HJ, Frank RG, Moulijn AC, van Capelle FJ, and Janse MJ (1991). Dispersion of refractoriness in canine ventricular myocardium. Effects of sympathetic stimulation. Circ Res 68, 1204–1215. [DOI] [PubMed] [Google Scholar]
- Paterick TE, and Tajik AJ (2012). Left ventricular noncompaction: a diagnostically challenging cardiomyopathy. Circ J 76, 1556–1562. [DOI] [PubMed] [Google Scholar]
- Pierpont GL, DeMaster EG, Reynolds S, Pederson J, and Cohn JN (1985). Ventricular myocardial catecholamines in primates. J Lab Clin Med 106, 205–210. [PubMed] [Google Scholar]
- Red-Horse K, Ueno H, Weissman IL, and Krasnow MA (2010). Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolio S, and Tamagnone L (2011). Multifaceted role of neuropilins in cancer. Curr Med Chem 18, 3563–3575. [DOI] [PubMed] [Google Scholar]
- Rohm B, Rahim B, Kleiber B, Hovatta I, and Puschel AW (2000). The semaphorin 3A receptor may directly regulate the activity of small GTPases. FEBS Lett 486, 68–72. [DOI] [PubMed] [Google Scholar]
- Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, and Bagnard D (2009). The many faces of semaphorins: from development to pathology. Cell Mol Life Sci 66, 649–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Molliver ME, Ginty DD, and Kolodkin AL (2003). Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci 23, 6671–6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Doci CL, and Gutkind JS (2012). Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell research 22, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra M, Ohnuki H, Maric D, Salvucci O, Hou X, Kumar A, Li X, and Tosato G (2012). Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood 120, 4104–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. (2003). Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391–397. [DOI] [PubMed] [Google Scholar]
- Shen J, Samul R, Zimmer J, Liu H, Liang X, Hackett S, and Campochiaro PA (2004). Deficiency of neuropilin 2 suppresses VEGF-induced retinal neovascularization. Mol Med 10, 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold C, and Jones EY (2013). Structural insights into semaphorins and their receptors. Semin Cell Dev Biol 24, 139–145. [DOI] [PubMed] [Google Scholar]
- Singh MK, and Epstein JA (2012). Epicardium-derived cardiac mesenchymal stem cells: expanding the outer limit of heart repair. Circ Res 110, 904–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Lu MM, Massera D, and Epstein JA (2011). MicroRNA-processing enzyme Dicer is required in epicardium for coronary vasculature development. J Biol Chem 286, 41036–41045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, and Klagsbrun M (1998). Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92, 735–745. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, and Epstein JA (2005). Cardiac neural crest. Semin Cell Dev Biol 16, 704–715. [DOI] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, et al. (2007). Interactions between plexin-A2, plexin-A4, and semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron 53, 535–547. [DOI] [PubMed] [Google Scholar]
- Ta-Shma A, Pierri CL, Stepensky P, Shaag A, Zenvirt S, Elpeleg O, and Rein AJ (2013). Isolated truncus arteriosus associated with a mutation in the plexin-D1 gene. Am J Med Genet A 161A, 3115–3120. [DOI] [PubMed] [Google Scholar]
- Takahashi T, and Strittmatter SM (2001). Plexina1 autoinhibition by the plexin sema domain. Neuron 29, 429–439. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, et al. (2010). Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol 11, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Kitakaze M, Asakura M, Asanuma H, Sanada S, Tashiro F, Niwa H, Miyazaki Ji J, Hirota S, Kitamura Y, et al. (2002). Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A 99, 3657–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, et al. (2006). Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 8, 615–622. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. (1999). Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99, 71–80. [DOI] [PubMed] [Google Scholar]
- Tammela T, and Alitalo K (2010). Lymphangiogenesis: Molecular mechanisms and future promise. Cell 140, 460–476. [DOI] [PubMed] [Google Scholar]
- Teekakirikul P, Kelly MA, Rehm HL, Lakdawala NK, and Funke BH (2013). Inherited cardiomyopathies: molecular genetics and clinical genetic testing in the postgenomic era. J Mol Diagn 15, 158–170. [DOI] [PubMed] [Google Scholar]
- Tong Y, and Buck M (2005). 1H, 15N and 13C Resonance assignments and secondary structure determination reveal that the minimal Rac1 GTPase binding domain of plexin-B1 has a ubiquitin fold. J Biomol NMR 31, 369–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Chugha P, Hota PK, Alviani RS, Li M, Tempel W, Shen L, Park HW, and Buck M (2007). Binding of Rac1, Rnd1, and RhoD to a novel Rho GTPase interaction motif destabilizes dimerization of the plexin-B1 effector domain. J Biol Chem 282, 37215–37224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, and Hori M (2007). Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. Embo J 26, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Yamamoto M, Makino N, Takamatsu H, Takegahara N, Suto F, Hori M, Fujisawa H, et al. (2008). Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev Biol 321, 251–262. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, et al. (2004a). Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev 18, 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Yabuki M, Harada K, Hori M, and Kikutani H (2004b). Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat Cell Biol 6, 1204–1211. [DOI] [PubMed] [Google Scholar]
- Varshavsky A, Kessler O, Abramovitch S, Kigel B, Zaffryar S, Akiri G, and Neufeld G (2008). Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res 68, 6922–6931. [DOI] [PubMed] [Google Scholar]
- Vieira JM, Schwarz Q, and Ruhrberg C (2007). Role of the neuropilin ligands VEGF164 and SEMA3A in neuronal and vascular patterning in the mouse. Novartis Found Symp 283, 230–235; discussion 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, Jiang J, Cowan CW, and Zhang X (2012). Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal 5, ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, and Taniguchi M (2008). PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci U S A 105, 2993–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worzfeld T, Swiercz JM, Senturk A, Genz B, Korostylev A, Deng S, Xia J, Hoshino M, Epstein JA, Chan AM, et al. (2014). Genetic dissection of plexin signaling in vivo. Proc Natl Acad Sci U S A 111, 2194–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, and Eichmann A (2002). Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 129, 4797–4806. [DOI] [PubMed] [Google Scholar]
- Yukawa K, Tanaka T, Kishino M, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, and Kumanogoh A (2010). Deletion of Sema4D gene reduces intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Int J Mol Med 26, 39–44. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Singh MK, Degenhardt KR, Lu MM, Bennett J, Yoshida Y, and Epstein JA (2009). Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev Biol 325, 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, et al. (2011). Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 121, 1894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Stalker TJ, Fong KP, Jiang H, Tran A, Crichton I, Lee EK, Neeves KB, Maloney SF, Kikutani H, et al. (2009). Disruption of SEMA4D ameliorates platelet hypersensitivity in dyslipidemia and confers protection against the development of atherosclerosis. Arterioscler Thromb Vasc Biol 29, 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipes DP (2008). Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleve Clin J Med 75 Suppl 2, S94–96. [DOI] [PubMed] [Google Scholar]
- Zygmunt T, Gay CM, Blondelle J, Singh MK, Flaherty KM, Means PC, Herwig L, Krudewig A, Belting HG, Affolter M, et al. (2011). Semaphorin-PlexinD1 signaling limits angiogenic potential via the VEGF decoy receptor sFlt1. Dev Cell 21, 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]


