Abstract
Background
Safe and effective vaccines against coronavirus disease 2019 (Covid-19) are urgently needed in young children.
Methods
We conducted a phase 1 dose-finding study and are conducting an ongoing phase 2–3 safety, immunogenicity, and efficacy trial of the BNT162b2 vaccine in healthy children 6 months to 11 years of age. We present results for children 6 months to less than 2 years of age and those 2 to 4 years of age through the data-cutoff dates (April 29, 2022, for safety and immunogenicity and June 17, 2022, for efficacy). In the phase 2–3 trial, participants were randomly assigned (in a 2:1 ratio) to receive two 3-μg doses of BNT162b2 or placebo. On the basis of preliminary immunogenicity results, a third 3-μg dose (≥8 weeks after dose 2) was administered starting in January 2022, which coincided with the emergence of the B.1.1.529 (omicron) variant. Immune responses at 1 month after doses 2 and 3 in children 6 months to less than 2 years of age and those 2 to 4 years of age were immunologically bridged to responses after dose 2 in persons 16 to 25 years of age who received 30 μg of BNT162b2 in the pivotal trial.
Results
During the phase 1 dose-finding study, two doses of BNT162b2 were administered 21 days apart to 16 children 6 months to less than 2 years of age (3-μg dose) and 48 children 2 to 4 years of age (3-μg or 10-μg dose). The 3-μg dose level was selected for the phase 2–3 trial; 1178 children 6 months to less than 2 years of age and 1835 children 2 to 4 years of age received BNT162b2, and 598 and 915, respectively, received placebo. Immunobridging success criteria for the geometric mean ratio and seroresponse at 1 month after dose 3 were met in both age groups. BNT162b2 reactogenicity events were mostly mild to moderate, with no grade 4 events. Low, similar incidences of fever were reported after receipt of BNT162b2 (7% among children 6 months to <2 years of age and 5% among those 2 to 4 years of age) and placebo (6 to 7% among children 6 months to <2 years of age and 4 to 5% among those 2 to 4 years of age). The observed overall vaccine efficacy against symptomatic Covid-19 in children 6 months to 4 years of age was 73.2% (95% confidence interval, 43.8 to 87.6) from 7 days after dose 3 (on the basis of 34 cases).
Conclusions
A three-dose primary series of 3-μg BNT162b2 was safe, immunogenic, and efficacious in children 6 months to 4 years of age. (Funded by BioNTech and Pfizer; ClinicalTrials.gov number, NCT04816643.)
Although coronavirus disease 2019 (Covid-19) is generally mild in children younger than 5 years of age, severe disease, hospitalizations, and postacute effects, including multisystem inflammatory syndrome in children (MIS-C), can occur.1-3 In the United States, rates of Covid-19−associated hospitalization among children younger than 5 years of age peaked at 14.5 per 100,000 in January 2022 during the early period of predominance of the B.1.1.529 (omicron) variant; this was approximately five times the rate during the period of predominance of the B.1.617.2 (delta) variant in 2021.4 It is notable that 63% of infants and children who had Covid-19–associated hospitalizations in 2022 did not have underlying medical conditions.4 Young children may also play an important role in spreading highly transmissible variants.5,6 Therefore, having safe and effective vaccines for children younger than 5 years of age is critical in curtailing the pandemic.
The BNT162b2 vaccine (Pfizer–BioNTech) has U.S. licensure for immunization against Covid-19 in persons 12 years of age or older and emergency use authorization in children 5 to 11 years of age.7,8 Results from ongoing phase 1 studies and phase 2–3 clinical trials involving healthy persons 5 years of age or older support the safety, immunogenicity, and efficacy of BNT162b2 administered as a two-dose series 21 days apart.9-12 A third dose (booster) is also authorized for persons 5 years of age or older.7 Because a two-dose primary series did not meet all criteria for immunobridging success in children 2 to 4 years of age and because emerging evidence indicates that three messenger RNA (mRNA) vaccine doses are needed to enhance immune responses against the omicron variant,13 we studied a third 3-μg BNT162b2 dose given at least 8 weeks after dose 2 in children 6 months to 4 years of age.
Methods
Objectives, Participants, and Oversight
The phase 1 dose-finding study and ongoing phase 2–3 safety, immunogenicity, and efficacy trial investigated BNT162b2 in healthy children 6 months to 11 years of age. Safety and immunogenicity results are presented for children 6 months to 4 years of age through the data-cutoff date (April 29, 2022). For the assessment of vaccine efficacy, we used a data-cutoff date of June 17, 2022. Participants in the phase 2–3 trial will be followed for 12 to 18 months after dose 3; follow-up will include monitoring for potential cases of Covid-19 and MIS-C. Details on eligibility criteria and ethical study conduct are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org. The protocol, which contains additional details, is also available at NEJM.org.
Pfizer was responsible for the design and conduct of the study; the collection, analysis, and interpretation of the data; and the writing of the manuscript. Pfizer and BioNTech manufactured BNT162b2 and placebo. BioNTech was the regulatory sponsor and contributed to data interpretation and manuscript writing. All data were available to the authors, who vouch for the accuracy and completeness of the data and for the adherence of the trial to the protocol.
Procedures
Phase 1, Open-Label, Dose-Finding Study
A vaccination regimen involving intramuscular injection (anterior thigh for children 6 months to <2 years of age and deltoid for those 2 to 4 years of age) of two BNT162b2 doses administered 21 days apart was initiated at a 10-μg dose level in children 2 to 4 years of age and subsequently at the 3-μg dose level in children 6 months to less than 2 years of age on the basis of the acceptable safety and immunogenicity of the 30-μg dose level in children 12 to 15 years of age (see the Supplementary Appendix for safety data review and stopping rules).9,11 On the basis of safety data as well as immunogenicity results at 1 week after dose 2, the 3-μg dose level was selected for all children 6 months to 4 years of age in the phase 2–3 trial.
Phase 2–3 Trial of the Selected Dose
Starting on June 21, 2021, participants were randomly assigned through an interactive Web-based system in a 2:1 ratio to receive two doses of 3-μg BNT162b2 or saline placebo 21 days apart. The third dose was administered at least 60 days after dose 2 for children 6 months to 4 years of age in January 2022. Data on children who reached their fifth birthday were unblinded, and these children received the 10-μg dose level for dose 2 or 3 on the basis of the emergency use authorization (these data are not included here). Participants and trial personnel, apart from those preparing or administering injections, were not aware of the trial-group assignments.
Immunogenicity
For all the participants in the phase 1 study and a subgroup of participants in the phase 2–3 trial (see the Supplementary Appendix), blood samples were collected for immunogenicity assessments, which included determination of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralization titers, as described previously.12,14,15 Neutralizing titers were measured for ancestral SARS-CoV-2 (USA-WA1/2020) (participants in the phase 1 study and the phase 2–3 trial) and omicron BA.1 variants (participants in the phase 2–3 trial).
Immunogenicity was analyzed in participants who were without serologic or virologic evidence of previous SARS-CoV-2 infection. In phase 1, SARS-CoV-2 neutralizing geometric mean titers (GMTs) were measured in serum samples that were obtained 7 days after dose 2. In phase 2–3, GMTs were measured before doses 1 and 3 and 1 month after doses 2 and 3. We calculated ratios of GMTs (i.e., geometric mean ratios) 1 month after doses 2 and 3 among children 6 months to less than 2 years of age and children 2 to 4 years of age in phase 2–3 as compared with GMTs 1 month after dose 2 among persons 16 to 25 years of age from the pivotal trial.10 Serum samples for each comparison were assayed in parallel to ensure comparability of titers. We also calculated differences in seroresponse (defined in the Supplementary Appendix) among children 6 months to less than 2 years of age and children 2 to 4 years of age 1 month after doses 2 and 3 as compared with seroresponse among persons 16 to 25 years of age 1 month after dose 2, as well as geometric mean fold rises from baseline to each subsequent time point after doses 2 and 3. Post hoc immunogenicity analyses included an assessment of SARS-CoV-2 neutralizing titers 1 month after dose 2 in a subgroup of children 2 to 4 years of age as compared with titers among persons 65 years of age or older from the pivotal trial.
Safety
Information on age-specific reactogenicity events was recorded by parents or guardians in an e-diary for 7 days after each dose. Data on adverse events were collected through 1 month after dose 2 for phase 1 and through 1 month after dose 3 for phase 2–3. Data on serious adverse events are being collected through 6 months after dose 3. Details about monitoring of potential cases of myocarditis or pericarditis are provided in the Supplementary Appendix.
Efficacy
Vaccine efficacy against the first occurrence of Covid-19 from at least 7 days after dose 3 to the data-cutoff date (June 17, 2022) was calculated. The methods that were used to identify SARS-CoV-2 infections, Covid-19, and MIS-C are outlined in the Supplementary Appendix.
Statistical Analysis
Study populations are defined in Table S1 in the Supplementary Appendix. In the phase 2–3 trial, effectiveness was inferred by an “immunobridging” approach, in which neutralizing titers elicited by 3 μg of BNT162b2 against the ancestral SARS-CoV-2 strain in a subgroup of children 6 months to less than 2 years of age and children 2 to 4 years of age were compared with titers elicited by 30 μg of BNT162b2 in a random sample of persons 16 to 25 years of age from the pivotal trial (see the Supplementary Appendix). Immunobridging success based on the geometric mean ratio of neutralizing titers of serum samples drawn 1 month after doses 2 and 3 was declared if the lower boundary of the 95% confidence interval for the geometric mean ratio (children 6 months to <2 years of age or children 2 to 4 years of age after dose 2 or after dose 3 as compared with persons 16 to 25 years of age after dose 2) was more than 0.67 and if the point estimate for the geometric mean ratio was at least 0.8 (specified in the protocol) or at least 1.0 (requested by the Food and Drug Administration [FDA]). Immunobridging success also required a lower boundary of the 95% confidence interval for the difference in the percentage of participants with seroresponse (children 6 months to <2 years of age or those 2 to 4 years of age as compared with persons 16 to 25 years of age) of greater than –10 percentage points. Immunobridging criteria were based on regulatory authority guidance, as previously reported for children 5 to 11 years of age.11 Calculations of sample size are provided in the Supplementary Appendix.
Safety end points are presented descriptively as counts and percentages with two-sided 95% Clopper–Pearson confidence intervals. Adverse events and serious adverse events are presented according to the terms in the Medical Dictionary for Regulatory Activities, version 25.0.
Vaccine efficacy, defined as 100×(1–illness rate ratio), was assessed for age groups in which immunobridging was successful after at least 21 cases of Covid-19 had accrued from at least 7 days after dose 3. The vaccine was considered to be effective if the lower boundary of the 95% confidence interval for vaccine efficacy was more than 30%. Efficacy evaluations for children 6 months to less than 2 years of age and those 2 to 4 years of age included both age groups for hypothesis testing and were performed separately in the two age groups for descriptive summaries because the same dose level was selected for both groups and both age groups met the criteria for immunobridging success. The illness rate ratio was the ratio of the rate of first cases of confirmed Covid-19 in the BNT162b2 group to the corresponding rate in the placebo group. Two-sided 95% confidence intervals for vaccine efficacy were derived with the use of the Clopper–Pearson method, with adjustment for surveillance time (total time in 1000 person-years for the given end point across all participants within each group at risk for the end point). The 95% confidence intervals were not adjusted for multiplicity. The hypotheses for immunobridging and vaccine efficacy were evaluated sequentially to control the type I error rate. Immunobridging based on geometric mean ratio and difference in seroresponse was assessed sequentially in the order specified for each age group. Immunobridging for the two age groups was assessed separately and not adjusted for multiplicity. The hypothesis for vaccine efficacy was tested if immunobridging criteria for the geometric mean ratio and seroresponse were met for both age groups.
Results
Participants
Phase 1
From April 5, 2021, a total of 16 children 6 months to less than 2 years of age and 48 children 2 to 4 years of age received two BNT162b2 doses (Fig. S1). Overall, 59% were male; 81% were White, and 6% were Hispanic or Latinx. The median age was 15.5 months among children 6 months to less than 2 years of age and 3.0 years among children 2 to 4 years of age (Table S2).
Phase 2–3
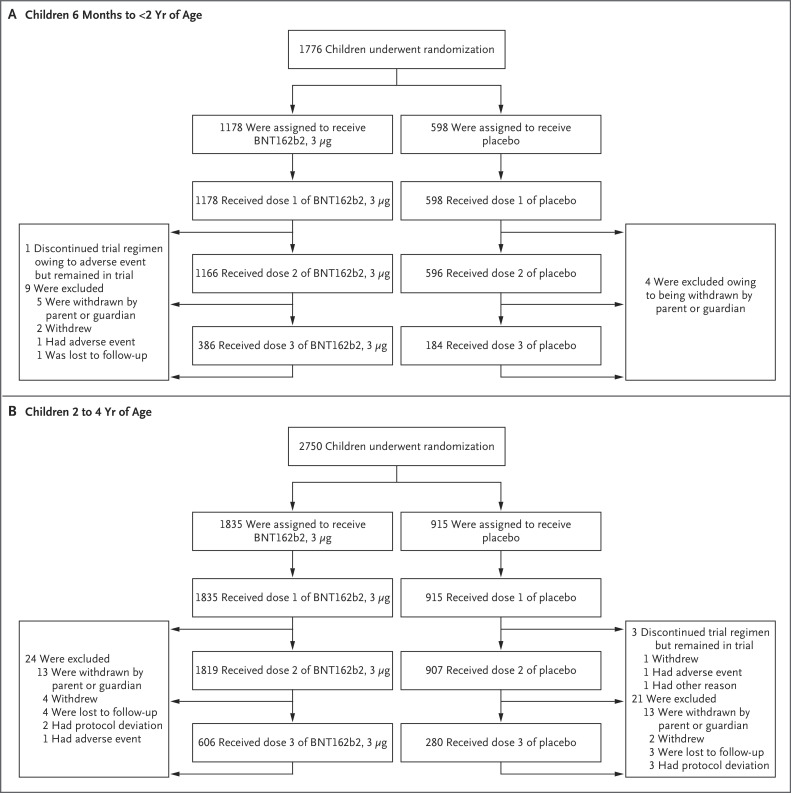
From June 21, 2021, a total of 1776 children 6 months to less than 2 years of age and 2750 children 2 to 4 years of age underwent randomization; 1178 and 1835, respectively, received BNT162b2, and 598 and 915, respectively, received placebo (Figure 1). More than 99% of the participants received dose 2. At the data-cutoff date (with recruitment and administration ongoing), some participants had not yet received dose 2, and 32.2% had received dose 3 (see Table 1 for time between dose 2 and dose 3). The median blinded follow-up after dose 3 was 1.3 months (range, 0 to 3.2) among children 6 months to less than 2 years of age and 1.4 months (range, 0 to 3.2) among children 2 to 4 years of age; 31.8% of children 6 months to less than 2 years of age and 35.0% of children 2 to 4 years of age had at least 2 months of follow-up safety data available after dose 3.
Figure 1. Screening, Randomization, and Vaccine and Placebo Administration in the Phase 2–3 Trial.
At the data-cutoff date (April 29, 2022), the trial was ongoing, and the analyses included all the participants who had received the first dose by March 31, 2022. Therefore, some participants had not received dose 2, and many had not received dose 3 by the data-cutoff date. The phase 2–3 trial was conducted across 65 sites in Brazil, Finland, Poland, Spain, and the United States. Withdrawal by participant describes the inability of the participant to complete trial procedures or visits.
Table 1. Demographic and Clinical Characteristics of the Participants in the Phase 2–3 Trial.*.
| Characteristic | Children 6 Mo to <2 Yr of Age | Children 2 to 4 Yr of Age | ||||
|---|---|---|---|---|---|---|
| BNT162b2 (N=1178) |
Placebo (N=598) |
Total (N=1776) |
BNT162b2 (N=1835) |
Placebo (N=915) |
Total (N=2750) |
|
| Age at vaccination | ||||||
| Mean | 15.2±5.0 mo | 15.4±5.1 mo | 15.3±5.0 mo | 3.0±0.8 yr | 3.0±0.8 yr | 3.0±0.8 yr |
| Median (range) | 16.0 mo (6–23) | 16.0 mo (6–23) | 16.0 mo (6–23) | 3.0 yr (2–4) | 3.0 yr (2–4) | 3.0 yr (2–4) |
| Male sex — no. (%) | 589 (50.0) | 291 (48.7) | 880 (49.5) | 901 (49.1) | 471 (51.5) | 1372 (49.9) |
| Race or ethnic group — no. (%)† | ||||||
| White | 922 (78.3) | 480 (80.3) | 1402 (78.9) | 1469 (80.1) | 720 (78.7) | 2189 (79.6) |
| Black | 42 (3.6) | 24 (4.0) | 66 (3.7) | 94 (5.1) | 41 (4.5) | 135 (4.9) |
| American Indian or Alaska Native | 3 (0.3) | 1 (0.2) | 4 (0.2) | 3 (0.2) | 4 (0.4) | 7 (0.3) |
| Asian | 91 (7.7) | 40 (6.7) | 131 (7.4) | 127 (6.9) | 76 (8.3) | 203 (7.4) |
| Native Hawaiian or other Pacific Islander | 0 | 0 | 0 | 2 (0.1) | 1 (0.1) | 3 (0.1) |
| Multiracial | 117 (9.9) | 49 (8.2) | 166 (9.3) | 131 (7.1) | 69 (7.5) | 200 (7.3) |
| Not reported | 3 (0.3) | 4 (0.7) | 7 (0.4) | 9 (0.5) | 4 (0.4) | 13 (0.5) |
| Hispanic or Latinx ethnic group — no. (%)† | ||||||
| Yes | 161 (13.7) | 64 (10.7) | 225 (12.7) | 264 (14.4) | 120 (13.1) | 384 (14.0) |
| No | 1014 (86.1) | 530 (88.6) | 1544 (86.9) | 1568 (85.4) | 795 (86.9) | 2363 (85.9) |
| Not reported‡ | — | — | 7 (0.4) | — | — | 3 (0.1) |
| Country — no. (%) | ||||||
| Brazil‡ | — | — | 2 (0.1) | 0 | 0 | 0 |
| Finland | 54 (4.6) | 26 (4.3) | 80 (4.5) | 63 (3.4) | 30 (3.3) | 93 (3.4) |
| Poland | 125 (10.6) | 63 (10.5) | 188 (10.6) | 205 (11.2) | 103 (11.3) | 308 (11.2) |
| Spain | 42 (3.6) | 22 (3.7) | 64 (3.6) | 73 (4.0) | 35 (3.8) | 108 (3.9) |
| United States | 957 (81.2) | 485 (81.1) | 1442 (81.2) | 1494 (81.4) | 747 (81.6) | 2241 (81.5) |
| Baseline SARS-CoV-2 infection status — no. (%)§ | ||||||
| Positive | 89 (7.6) | 44 (7.4) | 133 (7.5) | 233 (12.7) | 125 (13.7) | 358 (13.0) |
| Negative | 1078 (91.5) | 541 (90.5) | 1619 (91.2) | 1597 (87.0) | 783 (85.6) | 2380 (86.5) |
| Missing data | 11 (0.9) | 13 (2.2) | 24 (1.4) | 5 (0.3) | 7 (0.8) | 12 (0.4) |
| Obesity — no. (%)¶ | ||||||
| Yes | — | — | — | 120 (6.5) | 45 (4.9) | 165 (6.0) |
| No | — | — | — | 1712 (93.3) | 870 (95.1) | 2582 (93.9) |
| Missing data‡ | — | — | — | — | — | 3 (0.1) |
| Coexisting conditions — no. (%)‖ | ||||||
| Yes | 50 (4.2) | 34 (5.7) | 84 (4.7) | 222 (12.1) | 130 (14.2) | 352 (12.8) |
| No | 1128 (95.8) | 564 (94.3) | 1692 (95.3) | 1613 (87.9) | 785 (85.8) | 2398 (87.2) |
| Time between dose 2 and dose 3 — no. (%) | ||||||
| <8 wk | 0 | 0 | 0 | 2 (0.1) | 2 (0.2) | 4 (0.1) |
| 8–12 wk | 145 (12.3) | 76 (12.7) | 221 (12.4) | 405 (22.1) | 184 (20.1) | 589 (21.4) |
| 13–16 wk | 51 (4.3) | 27 (4.5) | 78 (4.4) | 89 (4.9) | 37 (4.0) | 126 (4.6) |
| 17–20 wk | 54 (4.6) | 21 (3.5) | 75 (4.2) | 21 (1.1) | 13 (1.4) | 34 (1.2) |
| 21–24 wk | 53 (4.5) | 33 (5.5) | 86 (4.8) | 45 (2.5) | 19 (2.1) | 64 (2.3) |
| 25–28 wk | 246 (20.9) | 24 (4.0) | 270 (15.2) | 215 (11.7) | 21 (2.3) | 236 (8.6) |
| 29–32 wk | 129 (11.0) | 2 (0.3) | 131 (7.4) | 169 (9.2) | 4 (0.4) | 173 (6.3) |
| 33–44 wk‡ | — | — | 81 (4.6) | — | — | 95 (3.5) |
Plus–minus values are means ±SD. Results are shown for the safety population (children who received at least one dose of BNT162b2 or placebo). Percentages may not total 100 because of rounding.
Race and ethnic group were reported by the parents or guardians.
Because of blinding, only the values for the total population are shown.
A positive status for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) required a positive N-binding antibody test at the first vaccination visit, a positive nucleic acid amplification test at the first vaccination visit, or a medical history of coronavirus disease 2019 (Covid-19).
Obesity is defined for children 2 to 4 years of age as a body-mass index (the weight in kilograms divided by the square of the height in meters) at or above the 95th percentile according to the Centers for Disease Control and Prevention growth charts (https://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm). Obesity is not defined for children younger than 2 years of age.
Coexisting conditions are those that increase the risk of severe Covid-19 (i.e., one or more prespecified underlying conditions defined by Kim et al.16). The most common medical history associated with increased risk of severe Covid-19 were obesity (2 to 4 years of age, 6.0%), asthma (6 months to <2 years of age, 0.8%; 2 to 4 years of age, 3.5%), and premature birth (6 months to <2 years of age, 1.7%; 2 to 4 years of age, 1.1%).
Half the participants were male; 79.3% were White, 4.4% Black, and 13.5% Hispanic or Latinx (Table 1). Among children 6 months to less than 2 years of age, the median age was 16.0 months, 4.7% had coexisting conditions, and 7.5% were SARS-CoV-2–positive at baseline. Corresponding values among children 2 to 4 years of age were 3.0 years, 12.8%, and 13.0%. Demographic characteristics were generally representative of the expected participant population (Table S3).
Phase 1 Dose Finding
For children 2 to 4 years of age, higher frequencies and greater severity of reactogenicity to the BNT162b2 10-μg dose level than to the 3-μg dose level were observed; fever was reported in approximately 19% of the participants who received 10 μg for both doses 1 and 2 (Fig. S2). Because postvaccination immunogenicity after dose 2 for the ancestral strain across dose levels was similar to that in older age groups (Fig. S3), a 3-μg dose level was selected for children 2 to 4 years of age. The reactogenicity profile of the 10-μg dose level in children 2 to 4 years of age resulted in forgoing evaluation of that dose level in children 6 months to less than 2 years of age. In children 6 months to less than 2 years of age, because immune responses at the 3-μg dose level were similar to those observed in children 2 to 4 years of age, and in light of the favorable safety and reactogenicity profile of the 3-μg dose level in this age group (Table S5), the 3-μg dose level was selected for phase 2–3 evaluation.
Phase 2–3 Immunogenicity
Immunobridging data after dose 2 met success criteria in children 6 months to less than 2 years of age and met success criteria for seroresponse but not for GMT in children 2 to 4 years of age (Table 2). Therefore, we assessed a third primary dose for both age groups.
Table 2. Results of Serum SARS-CoV-2 Neutralization Assay for the Ancestral Strain.*.
| Timing of Assay and Age Group | BNT162b2 Dose Level | No. of Participants | Geometric Mean 50% Neutralizing Titer (95% CI)† | Geometric Mean Ratio vs. 16 to 25 Yr of Age (95% CI)‡ | Seroresponse (95% CI)§ | Difference in Seroresponse vs. 16 to 25 Yr of Age (95% CI)¶ |
|---|---|---|---|---|---|---|
| μg | percent | percentage points | ||||
| 1 mo after BNT162b2 dose 3 (6 mo to 4 yr of age) or dose 2 (16 to 25 yr of age) | ||||||
| 6 mo to <2 yr | 3 | 80 to 82 | 1406.5 (1211.3 to 1633.1) |
1.19 (1.00 to 1.42) |
100.0 (95.5 to 100.0) |
1.2 (–3.4 to 4.2) |
| 2 to 4 yr | 3 | 141 to 143 | 1535.2 (1388.2 to 1697.8) |
1.30 (1.13 to 1.50) |
100.0 (97.4 to 100.0) |
1.2 (–1.5 to 4.2) |
| 16 to 25 yr | 30 | 170 | 1180.0 (1066.6 to 1305.4) |
— | 98.8 (95.8 to 99.9) |
— |
| 1 mo after BNT162b2 dose 2 (all age groups) | ||||||
| 6 mo to <2 yr and 16 to 25 yr | ||||||
| 6 mo to <2 yr | 3 | 245 | 979.7 (893.2 to 1074.6) |
1.03 (0.90 to 1.19) |
98.0 (95.3 to 99.3) |
1.7 (–1.4 to 5.2) |
| 16 to 25 yr | 30 | 238 | 946.8 (850.8 to 1053.7) |
— | 96.2 (92.9 to 98.3) |
— |
| 2 to 4 yr and 16 to 25 yr | ||||||
| 2 to 4 yr | 3 | 243 | 763.9 (688.5 to 847.5) |
0.61 (0.53 to 0.70) |
96.7 (93.6 to 98.6) |
–0.9 (–4.3 to 2.3) |
| 16 to 25 yr | 30 | 251 or 252 | 1255.4 (1131.2 to 1393.3) |
— | 97.6 (94.9 to 99.1) |
— |
Results are for the dose 3 and dose 2 immunogenicity population that could be evaluated of the immunobridging subgroup for participants 6 months to 4 years of age and the dose 2 immunogenicity population that could be evaluated for participants 16 to 25 years of age (Table S1) who had no serologic or virologic evidence of past or current SARS-CoV-2 infection up to the visit 1 month after dose 2 (16 to 25 years of age and 6 months to 4 years of age) or the visit 1 month after dose 3 (6 months to 4 years of age) and who had no history of Covid-19. Further details on the dose 3 population that could be evaluated and the timing intervals for dose 3 administration are provided in the Supplementary Appendix. Missing immunogenicity data were not imputed.
Geometric mean titers and two-sided 95% confidence intervals (CIs) were calculated by exponentiation of the mean logarithm of the titers and the corresponding confidence intervals (based on Student’s t distribution). Assay results below the lower limit of quantitation were set to 0.5 times the lower limit of quantitation.
The geometric mean ratio and two-sided 95% confidence intervals were calculated by exponentiation of the mean difference of the logarithms of the titers (children 6 months to <2 years of age minus persons 16 to 25 years of age, and children 2 to 4 years of age minus persons 16 to 25 years of age) and the corresponding confidence intervals (based on Student’s t distribution). The immunobridging criterion was met because the lower boundary of the two-sided confidence interval for the geometric mean ratio was more than 0.67, and the point estimate of the geometric mean ratio was at least 0.8.
Confidence intervals were calculated with the Clopper–Pearson method.
Confidence intervals were calculated with the Miettinen–Nurminen method. Immunobridging success also required a lower boundary of the 95% confidence interval for the difference in percentages of participants (between children 6 months to <2 years of age or those 2 to 4 years of age and persons 16 to 25 years of age) with a seroresponse to be greater than −10 percentage points.
Geometric mean ratios of neutralizing GMTs for 3 μg of BNT162b2 among children 6 months to less than 2 years of age and children 2 to 4 years of age 1 month after dose 3 to GMTs for 30 μg of BNT162b2 among persons 16 to 25 years of age 1 month after dose 2 were 1.19 (95% confidence interval [CI], 1.00 to 1.42) and 1.30 (95% CI, 1.13 to 1.50), respectively (Table 2). These findings met the immunobridging criterion of a lower boundary of the two-sided 95% confidence interval of more than 0.67; they also met the prespecified and FDA-requested point estimates for the geometric mean ratio of at least 0.8 and at least 1.0, respectively. The differences in the percentages of children 6 months to less than 2 years of age and children 2 to 4 years of age who had a seroresponse after dose 3 and the percentage of persons 16 to 25 years of age who had a seroresponse after dose 2 were 1.2 percentage points (95% CI, −3.4 to 4.2) and 1.2 percentage points (95% CI, −1.5 to 4.2), respectively; these findings also met this immunobridging criterion.
The serum-neutralizing GMTs against ancestral SARS-CoV-2 were 1407 among children 6 months to less than 2 years of age and 1535 among children 2 to 4 years of age 1 month after dose 3, as compared with 1180 among persons 16 to 25 years of age 1 month after dose 2 (Fig. S4). The geometric mean fold rises from before vaccination to 1 month after dose 3 were 68.4 among children 6 months to less than 2 years of age and 73.3 among children 2 to 4 years of age, as compared with a fold rise of 55.3 from before vaccination to 1 month after dose 2 among persons 16 to 25 years of age. In an exploratory analysis, omicron BA.1 SARS-CoV-2 serum-neutralizing GMTs in response to a third 3-μg BNT162b2 dose among 32 children 6 months to less than 2 years of age and 34 children 2 to 4 years of age were 129 and 114, respectively, as compared with 164 among adults 18 to 50 years of age who received a third 30-μg BNT162b2 dose at a similar time interval after dose 2 as the children in this trial — approximately 3 months (Fig. S5).
Ancestral SARS-CoV-2 serum-neutralizing GMTs 1 month after dose 2 in a subgroup of children 2 to 4 years of age and among adults 65 years of age who received two BNT162b2 doses were 830.0 and 431.8, respectively. The geometric mean ratio was 1.92 (two-sided 95% CI, 1.56 to 2.36).
Phase 2–3 Safety
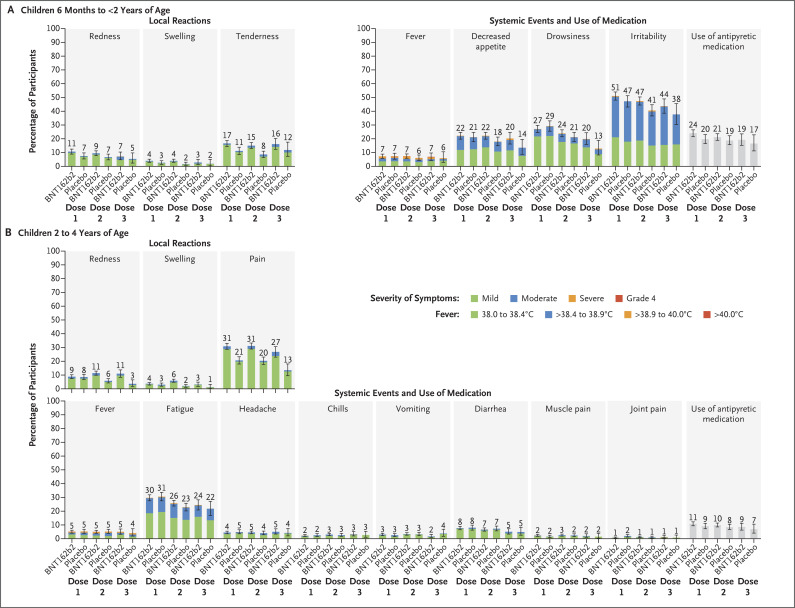
Local reactions were reported more frequently after BNT162b2 than after placebo, with most being mild to moderate; severe reactions after any dose were reported infrequently (≤0.3%) (Figure 2). No grade 4 local reactions were reported. Tenderness was the most frequent local reaction among children 6 months to less than 2 years of age, and pain was the most frequent among children 2 to 4 years of age; swelling and redness were less frequent. The median time of onset for all local reactions was 1 or 2 days after receipt of BNT162b2 or placebo; most reactions resolved within 1 to 2 days. The incidences of local reactions after dose 3 were generally similar to or lower than those after dose 1 or after dose 2.
Figure 2. Local Reactions and Systemic Events Reported within 7 Days after Administration of BNT162b2 or Placebo in the Phase 2–3 Trial.
Results are for the blinded, placebo-controlled follow-up period. Shown are local reactions and systemic events after dose 1, dose 2, and dose 3 in BNT162b2 recipients (children 6 months to <2 years of age: dose 1, 1159 to 1173 participants; dose 2, 1137 to 1147 participants; and dose 3, 362 to 365 participants; and children 2 to 4 years of age: dose 1, 1813 to 1825 participants; dose 2, 1772 to 1779 participants; and dose 3, 547 to 552 participants) and in placebo recipients (children 6 months to <2 years of age: dose 1, 591 to 595 participants; dose 2, 590 or 591 participants; and dose 3, 170 participants; and children 2 to 4 years of age: dose 1, 905 to 909 participants; dose 2, 877 or 878 participants; and dose 3, 262 participants). Age-specific severity scales and descriptions for the two age groups are summarized in Table S4. Fever categories are designated in the key. 𝙸 bars represent 95% confidence intervals. The numbers above the bars show the percentage of participants in each group with at least one “yes” or “no” response for the specified event after the specified dose. Four participants 6 months to less than 2 years of age had a fever with a body temperature of more than 40.0°C (3 BNT162b2 recipients: day 1 after dose 1, day 1 after dose 2, and day 3 after dose 3 [1 participant each]; and 1 placebo recipient: day 5 after dose 1); 2 BNT162b2 recipients who had a fever with a body temperature of more than 40.0°C had a concurrent viral infection (roseola on the basis of clinical presentation or concurrent exanthem due to unspecified viral infection). Three BNT162b2 recipients 2 to 4 years of age had a fever with a body temperature of more than 40.0°C (day 2 after dose 1, day 4 after dose 2, and day 6 after dose 2 [1 participant each]), 1 of whom had a clinical presentation suggestive of viral exanthem such as roseola. Missing reactogenicity data were not imputed.
Among children 6 months to less than 2 years of age, systemic events other than fever were generally reported more frequently after BNT162b2 than after placebo (Figure 2). Among children 2 to 4 years of age, systemic events were reported at generally similar frequencies after BNT162b2 and after placebo. Most systemic events were mild to moderate, with severe events after any dose reported infrequently (≤1.1%). No grade 4 systemic events were reported. Irritability was the most frequent systemic event among children 6 months to less than 2 years of age; fatigue was the most frequent among children 2 to 4 years of age. In BNT162b2 recipients, the median onset for most events was 2 to 3 days; most resolved within 1 to 2 days.
Among BNT162b2 recipients, no more than 2.1% of children 6 months to less than 2 years of age and no more than 1.2% of children 2 to 4 years of age had a fever with a body temperature higher than 38.9°C after any of the three doses. Most fevers resolved within 1 to 1.5 days. Among children 6 months to less than 2 years of age, a fever with a body temperature higher than 40.0°C occurred in three BNT162b2 recipients and one placebo recipient; these fevers resolved in 3 to 5 days. Among children 2 to 4 years of age, a fever with a body temperature higher than 40.0°C occurred in three BNT162b2 recipients; these fevers resolved in 1 to 4 days.
The frequencies of adverse events were similar among BNT162b2 recipients and placebo recipients in both age groups and were higher among children 6 months to less than 2 years of age than among those 2 to 4 years of age (Table S6). Few participants withdrew owing to adverse events, and no deaths occurred. No cases of myocarditis or pericarditis, Bell’s palsy (or facial paralysis or paresis), thromboembolic events, thrombocytopenic events, MIS-C, Kawasaki’s disease, acute respiratory distress syndrome, meningitis, myelitis or encephalomyelitis, or vaccine-related anaphylaxis were reported. No events consistent with demyelination, peripheral neuropathy, or vasculitis were identified.
Phase 2–3 Efficacy
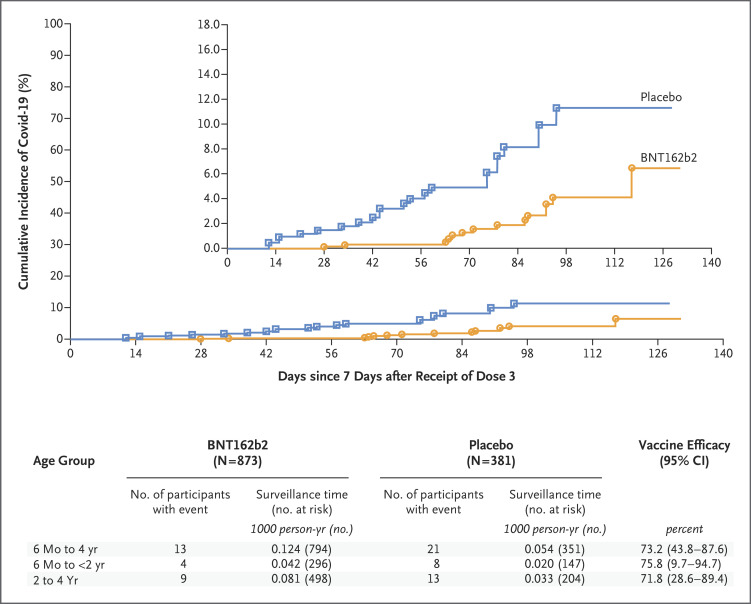
Among children 6 months to 4 years of age, 34 Covid-19 cases (13 in the BNT162b2 group and 21 in the placebo group) occurred from at least 7 days after dose 3 to the data-cutoff date (i.e., February 7 to June 17, 2022, which was entirely during the omicron-dominant phase), corresponding to an observed vaccine efficacy of 73.2% (95% CI, 43.8 to 87.6) (Figure 3). The efficacy of the vaccine was affirmed because the lower boundary of the 95% confidence interval was greater than 30%. Observed vaccine efficacy was 75.8% (95% CI, 9.7 to 94.7) among children 6 months to less than 2 years of age and 71.8% (95% CI, 28.6 to 89.4) among children 2 to 4 years of age.
Figure 3. Vaccine Efficacy in Participants 6 Months to 4 Years of Age.
The data-cutoff date was June 17, 2022. Data are for participants without evidence of infection with severe acute respiratory syndrome coronavirus 2 before 7 days after dose 3 in the efficacy population that could be evaluated (defined in Table S1). Two-sided 95% confidence intervals for vaccine efficacy were derived with the use of the Clopper–Pearson method, with adjustment for surveillance time. The percentage of participants who reported symptoms but had missing results of polymerase-chain-reaction testing was small and not imputed in the analysis. Surveillance time is the total time in 1000 person-years for the given end point across all participants within each group at risk for the end point. The time period for accrual of coronavirus disease 2019 (Covid-19) cases is from 7 days after dose 3 to the end of the surveillance period. The inset shows the same data on an enlarged y axis.
The majority of Covid-19 cases from 7 days after dose 3 were caused by omicron BA.2.12.1 and BA.2. Among children 6 months to 4 years of age, vaccine efficacy was 71.8% (95% CI, 40.5 to 87.1) against all omicron variants (33 cases). With respect to omicron sublineages, vaccine efficacy was 71.1% (95% CI, 9.1 to 91.5) against BA.2.12.1 (15 cases), 89.2% (95% CI, 45.7 to 98.9) against BA.2 (10 cases), and 13.3% (95% CI, −5016.9 to 95.5) against BA.4 (3 cases) (Table S7). There were 2 or fewer cases for each of the other omicron sublineages.
Discussion
Two 3-μg BNT162b2 doses that were administered 21 days apart followed by a third dose given at least 8 weeks later were safe and immunogenic in children 6 months to 4 years of age. Immunogenicity data showed robust vaccine-elicited immune responses to three doses in young children, including successful immunobridging to young adults from the original adult efficacy study on the basis of neutralizing titers against the ancestral SARS-CoV-2 strain.
Among children 2 to 4 years of age, immunobridging data after dose 2 only partially met success criteria. A descriptive analysis showed higher GMTs after dose 2 among children 2 to 4 years of age than among adults 65 years of age or older, for whom efficacy in the pivotal trial conducted before the omicron wave was shown.10 To achieve high effectiveness against symptomatic and severe Covid-19, including that caused by omicron BA.1 or BA.2 subvariants, real-world studies involving older populations, including adolescents, have also consistently shown the need for additional vaccine doses beyond the initial two doses used in these age groups.13,17-20 Given these real-world data in the omicron wave as well as clinical data, a third dose was authorized for persons 5 years of age or older,7,21,22 and a three-dose primary series was evaluated in children younger than 5 years of age. An exploratory analysis suggested that three 3-μg BNT162b2 doses induced neutralizing titers against the omicron BA.1 variant, with response patterns generally similar to those observed in adults 18 to 50 years of age administered a third dose (booster) at an interval similar to that used for doses 2 and 3 in young children (approximately 3 months), findings that further support the need for a three-dose primary series in children younger than 5 years of age.
Our data indicate that vaccinating young children affords efficacy against symptomatic Covid-19. This three-dose primary series was 73.2% effective (95% CI, 43.8 to 87.6) against Covid-19 from at least 7 days after dose 3, a period that was entirely within the omicron-dominant phase.
Availability of Covid-19 vaccines for young children is critical, because this population has had a surge in omicron-related cases and hospitalizations, and Covid-19 in this age group places a burden on families, including child care–related employment disruption.1-4,23 Protection against severe Covid-19 among young children is paramount, particularly with the unpredictability of new variants, including omicron BA.4 and BA.5 sublineages. As other mitigation measures are lifted (e.g., masking and restrictions on large gatherings), vaccinating this age group would also expand direct protection and add another layer of community protection against symptomatic and severe Covid-19.
The observed safety profile in this study did not suggest any concerns for BNT162b2 administered as three 3-μg doses in children 6 months to 4 years of age. Reactogenicity to BNT162b2, which included irritability as an adverse reaction in children 6 months to less than 2 years of age, was mostly mild to moderate and short-lived, with most events occurring at frequencies similar to or lower than those after dose 3 as compared with after dose 1 or after dose 2. No deaths were reported, and few adverse events led to withdrawal. No cases of myocarditis or pericarditis, MIS-C, or vaccine-related anaphylaxis were observed in the short term, and surveillance continues. BNT162b2 continues to demonstrate safety and an acceptable reactogenicity profile across age groups.
The trial has some limitations. It was not powered to assess efficacy against severe disease or against specific SARS-CoV-2 variants. We lacked data on omicron BA.4 and BA.5 serum-neutralizing activity and longer-term follow-up to assess immune-response duration as well as safety. In addition, the trial population was predominantly White. Like other phase 2–3 trials, the trial was not powered to detect potential rare side effects. However, follow-up from this trial (which will continue for ≥1 year after dose 3), together with effectiveness and safety data from real-world use (including from older age groups), should provide further information. Simultaneous administration of BNT162b2 with other vaccines that are administered in young children was also not assessed, although coadministration of Covid-19 vaccines with routine childhood immunizations is encouraged by the American Academy of Pediatrics and is endorsed by the Centers for Disease Control and Prevention (CDC).24 Data are available that suggest waning effectiveness of a third BNT162b2 dose in older adolescents and adults within a few months after administration, yet administration of a fourth dose given at least 4 months after dose 3 to older adults increases protection against SARS-CoV-2 infection, symptomatic and severe Covid-19, and Covid-19−associated hospitalizations and deaths.25,26 Therefore, additional doses, including doses beyond the primary series, may be required in all age groups to cover waning immunity, and variant-specific formulations might be needed to protect against emerging strains.
These data support vaccination of children 6 months to 4 years of age with three 3-μg primary BNT162b2 doses. BNT162b2 was recently granted emergency use authorization for this age group27; together with another Covid-19 mRNA vaccine, BNT162b2 is now recommended by the CDC.28
Acknowledgments
We thank Tricia Newell, Sheena Hunt, and Erin O’Keefe of ICON (Blue Bell, Pennsylvania), who wrote the first draft of the manuscript under direction from the authors. We thank all the participants who volunteered for this study and the caregivers who allowed them to participate. We also thank all the study site personnel for their contributions to this study. We especially acknowledge members of the data monitoring committee, who have been reviewing the trial safety data: Jonathan Zenilman, Kathryn Edwards, Lawrence Stanberry, Robert Belshe, Steven G. Self, Heather Lipkind, and Robert Phillips Heine. We also acknowledge the following persons for their contributions to this work: Pfizer colleagues Greg Adams, Priscilla Alba, Harshawardhan Anantaneni, Ayman Ayoub, Ruth Bailey, Vara Bandi, Molly Bennett, Pankaj Bhoir, Mark Boaz, Christopher Bowen, Donna Boyce, Michelle Bryson, Patrick Caubel, Andrea Cawein, Sherri Charlton, Darren Cowen, Kimberly Ann Cristall, Carmel Devlin, Julie Donato, Saumita Dubey, Camilla Farrell, Samantha Gault, Emily Graham, Tina Guina, Robin Hancock, Elisa Harkins Tull, Ahmed Hassan, Marie-Pierre Hellio Le Graverand-Gastineau, Zi Jia (Carrie) Jiang, Nai Chao Jin, Luis Jodar, Richa Kala, Hui Kim, Esther Ladipo, David Lambe, Venkateswarlu Layam, Rod MacKenzie, Jason McKinley, Robert Maroko, Ruchi Mathur, Zaynah Majid, Gosia Mineo, Shawn Musselman, Sagaya Mythili, Sheri Naly, Pascale Nantermet, Jason Painter, Marina Palombini, Elisabeth Pantazis-Butera, Vishal Patel, Elizabeth Paulukonis, Mark Pepin, Kellie Lynn Richardson, Laurel Rivera, Elizabeth Rogers, Christine Rossin, Melinda Rottas, Yanula Salamanca, Ian Schochet, Judy Sewards, Noushad Shahulhameed, Marianne Simone, Helen Smith, Naren Surampalli, Dina Tresnan, Sarah Tweedy, Sheela Veeramachaneni, Lina Wang, Xiang Wang, Xingbin Wang, Yingying Wang, Erica Weaver, Anil Kumar Yelike, Fang Yuan, Gabriel Zegrean, Liping Zhang, Ying Zhang, Mei Nan Zhuang, the members of the Vaccines Clinical Assay Team and the Vaccines Assay Development Team, and all the Pfizer colleagues not named here who contributed to this study; and BioNTech colleagues Meghan Bushway, Alexandra Kemmer Brück, Zakaria Khondker, Kimberly Krüger, Orkun Orzhelvaci, Ruben Rizzi, Svetlana Shpyro, and Anna Sokolowska.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by BioNTech and Pfizer.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org
References
- 1.Tsabouri S, Makis A, Kosmeri C, Siomou E. Risk factors for severity in children with coronavirus disease 2019: a comprehensive literature review. Pediatr Clin North Am 2021;68:321-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Laboratory-confirmed COVID-19-associated hospitalizations (https://gis.cdc.gov/grasp/COVIDNet/COVID19_5.html).
- 3.Centers for Disease Control and Prevention. COVID data tracker: health department-reported cases of multisystem inflammatory syndrome in children (MIS-C) in the United States (https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance).
- 4.Marks KJ, Whitaker M, Agathis NT, et al. Hospitalization of infants and children aged 0–4 years with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 2020–February 2022. MMWR Morb Mortal Wkly Rep 2022;71:429-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akaishi T, Ishii T. Coronavirus disease 2019 transmission and symptoms in young children during the severe acute respiratory syndrome coronavirus 2 delta variant and omicron variant outbreaks. J Int Med Res 2022;50:3000605221102079-3000605221102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul LA, Daneman N, Schwartz KL, et al. Association of age and pediatric household transmission of SARS-CoV-2 infection. JAMA Pediatr 2021;175:1151-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccines. December 22, 2022. (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine).
- 8.Comirnaty: COVID-19 vaccine, mRNA. New York: Pfizer, 2022. (https://www.fda.gov/media/153713/download). [Google Scholar]
- 9.Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med 2022;386:35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh EE, Frenck RW Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383:2439-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson MG, Natarajan K, Irving SA, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:139-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou J, Xia H, Xie X, et al. Neutralization against omicron SARS-CoV-2 from previous non-omicron infection. Nat Commun 2022;13:852-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurhade C, Zou J, Xia H, et al. Neutralization of omicron BA.1, BA.2, and BA.3 SARS-CoV-2 by 3 doses of BNT162b2 vaccine. Nat Commun 2022;13:3602-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim L, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of children aged <18 years hospitalized with laboratory-confirmed COVID-19 — COVID-NET, 14 states, March 1–July 25, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein NP, Stockwell MS, Demarco M, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years — VISION Network, 10 states, April 2021–January 2022. MMWR Morb Mortal Wkly Rep 2022;71:352-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Björk J, Bonander C, Moghaddassi M, et al. COVID-19 vaccine effectiveness against severe disease from SARS-CoV-2 omicron BA.1 and BA.2 subvariants — surveillance results from southern Sweden, December 2021 to March 2022. Euro Surveill 2022;27:2200322-2200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.UK Health Security Agency. COVID-19 vaccine surveillance report: week 13. March 31, 2022. (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1066759/Vaccine-surveillance-report-week-13.pdf).
- 21.Moreira ED Jr, Kitchin N, Xu X, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med 2022;386:1910-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Frenck RW Jr, Walsh EE, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med 2021;385:1627-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Easterly C, Kim D, Steiner MJ, deJong NA. Childcare and employment disruptions in 2020 among caregivers of children with and without special health care needs. JAMA Pediatr 2022;176:941-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Committee on Infectious Diseases. COVID-19 vaccines in infants, children, and adolescents. Pediatrics 2022;150(3):e2022058700-e2022058700. [DOI] [PubMed] [Google Scholar]
- 25.Patalon T, Saciuk Y, Peretz A, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun 2022;13:3203-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2022;386:1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age. June 17, 2022. (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children).
- 28.Centers for Disease Control and Prevention. Covid-19 vaccine: interim COVID-19 immunization schedule for persons 6 months of age and older. December 8, 2022. (https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.