Abstract

The accurate prediction of coal spontaneous combustion (CSC) in the goaf areas of coal mines is a vital aspect of the migration from passive to active fire prevention and control. However, CSC is highly complicated and existing technologies cannot accurately monitor coal temperatures over wide expanses. Thus, it may be beneficial to assess CSC based on various index gases produced by the reactions of coal. In the present study, the CSC process was simulated by temperature-programmed experiments, and the relationships between index gas concentrations with the coal temperature were determined using logistic fitting functions. CSC was divided into seven stages, and a coal seam spontaneous ignition early warning system involving six criteria was established. Field trials demonstrated that this system is a viable approach to predicting coal seam fires and meets the requirements for the active prevention and control of coal combustion. This work establishes an early warning system based on specific theoretical guidelines that permits the identification of CSC and the implementation of active fire prevention and extinguishing measures.
1. Introduction
Coal remains the most widely used fuel globally and global output has, in fact, increased significantly in recent years. As an example, in 2018, the global coal output was approximately 7813 Mt, representing an increase of close to 3.3% relative to the year prior, while China’s raw coal output was 3550 Mt, equivalent to a 4% increase.1 Although new environmentally friendly energy sources have been developed, the current global energy structure based on coal will not substantially change in the near future.2 In recent years, coal extraction in China has continually increased (Figure 1). As a consequence of this large-scale mining of coal, the prevention of coal spontaneous combustion (CSC) is important. CSC frequently occurs during coal mining, storage, and transportation and even in undisturbed underground coal seams. This process not only results in a loss of coal resources but can also threaten the health and safety of personnel.3 Taking China as an example, technologies such as mechanized top coal caving, retreat subsidence, high-rise grouting, and inclined longwall-coordinated mining have greatly increased efficiency.4−6 However, as the scale of the mining intensity has increased and mine depths have become greater, the underground environment has become both more complex and harsher, and coal fire disasters have occasionally occurred.8
Figure 1.

One obvious means of assessing the potential for CSC is to monitor the coal temperature. However, because coal is a poor conductor of heat, existing equipment cannot necessarily detect changes in coal temperature over a wide physical range with sufficient accuracy.9,10 The energy released by a coal fire is primarily generated by the sequential reactions of various functional groups in the molecular structure of the coal11,13 and various effects will cause these groups to react at different temperatures. Thus, during CSC, the various functional groups in coal are gradually activated and react with oxygen to release heat.12−17
For the above reasons, the development of means of predicting CSC has become vital to the active prevention and control of underground coal fires. In particular, the characteristics of coal seam fires have been widely studied, primarily based on the assessment of certain index gases. The specific early warning stages, early warning characteristics, and gaseous product indicators must be selected and further subdivided. These stages comprise latent heat storage (associated with temperatures in the range of 30–60 °C), self-heating (60–150 °C), and combustion (above 210 °C). Yu et al.18 used temperature-programmed experiments, thermogravimetric differential scanning calorimetry, and theoretical analysis models to determine the activation energies and temperatures that are characteristic of CSC stages for three coal types having different spontaneous combustion tendencies. This prior work devised relationships for the variations in temperature and activation energy and divided the low-temperature oxidation of coal into four stages, each with a specific temperature range. Xiao et al.19,20 employed thermogravimetric analysis together with a 15 Mt super-large spontaneous ignition experimental platform to determine seven characteristic temperatures for coal samples from the Yanzhou mining area. Specific temperature ranges were examined and the relationships between certain CSC index gases and the characteristic temperature were established. Guo et al. characterized CSC based on a coal–oxygen composite ignition mechanism.21−24 The relationship between various index gases and the temperature changes of the coal were analyzed with the aim of providing theoretical guidance for the optimization of CSC early warning systems. Zhang et al. established a natural ignition experimental platform and conducted research on the associations between various parameters and CSC. This prior work devised index gases and characteristic temperature ranges that allowed the prediction and prevention of CSC.25 Wen et al. studied variations in the gaseous products of CSC and oxygen consumption rates with changes in coal temperature while monitoring various complex parameters such as air leakage, coal moisture content, and oxygen content. A series of molecular models for coal was also used to study macroscopic properties, and a model for the physicochemical adsorption of oxygen was established and used to predict the CSC temperature.26−33 Tan et al.29 analyzed the relationship between index gases and temperature during CSC based on temperature-programmed experiments and proposed a four-stage early warning system. Guo et al.30 divided the CSC phenomenon into five risk levels through a series of experiments and analyses.
Based on prior research concerning evaluations of CSC stages and classification of warning criteria, the present work simulated the process of CSC experimentally and collected index gas data for different temperature stages of this process. These data were processed using logistic fitting functions to improve the early warning accuracy as a means of establishing a reliable coal seam combustion classification system. Field trials during which fire-fighting measures were employed were used to verify the feasibility of this new CSC early warning system.
2. Experimental Section
2.1. Experimental Apparatus
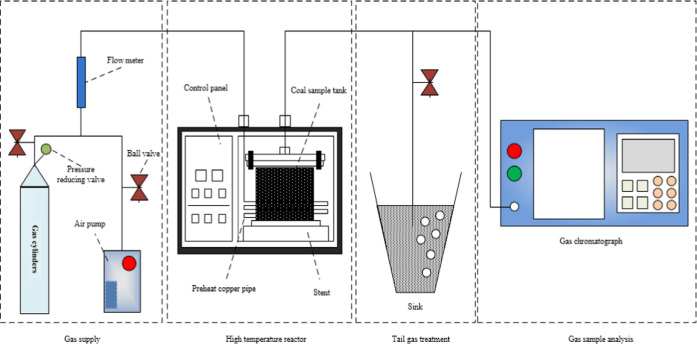
As noted, coal temperature is the most direct and accurate indicator of the potential for CSC. However, as a result of the physical characteristics of the goaf in a mine (meaning that this area is typically inaccessible and concealed and comprises coal and rock having poor thermal conductivity), it is almost impossible to directly and accurately determine the temperature of the coal. Therefore, it is necessary to ascertain the coal temperature indirectly by monitoring the gases in the goaf. As such, the most useful early warning systems for CSC are based on determining the relationship between the coal temperature and the amounts of certain index gases. In this study, the relationships between characteristic temperature points and the volume fractions of gaseous products during CSC were ascertained via temperature-programmed experiments. These relationships were then used to establish an early warning system.
A diagram of the experimental system used in the present study is shown in Figure 2. A DF13488 briquette-type self-ignition temperature-programmed device was employed that consisted of a gas source, reaction furnace, exhaust gas treatment unit, and gas sampling/analysis instrumentation. The gas source unit comprised a reaction gas source, a gas pretreatment apparatus, and a flow controller. The reaction furnace was composed of a preheating device, a reaction chamber, and a temperature display and control unit. The exhaust gas treatment unit processed the gaseous products generated by the reaction through absorption and extraction. The gas sample analysis system included a gas collection device and a gas chromatograph. Most of the gas generated by the reaction of coal and air in the reactor was discharged as waste gas, while a small portion was collected and analyzed.
Figure 2.
Temperature-programmed experimental apparatus.
2.2. Experimental Processes
Five types of coal samples obtained from different mining regions were selected for the experimental trials. Following a standard procedure for coal sampling,34 fresh coal specimens were collected from the working face in each case and transported to the laboratory in sealed packages. After unpacking and removing the outer oxide layer on the raw coal, each sample was crushed and then sieved to obtain particles in size ranges of 1–2.2, 2.2–3.4, 3.4–4.6, 4.6–6.8, and 6.8–8 mm that were then homogeneously mixed. During each experiment, the gas lines on the apparatus were connected and a tank holding a 1 kg coal sample was placed in the furnace, after which preheated dry air was introduced into the furnace, and the device was heated at a rate of 0.3 °C/min. When the temperature in the furnace reached 30 °C, aliquots of the gas in the tank were collected at intervals of 10 °C until the furnace reached 180 °C (the process is shown in Figure 3). The specific experimental conditions are summarized in Table 1.
Figure 3.

Flow chart summarizing the coal sample processing process.
Table 1. Temperature-Programmed Experimental Conditionsa.
| the average particle size | experimental coal height | experimental coal weight | coal sample volume | void ratio | heating rate | |
|---|---|---|---|---|---|---|
| experimental coal sample | d50 (mm) | (cm) | (kg) | (cm3) | (%) | (°C/min) |
| (A) Paner mine | 3.05 | 15 | 1.00 | 1295.25 | 0.45 | 0.30 |
| (B) Huangling mine | 4.43 | 18.5 | 1.00 | 1476.79 | 0.53 | 0.30 |
| (C) Fengchun mine | 4.25 | 18.29 | 1.00 | 1426.85 | 0.51 | 0.30 |
| (D) Shengli mine | 4.18 | 17.5 | 1.00 | 1327.54 | 0.48 | 0.30 |
| (E) Fengchun mine | 4.18 | 17.3 | 1.00 | 1358.05 | 0.47 | 0.30 |
Note: (1) d50: also called the median
particle size, the corresponding particle size when the cumulative
particle size distribution percentage of a sample reaches 50%; (2)
the void ratio was defined as 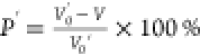 . Here, V0′
is the natural accumulation volume of the coal sample and V is the absolute compacted volume of the coal sample.
. Here, V0′
is the natural accumulation volume of the coal sample and V is the absolute compacted volume of the coal sample.
3. Analysis of Experimental Data
3.1. Characteristic Temperature Point Determination
3.1.1. Use of a Single Factor Index to Determine a Characteristic Temperature Range
During CSC, groups present on coal molecules, such as −CH2– and −CH2O–, will preferentially react with oxygen to generate CO and CO2 with the release of heat, and so the levels of these two gases will fluctuate throughout the entire CSC process.23−26,28,36,38 Therefore, the determination of the early warning characteristic temperature points in the low-temperature stage (below 110 °C) of CSC involves assessing CO and CO2 concentrations. In contrast, in the high-temperature stage (above 110 °C), aromatic ring structures and side chain bridging bonds will be broken to varying degrees. When numerous active groups on the coal surfaces come into contact with oxygen atoms, the concentration of the index gases generated during the high-temperature oxidation stage of CSC will increase sharply. Therefore, the concentrations of gaseous alkanes and olefins will reflect the degree of coal oxidation and its internal structural changes and determine the characteristic temperature points in the high-temperature stage. In the present work, variations in the release of CO and CO2 as well as alkanes and olefins with changes in coal temperature were assessed, as shown in Figure 4.
Figure 4.


Volume fractions of gaseous products as functions of coal temperature. (a) Carbon oxide volume fraction of A Mine; (b) alkane product volume fraction of A Mine; (c) carbon oxide volume fraction of B Mine; (d) alkane product volume fraction of B Mine; (e) carbon oxide volume fraction of C Mine; (f) alkane product volume fraction of C Mine; (g) carbon oxide volume fraction of D Mine; (h) alkane product volume fraction of D Mine; (i) carbon oxide volume fraction of E Mine; and (j) alkane product volume fraction of E Mine..
From these data, it is evident that CO and CO2 produced at low temperatures (30–50 °C) were primarily generated by desorption reactions of the coal and that the concentrations of CO and CO2 slowly increased over this temperature range. Between 50 and 70 °C, the coal molecules reacted with oxygen and increased amounts of CO and CO2 were generated. Over the range of 70–100 °C, within which the coal temperature exceeded the critical temperature (at which the reaction rate greatly increased), the chemical reactions intensified, and a greater quantity of CO was produced, indicating that the coal–oxygen reactions were accelerated. Between 100 and 150 °C, the coal reactions entered the pyrolysis stage and various microstructures in the fractured coal surfaces reacted with oxygen to generate a large amount of CO. Above 150 °C, the coal temperature was close to that associated with the fission stage in which the structures of various aromatic hydrocarbons are destroyed, and the CO2 concentration changed accordingly. At this point, the coal–oxygen recombination reactions proceeded rapidly to form a large amount of water–oxygen complexes and hydroxyl groups were converted into CO and CO2.21,32
CH4 is also generated during CSC as a result of desorption and pyrolysis and so can be used as an auxiliary predictor of this process. As the coal temperature rises from 30 to 50 °C, the intermolecular van der Waals forces are weakened. The desorption of the small amount of gases already present in the coal increases the CH4 concentration. Between 100 and 150 °C (that is, the high-temperature stage), the internal side chains of the coal molecules begin to rupture and the concentration of CH4 increases rapidly as the pyrolysis stage begins. Above 150 °C, the fragmentation of carbocyclic intermediates is accelerated and numerous unsaturated hydrocarbons are produced. The CSC reaction sequence is fully developed and the reaction of oxygen compounds with the coal proceeds vigorously. Therefore, the inflection point in the CH4 concentration plot can be used as a sign that the CSC has entered the fission stage.33−35
C2H4 and C2H6 and also important index gases that are well correlated with increases in the coal temperature. During the initial stage of each trial, C2H4 was not observed in the desorption gases. C2H6 is stable in coal seams over the temperature range of 30–50 °C, and so this gas primarily results from desorption below the pyrolysis temperature. Therefore, C2H6 can be used as an auxiliary gas for the monitoring of CSC at low temperatures. Within the range of 100–150 °C, the CSC process enters the pyrolysis stage such that the oxygen consumption rate increases rapidly and C2H4 and C2H6 are obtained. Above 150 °C, aromatic rings and aliphatic hydrocarbons are gradually cracked to create many free radicals that subsequently react to yield unsaturated hydrocarbons. The concentrations of C2H4 and C2H6 can thus be used as indicators of the progression of the cracking stage.36,37
3.1.2. Determination of the Characteristic Temperature Range using Index Gases
An analysis of the experimental data indicates that the early warning system can be broken down into several stages and that coal ignition can be accurately predicted by considering fluctuations in the proportions of various gases. The Graham coefficient is a highly sensitive predictor of CSC that eliminates the effect of air volume and allows the degree of fire spreading to be ascertained based on changes in gaseous products. The three-step chemical reactions of coal–oxygen complexes can be tracked by monitoring the CO generation rate and the corresponding changes in the oxygen concentration.6,14,26,36,39 The Graham coefficient, G, is calculated as
| 1 |
where φ(CO) and φ(O2) are the absolute values of the changes in the volume fractions of CO and O2 in the air flow. An analysis of the data shows that the φ(CO)/φ(O2) and φ(CH4)/φ(C2H4) ratios can be used as CSC indicators. These ratios can be used to determine characteristic temperature points for the early warning system on the basis of the single factors (such as the CO concentration) dividing the CSC process into separate stages.
The composite index graphs presented in Figure 5 indicate that the φ(CO)/φ(O2) ratio increased with temperature in each case. From 30 to 80 °C, this ratio increased slowly, suggesting that the coal–oxygen recombination reaction was not especially vigorous in this stage. Between 80 and 120 °C, the φ(CO)/φ(O2) value exhibited a more rapid increase as the oxidation reaction was gradually enhanced. Finally, above 120 °C, a very rapid increase occurred and so the oxidation reaction was evidently efficient in this stage such that oxygen was quickly consumed and the CO output was greatly increased. Interestingly, in all but one case, the φ(CH4)/φ(C2H4) ratio showed a downward trend at higher temperatures. In addition, because the point at which C2H4 was generated varied between samples, each specimen produced a different trend. Overall, this ratio decreased rapidly between 100 and 140 °C, indicating rapid spontaneous combustion and severe oxidation of the coal. Therefore, this range would be associated with the greatest danger of CSC.
Figure 5.

Composite index data curves: (a) A Mine; (b) B Mine; (c) C Mine; (d) D Mine; (e) E Mine.
3.2. Optimization of Early Warning Indicators for CSC
3.2.1. O2 Concentration
During CSC, coal undergoes heating caused by the adsorption of oxygen followed by oxidation reactions that eventually leads to spontaneous combustion. The so-called “three zones” method can be used to evaluate the potential for CSC in the goaf, in which the oxygen concentration is one of the main predictors (often in combination with other indicators). Therefore, the O2 concentration should be included in any coal spontaneous ignition warning system as an indicator gas.
3.2.2. CO and CO2 Concentrations
As coal begins to react, it will generate CO and CO2 during the low-temperature stage. During the high-temperature stage, the concentrations of these gases exhibited a nonlinear increase and the reaction rate increased almost exponentially. Considering that the amount of CO2 produced is affected by factors such as the coal structural characteristics, self-absorption by the coal, and the CSC stage, both desorption and the reaction of specific functional groups will lead to a surge in the CO2 concentration. Therefore, although this change in concentration is obvious, CO2 is not suitable for use as an early warning indicator gas. In contrast, the reactions that produce CO are affected by factors such as the degree of coal metamorphism and the coal porosity and sulfur content. Because fewer parameters are involved, the generation of CO can better characterize the low-temperature stage of spontaneous combustion. Therefore, CO concentration should be selected as an indicator gas for predicting CSC.
3.2.3. CH4, C2H4, and C2H6 Concentrations
Coal–oxygen reactions generate obvious fluctuations in the proportions of gaseous hydrocarbon products. CH4 is produced by desorption and pyrolysis throughout CSC. However, considering that the underground environment causes CH4 to be adsorbed at the onset of CSC, CH4 is not a viable indicator. C2H4 is an unsaturated compound that can further react with atmospheric oxygen to generate C2H6, and so not all C2H6 is produced by the combustion of coal. Therefore, considering that C2H6 is not suitable, C2H4 can be used as an early warning indicator.
3.2.4. φ(CO)/φ(O2) and φ(CH4)/φ(C2H4) Ratio
If the effect of air flow is ignored, the φ(CO)/φ(O2) index is a very effective predictor of spontaneous coal ignition and the changes in this ratio reflect the intensity of the coal reaction. Therefore, the φ(CO)/φ(O2) ratio should be included as a CSC indicator. Prior to the critical temperature, the φ(CH4)/φ(C2H4) ratio was relatively unchanged while, during the rapid oxidation stage, this value showed a rapid increase. For these reasons, the φ(CH4)/φ(C2H4) ratio was included as a prediction index.
3.3. Indicator Data Fitting
The volume fractions of the various index gases were correlated with the coal temperature. Therefore, the starting points (at which gas production is observed), mutation points (at which obvious changes in gas production occur), and inflection points of the various index gas data allowed levels in the early warning system to be developed. However, due to experimental errors, the volume fractions of the index gases corresponding to the characteristic temperature points of the coal had to be fit using mathematical functions. Ren et al. constructed a mathematical model for the gas temperature to predict CSC and determined that a logistic regression function was the most suitable means of processing CSC early warning indicators. Therefore, this paper uses the same process.
The logistic function equation is
| 2 |
where A1 is the minimum value of the data set corresponding to the function model, A2 is the maximum value of the data set corresponding to the function model, y is the ordinate of the curve, x is the abscissa of the curve, x0 is the abscissa corresponding to the inflection point of the curve, and P is a variable related to the slope at the inflection point of the curve. Figure 6 presents the fitting plots applied to the data based on logistic functions. These fitted data were used to determine the indicator thresholds for the CSC early warning system.
Figure 6.
Index gas fitting curves based on logistic functions.
4. Establishment of a Graded Early Warning System
4.1. Theoretical Classification and Early Warning Stage Divisions
With the ongoing development of ultrasonic extraction and quantum chemical simulations related to the study of CSC, the CSC mechanism has been examined in detail, and viable reaction mechanisms have gradually become clear.11,13,15−17 The CSC process can be further divided into seven theoretical stages (incubation, oxidation, self-heating, critical, pyrolysis, fission, and combustion),4,7,26,35 as shown in Figure 7. Specific early warning stages have to be determined according to the characteristic temperature points identified during the experimental data analysis. In this manner, indicators for each stage were devised based on the CO, CO2, and CnHm levels as well as the concentrations of other gases.6,12,27,36
Figure 7.
Theoretical classification of warning temperature ranges for CSC.
4.2. Establishment of a Grading Early Warning System for CSC
As noted, CSC is accompanied by the generation and release of CO, so this gas can be used to monitor the oxidation reactions of coal. However, during the CSC process, the temperature range over which CO can be detected is extremely wide such that the prediction range of this indicator is too large. This does not allow accurate determination of the relationship between the spontaneous combustion process and temperature. That is, CO values measured in the field may be lower than those produced by actual oxidation. At the beginning of CSC, there is usually no C2H4 in the adsorbed gases and so this compound is only generated during the coal oxidation process. Thus, this gas will predict different times and temperatures during combustion in mines and variations in the amount of C2H4 that is released should be considered.40,41
Because alkene ratios can only be calculated after the appearance of C2H4, the spontaneous ignition state before the critical temperature of C2H4 should be carefully considered. The C2H4/C2H6 ratio can be used as a predictor of the stage of CSC development after the appearance of C2H4 and before the production of C2H2. During the coal oxidation process, there will be a transition from the accelerated oxidation stage to the smoldering state, followed by open flame combustion. Based on the above, the relationships between the various gas parameters and coal temperature were quantitatively analyzed and a multistage early warning system suitable for CSC was devised. This system is based on CO, O2, and C2H4 proportions, the G value, and the φ(CH4)/φ(C2H4) ratio.39 The temperature intervals and gas concentration thresholds for this system were determined, and the CSC process was divided into seven stages with six warning points. This multistage warning system for CSC is summarized in Table 2.
Table 2. Early Warning System for CSC.

5. Example of a Field Application
5.1. Working Face Overview
The field work in this study was performed using the 12,205 first mining face having a strike length of 1600–1700 m and an inclined width of 240 m. The average thickness of the associated coal seam was 1.55 m, while the recoverable average thickness was 1.75 m. The width of the roadway along the trough of the working face was 5 m and the net height was 2.6 m. An anchor network is used for support in this mine. If the coal seam roof is not stable or the coal structure is poor, scaffolding is added for support. A mechanized coal mining method with a long-inclined arm is employed.
5.2. Field Trial Predictions
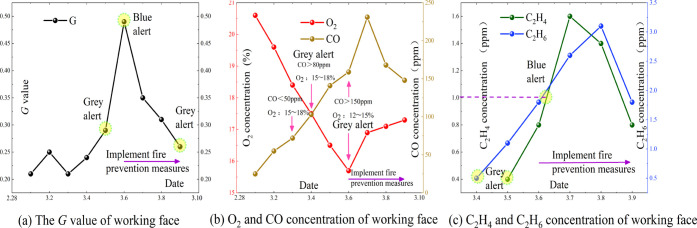
Figure 8 presents data acquired during the field trials.
Figure 8.
Various early warning system parameters obtained during field trials. (a) G value of the working face; (b) O2 and CO concentrations of the working face; and (c) C2H4 and C2H6 concentrations of the working face.
The analysis of the field trial data shows that the coal seam was in the critical stage of spontaneous combustion prior to March 5. On March 5, the CO concentration exceeded 100 ppm, while the O2 level was 16.5%, which met the initial warning conditions. At the same time, the G value reached 0.29 while varying between 0.2 and 0.3, equivalent to the first-level gray warning. These results indicated that the coal seam began to undergo low-temperature oxidation on March 5, with coal temperatures in the range of 30–40 °C. As a consequence of continuous air intrusion and oxidation, the CO concentration reached 159 ppm and the O2 concentration was 15.7% on March 6, which met the initial warning conditions, while the G value was 0.49. According to the newly developed system, the coal seam temperature was in the range of 50–60 °C at this time and so was in the second-level blue warning zone. Various fire-fighting measures were subsequently implemented and so the indicators remained in the blue early warning stage on March 7. There was no further development, indicating that the measures at this stage effectively controlled the occurrence of CSC. In the early morning of March 8, the G value decreased to 0.31 and was further reduced during the middle and evening shifts of that day. The CO and O2 concentrations remained in the first-level gray warning stage and the temperature dropped to 30–40 °C. These findings confirmed that the fire-fighting system that was employed effectively restrained the spontaneous combustion and oxidation of the coal. The specific collaborative prevention and control measures were guided by the hierarchical early warning system summarized in Table 3.
Table 3. Graded Early Warning Coordinated Prevention and Control Measures.
| date | stage name | temperature range (°C) | cooperative prevention and control measures |
|---|---|---|---|
| 3.1–3.4 | normal | <30–40 | dynamic advancement |
| 3.5–3.6 | gray alert | >30–40 | dynamic advancement ∩{seal blockage ∪ inert gases injection} |
| 3.6–3.7 | blue alert | >50–60 | dynamic advancement ∩{seal blockage ∩ inert gases injection} |
| 3.8 | gray alert | >30–40 | dynamic advancement ∩{seal blockage ∪ inert gases injection} |
5.3. Effectiveness Analysis
An analysis of variations in the on-site CO concentrations indicated that the CO concentration in the upper corner location of the mine did not exhibit an overall increase other than some large fluctuations at specific points in time. The concentration of O2 in the upper corner was relatively stable in the range of 17–18%. According to the early warning system, the coal seam underwent low-temperature oxidation on March 5 and reached the gray early warning stage, and then progressed to the blue early warning state on March 6. On March 7 and 8, the oxidation process was significantly suppressed and the situation returned to the gray warning zone, indicating that the fire prevention work had achieved good results.
6. Conclusions
At present, passive coal fire prevention and control systems are used in China and so the degree of spontaneous combustion cannot be precisely determined. Graded early warning indicators are not available and only basic stages in the CSC process have been determined. This work proposes a helpful categorization of the CSC stages. The relationships between the latent, oxidation, self-heating, critical, pyrolysis, fission, and combustion stages and the coal temperature were ascertained. Values of various index gas concentrations were then used to establish a graded CSC early warning system. The main conclusions of this work are as follows.
-
(1)
Expanding on the original three stages proposed for CSC, experimental data and field trials determined that the CSC process can be broken down into further stages associated with the temperature ranges of 30–50, 50–80, 80–110, 110–150, 150–210, and >210 °C.
-
(2)
The CO, O2, and C2H4 concentrations, the G value, and the φ(CH4)/φ(C2H4) ratio were found to provide suitable predictions of CSC progression. A relatively complete early warning system was established on this basis and the critical value of each level was determined.
-
(3)
Field trials using the working face of a coal mine were carried out to establish the viability of the proposed method. This new system was found to provide useful data regarding the state of CSC in the mine and the data reflected the effectiveness of fire-fighting measures. However, the system could be further improved by taking into account factors such as air leakage, coal type, geology, and gases that naturally occur in the coal seam.
Acknowledgments
This research is supported by the National Natural Science Foundation of China (52004209 and 52174198 and 52174197) and the Shaanxi Province Qin Chuangyuan “Scientist + Engineer” Innovation Team, Mine Fire Intelligent Monitoring, Early Warning and Prevention (2022KXJ-166).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c07401.
Statistics regarding coal usage (Figure S1); temperature-programmed experimental apparatus (Figure S2); low chart summarizing coal sample processing process (Figure S3); volume fractions of gaseous products as functions of coal temperature (Figure S4); composite index data curve (Figure S5); index gas fitting curves based on logistic functions (Figure S6); theoretical classification of warning temperature ranges for CSC (Figure S7); various early warning system parameters obtained during field trials (Figure S8) (PDF)
Author Contributions
J.G.: conceptualization, supervision, and formal analysis; Y.Q.: writing (original draft) and formal analysis; G.C.: supervision and formal analysis; Y.J.: methodology and investigation; X.Z.: methodology and investigation; and Y.L.: conceptualization and supervision.
The authors declare no competing financial interest.
Notes
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
References
- Key World Energy Statistics. https://www.iea.org/subscribe-to-data-services/world-energy-balances-and-statistics (accessed Apr 20, 2021).
- Xie H.; Wu X.; Zheng D. Prediction on the energy consumption and coal demand of China in 2025. J. China Coal Soc. 2019, 44, 1949–1960. [Google Scholar]
- Melody S. M.; Johnston F. H. Coal mine fires and human health: What do we know?. Int. J. Coal Geol. 2015, 152, 1–14. 10.1016/j.coal.2015.11.001. [DOI] [Google Scholar]
- Wang D. Thermodynamic disaster in coal mine and its characteristics. J. China Coal Soc. 2018, 43, 138–142. [Google Scholar]
- Wang J.; Wang F.; Xu Y.; Dong X. Study on current situation and development trend for coal mind fire prevention technology in China. Fresenius Environ. Bull. 2019, 28, 5010–5016. [Google Scholar]
- Guo J.; Yan H.; Liu Y.; Li S. Preventing spontaneous combustion of coal from damaging ecological environment based on thermogravimetric analysis. Appl. Ecol. Environ. Res. 2019, 17, 9031–9064. 10.15666/aeer/1704_90519064. [DOI] [Google Scholar]
- Moisés O. B. R.; Alan J. D.; Pablo B. B. A study of fire propagation in coal seam with numerical simulation of heat transfer and chemical reaction rate in mining field. Int. J. Min. Sci. Technol. 2019, 29, 873–879. [Google Scholar]
- Wang D.; Xin H.; Qi X.; Dou G.; Zhong X. Mechanism and relationship of elementary reactions in spontaneous combustion of coal: The coal oxidation kinetics theory and application. J. China Coal Soc. 2014, 39, 1668–1674. [Google Scholar]
- Zhang Y.; Yang C.; Li Y.; Huang Y.; Zhang J.; Zhang Y.; Li Q. Ultrasonic extraction and oxidation characteristics of functional groups during coal spontaneous combustion. Fuel 2019, 242, 287–294. 10.1016/j.fuel.2019.01.043. [DOI] [Google Scholar]
- Wen H.; Liu Y.; Jin Y.; Zhang D.; Guo J.; Li R.; Zheng X. Numerical simulation for mine oblique lane fire based on PDF non-premixed combustion. Combust. Sci. Technol. 2019, 193, 90–109. 10.1080/00102202.2019.1650271. [DOI] [Google Scholar]
- Jia T.; Lou H.; Liu J.; Qu G. Experimental study on thermal characteristics of spontaneous combustion process of coal with different moisture. J. China Coal Soc. 2019, 1080. [Google Scholar]
- Jin Y.; Guo J.; Wen H.; Liu W.; Wang K.; Ma X. Experimental study on the high temperature lean oxygen oxidation combustion characteristic parameters of coal spontaneous combustion. J. China Coal Soc. 2016, 40, 596–602. [Google Scholar]
- Lu Y.; Tang G.; Chen J.; Chen J.; Shao S.; Ding Y.; Li M.; Wu F.; Wang W. Development of composite phase change material for preventing spontaneous combustion of coal and its thermo-physical properties. Energy Fuels 2022, 36, 36. 10.1021/acs.energyfuels.1c04005. [DOI] [Google Scholar]
- Guo J.; Wen H.; Liu Y.; Jin Y. Data on analysis of temperature inversion during spontaneous combustion of coal. Data Brief 2019, 25, 104304 10.1016/j.dib.2019.104304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinov S. P.; Gonsalvesh L.; Stefanova M.; Yperman J.; Carleer R.; Reggers G.; Yürüm Y.; Groudeva V.; Gadjanov P. Combustion behavior of some biodesulphurized coals assessed by TGA/DTA. Thermochim. Acta 2010, 497, 46–51. 10.1016/j.tca.2009.08.012. [DOI] [Google Scholar]
- Lu J.; Li H.; Shi S.; Huang B.; Lu Y.; Li M.; Ye Q. Microwave-induced microstructure evolution of coal and its effects on the methane adsorption characteristic. Energy Fuels 2021, 35, 5. 10.1021/acs.energyfuels.0c04363. [DOI] [Google Scholar]
- Deng J.; Deng Y.; Zhang Y.; Wang C. Experiment study on airflow affected to generation of CO with low temperature oxidation of coal. Coal Sci. Technol. 2016, 44, 70–74. [Google Scholar]
- Yu M.; Yuan Z.; Zhu T.; Guo P.; Zheng K. Characterization differences of different spontaneous coal oxidation stages. J. Chongqing Univ. 2017, 40, 37–44. [Google Scholar]
- Xiao Y.; Ma L.; Wang Z.; Deng J.; Wang W.; Xiang X. Study on characteristic temperature of coal spontaneous combustion process by thermogravimetric analysis. Coal Sci. Technol. 2007, 05, 73–76. [Google Scholar]
- Xiao Y.; Wang Z.; Ma L.; Zhai X. Research on the corresponding relationship between coal spontaneous combustion index gas and characteristic temperature. Coal Sci. Technol. 2008, 6, 47–51. [Google Scholar]
- Guo J.; Wen H.; Zheng X.; Liu Y.; Cheng X. A method for evaluating the spontaneous combustion of coal by monitoring various gases. Process Saf. Environ. Prot. 2019, 126, 223–231. 10.1016/j.psep.2019.04.014. [DOI] [Google Scholar]
- Onifade M.; Genc B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. 10.1016/j.ijmst.2020.03.001. [DOI] [Google Scholar]
- Li B.; Li M.; Gao W.; Bi M.; Ma L.; Qin Q.; Shu C. Effects of particle size on the self-ignition behavior of a coal dust layer on a hot plate. Fuel 2020, 260, 116269 10.1016/j.fuel.2019.116269. [DOI] [Google Scholar]
- ISO 18283 . Hard Coal and Coke–Manual Sampling; ISO: Beijing, China, 2006. [Google Scholar]
- Zhang Y.Study on the Microcosmic Characteristics and Macro Parameters in the Process of Coal Oxidation and Spontaneous Combustion; D. Xi’an University of Science and Technology, 2012. [Google Scholar]
- GB 474-2008 . Method for Preparation of Coal Sample; Standard of China: Beijing, China, 2008. [Google Scholar]
- Xiao Y.; Ma L.; Wang Z.; Wen H.; Deng J. Law of index gases adsorption and condensability of coal spontaneous combustion. J. China Coal Soc. 2007, 32, 1015–1018. [Google Scholar]
- Janković B.; Manić N.; Stojiljković D.; Jovanović V. The assessment of spontaneous ignition potential of coals using TGA–DTG technique. Combust. Flame 2020, 211, 32–43. 10.1016/j.combustflame.2019.09.020. [DOI] [Google Scholar]
- Tan B.; Shao Z.; Guo Y.; Zhao T.; Zhu H.; Li C. Research on classification and early warning of coal spontaneous combustion based on index gas correlation analysis. Chin. J. Saf. Sci. 2021, 31, 33–39. [Google Scholar]
- Guo J.; Jin Y.; Wang F.; Yang P.; Sun M.. Research on Multi-Level Warning Method of Coal Spontaneous Combustion Based on Logistic Regression Analysis; China’s Safety Production Science and Technology, 2022; Vol. 18, pp 88–93. [Google Scholar]
- Chen X.; Zhao Y.; Liu L.; Zhang L.; Zhang Z.; Qiu P. Evaluation of chemical structure, pyrolysis reactivity and gaseous products of Shenmu coal of different particle sizes. J. Anal. Appl. Pyrolysis 2018, 130, 294–304. 10.1016/j.jaap.2017.12.019. [DOI] [Google Scholar]
- Deng J.; Zhao J.; Zhang Y.; Zhang Y.; Wang C. Micro-characteristics of spontaneous combustion of second oxidation with different rank coals. J. China Coal Soc. 2016, 41, 1165–1172. [Google Scholar]
- Li J.-l.; Lu W.; Kong B.; Cao Y.; Qi G.; Qin C. Mechanism of gas generation during low-temperature oxidation of coal and model compounds. Energy Fuels 2019, 33, 1527–1539. 10.1021/acs.energyfuels.8b03571. [DOI] [Google Scholar]
- Zheng Y.; Li Q.; Lin B.; Zhou Y.; Liu Q.; Zhang G.; Zhao Y. Real-time analysis of the changing trends of functional groups and corresponding gas generated law during coal spontaneous combustion. Fuel Process. Technol. 2020, 199, 10. 10.1016/j.fuproc.2019.106237. [DOI] [Google Scholar]
- Gürdal G.; Hoşgörmez H.; Özcan D.; Li X.; Liu H.; Song W. The properties of Çan Basin coals (Çanakkale—Turkey): Spontaneous combustion and combustion by-products. Int. J. Coal Geol. 2015, 138, 1–15. 10.1016/j.coal.2014.12.004. [DOI] [Google Scholar]
- Ma L.; Guo R.; Gao Y.; Ren L.; Wei G.; Li C. Study on coal spontaneous combustion characteristics under methane-containing atmosphere. Combust. Sci. Technol. 2019, 191, 1456–1472. 10.1080/00102202.2018.1531286. [DOI] [Google Scholar]
- Wen X.; Zhao J.; Zeng F. Distribution of polycyclic aromatic hydrocarbons in coal gangue and emitted gas with low-temperature spontaneous combustion in situ. Energy Fuels 2019, 33, 176–184. 10.1021/acs.energyfuels.8b03493. [DOI] [Google Scholar]
- Ma L.; Zou L.; Ren L.; Chung Y.; Zhang P.; Shu C. Prediction indices and limiting parameters of coal spontaneous combustion in the Huainan mining area in China. Fuel 2020, 264, 116883 10.1016/j.fuel.2019.116883. [DOI] [Google Scholar]
- Wen H.; Guo J.; Jin Y.; Wang K.; Zhang Y.; Zheng X. Experimental study on the influence of different oxygen concentrations on coal spontaneous combustion characteristic parameters. Int. J. Oil Gas Coal Technol. 2017, 16, 187–202. 10.1504/IJOGCT.2017.086320. [DOI] [Google Scholar]
- Deng J.; Liu L.; Lei C.; Wang C.; Xiao Y. Spatiotemporal distributions of the temperature and index gases during the dynamic evolution of coal spontaneous combustion. Combust. Sci. Technol. 2019, 10, 1679–1695. 10.1080/00102202.2019.1709178. [DOI] [Google Scholar]
- Li J.; Fu P.; Zhu Q.; Mao Y.; Yang C. A lab-scale experiment on low-temperature coal oxidation in context of underground coal fires. Appl. Therm. Eng. 2018, 141, 333–338. 10.1016/j.applthermaleng.2018.05.128. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.