Abstract

A redox electrolyte is a crucial part of dye-sensitized solar cells (DSSCs), which plays a significant role in the photovoltage and photocurrent of the DSSCs through efficient dye regeneration and minimization of charge recombination. An I–/I3– redox shuttle has been mostly utilized, but it limits the open-circuit voltage (Voc) to 0.7–0.8 V. To improve the Voc value, an alternative redox shuttle with more positive redox potential is required. Thus, by utilizing cobalt complexes with polypyridyl ligands, a significant power conversion efficiency (PCE) of above 14% with a high Voc of up to 1 V under 1-sun illumination was achieved. Recently, the Voc of a DSSC has exceeded 1 V with a PCE of around 15% by using Cu-complex-based redox shuttles. The PCE of over 34% in DSSCs under ambient light by using these Cu-complex-based redox shuttles also proves the potential for the commercialization of DSSCs in indoor applications. However, most of the developed highly efficient porphyrin and organic dyes cannot be used for the Cu-complex-based redox shuttles due to their higher positive redox potentials. Therefore, the replacement of suitable ligands in Cu complexes or an alternative redox shuttle with a redox potential of 0.45–0.65 V has been required to utilize the highly efficient porphyrin and organic dyes. As a consequence, for the first time, the proposed strategy for a PCE enhancement of over 16% in DSSCs with a suitable redox shuttle is made by finding a superior counter electrode to enhance the fill factor and a suitable near-infrared (NIR)-absorbing dye for cosensitization with the existing dyes to further broaden the light absorption and enhance the short-circuit current density (Jsc) value. This review comprehensively analyzes the redox shuttles and redox-shuttle-based liquid electrolytes for DSSCs and gives recent progress and perspectives.
1. Introduction
Solar energy, which comes from radiant light and heat from the sun, is the most powerful source of renewable energy. Solar energy is about 200 times greater than all renewable energy resources combined.1 The most efficient way to harness solar energy is through photovoltaic technology that directly converts sunlight into electrical energy. Among various emerging photovoltaic technologies, dye-sensitized solar cells (DSSCs) have received great attention due to several advantages, such as facile cell fabrication, low cost, multicoloration appropriate for building and automobile integration, high power conversion efficiency (PCE) under outdoor and indoor light, and so on. Figure 1 shows the device structure of a DSSC and its working principle. The working electrode, also known as the photoanode, consists of a dye-sensitized thin film of a wide-band-gap semiconducting material (mostly n-type TiO2) with a nanocrystalline morphology deposited on a transparent conducting oxide (TCO) coated glass substrate. The counter electrode consists of a TCO substrate coated with a suitable catalyst (usually Pt). Redox liquid electrolytes contain a redox couple as a redox shuttle in the most common organic solvent medium. A DSSC works through several charge transfer processes: dye excitation, charge injection and transportation, dye regeneration, and electrolyte regeneration. In the first step, the dye absorbs photons, and electrons from the HOMO (highest occupied molecular orbital) level move to the LUMO (lowest unoccupied molecular orbital) level. Then the excited dye injects electrons in the LUMO level into the conduction band of the TiO2 semiconductor. As a result, a charge separation occurs at the interface of the dye and the wide-band-gap nanostructured TiO2 semiconductor. Then, the TiO2 semiconductor transports electrons quickly to the FTO electrode through diffusion, and these electrons move to the counter electrode through an external circuit.
Figure 1.

DSSC device structure with an n-type TiO2 semiconductor and its working principle.
The oxidized dye regenerates by obtaining electrons from reduced species of electrolytes, which are oxidized. The oxidized species of electrolytes move toward the counter electrodes through diffusion, where these species receive electrons that come from the anode through an external circuit. This step is called electrolyte regeneration.
Besides the charge transfer processes required above, there is also the possibility of losing injected electrons by the oxidized dye and oxidized species of electrolytes. This undesirable back-charge-transfer process is called charge recombination. The dye regeneration should be fast to avoid injected electron recombination with the oxidized dye.
In indoor photovoltaics, DSSCs are promising candidates for commercialization due to the outstanding PCE (above 34%) and the great long-term device stability under ambient conditions. The Japanese electronics company Ricoh has already launched a 20% efficiency indoor photovoltaic solid-state dye-sensitized solar cell for integration with IoT sensors and autonomous electronic devices that require low electricity to power them.2 The Swedish company Exeger has already started the commercialization and integration of the DSSC technology within electronic devices that can generate power from indoor and outdoor light. By utilizing DSSC materials, this company makes Powerfoyle, a unique environment-friendly material that converts light into electricity and is capable of charging electronic devices with daylight and indoor light.3
In the last three decades, significant progress has been achieved in the DSSC field using D−π–A-structured organic and porphyrin dyes,4−7 cosensitized dyes,8−12 replacing redox mediators,13−18 solidifying liquid electrolytes,19−21 and developing counter electrode catalysts.22−27 A redox electrolyte is the vital component of a DSSC which affects the cell performance and long-term stability of the device. An electrolyte is accountable for the regeneration of photo-oxidized dye to dye and the transportation of the inner charge carrier between electrodes. Three parameters, photocurrent density (Jsc), photovoltage (Voc), and fill factor (FF), determine the PCE of the device. Electrolytes play a key role in contributing all three parameters (Jsc, Voc, and FF) of the device. Iodine electrolyte has been employed as the most widely used redox shuttle in DSSCs, and a maximum PCE of 12.4% under 1-sun illumination was achieved with concerted companion dyes.10,28 The low positive redox potential of the I–/I3– redox shuttle limits the open-circuit voltage (Voc) to 0.7–0.8 V since the Voc value is typically determined by the potential difference between the quasi-Fermi energy level of TiO2 and the redox potential of redox shuttles. Furthermore, competitive light absorption, the volatile nature of iodine, and corrosiveness toward metals, especially Ag, limit the iodine electrolytes for module development. Transition-metal complexes, especially Co complexes, have received great attention as redox shuttles due to the enhancement of the open-circuit voltage (Voc) of the device. Although the PCE of DSSCs under simulated 1-sun illumination has reached over 14% using cobalt electrolytes,9,11 sluggish mass transport and large reorganization between d7 (high-spin) and d6 (low-spin) in Co complexes limit a high Voc to 1 V. Recently, Cu complexes have been received great attention and recorded a PCE of 15.2% with a higher Voc of over 1 V obtained under simulated 1-sun irradiation by using a [Cu(tmby)2]+/[Cu(tmby)2]2+ redox shuttle with cosensitized organic dyes.29 Further improvement of PCE under 1-sun illumination is challenging by this [Cu(tmby)2]+/[Cu(tmby)2]2+ redox shuttle, because an improvement in Jsc value by using this redox shuttle is difficult.30 The competitive light absorption of Cu complexes with the dye in the blue region of the visible light spectrum and the use of a dye with a higher positive HOMO level reduce the entire visible light absorption range, which limits the Jsc value. Therefore, tuning the redox potential (Eredox ≈ 0.45–0.65 V vs NHE) of the Cu complexes by varying ligands, such as pentadentate ligands,30 can be a better solution so that currently developed porphyrin, organic, and NIR-absorbing dyes can be utilized for cosensitization with a Cu complex redox shuttle. Additionally, to enhance the long-term stability of the device, sealing with advanced techniques, such as glass frit encapsulation,31,32 and development of solidifying agents, such as polymeric gelating materials,19,33 to reduce the volatile organic solvent are also essential. This review comprehensively analyzes the DSSC redox liquid electrolytes (LEs) with advances and perspectives. According to our perspective, above 16% PCE under 1-sun is feasible to achieve for DSSCs, which is depicted and discussed in Summary and Outlook.
2. Solar Cell Evaluation
The dye-sensitized solar cell can be electrically modeled with the equivalent circuit shown in Figure 2, by including series resistance (RS) and shunt resistance (RSH).
Figure 2.
Modified diode equivalent circuit for the dye-sensitized solar cell.
The derived characteristic current density (J)–voltage (V) equation from the modified Shockley diode equation for the solar cell based on the equivalent circuit (see Figure 2) is expressed as
| 1 |
where JL is the photogenerated current density, Jo is the dark saturation current density, n is the diode ideality factor, RS and RSH are the series resistance and shunt resistance, respectively, q is the elementary charge (1.6 × 10–19 C), k is the Boltzmann constant (1.38 × 10–23 m2 kg s–2 K–1), and T is the absolute temperature.
The photovoltaic performance of DSSCs is estimated from J–V characteristics measurement under light illumination. The power conversion efficiency (PCE, denoted by η) of DSSC is evaluated from the J–V curve (Figure 3) by the eq 2
| 2 |
where Jsc is the short-circuit current density, Voc is the open-circuit voltage, FF is the fill factor, and Pin is the incident light power density for the J–V curve measurement.
Figure 3.
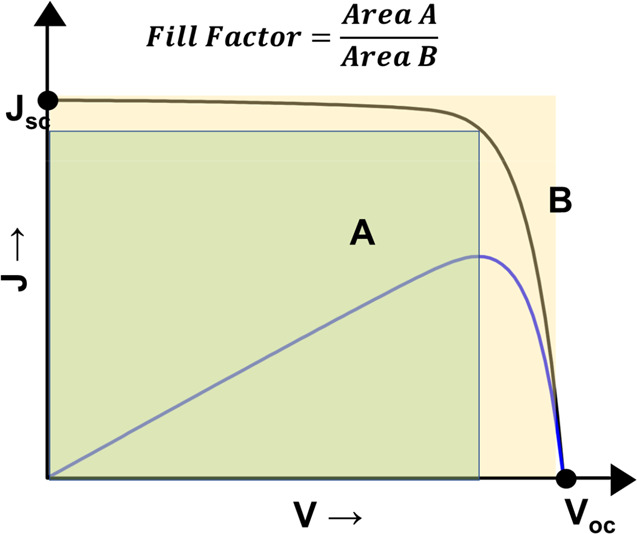
J–V curve of the DSSC.
2.1. Short-Circuit Current Density (Jsc)
Under short-circuit conditions, charges generated by photon absorption should flow into the external circuit with unit efficiency. The current density in the DSSC with incident light illumination of photon flux ϕ(λ) under a short-circuit condition is given by eq 3:34,35
| 3 |
Here, the monochromatic incident-photon-to-current conversion efficiency (IPCE(λ)) is related to the light-harvesting efficiency (LHE) of the dye, the electron injection efficiency (Φinj) in the excited state, the dye regeneration efficiency (ηreg) by electrolytes, and the change collection efficiency (ηcc) of the TiO2 electrode.
The LHE or absorptance (α(λ)) can be expressed by eq 4(36)
| 4 |
where A(λ) is the absorbance of the dye related to the molar extinction coefficient and the surface area of the loaded dye, as expressed in eq 5
| 5 |
where Io(λ) and I(λ) are the incident and transmitted light through the sample, respectively.
Thus, the LHE is influenced by the light absorption range of the dye, the amount of dye loaded in the mesoporous TiO2 surface, and the intensity of the incident light (see eqs 4 and 5). Usually, the surface area of the mesoporous TiO2 film is high; therefore, a large amount of dye can be loaded or adsorbed on the TiO2 film. Typically, the mesoporous TiO2 film has 18–20 nm diameter TiO2 nanoparticles that are much smaller than the wavelength of visible light. The scattering of light by the mesoporous TiO2 film can reduce the LHE by about 5%.37 Therefore, the optimization and improvement of the TiO2 film by having a scattering layer with larger TiO2 nanoparticles (typically ∼400 nm) can reduce light scattering and improve the LHE. Dye aggregation and charge recombination are two negative factors that can significantly reduce the LHE.
The electron injection efficiency (Φinj) depends on the free energy change (ΔGinj) for the electron transfer from the excited dye to the TiO2 semiconductor conduction band. ΔGinj is expressed by eq 6
| 6 |
where Edye*(LUMO) is the oxidation potential of the excited dye, i.e., the energy of the lowest unoccupied molecular orbital of the dye, and ECB is the energy of the conduction band edge of the semiconductor (typically 4.0 eV for anatase TiO2). For an efficient Φinj, ΔGinj should be >−0.2 eV.37
Dye aggregation reduces the electron injection efficiency. The recombination of the injected electrons with the oxidized dye can reduce Φinj. The dye regeneration efficiency (ηreg) depends on the free energy change (ΔGreg) in the reduction of the oxidized dye by the reduced species of electrolytes. ΔGreg is expressed by eq 7
| 7 |
where Edye(HOMO) is the ground-state oxidation potential, i.e., the energy of the highest occupied molecular orbital (HOMO) of the dye, and Eredox is the redox potential of the electrolytes. The dye regeneration by the reduced species of electrolytes should be fast for high-performance DSSCs. The redox potential of the redox shuttle should be higher (more negative) than the energy of the HOMO level of the dye to maintain the optimum driving force for efficient dye regeneration.
2.2. Open-Circuit Voltage (Voc)
Under an open-circuit condition, I = 0, the shunt resistance is assumed to be infinite. Thus, neglecting the final term of the J–V characteristics (eq 1), the open-circuit voltage (Voc), which is the maximum voltage obtained from the solar cell, is given by eq 8
| 8 |
Because JL ≫ Jo, therefore, JL + Jo ≈ JL.
The above equation for Voc derived from the modified Shockley diode equation for the solar cell is also relevant for the Voc value of the DSSC.
Under the open-circuit condition, the charges generated by the absorption of the photons recombine inside the cell. The photogenerated current density, JL, depends on the IPCE spectrum. The ideality factor, n, depends on the nonlinear recombination in the cell, and the inverse of the ideality factor is called the back-reaction order, β. The dark saturation current density, Jo, depends on the interfacial charge recombination of the cell and can be expressed by eq 9(38)
| 9 |
where no is the density of electrons in the conduction band state at thermal equilibrium accessible for the recombination under dark conditions, krec is the recombination rate, and na is the density of electron acceptors from the redox species of the electrolytes accessible for the recombination. Thus, by controlling or lowering the back-recombination (dark) reaction, it is possible to improve the Voc of the device.
The electron concentration in thermal equilibrium, no, can be expressed by eq 10(38)
| 10 |
where Nc is the concentration of electrons in the conduction band/trap state, Ea and Ec are the energy levels of the semiconductor conduction band and redox species, respectively, and α is the tailing parameter related to the trap states.
Under light illumination, the concentration of free electrons (n) increases around to its equilibrium value, no. The Voc value for the DSSC under constant light illumination can be obtained from the difference of the quasi-Fermi level (EFn) of the TiO2 semiconductor and redox potential (Eredox) of the electrolytes:39,40
| 11 |
According to eqs 8–11, charge recombination, electron density in the trap, state semiconductor Fermi level, and the redox potential of the electrolytes can affect the Voc value of the cell.
2.3. Fill Factor (FF)
The fill factor (FF) is defined as the ratio of the maximum power obtained from the solar cell to the product of the Jsc and Voc (see Figure 3). FF is regarded as the power efficiency of the solar cell. Electrical and electrochemical losses during in the solar cell operation influence FF.41 The shunt resistance (RSH) in the DSSC originates from the resistance for electron recombination (back electron transfer) across TiO2/electrolyte interfaces. The series resistance (RS) comes from the contribution of the sheet resistance of FTO, the electron transport resistance in TiO2, the mass transport of the electrolytes, and the charge transfer resistance at the counter electrode/electrolyte interfaces. The main contribution to the series resistance is the sheet resistance of the FTO substrate.42,43 A lower RS and higher RSH can give a higher fill factor. Generally, the fill factor of DSSCs varies from 0.6 to 0.8 because of the charge recombination and transport resistances.
3. Redox Electrolytes
The redox electrolytes are the key components of the DSSC and have a substantial impact on both the performance and long-term stability of the device.44 The liquid electrolytes (LEs) of DSSCs are liquid redox systems where the redox couple or shuttles are solvated in an aqueous or more common organic solvent medium.
3.1. Function of Redox Electrolytes in DSSCs
After photon absorption, the dye rapidly injects electrons from its excited state to the conduction band of the TiO2 and oxidizes (see Figure 1). The oxidized dye receives electrons from the reduced species in the electrolytes, which are oxidized by the following reaction:
In the counter electrode, a reverse reaction occurs:
The oxidized (Ox) species of the redox mediator migrate to the counter electrode and the reduced (Red) species of the redox mediator migrate from the counter electrode to the oxidized dye mainly through diffusion. The function of electrolytes in DSSCs is the dye regeneration and regeneration of reduced species of the redox couple in the counter electrode. The counter electrode requires the catalytic system to capture the electrons that come from the photoanode via the external circuit.
The photovoltaic performance of DSSCs strongly influences the kinetic competition of the dye regeneration reaction by the redox mediator and the injected electron recombination (dark reaction) by either the oxidized species of the redox mediator or the oxidized dye itself.
In solid-state DSSCs, the electrolyte in its solid state is the hole transporting material (HTM) or hole mediator. Dye regeneration is accomplished in solid-state DSSCs via the hole transfer from the oxidized state of the dye into the HOMO level of the HTM. The HTM is regenerated from the counter electrode by the charge transfer process.45
The state of the gel electrolyte (GE) is between the liquid and solid electrolyte. In quasi-solid-state DSSCs, the GEs contain a significant amount of liquid electrolyte retained by gelating materials, such as polymers,19 inorganic nanofillers,46 and nanocomposite polymers.47
3.2. Ion Transport in the Redox Electrolytes
Generally, the performance of electrolytes is evaluated by their ion transportability. Ionic conductivity is due to the movement of ionic charges. The ionic conductivity (σ) of any electrolyte (eq 12) is the product of the charge, concentration, and mobility of the carrier ions48−50
| 12 |
where μi, qi, and Ci are the mobility, charge, and concentration of the ionic specimen, i, respectively.
The mobility of the carrier ion is related to the diffusion coefficient (Di) of the carrier ion by the Einstein kinetic theory through eq 13
| 13 |
The diffusion coefficient is related to the viscosity of the medium by the Stokes–Einstein equation (eq 14)
| 14 |
where k is the Boltzmann constant, η is the viscosity of the medium, r is the radius of the ion, and T is the absolute temperature.
Therefore, the diffusion coefficient is inversely proportional to the viscosity of the medium (eq 3).
The general relationship between ionic conductivity and diffusion can be obtained from eq 15:
| 15 |
Therefore, with increasing diffusion of carrier ions in the electrolyte medium, the ionic conductivity increases, and with decreasing diffusion, the ionic conductivity decreases.
The concentration and mobility of carrier ions are thermally activated. The temperature dependence on the ionic conductivity or diffusion coefficient can be explained from the Arrhenius equation51,52
| 16 |
where σo is the pre-exponential factor linked to the concentration of the carrier ions, Ea is the activation energy, k is the Boltzmann constant, and T is the absolute temperature.
The activation energy (Ea) of the electrolyte can be obtained from the slope of the log(σ(T)) versus 1/T plot (see eq 16). The low activation energy of the electrolytes indicates that less energy is required by the carrier ions to initiate the migration process.
The Arrhenius equation explains the behavior of the ionic conductivity with temperature for homogeneous electrolytes very well. Usually, in all cases of LEs and most cases of GEs, the temperature-dependent ionic conductivity fits the Arrhenius equation well; however, the deviation from the Arrhenius behavior is observed for full-solid polymer electrolytes and some cases of GE.53
For nonhomogeneous electrolytes such as solid polymer electrolytes, the Vogel–Tammann–Fulcher (VTF) equation54−56 is generally used to clarify the conductivity behavior of the electrolytes with temperature (eq 17)
| 17 |
where B is the pseudo activation energy linked to the configuration entropy of the polymer chain and To is the equilibrium transition temperature (usually To ≈ Tg – 50 K).
The Dahms–Ruff equation (eq 18) can be utilized to explain the apparent diffusion coefficient for the concentrated system.57,58
| 18 |
Dphys is the viscosity dependent physical diffusion coefficient, which is related to the Stokes–Einstein equation (see eq 14). Dex is the electron exchange diffusion coefficient by the electron hopping process, which is related to the rate constant (kex) for electron exchange, concentration (c) of the redox species, and average center-to-center distance (δ) between the redox species.59 The nondiffusional hopping (e.g., Grotthus-like exchange) mechanism of triiodide (see eq 26) can be rationalized by the electron exchange diffusion coefficient (Dex).
3.2.1. Measurement of Ionic Conductivity from Bulk Resistance
Generally, the ionic conductivity of the electrolytes is obtained by estimating the bulk resistance of the electrolytes (Rb) in the Nyquist plot (also known as the Cole–Cole plot) using eq 19
| 19 |
where l is the electrolyte thickness, A is the electrolyte area, and Rb is the bulk resistance of the electrolyte obtained from the impedance. The Nyquist plot is obtained from the impedance measurement of the electrolyte-blocking electrode cell, placing or sandwiching electrolytes between two inert electrodes (usually stainless-steel electrodes).
To regenerate dye and electrolyte during operation of DSSCs under 1 sun, the ionic conductivity should be ≥10–3 S cm–1. However, the threshold value of ionic conductivity may vary with the light illumination, redox shuttle, additive, and medium of the electrolytes.
3.2.2. Evaluation of Charge Transfer Resistances by Impedance
To evaluate charge transfer resistance between electrodes and electrolytes, electrochemical impedance spectroscopy (EIS) was performed with a symmetrical dummy cell (counter electrode/electrolytes/counter electrode). In the Nyquist plot, two semicircles are found; the intercept in the real axis of the high-frequency region is regarded as the series resistance (Rs), the left semicircle is due to the charge transfer resistance (Rct) at the electrode–electrolyte interface, and the right semicircle arises due to the Nernst diffusion impedance (Zn) of the redox shuttle in the electrolytes (see Figure 4a).60
Figure 4.

(a) Charge transfer resistance of electrode/electrolytes determined from Nyquist plots in EIS measurement of a symmetrical dummy cell. Reprinted with permission from ref (60). Copyright 2014 Royal Society of Chemistry. (b) Apparent diffusion of I3– determined from the cathodic limiting current of J–V curves in LSV measurements (scan rate 10 mV s–1) of a symmetrical dummy cell. Reprinted with permission from ref(62). Copyright 2017 Royal Society of Chemistry.
3.2.3. Measurement of Apparent Diffusion Coefficient from Steady-State Limiting Current
Usually, the diffusion-limited current density (Jlim) can be obtained by measuring the cyclic voltammetry (CV) or linear sweep voltammetry (LSV) of a symmetrical dummy cell, counter electrode/electrolytes/counter electrode. In the case of CV, a slow scan rate (usually 5–10 mV s–1) is required to obtain steady-state conditions.61 The diffusion-limited current density is related to the apparent diffusion coefficient (Dapp) of the redox species of electrolytes by eq 20
| 20 |
where d is the electrolyte thickness, F is the Faraday constant, Co is the concentration of the redox species, and n is the number of electrons transferred for this process.
In LSV measurements (see Figure 4b),62 the anodic steady-state limiting current (upper plateau) is due to the electrochemical oxidation of the reduced species (I–), and the cathodic steady-state limiting current (lower plateau) is due to the electrochemical reduction of the oxidized species (I3–) in the electrolytes. Usually, due to the presence of excess I– ions in the electrolytes, I3– is the limiting ion. Under steady-state conditions, both the anodic and cathodic current plateaus are usually similar. Therefore, the obtained limited current from LSV is mainly valid for the calculation of the apparent diffusion of I3–.63,64 For efficient operation of the DSSC under 1 sun, the apparent diffusion coefficient should be ≥10–6 cm2 s–1.61
Fast dye generation and rapid diffusion minimize the recombination reaction (dark reaction) significantly; as a result, higher Jsc and Voc values can be obtained by the device.
4. Redox Electrolyte Components
4.1. Redox Shuttles
In DSSCs, the redox couple is used in liquid or gel electrolytes as a redox mediator or the redox shuttle and the HTM is used in solid electrolytes as a hole mediator. The role of the redox shuttle is to regenerate the dye by giving electrons to the photo-oxidized dye and transferring the electrons from the counter electrode to the oxidized dye to complete the circuit. The redox shuttle is the crucial part of the DSSC that strongly influences the photovoltage of the device. The redox shuttle sets the electrochemical potential at the counter electrode and influences the electrochemical potential of the TiO2 working electrode through the recombination of electrons in the TiO2 by their oxidized species.65 Under light illumination, the maximum theoretical Voc for the DSSC can be estimated from the difference between the quasi-Fermi level of electrons in the TiO2 semiconductor, which approaches the energy level of the TiO2 conduction band edge under intense light illumination, and the redox potential of the redox shuttle (see eq 11).
The redox potential of the electrolyte system can be obtained by the Nernst equation (eq 21)
| 21 |
where E°′ is the formal potential, R is the ideal gas constant, F is the Faraday constant, T is the absolute temperature, n is the number of electron transfers for the redox reaction (for I–/I3–, n = 2; for Co2+/Co3+ (complex) and Cu+/Cu2+ (complex), n = 1), and [Ox] and [Red] are the concentrations of oxidized and reduced species, respectively.
From the discovery of the DSSC up to now, I3–/ I–,66−72 Br3–/Br–,73,74 pseudohalogens such as Se(CN)3–/ Se(CN)−75 and (SCN)3–/SCN–,76 various transition-metal complexes such as Co3+/Co2+ (complex),4,15,77−80 Cu2+/Cu+ (complex),8,12,14,16,81−83 Fe3+/Fe2+ (complex),84,85 and ferrocenium/ferrocene (Fc+/Fc),13,86 and organic redox shuttles such as TEMPO+/TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy radical)87 and T2 (dimer of 5-mercapto-1-methyltetrazole ion)/T– (5-mercapto-1-methyltetrazole ion)88 have been employed and studied as redox shuttles in DSSC electrolytes. Standard redox potentials of some redox shuttles used in DSSCs are shown in Scheme 1.
Scheme 1. Structures of Some Important Redox Couples (R+/R) Used in DSSC Electrolytes as Redox Mediators and Their Standard Redox Potentials.

A redox couple with a higher redox potential may result in a high photovoltage of the device. To preserve the photocurrent, the dye regeneration by the redox mediator should be faster than the electron back transfer from the TiO2 to the oxidized dye.16 A sufficient driving force for dye regeneration is required to avoid the recombination of injected electrons with the oxidized dye. An inadequate driving force for dye regeneration can cause a lower photovoltaic performance by reducing the short-circuit current density (Jsc) and Voc, because of the recombination of injected electrons with the photo-oxidized dye. The high driving force for dye regeneration can also limit the Voc of the cell. Hence, an optimum driving force (around 20–25 kJ mol–1) is required for efficient dye regeneration; this is possible by selecting a suitable redox couple for the specific dye.89,90
Therefore, novel redox mediators with a more positive redox potential that maintain the optimum driving force for dye regeneration are the key to enhancing the open-circuit potential and photocurrent of the device. Hence, the general strategy for the Voc enhancement of a device is to simultaneously lower both the HOMO level of the dye and the reduction potential of the redox shuttle toward a positive potential (see Figure 5). The optimum driving force for dye regeneration can be varied with different redox couples.
Figure 5.
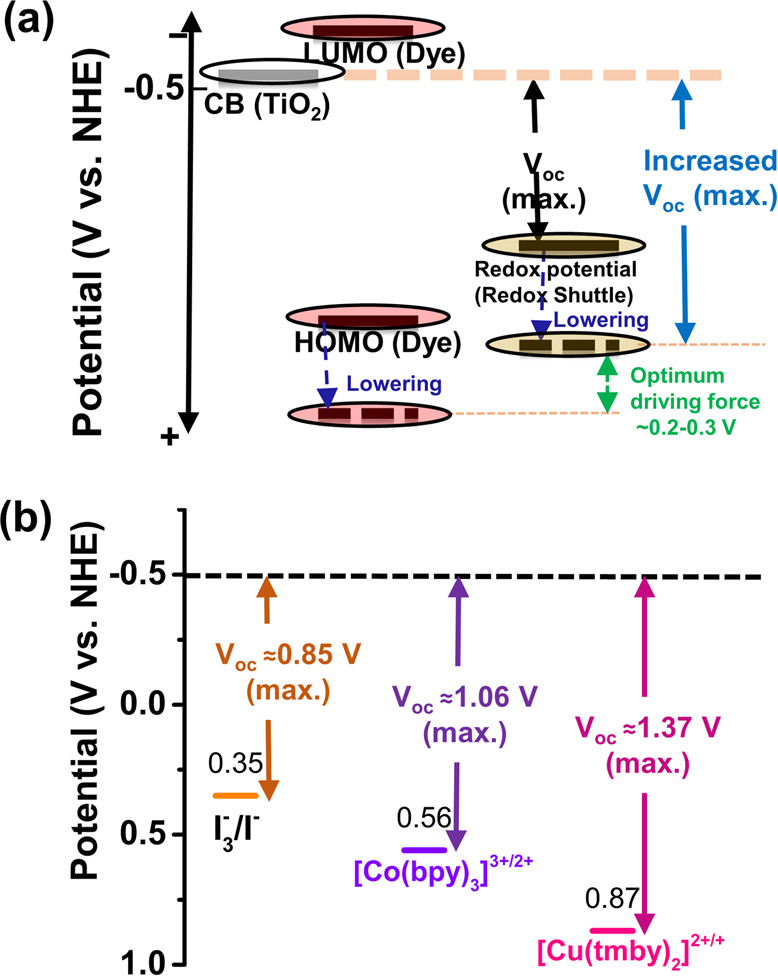
(a) Strategy for enhancing the maximum theoretical photovoltage (Voc) by simultaneously lowering the redox shuttle redox potential and the ground-state oxidation potential of the dye toward a more positive potential to ensure the optimum driving force for fast dye regeneration. (b) Comparison of the redox potentials and possible maximum open-circuit voltages among three of the most efficient redox couples used in the DSSC.
4.1.1. Halogenide Redox Shuttles
The I3–/I– redox shuttle shows an impressive performance in DSSCs and has been commonly used in the DSSC redox electrolytes since its invention. The standard redox potential of the I3–/I– redox couple is around 0.35 V (vs the normal hydrogen electrode (NHE));65 thus, there is a mismatch of more than 0.65 V with dyes having an oxidation potential above 1.0 V. This high driving force (>0.65 V) for the reduction of the oxidized dye allows for a rapid dye regeneration and slow recombination of injected electrons in TiO2 with I3–, which favor the high Jsc value of the device. However, the excess driving force for dye regeneration permits a significant Voc loss above 0.65 V for the I3–/I– electrolyte-based device.44,65 Furthermore, dye regeneration by I3–/I– electrolytes involves complex two-electron redox chemistry (eqs 22–24).65
| 22 |
| 23 |
| 24 |
The intermediate I2–• radical species formed in the dye regeneration process has a more negative redox potential than the standard redox potential of the I3–/I– redox species. Thus, the formation of intermediate radical species causes additional potential loss. Light absorption in the blue region of the visible spectrum by the I3–/I– redox electrolytes91 competes with the dye light absorption and causes a lower photocurrent. In addition, corrosiveness with the metal current collector, especially the Ag metal grid, by the I3–/I– redox electrolytes limits the commercial scale-up of the DSSC to a module.88,92 A study of various metal thin films with I3–/I– redox electrolytes by Okada et al. confirms that Ag, Au, and Al are highly corrosive with I3–/I–, while Pt, Ti, and Ni are less corrosive.92 Therefore, competitive visible light absorption, corrosion with metals, and limitation of the photovoltage are the major drawbacks of the I3–/I– redox couple. To overcome these limitations, a suitable alternative to the I3–/I– redox couple is needed.
The Br3–/Br– redox couple was also applied in the DSSC because of its higher positive reduction potential (∼1.1 V vs NHE) than the I3–/I– redox shuttle (∼0.35 V vs NHE).65,73,93 However, the Br3–/Br– redox electrolyte enhances the Voc up to 1 V for the carbazole dye74 but reduces Jsc. The dye regeneration by Br– is much slower than that of I– due to the low driving forces with most of the dyes. Besides, the Br3–/Br– redox couple has a problem similar to that of I3–/I–, including environmental impact.
A comparison study of pseudohalide redox couples and I3–/I– redox couples with the N3-dye was carried out by Oskam et al.76 They discovered that Se(CN)3–/Se(CN)−- and I3–/I–-based devices had similar Voc values. However, a significant decrease in Jsc for the Se(CN)3–/Se(CN)− redox couple based device was observed. The Jsc and Voc values were both the lowest for the (SCN)3–/SCN–-based device.76 Despite the more positive equilibrium potentials of pseudohalide redox couples than the I3–/I– redox couple, the Voc value did not improve.76 Therefore, a more positive equilibrium potential is not always applicable to account for the Voc enhancement of the device. Shifting of the FTO/dye-sensitized TiO2 and FTO/Pt electrode potential with the equilibrium potential of the redox couple is also required to account for the deviation of the Voc value. By transient absorbance studies, Oskam et al. concluded that dye regeneration is much slower for the Se(CN)3–/Se(CN)− and (SCN)3/SCN– redox couples than the I3–/I– redox couple, and the decreasing order of dye-regeneration rate is as follows: I3–/I– > Se(CN)3–/Se(CN)− > (SCN)3/SCN–.76
4.1.2. Organic Redox Shuttles
The stable organic radical 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and its ion TEMPO+ can also be used as redox mediators in DSSC electrolytes. The standard redox potential of TEMPO+/TEMPO (0.80 V vs NHE) is higher than that of the I3–/I– redox couple (0.35 V vs NHE). Zhang et al. reported a 5.4% power conversion efficiency under 1-sun conditions with a high photovoltage of 830 mV.87 However, the TEMPO+/TEMPO redox couple based device suffers from a low Jsc value due to the fast recombination (back electron transfer from TiO2). The long-term stability of the TEMPO+/TEMPO redox electrolyte based device also needs to be improved. Another iodine-free redox couple, 5-mercapto-1-methyltetrazole ion (T–) and its dimer (T2), was also used in DSSC electrolytes by Wang et al. due to advantages like noncorrosiveness, negligible visible light absorption, and a closer redox potential (∼0.48 V vs NHE) with the I3–/ I– redox couple. Wang et al. achieved a competitive power conversion efficiency of 6.4% under 1 sun illumination with Jsc of 16.18 mA cm–2, Voc of 681 mV, and FF of 0.58 using a Ru-based Z907Na dye.88 Despite the promising performances of the T2/T– redox couple, the long-term stability of the device still needs to be improved. A thiol-based self-assembled layer may form on the metal counter electrode, and devices with the T2/T– redox couple and the Pt counter electrode suffer from stability problems.44
4.1.3. Transition-Metal Complexes for Redox Shuttles
The ferrocenium hexafluorophosphate/ferrocene (Fc+/Fc) redox couple can be an alternative redox couple because of its noncorrosive nature, simple one-electron-transfer redox reaction chemistry, abundance, and suitable redox potential (0.62 V vs NHE).13,94 The redox potential of Fc+/Fc can be further tuned by introducing various substituents in the cyclopentadienyl ring. Various substituted Fc compounds are also commercially available.95,96 Daeneke et al. reported a 7.5% efficiency under 1-sun condition by using the Fc+/Fc redox couple and an organic sensitizer with the coadsorbent chenodeoxycholic acid (CDCA). A Voc enhancement of about 100 mV compared to I3–/I– was observed for the Fc+/Fc-based device due to the more positive redox potential of the Fc+/Fc redox couple.13 However, the charge recombination rate is faster for Fc+/Fc redox electrolytes than for the I3–/I– redox couple, which causes a reduction in the Jsc value for the Fc+/Fc-based device. Furthermore, an oxygen-free environment is required for both the electrolyte preparation and cell fabrication due to the instability of the Fc+ in the presence of O2. The Fe(bpy)33+/2+ redox couple can also be interesting due to its positive redox potential of around 1.37 V (vs NHE). However, a proper dye with a more positive oxidation potential is required for these redox electrolytes. Rodrigues et al. obtained a 1.4 V photovoltage by using Fe(bpy)33+/2+ redox electrolytes with the modified D−π–A structure based organic sensitizer RR9. However, the efficiency of the device was poor due to the very low Jsc value and fill factor.97 The stability of the Fe3+/Fe2+ electrolyte based device was also not reported.84,85,97 Ni- and Mn-based complexes were also utilized as redox shuttles for DSSCs; however, their photovoltaic performances were not significantly improved.98,99 The greatest advantage of transition-metal complexes as redox mediators is that the redox potential of the complexes and Voc of the device can be tuned by varying ligands of the complexes.
Among the various transition-metal complexes, cobalt complexes have received more attention as redox mediators in the DSSC. Negligible visible light absorption, less corrosiveness toward metals, outer-sphere one-electron-redox chemistry, and higher positive redox potential of cobalt polypyridine complexes make cobalt complexes promising as redox shuttles. Generally, the Co(II) complex exists in the high-spin (HS) state at room temperature, as a quartet state, while the Co(III) complex is in a low-spin (LS) state, as a triplet state (see Figure 6).100 The large reorganization energy between d7 (HS) and d6 (LS) causes slow regeneration of Co(II) species in the counter electrode and enhanced charge recombination with Co(III) species. Slow mass transfer due to the large molecular size,101,102 rapid back electron transfer from TiO2 to Co3+ species,103 and slow regeneration of Co2+ species in the Pt counter electrode103 are the major challenges of the cobalt complexes; these can be overcome by enhancing the solubility of the complex by introducing a suitable substituent in the ligand, optimizing the device structure (especially the TiO2 film thickness), reducing the spacer thickness between electrodes, introducing a passivation layer to inhibit the back transfer reaction, and using a suitable catalyst in the counter electrode.
Figure 6.
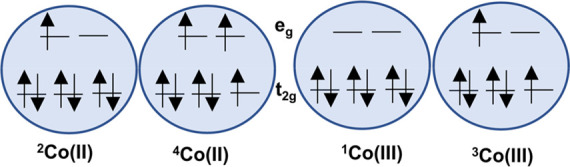
Electronic configurations of Co(II) species in doublet and quartet states and Co(III) species in singlet and triplet states.
The redox potential of the cobalt complexes can be tuned by using an appropriate ligand to match the oxidation potential of the dye. Feldt et al. tested a series of cobalt complexes with different redox potentials as a one-electron outer-sphere redox mediator in the DSSC.15 The standard redox potentials of cobalt(III/II) tris(2,2′-bipyridine), [Co(bpy)3]3+/2+, cobalt(III/II) tris(4,4′-dimethyl-2,2′-bipyridine), [Co(dmb)3]3+/2+, cobalt(III/II) tris(4,4′-di-tert-butyl-2,2′-bipyridine), [Co(dtb)3]3+/2+, and cobalt(III/II) tris(1,10-phenanthroline), [Co(phen)3]3+/2+, were 0.56, 0.43, 0.43, and 0.62 V, respectively. Here, for all the complexes, the counterion was hexafluorophosphate (PF6–). The Voc value of the devices should increase with increasing redox potential of the cobalt complexes; however, Feldt et al. obtained an optimum Voc and Jsc from the [Co(bpy)3]3+/2+ redox couple due to the optimum driving force for dye regeneration, less mass transport limitation due to the less bulky [Cu(bpy)3] complexes (see Figure 7), and reduction of recombination due to the introduction of steric bulk in the D35 dye.15
Figure 7.
Chemical structure of dyes and plots of current transients for various Co complexes with two different dyes: (a) D29; (b) D35. Mass transport can be avoided by using a less bulky Co(bpy)2 complex redox shuttle with the sterically bulky D35 dye. Reprinted with permission from ref (15). Copyright 2010 American Chemical Society.
The standard redox potential of the cobalt complex [Co(bpy)3]3+/2+(B(CN)4)−3/2 redox couple is 0.57 V (vs NHE).79 Here, 2,2′-bipyridine (bpy) is the bidentate ligand and tetracyanoborate (B(CN)4)− is the counterion of the cobalt complex. By a combination of the [Co(bpy)3]3+/2+(B(CN)4)−3/2 redox couple as the redox mediator and the donor−π-bridge–acceptor (D−π–A) structure-based Zn porphyrin dye YD2-o-C8 as a sensitizer, Yella et al. achieved a 11.9% efficiency with a Voc of 965 mV, Jsc of 17.3 mA cm–2, and FF of 0.71.79 By cosensitization with the organic dye, they further increased the efficiency to 12.5% under simulated 1-sun (AM 1.5G) illumination. The presence of octyloxy groups in the Zn porphyrin YD2-o-C8 sensitizer enables slower recombination of the injected electron in TiO2 with Co3+(bpy)3. This dye also absorbs light across the entire visible range.79 Kang et al. obtained a 12.1% efficiency under 1-sun illumination by using the D−π–A structured Zn-porphyrin SGT-021 dye with the cobalt complex redox shuttle [Co(bpy)3]3+/2+(B(CN)4)−3/2. In this case, the presence of the octyloxy group in the dye structure also prevented the electron back transfer recombination reaction and retained a Voc close to 1 V without sacrificing the Jsc value.4 The D−π–A structure based Zn-porphyrin SGT-025 also gave a promising efficiency of up to 11.0%77 with a coadsorbent π-conjugated phenyl linker (HC-A1).104 Therefore, the D−π–A structure based Zn-porphyrin sensitizer is a promising dye for cobalt complex redox electrolytes.
The standard redox potential of the tridentate ligand bpy-pz (6-(1H-pyrazol-1-yl)-2,2′-bipyridine) containing the [Co(bpy-pz)2]3+/2+(PF6–)3/2 redox couple is 0.86 V (vs NHE), which is higher than the standard redox potential of the bidentate 2,2′-bipyridine ligand based [Co(bpy)3]3+/2+(PF6–)3/2 redox couple (∼0.56 V vs NHE). Thus, the presence of pyrazole in the ligand bpy-pz stabilizes the HOMO level of the complexes more than does 2,2′-bipyridine (bpy). By using the Y123 organic sensitizer and [Co(bpy-pz)2]3+/2+(PF6–)3/2 redox electrolyte, Yum et al. achieved a 10% efficiency with a Voc above 1 V.78 In 2014, Mathew et al. reported a DSSC with a 13.0% efficiency using a D−π–A structured porphyrin dye, SM315, with the [Co(bpy)3]3+/2+ redox mediator.105 In 2015, Kakiage et al. reported a record efficiency of 14.3% under full sun illumination using a cosensitized organic dye, ADEKA-1 + LEG4, with the [Co(phen)3]3+/2+ redox couple.11 In 2018, Ren et al. reported a remarkable efficiency of 12.6% under full sun (AM 1.5G) illumination using the [Co(bpy)3]3+/2+ redox mediator and the D−π–A structured organic blue dye R6.80 Ji et al. reported an outstanding efficiency of 14.2% under 1-sun (AM 1.5G illumination) conditions by using the [Co(bpy)3]3+/2+ complex as the redox mediator and a cosensitized D−π–A structured organic dye, SGT-149, and a porphyrin dye, SGT-021, with the coadsorbent HC-A1.9
The driving force for the dye regeneration is sufficient for the [Co(phen)3]3+/2(PF6–)3/2 and [Co(bpy)3]3+/2(PF6–)3/2 complexes due to the moderate positive redox potential. Therefore, when [Co(phen)3]3+/2 and [Co(bpy)3]3+/2 complexes are used as redox mediators, a photovoltage of around 1 V should be obtained from the device if the recombination reaction can be minimized by optimizing the device and selecting the proper sensitizer. For efficient dye regeneration, a large driving force is usually required for the cobalt complexes due to the high reorganization energy between high-spin d7 states of Co(II) and low-spin d6 states of Co(III)-complexes,100,106 which is the major drawback of the Co complexes.
Copper complexes have received a great deal of attention as redox mediators due to their fast electron self-exchange property, which decreases the charge transport limitation.14,82 Copper complexes have been used both in liquid electrolytes as redox mediators and also in solid electrolytes as hole mediators.8,107 Like other transition-metal complexes, the redox potential of the copper complexes can also be tuned by changing the ligands in the complex. In addition, copper is nontoxic, abundant in the Earth, and comparatively cheaper than cobalt. In 2005, Hattori et al. first introduced various blue copper complexes as redox mediators in the DSSC. However, the efficiency of the device was poor at that time, but the Voc of the device using [Cu(dmp)2]2+/+ as the redox mediator was higher than the Voc of the device using the I3–/I– redox mediator due to the more positive standard redox potential of [Cu(dmp)2]2+/+ (0.93 vs NHE).14 In 2011, Bai et al. reported a C218 dye sensitized solar cell with 7.0% efficiency using [Cu(dmp)2]2+/+ complex redox shuttles.83 The enhancement in the efficiency of the copper complex redox couple based device was mainly due to the significant increase in the Voc (0.93 V) because of the higher positive redox potential of the copper complexes. The Jsc value for the copper electrolyte was lower than that for the iodine electrolyte. They also found a lower IPCE, especially in the blue region, for the copper electrolyte device than for the iodine electrolyte device. Their IPCE data indicate the stronger absorption of copper complexes than iodine electrolytes. They also reported that the copper redox shuttles may transfer electrons to a lesser extent to a noble metal, carbon black, and conducting oxides, which causes the poor fill factor. Therefore, careful selection of counter electrodes, copper complexes, and photosensitizers can further improve the efficiency of the device. Cong et al. compared the [Cu(bpye)2]2+/+ redox shuttle with the [Co(bpy)3]3+/2+ redox couple and found a higher PCE as well as a higher Voc for the [Cu(bpye)2]2+/+ redox couple based device than the [Co(bpy)3]3+/2+ redox couple based device due to the more positive redox potential (∼0.59 V) of [Cu(bpye)2]2+/+ complexes.82 The main disadvantage of the [Co(bpy)3]3+/2+ redox shuttle is the mass transport problem, which may be possible to overcome by changing the ligand of Co complexes to bpye. Pradhan et al. found in their study with different redox electrolytes that the [Cu(dmb)]2+/+ redox shuttle showed a lower mass transport, better diffusion, and comparatively low driving force (∼0.1 V) required for efficient dye regeneration compared to the [Co(bpy)]3+/2+ redox shuttles.108 Saygili et al. reported DSSCs with over 10% efficiency using three different copper complexes, [Cu(dmp)2]2+/+TFSI2/1, [Cu(dmby)2]2+/+TFSI2/1, and [Cu(tmby)2]2+/+TFSI2/1, as redox shuttles. The standard redox potential for [Cu(dmby)2]2+/+TFSI2/1 is 0.97 V (vs NHE), which is higher than the standard redox potential of the [Cu(tmby)2]2+/+TFSI2/1 complex (0.87 V vs NHE).16 Therefore, the ligand 6,6′-dimethyl-2,2′-bipyridine (dmby) in copper complexes reduces the redox potential toward a more positive potential than the ligand 4,4′,6,6′-tetramethyl-2,2′-bipyridine (tmby). However, the differences in the Voc values for the devices with these three different copper complexes were insignificant (Voc: 1.07 V for [Cu(dmby)2]2+/+, 1.04 V for [Cu(tmby)2]2+/+, and 1.06 V for [Cu(dmp)2]2+/+).16 If we consider the energy level of the TiO2 conduction band edge of −0.5 V (vs NHE), then the maximum theoretical Voc under an intense light condition for the device with the [Cu(tmby)2]2+/+ complex redox mediator (redox potential of 0.87 V vs NHE) should be 1.37 V. For the devices with [Cu(dmby)2]2+/+ and [Cu(dmp)2]2+/+, the maximum theoretical Voc values can be 1.47 and 1.43 V, respectively (see Figure 8).
Figure 8.
(a) Energy levels in DSSC for Y123 dye and Cu complexes, the chemical structures of (b) various Cu complexes and (c) Y123 dye, and (d) the minimum-energy structures of Cu(II) complexes. Reprinted with permission from ref (16). Copyright 2016 American Chemical Society.
The theoretical losses of Voc were around 400, 330, and 370 mV for the devices with [Cu(dmby)2]2+/+, [Cu(tmby)2]2+/+, and [Cu(dmp)2]2+/+, respectively. The lowest loss of Voc was for the device with the [Cu(tmby)2]2+/+ complex redox couple probably due to the slower back transfer recombination. There was an enhancement of the Jsc value for the device with the [Cu(tmby)2]2+/+TFSI2/1 redox couple. The small driving force is required to regenerate the dye efficiently by these Cu complexes due to the low reorganization energy, which may be attributed to the distorted-tetragonal structure of the copper(II) complexes (see Figure 8d) due to the steric hindrance of methyl groups in the 2,9-positions of the ligand.
Cao et al. reported an outstanding efficiency of 13.1% under standard AM 1.5G illumination for the [Cu(tmby)2]2+/+ redox couple by modifying the device architecture by direct contact between the dye-sensitized TiO2 photoanode and the electrically deposited poly(3,4-ethylenedioxythiophene) (PEDOT)/ FTO counter electrode (see Figure 9).12
Figure 9.
Modification of DSSC device structure by direct contact between the dye-sensitized TiO2 photoanode and the electrically deposited poly(3,4-ethylenedioxythiophene) (PEDOT) in the FTO. Reprinted with permission from ref (12). Copyright 2018 Elsevier.
There was no Voc drop due to the direct contact between the large-band-gap PEDOT conducting polymer (p-type) and TiO2 (n-type); this indicates the insignificant electrical shunt between them. Cao et al. achieved this outstanding performance by improving the light-harvesting efficiency using cosensitization of two D−π–A structured organic dyes, Y123 and XY1b, with the coadsorbent CDCA. The fill factor (0.79) was also noticeably high for copper electrolytes due to the improvement in the mass transport by using a high concentration of the redox couple and no TiCl4 post-treatment. In this direct contact device architecture, the redox shuttle diffuses through TiO2 nanopores and the Warburg resistance is significantly reduced due to the decrease in the diffusion path.12
In 2020, Ren et al. reported a DSSC with 12.7% efficiency with the [Cu(tmby)2]2+/+ redox electrolyte using the cosensitized organic blue dye R7 with the Y123 dye. In 2021, Zhang et al. introduced the Cu(tmby)2+/+ redox mediator in the D−π–A structure based organic dye MS5.8 They managed to maintain a theoretical Voc loss of only 130 mV for the MS5 dye, which may be due to the reduction of the back interfacial recombination and less driving force required for efficient dye regeneration by the Cu complexes. However, the Jsc value for the MS5-dye-based device was poor due to the low light-harvesting efficiency of that dye. By using the D−π–A structured organic dye XY1b with the adsorbent CDCA as a sensitizer and Cu(tmby)2+/+ complex as a redox mediator, they obtained an 11.8% efficiency under simulated 1-sun illumination with a comparatively high Jsc and fill factor. This may be due to the high LHE, efficient dye regeneration, and low back transfer recombination reaction. To further increase the LHE, they cosensitized the MS5 dye with the XY1b dye and reported a remarkable efficiency of 13.5% under standard AM 1.5G (1-sun) illumination and 34.5% under 1000 lx illumination (Osram 930 warm white daylight).8 In 2022, Ren et al. reported a record PCE of 15.2% under 1-sun condition by using cosensitization with the newly developed SL9 and SL10 organic dyes with [Cu(tmby)]2+/+ redox electrolytes.29 The device optimization by preabsorbing hydroxamic acid made it possible to achieve such a high PCE of 15.2% of the cosensitized device. [Cu(tmby)]2+/+ complexes were also used as hole mediators in a complete solid-state DSSC, and a record efficiency of 11.0% was obtained by Cao et al.107 Clearly, Cu(tmby)2+/+ complexes are more suitable as redox mediators due to several reasons: (1) the favorable redox potential (0.87 vs NHE) of Cu(tmby)2+/+ can minimize the photovoltage loss and a low driving force (∼0.1–0.2 V) is required for efficient dye regeneration due to the low internal reorganization energy of the complexes, (2) 6,6′-methyl substituents in the tmby ligand may protect copper complexes from oxidation and moisture,107 and (3) the 6,6′-methyl substituent in tmby may be the reason for the rigid structure of the Cu(tmby)]2+/+ complexes and prevent a structural change as much as possible, which may favor the rapid self-exchange electron transfer.107,109 It is observed that Co3+/2+ and Cu2+/+ complex redox electrolytes have already exceeded the performances of I3–/I– redox electrolytes. Metal complex redox shuttles are more promising in terms of enhancing the Voc of the device. In particular, Cu(tmby)22+/+ complexes are more suitable due to the low driving force required for efficient dye regeneration and minimal sacrifice of the Jsc value of the device. Judicious device optimization can play a key role in reducing the limitation of metal complex redox shuttles.
Both cobalt- and copper-based complexes can be potential redox shuttles for DSSCs by proper optimization of the electrolytes, tuning the redox potential by varying ligands to get an optimum driving force (∼0.2–0.3 V) for dye regeneration, using complexes with small reorganization energy between two states, optimization of the device to avoid charge recombination, and use of a suitable counter electrode for the specific redox shuttle.
4.1.4. Tandem or Mixed Redox Shuttles
Considering the synergistic effect of the mixed shuttles on the device performance, tandem or mixed redox shuttles, such as Co-complex-phenothiazine (PTZ)/Fc,110 sulfide/polysulfide-iodide,111 TEMPO-iodide,112 TEMPO-Co-complexes,113 and tris(4-alkoxyphenyl)amine-Co-complexes,114 were also used in DSSCs to enhance both Jsc and Voc simultaneously. A maximum PCE of 11% under 1-sun condition was reported by using tris(4-ethoxyphenyl)amine (TPEA)-[Co(bpy)3]3+/2+ tandem redox shuttles with an organic dye (see Figure 10).114 The maximum Voc value of around 1.03 V of the device was obtained by using the bulkier tris(butoxyphenyl)amine (TPBA) in this tris(4-alkoxyphenyl)amine-Co-complex tandem redox shuttle, which was attributed to the suppression of charge recombination by the bulkier butoxy groups in the tris(4-alkoxyphenyl)amine mediator.114 The highest PCE was achieved with the TPEA-[Co(bpy)3]3+/2+ redox system due to the balanced Jsc and Voc values.
Figure 10.

Effect of tris(4-alkoxyphenyl)amine (TPA) mediator on TPA-Co mixed redox shuttles. The recombination of TiO2 (e–) was retarded by the steric effect of the TPA mediator. Reprinted with permission from the graphical abstract of ref (114). Copyright 2018 American Chemical Society.
4.1.5. Redox Shuttle for Aqueous DSSCs
DSSCs using nitrile-based organic solvents can raise serious concerns about safety in practical applications, especially in indoor photovoltaics due to several drawbacks of organic solvents, such as high vapor pressure, toxicity, and acute environmental impact.115 Regarding the nontoxicity, safe, and eco-friendly nature of water, a 100% aqueous DSSC can be regarded to be a safe and eco-friendly energy source. To date, in most of the cases, the I3–/I– redox shuttle was utilized in aqueous DSSCs.116−118 The highest PCE of 7.02% for 100% aqueous DSSCs was achieved under 1-sun illumination by using the I3–/I– redox shuttle with an optimized counter electrode and sensitizer.118 However, there is an increasing issue about iodate formation and stability of the I3–/I– redox shuttle based aqueous DSSCs.119 Organic redox shuttles, such as TEMPO+/TEMPO120 and thiolate/disulfide (T–/DS),121 were also utilized to fabricate aqueous DSSCs, and the PCE under 1-sun condition was in the range of 4–5%. The most important issue about organic redox shuttle based aqueous DSSCs is that the stability of the device was not good.120,121 Among metal complexes, the [Fe(CN)6]4+/3+ redox couple can be a promising candidate due to its noncorrosiveness and similarity of its redox potential to that of I3–/I–. By using the [Fe(CN)6]4+/3+ redox shuttle and optimizing the device with a carbazole-based dye, Daeneke et al. reported a maximum PCE of 4.2% under 1-sun illumination. However, the photocatalytic degradation of the [Fe(CN)6]4+/3+ redox couple under white light illumination can cause instability of the device.122 Various Co complexes, such as [Co(bpy)3]3+/2+,123,124 [Co(phen)3]3+/2+,123 were also utilized as redox shuttles for aqueous DSSCs; however, the PCE under 1-sun illumination did not exceed 6% even by optimization of the device in various manners.
4.1.6. Redox Shuttles for p-Type DSSCs
In p-type DSSCs, a dye-sensitized p-type semiconductor film (e.g., NiO) on a TCO substrate works as a photocathode, where the photoexcited dye injects holes into the semiconductor. The reduced dye is then regenerated by the oxidized species of electrolytes. The reduced species of electrolytes inject electrons into the counter electrode. The injected electrons in the counter electrode move to the working electrode through the external circuit and complete the cycle (see Figure 11).
Figure 11.

p-Type DSSC device structure with a p-type semiconductor and its working principle.
Like n-type DSSCs, the I3–/I– redox couple is mostly used as a redox shuttle in p-type DSSCs. The PCEs of p-type DSSCs are much lower than those of n-type DSSCs due to the large amount of visible light absorbed by typical NiO semiconductors used in p-type DSSCs and small energy gap between I3–/I– (+0.35 V vs NHE) electrolytes and the NiO (+0.70 V vs NHE) semiconductor.125−127 Using the [Co(en)3]3+/2+ (en = 1,2-diaminoethane) redox shuttle, Powar et al. reported a 1.3% PCE with Voc = 0.709 V under 1-sun condition for p-type DSSCs. The enhanced Voc value was due to the use of the high negative redox potential [Co(en)3]3+/2+ redox shuttle.128 Using the same redox shuttle in an aqueous medium, the same research group further enhanced the maximum PCE to 1.6% under 1-sun condition by controlling pH ≈ 10 of the aqueous electrolyte solution.129 Perera et al. reported a PCE of up to 2.3% by optimizing a p-type device using an organic-solvent-based [Fe(acac)3]0/– (acac = acetylacetonato) redox shuttle with perylene-thiophene-triphenylamine dye (PMI-6T-TPA).127 They achieved a maximum Jsc of 7.65 mA cm–2 by optimizing electrolytes and using a blocking layer on the NiO working electrode.127 However, using the more negative redox potential [Fe(acac)3]0/– redox shuttle (Eredox = −0.2 V vs NHE), they did not achieve a high Voc compared to the [Co(en)3]3+/2+ redox shuttle (Eredox = −0.03 V vs NHE) device, because of the fast charge recombination across the electrolyte/FTO interface.127
4.2. Medium for the Redox Shuttle
The solvent is the medium for the transportation of ionic species in the electrolyte. The choice of the solvent in redox electrolytes depends on the solubility and fast diffusion of the redox shuttle through that medium. Viscosity, boiling point, dielectric constant, and donor number are the important parameters of the solvent when selecting a suitable solvent for the redox electrolytes in DSSCs. Table 1 gives the physical properties of some common solvents for the redox electrolyte system.
Table 1. Physical Properties of Some Common Solvents Used for DSSC Redox Liquid Electrolytesa.
| solvent | melting point (°C) | boiling point (° C) | viscosity (cP) | dielectric constant | donor number (kcal mol–1) |
|---|---|---|---|---|---|
| water | 0 | 100 | 0.89 | 78.4 | 18.0 |
| acetonitrile (ACN) | –44 | 81.6 | 0.33 (30 °C) | 37.5 (20 °C) | 14.1 |
| propionitrile (PN) | –93 | 97 | 0.39 (30 °C) | 27.7 | 16.1 |
| valeronitrile (VN) | –96.2 | 141 | 0.78 | ||
| 3-methoxypropionitrile (MPN) | –63 | 164 | 2.5 | 36 | 16.1 |
| tetrahydrofuran (THF) | –108.4 | 66 | 0.47 (30 °C) | 7.6 | 20.0 |
| N,N-dimethylformamide (DMF) | –78 | 133 | 0.80 | 36.7 | 26.6 |
| dimethyl sulfoxide (DMSO) | 19 | 189 | 2.0 | 46.5 | 29.8 |
| sulfolane | 27.5 | 285 | 10.07 | ||
| γ-butyrolactone (GBL) | –44 | 204 | 1.7 | 42 | 18.0 |
| N-methyl-2-pyrrolidone (NMP) | –24 | 203 | 1.65 | 32.2 | 27.3 |
| 3-methyl-2-oxazolidinone (NMO) | 15 | 266 | 2.5 | 77.5 | |
| Ethylene carbonate (EC) | 36 | 238 | 90 | 89.1 | 16.4 |
| Propylene carbonate (PC) | –49 | 241 | 2.5 | 64 | 15.1 |
All data at 25 °C and 760 mmHg except where otherwise specified.
The solvent should have low viscosity to enhance the mobility of the carrier ions, a high boiling point to reduce solvent leakage and evaporation problems, high polarity to dissociate the ionic salt, and inertness toward the dye attached on the TiO2 semiconductor surface or dye–metal oxide bond. In addition, solvents should have low toxicity and environmental impact. The dielectric constant of the solvent represents a rough estimation of its polarity. The higher the dielectric constant of the solvent, the higher the dissociation capacity of an ionic salt in that medium. The donor number is an empirical parameter to evaluate the nucleophilic property of the solvent. The donor number is defined as the negative ΔH value (kcal mol–1) for the interaction of the electron-pair donor with the standard acceptor SbCl5 in a diluted 1,2-dichloroethane (DN = 0) medium.130 With an increase in the donor number of the solvent, the Voc value significantly increases but Jsc decreases.131,132 A high donor number (large nucleophilic or basic property) of the solvent causes a negative shift of the TiO2 conduction band, resulting in the enhancement of the Voc. Due to the negative shift of the TiO2 conduction band, the driving force for the electron injection from the excited dye to the TiO2 semiconductor is reduced; as a result, the Jsc value decreases because of the reduction in the electron injection efficiency. Therefore, sometimes a mixed solvent can be used to tune the donor number of the medium. For instance, a 90/10 (v/v) mixture of acetonitrile (ACN) and 3-methyl-2-oxazolidinone (NMO) enhances the Voc with minimal loss in Jsc, leading to an overall increase in performance.133 From an analysis of efficient DSSCs (see Table 3), it is confirmed that nitrile-based solvents ACN and MPN are the most effective for redox electrolytes; however, they are highly volatile. Fukui et al. also found that an 80/20 (v/v) mixture of ACN and tetrahydrofuran (THF) in the I3–/I– electrolyte enhances the Voc with no loss in Jsc due to the favorable donating ability of the medium.131 However, THF is highly volatile and comparatively less polar and can solvate most organic sensitizers. Water was also used as a solvent in DSSCs, due to its safe and eco-friendly nature.117,134,135 However, in the I3–/I– redox system, the oxidation of iodide by water and oxygen may produce iodate (IO3–) rather than I3– (eq 25),119 which causes I3– depletion and decreases the device performance.
| 25 |
Table 3. Progress of the Photovoltaic Performances of DSSCs Using Various Redox Electrolytes under Simulated 1-Sun (Standard AM 1.5G, 100 mW cm–2) Conditions.
| redox shuttle (electrolyte composition) | sensitizer (type) | counter electrode catalyst | reported efficiency (Voc, Jsc, FF) | year, ref |
|---|---|---|---|---|
| I3–/I– (1.0 M DMII, 50 mM LiI, 30 mM I2, 1.0 M TBP, 0.1 M GNCS in the mixture of ACN and VN (85/15)) | C106 (Ru complex) + CDCA (coadsorbent) | Pt | 11.7% (0.758 V, 19.78 mA cm–2, 0.779) | 2010, Yu et al.68 |
| I3–/I– (0.6 M DMPII, 0.05 M I2, 0.1 M LiI and 0.4 M TBP in ACN) | Black dye (Ru complex) + Y1 (coadsorbent) | Pt | 11.4% (0.743 V, 21.3 mA cm–2, 0.770) | 2012, Han et al.69 |
| I3–/I– (0.1 M LiI, 0.05 M I2, 0.6 M PMII and 0.5 M TBP in ACN) | XW61 (D−π–A structure, organic and porphyrin dye linked with nonconjugated bridging group) + CDCA (coadsorbent) | Pt | 12.4% (0.775 V, 21.41 mA cm–2, 0.747) | 2020, Zeng et al.10 |
| Co3+/Co2+ (0.22 M [Co2+(bpy)3](BCN4)2, 0.05 M [Co3+(bpy)3](BCN4)3, 0.1 M LiClO4, and 0.8 M TBP in ACN) | SGT-021 (porphyrin, D−π–A structure) + HC-A4 (coadsorbent) | Pt | 12.1% (0.910 V, 17.50 mA cm–2, 0.753) | 2016, Kang et al.4 |
| Co3+/Co2+ (0.25 M [Co2+(bpy)3](TFSI)2, 0.06 M [Co3+(bpy)3](TFSI)3, 0.1 M LiTFSI, and 0.5 M TBP in ACN) | SGT-021 (porphyrin, D−π–A structure) + HC-A1 (coadsorbent) | Pt | 12.6% (0.849 V, 19.2 mA cm–2, 0.768) | 2019, Zhou et.al.77 |
| Co3+/Co2+ (0.25 M [Co2+(bpy)3](TFSI)2, 0.06 M [Co3+(bpy)3](TFSI)3, 0.1 M LiTFSI, and 0.5 M TBP in ACN) | SM315 (porphyrin, D−π–A structure) + CDCA (coadsorbent) | graphene nanoplatelet (GNP) | 13.0% (0.91 V, 18.1 mA cm–2, 0.78) | 2014, Mathew et al.105 |
| Co3+/Co2+ (0.22 M [Co2+(bpy)3](B(CN)4)2, 0.05 M [Co3+(bpy)3](B(CN)4)3, 0.1 M LiClO4, and 0.8 M TBP in ACN) | SM315 (porphyrin, D−π–A structure) + CDCA (coadsorbent) | N and S codoped mesoporous carbons | 12.72% (0.893 V, 18.78 mA cm–2, 0.759) | 2017, Kim et al.170 |
| Pt | 12.23% (0.873 V, 19.17 mA cm–2, 0.731) | |||
| Co3+/Co2+ (0.22 M [Co2+(bpy)3](B(CN)4)2, 0.05 M [Co3+(bpy)3](B(CN)4)3, 0.1 M LiClO4, and 0.85 M TBP in ACN) | ZL003 (organic, D−π–A structure) + CDCA (coadsorbent) | Pt | 13.6% (0.956 V, 20.73 mA cm–2, 0.69) | 2019, Zhang et al.171 |
| Co3+/Co2+ (0.20 M [Co2+(phen)3](PF6–)2, 0.05 M [Co3+(phen)3](PF6–)3, 0.07 M LiClO4, 0.02 M NaClO4, 0.03 M TBAPF, 0.01 M TBPPF, 0.01 M HMImPF, 0.30 M TBP, 0.10 M TMSP, 0.10 M MP, 0.05 M CPrBP, 0.10 M CPeBP, and 0.05 M COcBP in ACN) | ADEKA-1 + LEG4 (organic D−π–A structure) | Au/graphene nanoplatelet | 14.3% (1.013 V, 18.36 mA cm–2, 0.754) | 2015, Kakiage et al.11 |
| Co3+/Co2+ (0.22 M [Co2+(bpy)3](B(CN)4)2, 0.05 M [Co3+(bpy)3](B(CN)4)3, 0.1 M LiClO4, and 0.8 M TBP in ACN) | SGT-137 (organic D−π–A structure) + HC-A1 (coadsorbent) | Pt | 12.45% (0.884 V, 18.37 mA cm–2, 0.767) | 2017, Yom et al.5 |
| SGT-137 (organic D−π–A structure) + HC-A1 (coadsorbent)//SGT-021 (porphyrin D−π–A structure) + HC-A4 (coadsorbent) | parallel tandem cell 14.64% (0.878 V, 22.06 mA cm–2, 0.756) | |||
| Co3+/Co2+ (0.25 M [Co2+(bpy)3](TFSI)2, 0.10 M [Co3+(bpy)3](TFSI)3, 0.1 M TBP, and 0.1 M LiTFSI in ACN) | Blue dye, R6 (organic D−π–A structure) | Pt | 12.6% (0.850 V, 19.69 mA cm–2, 0.754) | 2018, Ren et al.80 |
| Co3+/Co2+ (0.22 M [Co2+(bpy)3](TFSI)2, 0.05 M [Co3+(bpy)3](TFSI)3, 0.1 M LiTFSI, and 0.8 M TBP in ACN) | SGT-149 (organic D−π–A structure) + SGT-021 (porphyrin D−π–A structure) + HC-A1 (coadsorbent) | Pt | 14.2% (0.919 V, 21.06 mA cm–2, 0.734) | 2020, Ji et al.9 |
| Co3+/Co2+/TPEA (0.22 M [Co2+(bpy)3](PF6)2, 0.05 M [Co3+(bpy)3](PF6)3, 0.1 M LiClO4, 0.2 M TBP, and 0.1 M TPEA in ACN) | AQ310 (organic D–A−π–A structure) | PEDOT | 11.0% (0.950 V, 15.5 mA cm–2, 0.745) | 2018, Hao et al.114 |
| Cu2+/Cu+ (0.09 M [Cu2+(tmby)2](TFSI)2, 0.20 M [Cu+(tmby)2]TFSI, 0.1 M LiTFSI, and 0.6 M NMB in ACN) | Y123 + XY1b (organic D−π–A structure) + CDCA (coadsorbent) | PEDOT | 13.1% (1.05 V, 15.74 mA cm–2, 0.79) | 2018, Cao et al.12 |
| Cu2+/Cu+ (0.2 M [Cu+(tmby)2] (TFSI), 0.09 M [Cu2+(tmby)2](TFSI)2, 0.1 M LiTFSI, and 0.6 M NMB in ACN) | R7 + Y123 (organic D−π–A structure) | PEDOT | 12.7% (1.04 V, 16.15 mA cm–2, 0.761) | 2020, Ren et al.172 |
| Cu2+/Cu+ (0.2 M [Cu+(tmby)2]TFSI, 0.1 M [Cu2+(tmby)2](TFSI)2, 0.1 M LiTFSI, and 0.6 M NMB in ACN) | MS5 + XY1b (organic, D−π–A structure) + CDCA (coadsorbent) | PEDOT | 13.5% (1.05 V, 15.84 mA cm–2, 0.813) | 2021, Zhang et al.8 |
| Cu2+/Cu+ (0.16 M [Cu+(tmby)2]TFSI, 0.08 M [Cu2+(tmby)2](TFSI)2, 0.1 M NaTFSI, and 0.45 M CEMI in ACN) | SL9 + SL10 (organic, D−π–A structure) + BPHA (preadsorbent) | PEDOT | 15.2% (1.04 V, 17.8 mA cm–2, 0.821) | 2022, Ren et al.29 |
The major limitation of aqueous DSSCs is the low photovoltaic performance due to the mass transport issue136 and significant dye desorption, especially with metal-based and hydrophilic dyes.137 Alcohols, such as methanol and ethanol, are not suitable as solvents in DSSC redox electrolytes due to their hydrophilicity, which can cause dye desorption. Ester- and lactone-based solvents, such as ethylene carbonate (EC), propylene carbonate (PC), and γ-butyrolactone (GBL), are also used in DSSCs due to their lower volatility and high dielectric constant.66,132,138 EC has a high melting point (∼36 °C) and high viscosity. Therefore, a cosolvent is required with EC. However, the performance of these solvents cannot overcome the performance of the ACN-based solvent due to the mass transport limitation of the redox shuttle in these media. GBL has potential as a solvent for DSSC redox electrolytes compared to other lactone-based solvents due to its low melting point (∼−44 °C), high boiling point (∼204 °C), moderate donor number (18), and comparatively low viscosity (∼1.7 cP). N-Methyl-2-pyrrolidone (NMP) has a high donor number (high basicity), so it can simultaneously enhance the Voc and decrease the Jsc value.
4.3. Ionic Liquids
An ionic liquid (IL) is a salt whose melting point is below 100 °C or even at room temperature. The IL is composed of at least one organic ion (usually a cation) with a delocalized charge to prevent a stable crystal lattice. Due to the loosely coordinating bonds between ions, the melting point is below 100 °C. An IL can be an alternative to volatile organic solvents due to several advantages, such as negligible vapor pressure even at the high temperature of the solar cell operation, high electrochemical stability, wide electrochemical window, and the ability to dissociate a wide range of organic and inorganic compounds. Generally, the viscosity of an IL is around 10–500 cP.139 Thus, ILs are far more viscous than the common organic solvents used in DSSC redox electrolytes. The IL is called a “tunable” solvent because its physicochemical properties can be varied by modifying the cationic or anionic components.140 The high viscosity of ILs causes a mass transport limitation of the redox shuttle. A relatively low viscosity and hydrophobicity of ILs are essential for their use in redox electrolytes for DSSCs. Table 2 shows the structure and physical properties of the most common ILs used in DSSC redox electrolytes.
Table 2. Structures and Physical Properties of Ionic Liquids Used in DSSC Redox Electrolytes.
In the 1-alkyl-3-methylimidazolium iodide series, compounds with an alkyl chain from propyl (C3) to nonyl (C9) are viscous liquids, and their viscosity increases with increasing alkyl chain length due to the increasing van der Waals interactions. Kubo et al. studied a series of 1-alkyl-3-methylimidazolium iodide ionic liquids with alkyl chain lengths from C3 to C9 as molten salts in iodine electrolytes and found that the 1-hexyl-3-methylimidazolium iodide molten salt electrolyte had the best photovoltaic performance despite having a higher viscosity and conductivity than the lower alkyl chain length members.141 The Jsc value increases with increasing alkyl chain length from propyl (C3) to heptyl (C7) and then decreases for longer chain lengths. They reported that the reason for the Jsc value increasing with increasing alkyl chain length from C3 to C7 is due to the enhanced diffusion coefficient of electrons in the TiO2/electrolyte system. The increasing alkyl chain length increases the van der Waals interaction and aggregation of the 1-alkyl-3-methylimidazolium cation, which could enhance the adsorption of this cation onto the TiO2 surfaces. As a consequence, the Jsc value may increase with increasing diffusion of electrons in TiO2 due to the enhanced adsorption of the 1-alkyl-3-methylimidazolium cation.141,142 In the imidazolium-based ionic liquid, the viscosity can be reduced by changing its counteranion to a loosely coordinating anion.139 1-Ethyl-3-methylimidazolium iodide (EMII) is solid at room temperature, and the viscosity of the 1-ethyl-3-methylimidazolium (EMI) cation based ionic liquid decreased in the following order: EMI+(PF6)− < EMI+(BF4)− < EMI+[N(CF3SO2)2]− < EMI+(CF3COO)− < EMI+[B(CN)4]− or EMI+[C(CN)3]−.143−145 Wang et al. achieved a remarkable efficiency of 7.4% under 1-sun illumination completely using ionic liquids as the solvent.143 They used binary ionic liquids, 1-propyl-3-methylimidazolium iodide (PMII) and 1-ethyl-3-methylimidazolium tricyanomethanide (EMITCM), as nonvolatile solvents. The viscosities of EMITCM and PMII are around 18 cP (at 22 °C) and 865 cP (at 25 °C), respectively.141,146 Thus, due to the loosely coordinating bond between the tricyanomethanide (TCM) anion and the 1-ethyl-3-methylimidazolium (EMI) cation, the viscosity of the EMITCM ionic liquid is significantly reduced. The reason for using binary ionic liquids is to reduce the viscosity of the media; therefore, the mass transport limitation of the redox shuttle can be suppressed. Kuang et al. reported a 7.6% efficiency at simulated 1 sun illumination using 65% PMII with 35% 1-ethyl-3-methylimidazolium tetracyanoborate (EMIB(CN)4) as binary ionic liquids in organic-solvent-free I3–/I– redox electrolytes. They also claimed this binary ionic liquid based device was highly stable even at 80 °C in the dark. During a 1000 h accelerated test under 80 °C and dark conditions, they claimed that their device was able to retain more than 90% of its initial efficiency.147 Due to the high viscosity of the ionic liquid, when the ionic liquid is used as a solvent in redox electrolytes, a high concentration of the redox shuttle is required to compensate for the mass transport limitation for fast dye regeneration. The Grotthus-type charge transport mechanism (eq 26) may affect the overall transport with increasing iodide concentration in ionic-liquid-based I3–/I– redox electrolytes.63
| 26 |
Thus, triiodide might transfer to the counter electrode not only by diffusion but also by other nondiffusional hopping type mechanisms.63,148 However, a high concentration of the redox shuttle (iodide species) can increase the probability of the reductive quenching of the excited dye.149 Wang et al. discovered evidence of the reductive quenching of the dye excited state by a very high iodide concentration (eq 27) in PMII ionic-liquid-based I3–/I– redox electrolytes. They also reported from their transient laser experiment that reduced dye species (S–) do not inject electrons into the TiO2 conduction band; rather, they decay by a slow reaction process (t1/2 ≈ 1 ms) with oxidized species of electrolytes (I3–).143
| 27 |
Thus, reductive quenching of the excited dye can cause a low photocurrent of the device. The short-circuit current density and PCE of the device decrease with increasing viscosity of the ionic liquid.148 From a comparison of the impedance of the room-temperature ionic liquid (RTIL) and liquid solvent based cells, it was determined that a higher recombination and lower injection efficiency are the main causes of the limiting performance of RTIL-based cells.147 Due to the limitation of the performance, the ionic liquid is not suitable as a single solvent in redox electrolytes; rather, it can be mixed with an organic solvent to enhance the performance of the device. Despite the high viscosity and mass transport limitation of the RTILs, several attempts have been made to use an RTIL as a single nonvolatile solvent in DSSCs to enhance both the performance and stability of the devices. Shi et al. tested two types of redox liquid electrolytes: a low-volatility electrolyte (1) organic solvent MPN with a combination of an ionic liquid salt DMII and additives in the I3–/I– redox couple and a nonvolatile electrolyte (2) fully organic-solvent-free mixed ionic liquids EMII, EMIB(CN)4 with a combination of DMII and the same additives.150 They obtained an efficiency of 9.6% for the organic solvent based low-volatility electrolytes and 8.6% for the fully ionic liquid based nonvolatile electrolytes under standard AM 1.5G illumination. However, in a long-term stability test, both low-volatility organic solvent based and nonvolatile ionic liquid based devices were stable with an insignificant difference (efficiency dropping 9% for the low-volatility electrolytes and 6% for the ionic liquid electrolytes) under 60 °C and full sunlight for 1000 h.150 Recently, Bousrez et al. reported that the 1-alkyl-3-methyltriazolium iodide ionic liquids can be more suitable in DSSC electrolytes over imidazolium iodides due to their less hygroscopic nature. In the 1-alkyl-3-methyltriazolium iodide series, compounds with an alkyl chain length from C4 to C10 exhibit the characteristics of ionic liquids. They found that the 1-butyl-3-methyltriazolium iodide ionic liquid based device showed the best efficiency of 6.63% under 40 °C and 1-sun conditions.151
Although ionic liquids limit mass transport due to their high viscosity, this class of compounds has great potential due to the enhancement of the stability of the device. Under ambient light conditions, the operation of the device requires a lower mass transport of the redox shuttle due to the lower photocurrent generated by the dye in low light. Therefore, in indoor photovoltaics, the ionic liquid can play a significant role as a nonvolatile solvent. By proper optimization of the device, the PCE under 1 sun may also be enhanced to a certain extent. The most important use of this class of compounds can be as additives in organic solvent based liquid redox electrolytes and iodide sources of I–/I3– redox electrolytes.
4.4. Additives for the Redox Electrolytes
Additives are certain compounds whose presence in electrolytes can improve the photovoltaic performance of the solar cell. The common additives used in DSSC redox electrolytes are shown in Scheme 2.
Scheme 2. Structures of the Most Effective Additives in DSSC Redox Electrolytes.
Nazeeruddin et al. first reported that a TBP treatment in a dye-sensitized TiO2 electrode significantly enhanced the Voc and fill factor of the device due to the suppression of the interfacial recombination reaction between the injected electrons in TiO2 and the oxidized species of the redox couple.133 The Voc value for DSSCs with the I3–/I– redox electrolyte can be expressed by eq 28(133)
| 28 |
where Iinj is the flux of the charge from injection by the sensitizer to the TiO2 semiconductor, ncb is the carrier electron density on the conduction band of TiO2, and krec is the rate constant of the interfacial recombination reaction (back electron transfer from TiO2 to I3–; see eq 29).
| 29 |
Therefore, the Voc value can be enhanced by reducing the rate of the interfacial recombination reaction (eq 28). Adsorption of electron-donating TBP at the electron-accepting TiO2 sites causes passivation at the TiO2 surface, which suppresses the back electron transfer from TiO2 to the oxidized species (electron acceptor) of the electrolytes and reduction in the electron injection efficiency from the excited dye. Boschloo et al. quantified the effect of the addition of TBP to the I3–/I– redox electrolytes in DSSCs. From the charge extraction and electron lifetime measurement, they concluded that the increase in the Voc of around 260 mV due to the addition of 0.5 M TBP in MPN solvent based I3–/I– electrolytes could be attributed to both a shift of the TiO2 conduction band toward negative potential (∼60% contribution) and an enhancement of the electron lifetime (∼40% contribution).152 The adsorption of TBP on the TiO2 surface may decrease the chance of I3– accessing the TiO2 surface. The increase in the electron lifetime may be attributed to the blocking effect of TBP and the complexation of free iodine with TBP, which is much more pronounced at higher TBP concentrations and for the MPN-based solvent than for the acetonitrile solvent.152 However, the major factors for Voc enhancement can be attributed to the shifting of the TiO2 conduction band edge toward negative potential153,154 to suppress the interfacial recombination reaction of the injected electron and oxidized species (electron acceptor) of the redox electrolytes.133,155 Besides, the presence of TBP may decrease the amount of adsorbed protons and cations on the TiO2 surface. However, TBP and pyridine can be adsorbed readily on the Pt surface by its nitrogen atom in the aromatic ring.156,157 Kim et al. studied the effect of TBP on DSSC electrodes. They found from the EIS measurement of the Pt-FTO/iodine electrolytes/Pt-FTO cell that the catalytic effect of Pt can deteriorate and the diffusion resistance of electrolytes can be increased by a higher TBP concentration (>0.5 M).158 There is no measurable influence on the charge transfer resistance in the counter electrode and the diffusion due to the addition of TBP in Co3+/Co2+ complex redox electrolytes. However, TBP harms the charge transfer in the counter electrode and the diffusion in the Cu2+/Cu+ complex redox system.106 The Cu(II) species is the diffusion-controlling ion (low concentration of Cu(II) species) in the Cu(II)/Cu(I) redox system. The Cu(II) species is susceptible to altering its coordination sphere, which may influence its coordination geometry and increase its coordination number from 4 to 6. The substitution of the ligand by TBP, solvent, and/or counterions in the Cu(II) complex may happen.159 The Cu(II) species in the redox solution can become bulky and hydrophobic due to this change.106 Therefore, the reduced charge transfer and diffusion can be observed due to the changes of the Cu(II) species. Ferdowsi et al. found correlations of the basicity (pKa) of the pyridine bases with the charge transfer rate in the PEDOT counter electrode and the diffusion resistances of the copper redox mediator. By studying the series of pyridine bases, they found that an increase in the basicity of the pyridine bases leads to a decrease in the formal rate constant of the charge transfer and an increase in the diffusion coefficient.160 However, a weak Lewis base like 2,6-bis-tert-butylpyridine (pKa ≈ 3–4) is ineffective in increasing Voc by shifting the TiO2 conduction band edge upward. Therefore, bases with a pKa value near 6.0 like TBP and 4-(5-nonyl)pyridine can be effective in enhancing the Voc and the overall performance of the solar cell.
The pKa value of 1-methylbenzimidazole or N-methylbenzimidazole (MBI or NMB) (∼6.0)161 is almost the same as that of TBP. The role of MBI as an additive in DSSC redox electrolytes is also the same as that of TBP: i.e., an improvement in the Voc value by shifting the TiO2 conduction band edge to a more negative potential and enhancing the electron lifetime on the TiO2 surface.162 Like TBP, MBI may also affect the TiO2 surface charge by reducing the Li+ ion adsorption on the TiO2 surface and may suppress the back electron transfer to the electron acceptor species of electrolytes by interacting with them.163 Recently, it was found that the effect of MBI in enhancing Voc is much more pronounced than that of TBP in I3–/I– electrolytes.19 However, MBI reduces the Jsc value more in comparison with TBP probably due to the lower electron injection efficiency by a higher upshift of the TiO2 conduction band. There was no significant difference in the overall PCE and stability of the device due to the use of either the TBP or MBI additive in I3–/I– electrolytes.19 The best power conversion efficiencies for copper complex redox mediators have been achieved by using the MBI additive (see Table 3). Therefore, MBI may be more suitable as a Lewis base additive in copper electrolytes over TBP. Recently, Fürer et al. studied the role of Lewis base additives in the copper redox mediator.164 They concluded from their CV analysis that the coordination of strong Lewis bases like TBP and MBI with the Cu(II) complex (coordination number 4) is the main factor for efficient blocking of the electron recombination with the Cu(II) species (see Figure 12).
Figure 12.

Effect of the coordination of an additional Lewis base (LB) on the oxidation and reduction in copper complexes, with the most favorable paths for oxidation and reduction being ACD and DBA, respectively, and the possible mechanism of dye regeneration and electrolyte regeneration by copper complexes in the presence of LB. Reprinted with permission from ref (164). Copyright 2020 Wiley.
Fürer et al. achieved the best efficiency with the MBI additive in [Cu(dmp)2]2+/+ redox mediators mainly due to the improvement in Voc with minimal sacrifice of the Jsc value.164 However, it is still unclear why the Jsc value is more reduced in the case of TBP rather than the MBI additive in Cu(dmp)2+/+ redox electrolytes. They also claimed that a weak Lewis base additive, which may not coordinate with the Cu(II) center, can reduce the photovoltaic performance due to a higher charge recombination. The possibly rational reasons for the low cell performance with weak LB additive are the inability of a weak LB additive to increase Voc by shifting the TiO2 conduction band edge upward160 and the ineffectiveness in blocking charge recombination processes. The binding or coordination of Cu(II) complexes with the additional Lewis base additive TBP or MBI may slow down the reduction of the Cu(II) species at the counter electrode164 and complicate the redox process. It should be noted that the binding or coordination of Cu(II) complexes can negatively shift the redox potential,165,166 which can enhance the rate of the charge recombination reaction due to the lower driving force for recombination.166 Recently, Ren et al. obtained a high FF without sacrificing Jsc and Voc by using a 5-chloro-1-ethyl-2-methylimidazole (CEMI) additive in [Cu(tmby)2]2+/+ redox electrolytes instead of using MBI.29 The high FF may be due to the low interfacial transport resistance between the electrolyte and the counter electrode. The comparable Jsc and Voc values with MBI additive indicate a similar interaction of [Cu(tmby)2]2+/+and CEMI.
The adoption of certain cations also affects the performance of the DSSC. From laser-pulse-induced photocurrent transient measurements, Kambe et al. found that the photogenerated electron in the TiO2 electrode increases in the order DMHI+ < TBA+ < Na+ < Li+.142 At high concentrations, the adsorption of Li+ on the TiO2 surface boosts the photogeneration of electrons and also increases the electron diffusion coefficient in the TiO2 electrode. The multilayer adsorption of the imidazolium cation, DHMI+, increases electron diffusion to a great extent but lowers the photogeneration of electrons.142 The presence of a high concentration of both Li+ and imidazolium cations in the electrolyte solution may be beneficial due to the increase of electron diffusion and the optimum photogeneration of electrons. Guanidinium thiocyanate (GuSCN) is also used as an additive in DSSC redox electrolytes. The guanidinium cation affects the photocurrent in the same way as other cations, shifting the TiO2 conduction band toward a positive potential, which increases the electron injection efficiency.167 The adsorbent Gu+ suppresses the electron recombination at the TiO2/electrolyte surface by passivating recombination sites. The net effect of the downward shift of the TiO2 conduction and slower recombination due to the adsorbent Gu+ may increase the overall Voc up to 20 mV.168 Like Li+, intercalation of Gu+ into TiO2 is not anticipated. However, Kopidakis et al. reported that the Li+ interaction in the TiO2 film does not affect the photovoltaic performance of the DSSC and only has a small influence on the charge collection efficiency and the rate of recombination.169
5. Progress of Photovoltaic Performance of DSSCs by Liquid Redox Electrolytes
5.1. Under 1-Sun Illumination
In Table 3, the outstanding photovoltaic performances of DSSCs by using redox liquid electrolytes under standard AM 1.5G (100 mW cm–2) illumination are summarized. The significant PCEs of 11.7% and 12.4% with a ruthenium dye68 and concerted companion dye,10 respectively, for I3–/I– redox shuttles, 14.3% with two cosensitized organic dyes, for a Co3+/Co2+ redox shuttle, and 15.2% with two cosensitized organic dyes8 for a Cu2+/Cu+ redox shuttle have been reported under standard AM 1.5G (1-sun) illumination by optimizing the dye structure, the counter electrode, and device fabrication.
5.2. Under Ambient Light Conditions
The intensity of indoor light is much lower than that of outdoor light. The spectra of ambient light also vary significantly with the solar emission spectra. In Figure 13, a comparison of the emission spectra of fluorescence and LED bulbs with the spectra of AM 1.5G standard is shown.173 The intensity of standard indoor light is around 200–2000 lx, which is around 0.2–2% of the full sun (100 mW cm–2, AM 1.5G) illumination (100000–110000 lx).
Figure 13.

Normalized emission spectra of different ambient light bulbs and AM 1.5G standard. Reprinted with permission from ref (172). Copyright 2021 Royal Society of Chemistry.
The low photocurrent is generated by indoor light conditions. Therefore, a low concentration of the redox couple is required to operate the solar cell and for efficient dye regeneration. The reduced concentration of redox species in electrolytes for indoor light conditions suppresses the interfacial charge recombination.8 Outstanding performances under ambient light have already been achieved for DSSCs. The exciting performances for liquid-state DSSCs under ambient light conditions reported in the literature are shown in Table 4. A record power conversion efficiency of 34.5% was reported for DSSCs by using copper complex redox shuttles.8 The outstanding performances of DSSCs under indoor light conditions make this technology a promising and clean energy source to integrate in IoT and low-energy-consumption electronic devices.
Table 4. Progress of the Photovoltaic Performances for DSSCs Using Various Redox Electrolytes under Ambient Light Illumination.
| liquid electrolyte composition | sensitizer | counter electrode catalyst | light source | illumination | reported efficiency (Voc, Jsc, FF) | year, ref. |
|---|---|---|---|---|---|---|
| I3–/I– (0.07 M LiI, 1 M PMII, 0.05 M I2, 0.05 M TBP in ACN/VN (85:15, v/v) | anthracene-based D–A−π–A sensitizer (TY6) + CDCA (coadsorbent) | PVP-Pt | T5-fluorescent lamp | 1200 lx, Pin = 345 μW cm–2 | 24.4% (0.671 V, 164 μA cm–2, 0.778) | 2017, Tingare et al.174 |
| I3–/I– (EL-HPE; iodine, inorganic iodide salt, organic iodide salt, pyridine derivatives in ACN and VN solvent mixture) | N719 + CDCA (coadsorbent) | Pt | LED (Osram, class A, 86 W, 2700 K) | Pin = 1577 μW cm–2 | 28.7% (0.640 V, 910.8 μA cm–2, 0.78) | 2019, Hora et al.175 |
| Co3+/Co2+ (0.11 M [Co2+(bpy)3](PF6)2, 0.025 M [Co3+(bpy)3](PF6)3, 0.1 M LiClO4, and 1.2 M TBP in MPN) | Y123 | Pt | T5-fluorescent lamp | 999.6 lx, Pin = 311.47 μW cm–2 | 24.5% (0.846 V, 124.38 μA cm–2, 0.725) | 2019, Venkatesan et al.176 |
| Cu2+/Cu+ (0.04 M [Cu(tmby)2](TFSI)2, 0.20 M [Cu(tmby)2]TFSI, 0.1 M LiTFSI and 0.6 M tributyl phosphate in ACN) | XY1 + D35 (organic, D–A−π–A, and D−π–A structure) | PEDOT | Osram 930 warm white fluorescent light | 1000 lx, Pin = 306.6 μW cm–2 | 28.9% (0.797 V, 138 μA cm–2, 0.80) | 2017, Freitag et al.177 |
| Cu2+/Cu+ (0.04 M [Cu(tmby)2](TFSI)2, 0.20 M [Cu(tmby)2]TFSI, 0.1 M LiTFSI, and 0.6 M MBI in ACN) | Y123 + XY1b (organic D−π–A structure) + CDCA (coadsorbent) | PEDOT | Osram 930 warm white fluorescent light | 1000 lx, Pin = 318.2 μW cm–2 | 31.8% (0.878 V, 0.418 mA, 0.773) | 2018, Cao et al.12 |
| Cu2+/Cu+ (0.2 M [Cu(tmby)2]TFSI and 0.04 M [Cu(tmby)2][TFSI]2, 0.1 M LiTFSI, and 0.6 M TBP in ACN) | XY1 + L1 (organic D–A−π–A and D−π–A structure) + CDCA (coadsorbent) | PEDOT | Osram 930 warm white fluorescent light | 1000 lx, Pin = 303.1 μW cm–2 | 34.0% (0.910 V, 147 μA cm–2, 0.77) | 2020, Michaels et al.81 |
| Cu2+/Cu+ (0.1 M [Cu(tmby)2]TFSI, 0.04 M [Cu(tmby)2](TFSI)2, 0.1 M LiTFSI, and 0.6 M MBI in ACN) | MS5 + XY1b (organic D−π–A and D–A−π–A structure) + CDCA (coadsorbent) | PEDOT | Osram 930 warm white fluorescent light | 1000 lx, Pin = 318.2 μW cm–2 | 34.5% (0.98 V, 0.387 mA, 0.815) | 2021, Zhang et al.8 |
6. Summary and Outlook
The redox electrolyte is the major component of a DSSC, which affects the PCE and long-term stability of the device. By using an I3–/I– redox shuttle, a remarkable PCE of up to 12.4% has been achieved under 1-sun illumination. However, its low positive redox potential restricts the Voc of the device to 0.8 V. Also, its drawbacks, such as competitive visible light absorption with dye, corrosiveness toward metal, and volatile nature of iodine, limit the large-scale module development by using the I3–/I– redox shuttle. Thus, Co complex based redox shuttles with a high Voc value have received great attention due to the less competitive visible light absorption, with tuning of the redox potential by replacing ligands. An outstanding PCE of over 14% has been achieved by using the [Co(bpy)3]3+/[Co(bpy)3]2+ redox shuttle, however, slow mass transport and large reorganization energy in the counter electrode still limit the Voc value to 1 V. Recently, using a Cu complex, a [Cu(tmby)2]2+/[Cu(tmby)2]+ redox shuttle, the PCE of DSSC under 1-sun illumination has reported to be 15.2% with a Voc of over 1 V. Although the Voc value was improved significantly by using the [Cu(tmby)2]2+/[Cu(tmby)2]+ redox shuttle, further improvement of Jsc value using this redox shuttle is difficult, due to the competition of the visible light absorption of Cu complexes and the requirement of using a dye with a higher positive HOMO level, which limits the scope of the entire range of visible light utilization. Due to the higher positive redox potential of [Cu(tmby)]2+/[Cu(tmby)]+ complexes (∼0.87 V vs NHE), most of the developed high-efficiency porphyrin dyes, such as SM315 (EHOMO ≈ 0.89 V),105 SGT-021 (EHOMO ≈ 0.82 V),4 and XW17 (EHOMO ≈ 0.68 V),178 some organic dyes, such as SGT-149 (EHOMO ≈ 0.84),9 and near-infrared (NIR) absorbing organic dyes, such as ISQ1 (EHOMO ≈ 0.62 V)179 and AP3 (EHOMO ≈ 0.74 V),178 cannot be explored with this Cu redox shuttle because of the insufficient driving force for dye regeneration. Therefore, research is needed to find a suitable redox shuttle with a redox potential of 0.45–0.65 V or replacement of a ligand to tune the redox potential of the Cu complexes, so that most of the porphyrin and organic dyes can be utilized.
It has been assessed that the commercial application of DSSCs for outdoor applications may be possible and economically beneficial if the PCE of the device exceeds 15% under 1-sun illumination30 and the device retains high stability under light-soaking at a high temperature (∼60 °C).180 Therefore, as proposed and depicted in Scheme 3 for the PCE enhancement of over 16% (1) simultaneously improving Jsc to over 21 mA/cm2 and Voc to 1.0 V of the device can be feasible by utilizing cosensitization of currently developed organic/porphyrin dyes with a NIR-absorbing dye and redox shuttles with a redox potential around 0.45–0.65 V and (2) improving the FF to 0.80 can be possible by using a superior counter electrode catalyst with a specific redox shuttle and proper device optimization.29,170,181,182
Scheme 3. Proposed Strategy for the PCE Enhancement of over 16% in DSSCs under 1-Sun Illumination.

Redox shuttles with a redox potential around 0.45–0.65 V can give a theoretical Voc in the range of 0.95–1.15 V. The use of cosensitization of previously developed porphyrin, organic, and NIR absorbing dyes can broaden whole visible light absorption, which surely can enhance the Jsc value by entire visible light utilization. Depending on the cosensitization system and maintenance of an optimum driving force of ∼0.2–0.3 V for dye regeneration, the use of a metal complex (especially, Co or Cu complexes) redox shuttle with redox potential of ∼0.45–0.65 V may enhance the Voc and Jsc values of the device simultaneously by reducing small potential loss and increasing the LHE. The proper optimization of the device and use of a superior catalyst for the specific redox shuttle can reduce the charge transfer resistances and enhance the fill factor of the devices.
In DSSC redox LEs, ACN-based solvents were highly effective; however, they are volatile. Ionic liquids can play a significant role as nonvolatile additives in the redox LEs to improve the stability of the device. The improvement of sealing by using advanced techniques and the development of semisolid electrolytes are also demanded for the long-term stability of the devices. Both TBP and MBI were effective as Lewis base additives in DSSC redox electrolytes; however, MBI was more effective for copper electrolytes than TBP. The additional coordination of Cu(II) complexes with the strong Lewis base TBP can slow down the reduction of Cu(II) species in the counter electrode, complicate the redox process, and negatively shift the redox potential. The record PCE of over 34% under ambient light was obtained by using a [Cu(tmby)2]2+/+ complex redox shuttle, which ensures the great potential of copper-based redox electrolytes for commercial application in DSSCs to power IoT and low-power-consumption electronic devices.
Acknowledgments
This work was supported by the Korean government (the Ministry of Science, ICT, and Future Planning) through the Midcareer Researcher Program (NRF-2021R1A2C2006328) and “Human Resources Program in Energy Technology” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resources from the Ministry of Trade, Industry & Energy, Republic of Korea (KETEP-20204030200070).
The authors declare no competing financial interest.
References
- Perez R.; Zweibel K.; Hoff T. E. Solar Power Generation in the US: Too Expensive, or a Bargain?. Energy Policy 2011, 39 (11), 7290–7297. 10.1016/j.enpol.2011.08.052. [DOI] [Google Scholar]
- Launch of new RICOH EH DSSC modules with 20% increase in power generation | Global | Ricoh. https://www.ricoh.com/release/2021/0513_1/ (accessed 2021-05-31).
- News - Exeger. https://www.exeger.com/news/ (accessed 2021-08-11).
- Kang S. H.; Jeong M. J.; Eom Y. K.; Choi I. T.; Kwon S. M.; Yoo Y.; Kim J.; Kwon J.; Park J. H.; Kim H. K. Porphyrin Sensitizers with Donor Structural Engineering for Superior Performance Dye-Sensitized Solar Cells and Tandem Solar Cells for Water Splitting Applications. Adv. Energy Mater. 2017, 7 (7), 1602117. 10.1002/aenm.201602117. [DOI] [Google Scholar]
- Eom Y. K.; Kang S. H.; Choi I. T.; Yoo Y.; Kim J.; Kim H. K. Significant Light Absorption Enhancement by a Single Heterocyclic Unit Change in the π-Bridge Moiety from Thieno[3,2-b]Benzothiophene to Thieno[3,2-b]Indole for High Performance Dye-Sensitized and Tandem Solar Cells. J. Mater. Chem. A 2017, 5 (5), 2297–2308. 10.1039/C6TA09836C. [DOI] [Google Scholar]
- Eom Y. K.; Choi I. T.; Kang S. H.; Lee J.; Kim J.; Ju M. J.; Kim H. K. Thieno[3,2-b][1]Benzothiophene Derivative as a New π-Bridge Unit in D−π–A Structural Organic Sensitizers with Over 10.47% Efficiency for Dye-Sensitized Solar Cells. Adv. Energy Mater. 2015, 5 (15), 1500300. 10.1002/aenm.201500300. [DOI] [Google Scholar]
- Ji J. M.; Zhou H.; Kim H. K. Rational Design Criteria for D-π-A Structured Organic and Porphyrin Sensitizers for Highly Efficient Dye-Sensitized Solar Cells. J. Mater. Chem. A 2018, 6 (30), 14518–14545. 10.1039/C8TA02281J. [DOI] [Google Scholar]
- Zhang D.; Stojanovic M.; Ren Y.; Cao Y.; Eickemeyer F. T.; Socie E.; Vlachopoulos N.; Moser J.-E. E.; Zakeeruddin S. M.; Hagfeldt A.; Grätzel M. A Molecular Photosensitizer Achieves a V Oc of 1.24 V Enabling Highly Efficient and Stable Dye-Sensitized Solar Cells with Copper(II/I)-Based Electrolyte. Nat. Commun. 2021, 12 (1), 1777. 10.1038/s41467-021-21945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J. M.; Zhou H.; Eom Y. K.; Kim C. H.; Kim H. K. 14.2% Efficiency Dye-Sensitized Solar Cells by Co-Sensitizing Novel Thieno[3,2-b]Indole-Based Organic Dyes with a Promising Porphyrin Sensitizer. Adv. Energy Mater. 2020, 10 (15), 2000124. 10.1002/aenm.202000124. [DOI] [Google Scholar]
- Zeng K.; Chen Y.; Zhu W. H.; Tian H.; Xie Y. Efficient Solar Cells Based on Concerted Companion Dyes Containing Two Complementary Components: An Alternative Approach for Cosensitization. J. Am. Chem. Soc. 2020, 142 (11), 5154–5161. 10.1021/jacs.9b12675. [DOI] [PubMed] [Google Scholar]
- Kakiage K.; Aoyama Y.; Yano T.; Oya K.; Fujisawa J. I.; Hanaya M. Highly-Efficient Dye-Sensitized Solar Cells with Collaborative Sensitization by Silyl-Anchor and Carboxy-Anchor Dyes. Chem. Commun. 2015, 51 (88), 15894–15897. 10.1039/C5CC06759F. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Liu Y.; Zakeeruddin S. M.; Hagfeldt A.; Grätzel M. Direct Contact of Selective Charge Extraction Layers Enables High-Efficiency Molecular Photovoltaics. Joule 2018, 2 (6), 1108–1117. 10.1016/j.joule.2018.03.017. [DOI] [Google Scholar]
- Daeneke T.; Kwon T. H.; Holmes A. B.; Duffy N. W.; Bach U.; Spiccia L. High-Efficiency Dye-Sensitized Solar Cells with Ferrocene-Based Electrolytes. Nat. Chem. 2011, 3 (3), 211–215. 10.1038/nchem.966. [DOI] [PubMed] [Google Scholar]
- Hattori S.; Wada Y.; Yanagida S.; Fukuzumi S. Blue Copper Model Complexes with Distorted Tetragonal Geometry Acting as Effective Electron-Transfer Mediators in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2005, 127 (26), 9648–9654. 10.1021/ja0506814. [DOI] [PubMed] [Google Scholar]
- Feldt S. M.; Gibson E. A.; Gabrielsson E.; Sun L.; Boschloo G.; Hagfeldt A. Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2010, 132 (46), 16714–16724. 10.1021/ja1088869. [DOI] [PubMed] [Google Scholar]
- Saygili Y.; Söderberg M.; Pellet N.; Giordano F.; Cao Y.; Munoz-García A. B.; Zakeeruddin S. M.; Vlachopoulos N.; Pavone M.; Boschloo G.; Kavan L.; Moser J. E.; Grätzel M.; Hagfeldt A.; Freitag M. Copper Bipyridyl Redox Mediators for Dye-Sensitized Solar Cells with High Photovoltage. J. Am. Chem. Soc. 2016, 138 (45), 15087–15096. 10.1021/jacs.6b10721. [DOI] [PubMed] [Google Scholar]
- Yang W.; Vlachopoulos N.; Hao Y.; Hagfeldt A.; Boschloo G. Efficient Dye Regeneration at Low Driving Force Achieved in Triphenylamine Dye LEG4 and TEMPO Redox Mediator Based Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2015, 17 (24), 15868–15875. 10.1039/C5CP01880C. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Chen P.; Murakami T. N.; Zakeeruddin S. M.; Grätzel M. The 2,2,6,6-Tetramethyl-l-Piperidinyloxy Radical: An Efficient, Iodine-Free Redox Mediator for Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2008, 18 (2), 341–346. 10.1002/adfm.200701041. [DOI] [Google Scholar]
- Masud; Kim K. M.; Kim H. K. Highly Efficient Gel Electrolytes by End Group Modified PEG-Based ABA Triblock Copolymers for Quasi-Solid-State Dye-Sensitized Solar Cells. Chem. Eng. J. 2021, 420, 129899. 10.1016/j.cej.2021.129899. [DOI] [Google Scholar]
- Suzuka M.; Hayashi N.; Sekiguchi T.; Sumioka K.; Takata M.; Hayo N.; Ikeda H.; Oyaizu K.; Nishide H. A Quasi-Solid State DSSC with 10.1% Efficiency through Molecular Design of the Charge-Separation and -Transport. Sci. Rep. 2016, 6 (June), 1–7. 10.1038/srep28022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.-L.; Chang T.-W.; Teng H.; Wu C.-G.; Chen C.-Y.; Yang Y.-M.; Lee Y.-L. Highly Efficient Gel-State Dye-Sensitized Solar Cells Prepared Using Poly(Acrylonitrile-Co-Vinyl Acetate) Based Polymer Electrolytes. Phys. Chem. Chem. Phys. 2013, 15 (10), 3640–3645. 10.1039/c3cp50170a. [DOI] [PubMed] [Google Scholar]
- Jeon I.-Y.; Kim H. M.; Kweon D. H.; Jung S.-M.; Seo J.-M.; Shin S.-H.; Choi I. T.; Eom Y. K.; Kang S. H.; Kim H. K.; Ju M. J.; Baek J.-B. Metalloid Tellurium-Doped Graphene Nanoplatelets as Ultimately Stable Electrocatalysts for Cobalt Reduction Reaction in Dye-Sensitized Solar Cells. Nano Energy 2016, 30, 867–876. 10.1016/j.nanoen.2016.09.001. [DOI] [Google Scholar]
- Ju M. J.; Jeon I.-Y.; Kim H. M.; Choi J. I.; Jung S.-M.; Seo J.-M.; Choi I. T.; Kang S. H.; Kim H. S.; Noh M. J.; Lee J.-J.; Jeong H. Y.; Kim H. K.; Kim Y.-H.; Baek J.-B. Edge-Selenated Graphene Nanoplatelets as Durable Metal-Free Catalysts for Iodine Reduction Reaction in Dye-Sensitized Solar Cells. Sci. Adv. 2016, 2 (6), e1501459. 10.1126/sciadv.1501459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M. J.; Jeon I.-Y.; Kim J. C.; Lim K.; Choi H.-J.; Jung S.-M.; Choi I. T.; Eom Y. K.; Kwon Y. J.; Ko J.; Lee J.-J.; Kim H. K.; Baek J.-B. Graphene Nanoplatelets Doped with N at Its Edges as Metal-Free Cathodes for Organic Dye-Sensitized Solar Cells. Adv. Mater. 2014, 26 (19), 3055–3062. 10.1002/adma.201304986. [DOI] [PubMed] [Google Scholar]
- Ju M. J.; Jeon I.-Y.; Lim K.; Kim J. C.; Choi H.-J.; Choi I. T.; Eom Y. K.; Kwon Y. J.; Ko J.; Lee J.-J.; Baek J.-B.; Kim H. K. Edge-Carboxylated Graphene Nanoplatelets as Oxygen-Rich Metal-Free Cathodes for Organic Dye-Sensitized Solar Cells. Energy Environ. Sci. 2014, 7 (3), 1044–1052. 10.1039/C3EE43732A. [DOI] [Google Scholar]
- Ellis H.; Vlachopoulos N.; Häggman L.; Perruchot C.; Jouini M.; Boschloo G.; Hagfeldt A. PEDOT Counter Electrodes for Dye-Sensitized Solar Cells Prepared by Aqueous Micellar Electrodeposition. Electrochim. Acta 2013, 107, 45–51. 10.1016/j.electacta.2013.06.005. [DOI] [Google Scholar]
- Aftabuzzaman M.; Lu C.; Kim H. K. Recent Progress on Nanostructured Carbon-Based Counter/Back Electrodes for High-Performance Dye-Sensitized and Perovskite Solar Cells. Nanoscale 2020, 12 (34), 17590–17648. 10.1039/D0NR04112B. [DOI] [PubMed] [Google Scholar]
- Zou J.; Wang Y.; Baryshnikov G.; Luo J.; Wang X.; Ågren H.; Li C.; Xie Y. Efficient Dye-Sensitized Solar Cells Based on a New Class of Doubly Concerted Companion Dyes. ACS Appl. Mater. Interfaces 2022, 14 (29), 33274–33284. 10.1021/acsami.2c07950. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Zhang D.; Suo J.; Cao Y.; Eickemeyer F. T.; Vlachopoulos N.; Zakeeruddin S. M.; Hagfeldt A.; Grätzel M. Hydroxamic Acid Preadsorption Raises Efficiency of Cosensitized Solar Cells. Nature 2023, 613, 60. 10.1038/s41586-022-05460-z. [DOI] [PubMed] [Google Scholar]
- Higashino T.; Imahori H. Emergence of Copper(I/II) Complexes as Third-Generation Redox Shuttles for Dye-Sensitized Solar Cells. ACS Energy Lett. 2022, 7 (6), 1926–1938. 10.1021/acsenergylett.2c00716. [DOI] [Google Scholar]
- Sastrawan R.; Beier J.; Belledin U.; Hemming S.; Hinsch A.; Kern R.; Vetter C.; Petrat F. M.; Prodi-Schwab A.; Lechner P.; Hoffmann W. A Glass Frit-Sealed Dye Solar Cell Module with Integrated Series Connections. Sol. Energy Mater. Sol. Cells 2006, 90 (11), 1680–1691. 10.1016/j.solmat.2005.09.003. [DOI] [Google Scholar]
- Martins J.; Emami S.; Ivanou D.; Mendes A. Ultralow Temperature Glass Frit Encapsulation for Stable Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5 (11), 14185–14192. 10.1021/acsaem.2c02714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masud; Kim K. M.; Kim H. K. Polymer Gel Electrolytes Based on PEG-Functionalized ABA Triblock Copolymers for Quasi-Solid-State Dye-Sensitized Solar Cells: Molecular Engineering and Key Factors. ACS Appl. Mater. Interfaces 2020, 12 (37), 42067–42080. 10.1021/acsami.0c09519. [DOI] [PubMed] [Google Scholar]
- Hagfeldt A.; Boschloo G.; Sun L.; Kloo L.; Pettersson H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110 (11), 6595–6663. 10.1021/cr900356p. [DOI] [PubMed] [Google Scholar]
- Sun C.; Li Y.; Song P.; Ma F. An Experimental and Theoretical Investigation of the Electronic Structures and Photoelectrical Properties of Ethyl Red and Carminic Acid for DSSC Application. Materials (Basel). 2016, 9 (10), 813. 10.3390/ma9100813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardo S.; Meyer G. J. Photodriven Heterogeneous Charge Transfer with Transition-Metal Compounds Anchored to TiO2 Semiconductor Surfaces. Chem. Soc. Rev. 2009, 38 (1), 115–164. 10.1039/B804321N. [DOI] [PubMed] [Google Scholar]
- Katoh R.; Furube A. Electron Injection Efficiency in Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 1–16. 10.1016/j.jphotochemrev.2014.02.001. [DOI] [Google Scholar]
- Zhang S.; Yang X.; Numata Y.; Han L. Highly Efficient Dye-Sensitized Solar Cells: Progress and Future Challenges. Energy Environ. Sci. 2013, 6 (6), 1443–1464. 10.1039/c3ee24453a. [DOI] [Google Scholar]
- Bisquert J.; Zaban A.; Salvador P. Analysis of the Mechanisms of Electron Recombination in Nanoporous TiO2 Dye-Sensitized Solar Cells. Nonequilibrium Steady-State Statistics and Interfacial Electron Transfer via Surface States. J. Phys. Chem. B 2002, 106 (34), 8774–8782. 10.1021/jp026058c. [DOI] [Google Scholar]
- Zaban A.; Greenshtein M.; Bisquert J. Determination of the Electron Lifetime in Nanocrystalline Dye Solar Cells by Open-Circuit Voltage Decay Measurements. ChemPhysChem 2003, 4 (8), 859–864. 10.1002/cphc.200200615. [DOI] [PubMed] [Google Scholar]
- Nazeeruddin M. K.; Baranoff E.; Grätzel M. Dye-Sensitized Solar Cells: A Brief Overview. Sol. Energy 2011, 85 (6), 1172–1178. 10.1016/j.solener.2011.01.018. [DOI] [Google Scholar]
- Aftabuzzaman M.; Sarker S.; Lu C.; Kim H. K. In-Depth Understanding of the Energy Loss and Efficiency Limit of Dye-Sensitized Solar Cells under Outdoor and Indoor Conditions. J. Mater. Chem. A 2021, 9 (44), 24830–24848. 10.1039/D1TA03309C. [DOI] [Google Scholar]
- Liu X.; Zhang Q.; Li J.; Valanoor N.; Tang X.; Cao G. Increase of Power Conversion Efficiency in Dye-Sensitized Solar Cells through Ferroelectric Substrate Induced Charge Transport Enhancement. Sci. Rep. 2018, 8 (1), 17389. 10.1038/s41598-018-35764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Grätzel C.; Zakeeruddin S. M.; Grätzel M. Recent Developments in Redox Electrolytes for Dye-Sensitized Solar Cells. Energy Environ. Sci. 2012, 5 (11), 9394–9405. 10.1039/c2ee23081j. [DOI] [Google Scholar]
- Benesperi I.; Michaels H.; Freitag M. The Researcher’s Guide to Solid-State Dye-Sensitized Solar Cells. J. Mater. Chem. C 2018, 6 (44), 11903–11942. 10.1039/C8TC03542C. [DOI] [Google Scholar]
- Ma P.; Fang Y.; Li A.; Wen B.; Cheng H.; Zhou X.; Shi Y.; Yang H. Y.; Lin Y. Highly Efficient and Stable Ionic Liquid-Based Gel Electrolytes. Nanoscale 2021, 13 (15), 7140–7151. 10.1039/D0NR08765C. [DOI] [PubMed] [Google Scholar]
- Saidi N. M.; Omar F. S.; Numan A.; Apperley D. C.; Algaradah M. M.; Kasi R.; Avestro A.-J.; Subramaniam R. T. Enhancing the Efficiency of a Dye-Sensitized Solar Cell Based on a Metal Oxide Nanocomposite Gel Polymer Electrolyte. ACS Appl. Mater. Interfaces 2019, 11 (33), 30185–30196. 10.1021/acsami.9b07062. [DOI] [PubMed] [Google Scholar]
- Bard J. A.; Faulkner L. R.. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: 2000. [Google Scholar]
- Owen J.Ionic Conductivity. In Comprehensive Polymer Science and Supplements; Elsevier: 1989; pp 669–686. 10.1016/b978-0-08-096701-1.00058-6. [DOI] [Google Scholar]
- Ratner M. A.; Shriver D. F. Ion Transport in Solvent-Free Polymers. Chem. Rev. 1988, 88 (1), 109–124. 10.1021/cr00083a006. [DOI] [Google Scholar]
- Menzinger M.; Wolfgang R. The Meaning and Use of the Arrhenius Activation Energy. Angew. Chem., Int. Ed. Engl. 1969, 8 (6), 438–444. 10.1002/anie.196904381. [DOI] [Google Scholar]
- Arrhenius S. Über Die Reaktionsgeschwindigkeit Bei Der Inversion von Rohrzucker Durch Säuren. Zeitschrift für Phys. Chemie 1889, 4U (1), 226–248. 10.1515/zpch-1889-0416. [DOI] [Google Scholar]
- Bandara T. M. W. J.; Senavirathna S. L. N.; Wickramasinghe H. M. N.; Vignarooban K.; De Silva L. A.; Dissanayake M. A. K. L.; Albinsson I.; Mellander B. Binary Counter Ion Effects and Dielectric Behavior of Iodide Ion Conducting Gel-Polymer Electrolytes for High-Efficiency Quasi-Solid-State Solar Cells. Phys. Chem. Chem. Phys. 2020, 22 (22), 12532–12543. 10.1039/D0CP01547D. [DOI] [PubMed] [Google Scholar]
- Vogel H. Das Temperaturabhaengigkeitsgesetz Der Viskositaet von Fluessigkeiten. Phys. Zeitschrift 1921, 22, 645. [Google Scholar]
- Fulcher G. S. ANALYSIS OF RECENT MEASUREMENTS OF THE VISCOSITY OF GLASSES. J. Am. Ceram. Soc. 1925, 8 (6), 339–355. 10.1111/j.1151-2916.1925.tb16731.x. [DOI] [Google Scholar]
- Tammann G.; Hesse W. Die Abhängigkeit Der Viscosität von Der Temperatur Bie Unterkühlten Flüssigkeiten. Zeitschrift für Anorg. und Allg. Chemie 1926, 156 (1), 245–257. 10.1002/zaac.19261560121. [DOI] [Google Scholar]
- Dahms H. Electronic Conduction in Aqueous Solution. J. Phys. Chem. 1968, 72 (1), 362–364. 10.1021/j100847a073. [DOI] [Google Scholar]
- Ruff I.; Friedrich V. J. Transfer Diffusion. I. Theoretical. J. Phys. Chem. 1971, 75 (21), 3297–3302. 10.1021/j100690a016. [DOI] [Google Scholar]
- Tahara H.; Tanaka Y.; Yamamoto S.; Yonemori S.; Chan B.; Murakami H.; Sagara T. A Redox-Active Ionic Liquid Manifesting Charge-Transfer Interaction between a Viologen and Carbazole and Its Effect on the Viscosity, Ionic Conductivity, and Redox Process of the Viologen. Chem. Sci. 2021, 12 (13), 4872–4882. 10.1039/D0SC06244H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.; Zhang L.; Wang Z.-S. Single-Crystal CoSe2 Nanorods as an Efficient Electrocatalyst for Dye-Sensitized Solar Cells. J. Mater. Chem. A 2014, 2 (38), 16023–16029. 10.1039/C4TA02238F. [DOI] [Google Scholar]
- Hauch A.; Georg A. Diffusion in the Electrolyte and Charge-Transfer Reaction at the Platinum Electrode in Dye-Sensitized Solar Cells. Electrochim. Acta 2001, 46 (22), 3457–3466. 10.1016/S0013-4686(01)00540-0. [DOI] [Google Scholar]
- Girma W. M.; Chen C. H.; Yang C. H.; Wang P. I.; Ou K. L.; Liaw D. J.; Chang J. Y. A Low Molecular Mass Organogelator Electrolyte with TiO2 Nanoparticles for Stable and Efficient Quasi-Solid-State Dye Sensitized Solar Cells. RSC Adv. 2017, 7 (13), 7671–7678. 10.1039/C6RA27203G. [DOI] [Google Scholar]
- Papageorgiou N.; Athanassov Y.; Armand M.; Bonhote P.; Pettersson H.; Azam A.; Grätzel M. The Performance and Stability of Ambient Temperature Molten Salts for Solar Cell Applications. J. Electrochem. Soc. 1996, 143 (10), 3099–3108. 10.1149/1.1837171. [DOI] [Google Scholar]
- Chatzivasiloglou E.; Stergiopoulos T.; Kontos A. G.; Alexis N.; Prodromidis M.; Falaras P. The Influence of the Metal Cation and the Filler on the Performance of Dye-Sensitized Solar Cells Using Polymer-Gel Redox Electrolytes. J. Photochem. Photobiol. A Chem. 2007, 192 (1), 49–55. 10.1016/j.jphotochem.2007.05.003. [DOI] [Google Scholar]
- Boschloo G.; Hagfeldt A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42 (11), 1819–1826. 10.1021/ar900138m. [DOI] [PubMed] [Google Scholar]
- O’Regan B.; Grätzel M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353 (6346), 737–740. 10.1038/353737a0. [DOI] [Google Scholar]
- Chen C.-Y.; Wang M.; Li J.-Y.; Pootrakulchote N.; Alibabaei L.; Ngoc-le C.; Decoppet J.-D.; Tsai J.-H.; Grätzel C.; Wu C.-G.; Zakeeruddin S. M.; Grätzel M. Highly Efficient Light-Harvesting Ruthenium Sensitizer for Thin-Film Dye-Sensitized Solar Cells. ACS Nano 2009, 3 (10), 3103–3109. 10.1021/nn900756s. [DOI] [PubMed] [Google Scholar]
- Yu Q.; Wang Y.; Yi Z.; Zu N.; Zhang J.; Zhang M.; Wang P. High-Efficiency Dye-Sensitized Solar Cells: The Influence of Lithium Ions on Exciton Dissociation, Charge Recombination, and Surface States. ACS Nano 2010, 4 (10), 6032–6038. 10.1021/nn101384e. [DOI] [PubMed] [Google Scholar]
- Han L.; Islam A.; Chen H.; Malapaka C.; Chiranjeevi B.; Zhang S.; Yang X.; Yanagida M. High-Efficiency Dye-Sensitized Solar Cell with a Novel Co-Adsorbent. Energy Environ. Sci. 2012, 5 (3), 6057–6060. 10.1039/c2ee03418b. [DOI] [Google Scholar]
- Zhou D.; Xia Z.; Shang H.; Xiao H.; Jiang Z.; Li H.; Zheng L.; Dong J.; Chen W. A Rational Design of an Efficient Counter Electrode with the Co/Co1P1N3 Atomic Interface for Promoting Catalytic Performance. Mater. Chem. Front. 2021, 5 (7), 3085–3092. 10.1039/D0QM00806K. [DOI] [Google Scholar]
- Wang Q.; Ito S.; Grätzel M.; Fabregat-Santiago F.; Mora-Seró I.; Bisquert J.; Bessho T.; Imai H. Characteristics of High Efficiency Dye-Sensitized Solar Cells. J. Phys. Chem. B 2006, 110 (50), 25210–25221. 10.1021/jp064256o. [DOI] [PubMed] [Google Scholar]
- Nazeeruddin M. K.; Kay A.; Rodicio I.; Humphry-Baker R.; Mueller E.; Liska P.; Vlachopoulos N.; Graetzel M. Conversion of Light to Electricity by Cis-X2bis(2,2′-Bipyridyl-4,4′-Dicarboxylate)Ruthenium(II) Charge-Transfer Sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115 (14), 6382–6390. 10.1021/ja00067a063. [DOI] [Google Scholar]
- Wang Z.-S.; Sayama K.; Sugihara H. Efficient Eosin Y Dye-Sensitized Solar Cell Containing Br-/Br3- Electrolyte. J. Phys. Chem. B 2005, 109 (47), 22449–22455. 10.1021/JP053260H. [DOI] [PubMed] [Google Scholar]
- Teng C.; Yang X.; Yuan C.; Li C.; Chen R.; Tian H.; Li S.; Hagfeldt A.; Sun L. Two Novel Carbazole Dyes for Dye-Sensitized Solar Cells with Open-Circuit Voltages up to 1 V Based on Br–/Br3– Electrolytes. Org. Lett. 2009, 11 (23), 5542–5545. 10.1021/ol9022936. [DOI] [PubMed] [Google Scholar]
- Wang P.; Zakeeruddin S. M.; Moser J.-E.; Humphry-Baker R.; Gratzel M. A Solvent-Free, SeCN-/(SeCN)3- Based Ionic Liquid Electrolyte for High-Efficiency Dye-Sensitized Nanocrystalline Solar Cells. J. Am. Chem. Soc. 2004, 126 (23), 7164–7165. 10.1021/JA048472R. [DOI] [PubMed] [Google Scholar]
- Oskam G.; Bergeron B. V.; Meyer G. J.; Searson P. C. Pseudohalogens for Dye-Sensitized TiO2 Photoelectrochemical Cells. J. Phys. Chem. B 2001, 105 (29), 6867–6873. 10.1021/jp004411d. [DOI] [Google Scholar]
- Zhou H.; Ji J.-M.; Kang S. H.; Kim M. S.; Lee H. S.; Kim C. H.; Kim H. K. Molecular Design and Synthesis of D−π–A Structured Porphyrin Dyes with Various Acceptor Units for Dye-Sensitized Solar Cells. J. Mater. Chem. C 2019, 7 (10), 2843–2852. 10.1039/C8TC05283B. [DOI] [Google Scholar]
- Yum J.-H.; Baranoff E.; Kessler F.; Moehl T.; Ahmad S.; Bessho T.; Marchioro A.; Ghadiri E.; Moser J.-E.; Yi C.; Nazeeruddin M. K.; Grätzel M. A Cobalt Complex Redox Shuttle for Dye-Sensitized Solar Cells with High Open-Circuit Potentials. Nat. Commun. 2012 31 2012, 3 (1), 1–8. 10.1038/ncomms1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yella A.; Lee H.-W. W.; Tsao H. N.; Yi C.; Chandiran A. K.; Nazeeruddin M. K. K.; Diau E. W.-G. G.; Yeh C.-Y. Y.; Zakeeruddin S. M.; Grätzel M. Porphyrin-Sensitized Solar Cells with Cobalt (II/III)–Based Redox Electrolyte Exceed 12% Efficiency. Science (80-.) 2011, 334 (6056), 629–634. 10.1126/science.1209688. [DOI] [PubMed] [Google Scholar]
- Ren Y.; Sun D.; Cao Y.; Tsao H. N.; Yuan Y.; Zakeeruddin S. M.; Wang P.; Grätzel M. A Stable Blue Photosensitizer for Color Palette of Dye-Sensitized Solar Cells Reaching 12.6% Efficiency. J. Am. Chem. Soc. 2018, 140 (7), 2405–2408. 10.1021/jacs.7b12348. [DOI] [PubMed] [Google Scholar]
- Michaels H.; Rinderle M.; Freitag R.; Benesperi I.; Edvinsson T.; Socher R.; Gagliardi A.; Freitag M. Dye-Sensitized Solar Cells under Ambient Light Powering Machine Learning: Towards Autonomous Smart Sensors for the Internet of Things. Chem. Sci. 2020, 11 (11), 2895–2906. 10.1039/C9SC06145B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong J.; Kinschel D.; Daniel Q.; Safdari M.; Gabrielsson E.; Chen H.; Svensson P. H.; Sun L.; Kloo L. Bis(1,1-Bis(2-Pyridyl)Ethane)Copper(i/II) as an Efficient Redox Couple for Liquid Dye-Sensitized Solar Cells. J. Mater. Chem. A 2016, 4 (38), 14550–14554. 10.1039/C6TA06782D. [DOI] [Google Scholar]
- Bai Y.; Yu Q.; Cai N.; Wang Y.; Zhang M.; Wang P. High-Efficiency Organic Dye-Sensitized Mesoscopic Solar Cells with a Copper Redox Shuttle. Chem. Commun. 2011, 47 (15), 4376–4378. 10.1039/c1cc10454c. [DOI] [PubMed] [Google Scholar]
- Rodrigues R. R.; Peddapuram A.; Dorris A. L.; Hammer N. I.; Delcamp J. H. Thienopyrroledione-Based Photosensitizers as Strong Photoinduced Oxidants: Oxidation of Fe(Bpy)32+ in a > 1.3 V Dye-Sensitized Solar Cell. ACS Appl. Energy Mater. 2019, 2 (8), 5547–5556. 10.1021/acsaem.9b00730. [DOI] [Google Scholar]
- Curiac C.; Rodrigues R. R.; Watson J.; Hunt L. A.; Devdass A.; Jurss J. W.; Hammer N. I.; Fortenberry R. C.; Delcamp J. H. Iron Redox Shuttles with Wide Optical Gap Dyes for High-Voltage Dye-Sensitized Solar Cells. ChemSusChem 2021, 14, 3084. 10.1002/cssc.202100884. [DOI] [PubMed] [Google Scholar]
- Hamann T. W.; Farha O. K.; Hupp J. T. Outer-Sphere Redox Couples as Shuttles in Dye-Sensitized Solar Cells. Performance Enhancement Based on Photoelectrode Modification via Atomic Layer Deposition. J. Phys. Chem. C 2008, 112 (49), 19756–19764. 10.1021/jp807395g. [DOI] [Google Scholar]
- Zhang Z.; Chen P.; Murakami T. N.; Zakeeruddin S. M.; Grätzel M. The 2,2,6,6-Tetramethyl-1-Piperidinyloxy Radical: An Efficient, Iodine- Free Redox Mediator for Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2008, 18 (2), 341–346. 10.1002/adfm.200701041. [DOI] [Google Scholar]
- Wang M.; Chamberland N.; Breau L.; Moser J.-E.; Humphry-Baker R.; Marsan B.; Zakeeruddin S. M.; Grätzel M. An Organic Redox Electrolyte to Rival Triiodide/Iodide in Dye-Sensitized Solar Cells. Nat. Chem. 2010 25 2010, 2 (5), 385–389. 10.1038/nchem.610. [DOI] [PubMed] [Google Scholar]
- Daeneke T.; Mozer A. J.; Kwon T. H.; Duffy N. W.; Holmes A. B.; Bach U.; Spiccia L. Dye Regeneration and Charge Recombination in Dye-Sensitized Solar Cells with Ferrocene Derivatives as Redox Mediators. Energy Environ. Sci. 2012, 5 (5), 7090–7099. 10.1039/c2ee21257a. [DOI] [Google Scholar]
- Daeneke T.; Mozer A. J.; Uemura Y.; Makuta S.; Fekete M.; Tachibana Y.; Koumura N.; Bach U.; Spiccia L. Dye Regeneration Kinetics in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134 (41), 16925–16928. 10.1021/ja3054578. [DOI] [PubMed] [Google Scholar]
- Tian H.; Jiang X.; Yu Z.; Kloo L.; Hagfeldt A.; Sun L. Efficient Organic-Dye-Sensitized Solar Cells Based on an Iodine-Free Electrolyte. Angew. Chemie Int. Ed. 2010, 49 (40), 7328–7331. 10.1002/anie.201003740. [DOI] [PubMed] [Google Scholar]
- Okada K.; Matsui H.; Kawashima T.; Ezure T.; Tanabe N. 100 Mm × 100 Mm Large-Sized Dye Sensitized Solar Cells. J. Photochem. Photobiol. A Chem. 2004, 164 (1–3), 193–198. 10.1016/j.jphotochem.2004.01.028. [DOI] [Google Scholar]
- Yu Z.; Vlachopoulos N.; Gorlov M.; Kloo L. Liquid Electrolytes for Dye-Sensitized Solar Cells. Dalt. Trans. 2011, 40 (40), 10289–10303. 10.1039/c1dt11023c. [DOI] [PubMed] [Google Scholar]
- Pavlishchuk V. V.; Addison A. W. Conversion Constants for Redox Potentials Measured versus Different Reference Electrodes in Acetonitrile Solutions at 25°C. Inorg. Chim. Acta 2000, 298 (1), 97–102. 10.1016/S0020-1693(99)00407-7. [DOI] [Google Scholar]
- Brown K. N.; Gulyas P. T.; Lay P. A.; McAlpine N. S.; Masters A. F.; Phillips L. Electrochemistry of Chlorinated Ferrocenes: Stability of Chlorinated Ferrocenium Ions. J. Chem. Soc. Dalt. Trans. 1993, 6, 835–840. 10.1039/dt9930000835. [DOI] [Google Scholar]
- Noviandri I.; Brown K. N.; Fleming D. S.; Gulyas P. T.; Lay P. A.; Masters A. F.; Phillips L. The Decamethylferrocenium/Decamethylferrocene Redox Couple: A Superior Redox Standard to the Ferrocenium/Ferrocene Redox Couple for Studying Solvent Effects on the Thermodynamics of Electron Transfer. J. Phys. Chem. B 1999, 103 (32), 6713–6722. 10.1021/jp991381+. [DOI] [Google Scholar]
- Rodrigues R. R.; Cheema H.; Delcamp J. H. A High-Voltage Molecular-Engineered Organic Sensitizer–Iron Redox Shuttle Pair: 1.4 V DSSC and 3.3 V SSM-DSSC Devices. Angew. Chemie - Int. Ed. 2018, 57 (19), 5472–5476. 10.1002/anie.201712894. [DOI] [PubMed] [Google Scholar]
- Spokoyny A. M.; Li T. C.; Farha O. K.; Machan C. W.; She C.; Stern C. L.; Marks T. J.; Hupp J. T.; Mirkin C. A. Electronic Tuning of Nickel-Based Bis(Dicarbollide) Redox Shuttles in Dye-Sensitized Solar Cells. Angew. Chemie Int. Ed. 2010, 49 (31), 5339–5343. 10.1002/anie.201002181. [DOI] [PubMed] [Google Scholar]
- Perera I. R.; Gupta A.; Xiang W.; Daeneke T.; Bach U.; Evans R. A.; Ohlin C. A.; Spiccia L. Introducing Manganese Complexes as Redox Mediators for Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2014, 16 (24), 12021–12028. 10.1039/c3cp54894e. [DOI] [PubMed] [Google Scholar]
- Mosconi E.; Yum J.-H.; Kessler F.; Gómez García C. J.; Zuccaccia C.; Cinti A.; Nazeeruddin M. K.; Grätzel M.; De Angelis F. Cobalt Electrolyte/Dye Interactions in Dye-Sensitized Solar Cells: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2012, 134 (47), 19438–19453. 10.1021/ja3079016. [DOI] [PubMed] [Google Scholar]
- Nusbaumer H.; Zakeeruddin S. M.; Moser J.-E. E.; Grätzel M. An Alternative Efficient Redox Couple for the Dye-Sensitized Solar Cell System. Chem. - Eur. J. 2003, 9 (16), 3756–3763. 10.1002/chem.200204577. [DOI] [PubMed] [Google Scholar]
- Nusbaumer H.; Moser J.-E. E.; Zakeeruddin S. M.; Nazeeruddin M. K.; Grätzel M. CoII(Dbbip)22+ Complex Rivals Tri-Iodide/Iodide Redox Mediator in Dye-Sensitized Photovoltaic Cells. J. Phys. Chem. B 2001, 105 (43), 10461–10464. 10.1021/jp012075a. [DOI] [Google Scholar]
- Wang H.; Nicholson P. G.; Peter L.; Zakeeruddin S. M.; Grätzel M. Transport and Interfacial Transfer of Electrons in Dye-Sensitized Solar Cells Utilizing a Co(Dbbip)2 Redox Shuttle. J. Phys. Chem. C 2010, 114 (33), 14300–14306. 10.1021/jp105753k. [DOI] [Google Scholar]
- Song B. J.; Song H. M.; Choi I. T.; Kim S. K.; Seo K. D.; Kang M. S.; Lee M. J.; Cho D. W.; Ju M. J.; Kim H. K. A Desirable Hole-Conducting Coadsorbent for Highly Efficient Dye-Sensitized Solar Cells through an Organic Redox Cascade Strategy. Chem. - A Eur. J. 2011, 17 (40), 11115–11121. 10.1002/chem.201100813. [DOI] [PubMed] [Google Scholar]
- Mathew S.; Yella A.; Gao P.; Humphry-Baker R.; Curchod B. F. E.; Ashari-Astani N.; Tavernelli I.; Rothlisberger U.; Nazeeruddin M. K.; Grätzel M. Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers. Nat. Chem. 2014, 6 (3), 242–247. 10.1038/nchem.1861. [DOI] [PubMed] [Google Scholar]
- Kavan L.; Saygili Y.; Freitag M.; Zakeeruddin S. M.; Hagfeldt A.; Grätzel M. Electrochemical Properties of Cu(II/I)-Based Redox Mediators for Dye-Sensitized Solar Cells. Electrochim. Acta 2017, 227, 194–202. 10.1016/j.electacta.2016.12.185. [DOI] [Google Scholar]
- Cao Y.; Saygili Y.; Ummadisingu A.; Teuscher J.; Luo J.; Pellet N.; Giordano F.; Zakeeruddin S. M.; Moser J.-E.; Freitag M.; Hagfeldt A.; Grätzel M. 11% Efficiency Solid-State Dye-Sensitized Solar Cells with Copper(II/I) Hole Transport Materials. Nat. Commun. 2017, 8 (1), 15390. 10.1038/ncomms15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan S. C.; Hagfeldt A.; Soman S. Resurgence of DSCs with Copper Electrolyte: A Detailed Investigation of Interfacial Charge Dynamics with Cobalt and Iodine Based Electrolytes. J. Mater. Chem. A 2018, 6 (44), 22204–22214. 10.1039/C8TA06948D. [DOI] [Google Scholar]
- Davies K.; Byers B. Kinetic Study of Electron-Transfer of Sterically Constrained Bis(Diimine) Complexes of Copper(II) and Copper (i) with Ruthenium Ammine Complexes. Inorg. Chem. 1987, 26 (22), 3823–3825. 10.1021/ic00269a600. [DOI] [Google Scholar]
- Cazzanti S.; Caramori S.; Argazzi R.; Elliott C. M.; Bignozzi C. A. Efficient Non-Corrosive Electron-Transfer Mediator Mixtures for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2006, 128 (31), 9996–9997. 10.1021/ja062087f. [DOI] [PubMed] [Google Scholar]
- Liu J.; Yang X.; Cong J.; Kloo L.; Sun L. Solvent-Free Ionic Liquid Electrolytes without Elemental Iodine for Dye-Sensitized Solar Cells. Phys. Chem. Chem. Phys. 2012, 14 (33), 11592–11595. 10.1039/c2cp42234d. [DOI] [PubMed] [Google Scholar]
- Chu T.-C.; Lin R. Y.-Y.; Lee C.-P.; Hsu C.-Y.; Shih P.-C.; Lin R.; Li S.-R.; Sun S.-S.; Lin J. T.; Vittal R.; Ho K.-C. Ionic Liquid with a Dual-Redox Couple for Efficient Dye-Sensitized Solar Cells. ChemSusChem 2014, 7 (1), 146–153. 10.1002/cssc.201301015. [DOI] [PubMed] [Google Scholar]
- Cong J.; Hao Y.; Boschloo G.; Kloo L. Electrolytes Based on TEMPO–Co Tandem Redox Systems Outperform Single Redox Systems in Dye-Sensitized Solar Cells. ChemSusChem 2015, 8 (2), 264–268. 10.1002/cssc.201402780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y.; Yang W.; Karlsson M.; Cong J.; Wang S.; Li X.; Xu B.; Hua J.; Kloo L.; Boschloo G. Efficient Dye-Sensitized Solar Cells with Voltages Exceeding 1 V through Exploring Tris(4-Alkoxyphenyl)Amine Mediators in Combination with the Tris(Bipyridine) Cobalt Redox System. ACS Energy Lett. 2018, 3 (8), 1929–1937. 10.1021/acsenergylett.8b00872. [DOI] [Google Scholar]
- Mariotti N.; Bonomo M.; Fagiolari L.; Barbero N.; Gerbaldi C.; Bella F.; Barolo C. Recent Advances in Eco-Friendly and Cost-Effective Materials towards Sustainable Dye-Sensitized Solar Cells. Green Chem. 2020, 22 (21), 7168–7218. 10.1039/D0GC01148G. [DOI] [Google Scholar]
- Law C.; Pathirana S. C.; Li X.; Anderson A. Y.; Barnes P. R. F.; Listorti A.; Ghaddar T. H.; O'Regan B. C. Water-Based Electrolytes for Dye-Sensitized Solar Cells. Adv. Mater. 2010, 22 (40), 4505–4509. 10.1002/adma.201001703. [DOI] [PubMed] [Google Scholar]
- Bella F.; Galliano S.; Falco M.; Viscardi G.; Barolo C.; Grätzel M.; Gerbaldi C. Approaching Truly Sustainable Solar Cells by the Use of Water and Cellulose Derivatives. Green Chem. 2017, 19 (4), 1043–1051. 10.1039/C6GC02625G. [DOI] [Google Scholar]
- Bella F.; Porcarelli L.; Mantione D.; Gerbaldi C.; Barolo C.; Grätzel M.; Mecerreyes D. A Water-Based and Metal-Free Dye Solar Cell Exceeding 7% Efficiency Using a Cationic Poly(3,4-Ethylenedioxythiophene) Derivative. Chem. Sci. 2020, 11 (6), 1485–1493. 10.1039/C9SC05596G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tributsch H. Dye Sensitization Solar Cells: A Critical Assessment of the Learning Curve. Coord. Chem. Rev. 2004, 248 (13–14), 1511–1530. 10.1016/j.ccr.2004.05.030. [DOI] [Google Scholar]
- Yang W.; Söderberg M.; Eriksson A. I. K.; Boschloo G. Efficient Aqueous Dye-Sensitized Solar Cell Electrolytes Based on a TEMPO/TEMPO+ Redox Couple. RSC Adv. 2015, 5 (34), 26706–26709. 10.1039/C5RA03248B. [DOI] [Google Scholar]
- Fayad R.; Shoker T. A.; Ghaddar T. H. High Photo-Currents with a Zwitterionic Thiocyanate-Free Dye in Aqueous-Based Dye Sensitized Solar Cells. Dalt. Trans. 2016, 45 (13), 5622–5628. 10.1039/C6DT00071A. [DOI] [PubMed] [Google Scholar]
- Daeneke T.; Uemura Y.; Duffy N. W.; Mozer A. J.; Koumura N.; Bach U.; Spiccia L. Aqueous Dye-Sensitized Solar Cell Electrolytes Based on the Ferricyanide–Ferrocyanide Redox Couple. Adv. Mater. 2012, 24 (9), 1222–1225. 10.1002/adma.201104837. [DOI] [PubMed] [Google Scholar]
- Ellis H.; Jiang R.; Ye S.; Hagfeldt A.; Boschloo G. Development of High Efficiency 100% Aqueous Cobalt Electrolyte Dye-Sensitised Solar Cells. Phys. Chem. Chem. Phys. 2016, 18 (12), 8419–8427. 10.1039/C6CP00264A. [DOI] [PubMed] [Google Scholar]
- Dong C.; Xiang W.; Huang F.; Fu D.; Huang W.; Bach U.; Cheng Y.-B.; Li X.; Spiccia L. Controlling Interfacial Recombination in Aqueous Dye-Sensitized Solar Cells by Octadecyltrichlorosilane Surface Treatment. Angew. Chemie Int. Ed. 2014, 53 (27), 6933–6937. 10.1002/anie.201400723. [DOI] [PubMed] [Google Scholar]
- Li L.; Gibson E. A.; Qin P.; Boschloo G.; Gorlov M.; Hagfeldt A.; Sun L. Double-Layered NiO Photocathodes for p-Type DSSCs with Record IPCE. Adv. Mater. 2010, 22 (15), 1759–1762. 10.1002/adma.200903151. [DOI] [PubMed] [Google Scholar]
- Zhu H.; Hagfeldt A.; Boschloo G. Photoelectrochemistry of Mesoporous NiO Electrodes in Iodide/Triiodide Electrolytes. J. Phys. Chem. C 2007, 111 (47), 17455–17458. 10.1021/jp077134k. [DOI] [Google Scholar]
- Perera I. R.; Daeneke T.; Makuta S.; Yu Z.; Tachibana Y.; Mishra A.; Bäuerle P.; Ohlin C. A.; Bach U.; Spiccia L. Application of the Tris(Acetylacetonato)Iron(III)/(II) Redox Couple in p-Type Dye-Sensitized Solar Cells. Angew. Chemie Int. Ed. 2015, 54 (12), 3758–3762. 10.1002/anie.201409877. [DOI] [PubMed] [Google Scholar]
- Powar S.; Daeneke T.; Ma M. T.; Fu D.; Duffy N. W.; Götz G.; Weidelener M.; Mishra A.; Bäuerle P.; Spiccia L.; Bach U. Highly Efficient P-Type Dye-Sensitized Solar Cells Based on Tris(1,2-Diaminoethane)Cobalt(II)/(III) Electrolytes. Angew. Chemie Int. Ed. 2013, 52 (2), 602–605. 10.1002/anie.201206219. [DOI] [PubMed] [Google Scholar]
- Xiang W.; Marlow J.; Bäuerle P.; Bach U.; Spiccia L. Aqueous P-Type Dye-Sensitized Solar Cells Based on a Tris(1,2-Diaminoethane)Cobalt(Ii)/(Iii) Redox Mediator. Green Chem. 2016, 18 (24), 6659–6665. 10.1039/C6GC02001A. [DOI] [Google Scholar]
- Gutmann V. Solvent Effects on the Reactivities of Organometallic Compounds. Coord. Chem. Rev. 1976, 18 (2), 225–255. 10.1016/S0010-8545(00)82045-7. [DOI] [Google Scholar]
- Fukui A.; Komiya R.; Yamanaka R.; Islam A.; Han L. Effect of a Redox Electrolyte in Mixed Solvents on the Photovoltaic Performance of a Dye-Sensitized Solar Cell. Sol. Energy Mater. Sol. Cells 2006, 90 (5), 649–658. 10.1016/j.solmat.2005.01.020. [DOI] [Google Scholar]
- Hara K.; Horiguchi T.; Kinoshita T.; Sayama K.; Arakawa H. Influence of Electrolytes on the Photovoltaic Performance of Organic Dye-Sensitized Nanocrystalline TiO2 Solar Cells. Sol. Energy Mater. Sol. Cells 2001, 70 (2), 151–161. 10.1016/S0927-0248(01)00019-8. [DOI] [Google Scholar]
- Nazeeruddin M. K.; Kay A.; Rodicio I.; Humphry-Baker R.; Mueller E.; Liska P.; Vlachopoulos N.; Graetzel M. Conversion of Light to Electricity by Cis-X2Bis(2,2′-Bipyridyl-4,4′-Dicarboxylate)Ruthenium(11) Charge-Transfer Sensitizers (X=C1-, Br-, I-, CN-, and Cis-XSCN-) on Nanocrystalline Ti02 Electrodes. J. Am. Chem. Soc. 1993, 115 (14), 6382–6390. 10.1021/ja00067a063. [DOI] [Google Scholar]
- Choi H.; Han J.; Kang M. S.; Song K.; Ko J. Aqueous Electrolytes Based Dye-Sensitized Solar Cells Using I -/I3- Redox Couple to Achieve ≥ 4% Power Conversion Efficiency. Bull. Korean Chem. Soc. 2014, 35 (5), 1433–1439. 10.5012/bkcs.2014.35.5.1433. [DOI] [Google Scholar]
- Zhang H.; Qiu L.; Xu D.; Zhang W.; Yan F. Performance Enhancement for Water Based Dye-Sensitized Solar Cells via Addition of Ionic Surfactants. J. Mater. Chem. A 2014, 2 (7), 2221–2226. 10.1039/C3TA14571A. [DOI] [Google Scholar]
- Bella F.; Galliano S.; Falco M.; Viscardi G.; Barolo C.; Grätzel M.; Gerbaldi C. Unveiling Iodine-Based Electrolytes Chemistry in Aqueous Dye-Sensitized Solar Cells. Chem. Sci. 2016, 7 (8), 4880–4890. 10.1039/C6SC01145D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano S.; Bella F.; Gerbaldi C.; Falco M.; Viscardi G.; Grätzel M.; Barolo C. Photoanode/Electrolyte Interface Stability in Aqueous Dye-Sensitized Solar Cells. Energy Technol. 2017, 5 (2), 300–311. 10.1002/ente.201600285. [DOI] [Google Scholar]
- Kato N.; Takeda Y.; Higuchi K.; Takeichi A.; Sudo E.; Tanaka H.; Motohiro T.; Sano T.; Toyoda T. Degradation Analysis of Dye-Sensitized Solar Cell Module after Long-Term Stability Test under Outdoor Working Condition. Sol. Energy Mater. Sol. Cells 2009, 93 (6–7), 893–897. 10.1016/j.solmat.2008.10.022. [DOI] [Google Scholar]
- Anthony J. L.; Brennecke J. F.; Holbrey J. D.; Maginn E. J.; Mantz R. A.; Rogers R. D.; Trulove P. C.; Visser A. E.; Welton T. Physicochemical Properties of Ionic Liquids. Ionic Liquids in Synthesis 2002, 41–126. 10.1002/3527600701.ch3. [DOI] [Google Scholar]
- Gorlov M.; Kloo L. Ionic Liquid Electrolytes for Dye-Sensitized Solar Cells. Dalt. Trans. 2008, 20, 2655–2666. 10.1039/b716419j. [DOI] [PubMed] [Google Scholar]
- Kubo W.; Kitamura T.; Hanabusa K.; Wada Y.; Yanagida S. Quasi-Solid-State Dye-Sensitized Solar Cells Using Room Temperature Molten Salts and a Low Molecular Weight Gelator. Chem. Commun. 2002, 4, 374–375. 10.1039/b110019j. [DOI] [PubMed] [Google Scholar]
- Kambe S.; Nakade S.; Kitamura T.; Wada Y.; Yanagida S. Influence of the Electrolytes on Electron Transport in Mesoporous TiO2–Electrolyte Systems. J. Phys. Chem. B 2002, 106 (11), 2967–2972. 10.1021/jp013397h. [DOI] [Google Scholar]
- Wang P.; Wenger B.; Humphry-Baker R.; Moser J.-E.; Teuscher J.; Kantlehner W.; Mezger J.; Stoyanov E. V.; Zakeeruddin S. M.; Grätzel M. Charge Separation and Efficient Light Energy Conversion in Sensitized Mesoscopic Solar Cells Based on Binary Ionic Liquids. J. Am. Chem. Soc. 2005, 127 (18), 6850–6856. 10.1021/ja042232u. [DOI] [PubMed] [Google Scholar]
- Hao F.; Lin H.; Liu Y.; Li J. Anionic Structure-Dependent Photoelectrochemical Responses of Dye-Sensitized Solar Cells Based on a Binary Ionic Liquid Electrolyte. Phys. Chem. Chem. Phys. 2011, 13 (14), 6416–6422. 10.1039/c0cp02704a. [DOI] [PubMed] [Google Scholar]
- Kuang D.; Klein C.; Zhang Z.; Ito S.; Moser J.-E.; Zakeeruddin S. M.; Grätzel M. Stable, High-Efficiency Ionic-Liquid-Based Mesoscopic Dye-Sensitized Solar Cells. Small 2007, 3 (12), 2094–2102. 10.1002/smll.200700211. [DOI] [PubMed] [Google Scholar]
- Yoshida Y.; Muroi K.; Otsuka A.; Saito G.; Takahashi M.; Yoko T. 1-Ethyl-3-Methylimidazolium Based Ionic Liquids Containing Cyano Groups: Synthesis, Characterization, and Crystal Structure. Inorg. Chem. 2004, 43 (4), 1458–1462. 10.1021/ic035045q. [DOI] [PubMed] [Google Scholar]
- Fabregat-Santiago F.; Bisquert J.; Palomares E.; Otero L.; Kuang D.; Zakeeruddin S. M.; Grätzel M. Correlation between Photovoltaic Performance and Impedance Spectroscopy of Dye-Sensitized Solar Cells Based on Ionic Liquids. J. Phys. Chem. C 2007, 111 (17), 6550–6560. 10.1021/jp066178a. [DOI] [Google Scholar]
- Matsumoto H.; Matsuda T.; Tsuda T.; Hagiwara R.; Ito Y.; Miyazaki Y. The Application of Room Temperature Molten Salt with Low Viscosity to the Electrolyte for Dye-Sensitized Solar Cell. Chem. Lett. 2001, 30 (1), 26–27. 10.1246/cl.2001.26. [DOI] [Google Scholar]
- Sun H.; Hoffman M. Z. Reductive Quenching of the Excited States of Ruthenium(II) Complexes Containing 2,2′-Bipyridine, 2,2′-Bipyrazine, and 2,2′-Bipyrimidine Ligands. J. Phys. Chem. 1994, 98 (45), 11719–11726. 10.1021/j100096a015. [DOI] [Google Scholar]
- Shi D.; Pootrakulchote N.; Li R.; Guo J.; Wang Y.; Zakeeruddin S. M.; Grätzel M.; Wang P. New Efficiency Records for Stable Dye-Sensitized Solar Cells with Low-Volatility and Ionic Liquid Electrolytes. J. Phys. Chem. C 2008, 112 (44), 17046–17050. 10.1021/jp808018h. [DOI] [Google Scholar]
- Bousrez G.; Renier O.; Adranno B.; Smetana V.; Mudring A.-V. Ionic Liquid-Based Dye-Sensitized Solar Cells—Insights into Electrolyte and Redox Mediator Design. ACS Sustain. Chem. Eng. 2021, 9 (24), 8107–8114. 10.1021/acssuschemeng.1c01057. [DOI] [Google Scholar]
- Boschloo G.; Häggman L.; Hagfeldt A. Quantification of the Effect of 4-Tert-Butylpyridine Addition to I-/I3- Redox Electrolytes in Dye-Sensitized Nanostructured TiO2 Solar Cells. J. Phys. Chem. B 2006, 110 (26), 13144–13150. 10.1021/jp0619641. [DOI] [PubMed] [Google Scholar]
- Nakade S.; Kanzaki T.; Kubo W.; Kitamura T.; Wada Y.; Yanagida S. Role of Electrolytes on Charge Recombination in Dye-Sensitized TiO2 Solar Cell (1): The Case of Solar Cells Using the I-/I3- Redox Couple. J. Phys. Chem. B 2005, 109 (8), 3480–3487. 10.1021/jp0460036. [DOI] [PubMed] [Google Scholar]
- Schlichthörl G.; Huang S. Y.; Sprague J.; Frank A. J. Band Edge Movement and Recombination Kinetics in Dye-Sensitized Nanocrystalline TiO2 Solar Cells: A Study by Intensity Modulated Photovoltage Spectroscopy. J. Phys. Chem. B 1997, 101 (41), 8141–8155. 10.1021/jp9714126. [DOI] [Google Scholar]
- Huang S. Y.; Schlichthörl G.; Nozik A. J.; Grätzel M.; Frank A. J. Charge Recombination in Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 1997, 101 (14), 2576–2582. 10.1021/jp962377q. [DOI] [Google Scholar]
- Johnson A. L.; Muetterties E. L.; Stohr J.; Sette F. Chemisorption Geometry of Pyridine on Platinum(111) by NEXAFS. J. Phys. Chem. 1985, 89 (19), 4071–4075. 10.1021/j100265a029. [DOI] [Google Scholar]
- Krauskopf E. K.; Rice-Jackson L. M.; Wieckowski A. Pyridine Adsorption on Polycrystalline Platinum Studied by the Radioactive-Labeling Method. Langmuir 1990, 6 (5), 970–973. 10.1021/la00095a014. [DOI] [Google Scholar]
- Kim J.-Y.; Kim J. Y.; Lee D.-K.; Kim B.; Kim H.; Ko M. J. Importance of 4-Tert-Butylpyridine in Electrolyte for Dye-Sensitized Solar Cells Employing SnO2 Electrode. J. Phys. Chem. C 2012, 116 (43), 22759–22766. 10.1021/jp307783q. [DOI] [Google Scholar]
- Hoffeditz W. L.; Katz M. J.; Deria P.; Cutsail G. E. III; Pellin M. J.; Farha O. K.; Hupp J. T. One Electron Changes Everything. A Multispecies Copper Redox Shuttle for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120 (7), 3731–3740. 10.1021/acs.jpcc.6b01020. [DOI] [Google Scholar]
- Ferdowsi P.; Saygili Y.; Zakeeruddin S. M.; Mokhtari J.; Grätzel M.; Hagfeldt A.; Kavan L. Alternative Bases to 4-Tert-Butylpyridine for Dye-Sensitized Solar Cells Employing Copper Redox Mediator. Electrochim. Acta 2018, 265, 194–201. 10.1016/j.electacta.2018.01.142. [DOI] [Google Scholar]
- Buncel E.; Joly H. A.; Jones J. R. Proton Transfer from Imidazole, Benzimidazole, and Their 1-Alkyl Derivatives. FMO Analysis of the Effect of Methyl and Benzo Substitution. Can. J. Chem. 1986, 64 (6), 1240–1245. 10.1139/v86-206. [DOI] [Google Scholar]
- Yu Z.; Gorlov M.; Boschloo G.; Kloo L. Synergistic Effect of N-Methylbenzimidazole and Guanidinium Thiocyanate on the Performance of Dye-Sensitized Solar Cells Based on Ionic Liquid Electrolytes. J. Phys. Chem. C 2010, 114 (50), 22330–22337. 10.1021/jp1073686. [DOI] [Google Scholar]
- Zhang C.; Dai J.; Huo Z.; Pan X.; Hu L.; Kong F.; Huang Y.; Sui Y.; Fang X.; Wang K.; Dai S. Influence of 1-Methylbenzimidazole Interactions with Li+ and TiO2 on the Performance of Dye-Sensitized Solar Cells. Electrochim. Acta 2008, 53 (17), 5503–5508. 10.1016/j.electacta.2008.03.016. [DOI] [Google Scholar]
- Fürer S. O.; Milhuisen R. A.; Kashif M. K.; Raga S. R.; Acharya S. S.; Forsyth C.; Liu M.; Frazer L.; Duffy N. W.; Ohlin C. A.; Funston A. M.; Tachibana Y.; Bach U. The Performance-Determining Role of Lewis Bases in Dye-Sensitized Solar Cells Employing Copper-Bisphenanthroline Redox Mediators. Adv. Energy Mater. 2020, 10 (37), 2002067. 10.1002/aenm.202002067. [DOI] [Google Scholar]
- Kannankutty K.; Chen C.-C.; Nguyen V. S.; Lin Y.-C.; Chou H.-H.; Yeh C.-Y.; Wei T.-C. Tert-Butylpyridine Coordination with [Cu(Dmp)2]2+/+ Redox Couple and Its Connection to the Stability of the Dye-Sensitized Solar Cell. ACS Appl. Mater. Interfaces 2020, 12 (5), 5812–5819. 10.1021/acsami.9b19119. [DOI] [PubMed] [Google Scholar]
- Saygili Y.; Stojanovic M.; Michaels H.; Tiepelt J.; Teuscher J.; Massaro A.; Pavone M.; Giordano F.; Zakeeruddin S. M.; Boschloo G.; Moser J.-E.; Grätzel M.; Muñoz-García A. B.; Hagfeldt A.; Freitag M. Effect of Coordination Sphere Geometry of Copper Redox Mediators on Regeneration and Recombination Behavior in Dye-Sensitized Solar Cell Applications. ACS Appl. Energy Mater. 2018, 1 (9), 4950–4962. 10.1021/acsaem.8b00957. [DOI] [Google Scholar]
- Zhang C.; Huang Y.; Huo Z.; Chen S.; Dai S. Photoelectrochemical Effects of Guanidinium Thiocyanate on Dye-Sensitized Solar Cell Performance and Stability. J. Phys. Chem. C 2009, 113 (52), 21779–21783. 10.1021/jp909732f. [DOI] [Google Scholar]
- Kopidakis N.; Neale N. R.; Frank A. J. Effect of an Adsorbent on Recombination and Band-Edge Movement in Dye-Sensitized TiO2 Solar Cells: Evidence for Surface Passivation. J. Phys. Chem. B 2006, 110 (25), 12485–12489. 10.1021/jp0607364. [DOI] [PubMed] [Google Scholar]
- Kopidakis N.; Benkstein K. D.; van de Lagemaat J.; Frank A. J. Transport-Limited Recombination of Photocarriers in Dye-Sensitized Nanocrystalline TiO2 Solar Cells. J. Phys. Chem. B 2003, 107 (41), 11307–11315. 10.1021/jp0304475. [DOI] [Google Scholar]
- Kim C. K.; Choi I. T.; Kang S. H.; Kim H. K. Anchovy-Derived Nitrogen and Sulfur Co-Doped Porous Carbon Materials for High-Performance Supercapacitors and Dye-Sensitized Solar Cells. RSC Adv. 2017, 7 (57), 35565–35574. 10.1039/C7RA06102A. [DOI] [Google Scholar]
- Zhang L.; Yang X.; Wang W.; Gurzadyan G. G.; Li J.; Li X.; An J.; Yu Z.; Wang H.; Cai B.; Hagfeldt A.; Sun L. 13.6% Efficient Organic Dye-Sensitized Solar Cells by Minimizing Energy Losses of the Excited State. ACS Energy Lett. 2019, 4 (4), 943–951. 10.1021/acsenergylett.9b00141. [DOI] [Google Scholar]
- Ren Y.; Flores-Díaz N.; Zhang D.; Cao Y.; Decoppet J. D.; Fish G. C.; Moser J. E.; Zakeeruddin S. M.; Wang P.; Hagfeldt A.; Grätzel M. Blue Photosensitizer with Copper(II/I) Redox Mediator for Efficient and Stable Dye-Sensitized Solar Cells. Adv. Funct. Mater. 2020, 30 (50), 2004804. 10.1002/adfm.202004804. [DOI] [Google Scholar]
- Michaels H.; Benesperi I.; Freitag M. Challenges and Prospects of Ambient Hybrid Solar Cell Applications. Chem. Sci. 2021, 12 (14), 5002–5015. 10.1039/D0SC06477G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingare Y. S.; Vinh N. S.; Chou H.-H.; Liu Y.-C.; Long Y.-S.; Wu T.-C.; Wei T.-C.; Yeh C.-Y. New Acetylene-Bridged 9,10-Conjugated Anthracene Sensitizers: Application in Outdoor and Indoor Dye-Sensitized Solar Cells. Adv. Energy Mater. 2017, 7 (18), 1700032. 10.1002/aenm.201700032. [DOI] [Google Scholar]
- Hora C.; Santos F.; Sales M. G. F.; Ivanou D.; Mendes A. Dye-Sensitized Solar Cells for Efficient Solar and Artificial Light Conversion. ACS Sustain. Chem. Eng. 2019, 7 (15), 13464–13470. 10.1021/acssuschemeng.9b02990. [DOI] [Google Scholar]
- Venkatesan S.; Lin W.-H.; Teng H.; Lee Y.-L. High-Efficiency Bifacial Dye-Sensitized Solar Cells for Application under Indoor Light Conditions. ACS Appl. Mater. Interfaces 2019, 11 (45), 42780–42789. 10.1021/acsami.9b14876. [DOI] [PubMed] [Google Scholar]
- Freitag M.; Teuscher J.; Saygili Y.; Zhang X.; Giordano F.; Liska P.; Hua J.; Zakeeruddin S. M.; Moser J.-E.; Grätzel M.; Hagfeldt A. Dye-Sensitized Solar Cells for Efficient Power Generation under Ambient Lighting. Nat. Photonics 2017, 11 (6), 372–378. 10.1038/nphoton.2017.60. [DOI] [Google Scholar]
- Tang Y.; Wang Y.; Li X.; Ågren H.; Zhu W.-H.; Xie Y. Porphyrins Containing a Triphenylamine Donor and up to Eight Alkoxy Chains for Dye-Sensitized Solar Cells: A High Efficiency of 10.9%. ACS Appl. Mater. Interfaces 2015, 7 (50), 27976–27985. 10.1021/acsami.5b10624. [DOI] [PubMed] [Google Scholar]
- Bisht R.; Mele Kavungathodi M. F.; Nithyanandhan J. Indenoquinaldine-Based Unsymmetrical Squaraine Dyes for Near-Infrared Absorption: Investigating the Steric and Electronic Effects in Dye-Sensitized Solar Cells. Chem. - A Eur. J. 2018, 24 (61), 16368–16378. 10.1002/chem.201803062. [DOI] [PubMed] [Google Scholar]
- Wang P.; Yang L.; Wu H.; Cao Y.; Zhang J.; Xu N.; Chen S.; Decoppet J. D.; Zakeeruddin S. M.; Grätzel M. Stable and Efficient Organic Dye-Sensitized Solar Cell Based on Ionic Liquid Electrolyte. Joule 2018, 2 (10), 2145–2153. 10.1016/j.joule.2018.07.023. [DOI] [Google Scholar]
- Kim C. K.; Kim H. M.; Aftabuzzaman M.; Jeon I.-Y.; Kang S. H.; Eom Y. K.; Baek J. B.; Kim H. K. Comparative Study of Edge-Functionalized Graphene Nanoplatelets as Metal-Free Counter Electrodes for Highly Efficient Dye-Sensitized Solar Cells. Mater. Today Energy 2018, 9, 67–73. 10.1016/j.mtener.2018.05.003. [DOI] [Google Scholar]
- Ji J.-M.; Kim C. K.; Kim H. K. Well-Dispersed Te-Doped Mesoporous Carbons as Pt-Free Counter Electrodes for High-Performance Dye-Sensitized Solar Cells. Dalt. Trans. 2021, 50 (27), 9399–9409. 10.1039/D0DT04372A. [DOI] [PubMed] [Google Scholar]