Abstract
Background:
People with cocaine use disorder (CUD) often have abnormal cognitive function and brain structure. Cognition is supported by brain networks that typically have characteristics like rich-club organization, which is a group of regions that are highly connected across the brain and to each other, and small worldness, which is a balance between local and long-distance connections. However, it is unknown whether there are abnormalities in structural brain network connectivity of CUD.
Methods:
Using diffusion-weighted imaging, we measured structural connectivity in 37 people with CUD and 38 age-matched controls. We identified differences in rich-club organization and whether such differences related to small worldness and behavior. We also tested whether rich-club reorganization was associated with caudate and putamen structural connectivity due to the relevance of the dopamine system to cocaine use.
Results:
People with CUD had a higher normalized rich-club coefficient than controls, more edges connecting rich-club nodes to each other and to non-rich-club nodes, and fewer edges connecting non-rich-club nodes. Rich-club nodes were shifted posterior and lateral. Rich-club reorganization was related to lower clustered connectivity around individual nodes found in CUD, to increased impulsivity, and to a decrease in caudate connectivity.
Conclusions:
These findings are consistent with previous work showing increased rich-club connectivity in conditions associated with a hypofunctional dopamine system. The posterior shift in rich-club nodes in CUD suggests that the structural connectivity of posterior regions may be more impacted than previously recognized in models based on brain function and morphology.
Keywords: Brain networks, Diffusion-weighted imaging, Structural MRI, Cocaine, Impulsivity, Rich club
1. INTRODUCTION
Cocaine use across the world has been regaining popularity (United Nations Office on Drugs and Crime, 2020) and is associated with increased risk of mortality, infections, and serious psychological distress (Butler et al., 2017; Mustaquim et al., 2021; Peacock et al., 2021). Chronic cocaine use alters dopamine system functioning (Mash et al., 2002; Volkow et al., 1993; Volkow et al., 2006) and is associated with a range of cognitive deficits (Potvin et al., 2014), which are accompanied by abnormal brain structure and function (Hester and Garavan, 2004; Kubler et al., 2005; Ma et al., 2009; Moeller et al., 2010). Healthy structural brain networks are organized with features of complex networks, including hubs (regions that are highly connected across the brain), some of which are highly connected to one another to form what is called a rich-club (Fornito and Bullmore, 2015; van den Heuvel and Sporns, 2013), and small worldness, which is a balance between long distance and local connections (Bullmore and Sporns, 2009; Sporns, 2013; Watts and Strogatz, 1998). Such network characteristics are critical for healthy cognitive function (Caeyenberghs et al., 2014; Crossley et al., 2016; Misic and Sporns, 2016) by shaping the functioning of the network (Betzel et al., 2013; Bullmore and Sporns, 2009; Honey et al., 2009). Emerging evidence suggests that individuals with substance use and other addictive disorders have abnormal rich-club organization and small worldness (Park et al., 2018; Zorlu et al., 2019); however, whole brain structural brain networks have not been mapped in people with cocaine use disorder (CUD).
Neuroimaging studies have revealed localized effects of cocaine on the structural integrity of the brain. Generally, cocaine use is associated with reduced white matter integrity, typically in frontal/anterior structures (Hanlon et al., 2011a; Lim et al., 2002; Lim et al., 2008; Lyoo et al., 2004; Moeller et al., 2005; Romero et al., 2010) and white matter tracts that connect such regions (Bell et al., 2011; He et al., 2020), as well as subcortical regions (Hanlon et al., 2011a). Several recent meta-analyses and reviews have shown reduced white matter integrity in the frontal lobes and in the anterior corpus callosum, which connects the frontal hemispheres (Beard et al., 2019; Hampton et al., 2019; Suchting et al., 2021). Other studies found increased integrity in posterior white matter tracts connected to the parietal lobes associated with recent cocaine use (Bell et al., 2011), and people with CUD have greater gray matter volume in the posterior parietal cortex (Romero et al., 2010). However, other work has shown decreased integrity in posterior regions (Hodges et al., 2021; Ma et al., 2017; Narayana et al., 2009; Narayana et al., 2014). Identifying changes in whole-brain network organization in CUD will clarify whether these local anatomical changes relate to abnormalities in brain network connectivity, as they do in non-cocaine-using populations (Irimia and Van Horn, 2014), and the specific pattern of network reorganization in CUD.
The use of graph theory to examine whole-brain abnormalities in structural connectivity in brain disorders is becoming increasingly common in emerging clinical paradigms (Fornito and Bullmore, 2015; Fornito et al., 2015; Zalesky et al., 2010). Highly connected hub regions are biologically costly and are particularly vulnerable to damage in neurocognitive disorders (Crossley et al., 2014). In non-clinical populations, these hubs are commonly identified as including the precuneus, superior frontal cortex, anterior and posterior cingulate cortex, insula, superior parietal lobule, putamen, hippocampus, and thalamus (Kocher et al., 2015; van den Heuvel and Sporns, 2011). Accordingly, abnormal structural rich-club organization has been found across multiple neurocognitive conditions, including decreased rich-club connectivity strength in alcohol use disorder (Zorlu et al., 2019), multiple sclerosis (Shu et al., 2018), siblings of people with schizophrenia (Collin et al., 2014a), and attention-deficit/hyperactivity disorder (Ray et al., 2014) compared with controls. Interestingly, there is increased rich-club connectivity in early Parkinson’s disease (Mishra et al., 2020) and in people with Alzheimer’s disease (Daianu et al., 2013; Lee et al., 2018), both of which have etiologies that include decreased dopamine system functionality (Lotharius and Brundin, 2002; Nobili et al., 2017; Pan et al., 2019), like CUD. This increase in connectivity strength may occur when brain damage is restricted enough that there can be compensatory hyperconnectivity.
The present study compared the structural connectome based on diffusion-weighted imaging (DWI) data of CUD to that of non-cocaine using controls. We hypothesized that CUD would have abnormal rich-club organization compared to controls. Though the increased rich-club connectivity in alcohol use disorder may suggest a similar pattern in CUD, alcohol facilitates inhibitory GABA transmission (Nevo and Hamon, 1995) whereas cocaine acts as a stimulant (Withers et al., 1995). Furthermore, other neuropsychiatric disorders with dopaminergic etiology, such as early Parkinson’s disease and Alzheimer’s disease, are associated with increased rich-club connectivity. Second, we predicted that abnormal rich-club organization would be related to impulsivity, abnormal small world properties, and to structural abnormalities in dopamine-rich regions.
2. METHODS AND MATERIALS
2.1. Participants and procedures
The study recruited adults aged 18-55 years who either had a current diagnosis of CUD or no history of cocaine use (controls). The CUD group met the following criteria: current cocaine dependence as defined by the DSM-IV-TR, regular cocaine use for ≥1 year, ≥2 days of use in the past 30 days, and cocaine as a principal drug of abuse. The control group met the following criteria: no lifetime CUD (abuse or dependence), no history of regular cocaine use, no cocaine use in the past year, and a cocaine-negative urine drug screen. Alcohol, marijuana, and nicotine use were permitted in both groups, but participants could not have a current primary alcohol or marijuana use disorder (i.e., alcohol or marijuana dependence secondary to a CUD was acceptable). For all other illicit drugs, individuals were excluded for any history of dependence, lifetime regular use for >2 years, regular use in the past 25 years, and use in the past 30 days. Additional exclusion criteria were: indicators of severe mental illness or impaired mental status; <8th grade education; English non-fluency or illiteracy; severe learning disability with functional impairment; serious neurological disorders; acute opportunistic brain infections or a history of such infections without return to normal cognition; severe head trauma with loss of consciousness >30 minutes and persistent functional decline; and any MRI contraindications.
Study procedures were approved by the institutional review board at Duke University Health System. Informed consent was obtained from all participants. The screening included clinical interviews, computerized surveys, a urine drug screen, and pregnancy testing. The Mini International Neuropsychiatric Interview was used to confirm the absence of disqualifying psychiatric disorders and conditions (Sheehan et al., 1998). The Addiction Severity Index-Lite (McLellan et al., 1992) and the Structured Clinical Interview for DSM-IV-TR (First et al., 1996) were used to assess lifetime substance use and substance use disorders, respectively. At each visit, urine toxicology screens were used to corroborate self-report of recent drug use. Participants also completed an audio computer-assisted self-interview that included a demographics survey and medical history. HIV-negative status was verified using an OraQuick© rapid test. After the visit, healthcare records were reviewed to ensure no exclusionary medical history, including substance abuse.
Participants completed the Barret Impulsiveness Scale, 11th revision (BIS-11; Barratt, 1959; Stanford et al., 2009). The BIS is a 30-item self-report instrument, all rated on a scale from 1-4, designed to assess the personality/behavioral construct of impulsiveness. Additional details about procedures and data acquisition, and processing are described in detail in our previous work (Meade et al., 2020) and in the Supplementary Materials.
2.2. MRI data acquisition and processing
All scans were performed on the same 3T GE Discovery MR750 imaging system with an 8-channel head coil at Duke University Hospital. Data were collected using three protocols with slightly different acquisition parameters and were harmonized during processing. Diffusion-weighted images (DWI) were acquired in the axial plane using a single shot spin-echo EPI sequence (FOV= 256 mm2, voxel size = 2mm3,128*128 matrix, 90° flip angle, interleaved slices of 2mm thickness). Data were acquired in 30 directions for protocols 1 and 2, and 64 directions for the third protocol. TE was set to use the minimum to maximize the signal-to-noise ratio and the time ranged across participants. Additional parameters differed slightly between protocol 1 (b-factor= 900 s/mm2, TR/TE= 10,000/81.5-84.6, 73 slices), protocol 2 (b-factor= 800 s/mm2, TR/TE= 8000/77.9-83.9, 67 slices), and protocol 3 (b-factor=800 s/mm2, TR/TE=8000/77.9-81.0, 67 slices). To harmonize the DWI data, we implemented a downsampling procedure using a matlab dot() function (inner product) that identified the diffusion-encoding directions that were most similar to those in the protocol with 30 directions (Chen et al., 2021). Details on DWI data processing are provided in our previous work (Hall et al., 2021) and in the Supplementary Materials. Protocol was included as a dummy coded covariate in all analyses. A visual check ensured whole-brain coverage and absence of uncorrectable motion or scanner artifacts.
2.3. Whole-brain tractography
Deterministic tractography was conducted using MRtrix3’s tckgen, implementing an anatomically-constrained (ACT) procedure (Smith et al., 2012) with a five-tissue-type (5TT) segmentation of the T1 image using the Freesurfer-based algorithm in 5ttgen (Smith et al., 2012). One hundred thousand tracks were generated from random points in the mask image at a step size of 0.2mm, constrained to a 60-degree maximum angle between steps and excluding tracks whose lengths were outside the range of 4mm-200mm. Ten percent of the tracks were overlaid on each participant’s MNI-registered T1 image and visually inspected to ensure tracts fell within white matter. The Human Connectome Project multi-modal parcellation (HCPMMP) atlas (Glasser et al., 2016) was used to define nodes and included subcortical regions from the Harvard-Oxford atlas (Desikan et al., 2006; Frazier et al., 2005; Goldstein et al., 2007; Makris et al., 2006). The HCPMMP atlas consists of 360 neuroanatomical regions delineated using multimodal MRI and machine learning and provides complete cortical and subcortical coverage. We also created a connectome using the Desikan-Killiany atlas that was used for replication (Desikan et al., 2006). The Desikan-Killiany atlas consists of 68 cortical regions based on probabilistic information estimated from a manually labeled training set and 16 subcortical volumes based on an atlas containing probabilistic information on the location of structures. Both atlases that were utilized in this investigation contained nodes that corresponded to the non-clinical rich club nodes previously identified. Streamline count between every pair of nodes was extracted into weighted, undirected connectomes using tck2connectome (Tournier et al., 2019). We excluded edges consisting of fewer than three streamlines. Furthermore, to eliminate the effect of region size, the number of streamlines was normalized by the mean of the sizes of the two regions for each edge. To ensure that the connectomes in both groups had the same density, we applied a density threshold, retaining only the top x% of connections. For the HCPMMP and Desikan-Killiany atlases respectively, every individual had a density of at least 2% and 12%, so these were used as the respective thresholds. Degree distributions for each group were visually inspected to confirm similarity between groups (Figure S1).
2.4. Rich-club organization
Rich-club organization, and all graph metrics, were computed from the weighted structural connectivity matrices from the in-house scripts. The weighted rich-club coefficient φk was computed at all node degrees k:
where w is a vector of all connection weights, E>k is the number of links between the nodes with a degree greater than k, and W>k is the sum of the weights between the same nodes (van den Heuvel and Sporns, 2011). Normalized rich-club coefficients (nRCC) were calculated for each participant by dividing their RCCs by the average RCC of 1000 random graphs constructed by randomly rewiring nodes in a manner that preserves degree distribution at all nodes (Maslov and Sneppen, 2002). The network was said to have a rich-club organization at a given node degree k when the nRCC was greater than 1. Significance was assessed using one-sample t-tests against 1 in each group. Rich-club results were analyzed when all participants had edges at a given k.
Between-group differences in rich-club organization were conducted on the individual nRCCs using PALM permutation testing (Winkler et al., 2014) at each degree level with 1000 permutations. The significance threshold was set at FDR-corrected p < 0.05. Rich-club organization was verified using the Desikan-Killiany atlas.
Individual-level rich-club nodes were ranked by determining the highest degree level at which a node participated in a rich-club, which were then averaged in each group. The top 12% of nodes were considered as a group-level rich-club (Collin et al., 2014b; Daianu et al., 2016; van den Heuvel and Sporns, 2011; van den Heuvel et al., 2013). Using this rich-club node designation, we calculated the percent of rich-club edges (connections between two rich-club nodes), feeder edges (connections between a rich-club node to a non-rich-club node), and local edges (connections between two non-rich-club nodes), out of all existing edges. Those percentages were compared between groups using ANCOVAs. Due to group differences in years of education (mean-centered), alcohol use in the past 30 days (yes/no), cannabis use in the past 30 days (yes/no), and daily cigarette (yes/no), these were included as covariates in all models unless otherwise noted. These ANCOVAs, and all statistics reported, were conducted using SPSS 28.
2.5. Brain network metrics
Other brain network metrics were obtained using the Brain Connectivity Toolbox (http://www.brain-connectivity-toolbox.net; Rubinov and Sporns, 2010) and in-house scripts. The small world coefficient, which is the ratio of the normalized clustering coefficient (the fraction of triangles around a node normalized by the clustering coefficient for a random graph) to the normalized characteristic path length (the average distance between a node and all other nodes in the brain; formal equations defined in Rubinov and Sporns, 2010) was > 1 for all participants. This confirmed that the threshold applied allowed brain networks to maintain the standard organization required for functioning (Bassett and Bullmore, 2006; Watts and Strogatz, 1998). We report the normalized transitivity, which is the ratio of triangles to triplets in a network and is an alternative to clustering coefficient (defined in Rubinov and Sporns, 2010), and normalized characteristic path length for each group. Group differences in normalized transitivity and normalized characteristic path length were examined using two ANCOVAs.
2.6. Relationship between rich-club and small world characteristics and impulsivity
To determine whether abnormal rich-club characteristics relate to other abnormal brain network properties, we ran a series of four linear regression models predicting normalized transitivity as a function of each of four rich-club characteristics (nRCC, % rich-club edges, % feeder edges, and % local edges). A parallel set of analyses were run predicting normalized characteristic path length. Cocaine group was entered as a control variable as we did not have an a priori reason to expect that the relationship between rich-club characteristics and small world characteristics would differ between CUD and controls. A similar set of regression models were run using total BIS scores as a function of the four rich-club characteristics in the CUD group only.
2.7. Exploratory relationship between striatal integrity and rich-club characteristics
Due to the relationship between cocaine use and striatal dopamine system abnormalities, we identified group differences in caudate and putamen connectivity strength (the sum of the normalized number of tracts; Rubinov and Sporns, 2010) with the rest of the brain, averaged across hemispheres, using ANCOVAs. Cocaine group was entered as a control variable.
We then investigated the relationship between the striatal connectivity strength and rich-club characteristics. The connectivity strength of the structure(s) that significantly differed between CUD and controls were used as independent variables in regression models with rich-club characteristics as dependent variables. To verify that the relationship between striatal connectivity and rich-club characteristics was not driven by average whole-brain connectivity strength, we identified group differences in whole-brain connectivity strength and ran parallel regression models with whole-brain connectivity strength as the independent variable.
3. RESULTS
3.1. Sample characteristics
The sample included 37 people with CUD and 38 control age-matched participants (Table 1). Participants were primarily male and African-American, ranging in age from 28 to 55. Participants were well matched on gender and race, but the CUD group had significantly fewer years of education and a greater mean total BIS score than controls.
Table 1.
Demographic information
| CUD (N = 37) | Control (N = 38) | Statistic | p-value | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Sex | 0.706 | |||
| Male, %, N | 62.16%, 23 | 57.89%, 22 | χ2(1) = 0.14 | |
| Female, %, N | 37.84%, 14 | 42.11%, 16 | ||
| Age in years, M (SD) | 45.59 (6.32) | 43.13 (7.43) | t(73) = −1.55 | 0.127 |
| Race | χ2(2) = 1.53 | 0.465 | ||
| African American, %, N | 81.08% 30 | 73.68%, 28 | ||
| White, %, N | 13.51%, 5 | 23.68%, 9 | ||
| Other/Mixed, %, N | 5.41%, 2 | 2.63%, 1 | ||
| Education in years, M (SD) | 12.51 (2.51) | 13.92 (2.03) | t(73) = 2.67 | 0.009 |
| Barratt Impulsiveness Scale, M (SD) | 67.27 (11.60) | 54.13 (7.83) | t(73) = −5.77 | 0.0000002 |
| Substance Use Characteristics | ||||
| Alcohol use in past 30 days, %, N | 86.49%, 32 | 55.26%, 21 | χ2(1) = 8.82 | 0.003 |
| Marijuana use in past 30 days, %, N | 45.95%, 17 | 26.32%, 10 | χ2(1) = 3.14 | 0.077 |
| Daily cigarette use in past 30 days, %, N | 62.16%, 23 | 34.21%, 13 | χ2(1) = 5.87 | 0.015 |
The CUD group had been using cocaine regularly for an average of 17.68 years (SD = 8.28), with the majority (92%) reporting smoking as their primary route of administration. In the 30 days prior to screening, they had used on an average of 10.35 days (SD = 7.09). On the day of the MRI, 70% had a urine toxicology screen positive for cocaine. The mean number of days since last use was 3.43 (SD = 4.20), with 73% reporting use within the past 3 days. Other substance use was common in both groups, although people with CUD generally were more likely to use cigarettes, alcohol, and cannabis (Table 1).
3.2. Rich-club organization
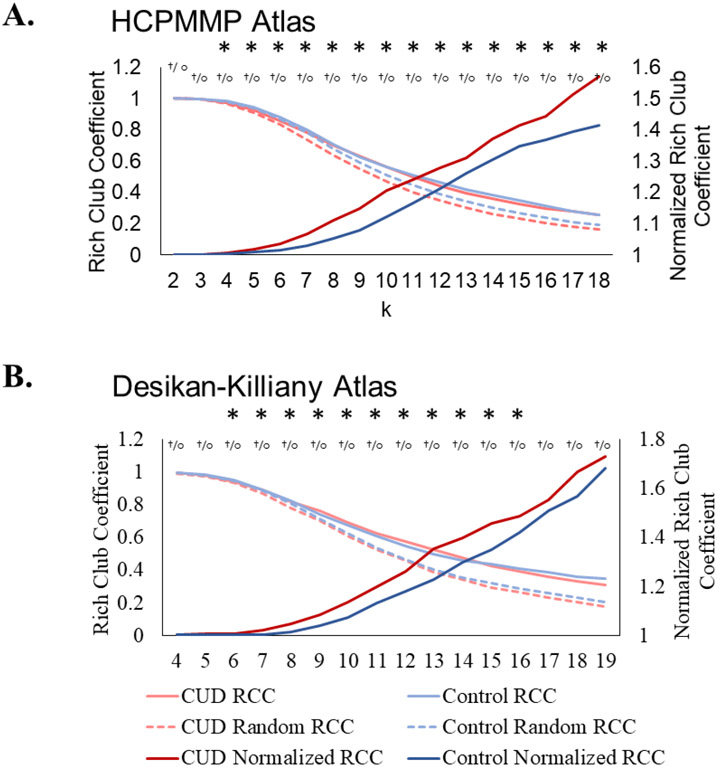
Rich-club organization was present at degrees ranging from 2-18 for both the CUD and the control groups. At k = 4-18, the CUD group had higher nRCCs than controls (Figure 1A, Table S1). Results were replicated using the Desikan-Killiany atlas (Figure 1B, Table S1).
Figure 1. Rich-club coefficients.
A. These graphs show the raw RCC for each group graph (light solid lines), the RCC for the random graphs (dotted lines), and the mean nRCC (normalized RCC; dark lines). Using both atlases, the CUD group had a higher nRCC than the controls. Significant group differences are indicated with asterisks. Significant rich-club organization in the CUD (or control group) is indicated with crosses (or open circles). Using the HCPMMP atlas, the CUD group had a higher nRCC for all ks between 4 and 18. Both groups had significant rich-club organization between k = 2-18. B. Using the Desikan-Killiany atlas, the CUD group had a higher nRCC for all ks between 6 and 16. The CUD and control groups both had significant rich-club organization between k = 4-19.
3.3. Rich-club nodes
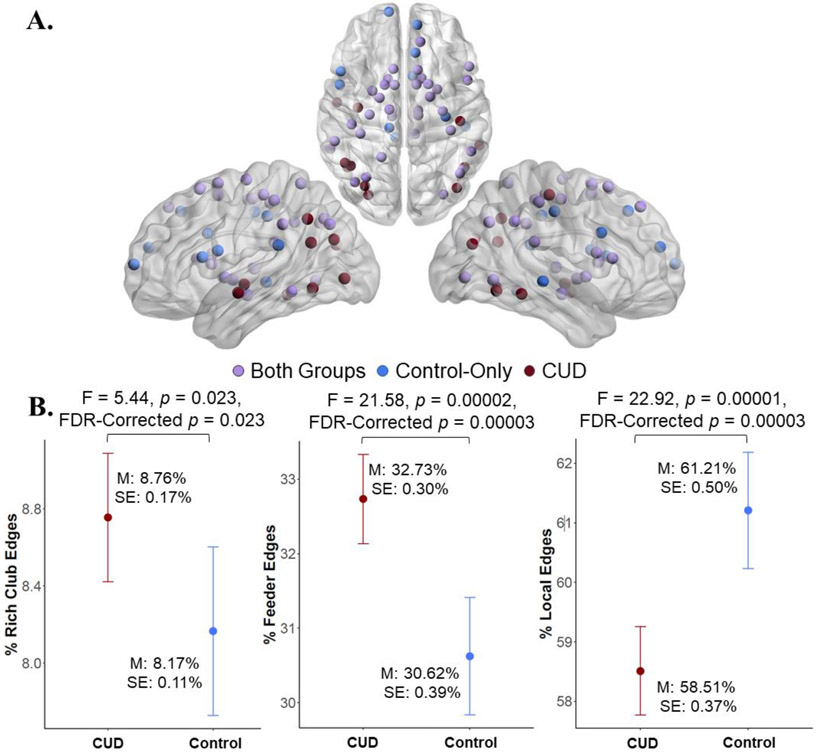
Nodes in the rich-club in both groups were similar to those typically found to be in the rich-club in non-clinical populations, including superior frontal, putamen, and thalamus (van den Heuvel and Sporns, 2011). Rich-club nodes that were specific to the control group were largely in frontal regions, including medial and inferior frontal regions, anterior frontal regions, retrosplenial cortex, and posterior cingulate gyrus, whereas rich-club nodes that were specific to the CUD group were largely in parietal and temporal regions, including inferior parietal cortex, insula, and lateral temporal regions (Figure 2A, Table S2).
Figure 2. Rich-club coefficients and nodes.
A. Rich-club nodes common to both groups included frontal, parietal, and subcortical regions. Nodes specific to the controls were largely in frontal regions whereas nodes specific to CUD were in temporoparietal regions. B. There were a greater percent of rich-club and feeder edges but fewer local edges in CUD compared to the control group. All analyses controlled for protocol, education, 30-day alcohol use, 30-day cannabis, and daily cigarette smoking. Degrees of freedom = 1,67.
3.4. Group differences in edge class distribution and small world characteristics
There was a greater percent of rich-club edges and feeder edges in the CUD than the control group. In contrast, there were fewer local edges in the CUD group than in the controls (Figure 2B).
3.5. Relationship between rich-club characteristics and small world characteristics
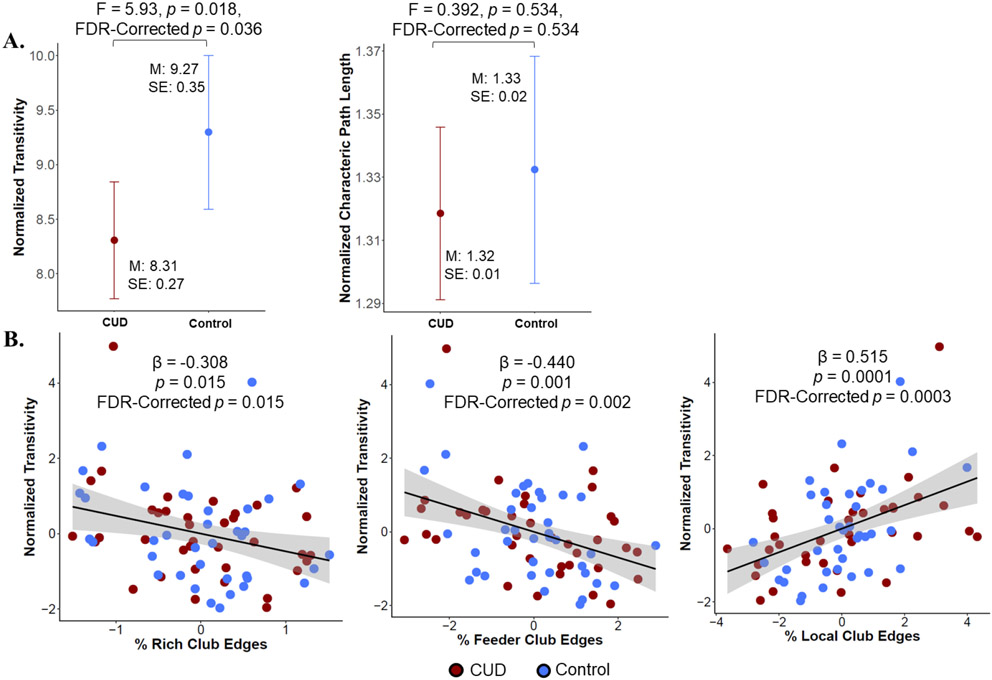
People with CUD had significantly lower normalized transitivity than controls but no significant difference in normalized characteristic path length (see Figure 3A). There were relationships between the percent of rich-club, feeder, and local edges and normalized transitivity, with a greater percent of rich and feeder edges being associated with lower transitivity and a greater percent of local edges being associated with higher transitivity (Figure 3B, Table S3). There were no relationships between rich-club characteristics and characteristic path length.
Figure 3. Edge classes and small world characteristics.
A. Estimated marginal means (controlling for protocol, education, 30-day alcohol use, 30-day cannabis use, and daily cigarette smoking) showing that the CUD group had significantly lower normalized transitivity than controls but no significant difference in normalized characteristic path length. Degrees of freedom = 1,67. B. Partial correlation plots showing a negative relationship between the % of rich-club and feeder edges and normalized transitivity, and a positive relationship between the % of local edges and normalized transitivity. Protocol, education, 30-day alcohol use, 30-day cannabis use, daily cigarette smoking, and cocaine use status were included as covariates of no interest.
3.6. Relationship between rich-club characteristics and impulsivity
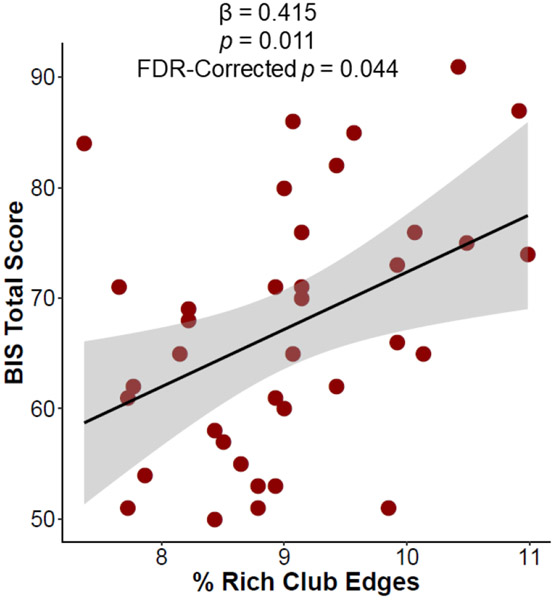
There was a significant relationship between percent rich club edges and BIS score among persons with CUD, where more rich club edges were associated with a higher BIS score (Figure 4, Table S4). There was no relationship between the nRCC, percent of feeder edges, or local edges and BIS score.
Figure 4. Relationship between rich club edges and impulsivity in CUD.
There is a significant positive relationship between the proportion of rich club edges and total BIS score in persons with CUD.
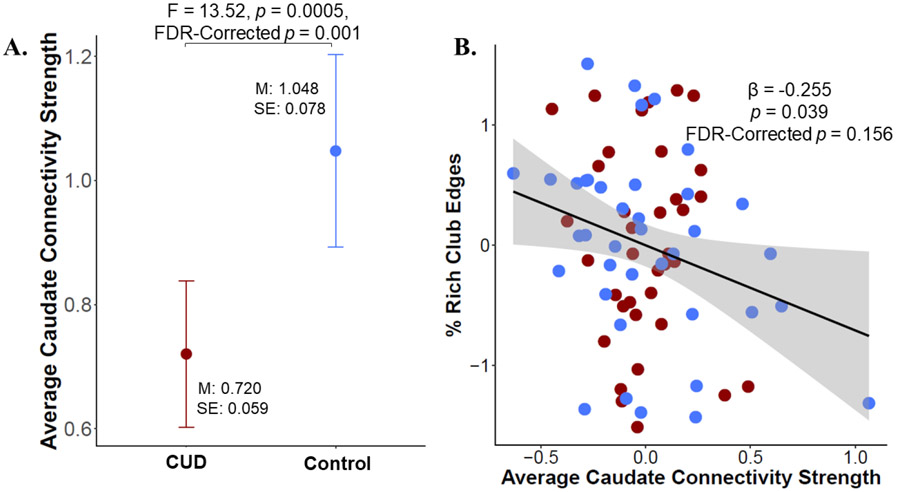
3.7. Relationship between striatal structural integrity and rich-club characteristics
We first found that the caudate had lower connectivity strength in CUD compared to controls but that there were no group differences in putamen connectivity strength (Figure 5A, Table S5). Then, examining the relationship between caudate connectivity strength and rich-club characteristics, we found a significant relationship between caudate connectivity strength and % of rich-club edges, with more caudate connectivity strength being associated with fewer rich-club edges, although this relationship did not survive FDR multiple comparisons correction (Figure 5B, Table S6). Though there was a significant decrease in whole-brain connectivity strength in CUD compared to controls (CUD estimated marginal mean [EMM] = 0.308 (0.010), control EMM = 0.389 (0.013), F(1,67) = 29.935, p = 0.0000007; Table S5), there was no relationship between whole-brain connectivity strength and rich-club characteristics (Table S6), suggesting that this is not a general relationship between connectivity strength and % of rich-club edges.
Figure 5. Striatal – rich-club properties relationship.
A. Estimated marginal means showing decreased connectivity strength between the caudate and the rest of the brain in CUD compared to controls, controlling for protocol, education, 30-day alcohol use, 30-day cannabis use, and daily cigarette smoking. B. Partial correlation plots showing the relationship between rich-club properties and caudate connectivity strength. There was a negative relationship between caudate connectivity strength and the % of rich-club edges across both groups. Protocol, education, 30-day alcohol use, 30-day cannabis use, daily cigarette smoking, and cocaine use status were included as covariates of no interest.
4. DISCUSSION
To our knowledge, this is the first study to investigate differences in the structural connectome of people with CUD compared to controls. We found that the CUD group had a higher rich-club coefficient at k levels ranging from 4-18, indicating greater connectivity amongst rich-club nodes compared to controls. They also had a greater percentage of rich-club and feeder edges, and a lower percentage of local edges. These abnormal rich-club properties were related to abnormal small world properties and increased impulsivity. Rich-club nodes common to both groups were largely concentrated in medial and lateral frontal regions, but there were fewer in the CUD group compared to controls. Rich-club nodes specific to CUD were largely concentrated in temporoparietal regions but were missing from the posterior midline regions where they were found in controls. This suggests a lateral posterior shift in rich-club nodes in CUD. Finally, abnormal rich-club properties were associated with decreased striatal structural connectivity.
As hypothesized, we found that people with CUD had abnormal rich-club connectivity. Similar to studies on Parkinson’s disease and Alzheimer’s disease, people with CUD had increased rich club connectivity (Daianu et al., 2013; Lee et al., 2018; Mishra et al., 2020). The increased rich-club organization we find in CUD, coupled with the increase in the percent of rich-club and feeder edges, complements previous work showing increased connectivity in CUD. Wang et al. (Wang et al., 2015) show global increases in functional connectivity in CUD compared to controls during resting state, and Konova et al. (Konova et al., 2015) show increased hub node functional connectivity density in CUD, with hub nodes consisting of default mode network nodes. Our findings of decreased local clustering in CUD and fewer local edges are similar to work showing decreased functional local efficiency in CUD (Wang et al., 2015). The increased rich-club organization in CUD may signify inefficient network connectivity caused by the shift in rich club nodes from more frontal to posterior regions. It should be noted that for the covariates entered in our ANCOVA models, only years of education was also a predictor of rich-club organization. Studies examining socioeconomic status that include years of education have found a positive relationship between greater years of education and white matter integrity (Johnson et al., 2013; Shaked et al., 2019). However, as the CUD group had lower years of education, and greater rich club coefficient, if this variable was driving the results we would have expected the opposite effect. We also show a relationship between rich-club/feeder/local edge redistribution and local clustering in both people with CUD and controls but no relationship with long distance connections, measured with characteristic path length. Damaged rich-club edge connectivity has been shown to have a larger impact on local connectivity than global, possibly because rich-club edges are responsible for establishing diverse connections across the brain that integrate multiple cognitive functions (de Reus and van den Heuvel, 2014).
The posterior shift in rich-club nodes that we find in CUD is consistent with theories about addiction that postulate that in prolonged drug use, posterior default mode network regions, including the posterior parietal cortex, become more active as self-referential processing intensifies. In contrast, regions in the anterior default mode network, including medial frontal regions, become less active as emotion regulation abilities become less intact (Zhang and Volkow, 2019). Interestingly, lateral posterior parietal regions are not implicated in prevailing models of addiction (Everitt and Robbins, 2016; Volkow et al., 2019). However, these models are largely based on work investigating brain function and morphology; the addition of structural connectivity to the literature may enhance these models. Though medial parietal structures are more frequently implicated in CUD (Lebel et al., 2013; Narayana et al., 2009) than lateral parietal cortical structures (e.g., Grewen et al., 2014), there is evidence showing posterior parietal regions in CUD have elevated creatine, which has been attributed to the establishment of white-matter tract connectivity (O'Neill et al., 2001). Additionally, people with CUD have been shown to have increased activity in parietal regions in response to reward and reward cues, especially cocaine (Costumero et al., 2018; Porrino et al., 2007; Tomasi et al., 2015), and have increased parietal connectivity during rest (Ray et al., 2015). Furthermore, people with CUD have decreased parietal activity during inhibition (Barros-Loscertales et al., 2011; Elton et al., 2014; Li et al., 2008), and reduced parietal volume related to impulsivity (Meade et al., 2020). This suggests that there may be some extended impacts of cocaine beyond the regions identified in classic models of addiction, and that identifying abnormalities in structural connectivity may reveal such impacts.
Additionally, the decreased connectivity that we find in CUD in frontal regions, and orbitofrontal regions in particular, is in line with much of the addiction literature. Models of cocaine use postulate that the medial/orbitofrontal cortex regions facilitate control over drug use and when lesioned, drug use increases (Everitt and Robbins, 2005; Grakalic et al., 2010). Furthermore, as drug use becomes more compulsive, frontal regions are thought to disengage as the role of striatal regions in mediating drug-taking behavior increases (Everitt and Robbins, 2016). Accordingly, decreases in frontal white matter integrity in CUD compared to controls are commonly reported (He et al., 2020; Lim et al., 2002), as well as decreased frontal functional connectivity (Gu et al., 2010; Kelly et al., 2011). As the people with CUD in our sample had been using for an average of >15 years, the decrease in frontal connectivity is aligned with this model of compulsive drug use.
The relationship we show between the percent of rich club edges and impulsivity in CUD elucidates a potential mechanism through which CUD may be susceptible to such use. People with CUD are known to be impulsive (Moeller et al., 2001; Molander et al., 2011); further, impulsivity is associated with other cocaine-related outcomes, like compulsive cocaine-taking (Belin et al., 2008), and cocaine-seeking after abstinence (Economidou et al., 2009). Impulsivity has been shown to be related to whole-brain resting state network organization, with increased connectivity between frontal and subcortical regions being associated with less impulsivity (Davis et al., 2013). In CUD, impulsivity is associated with decreased frontal grey matter volume (Ersche et al., 2011; Meade et al., 2020) and white matter integrity (Romero et al., 2010). Our work contributes to this literature by showing that whole-brain structural reorganization of edges in CUD to prioritize rich club node connectivity, which largely exclude frontal regions, is also related to increased impulsivity.
Finally, we present evidence showing that abnormal rich-club organization is associated with abnormalities in the striatal dopamine system, with lower caudal structural connectivity being associated with a greater percent of rich-club edges. People with CUD have a hypofunctional dopamine system (Volkow et al., 1990) and the caudate is commonly impacted by cocaine use, with lower structural integrity in the white matter surrounding it (Vaquero et al., 2017), and decreased functional connectivity (Hanlon et al., 2011b). Dopamine system functionality is associated with decreased functional integration across the brain and increased segregation, as well as decreased default mode network connectivity (Carbonell et al., 2014; Kelly et al., 2009; Luo et al., 2015; Nagano-Saito et al., 2017; Shine et al., 2019; Weingarten et al., 2015). This suggests that damage to the dopamine system may lead to increased default mode network function and increased global connectivity. Mishra and colleagues show that individuals with Parkinson’s disease, a neurologic condition associated with a hypofunctional dopamine system (Lotharius and Brundin, 2002), had increased structural rich-club organization compared to controls (Mishra et al., 2020). In contrast, a hyperfunctional dopamine system, exemplified in schizophrenia, is associated with the converse: decreased integration and increased segregation (Hadley et al., 2016). The caudate is involved in goal-directed and motivated behavior (Balleine et al., 2007) and cognitive flexibility (Klanker et al., 2013). Therefore, decreased caudal connectivity in CUD and its relationship with fewer rich-club edges in CUD could reflect a shift from goal-directed functioning to a more self-focused state with increased rumination, negative affect, or drug-related cognition, supported by the posterior shift in rich-club nodes away from frontal regions and into posterior default mode network regions (Zhang and Volkow, 2019).
4.1. Limitations/Conclusions
This work is the first to demonstrate abnormal whole-brain structural connectivity in CUD, the relationship with impaired cognition, and shows a potential mechanism to explain such abnormalities. However, we note limitations to this work. 1. Due to the relatively low number of diffusion directions in our data (n=30) we used deterministic tractography rather than probabilistic tractography. It has been suggested that deterministic tractography results in greater specificity with a higher number of false negative connections relative to probabilistic tractography, which has greater sensitivity but more false positive connections (Zalesky et al., 2016). When building structural connectomes, Zalesky et al. (2016) concluded that specificity is more important than sensitivity. 2. Though we showed that increased rich-club connectivity in CUD is robust to the selection of atlas, due to the modest sample size, a replication is needed in an independent sample. 3. We do not have a direct measure of dopamine system functionality. Future work will be necessary to validate the relationship between dopamine system functionality and structural connectivity in CUD. Despite these limitations, this work shows that prolonged cocaine use has a wide-spread impact on brain structure, which may encompass regions that are not typically discussed in neurologic models of CUD. If supported by future work, this suggests that such models may be updated to include the downstream effects of cocaine use and how those impact behavioral outcomes. Understanding the shift in hub regions in cocaine use disorder and how this relates to abnormal dopamine circuitry could provide a potential target for interventions that could improve decrease impulsive behaviors and potentially help treat CUD.
Supplementary Material
Highlights.
People with cocaine use disorder (CUD) have increased structural rich club connectivity
Rich club nodes are shifted to the posterior in CUD
People with CUD have more rich-club and feeder edges, but fewer local edges than controls
Rich-club abnormalities are related to lower clustering and striatal connectivity
Rich-club abnormalities are associated with increased impulsivity in CUD
Acknowledgments
We would like to thank all participants for their time and help with our research.
Funding
This work was supported by the National Institutes of Health [grant number R01-DA045565].
Abbreviations:
- CUD
Cocaine use disorder
- nRCC
Normalized rich club coefficient
- BIS
Barratt Impulsiveness Scale
Footnotes
Conflict of Interest
No conflict declared
References
- Balleine BW, Delgado MR, Hikosaka O, 2007. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience 27(31), 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt ES, 1959. Anxiety and impulsiveness related to psychomotor efficiency. PMS 9, 191–198. [Google Scholar]
- Barros-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, Avila C, 2011. Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Res 194(2), 111–118. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, 2006. Small-world brain networks. Neuroscientist 12(6), 512–523. [DOI] [PubMed] [Google Scholar]
- Beard CL, Schmitz JM, Soder HE, Suchting R, Yoon JH, Hasan KM, Narayana PA, Moeller FG, Lane SD, 2019. Regional differences in white matter integrity in stimulant use disorders: A meta-analysis of diffusion tensor imaging studies. Drug Alcohol Depend. 201, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ, 2008. High impulsivity predicts the switch to compulsive cocaine-taking. Science 320(5881), 1352–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H, 2011. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 114(2–3), 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Griffa A, Avena-Koenigsberger A, Goñi J, Thiran JP, Hagmann P, Sporns O, 2013. Multi-scale community organization of the human structural connectome and its relationship with resting-state functional connectivity. Network Science 1(3), 353–373. [Google Scholar]
- Bullmore E, Sporns O, 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience 10(3), 186–198. [DOI] [PubMed] [Google Scholar]
- Butler AJ, Rehm J, Fischer B, 2017. Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug and Alcohol Dependence 180, 401–416. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K, Leemans A, Leunissen I, Gooijers J, Michiels K, Sunaert S, Swinnen SP, 2014. Altered structural networks and executive deficits in traumatic brain injury patients. Brain Structure & Function 219(1), 193–209. [DOI] [PubMed] [Google Scholar]
- Carbonell F, Nagano-Saito A, Leyton M, Cisek P, Benkelfat C, He Y, Dagher A, 2014. Dopamine precursor depletion impairs structure and efficiency of resting state brain functional networks. Neuropharmacology 84, 90–100. [DOI] [PubMed] [Google Scholar]
- Chen N.-k., Bell RP, Meade CS, 2021. On the down-sampling of diffusion MRI data along the angular dimension. Magn. Reson. Imaging 82, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Kahn RS, de Reus MA, Cahn W, van den Heuvel MP, 2014a. Impaired Rich Club Connectivity in Unaffected Siblings of Schizophrenia Patients. Schizophr. Bull 40(2), 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Sporns O, Mandl RCW, van den Heuvel MP, 2014b. Structural and Functional Aspects Relating to Cost and Benefit of Rich Club Organization in the Human Cerebral Cortex. Cereb. Cortex 24(9), 2258–2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costumero V, Rosell-Negre P, Bustamante JC, Fuentes-Claramonte P, Llopis JJ, Avila C, Barros-Loscertales A, 2018. Left frontoparietal network activity is modulated by drug stimuli in cocaine addiction. Brain Imaging and Behavior 12(5), 1259–1270. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Fox PT, Bullmore ET, 2016. Meta-connectomics: human brain network and connectivity meta-analyses. Psychological Medicine 46(5), 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET, 2014. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137(Pt 8), 2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daianu M, Dennis EL, Jahanshad N, Nir TM, Toga AW, Jack CR, Weiner MW, Thompson PM, Initia ADN, 2013. Alzheimer's Disease Disrupts Rich Club Organization in Brain Connectivity Networks. 2013 Ieee 10th International Symposium on Biomedical Imaging (Isbi), 266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daianu M, Mezher A, Mendez MF, Jahanshad N, Jimenez EE, Thompson PM, 2016. Disrupted rich club network in behavioral variant frontotemporal dementia and early-onset Alzheimer's disease. Hum. Brain Mapp 37(3), 868–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Knodt AR, Sporns O, Lahey BB, Zald DH, Brigidi BD, Hariri AR, 2013. Impulsivity and the modular organization of resting-state neural networks. Cereb Cortex 23(6), 1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reus MA, van den Heuvel MP, 2014. Simulated rich club lesioning in brain networks: a scaffold for communication and integration? Frontiers in Human Neuroscience 8, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31(3), 968–980. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ, 2009. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol. Psychiatry 65(10), 851–856. [DOI] [PubMed] [Google Scholar]
- Elton A, Young J, Smitherman S, Gross RE, Mletzko T, Kilts CD, 2014. Neural network activation during a stop-signal task discriminates cocaine-dependent from non-drug-abusing men. Addict. Biol 19(3), 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET, 2011. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134(Pt 7), 2013–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci 8(11), 1481–1489. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2016. Drug addiction: updating actions to habits to compulsions ten years on. Annual Review of Psychology 67, 23–50. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient/Non-patient Edition. Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Fornito A, Bullmore ET, 2015. Connectomics: a new paradigm for understanding brain disease. European Neuropsychopharmacology 25(5), 733–748. [DOI] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M, 2015. The connectomics of brain disorders. Natural Reviews Neuroscience 16(3), 159–172. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J, 2005. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. The American Journal of Psychiatry 162(7), 1256–1265. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016. A multi-modal parcellation of human cerebral cortex. Nature 536(7615), 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS Jr, Kennedy DN, Faraone SV, Tsuang MT, 2007. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biological Psychiatry 61(8), 935–945. [DOI] [PubMed] [Google Scholar]
- Grakalic I, Panlilio LV, Quiroz C, Schindler CW, 2010. Effects of Orbitofrontal Cortex Lesions on Cocaine Self-Administration. Neuroscience 165(2), 313–324. [DOI] [PubMed] [Google Scholar]
- Grewen K, Burchinal M, Vachet C, Gouttard S, Gilmore JH, Lin WL, Johns J, Elam M, Gerig G, 2014. Prenatal cocaine effects on brain structure in early infancy. NeuroImage 101, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y, 2010. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. NeuroImage 53(2), 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley JA, Kraguljac NV, White DM, Hoef LV, Tabora J, Lahti AC, 2016. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. Npj Schizophrenia 2, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SA, Bell RP, Davis SW, Towe SL, Ikner TP, Meade CS, 2021. HIV-Related Decreases in Corpus Callosal Integrity and Corresponding Increases in Functional Connectivity Hum. Brain Mapp. 42(15), 4958–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton WH, Hanik IM, Olson IR, 2019. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug and Alcohol Dependence 197, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ, 2011a. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology 218(4), 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ, 2011b. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 115(3), 240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He QH, Li DD, Turel O, Bechara A, Hser YI, 2020. White matter integrity alternations associated with cocaine dependence and long-term abstinence: Preliminary findings. Behav. Brain Res 379. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H, 2004. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience 24(49), 11017–11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges CB, Steinberg J, Ziniga E, Ma L, Bjork JM, Moeller FG, 2021. Chronic cocaine use and white matter integrity: A diffusion tensor imaging study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P, 2009. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences of the United States of America 106(6), 2035–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A, Van Horn JD, 2014. Systematic network lesioning reveals the core white matter scaffold of the human brain. Frontiers in Human Neuroscience 8, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NF, Kim C, Gold BT, 2013. Socioeconomic status is positively correlated with frontal white matter integrity in aging. AGE 35(6), 2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K, 2009. L-Dopa Modulates Functional Connectivity in Striatal Cognitive and Motor Networks: A Double-Blind Placebo-Controlled Study. J. Neurosci 29(22), 7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP, 2011. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biological Psychiatry 69(7), 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klanker M, Feenstra M, Denys D, 2013. Dopaminergic control of cognitive flexibility in humans and animals. Frontiers in Neuroscience 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher M, Gleichgerrcht E, Nesland T, Rorden C, Fridriksson J, Spampinato MV, Bonilha L, 2015. Individual variability in the anatomical distribution of nodes participating in rich club structural networks. Frontiers in Neural Circuits 9, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Goldstein RZ, 2015. Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Research 1628(A), 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H, 2005. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. European Journal of Neuroscience 21(7), 1984–1992. [DOI] [PubMed] [Google Scholar]
- Lebel C, Warner T, Colby J, Soderberg L, Roussotte F, Behnke M, Davis Eyler F, Sowell ER, 2013. White matter microstructure abnormalities and executive function in adolescents with prenatal cocaine exposure. Psychiatry Res. 213(2), 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Han CE, Aganj I, Seo SW, Seong JK, 2018. Distinct Patterns of Rich Club Organization in Alzheimer's Disease and Subcortical Vascular Dementia: A White Matter Network Study. Journal of Alzheimers Disease 63(3), 977–987. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R, 2008. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology 33(8), 1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP, 2002. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biological Psychiatry 51(11), 890–895. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP, 2008. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug and Alcohol Dependence 92(1–3), 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotharius J, Brundin P, 2002. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci 3(12), 932–942. [DOI] [PubMed] [Google Scholar]
- Luo CY, Guo XY, Song W, Chen Q, Cao B, Yang J, Gong QY, Shang HF, 2015. Functional connectome assessed using graph theory in drug-naive Parkinson's disease. J. Neurol 262(6), 1557–1567. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Streeter CC, Ahn KH, Lee HK, Pollack MH, Silveri MM, Nassar L, Levin JM, Sarid-Segal O, Ciraulo DA, Renshaw PF, Kaufman MJ, 2004. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Research-Neuroimaging 131(2), 135–145. [DOI] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG, 2009. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug and Alcohol Dependence 104(3), 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Wang Q, Schmitz JM, Boone EL, Narayana PA, Moeller FG, 2017. A preliminary longitudinal study of white matter alteration in cocaine use disorder subjects. Drug Alcohol Depend 173, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ, 2006. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research 83(2–3), 155–171. [DOI] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S, 2002. Dopamine transport function is elevated in cocaine users. J Neurochem 81(2), 292–300. [DOI] [PubMed] [Google Scholar]
- Maslov S, Sneppen K, 2002. Specificity and stability in topology of protein networks. Science 296(5569), 910–913. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment 9(3), 199–213. [DOI] [PubMed] [Google Scholar]
- Meade CS, Bell RP, Towe SL, Hall SA, 2020. Cocaine-related alterations in fronto-parietal gray matter volume correlate with trait and behavioral impulsivity. Drug Alcohol Depend. 206, 107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra VR, Sreenivasan KR, Yang ZS, Zhuang XW, Cordes D, Mari Z, Litvan I, Fernandez HH, Eidelberg D, Ritter A, Cummings JL, Walsh RR, 2020. Unique white matter structural connectivity in early-stage drug-naive Parkinson disease. Neurology 94(8), E774–E784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic B, Sporns O, 2016. From regions to connections and networks: new bridges between brain and behavior. Current Opinion in Neurobiology 40, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J, 2001. The impact of impulsivity on cocaine use and retention in treatment. J. Subst. Abuse Treat 21(4), 193–198. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA, 2005. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology 30(3), 610–617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma LS, Liu SJ, Kjome KL, Rathnayaka N, Kramer LA, Narayana PA, 2010. Working memory fMRI activation in cocaine-dependent subjects: Association with treatment response. Psychiatry Research-Neuroimaging 181(3), 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, Dalley JW, 2011. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology (Berl). 215(4), 721–731. [DOI] [PubMed] [Google Scholar]
- Mustaquim D, Jones CM, Compton WM, 2021. Trends and correlates of cocaine use among adults in the United States, 2006–2019. Addict. Behav 120, 106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Lissemore JI, Gravel P, Leyton M, Carbonell F, Benkelfat C, 2017. Posterior dopamine D2/3 receptors and brain network functional connectivity. Synapse 71(11). [DOI] [PubMed] [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG, 2009. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 171(3), 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, Moeller FG, 2014. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 221(3), 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo I, Hamon M, 1995. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int 26(4), 305–336; discussion 337-342. [DOI] [PubMed] [Google Scholar]
- Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, Krashia P, Rizzo FR, Marino R, Federici M, De Bartolo P, Aversa D, Dell'Acqua MC, Cordella A, Sancandi M, Keller F, Petrosini L, Puglisi-Allegra S, Mercuri NB, Coccurello R, Berretta N, D'Amelio M, 2017. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease. Nature Communications 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill J, Cardenas VA, Meyerhoff DJ, 2001. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addiction Biology 6(4), 347–361. [DOI] [PubMed] [Google Scholar]
- Pan XF, Kaminga AC, Wen SW, Wu XY, Acheampong K, Liu AZ, 2019. Dopamine and Dopamine Receptors in Alzheimer's Disease: A Systematic Review and Network Meta-Analysis. Frontiers in Aging Neuroscience 11, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Chun JW, Cho H, Kim DJ, 2018. Alterations in the connection topology of brain structural networks in Internet gaming addiction. Scientific Reports 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A, Tran LT, Larney S, Stockings E, Santo T, Jones H, Santomauro D, Degenhardt L, 2021. All-cause and cause-specific mortality among people with regular or problematic cocaine use: a systematic review and meta-analysis. Addiction 116(4), 725–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJR, 2007. The effects of cocaine: A shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry 31(8), 1593–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, Stavro K, Rizkallah E, Pelletier J, 2014. Cocaine and cognition: A systematic quantitative review. Journal of Addiction Medicine 8(5), 368–376. [DOI] [PubMed] [Google Scholar]
- Ray S, Gohel S, Biswal BB, 2015. Altered Functional Connectivity Strength in Abstinent Chronic Cocaine Smokers Compared to Healthy Controls. Brain Connectivity 5(8), 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, Hawkey E, Painter JG, Kriz D, Fombonne E, Nigg JT, Fair DA, 2014. Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: a rich club-organization study. Hum. Brain Mapp. 35(12), 6032–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ, 2010. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Research: Neuroimaging 181(1), 57–63. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O, 2010. Complex network measures of brain connectivity: uses and interpretations. NeuroImage 52(3), 1059–1069. [DOI] [PubMed] [Google Scholar]
- Shaked D, Leibel DK, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, Erus G, Evans MK, Zonderman AB, Waldstein SR, 2019. Disparities in Diffuse Cortical White Matter Integrity Between Socioeconomic Groups. Frontier in Human Neuroscience 13, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59(Suppl 30), 22–33. [PubMed] [Google Scholar]
- Shine JM, Bell PT, Matar E, Poldrack RA, Lewis SJG, Halliday GM, O'Callaghan C, 2019. Dopamine depletion alters macroscopic network dynamics in Parkinson's disease. Brain 142, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu N, Duan YY, Huang J, Ren ZQ, Liu Z, Dong HQ, Barkhof F, Li KC, Liu Y, 2018. Progressive brain rich-club network disruption from clinically isolated syndrome towards multiple sclerosis. Neuroimage-Clinical 19, 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Tournier J-D, Calamante F, Connelly A, 2012. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage 62(3), 1924–1938. [DOI] [PubMed] [Google Scholar]
- Sporns O, 2013. Structure and function of complex brain networks. Dialogues in Clinical Neuroscience 15(3), 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH, 2009. Fifty years of the Barratt Impulsiveness Scale: An update and review. Personality and Individual Differences 47(5), 385–395. [Google Scholar]
- Suchting R, Beard CL, Schmitz JM, Soder HE, Yoon JH, Hasan KM, Narayana PA, Lane SD, 2021. A meta-analysis of tract-based spatial statistics studies examining white matter integrity in cocaine use disorder. Addict. Biol 26(2), e12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang RL, Caparelli EC, Logan J, Volkow ND, 2015. Overlapping Patterns of Brain Activation to Food and Cocaine Cues in Cocaine Abusers: Association to Striatal D2/D3 Receptors. Hum. Brain Mapp 36(1), 120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M, Christiaens D, Jeurissen B, Yeh CH, Connelly A, 2019. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202, 116137. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime, 2020. World Drug Report 2020. United Nations, Vienna. [Google Scholar]
- van den Heuvel MP, Sporns O, 2011. Rich-club organization of the human connectome. The Journal of Neuroscience 31(44), 15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, 2013. Network hubs in the human brain. Trends in Cognitive Sciences 17(12), 683–696. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RCW, Cahn W, Goni J, Pol HEH, Kahn RS, 2013. Abnormal Rich Club Organization and Functional Brain Dynamics in Schizophrenia. JAMA Psychiatry 70(8), 783–792. [DOI] [PubMed] [Google Scholar]
- Vaquero L, Camara E, Sampedro F, Perez de Los Cobos J, Batlle F, Fabregas JM, Sales JA, Cervantes M, Ferrer X, Lazcano G, Rodriguez-Fornells A, Riba J, 2017. Cocaine addiction is associated with abnormal prefrontal function, increased striatal connectivity and sensitivity to monetary incentives, and decreased connectivity outside the human reward circuit. Addict. Biol 3, 844–856. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP, 1993. Decreased Dopamine-D(2) Receptor Availability Is Associated with Reduced Frontal Metabolism in Cocaine Abusers. Synapse 14(2), 169–177. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, Hitzemann R, Henn F, 1990. Effects of Chronic Cocaine Abuse on Postsynaptic Dopamine-Receptors. Am. J. Psychiatry 147(6), 719–724. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, Baler R, 2019. The Neuroscience of Drug Reward and Addiction. Physiol Rev 99(4), 2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma YM, 2006. Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J. Neurosci 26(27), 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Suh J, Li Z, Li Y, Franklin T, O'Brien C, Childress AR, 2015. A hyper-connected but less efficient small-world network in the substance-dependent brain. Drug and Alcohol Dependence 152, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH, 1998. Collective dynamics of 'small-world' networks. Nature 393(6684), 440–442. [DOI] [PubMed] [Google Scholar]
- Weingarten CP, Sundman MH, Hickey P, Chen NK, 2015. Neuroimaging of Parkinson's disease: Expanding views. Neurosci. Biobehav. Rev 59, 16–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE, 2014. Permutation inference for the general linear model. NeuroImage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers NW, Pulvirenti L, Koob GF, Gillin JC, 1995. Cocaine abuse and dependence. J. Clin. Psychopharmacol 15(1), 63–78. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET, 2010. Network-based statistic: identifying differences in brain networks. NeuroImage 53(4), 1197–1207. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Cocchi L, Gollo LL, van den Heuvel MP, Breakspear M, 2016. Connectome sensitivity or specificity: which is more important? NeuroImage 142, 397–410. [DOI] [PubMed] [Google Scholar]
- Zhang R, Volkow ND, 2019. Brain default-mode network dysfunction in addiction. NeuroImage 200, 313–331. [DOI] [PubMed] [Google Scholar]
- Zorlu N, Capraz N, Oztekin E, Bagci B, Di Biase MA, Zalesky A, Gelal F, Bora E, Durmaz E, Besiroglu L, Saricicek A, 2019. Rich club and reward network connectivity as endophenotypes for alcohol dependence: a diffusion tensor imaging study. Addict. Biol 24(2), 265–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.