Abstract
Background
Gastrointestinal strictures impact clinical presentation in abdominal tuberculosis and are associated with significant morbidity.
Aim
To conduct a systematic review of the prevalence of stricturing disease in abdominal and gastrointestinal tuberculosis and response to antitubercular therapy (ATT).
Methods
We searched Pubmed and Embase on 13th January 2022, for papers reporting on the frequency and outcomes of stricturing gastrointestinal tuberculosis. The data were extracted, and pooled prevalence of stricturing disease was estimated in abdominal tuberculosis and gastrointestinal (intestinal) tuberculosis. The pooled clinical response and stricture resolution (endoscopic or radiologic) rates were also estimated. Publication bias was assessed using the Funnel plot and Egger test. The risk of bias assessment was done using a modified Newcastle Ottawa Scale.
Results
Thirty-three studies reporting about 1969 patients were included. The pooled prevalence of intestinal strictures in abdominal tuberculosis and gastrointestinal TB was 0.12 (95%CI 0.07–0.20, I2 = 89%) and 0.27 (95% CI 0.21–0.33, I2 = 85%), respectively. The pooled clinical response of stricturing gastrointestinal tuberculosis to antitubercular therapy was 0.77 (95%CI 0.65–0.86, I2 = 74%). The pooled stricture response rate (endoscopic or radiological) was 0.66 (95%CI 0.40–0.85, I2 = 91%). The pooled rate of need for surgical intervention was 0.21 (95%CI 0.13–0.32, I2 = 70%), while endoscopic dilatation was 0.14 (95%CI 0.09–0.21, I2 = 0%).
Conclusion
Stricturing gastrointestinal tuberculosis occurs in around a quarter of patients with gastrointestinal tuberculosis, and around two-thirds of patients have a clinical response with antitubercular therapy. A subset of patients may need endoscopic or surgical intervention. The estimates for the pooled prevalence of stricturing disease and response to ATT had significant heterogeneity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12876-023-02682-x.
Keywords: Intestinal tuberculosis, Crohn's disease, Tuberculous peritonitis, Peritoneal tuberculosis, Gastrointestinal tuberculosis, Abdominal tuberculosis
Introduction
Abdominal tuberculosis is an important form of extra-pulmonary tuberculosis. It has a varied clinical presentation depending on the site of involvement: peritoneum, intestines, visceral organs, and/or abdominal lymph nodes. Tuberculous peritonitis and gastrointestinal tuberculosis (GITB) are the two most frequent patterns. The ileocecal region is the most common site of tuberculosis involvement in the intestine (25 to 90%). The morphologic patterns of GITB include ulcerative, hypertrophic, stricturing, or a combination of these. [1, 2] For the purpose of this systematic review we have used the ‘abdominal tuberculosis’ as an umbrella term that encompasses both the luminal (intestinal or gastrointestinal tuberculosis) and peritoneal tuberculosis (tuberculous peritonitis). While strictures are more frequent in intestinal tuberculosis, they may also occasionally occur in peritoneal tuberculosis due to peritoneal fibrosis and adhesions.
Mycobacterium tuberculosis, upon penetration of the intestinal mucosa, initiates a local inflammatory reaction in the submucosal lymphoid tissue. This leads to lymphangitis, granuloma formation, caseation necrosis, mucosal ulceration, and scarring [3]. The clinical presentation of abdominal tuberculosis depends on the underlying morphology: extensive ulcerations are usually associated with diarrhea, while stricture and hypertrophic forms may present with abdominal pain and intestinal obstruction features [1–4]. The reasons for the predominance of a particular morphologic pattern in an individual patient are unclear. Recurrent episodes of pain and obstruction may lead to frequent hospitalizations, poor quality of life, and the need for surgical interventions amongst this subset of patients. Gastrointestinal strictures are reported in a variable number of patients with tuberculosis: the variations are due to differing populations (intestinal or peritoneal or both) or selection bias (surgical series versus medically managed patients) in the published reports. Strictures in GITB may be inflammatory or fibrotic, depending predominantly on the activity and duration of the disease. Response of the intestinal strictures to anti-tubercular therapy (ATT) is varied as the inflammatory component may get resolved with treatment but also lead to healing and scarring with subsequent persistence of the fibrotic stricture. The response of tubercular strictures to ATT could be a clinical response (resolution of symptoms of stricture like intestinal obstruction or pain) or stricture response (resolution of stricture as assessed using radiology or endoscopy).
Therefore, we planned a systematic review to study the frequency of stricturing GITB in patients with abdominal TB and GITB, response to ATT and need for intervention (endoscopic dilatation or surgery) in these patients.
Methods
This meta-analysis was conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group recommendations and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance. [5, 6]
Search strategy
We searched Pubmed and Embase for articles reporting on frequency, clinical outcomes, and the need for intervention (surgery or endoscopic dilatation) in patients with stricturing gastrointestinal tuberculosis. The search was recent till 13th January, 2022. The search strategy combined the terms “Intestinal Tuberculosis” OR “Gastrointestinal Tuberculosis” OR “Peritoneal Tuberculosis” OR “Tuberculous peritonitis” OR “Abdominal Tuberculosis” with 'stricture’ OR ‘fibrosis’ OR ‘stenosis’ OR ‘surgery' using the operator ‘AND’. The detailed search strategy is depicted in Additional file 1: Table S1. The results were combined, and duplicates were removed. The title and abstract screening were done by two reviewers (RM and KR) independently. The titles selected underwent full-text screening.
Study selection and data extraction
All articles, irrespective of article type or the language of publication, which provided data relevant to the study question were included. This included one or more of the following.
Frequency of intestinal strictures or stricturing disease in patients with intestinal or abdominal tuberculosis
Frequency of clinical response, stricture improvement (as determined using radiological and endoscopic assessment) in stricturing intestinal tuberculosis
We excluded studies that reported on a series of < 10 patients, those which did not provide clear data for stricturing disease separately, and series which reported predominantly or solely on surgically managed patients and studies. For each planned analysis, we excluded those studies with a total patient number of 5 or less eligible for that analysis. We also excluded those study types which did not provide original data like reviews, letters, and guidelines. Abstracts were included if they provided relevant information.
The data were extracted from each of the studies for the type of study population (abdominal TB or intestinal TB or both), mean age and gender, frequency of stricturing disease in the subset of abdominal TB and gastrointestinal tuberculosis, clinical response (and its definition) to antitubercular therapy (ATT), stricture resolution (endoscopic or radiologic) and requirement of intervention (surgery or endoscopic balloon dilatation). Data extraction was done by two reviewers independently (AJ, RM) and any discrepancies were resolved by mutual discussion with a third reviewer (VS).
Definitions
For the purpose of this systematic review, we have used the ‘abdominal tuberculosis’ as an umbrella term that encompasses both the luminal (intestinal or gastrointestinal tuberculosis) and peritoneal tuberculosis (tuberculous peritonitis). Gastrointestinal tuberculosis specifically refers to intestinal (i.e. luminal) involvement.
Outcomes
We calculated the pooled prevalence of stricturing GITB in patients with abdominal TB. We also calculated the pooled prevalence of stricturing GITB in patients with intestinal TB. We calculated pooled clinical response rate and pooled stricture response (endoscopic and radiologic) rates after ATT. We calculated the pooled rates of intervention required in stricturing GITB i.e. surgery or endoscopic dilatation.
Analysis
We used the R statistical software version 4.1.2 for the analysis and in addition to the base package, meta and metafor packages were used. [7, 8] We calculated the pooled prevalence rates using a random effect method with an inverse variance approach. Logit transformations were made for the individual rates before computation of the pooled summary.
The heterogeneity was assessed using the I2 statistic, and heterogeneity of > 50% was considered as high. We performed subgroup analyses based on the site of disease, type of studies (prospective, retrospective), and the duration of ATT to evaluate the heterogeneity. Sensitivity analysis was also performed after excluding studies with a high or fair risk of bias. Baujat plots were constructed to identify studies contributing to heterogeneity.
Risk of bias
Two of the investigators (AJ and PB) independently assessed the methodological quality and risk of bias of studies using a modified Newcastle Ottawa Scale. [9] Any discordance in risk of bias, was settled with mutual agreement with a third reviewer (VS). Since no comparative analyses was performed for this proportional meta-analysis, we removed the comparability domain in the modified scale. We considered only those studies to be of good quality if the score was seven. Publication bias was assessed using Funnel plot (standard and Trimfill) and Egger test. [10]
Results
Study selection
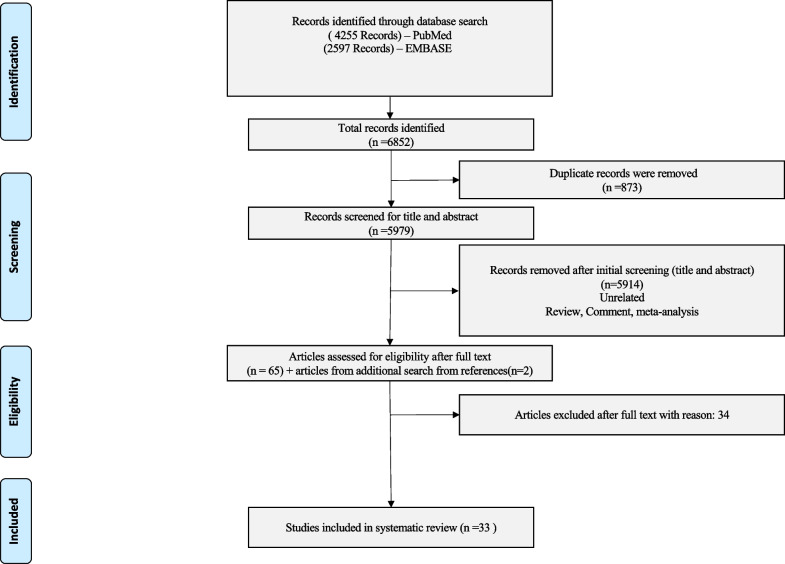
The result of the search yielded 6852 citations. (Fig. 1, PRISMA flow chart) Of the total of 6852 studies, there were 873 duplicates. We excluded 5914 citations after the abstract screening, and 65 citations were screened for full text. We obtained 2 further studies after manually searching the references of included studies. After full-text screening, we excluded 34 studies that did not fulfill the inclusion criteria. Eventually, 33 studies (30 full texts and 3 abstracts) were included in the final analysis. The details of the included studies are illustrated in Table 1.[11–43] The details of the excluded studies are illustrated in Additional file 1: Table S2.
Fig. 1.
The PRISMA flow chart showing the process of screening and selection of eligible studies
Table 1.
Details of the included studies in the meta-analysis
| Authors with year | Country | Type of study | ATB and GITB | Associated Extra–intestinal involvement | Duration of symptoms | Associated conditions | Stricturing TB (n) | Age, Number of males | Location | Clinical features | Clinical Response | ATT | Endoscopic Intervention | Surgery | Stricture resolution | Stricture Response |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anand BS et al., 1998 | India | Prospective | – | – | 24.4 (33.3) months | – | 39 |
33.4 ± 15.1yrs, 25 |
Duodenum (n = 1), Small intestine (n = 15), Ileocecal area (n = 10), Colon (n = 9), multiple sites (n = 4) |
Pain (n = 36), Obstruction(n = 34), weight loss (n = 36), diarrhea (n = 12), bleeding(n = 4) |
31 of 34 |
3 months HRS + 9 months HR (E if reaction to any) |
– | 3 | 16 of 23 | |
| Alvares JF et al., 2005 | India | Retrospective | GITB (n = 43) | 11 | – | – | 10 | – | Colonic (n = 10) | – | 8 of 10 | 2 months HRZES + 7 months months HR | – | 2 | 8 of 10 | |
| Aggarwal P et al., 2017 | India | Ambispective | GITB (n = 286) | – | 12 (6–24) months | – | 128 |
Median age: 35 years(n = 106), 63 |
Duodenum (n = 4), Small intestine (n = 10), Ileocecal area (n = 52), Colon (n = 37), Multiple sites (n = 4) |
Fever (n = 44), Pain (n = 99), Obstruction (n = 80), Weight loss(n = 86) |
52 of 104 | 2 months HRZE + 4–7 months HR | 12 | 7 | 25 of 106 | – |
| Amrapurkar DN et al., 2008 | India | Prospective | GITB (n = 26) | 6 | 7.2 (3.4) months | – | 5 | – | – | – | 1 of 5 | 2 months HRZE + 10 months HR | 0 | 4 | 1 of 5 | – |
| Bhargava DK et al. 1992 | India | Retrospective |
GITB (n = 29) |
10 | – | – | 10 | Mean age– 38.4 years, Males (n = 4) | Colonic (n = 10) | – | 7 of 10 | Regimen not mentioned | – | 3 | – | – |
| Cheng W et al. 2019 | China | Retrospective |
GITB (n = 49) |
Hepatic– 8, Cervical TB–4, Renal–3, Ovarian–1 |
102 (3–7300) days | 3 on immunosuppressants | 11 | – | – | – | – | – | – | – | – | – |
| Das HS et al., 2000 | India | Retrospective (abstract) | GITB (n = 21) | – | – | – | 3 | – | Colonic (n = 3) | – | – | – | – | – | – | – |
| Deka UJ et al., 2012 | India | –(abstract) | GITB (n = 44) | – | – | – | 7 | – | – | – | 7 of 7 | 6 months course | – | – | – | – |
| Dutta AK et al., 2011 | India | Prospective | GITB (n = 24) | – | 3 months (1 month to 2 years) | – | 4 | – | – | – | – | – | – | 1 | – | – |
| Fillion A et al., 2015 | France | Retrospective | ATB (n = 21), GITB (n = 7) | – | 13 months | 2 coexisting immunosuppressed conditions | 1 | – | – | – | – | 6 months four drug regimen | – | 1 | – | – |
| Gan H et al., 2016 | China | Retrospective | GITB (n = 81) | TB pleuritis: 14, lymph node TB: 7, uro– genital TB: 4, bone TB: 2 | 8 months | – | 16 | – | – | – | – | 3 months HRZE + 9–15 months HR | – | – | – | – |
| Hu ML et al., 2009 | Taiwan | Retrospective |
ATB(n = 14), GITB (n = 3) |
TB meningitis– 3 | – | 1 had Cancer | 1 | – | – | – | – | – | – | – | – | – |
| Jung Y et al., 2016 | South Korea | Retrospective | GITB (n = 98) | – | – | 2 had malignancy | 9 | – | – | – | – | – | – | – | – | – |
| Kentley J et al., 2017 | UK | Retrospective |
ATB (n = 147), GITB (n = 61) |
Appendiceal TB–2 | 13 (2–16) weeks | – | 9 | – | – | – | – | – | – | – | – | – |
| Khan R et al., 2006 | Pakistan | Retrospective |
ATB (n = 209), GITB (n = 102) |
Past TB– 13 | – | 0 | 17 | – | – | – | – | 9–12 months various combination of HRZES | – | 11 | – | – |
| Kim KM et al., 1998 | Korea | Retrospective | GITB (n = 42) | – | – | – | 3 | – | – | – | – | – | – | – | – | – |
| Larsson G et al., 2015 | India | Prospective | GITB (n = 30) | – | – | – | 3 | – | – | – | – | – | – | – | – | – |
| Lee YJ et al., 2006 | Korea | Prospective | GITB (n = 44) | – | – | – | 8 | – | – | – | – | – | – | – | – | – |
| Lu S et al., 2020 | China | Retrospective | GITB (n = 10) | – | – | – | 6 | – | – | Pain (n = 6) | – | 3 months intensive + 9–15 months consolidation | – | 2 | – | – |
| Lu Y et al., 2021 | China | Retrospective | GITB (n = 84) | – | – | – | 24 | – | – | – | – | – | – | – | – | – |
| Makanjuola D et al., 1998 | Saudi Arabia | Retrospective | GITB (n = 21) | liver and pancre in one and pancreas alone in one patient | – | – | 8 | – | – | Obstruction (n = 6), Diarrhea (n = 1) | – | – | – | – | – | – |
| Millar AJW et al., 1990 | South Africa | Retrospective | ATB(n = 95) children | – | – | Factor V deficiency in one | 16 | – | – | – | – | Quadruple HRZE till discharge or TB hospital + triple therapy total 6 months | – | 4 | – | – |
| Misra SP et al., 1999 | India | Retrospective | GITB (n = 50) | Already on ATT: 13 patients | – | 0 | 12 | – | Colonic (n = 12) | – | 10 of 12 | 2 months HRZE + 10 months HR | 1 | 2 | 4 | 10 |
| Mukewar S et al., 2012 | India | Prospective | GITB (n = 69) | – | – | 0 | 30 | – | Colonic (n = 30) | – | 26 of 30 | 2 months HRZE + 7 months HR | 0 | 4 | 16 of 21 | 17 of 21 |
| Nagi B et al., 2003 | India | Retrospective | GITB (n = 74) | 10 | – | 0 | 40 | – |
Colonic (n = 40) Transverse–20 Rectum–13 Ascending–9 Sigmoid–3 |
– | – | – | – | – | – | – |
| Palmer KR et al., 1985 | UK | Retrospective |
ATB (n = 90), GITB (n = 42) |
Liver– 8 | 4 ± 0.9 years | – | 10 | – | – | – | – | Variable regimens of HRZE and PAS | – | – | – | – |
| Singh H et al., 2018 | India | Retrospective | ATB (n = 119), GITB (n = 75) | Prior ATT: 16 | – | 0 | 48 | – | – | – | 26 of 48 | 2 months HRZE + 4 months HRE | 10 | 14 | – | – |
| Singh V et al., 1996 | India | Retrospective | GITB (n = 62) | – | 15 days– 10 years | – | 17 | – | Colonic (n = 17) | – | 17 | Standard regimen | – | – | – | – |
| Sinha S et al., 2017 | India | Prospective (abstract) | GITB (n = 32) | – | – | – | 13 | – | – | – | 9 of 13 | 9 months ATT | – | 4 | – | – |
| Tripathi PB et al., 2009 | India | Retrospective | GITB (n = 110) | 14 | – | – | 57 | – | – | – | – | – | – | – | – | – |
| Udgirkar S et al., 2019 | India | Prospective |
ATB (n = 176), GITB (n = 162) |
TB Meningitis | 165 + /–23 days | – | 29 | – |
Terminal ileum (n = 6), Ileocecal area (n = 13), Ascending colon (n = 4), Hepatic flexure (n = 2) Transverse colon (n = 3), Descending (n = 1) |
– | 23 of 28 |
6 months four drug followed by two, MDR–total 18 months with second line |
3 | 2 | 3 | 21 |
| Uygur–Bayramicli O et al., 2003 | Turkey | Prospective |
ATB (n = 31), GITB (n = 19) |
Bone TB–2 | 1 month–1 1 years | – | 1 | – | Colon (n = 1) | – | – | Four drug regimen for 9 months | – | – | – | – |
| Zhu QQ et al., 2014 | China | Retrospective | GITB (n = 35) | – | – | – | 22 | – | – | – | – | – | – | – | – | – |
ATB Abdominal Tuberculosis, ATT Anti-tubercular therapy, E Ethambutol, GITB Gastro-intestinal Tuberculosis, H Isoniazid, MDR Multidrug resistant, PAS Para-aminosalicylic acid, R Rifampicin, S Streptomycin, Z Pyrazinamide
Prevalence of stricturing GITB
Overall, 9 studies (902 patients) reported the frequency of stricturing GITB in the setting of abdominal TB. The pooled prevalence of intestinal strictures in abdominal TB was 0.12 (95% CI 0.07–0.20, I2 = 89%) (Additional file 1: Fig. S1) (Fig. 2).
Fig. 2.
Forest Plot showing the pooled prevalence of stricturing disease in patients with gastro-intestinal tuberculosis
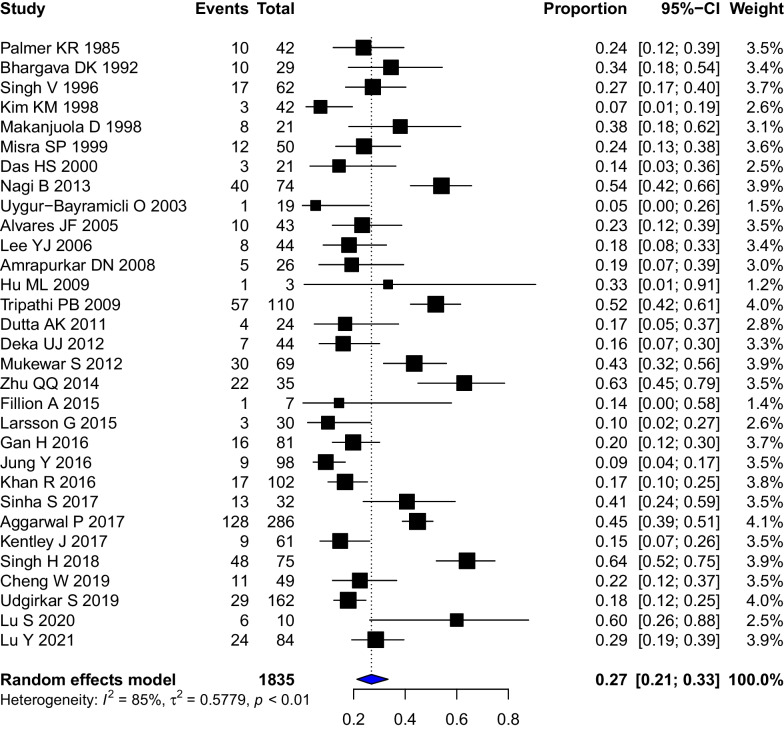
For gastrointestinal tuberculosis, 31 studies (1835 patients) reported the frequency of intestinal strictures. The pooled prevalence of intestinal strictures in gastro-intestinal TB was 0.27 (95%CI 0.21–0.33, I2 = 85%) (Fig. 3). The Baujat plot constructed for studies suggested that the studies by Singh H et al. 2018, Jung Y et al. 2016, Agarwal P et al. 2017 contributed the maximum to the heterogeneity (Additional file 1: Fig. S2). [13, 23, 37] However, for the lack of clear reasoning to exclude these we did not perform a sensitivity analysis after removing these studies. To evaluate heterogeneity, we conducted a subgroup analysis by stratifying the studies by stricture site. However, the heterogeneity remained high. There were 7 studies (348 patients) reporting the frequency of strictures in colonic tuberculosis. The pooled prevalence of stricturing disease in colonic TB was 0.32 (95%CI 0.23–0.43, I2 = 74%) (Additional file 1: Fig. S3). Subgroup analysis based on the study types found that one study with an unclear design had the lowest prevalence (0.16, 0.7–0.30) while one with ambispective design had the highest prevalence (0.45, 0.39–0.51) of stricturing disease. The prevalence of strictures was higher in retrospective studies (0.29, 0.21–0.37) and compared to prospective studies (0.22, 0.14–0.32) (Additional file 1: Fig. S4). The subgroup analysis on the basis of the duration of ATT did not suggest any differences in stricturing disease (P = 0.9677) (Additional file 1: Fig. S5).
Fig. 3.
Forest Plot showing the pooled clinical response rates to anti-tubercular therapy in patients with stricturing gastrointestinal tuberculosis
Sensitivity analysis by including only the six studies deemed low risk of bias, suggested that pooled estimates of stricturing disease were similar (0.36, 95% CI 0.24; 0.49) (Additional file 1: Fig. S6).
Response to therapy
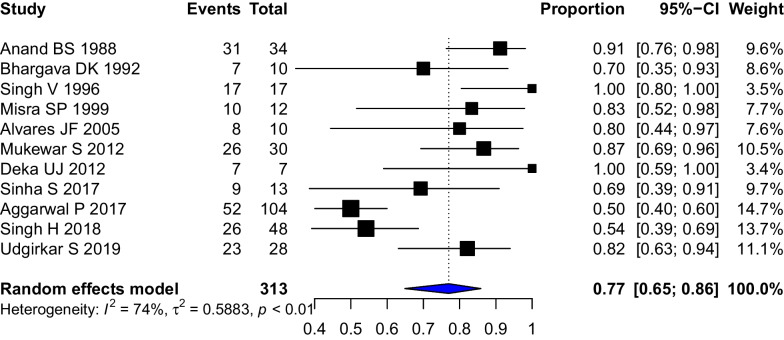
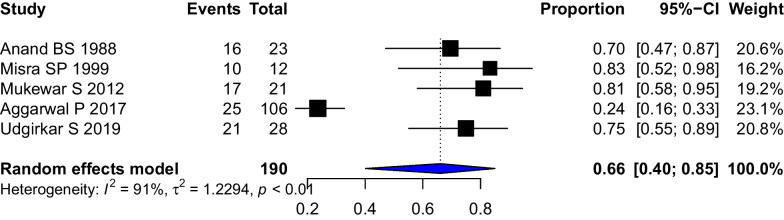
The definitions of clinical response and clinical cure in each study have been provided in Additional file 1: Table S3. For the purpose of analysis, we used the clinical response rates wherever available. Eleven studies (313 patients) of tubercular intestinal strictures reported clinical responses to therapy. The pooled clinical response of strictures to therapy was 0.77 (95%CI 0.65–0.86, I2 = 74%) (Fig. 4). The stricture response/resolution as defined on the basis of endoscopic or radiological criteria in each study has been provided in Additional file 1: Table S3. The pooled stricture response rate (5 studies, 190 patients) was 0.66 (95%CI 0.40–0.85, I2 = 91%) (Fig. 5). The differing definitions of stricture response and differing modalities (endoscopic/radiologic) contributed to the heterogeneity. A leave-one-out analysis was performed, and on omitting Aggarwal P 2017, the stricture response was 0.76 [0.65; 0.84] with I2 = 0% (Additional file 1: Fig. S7).
Fig. 4.
Forest Plot showing the pooled stricture response rates to anti-tubercular therapy in patients with stricturing gastrointestinal tuberculosis
Fig. 5.
Forest plot showing the pooled rates of a endoscopic dilatation b surgery in patients with stricturing gastrointestinal tuberculosis
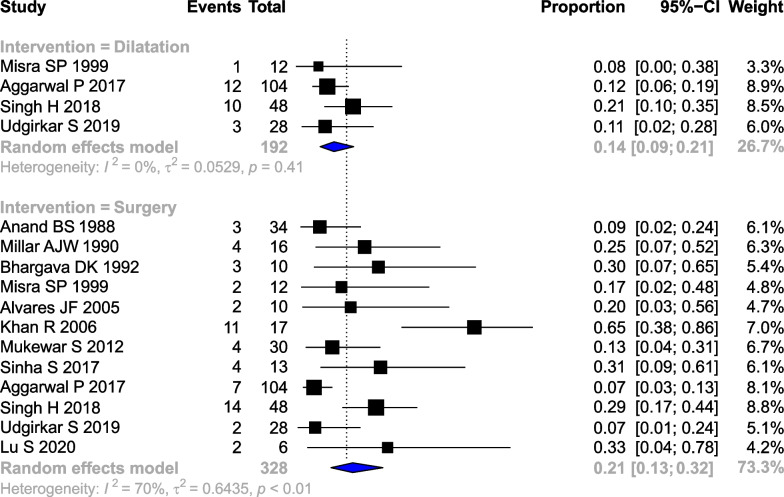
Need for intervention
The pooled rate of surgery (12 studies, 328 patients) was 0.21 (95% CI 0.13–0.32, I2 = 70%). The pooled rate of endoscopic dilatation (4 studies, 192 patients) was 0.14 (95%CI 0.09–0.21, I2 = 0%) (Fig. 6).
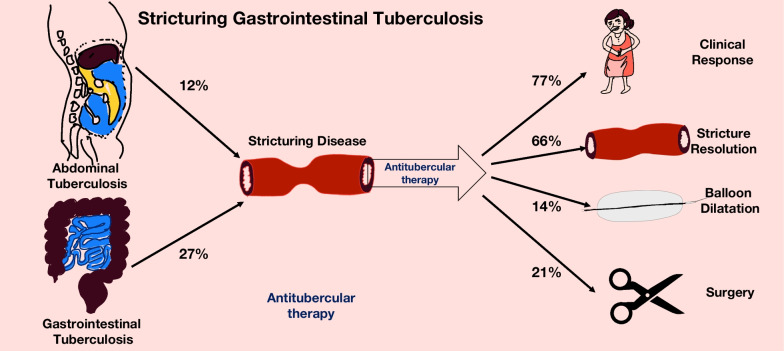
Fig. 6.
Pictorial depiction of the summary of findings of the systematic review
Risk of bias
The results of the risk of bias assessment are shown in Additional file 1: Table S4. Of the included studies, six were of good quality, fifteen were of fair quality, and the remaining were of poor quality.
The publication bias for the studies reporting the frequency of stricturing disease in patients of gastro-intestinal TB was assessed using Funnel plot and Eggers’ test (Additional file 1: Fig. S8). The Eggers’ test suggests the presence of publication bias (t statistic = − 2.91, p = 0.007). However, the visual interpretation of the funnel plot suggests a significant horizontal scatter of even the powerful studies suggesting that the results may be due to underlying heterogeneity. The use of trimfill method made the plot more symmetrical but with many studies still outside the funnel. The adjusted pooled estimate of stricturing in abdominal tuberculosis was 0.36 [0.28; 0.44] (with 10 additional ‘missing’ studies).
Discussion
The results of the present systematic review suggest that stricturing GITB is a significant problem that could be encountered in around a quarter of the patients with gastrointestinal tuberculosis. The findings also suggest that while most patients have a clinical response and improvement in strictures with antitubercular therapy, around 21% of stricturing GITB may need surgical intervention to alleviate the persistent symptoms (Fig. 6). The rates of endoscopic interventions are lower than those of surgery; this may be due to lack of access or feasibility of endoscopic dilatation. Endoscopic dilatation is feasible only in relatively shorter strictures, which can be accessed using colonoscopy.
Gastrointestinal strictures are one of the morphological patterns which are seen in the spectrum of abdominal tuberculosis. They are important, although not the only, cause of abdominal pain and intestinal obstruction in these patients. Other important causes of intestinal obstruction could be mass-forming (pseudo-tumoral or hypertrophic) intestinal tuberculosis, adhesions due to peritoneal involvement, or the formation of abdominal cocoon [1–4, 44, 45]. Nevertheless, strictures are one of the potentially treatable causes of symptoms because part of the pathophysiological processes is potentially reversible. Our analysis demonstrates that symptomatic improvement occurs in most patients with ATT. Some amount of stricture resolution also occurs in most of the patients. However, a complete resolution of the strictures is infrequent. This correlates with the pathophysiological understanding of two dominant phenomena participating in stricture formation: inflammatory narrowing and fibrosing stenosis. Often the tubercular strictures are associated with ulcerations, and with antitubercular therapy, there is a healing of the ulcers [46, 47]. As against the lesions in Crohn’s disease, tubercular ulcers are typically non-penetrating, and the associated edema is also less than in CD. The degree of fibrosis is variable and possibly relates to the duration of the disease process [48]. This suggests that the narrowing may be reversible at least early in the disease course. This would resolve symptoms in most patients, but morphological anomalies may persist.
Although we had planned for analysis of the clinical presentation of stricturing GITB, most of the studies reported the clinical presentation of the entire subset of the GITB (with or without strictures). Nevertheless, most studies suggest abdominal pain and features of intestinal obstruction dominate the clinical presentation of stricturing GITB [11, 13, 31]. The predictors of clinical outcomes, need of endoscopic dilatation or surgery are unclear. In a study by Anand BS et al., young females with longer duration of symptoms were less likely to have a radiological response [11]. The site of the stricture did not seem to impact the outcomes. In contrast, a large recent study from India suggested that colonic strictures are less likely to respond to ATT [13]. Understandably, the resolution rates were also worse in the patients with longer (> 3 cm long) or multiple strictures [13]. The present systematic review also provides estimates of the need for interventions in these patients with around one-fifths of the patients requiring surgery. This suggests that a fraction of the patients might have dominant fibrosis related strictures and do not improve with ATT. It is unclear if preoperative evaluation using imaging could differentiate inflammatory strictures from fibrotic strictures and therefore predict response to ATT. This differentiation has been reported in the setting of CD but not in GITB [49].
Our systematic review has certain limitations: we could not analyze the frequency of the involvement of various sites and response to ATT because of variable definitions of the site and lack of data regarding response (Additional file 1: Table S5). The diagnostic criteria used in various studies were also different and could be responsible for the heterogeneity (Additional file 1: Table S6). We attempted to evaluate high heterogeneity using subgroup analyses and sensitivity analyses, but these could not explain the heterogeneity completely. Also, we did not have data regarding the clinical features, as most studies reported clinical features for the entire subset of GITB. This was because the reporting was variable: some studies reported distal ileum and ileocecal together, while others reported terminal ileum strictures with small bowel. We could not calculate the frequency of clinical symptoms of stricturing GITB as most studies provided clinical features for the entire group of patients with GITB. The impact of the site of involvement on clinical improvement or stricture resolution could also not be estimated because only a few studies provided data separately for resolution rates depending on the site. We had to exclude a large number of studies that provided data only from surgical series because of selection bias towards ‘severely symptomatic’ GITB requiring surgical intervention. In addition, while we have pooled the need for surgery and endoscopic dilatation- the standards for these therapies could be variable between various centers, and the choice of therapy may depend on the local preferences and expertise. The impact of disease duration on the degree of strictures and, eventually, the impact of the degree of strictures on response to ATT may be better estimated by an individual participant meta-analysis with complete details of stricture estimates based on radiological or endoscopic criteria. Because of the heterogeneity in study design, participants, and outcomes estimation, we used a random effects model- however, such a model tends to weigh the study effects more equally and provide more conservative estimates. The study has multiple strengths apart from being the first such analysis of the frequency and impact of stricturing GITB. The analysis included a large number of studies, and we could analyze the frequency of stricturing disease separately for colonic tuberculosis. The study also provided estimates on clinical improvement and stricture response which could help the clinicians in appropriate prognostication of such patients.
Future studies should try to address the issues of heterogenity in disease definitions, study populations, response assessment. This can be accomplished by clear case definitions (microbiologically diagnosed or clinically diagnosed case) of tuberculosis, clear definition of site of involvement, timing of stricture development (symptom duration and relationship with ATT), standard therapy in all cases and clear criteria to define strictures and response (imaging for small bowel and colonoscopy for large intestine) and homogenous assessment of timing of response assessment.
Conclusion
The present systematic review found that stricturing disease occurs in around a quarter of patients with gastrointestinal tuberculosis. Most patients (three-fourths) have a symptomatic improvement with antitubercular therapy, while the response of strictures is slightly lower (two-thirds). A substantial number of patients require intervention, including endoscopic dilatation or surgical intervention (one-fifth). Although the present systematic review reports these clinically relevant estimates, these should be interpreted cautiously because of the significant heterogeneity in the analyses especially in relation to the pooled prevalence of stricturing disease and clinical response of stricturing disease to ATT.
Supplementary Information
Acknowledgements
None
Author contributions
AJ: Screening, Study Selection, Data Extraction, ROB, First Draft; RM: Screening and Study Selection; KR: Screening and Study Selection; PB N: Screening, Study Selection, Data extraction, RoB; DCT:Screening and Study Selection; HS: Important intellectual inputs and Manuscript revision; PG: Important intellectual inputs and Manuscript revision; VS: Important intellectual inputs and Manuscript revision; VS: Conception, Search, Data Extraction, Analysis, Important intellectual inputs, Manuscript writing and revision. All authors read and approved by the final manuscript.
Funding
None.
Availability of data and materials
No new data were created for this manuscript and this meta-analysis used the data available in public domain.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anuraag Jena, Email: anuraag2destiny@gmail.com.
Ritin Mohindra, Email: ritin.mohindra@gmail.com.
Kirtan Rana, Email: ranakirtan006@gmail.com.
Pardhu B. Neelam, Email: drpardhu.bharath@gmail.com
Dhuni Chand Thakur, Email: drduni816@gmail.com.
Harjeet Singh, Email: harjeetsingh1982@gmail.com.
Pankaj Gupta, Email: pankajgupta959@gmail.com.
Vikas Suri, Email: surivikas9479@gmail.com.
Vishal Sharma, Email: sharma.vishal@pgimer.edu.in.
References
- 1.Sharma V, Debi U, Mandavdhare HS, Prasad KK. Tuberculosis and other mycobacterial infections of the abdomen. In: Kuipers EJ. Encyclopedia of gastroenterology (Second Edition). Academic Press 2020; Pp 646–659
- 2.Sharma MP, Bhatia V. Abdominal tuberculosis. Indian J Med Res. 2004;120(4):305–315. [PubMed] [Google Scholar]
- 3.Rathi P, Gambhire P. Abdominal tuberculosis. J Assoc Physicians India. 2016;64(2):38–47. [PubMed] [Google Scholar]
- 4.Al-Zanbagi AB, Shariff MK. Gastrointestinal tuberculosis: a systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol: Off J Saudi Gastroenterol Assoc. 2021;27(5):261. doi: 10.4103/sjg.sjg_148_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 6.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2022;36(3):1–48. [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anand BS, Nanda R, Sachdev GK. Response of tuberculous stricture to antituberculous treatment. Gut. 1988;29(1):62–69. doi: 10.1136/gut.29.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005;37(4):351–356. doi: 10.1055/s-2005-861116]. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal P, Kedia S, Sharma R, et al. Tubercular intestinal strictures show a poor response to anti-tuberculous therapy. Dig Dis Sci. 2017;62(10):2847–2856. doi: 10.1007/s10620-017-4727-3. [DOI] [PubMed] [Google Scholar]
- 14.Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol. 2008;14(5):741–746. doi: 10.3748/wjg.14.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhargava DK, Kushwaha AK, Dasarathy S, Chopra P. Endoscopic diagnosis of segmental colonic tuberculosis. Gastrointest Endosc. 1992;38(5):571–574. doi: 10.1016/s0016-5107(92)70519-7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng W, Zhang S, Li Y, Wang J, Li J. Intestinal tuberculosis: clinico-pathological profile and the importance of a high degree of suspicion. Trop Med Int Health. 2019;24(1):81–90. doi: 10.1111/tmi.13169. [DOI] [PubMed] [Google Scholar]
- 17.Das HS, Rathi P, Sawant P, et al. Colonic tuberculosis: colonoscopic appearance and clinico-pathologic analysis. J Assoc Physicians India. 2000;48(7):708–710. [PubMed] [Google Scholar]
- 18.Deka UJ, Dasgupta JK, Sarkar R, Das K, Banerjee S, Ahmed M, Bhattacharyya A, Basu K. A clinical study of intestinal tuberculosis with special reference to colonic involvement and response to short course anti-tuberculosis therapy. Indian Society of Gastroenterology. Indian J Gastroenterol. 2012; 31:1–114. 10.1007/s12664-012-0264-3
- 19.Dutta AK, Sahu MK, Gangadharan SK, Chacko A. Distinguishing Crohn's disease from intestinal tuberculosis–a prospective study. Trop Gastroenterol. 2011;32(3):204–209. [PubMed] [Google Scholar]
- 20.Fillion A, Ortega-Deballon P, Al-Samman S, et al. Abdominal tuberculosis in a low prevalence country. Med Mal Infect. 2016;46(3):140–145. doi: 10.1016/j.medmal.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Gan H, Mely M, Zhao J, Zhu L. An analysis of the clinical, endoscopic, and pathologic features of intestinal tuberculosis. J Clin Gastroenterol. 2016;50(6):470–475. doi: 10.1097/MCG.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 22.Hu ML, Lee CH, Kuo CM, et al. Abdominal tuberculosis: analysis of clinical features and outcome of adult patients in southern Taiwan. Chang Gung Med J. 2009;32(5):509–516. [PubMed] [Google Scholar]
- 23.Jung Y, Hwangbo Y, Yoon SM, et al. Predictive factors for differentiating between Crohn's disease and intestinal tuberculosis in Koreans. Am J Gastroenterol. 2016;111(8):1156–1164. doi: 10.1038/ajg.2016.212. [DOI] [PubMed] [Google Scholar]
- 24.Kentley J, Ooi JL, Potter J, et al. Intestinal tuberculosis: a diagnostic challenge. Trop Med Int Health. 2017;22(8):994–999. doi: 10.1111/tmi.12908. [DOI] [PubMed] [Google Scholar]
- 25.Khan R, Abid S, Jafri W, Abbas Z, Hameed K, Ahmad Z. Diagnostic dilemma of abdominal tuberculosis in non-HIV patients: an ongoing challenge for physicians. World J Gastroenterol. 2006;12(39):6371–6375. doi: 10.3748/wjg.v12.i39.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KM, Lee A, Choi KY, Lee KY, Kwak JJ. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am J Gastroenterol. 1998;93(4):606–609. doi: 10.1111/j.1572-0241.1998.173_b.x. [DOI] [PubMed] [Google Scholar]
- 27.Larsson G, Shenoy KT, Ramasubramanian R, et al. High faecal calprotectin levels in intestinal tuberculosis are associated with granulomas in intestinal biopsies. Infect Dis (Lond). 2015;47(3):137–143. doi: 10.3109/00365548.2014.974206. [DOI] [PubMed] [Google Scholar]
- 28.Lee YJ, Yang SK, Byeon JS, et al. Analysis of colonoscopic findings in the differential diagnosis between intestinal tuberculosis and Crohn's disease. Endoscopy. 2006;38(6):592–597. doi: 10.1055/s-2006-924996. [DOI] [PubMed] [Google Scholar]
- 29.Lu S, Fu J, Guo Y, Huang J. Clinical diagnosis and endoscopic analysis of 10 cases of intestinal tuberculosis. Medicine (Baltimore) 2020;99(28):e21175. doi: 10.1097/MD.0000000000021175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Chen Y, Peng X, et al. Development and validation of a new algorithm model for differential diagnosis between Crohn's disease and intestinal tuberculosis: a combination of laboratory, imaging and endoscopic characteristics. BMC Gastroenterol. 2021;21(1):291. doi: 10.1186/s12876-021-01838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makanjuola D, al Orainy I, al Rashid R, Murshid K. Radiological evaluation of complications of intestinal tuberculosis. Eur J Radiol. 1998;26(3):261–268. 10.1016/s0720-048x(96)01091-1PMID: 9587753 [DOI] [PubMed]
- 32.Millar AJW, Rode H, Cywes S. Abdominal tuberculosis in children surgical management. Pediatr SurgInt. 1990;5:392–396. [Google Scholar]
- 33.Misra SP, Misra V, Dwivedi M, Gupta SC. Colonic tuberculosis: clinical features, endoscopic appearance and management. J Gastroenterol Hepatol. 1999;14(7):723–729. doi: 10.1046/j.1440-1746.1999.01940.x. [DOI] [PubMed] [Google Scholar]
- 34.Mukewar S, Mukewar S, Ravi R, Prasad A, S Dua K. Colon tuberculosis: endoscopic features and prospective endoscopic follow-up after anti-tuberculosis treatment. Clin Transl Gastroenterol. 2012;3(10):e24. 10.1038/ctg.2012.19PMID: 23238066 [DOI] [PMC free article] [PubMed]
- 35.Nagi B, Kochhar R, Bhasin DK, Singh K. Colorectal tuberculosis. Eur Radiol. 2003;13(8):1907–1912. doi: 10.1007/s00330-002-1409-z. [DOI] [PubMed] [Google Scholar]
- 36.Palmer KR, Patil DH, Basran GS, Riordan JF, Silk DB. Abdominal tuberculosis in urban Britain–a common disease. Gut. 1985;26(12):1296–1305. doi: 10.1136/gut.26.12.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh H, Krishnamurthy G, Rajendran J, et al. Surgery for abdominal tuberculosis in the present Era: experience from a tertiary-care center. Surg Infect (Larchmt) 2018;19(6):640–645. doi: 10.1089/sur.2018.077. [DOI] [PubMed] [Google Scholar]
- 38.Singh V, Kumar P, Kamal J, Prakash V, Vaiphei K, Singh K. Clinicocolonoscopic profile of colonic tuberculosis. Am J Gastroenterol. 1996;91(3):565–568. [PubMed] [Google Scholar]
- 39.Sinha S, Malik S, Kochhar R, Vaiphei K, Sharma K, Koshy A, Berry N, Dhaka N. Narrow band imaging guided biopsy improves yield of histology for diagnosis of gastrointestinal tuberculosis. Gastroenterology. 2017;152(5):S428–S429. doi: 10.1016/S0016-5085(17)31651-7. [DOI] [Google Scholar]
- 40.Tripathi PB, Amarapurkar AD. Morphological spectrum of gastrointestinal tuberculosis. Trop Gastroenterol. 2009;30(1):35–39. [PubMed] [Google Scholar]
- 41.Udgirkar S, Jain S, Pawar S, Chandnani S, Contractor Q, Rathi P. Clinical profile, drug resistance pattern and treatment outcomes of abdominal tuberculosis patients in Western India. Arq Gastroenterol. 2019;56(2):178–183. doi: 10.1590/S0004-2803.201900000-35. [DOI] [PubMed] [Google Scholar]
- 42.Uygur-Bayramicli O, Dabak G, Dabak R. A clinical dilemma: abdominal tuberculosis. World J Gastroenterol. 2003;9(5):1098–1101. doi: 10.3748/wjg.v9.i5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu QQ, Zhu WR, Wu JT, Chen WX, Wang SA. Comparative study of intestinal tuberculosis and primary small intestinal lymphoma. World J Gastroenterol. 2014;20(15):4446–4452. doi: 10.3748/wjg.v20.i15.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma V, Singh H, Mandavdhare HS. Tubercular abdominal cocoon: systematic review of an uncommon form of tuberculosis. Surg Infect (Larchmt) 2017;18(6):736–741. doi: 10.1089/sur.2017.110. [DOI] [PubMed] [Google Scholar]
- 45.Ahamed ZR, Shah J, Agarwala R, Kumar-M P, Mandavdhare HS, Gupta P, Singh H, Sharma A, Dutta U, Sharma V. Controversies in classification of peritoneal tuberculosis and a proposal for clinico-radiological classification. Expert Rev Anti Infect Ther. 2019;17(8):547–555. doi: 10.1080/14787210.2019.1642746. [DOI] [PubMed] [Google Scholar]
- 46.Dasgupta A, Singh N, Bhatia A. Abdominal tuberculosis: a histopathological study with special reference to intestinal perforation and mesenteric vasculopathy. J Lab Phys. 2009;1(2):56–61. doi: 10.4103/0974-2727.59700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma V, Mandavdhare HS, Dutta U. Letter: mucosal response in discriminating intestinal tuberculosis from Crohn's disease-when to look for it? Aliment Pharmacol Ther. 2018;47(6):859–860. doi: 10.1111/apt.14495. [DOI] [PubMed] [Google Scholar]
- 48.Tandon HD, Prakash A. Pathology of intestinal tuberculosis and its distinction from Crohn's disease. Gut. 1972;13(4):260–269. doi: 10.1136/gut.13.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rimola J, Capozzi N. Differentiation of fibrotic and inflammatory component of Crohn's disease-associated strictures. Intest Res. 2020;18(2):144–150. doi: 10.5217/ir.2020.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created for this manuscript and this meta-analysis used the data available in public domain.