Abstract
Periprosthetic joint infection (PJI) is a difficult complication requiring a comprehensive eradication protocol. Cure rates have essentially stalled in the last two decades, using methods of antimicrobial cement joint spacers and parenteral antimicrobial agents. Functional spacers with higher-dose antimicrobial-loaded cement and antimicrobial-loaded calcium sulphate beads have emphasized local antimicrobial delivery on the premise that high-dose local antimicrobial delivery will enhance eradication. However, with increasing antimicrobial pressures, microbiota have responded with adaptive mechanisms beyond traditional antimicrobial resistance genes. In this review we describe adaptive resistance mechanisms that are relevant to the treatment of PJI. Some mechanisms are well known, but others are new. The objective of this review is to inform clinicians of the known adaptive resistance mechanisms of microbes relevant to PJI. We also discuss the implications of these adaptive mechanisms in the future treatment of PJI.
Cite this article: Bone Joint J 2022;104-B(5):575–580.
Keywords: Microbial variants, Periprosthetic joint infection, PJI, Transient hypermutability, Small colony variants, Persister cells, Adaptive resistance, Transient antimicrobial resistance, Phoenix colonies, Variant selection, Periprosthetic joint infection (PJI), antimicrobial agents, calcium sulphate, calcium sulphate, clinicians, biofilms, organism(s), antibiotic, surgical debridement, toxin
Introduction
The rise in the number of arthroplasties performed worldwide suggests a concordant rise of associated complications. 1 One of the most dreaded complications is periprosthetic joint infection (PJI). Unfortunately, the incidence of PJI in hip and knee arthroplasty has remained relatively steady in the last two decades. 2 Treatment of an established chronic PJI is an exchange protocol whereby all prosthetic implants, foreign materials, and surrounding fibro-inflammatory tissues are removed, with vigorous lavage of the periprosthetic region. The exchange protocols include either a single-stage or multistage exchange. 3,4 The choice takes into consideration variables that include host health, limb health, microbe virulence, and surgeon treatment philosophy. In two- and multistage exchange protocols, an intermediate poly(methyl methacrylate) (PMMA) cement spacer construct is placed that contains high doses of surgeon-added antimicrobial agents. Furthermore, in the last two decades, adjuvant treatment with surgeon-fabricated antimicrobial-loaded calcium sulphate beads delivered into the joint has been used. 5 This technique, in laboratory models, has been shown to kill microbiota within a biofilm up to 5 mm to 10 mm from a bead. Notably, a common theme in local antimicrobial delivery is the use of multiple antimicrobial agents with ever-increasing doses. 6,7 Published antimicrobial formulae report as much as 8 gm of surgeon-added antimicrobial agents within one 40 gm pack of PMMA. 8 In calcium sulphate beads, 2 gm to 3 gm of antimicrobials are commonly impregnated in 10 cc of beads. 5 It would seem logical that such supraphysiological local doses would provide an extended microbial killing zone, and eradication rates would be improved. However, microbiota in stressed conditions respond with adaptive mechanisms to thwart eradication. 9 Adaptation is marked by a variety of transient and permanent resistance mechanisms that extend beyond traditional antimicrobial resistance genes. Some mechanisms have existed since the advent of penicillin, but newer adaptive variants are now being recognized. 10
In this review, we present an overview of adaptive microbial responses relevant to PJI treatment. Permanent adaptive mechanisms include hypermutability and small colony variants (SCVs), whereas transient adaptive mechanisms are observed in persister cells, adaptive resistance, and phoenix colonies. The common goal of these mechanisms is survival of the organism(s) upon exposure to antimicrobial agents above mean minimum inhibitory concentration (MIC), the surgical debridement stress, and changes within the microenvironment. The objective of this review is to share information about these adaptive mechanisms. We also discuss the implications of these adaptive mechanisms in the future treatment of PJI.
Transient hypermutability
In microbial replication, there is an expected rate of stochastic genetic mutations. In hypermutable variants, the rate of generated genetic mutations multiplies (from 10- to 1,000-fold), providing variants with a selective advantage compared to wild-type strains. 11 There are multiple mechanisms triggering this phenomenon, including antimicrobial selection, endogenous stress responses, and heritable defects in mismatch repair genes. 11 Hypermutators affect a small subset of the infecting colony and this mechanism imparts a survival advantage, leaving wild-type strains susceptible to killing. Mutations that form under hypermutability can be neutral or deleterious. When selective pressures are removed, neutral mutators persist, whereas deleterious mutators (adverse to the organism) are outcompeted by reconfigured wild-type organisms. 12,13
Small colony variants
On blood agar plates, SCVs are seen as small irregular pinpoint colonies compared to wild-type colonies. SCVs are slow growing as they develop metabolic mutations from selective pressures. Exposure to specific antimicrobials such as the aminoglycosides, commonly used in PJI, often creates reproducible mutants. 14 Less commonly, the mere intracellular location of bacteria can trigger SCVs with similar metabolic mutants due to the presence of host antimicrobial cationic peptides. 15 These variants often lead to subclinical chronic infections until supplementation of nutrients, which circumvent the cellular metabolic mutations, restoring wild-type growth. 16-18 This can occur during any intervention that supplies fresh blood to an infected region, such as incision and drainage or implant exchange, and provides a sobering realization that surgical attempts to eradicate infection might actually exacerbate a patient’s joint infection. 17
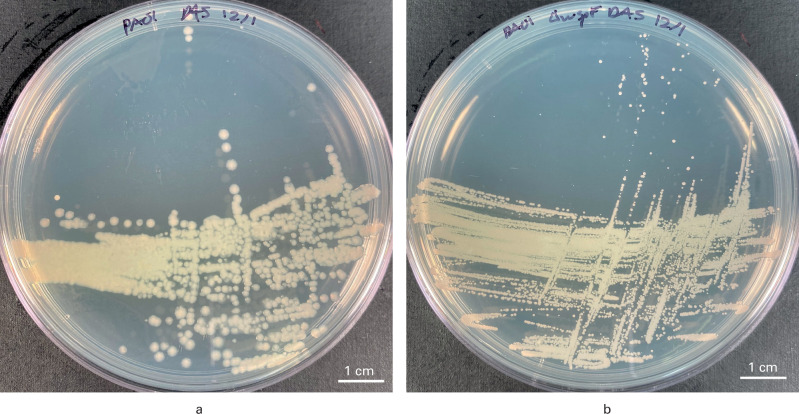
SCVs possess mutations in respiratory metabolism that limit the Krebs cycle. The residual metabolism relies on glycolysis and fermentation for energy and lead to a significant reduction in adenosine triphosphate (ATP). 17 Thus, these colonies are much smaller, compared to their wild-type form. In addition, they display atypical colony morphology secondary to alterations in metabolism, which help clinical microbiologists identify SCVs on blood agar plates held for > 72 hours (Figure 1).
Fig. 1.
Differences in morphotype wild-type and small colony variants (SCVs) of Pseudomonas aeruginosa. a) The Luria Broth (LB) agar plate shows large, round homogenous colonies of wild-type P. aeruginosa. b) The LB agar plate shows very small, pinpoint SVCs.
The phylogenic advantages of SCVs are numerous. SCVs are unaffected by antimicrobials that target cell wall and protein synthesis due to their decreased metabolism. In addition, SCVs have a proclivity to exist within human cells, specifically host immune and nonimmune musculoskeletal cells. 19 The intracellular location of SCVs shield them from established extracellular antimicrobial gradients within the periprosthetic joint space and from host immunity. 20-22 Hence, the ideal anti-SCV therapy would require an antimicrobial that can penetrate a host cell and maintain its activity. In the setting of intracellular Staphylococcus aureus SCVs, clinicians are left with few antibiotic options such as rifamycins (with monotherapy inducing high mutational rates and consequently not recommended), and specific glycopeptides. 20,21,23 Lastly, SCVs, through cytokines, are able to manipulate host immune cells into an anti-inflammatory state, contributing to the immunosuppressive microenvironment surrounding a chronically infected prosthetic joint. 24
Once a patient is colonized by SCVs, eradication is an arduous task. Sometimes, this can only be achieved by amputation. This is because SCVs are inclined to colonize the osteolacunar canalicular network (OLCN) of bone, rendering bacteria inaccessible to host immunity. 19 The unanswered question remains: how long can they persist within cells and the OLCN? It may be that SCVs, once established, are permanent. Eradication might thus require significant segmental bone removal.
Persister cells
Persister cells are a phenotypic variant that occur in a very small subset of the wild-type colonizers (< 1%). 17,25 These cells go into dormancy despite nutrients being available to them. Upon exposure to antimicrobials above MIC, while the fast-growing microbes may be killed, persister cells, due to their cessation of cell cycle activity (replication) can persist in the G0 phase. Thus, antimicrobial agents that impede cell cycle replication or interfere with metabolic activity (including most antimicrobial agents) are rendered ineffective. Once the antimicrobial challenge is reduced below MIC, persister cells can re-enter the growth cycle. After returning from G0 dormancy, persister cells are unique, as they maintain their wild-type metabolism and antimicrobial susceptibility. Not all wild-type colonies have persister cell variants.
One known mechanism by which persister cell variants enable G0 phase is through a toxin/antitoxin (TA) system that is present in various forms and exists within numerous microbiota species. 26-28 A TA system is composed of a stable toxin and a labile antitoxin. The toxin represses bacterial growth by inhibiting important bacterial physiological processes, including DNA replication, transcription, protein synthesis, cell wall synthesis, and cell division. Normally, the function of a toxin is neutralized by its cognate antitoxin. It is proposed that the antitoxin is degraded in response to environmental stresses including: antimicrobial agents exceeding MIC, altered local pH, and nutrient depletion. The unchecked toxin then enacts its effects on the cell to induce dormancy.
The foremost unanswered question is how long can a persister cell exist in G0 phase within the periprosthetic region? Microbiota in G0 phase replicate at a very slow rate. It is suggested that G0 phase turnover rate is approximately six to eight weeks, but it may be even longer. 29 A theoretical question that concerns many: can G0 phase microbiota exist for an extended time within the human host? If true, then it needs to be established what triggers reactivation, and whether persister cells can be coaxed (via signalling molecules) out of dormancy.
Adaptive resistance
Adaptive resistance, better described as transient antimicrobial resistance (TAR), involves metabolic adaptation limiting the capacity for antimicrobials to kill infecting organisms. As the name implies, a small subset of the wild-type colony is enabled with a multidrug efflux pump mechanism, to pump the affecting antimicrobial out of the cell, thus limiting its effect. 30 This efflux pump mechanism is transiently induced and upregulated when exposed to an antimicrobial challenge exceeding MIC. Characteristically, once the antimicrobial pressure is removed, the efflux pump is downregulated, and organisms revert to wild-type characteristics and antimicrobial sensitivity. At present, there is no established clinical test to detect this variant. In the future, it may be possible with next-generation DNA sequencing, to identify microbiota having the adaptive efflux pump regulatory mechanism.
Phoenix colonies
The phoenix colony is a novel variant described by Sindeldecker et al. 31 These variants represent a small subset (< 0.1%) of the wild-type population. Phoenix colonies were initially identified on in vitro Pseudomonas biofilm lawn plates treated with high-dose aminoglycoside mixed into an antimicrobial loaded calcium sulphate (ALCS) bead. The original agar lawn of biofilm population was completely eradicated, but on day 4 of observation, colonies of Pseudomonas grew from “the ashes” of the dead biofilm (Figure 2). The mechanism of phoenix colony emergence is unique from other variants. It is not clear whether the phoenix variant resides within the biofilm at all times or if it is triggered by exposure to an increasing antibiotic gradient.
Fig. 2.
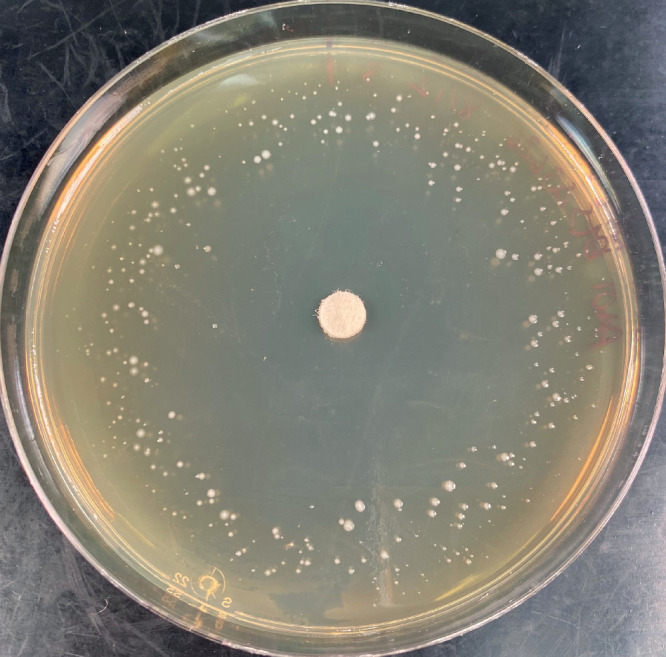
Photograph demonstrating the appearance of phoenix colony variants of Pseudomonas aeruginosa in a biofilm lawn plate at four days. In the centre is a disc containing tobramycin, which has created an antibiotic gradient radiating from the centre of the plate. The clear zone in the central region is where the antibiotic gradient has killed all biofilm bacteria, including resistant and variant phenotypes. At the rim of the plate is the remains of the wild-type lawn (diffuse light tan hue) where the antibiotic gradient remains below the minimum inhibitory concentration. In between the peripheral lawn and the cleared area are small, white, pinpoint “phoenix” colonies of Pseudomonas that have slowly emerged within from what first appeared to be the completely killed biofilm lawn (clear zone).
There are several characteristic features of phoenix colonies. First, they remain metabolically active and do not enter a G0 phase, and thus are not persister cells. There are no differences in structural morphology compared to their wild-type form. Phoenix variants have not acquired additional antimicrobial resistance genes compared to their wild-type form. Furthermore, phoenix variants have no alterations in metabolism or growth rates. These colonies continue to grow despite being exposed to extremely high concentrations of aminoglycosides. Lastly, phoenix colonies revert to their wild-type susceptibility when the aminoglycoside is removed.
The mechanism of tolerance of phoenix colonies likely lies with the mechanism of action of aminoglycosides which act upon the 30s ribosome-altering protein synthesis. Although not thoroughly understood, Sindeldecker and Stoodley 32 propose that tolerance results from an increased mRNA transcriptional plasticity during protein synthesis. Aminoglycosides impede mRNA translation and are thought to act as road bumps affecting protein synthesis. Phoenix variant microbiota, via a more flexible mRNA structure, may continue with the translation process without premature translation termination. Ongoing work seeks to determine if this mechanism of flexible translation is heritable.
Clinical relevance of adaptive microbial variants in treatment of PJI
The unanswered matter in PJI treatment is the dose of antimicrobial agents versus their duration. Those espousing high-dose treatments believe that local doses of antimicrobial agents that are multiples higher than established MICs will kill microbiota and their associated biofilms, and provide a high antimicrobial gradient to diffuse to all affected areas within the PJI space. This includes the OLCN and its associated intracellular bacteria. 19 Laboratory studies by Sindeldecker et al 31 have suggested that high local antimicrobial concentrations with substantial antimicrobial gradients can eradicate/exhaust all variants. Thus, surgeons should incorporate an emphasis on local antimicrobials in PJI hardware exchange protocols. The clinical data supporting this concept are limited, and for future clinical investigation, randomized controlled trials (RCTs) are needed. A benefit of using a local high-dose antimicrobial delivery technique is that it limits the human host to systemic antimicrobial exposure, which theoretically reduces antimicrobial resistance gene production within existing human biomes. Antimicrobial resistance, conferred through trans-species horizontal gene transfer via plasmids, is a major concern. Thus, limiting microbiome exposure to parenteral antimicrobials may be valuable. The weakness of this treatment strategy is the temporal component. It is still unknown how long persister cells and SCVs can survive in their recalcitrant forms. Longitudinal data are needed to answer this question.
If time is the primary factor to exhaust adaptive variant colonies, then antimicrobial duration should be emphasized. It is thought that persister cells replicate at a very slow rate (around six to eight weeks), but this is not well-defined. It is theoretically possible for persister cells and SCVs to exist for long periods of time. Thus, in exchange protocols, extended antimicrobial treatment would be required. The question is whether antimicrobial gradients within the PJI region can be adequately maintained for an extended period. This would potentially require months of treatment, if not longer. Furthermore, the use of oral antimicrobial treatment for the extended term will be unlikely to deliver an effective antimicrobial gradient throughout the entire PJI space. Thus, resistant variants would be allowed to flourish and acquire traditional antimicrobial resistance genes. Furthermore, the extended use of antimicrobial agents favours the SCV adaptive mechanism. As long as there is a significant antimicrobial pressure, the SCV will persist. The longer the exposure, the more likely the chance for SCV selection. 33
Because of the weaknesses with each of the above treatment philosophies, we believe adjuvant treatment other than antimicrobials should be developed and used concomitantly to provide complete termination of microbiota variant reserves. To improve the stubbornly persistent reinfection rate seen with current exchange protocols, we foresee multimodal protocols that specifically treat all microbiota, including all adaptive variants discussed above. Strategies include: use of novel antimicrobials; repurposing clinically approved medications (i.e. immunotherapy and chemotherapeutics) to treat PJI; neoadjuvant antibody therapy against biofilm structures; biofilm-disrupting agents; immunomodulation therapy to overcome an acquired local host immune suppressive state; phage therapy; new agents/combinations that promote mechanical removal of microbiota and remnant biofilm islands from the interstices of bone; and implant coatings that prevent recolonization of revision implants during reimplantation.
Lastly, surgeons must improve methods to identify intraoperatively regions infected by microbiota. Removal of microbially contaminated tissues is difficult and remains in the realm of “art”. Specialist PJI surgeons are experts at debridement but the naked eye cannot recognize microscopic extensions of infection. New methodologies should be developed to assist in intraoperative surgical debridement of PJI tissues via fluorescence or bioluminescence.
Human pressures forcing variant selection
To mitigate further microbial variant selection, we must understand the human pressures selecting for these organisms. First, every care should be taken to minimize direct contamination of the periprosthetic space at the time of joint implantation. These include preoperative host optimization and skin cleaning, timely perioperative prophylactic antimicrobials, improved intraoperative field sterility, and postoperative wound care. 34 However, while susceptible organisms can be eliminated, recalcitrant organisms may multiply and reconstitute this gap. 35 Second, the increasing prevalence of comorbidities such as diabetes, and the use of specialized systemic immune modulators for inflammatory conditions, is creating a larger immunocompromised population. This raises the risk of other microbiota populating the internal and external human microbiome, creating dysbiosis. Third, we are learning of medical treatments, considered routine and safe, as causing unintended consequences. For example, the routine use of proton pump inhibitors increases proximal gut pH, changing gut microbiome populations. 36,37 In concert, the regular intake of non-steroidal anti-inflammatory drugs can disrupt colonic tight junctions, allowing microbiota to leak into the vasculature, which may allow prosthetic joint inoculation by haematogenous seeding. 36,37 Finally, the liberal use of powerful antimicrobial agents at high doses and extended intervals selects for the most elusive and persistent microorganisms. Taken altogether, antibiotic tolerant microbial variants have selectively come to the forefront. In order to shift stresses away from variant selection, we must strategically rethink all treatment methods to rebalance humans with their microbiome partners.
To summarize, in the last two decades, we have come to understand better the challenges for effective cure of PJI. Foremost, microbial biofilms composed of highly rigorous extracellular polymericsubstances with their microbiota, envelope implants, and periarticular tissues, prohibiting infection eradication. 38 Second, we know that compromises in host immunity and limb health can limit effective treatment. 39,40 Third, we are identifying microbial variants with newer genetic mechanisms thwarting antimicrobial agents and host immune defenses. The result is PJI persistence. Table I summarizes the currently understood microbial defense mechanisms that we believe are pertinent to PJI. These adaptive mechanisms must be collectively addressed when developing future PJI treatment strategies. Surgical debridement must be comprehensive, and methods to enable visualization of microbiota must be developed to allow removal of microscopic extensions. Adjuvant treatments other than antimicrobials must be developed and used concomitantly to achieve complete microbial eradication. To improve the stubbornly persistent reinfection rate seen with current exchange protocols, we anticipate multimodal interventions to improve cure rates and specifically address variant reserves.
Table I.
Current summary of microbial defense mechanisms relevant to periprosthetic joint infection.
| Defense mechanism | Definition |
|---|---|
| Biofilm production | Formation of extracellular polymeric substances encompassing microbiota that greatly reduces ability of host immune mechanisms and antimicrobial agents to eradicate the pathogenic organism(s). |
| Multidrug resistance | Resistance genes that are acquired and incorporated into a microbial gene set that thwart microbial kill by antimicrobial agents. This is termed traditional resistance. |
| Hypermutability | In a stress state (for example, antimicrobial challenge) the ability of some microbiota to increase rate of gene mutation by multiples to acquire mutations that confer antimicrobial resistance. |
| Small colony variants | SCVs have genetically altered metabolism with reduced respiratory metabolism. SCVs take advantage of host intracellular colonization to protect from antimicrobials and host immunity, resulting in resistance and persistent infection. Microorganisms maintain minimal metabolic activity and revert to wild-type form only upon specific nutritional supplementation. |
| Persister cells | Microbes that enter a state of dormancy in which the microbes become metabolically inactive when exposed to antimicrobial loads above MIC. Also known as G0 phase existence. Metabolism resumes once antimicrobial levels drop below the MIC threshold. |
| Adaptive resistance | More accurately termed TAR, this is the ability of microbiota transiently to activate intracellular mechanisms to resist an antimicrobial threat. These variants are enabled with a multidrug efflux pump mechanism to pump the affecting antimicrobial out of the cell, thus limiting its effect. Once the antimicrobial pressure is removed, the efflux pump is downregulated, and organisms revert to wild-type characteristics and antimicrobial sensitivity. |
| Phoenix colonies | Microbiota that develop transient resistance to supraphysiological concentrations of antimicrobials (currently aminoglycosides) via a proposed mechanism of modified mRNA transcription. Phoenix variants continue with wild-type growth and resistance upon removal of antimicrobial pressure. |
MIC, minimal inhibitory concentration; SCV, small colony variant; TAR, transient antimicrobial resistance.
Take home message
- Periprosthetic joint infection (PJI) treatments have used increasing doses and durations of antimicrobial agents. Microbiota have responded with adaptive mechanisms beyond biofilm formation and traditional antimicrobial resistance genes.
- These adaptive mechanisms include transient hypermutability, small colony variants, persister cells, transient antimicrobial resistance, and phoenix colonies.
- Knowledge of these mechanisms and their genesis is critical in developing reconfigured strategies for microbial eradication. We have summarized these adaptive mechanisms and suggest tactical approaches for future PJI treatment.
Author contributions
C. Hamad: Methodology, Resources, Investigation, Validation, Visualization, Writing – original draft.
M. Chowdhry: Conceptualization, Methodology, Project administration, Resources, Investigation, Validation, Visualization, Writing – review & editing.
D. Sindeldecker: Methodology, Resources, Validation, Visualization, Writing – review & editing.
N. M. Bernthal: Methodology, Resources, Validation, Visualization.
P. Stoodley: Conceptualization, Methodology, Resources, Investigation, Supervision, Visualization, Writing – review & editing.
E. J. McPherson: Conceptualization, Methodology, Project administration, Resources, Supervision, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
Open access funding
The authors confirm that the open access fee for this study was self-funded.
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
This article was primary edited by G. Scott.
Contributor Information
Christopher Hamad, Email: chamad@mednet.ucla.edu.
Madhav Chowdhry, Email: madhav.chowdhry@kellogg.ox.ac.uk, madhav.chowdhry@gmail.com.
Devin Sindeldecker, Email: devin.sindeldecker@osumc.edu.
Nicholas M. Bernthal, Email: NBernthal@mednet.ucla.edu.
Paul Stoodley, Email: Paul.stoodley@osumc.edu.
Edward J. McPherson, Email: edmcpherson@gmail.com.
References
- 1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89-A(4):780–785. 10.2106/JBJS.F.00222 [DOI] [PubMed] [Google Scholar]
- 2. Xu C, Goswami K, Li WT, et al. Is treatment of periprosthetic joint infection improving over time? J Arthroplasty. 2020;35(6):1696–1702. 10.1016/j.arth.2020.01.080 [DOI] [PubMed] [Google Scholar]
- 3. Izakovicova P, Borens O, Trampuz A. Periprosthetic joint infection: current concepts and outlook. EFORT Open Rev. 2019;4(7):482–494. 10.1302/2058-5241.4.180092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griseti Q, Jacquet C, Sautet P, et al. Antimicrobial properties of antibiotic-loaded implants. Bone Joint J. 2020;102-B(6_Supple_A):158–162. 10.1302/0301-620X.102B6.BJJ-2019-1636.R1 [DOI] [PubMed] [Google Scholar]
- 5. Kallala R, Harris WE, Ibrahim M, Dipane M, McPherson E. Use of Stimulan absorbable calcium sulphate beads in revision lower limb arthroplasty: Safety profile and complication rates. Bone Joint Res. 2018;7(10):570–579. 10.1302/2046-3758.710.BJR-2017-0319.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park KJ, Chapleau J, Sullivan TC, Clyburn TA, Incavo SJ. 2021 Chitranjan S. Ranawat Award: Intraosseous vancomycin reduces periprosthetic joint infection in primary total knee arthroplasty at 90-day follow-up. Bone Joint J. 2021;103-B(6 Supple A):13–17. 10.1302/0301-620X.103B6.BJJ-2020-2401.R1 [DOI] [PubMed] [Google Scholar]
- 7. Lawrie CM, Kazarian GS, Barrack T, Nunley RM, Barrack RL. Intra-articular administration of vancomycin and tobramycin during primary cementless total knee arthroplasty: determination of intra-articular and serum elution profiles. Bone Joint J. 2021;103-B(11):1702–1708. 10.1302/0301-620X.103B11.BJJ-2020-2453.R1 [DOI] [PubMed] [Google Scholar]
- 8. McPherson EJ, Dipane MV, Chowdhry M, Wassef AJ. Fabrication of antibiotic-loaded dissolvable calcium sulfate beads: an in vitro mixing lab utilizing various antibiotic mixing formulas. J Bone Jt Infect. 2021;6(9):405–412. 10.5194/jbji-6-405-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsang S-TJ, Simpson AHRW. Antimicrobial rationing in orthopaedic surgery. Bone Joint Res. 2020;9(12):870–872. 10.1302/2046-3758.912.BJR-2020-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bigger JW. Treatment of Staphylococcal infections with penicillin by intermittent sterilisation. Lancet. 1944;244(6320):497–500. 10.1016/S0140-6736(00)74210-3 [DOI] [Google Scholar]
- 11. Swings T, Van den Bergh B, Wuyts S, et al. Adaptive tuning of mutation rates allows fast response to lethal stress in Escherichia coli. Elife. 2017;6(6):e22939. 10.7554/eLife.22939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Loh E, Salk JJ, Loeb LA. Optimization of DNA polymerase mutation rates during bacterial evolution. Proc Natl Acad Sci U S A. 2010;107(3):1154–1159. 10.1073/pnas.0912451107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bjedov I, Tenaillon O, Gérard B, et al. Stress-induced mutagenesis in bacteria. Science. 2003;300(5624):1404–1409. 10.1126/science.1082240 [DOI] [PubMed] [Google Scholar]
- 14. Proctor RA, van Langevelde P, Kristjansson M, Maslow JN, Arbeit RD. Persistent and relapsing infections associated with small-colony variants of Staphylococcus aureus. Clin Infect Dis. 1995;20(1):95–102. 10.1093/clinids/20.1.95 [DOI] [PubMed] [Google Scholar]
- 15. Martínez JL, Rojo F. Metabolic regulation of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):768–789. 10.1111/j.1574-6976.2011.00282.x [DOI] [PubMed] [Google Scholar]
- 16. Bhattacharyya S, Roy S, Mukhopadhyay P, et al. Small colony variants of Staphylococcus aureus isolated from a patient with infective endocarditis: a case report and review of the literature. Iran J Microbiol. 2012;4(2):98–99. [PMC free article] [PubMed] [Google Scholar]
- 17. Proctor RA, von Eiff C, Kahl BC, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(4):295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 18. Tande AJ, Osmon DR, Greenwood-Quaintance KE, Mabry TM, Hanssen AD, Patel R. Clinical characteristics and outcomes of prosthetic joint infection caused by small colony variant staphylococci. mBio. 2014;5(5):e01910-14. 10.1128/mBio.01910-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zoller SD, Hegde V, Burke ZDC, et al. Evading the host response: Staphylococcus “hiding” in cortical bone canalicular system causes increased bacterial burden. Bone Res. 2020;8(1):43. 10.1038/s41413-020-00118-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen TK, Argudín MA, Deplano A, et al. Antibiotic resistance, biofilm formation, and intracellular survival as possible determinants of persistent or recurrent infections by Staphylococcus aureus in a Vietnamese tertiary hospital: focus on bacterial response to Moxifloxacin. Microb Drug Resist. 2020;26(6):537–544. 10.1089/mdr.2019.0282 [DOI] [PubMed] [Google Scholar]
- 21. Lamret F, Colin M, Mongaret C, Gangloff SC, Reffuveille F. Antibiotic tolerance of Staphylococcus aureus biofilm in periprosthetic joint infections and antibiofilm strategies. Antibiotics (Basel). 2020;9(9):E547. 10.3390/antibiotics9090547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barcia-Macay M, Seral C, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother. 2006;50(3):841–851. 10.1128/AAC.50.3.841-851.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abad LA, Josse J, Tasse J, et al. Staphylococcus aureus bone and joint infection: comparison of rifamycin intraosteoblastic activity and impact on intracellular emergence of small colony variants. Orthopaedic Proceedings. 2018;100-B(SUPP-17). [Google Scholar]
- 24. Seebach E, Kubatzky KF. Chronic implant-related bone infections-can immune modulation be a therapeutic strategy? Front Immunol. 2019;10:1724. 10.3389/fimmu.2019.01724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Appl Environ Microbiol. 2013;79(23):7116–7121. 10.1128/AEM.02636-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amraei F, Narimisa N, Sadeghi Kalani B, Lohrasbi V, Masjedian Jazi F. Persister cells formation and expression of type II Toxin-Antitoxin system genes in Brucella melitensis (16M) and Brucella abortus (B19). Iran J Pathol. 2020;15(2):127–133. 10.30699/ijp.2020.118902.2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muthuramalingam M, White JC, Bourne CR. Toxin-antitoxin modules are pliable switches activated by multiple protease pathways. Toxins (Basel). 2016;8(7):E214. 10.3390/toxins8070214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Habib G, Zhu J, Sun B. A novel type I toxin-antitoxin system modulates persister cell formation in Staphylococcus aureus . Int J Med Microbiol. 2020;310(2):151400. 10.1016/j.ijmm.2020.151400 [DOI] [PubMed] [Google Scholar]
- 29. Kumar R, Srivastava S. Quantitative proteomic comparison of stationary/G0 phase cells and tetrads in budding yeast. Sci Rep. 2016;6:32031. 10.1038/srep32031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fernández L, Hancock REW. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev. 2012;25(4):661–681. 10.1128/CMR.00043-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sindeldecker D, Moore K, Li A, et al. Novel aminoglycoside-tolerant phoenix colony variants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2020;64(9):e00623-20. 10.1128/AAC.00623-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sindeldecker D, Stoodley P. The many antibiotic resistance and tolerance strategies of Pseudomonas aeruginosa. Biofilm. 2021;3:100056. 10.1016/j.bioflm.2021.100056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. von Eiff C, Bettin D, Proctor RA, et al. Recovery of small colony variants of Staphylococcus aureus following gentamicin bead placement for osteomyelitis. Clin Infect Dis. 1997;25(5):1250–1251. 10.1086/516962 [DOI] [PubMed] [Google Scholar]
- 34. Iannotti F, Prati P, Fidanza A, et al. Prevention of periprosthetic joint infection (PJI): a clinical practice protocol in high-risk patients. Trop Med Infect Dis. 2020;5(4):E186. 10.3390/tropicalmed5040186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiu L, Bazin T, Truchetet ME, Schaeverbeke T, Delhaes L, Pradeu T. Protective microbiota: from localized to long-reaching co-immunity. Front Immunol. 2017;8:1678. 10.3389/fimmu.2017.01678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bruin MM, Deijkers RLM, Bazuin R, Elzakker EPM, Pijls BG. Proton-pump inhibitors are associated with increased risk of prosthetic joint infection in patients with total hip arthroplasty: a case-cohort study. Acta Orthop. 2021;92(4):431–435. 10.1080/17453674.2021.1920687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141(4):1314–1322. 10.1053/j.gastro.2011.06.075 [DOI] [PubMed] [Google Scholar]
- 38. Geesey GG, Mutch R, Costerton JW, Green RB. Sessile bacteria: An important component of the microbial population in small mountain streams 1. Limnol Oceanogr. 1978;23(6):1214–1223. 10.4319/lo.1978.23.6.1214 [DOI] [Google Scholar]
- 39. McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. [PubMed] [Google Scholar]
- 40. McPherson EJ, Tontz W, Patzakis M, et al. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop (Belle Mead NJ). 1999;28(3):161–165. [PubMed] [Google Scholar]