Abstract
Aims
The aim of this study was to estimate the 90-day periprosthetic joint infection (PJI) rates following total knee arthroplasty (TKA) and total hip arthroplasty (THA) for osteoarthritis (OA).
Methods
This was a data linkage study using the New South Wales (NSW) Admitted Patient Data Collection (APDC) and the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), which collect data from all public and private hospitals in NSW, Australia. Patients who underwent a TKA or THA for OA between 1 January 2002 and 31 December 2017 were included. The main outcome measures were 90-day incidence rates of hospital readmission for: revision arthroplasty for PJI as recorded in the AOANJRR; conservative definition of PJI, defined by T84.5, the PJI diagnosis code in the APDC; and extended definition of PJI, defined by the presence of either T84.5, or combinations of diagnosis and procedure code groups derived from recursive binary partitioning in the APDC.
Results
The mean 90-day revision rate for infection was 0.1% (0.1% to 0.2%) for TKA and 0.3% (0.1% to 0.5%) for THA. The mean 90-day PJI rates defined by T84.5 were 1.3% (1.1% to 1.7%) for TKA and 1.1% (0.8% to 1.3%) for THA. The mean 90-day PJI rates using the extended definition were 1.9% (1.5% to 2.2%) and 1.5% (1.3% to 1.7%) following TKA and THA, respectively.
Conclusion
When reporting the revision arthroplasty for infection, the AOANJRR substantially underestimates the rate of PJI at 90 days. Using combinations of infection codes and PJI-related surgical procedure codes in linked hospital administrative databases could be an alternative way to monitor PJI rates.
Cite this article: Bone Joint J 2022;104-B(9):1060–1066.
Keywords: Arthroplasty, Osteoarthritis, Infection, osteoarthritis (OA), periprosthetic joint infection (PJI), hip and knee arthroplasty, hip and knee arthroplasty, revision arthroplasties, infections, total knee and total hip arthroplasty, Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), total knee and total hip arthroplasty, arthroplasty registries
Introduction
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are effective treatments for late-stage osteoarthritis (OA), and have the potential to substantially increase patient quality of life after the surgery. 1 The rising demand for TKA and THA to treat OA is expected to continue in the next decade due to increasing levels of obesity, an ageing population, and growth in sports-related injuries. 2,3 However, the success of both interventions can be undermined by the increasing incidence of periprosthetic joint infection (PJI), 4 which is one of the most devastating and costly complications following arthroplasty surgery. The infection can leave patients in a worse condition than their preoperative state. 5 Aside from the health impact, PJI often leads to readmissions to hospital and in some cases revision surgeries, which add to the significant economic burden of PJI. 6-8
Despite the clinical and health economic significance of PJI, the exact incidence rate is unknown. Arthroplasty registries have been used to estimate the incidence rate of PJI, but these registries only report a revision if a component of the implant was removed or added. Relying on revision for infection reported in an arthroplasty registry is likely to underestimate the true incidence rate of PJI, because not all PJIs require revision arthroplasty, and the first treatment option for many PJIs is non-surgical antibiotic treatment, surgical debridement, and irrigation. 9 Routinely collected administrative databases capturing hospital diagnosis and procedure codes are a potential data source for identifying PJIs that did not undergo revision surgery. 10 While such databases could serve as valuable sources of information to study the incidence rate of PJI, there is no consensus on what codes should be used to identify PJIs following TKA and THA. Recent international studies combining a PJI diagnosis code with a relevant surgical procedure code have shown promising accuracy in the detection of PJIs from administrative databases when referencing to standard medical chart review. 11,12 In Australia, the occurrence of PJI after TKA and THA was previously reported to be 1.7% over two years using administrative admission data from four hospitals. 13 However, incidence rates of PJI identified in state-wide administrative databases, using different combinations of diagnosis and procedure codes, have not been compared against reference cases of known revision arthroplasty performed for PJI in linked arthroplasty registry data. Estimating the rate of PJI using different strategies will help facilitate future studies to identify risk factors for PJI using linked administrative databases.
This study aimed to develop coding algorithms which combine diagnosis codes according to the International Classification of Disease, 10th revision, Australian Modification (ICD-10-AM) with Australian Classification of Health Intervention (ACHI) procedure codes to identify 90-day hospital readmissions for PJI, 14 and compare the resulting incidence rate with the rate identified by the PJI diagnosis code (T84.5) only and the rate of revision arthroplasty for infection as determined by a national arthroplasty registry.
Methods
We used records from the New South Wales (NSW) Admitted Patient Data Collection (APDC) and the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR), linked probabilistically by the Centre for Health Record Linkage with rates of false positive and false negative links estimated at 0.5%. 15 The APDC data collection records all admitted patient services provided by public and private hospitals in NSW, Australia. The AOANJRR records details of all primary and revision knee and hip arthroplasties performed in Australia. We included patients who underwent a TKA or a THA with a primary diagnosis of OA in NSW, Australia between 1 January 2002 and 31 December 2017.
Data linkage procedure for index arthroplasty
Primary TKA and THA procedures were identified from the AOANJRR. Procedure records were restricted to those with a primary diagnosis of OA and those performed in a hospital in NSW. These records were subsequently matched to the hospital admissions for the primary procedure recorded in the APDC. Records were retained in the study if they demonstrated good matching quality, which was defined by the following criteria: the surgical operation date recorded in the AOANJRR fell within the date range of the closest matched hospital stay recorded in the APDC; or the matched hospital stay had a procedure code of TKA or THA that corresponded to the same joint and procedure type information recorded in the AOANJRR. The ACHI procedure codes used for TKA and THA are listed in Supplementary Table i.
Definitions of readmission for PJI
The primary and secondary diagnosis and procedure codes of all hospital admissions within 90 days of the initial admission for the primary TKA or THA were examined for any codes indicating a PJI. The 90-day period was suggested as the minimum follow-up period to track surgical site infection rates for TKA and THA from linked administrative data. 16,17 Given that there is no gold-standard definition for PJI, we estimated the incidence of 90-day readmission for PJI using three levels of definitions: revision arthroplasty for PJI, as recorded by the AOANJRR; conservative definition of PJI, as defined by the presence of T84.5 as a primary or secondary diagnosis in the APDC irrespective of the presence of an associated surgical procedure code; and extended definition of PJI, as defined by either T84.5 being recorded as a primary or secondary diagnosis in the APDC, or one or more readmissions in the APDC identified by a combination of diagnosis and procedure code groups determined via expert review and machine learning algorithms, as described below.
Developing algorithms for the extended definition of PJI
Algorithms for detecting 90-day readmissions for PJI in this study were developed in two steps. First, we conducted an expert review process with three experienced orthopaedic surgeons (IH, RS, SEG) who specialize in hip or knee arthroplasty to select a list of potential ICD-10-AM diagnosis codes and ACHI procedure codes that could indicate a joint infection. The presence of each selected code group was flagged in hospital admission data within 90 days of the index arthroplasty. Then, binary recursive partitioning was applied to the TKA and THA data separately to derive code groups that were predictive of PJIs. Binary recursive partitioning, also known as classification and regression tree, is a non-parametric method used to predict dichotomous outcome. The method creates a decision tree by repeatedly dividing the sample into subgroups, with each subdivision being formed by separating the sample on the value of one of the predictor variables. 18 All diagnosis and procedure code groups identified in the expert review were used as predictor variables with the reference outcome being a revision arthroplasty for infection confirmed by the records in the AOANJRR, which had been used internationally to estimate infection burden in total joint replacement. 19 Multiple decision trees were created with ten-fold cross-validation and hyperparameter tuning. Because it is impractical to verify a PJI that was not treated with revision arthroplasty in this large-scale linkage study, the best decision tree was selected based on the highest positive predictive value (PPV), which is the proportion of true positive (i.e. revision for infection) in the total number of test positives (i.e. model-predicted revision for infection). The code groups included in the best decision tree were subsequently used in the extended definition of the 90-day readmission for PJI.
Statistical analysis
Statistical analyses were performed using R (v. 4.0.4, R Foundation for Statistical Computing, Austria). Binary recursive partitioning was performed using the R package ‘rpart’ (v. 4.1.15). Importance scores of the identified code groups from the best decision tree were calculated based on the reduction of Gini impurity index, which measured the sum of probability of the reference outcome (i.e. revision arthroplasty for infection) being wrongly classified by a code group when randomly selected. 20 The incidence rates of 90-day readmission for PJI following TKA and THA over the study period were calculated for the three definitions mentioned earlier and compared on a yearly basis. The 95% confidence intervals for incidence rates were estimated using the Clopper-Pearson interval.
Results
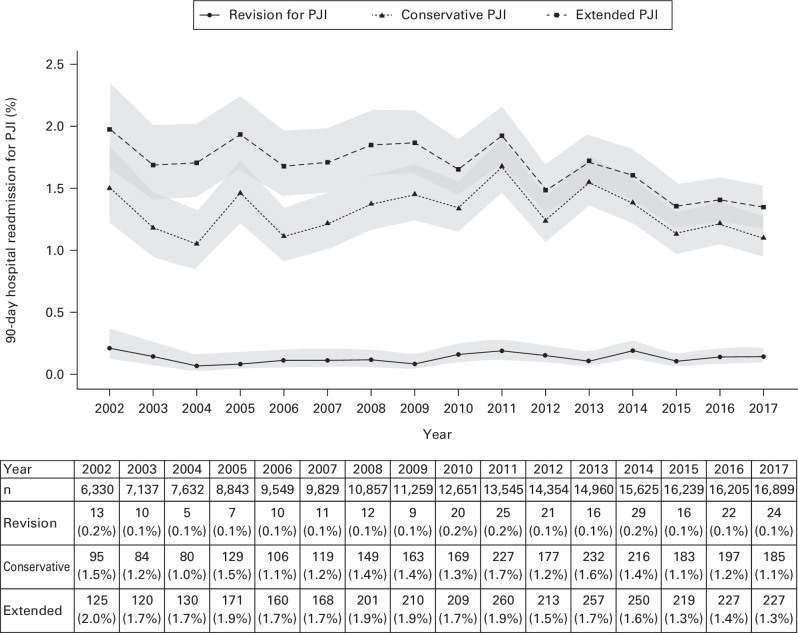
Data on 301,326 primary total arthroplasty procedures (191,914 TKAs and 109,412 THAs) performed in 107 hospitals from 1 January 2002 to 31 December 2017 were included. Figure 1 and Figure 2 compare the 90-day incidence rates of PJI across the three definitions following primary TKA and THA, respectively.
Fig. 1.
Annual 90-day hospital readmission rate for periprosthetic joint infection (PJI) following primary total knee arthroplasty for osteoarthritis.
Fig. 2.
Annual 90-day hospital readmission rate for periprosthetic joint infection (PJI) following primary total hip arthroplasty for osteoarthritis.
Revision for infection
The mean 90-day revision for infection rate was 0.1% (0.1% to 0.2%) following primary TKA and 0.3% (0.1% to 0.5%) following primary THA. The revision for infection rate after THA appeared to have increased in recent years, while the rate after TKA remained stable over time.
Conservative definition of PJI
When hospital readmission for PJI was defined by T84.5 diagnosis code alone, the mean 90-day PJI rate was 1.3% (1.1% to 1.7%) after primary TKA and 1.1% (0.8% to 1.3%) after primary THA.
Extended definition of PJI: binary recursive partitioning algorithm
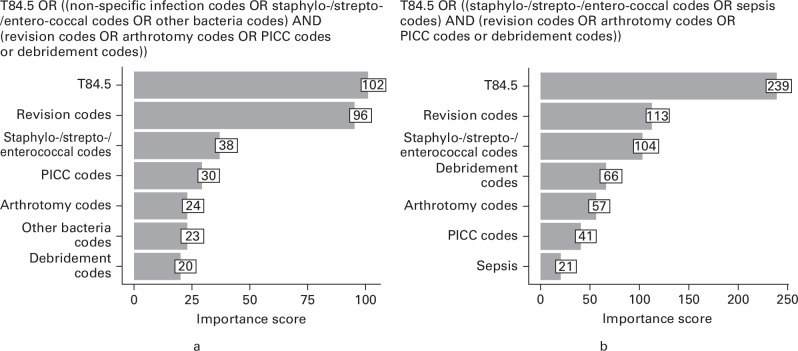
The binary recursive partitioning model identified six code groups which were important to detect PJI following TKA (non-specific postprocedural infection, strepto-/staphylo-/enterococcus infection, other bacterial infection, peripherally inserted central catheter, debridement, and arthrotomy) and five code groups for THA (strepto-/staphylo-/enterococcus infection, sepsis, peripherally inserted central catheter, debridement, and arthrotomy). Figure 3 shows the extended definition of PJI for TKA and THA and the calculated variable importance score of the identified code groups in detecting revision for PJI.
Fig. 3.
Extended definition of periprosthetic joint infection and importance score of code groups identified from binary recursive partitioning for a) total knee arthroplasty and b) total hip arthroplasty. PICC, peripherally inserted central catheter.
Annual readmission rates for PJI
The mean annual readmission rates for PJI identified by the extended definition were 1.9% (1.5% to 2.2%) and 1.5% (1.3% to 1.7%) within 90 days following TKA (Figure 1) and THA (Figure 2), respectively. These rates were higher than the rates identified by the conservative definition of PJI using the diagnosis code T84.5 alone.
Discussion
To our knowledge, this is the first study to compare 90-day incidence rates of PJI by refined use of hospital administrative data linked to an arthroplasty registry in Australia. The yearly 90-day hospital readmission rates for PJI ranged from 1.0% to 2.0% following TKA for OA, and from 0.8% to 1.7% following THA for OA, depending on the coding definitions. We found that revision arthroplasties for infection recorded in the AOANJRR only made up a small proportion of the 90-day hospital readmissions for PJI. At 90 days, the actual incidence rate of PJI may be more than ten times higher than the revision arthroplasty rate for infection after TKA, and more than four times higher than that after THA.
The PJI rates estimated from the present study are close to the findings from a previous study, which reported a PJI occurrence rate of 1.7% within two years following a TKA or THA in four hospitals in Australia, 13 using routinely collected administrative admission data. The subtle difference in the incidence rates between the present study and the previous one could be attributable to the different PJI definitions used, different duration of follow-up, different patient characteristics in the included hospitals, and the inclusion of reasons other than OA for the primary arthroplasty, such as rheumatoid arthritis, which is an independent risk factor for PJI. 21,22 Nevertheless, our findings of 90-day readmissions for PJI being higher after primary TKA compared to THA are consistent with those that have been previously reported. 13,23,24
A few international studies have previously reported the underestimation of PJI rates using joint arthroplasty registry data alone. 25-28 The ‘true’ incidence rates of PJI were underestimated by approximately 40% in the Danish Arthroplasty Register 26 and 33% in the Swedish Hip Arthroplasty Register. 27 The New Zealand Joint Registry reported a sensitivity of only 63% when using revision arthroplasty rates for PJI as compared to the audit of hospital records. 25 A recent cross-validation study also reported that the 90-day incidence of revision arthroplasty due to infection from the Dutch Arthroplasty Register was 0.6% compared to the true PJI rate of 1% in a cohort of nine hospitals. 29 Our results are in line with the findings of these studies, showing that the revision arthroplasty rate for infection recorded in the AOANJRR significantly underestimated 90-day readmissions for PJI.
The main strength of the present study rests on the refined use of linked data between hospital administrative data and the national arthroplasty registry to estimate the ‘true’ incidence rates of PJI. The linked data created a state-wide cohort representative of all primary TKA and THA procedures performed for OA. In addition, we used an innovative machine-learning method to develop an extended definition of PJI, which could be used in future registry-based studies on PJI after arthroplasty for OA. Intuitively, using combinations of infection diagnosis codes and surgical procedure codes in the extended PJI definition should deliver an improvement in detection over a PJI code alone. A definitive PJI diagnosis is usually not made at the time of hospital admission and it requires subsequent laboratory tests such as periprosthetic cultures. In contrast, surgical procedures such as peripheral insertion of a central catheter, which facilitates extended administration of antibiotics, are unlikely to be performed for reasons other than treatment for PJI.
We acknowledge there are limitations in the present study. First, the use of routinely collected administrative data to detect a PJI may underestimate or overestimate the incidence rates of PJI due to variation in recording PJI across different hospitals. 30 Because of the large scale of data in this study, it was not practical to validate the identified hospital admissions for PJI by medical chart reviews or pathology reports. However, previous international studies have reported results of promising accuracy in identifying PJI using administrative data. For example, a Danish study using data from administrative discharge registers was able to achieve a PPV of 86% in PJI detection after primary hip arthroplasty when the detection algorithm combined T84.5 with an infection-related surgical procedure code. 31 Similarly, a recent Canadian study also demonstrated a sensitivity of 88% when using the T84.5 alone and a sensitivity of 95% when combing with a joint arthroplasty procedure code or a peripherally inserted central catheter code. 12 Lastly, the extended definition of PJI was developed from decision trees which predict infections leading to a revision arthroplasty, rather than PJIs in general. As a result, the extended definition may still underestimate the actual rate of PJIs.
Further cross-validation of the extended definition of PJIs is required in the future to enable register-based studies on PJI after joint arthroplasty. Ideally, the diagnosis of PJI should be ascertained by clinical notes review, microbiological culture and histology, according to the latest international guidelines. 32,33 Similar studies with data linkage between hospital administrative data and the joint arthroplasty in other jurisdictions in Australia will allow standardized comparison of 90-day readmission rate for PJI across states.
Using revision arthroplasty for infection alone for monitoring acute PJI following TKA and THA substantially underestimates the incident rate of 90-day readmission for PJI. Using combinations of infection diagnosis codes and PJI-related surgical procedure codes in linked hospital administrative databases can be an alternative way to monitor PJI rates.
Take home message
- Records of revision arthroplasty for infection from arthroplasty registries underestimate the true incidence rate of periprosthetic joint infection (PJI).
- National arthroplasty registry data linked to hospital administrative data indicated that the 90-day hospital readmission rate for PJI could be ten times and four times higher than the revision arthroplasty rate for infection after total knee and total hip arthroplasty, respectively.
- Algorithms combining infection codes and PJI-related surgical procedure codes in linked hospital administrative databases may improve the detection of PJI.
Author contributions
X. Jin: Conceptualization, Methodology, Project administration, Investigation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing.
B. Gallego Luxan: Methodology, Supervision, Software, Formal analysis, Validation, Writing – original draft, Writing – review & editing.
M. Hanly: Methodology, Resources, Software, Data curation, Formal analysis, Validation, Visualization, Writing – review & editing.
N. L. Pratt: Methodology, Funding acquisition, Project administration, Resources, Data curation, Formal analysis, Validation, Writing – review & editing.
I. Harris: Conceptualization, Funding acquisition, Validation, Writing – original draft, Writing – review & editing.
R. de Steiger: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Writing – review & editing.
S. E. Graves: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
L. Jorm: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Investigation, Data curation, Formal analysis, Writing – review & editing.
Funding statement
The authors disclose receipt of the following financial or material support for the research, authorship, and/or publication of this article: This project is funded by the Australian National Health and Medical Research Centre (NHMRC) Grant (APP1148106). XJ is supported by an Australian NHMRC Early Career Fellowship (APP1104600).
Acknowledgements
We thank the Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) and the hospitals, orthopaedic surgeons, and patients whose data made this work possible. The Australian Government funds the AOANJRR through the Department of Health and Ageing.
Ethical review statement
The use of the data for the study was approved by the New South Wales Population Health Services Research Ethics Committee (Ref: 2019/ETH00436). A waiver of individual informed consent by patients was granted for our analysis of de-identified data.
Open access funding
Open access fee was funded by the Australian NHMRC Early Career Fellowship (APP1104600).
Open access statement
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/
Follow X. Jin @XingzhongJ
Follow N. L. Pratt @nicolepratt_
Follow I. Harris @DrIanHarris
Follow L. Jorm @Louisa_Jorm
Follow University of New South Wales @UNSW
Supplementary material
Table of diagnostic and procedure codes used to develop periprosthetic joint infection detection algorithms.
This article was primary edited by A. D. Liddle.
Contributor Information
Xingzhong Jin, Email: xingzhong.jin@unsw.edu.au.
Blanca Gallego Luxan, Email: b.gallego@unsw.edu.au.
Mark Hanly, Email: m.hanly@unsw.edu.au.
Nicole L. Pratt, Email: Nicole.pratt@unisa.edu.au.
Ian Harris, Email: ianharris@unsw.edu.au.
Richard de Steiger, Email: Richard.Desteiger@epworth.org.au.
Stephen E. Graves, Email: Segraves@aoanjrr.org.au.
Louisa Jorm, Email: l.jorm@unsw.edu.au.
References
- 1. Konopka JF, Lee Y, Su EP, McLawhorn AS. Quality-adjusted life years after hip and knee arthroplasty. JBJS OA. 2018;3(3):e0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vos T, Allen C, Arora M, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackerman IN, Pratt C, Gorelik A, Liew D. Projected burden of osteoarthritis and rheumatoid arthritis in Australia: a population-level analysis. Arthritis Care Res (Hoboken). 2018;70(6):877–883. [DOI] [PubMed] [Google Scholar]
- 4. Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27(2):302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cahill JL, Shadbolt B, Scarvell JM, Smith PN. Quality of life after infection in total joint replacement. J Orthop Surg (Hong Kong). 2008;16(1):58–65. [DOI] [PubMed] [Google Scholar]
- 6. Peel TN, Dowsey MM, Buising KL, Liew D, Choong PFM. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin Microbiol Infect. 2013;19(2):181–186. [DOI] [PubMed] [Google Scholar]
- 7. Kapadia BH, Banerjee S, Cherian JJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections after total hip arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2016;31(7):1422–1426. [DOI] [PubMed] [Google Scholar]
- 8. Kerzner B, Kunze KN, O’Sullivan MB, Pandher K, Levine BR. An epidemiological analysis of revision aetiologies in total hip arthroplasty at a single high-volume centre. Bone Jt Open. 2021;2(1):16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sousa R, Abreu MA. Treatment of prosthetic joint infection with debridement, antibiotics and irrigation with implant retention - a narrative review. J Bone Jt Infect. 2018;3(3):108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rusk A, Bush K, Brandt M, et al. Improving surveillance for surgical site infections following total hip and knee arthroplasty using diagnosis and procedure codes in a provincial surveillance network. Infect Control Hosp Epidemiol. 2016;37(6):699–703. [DOI] [PubMed] [Google Scholar]
- 11. Weinstein EJ, Stephens-Shields A, Loabile B, et al. Development and validation of case-finding algorithms to identify prosthetic joint infections after total knee arthroplasty in Veterans Health Administration data. Pharmacoepidemiol Drug Saf. 2021;30(9):1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandel CE, Jenkinson R, Widdifield J, et al. Identification of prosthetic hip and knee joint infections using administrative databases-A validation study. Infect Control Hosp Epidemiol. 2021;42(3):325–330. [DOI] [PubMed] [Google Scholar]
- 13. Marang-van de Mheen PJ, Bragan Turner E, Liew S, et al. Variation in prosthetic joint infection and treatment strategies during 4.5 years of follow-up after primary joint arthroplasty using administrative data of 41397 patients across Australian, European and United States hospitals. BMC Musculoskelet Disord. 2017;18(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elsworthy AM, Claessen SM, Graham B, et al. ICD-10-AM: the International statistical Classification of Diseases and related health problems, 10th revision, Australian modification: tabular list of diseases. 8th ed. Wollongong, Australia: National Casemix & Classification Centre, Australian Health Services Research Institute, 2013. [Google Scholar]
- 15. No authors listed . Quality assurance. Centre for Health Record Linkage. 2012. https://www.cherel.org.au/quality-assurance(date last accessed 20 June 2022).
- 16. Lethbridge LN, Richardson CG, Dunbar MJ. Measuring surgical site infection from linked administrative data following hip and knee replacement. J Arthroplasty. 2020;35(2):528–533. [DOI] [PubMed] [Google Scholar]
- 17. Edwards NM, Varnum C, Nelissen RGHH, Overgaard S, Pedersen AB. The association between socioeconomic status and the 30- and 90-day risk of infection after total hip arthroplasty: a registry-based cohort study of 103,901 patients with osteoarthritis. Bone Joint J. 2022;104-B(2):221–226. [DOI] [PubMed] [Google Scholar]
- 18. Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods. 2009;14(4):323–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Springer BD, Cahue S, Etkin CD, Lewallen DG, McGrory BJ. Infection burden in total hip and knee arthroplasties: an international registry-based perspective. Arthroplast Today. 2017;3(2):137–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nembrini S, König IR, Wright MN. The revival of the Gini importance? Bioinformatics. 2018;34(21):3711–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsieh P-H, Huang K-C, Shih H-N. Prosthetic joint infection in patients with rheumatoid arthritis: an outcome analysis compared with controls. PLoS ONE. 2013;8(8):e71666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schrama JC, Fenstad AM, Dale H, et al. Increased risk of revision for infection in rheumatoid arthritis patients with total hip replacements. Acta Orthop. 2015;86(4):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Y, Zhang F, Chen W, Liu S, Zhang Q, Zhang Y. Risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. J Hosp Infect. 2015;89(2):82–89. [DOI] [PubMed] [Google Scholar]
- 24. Aggarwal VK, Rasouli MR, Parvizi J. Periprosthetic joint infection: current concept. Indian J Orthop. 2013;47(1):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhu M, Ravi S, Frampton C, Luey C, Young S. New Zealand Joint Registry data underestimates the rate of prosthetic joint infection. Acta Orthop. 2016;87(4):346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gundtoft PH, Overgaard S, Schønheyder HC, Møller JK, Kjærsgaard-Andersen P, Pedersen AB. The “true” incidence of surgically treated deep prosthetic joint infection after 32,896 primary total hip arthroplasties: a prospective cohort study. Acta Orthop. 2015;86(3):326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lindgren JV, Gordon M, Wretenberg P, Kärrholm J, Garellick G. Validation of reoperations due to infection in the Swedish Hip Arthroplasty Register. BMC Musculoskelet Disord. 2014;15(1):384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dale H, Skråmm I, Løwer HL, et al. Infection after primary hip arthroplasty: a comparison of 3 Norwegian health registers. Acta Orthop. 2011;82(6):646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamp MC, Liu W-Y, Goosen JHM, et al. Mismatch in capture of periprosthetic joint infections between the Dutch Arthroplasty Register (LROI) and a detailed regional periprosthetic joint infection registry. J Arthroplasty. 2022;37(1):126–131. [DOI] [PubMed] [Google Scholar]
- 30. Lujic S, Watson DE, Randall DA, Simpson JM, Jorm LR. Variation in the recording of common health conditions in routine hospital data: study using linked survey and administrative data in New South Wales, Australia. BMJ Open. 2014;4(9):e005768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lange J, Pedersen AB, Troelsen A, Søballe K. Do hip prosthesis related infection codes in administrative discharge registers correctly classify periprosthetic hip joint infection? Hip Int. 2015;25(6):568–573. [DOI] [PubMed] [Google Scholar]
- 32. Tubb CC, Polkowksi GG, Krause B. Diagnosis and prevention of periprosthetic joint infections. J Am Acad Orthop Surg. 2020;28(8):e340–e348. [DOI] [PubMed] [Google Scholar]
- 33. Parvizi J, Gehrke T, International Consensus Group on Periprosthetic Joint Infection . Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. [DOI] [PubMed] [Google Scholar]