Abstract
Powdery mildew, caused by Blumeria graminis f. sp. tritici (Bgt), is a serious fungal disease that critically threatens the yield and quality of wheat. Utilization of host resistance is the most effective and economical method to control this disease. In our study, a wheat breeding line ShiCG15–009, released from Hebei Province, was highly resistant to powdery mildew at all stages. To dissect its genetic basis, ShiCG15–009 was crossed with the susceptible cultivar Yannong 21 to produce F1, F2 and F2:3 progenies. After genetic analysis, a single dominant gene, tentatively designated PmCG15–009, was proved to confer resistance to Bgt isolate E09. Further molecular markers analysis showed that PmCG15–009 was located on chromosome 2BL and flanked by markers XCINAU130 and XCINAU143 with the genetic distances 0.2 and 0.4 cM, respectively, corresponding to a physic interval of 705.14–723.48 Mb referred to the Chinese Spring reference genome sequence v2.1. PmCG15–009 was most likely a new gene differed from the documented Pm genes on chromosome 2BL since its different origin, genetic diversity, and physical position. To analyze and identify the candidate genes, six genes associated with disease resistance in the candidate interval were confirmed to be associated with PmCG15–009 via qRT-PCR analysis using the parents ShiCG15–009 and Yannong 21 and time-course analysis post-inoculation with Bgt isolate E09. To accelerate the transfer of PmCG15–009 using marker-assisted selection (MAS), 18 closely or co-segregated markers were evaluated and confirmed to be suitable for tracing PmCG15–009, when it was transferred into different wheat cultivars.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-023-04132-y.
Keywords: Triticum aestivum L., Powdery mildew, Molecular mapping, PmCG15–009, MAS
Background
Common wheat (Triticum aestivum L., 2n = 6x = 42, AABBDD) is the most widely grown cereal crop throughout the world, which provides approximately 20% of calories for humans [1]. However, the yield and quality of wheat are affected by multiple pathogens. Powdery mildew, caused by Blumeria graminis f. sp. tritici (Bgt), is one of the most common diseases of wheat, with the potential to cause up to 40% grain loss or even worse during severe epidemics [2, 3]. Therefore, it’s significantly important to control the occurrence of powdery mildew. Although chemical and agricultural treatments are the mostly used methods for disease control, resistant cultivars are preferred because of high-efficiency and environmental-friendly therefore their breeding is one of objectives that breeders pursue.
Up to now, 68 formally designated powdery mildew resistance genes at 63 loci (Pm1-Pm68, Pm8 = Pm17, Pm18 = Pm1c, Pm22 = Pm1e, Pm23 = Pm4c, Pm31 = Pm21) have been reported [4, 5]. Most of these genes are race-specific, which is easy to lose resistance with the large-scale deployment in production due to evolution of the pathogen. Although a number of resistance genes have been identified in wheat and its relatives [6], new Bgt isolates continue to emerge to defeat deployed Pm genes. Recent studies indicate that Pm2, Pm3a, Pm3b, Pm3f, Pm4a, Pm6, Pm8, and Pm17 have been overcome in part or all of the USA, while Pm1a, Pm3a, and Pm8 were defeated in Australia, China, and Egypt [7, 8]. Therefore, it is necessary to continuously search for new Pm genes from various resistance sources to reply to the constantly evolved Bgt isolates.
Pm genes currently reported are derived from common wheat or its relatives, including Aegilops squarrosa, Ae. speltoides, Ae. longissima, Ae. ovata, Dasypyrum villosum, T. urartu, T. turgidum var. dicoccoides, T. turgidum var. dicoccum, T. turgidum var. durum, T. timopheevii, T. monococcum, Thinopyrum intermedium, and rye (Secale cereale L.) (http://wheat.pw.usda.gov/). Generally, the genes derived from wild relatives of wheat cannot be directly applied in wheat production due to the poor agronomic traits or other undesirable linkage drag, such as Pm6 derived from T. timopheevii and Pm8 from rye [9, 10], have been widely used in wheat powdery mildew resistance improvement. However, it is a great challenge to eliminate linkage drag associated with alien genes. In fact, nearly half of the reported Pm genes are derived from common wheat, such as Pm52 [11], Pm59 [12] and Pm65 [13]. These genes could be directly applied to breeding practices through conventional cross and backcross ways. Therefore, mining and utilizing novel genes/alleles from common wheat is more attractive to balance resistance and applicability.
Once the novel disease-resistance gene(s) is identified, its accurate and efficient transfer or pyramiding is important in breeding programs. Marker-assisted selection (MAS) based on the gene-linked DNA markers matching the target phenotype is routinely used in the selection of desired characteristics, which is more effective than conventional breeding because it can accelerate the breeding process [14]. In view of this technology, reliable markers are the key factor. So far, although numerous molecular markers related to Pm genes have been developed and identified, most of them are commonly used for gene mapping or cloning, and their effectiveness in different genetic backgrounds needs to be further verified.
Wheat breeding line ShiCG15–009, released from Hebei Province, showed high resistance at the seedling and adult stages to powdery mildew and elite agronomic traits for consecutive years. In the present study, to better clarify and use the powdery mildew resistance in ShiCG15–009, the objectives of this study were to (i) characterize the powdery mildew resistance gene(s) and determine its inheritance; (ii) rapidly map the Pm gene(s); (iii) predict and analyze the candidate genes in the targeted interval; (iv) evaluate and develop the tightly linked or co-segregated markers suitable for MAS.
Results
Inheritance of powdery mildew resistance in ShiCG15–009
When inoculated with isolate E09, ShiCG15–009 was highly resistant with IT 0, whereas Yannong 21 was highly susceptible with IT 4 (Fig. 1; Table 1). All the 10 F1 seedlings of the cross ShiCG15–009 × Yannong 21 were resistant with ITs 0–1, indicating the resistance of ShiCG15–009 to Bgt isolate E09 was controlled by dominant Pm gene(s). Among 115 F2 plants, 79 were resistant with ITs 0–2 and 36 were susceptible with ITs 3–4, fitting a 3:1 ratio (χ2 = 2.11, P = 0.15). Subsequently, all 115 F2 plants were transplanted in the field to generate F2:3 families for the confirmation of the homozygous or heterozygous genotype of the resistant F2 plants. Twenty plants of each F2:3 family were evaluated for powdery mildew response. The ratios of homozygous resistant (RR): segregating (Rr): homozygous susceptible (rr) families from the cross ShiCG15–009 × Yannong 21 were consistent with the expected 1:2:1 (χ2 = 3.42; P = 0.18) (Fig. 1; Table 1). Therefore, we concluded that the resistance to Bgt isolate E09 in ShiCG15–009 was controlled by a single dominant gene, tentatively designated as PmCG15–009. More importantly, wheat line ShiCG15–009 was also resistant to the highly virulent isolates E20 and E31 with IT 0 and IT1, respectively (Table S1).
Fig. 1.
The phenotype of resistant parent ShiCG15–009, susceptible parent Yannong 21, and part of F2 plants about 14 days after inoculation with powdery mildew Blumeria graminis f. sp. tritici (Bgt) isolate E09
Table 1.
Genetic analysis of resistance to Blumeria graminis f. sp. tritici (Bgt) isolate E09 in F1, F2 and F2:3 population from cross ShiCG15–009 and susceptible parent Yannong 21
| Parent and Cross | Generation | Observed ratio | Expected ratio | χ2a | P | ||
|---|---|---|---|---|---|---|---|
| HR | Seg | HS | |||||
| ShiCG15–009 | PR | 10 | |||||
| Yannong 21 | PS | 10 | |||||
| ShiCG15–009 × Yannong 21 | F1 | 10 | |||||
| ShiCG15–009 × Yannong 21 | F2 | 79 | 36 | 3:1 | 2.11 | 0.15 | |
| ShiCG15–009 × Yannong 21 | F2:3 | 22 | 57 | 36 | 1:2:1 | 3.42 | 0.18 |
PR Resistant parent, Ps Susceptible parent, HR Homozygous resistant, Seg Segregating, HS Homozygous susceptible
aValues for significance at P = 0.05 are 3.84 (df = 1) and 5.99 (df = 2)
Molecular mapping of PmCG15–009
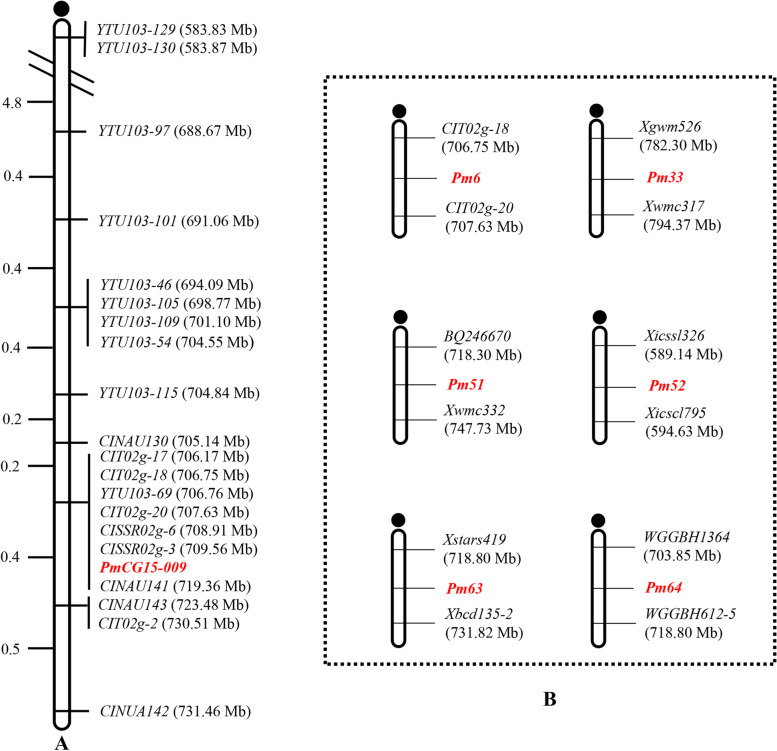
In an initial survey of polymorphism between ShiCG15–009 and Yannong 21 and two DNA bulks with 321 molecular markers distributed across the wheat genome only ten markers which were located on chromosome 2BL amplified consistent polymorphisms between the parents and bulks. Then, these ten markers were genotyped on the entire 115 F2:3 families to map PmCG15–009. To further narrow the mapping interval, based on the Chinese Spring reference genome sequence v2.1 in the targeted region, ten developed SSR markers showed identical polymorphisms between the two parents and two DNA bulks and were also used to genotype the F2:3 families. Finally, PmCG15–009 was flanked by the markers CINAU130 and CINAU143/CIT02g-2 with genetic distances of 0.2 cM and 0.4 cM, respectively, corresponding to 705.14–723.48 Mb physic interval according to the IWGSC Chinese Spring reference genome v2.1 (Figs. 2 and 3, Table 2).
Fig. 2.
Linkage map of PmCG15–009 using the F2:3 families of ShiCG15–009 × Yannong 21 (A) and the physical intervals of documented formally designated powdery mildew resistance genes on chromosome arm 2BL (B). Genetic distances in cM are showed to the left. The black filled circle represents the centromere
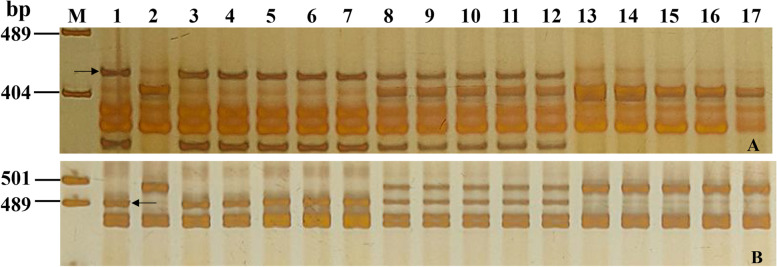
Fig. 3.
Amplification patterns of PmCG15–009-linked markers YTU103–101 (A) and CIT02g–17 (B) in genotyping resistant parent ShiCG15–009, susceptible parent Yannong 21, and randomly selected F2:3 families of ShiCG15–009 × Yannong 21. Lane M: pUC19/MspI; 1: ShiCG15–009; 2: Yannong 21; 3–7: homozygous resistant F2:3 families; 8–12: heterozygous F2:3 families; 13–17: homozygous susceptible F2:3 families. The black arrows were used to indicate the polymorphic bands linked to PmCG15–009
Table 2.
Polymorphic and linkage analysis of the markers linked to the powdery mildew resistance genes located on chromosome arm 2BL using the mapping population derived from the cross of ShiCG15–009 × Yannong 21
| Marker | Resistance genes | Polymorphism | Linkage to PmCG15–009 | Forward primer (5′–3′) | Reverse primer (5′–3′) | Cultivars | References | |
|---|---|---|---|---|---|---|---|---|
| Parents | F2:3 bulks | |||||||
| CIT02g-1 | Pm6 | – | – | – | TGTCACCTACCCATTCAGCT | TTCTCCAATGCTTCGAGTGC | Coker747 | [15] |
| CIT02g-2 | Pm6 | + | + | + | GAGAGCATTCGTCGGTTTCC | ATTCGACCGCCTCAAATCCA | ||
| CIT02g-3 | Pm6 | – | – | – | GACCGTGCCTTCCATTGTTG | TGTTCACACAAGCAGCAAGT | ||
| CIT02g-4 | Pm6 | – | – | – | TGACCCTAAAACAGTCTCAAAGA | TGTTGTAAATGAGAAGTGCACCT | ||
| CIT02g-5 | Pm6 | – | – | – | GGTCACCTTCTTCATAGCGC | GGTCACCTTCTTCATAGCGC | ||
| CIT02g-6 | Pm6 | – | – | – | CGGCATCGTCCAGGAAATG | TGCTTTGGTTCGAGTTGGTG | ||
| CIT02g-7 | Pm6 | – | – | – | CCTCTCTTCCTGTCCCTTATGG | ACTACCGATGAGAGTTCCAGA | ||
| CIT02g-8 | Pm6 | – | – | – | AAGAAAGCGCGCACCATG | GCAGTCCACGAACCGCTC | ||
| CIT02g-9 | Pm6 | – | – | – | AAATCGAAGCCTTGCACCAA | GGACAAAGTGCGCGAAGT | ||
| CIT02g-10 | Pm6 | – | – | – | TGGGACTGGTTAGCACTTGA | CGATGAGGAATAAGTGGGCA | ||
| CIT02g-11 | Pm6 | – | – | – | CAAAGCTTGCAAGATGGGTG | TTCCAGCCCCTCTAGTGATC | ||
| CIT02g-12 | Pm6 | – | – | – | TGGAACGTCTAGACCACAGG | TGGAACGTCTAGACCACAGG | ||
| CIT02g-13 | Pm6 | – | – | – | AGAGAAGTGGAGGTGATGGC | CACGGAGGCTGGGTTCAC | ||
| CIT02g-14 | Pm6 | – | – | – | TCTTCCTCTCTTCCTGTCCC | ACTACCGATGAGAGTTCCAGA | ||
| CIT02g-15 | Pm6 | – | – | – | GAGAGCATTCGTCGGTTTCC | GCTTCCTGGATCATCTGAGC | ||
| CIT02g-16 | Pm6 | – | – | – | GCATCAATAAATCCCTTTCTGCA | TTTCCTCCAGTTCATCGCCC | ||
| CIT02g-17 | Pm6 | + | + | + | CTGGATGAACTTCCCCAAAA | TCAATCTTGAACATCTCCCTCA | ||
| CIT02g-18 | Pm6 | + | + | + | GGCCTTAGTGGTGATGCAGT | GCGGCTTGTCGGTGTATAG | ||
| CIT02g-19 | Pm6 | – | – | – | TCGTTCACACTCAACTCCCA | AGCGAGATCCCATGACTGAC | ||
| CIT02g-20 | Pm6 | + | + | + | CGTGCCTTCCATTGTTGTAT | TGTTCACACAAGCAGCAAGTT | ||
| CIT02g-21 | Pm6 | – | – | – | TTTGGGCCTGCGACGATC | ACGGTGTTATTCCTAGCATGC | ||
| CIT02g-22 | Pm6 | – | – | – | CTCTACGAGCTGTCTTCGCT | TCCCTTGGTAGTACTTGGACA | ||
| CISSR02g-1 | Pm6 | – | – | – | TGTCATTTACTCGTGTGCTTCA | CCTTACGCTTTCCTCATAAACC | ||
| CISSR02g-2 | Pm6 | – | – | – | GACTACAACTACCTTCCCGTGG | AGGATGAAAACCTCGACACACT | ||
| CISSR02g-3 | Pm6 | + | + | + | CTAAACCATAAGCAATCCCCTG | GTCTACAACTACCTTCCCGTGG | ||
| CISSR02g-4 | Pm6 | – | – | – | TTCGTAGGTTTTGTGCATGTTC | AGTTAGGGTAGGAAGAGGTGGG | ||
| CISSR02g-5 | Pm6 | – | – | – | ACTTCCAGCAAATGTTGTAGCC | GTCGAGAGTTGAGGGTCGTC | ||
| CISSR02g-6 | Pm6 | + | + | + | TAAGCAACATCTCATCCCCTTT | GAATACGCCTCCACTCATACCT | ||
| CINAU117 | Pm6 | – | – | – | GACCCAAGAGGCGTTGATTA | CATGTGTGCCAAATTCAAGC | [16] | |
| CINAU118 | Pm6 | – | – | – | GCTGTGACTGCTGGATTCAA | ACCGGGACTGTGTAGACTGG | ||
| CINAU119 | Pm6 | – | – | – | CTTCGTTGCTCGAAAGGTTC | CGGGTGAAACATCTTCTGGT | ||
| CINAU120 | Pm6 | – | – | – | GCCATGGCTAAGGAAGAAGA | ACCTTGGCGAGCTTCTTGAC | ||
| CINAU121 | Pm6 | – | – | – | CCTAGACTGGCCAAGACGAT | ATGGTTTGATTCACCAGCAA | ||
| CINAU122 | Pm6 | – | – | – | CACCTACCTCGTCAACGG | GAGTGCTCCACTGTAAAGCC | ||
| CINAU123 | Pm6 | – | – | – | TTGTACGCCATCGACACATT | CCGAACAGAGTTTTGCCTTC | ||
| CINAU124 | Pm6 | – | – | – | GAGTGCTCCACTGTAAAGCC | CACCTTTGTAGACAGTCCCG | ||
| CINAU125 | Pm6 | – | – | – | CCTCTTCCTGACCATCTTCC | TGACAGTCACTCCAATCACG | ||
| CINAU126 | Pm6 | – | – | – | TCATTTGGTTGCATAGTTGC | AATTTAGCAGTATTCTTAGCTTCCC | ||
| CINAU127 | Pm6 | – | – | – | AATTTAGCAGTATTCTTAGCTTCCC | ATGGGCCGTACAAGAAAGTG | ||
| CINAU128 | Pm6 | – | – | – | TCGAACATGGCTGTGATGAT | GGCTCAGCTTTACCAAGAGC | ||
| CINAU129 | Pm6 | – | – | – | ATCTTGCAGCTTTTGCGTTT | GCTCCCTGACACTCTTGAGG | ||
| CINAU130 | Pm6 | + | + | + | GGCGAGAAAATGTTGTCCAT | AGAAGAGCTGGAGCACCTTG | ||
| CINAU131 | Pm6 | – | – | – | CAACTGCTGGCTCTTCTTCC | GGAACAGCAGCGTCTTCTTC | ||
| CINAU132 | Pm6 | – | – | – | GTGGCTACACCCAAACGG | CAGATCAACGGGAGACATCAC | ||
| CINAU133 | Pm6 | – | – | – | AAGAACCATATCTGGGCTGTC | TACAACAAGATGCCGCAGGCTAACA | ||
| CINAU134 | Pm6 | – | – | – | ATCAACAAGATCTTCGACGG | CTTTGTCTGAACATTGCTGC | ||
| CINAU135 | Pm6 | – | – | – | TTGGTGACGCAGTAATGGAA | TGTGACAGAGCTAGGGCAAG | ||
| CINAU136 | Pm6 | – | – | – | CTGACTGCGCCTTATGTTGA | CCGTGGCTTGATGGAGTCATA | ||
| CINAU137 | Pm6 | – | – | – | GGACAATGAGAAAGCAAAGG | CTTTGCAAGAGCATCAGAGG | ||
| CINAU138 | Pm6 | – | – | – | TTCCCGAAGGACTACCATTG | TCCAGTCACCTCTGGAGCTT | ||
| CINAU139 | Pm6 | – | – | – | CAAAGGAGCCTTTCGATGAG | GGATTCGGGTAGCTTGCATA | ||
| CINAU140 | Pm6 | – | – | – | CACGGTGGAAGTCACTAACC | CAGTTTCCAAGGCATAGGG | ||
| CINAU141 | Pm6 | + | + | + | CACACATGGCAAGTTACAGG | ATCAGACTTGCTTGCTCACC | ||
| CINAU142 | Pm6 | + | + | + | CGACTACGTGACGCTCAAGA | ACTTGTCGTCGAGGAGGATG | ||
| CINAU143 | Pm6 | + | + | + | GTTGGTGGTTGAAAAGATGG | AGTATGCACCTTCGATTTGC | ||
| CINAU144 | Pm6 | – | – | – | GCTCCTCAGCAAATGCCTAC | GATGAAGTGGTGAGCAAGCA | ||
| NAU/STSBCD135–2 | Pm6 | – | – | – | GCTCCGAAGCAAGAGAAGAA | TCTGCTGGTCCTCTGATGTG | [17] | |
| Xwmc317 | Pm33 | – | – | – | TGCTAGCAATGCTCCGGGTAAC | TCACGAAACCTTTTCCTCCTCC | Am9/3 | [18] |
| Xgwm526 | Pm33 | – | – | – | CAATAGTTCTGTGAGAGCTGCG | CCAACCCAAATACACATTCTCA | ||
| BQ246670 | Pm51 | – | – | – | ACATGAGTGAGTTGTGAGTC | AGAAGGCACACTGCTGGAAC | CH7086 | [19] |
| BE444894 | Pm51 | – | – | – | CAATGGGGGTCTTATGGATG | GATGTTGCAGACGGGGTAGT | ||
| BE405017 | Pm51 | – | – | – | CTTACTGGTGGACATGGGCT | CGCAGGGCTATCTTGTTCTC | ||
| Xbarc159 | Pm51 | – | – | – | CGCAATTTATTATCGGTTTTAGGAA | CGCCCGATAGTTTTTCTAATTTCTGA | ||
| Xwmc332 | Pm51 | – | – | – | CATTTACAAAGCGCATGAAGCC | GAAAACTTTGGGAACAAGAGCA | ||
| Cos66 | Pm51 | – | – | – | CACGGTGGAAGTCACTAACC | CAGTTTCCAAGGCATAGGG | ||
| Xicsl34 | Pm52 | – | – | – | GTCCAATCGATCAACTTCAG | GACTAGCTCGCTCTGGATTA | Liangxing 99 | [20] |
| Xicsl62 | Pm52 | – | – | – | AGCAAAGCAATTAGGAGAGTT | CTGCGACTGTTTTCTTTTAAC | ||
| Xicsl90 | Pm52 | – | – | – | AGACTGGGTGCTAGTTGTGT | TGACTTGTCACTGGTTTTCTC | ||
| Xicsl163 | Pm52 | – | – | – | GAGAGTACAAAAGGCAGAGG | ACATAGGGAAATCGAATAAGG | ||
| Xicsl224 | Pm52 | – | – | – | TGCTGTGCTACTTTTGCTACT | TCTCCCAATCTATCAACGTAA | ||
| Xicsl234 | Pm52 | – | – | – | TCTCAGTTTTCACCTCCACTA | CCTTGCTAGAAAAAGGAGAAT | ||
| Xicsl275 | Pm52 | – | – | – | CCGTCCGTATATTCAATTACTC | GCGTTTGCAAGTACAGACTAC | ||
| Xicsl306 | Pm52 | – | – | – | GCGTTTGCAAGTACAGACTAC | GTAGTAAAATGGCAGCAGAGA | ||
| Xicscl437 | Pm52 | – | – | – | CTGTTAGCAAGAACCATTAGG | GGAATAGCTGGAAGTCTTCTG | ||
| Xicscl445 | Pm52 | – | – | – | GGAATAGCTGGAAGTCTTCTG | TAAACAACTCCATGGTTCAGT | ||
| Xicscl726 | Pm52 | – | – | – | GCTGCTGAGTAGCTGTATGAG | CTATCATGGAACTTGCAAAAC | ||
| Xicscl795 | Pm52 | – | – | – | GTCAACCTCATCTTCTCCTG | GTCAACCTCATCTTCTCCTG | ||
| Xicssl173 | Pm52 | – | – | – | GGAAACTCAATTCATCACAAG | GGCTGAGGGTATGTACAAGTAG | ||
| Xicssl174 | Pm52 | – | – | – | AACAAGCTTAACGTGTACCAA | AAAGCTTGCATGCTATAATGT | ||
| Xicssl326 | Pm52 | – | – | – | AAGATGCACTTACCCAAAAAC | TGCTACATATAACTGCTGCTG | ||
| Xwmc175 | Pm52 | – | – | – | GCTCAGTCAAACCGCTACTTCT | CACTACTCCAATCTATCGCCGT | [21] | |
| Xwmc441 | Pm52 | – | – | – | TCCAGTAGAGCACCTTTCATT | ATCACGAAGATAAACAAACGG | ||
| Xgwm120 | Pm52 | – | – | – | GATCCACCTTCCTCTCTCTC | GATTATACTGGTGCCGAAAC | ||
| Xbcd135–2 | Pm63 | – | – | – | GCTCCGAAGCAAGAGAAGAA | TCTGCTGGTCCTCTGATGTG | PI 628024 | [22] |
| Xstars419 | Pm63 | – | – | – | GCCCTTGTCAGTTTCAGTCC | GTCGATCGCTCCACCTCTAC | ||
| Xgwm120 | Pm63 | – | – | – | GATCCACCTTCCTCTCTCTC | GATTATACTGGTGCCGAAAC | ||
| Xwmc175 | Pm63 | – | – | – | GCTCAGTCAAACCGCTACTTCT | CACTACTCCAATCTATCGCCGT | ||
| Xwmc441 | Pm63 | – | – | – | TCCAGTAGAGCACCTTTCATT | ATCACGAAGATAAACAAACGG | ||
| Xwmc332 | Pm63 | – | – | – | CATTTACAAAGCGCATGAAGCC | GAAAACTTTGGGAACAAGAGCA | ||
| WGGBH1364 | Pm64 | – | – | – | CCAAGAAATGGAGTGTTTGA | CAATTATTGGGATCAACACC | WE35 | [23] |
| WGGBH218 | Pm64 | – | – | – | CCTTCCTCCGGTAACTCATA | CGAGCTAGCAATCAGAGAAG | ||
| WGGBH1099 | Pm64 | – | – | – | CGAGCTAGCAATCAGAGAAG | AGGCGGTCTACTGGATTATATGT | ||
| WGGBH913 | Pm64 | – | – | – | ACTGAAACGACAGCTTTTAGG | GGTGAGCTAGTTTGCTCTGTT | ||
| WGGBH252 | Pm64 | – | – | – | GGTGAGCTAGTTTGCTCTGTT | GGATTGGACTATTAGTCAACG | ||
| WGGBH1212 | Pm64 | – | – | – | AACCTCAGTAACCATTGCCAAG | CTCACGCCTTCAACTCATCAG | ||
| WGGBH612–5 | Pm64 | – | – | – | TCTTGCCCTTGTCAGTTTCAG | TACGTGCGAGTAAGAGTAGGAG | ||
| WGGBH134 | Pm64 | – | – | – | AGCTTGAATGAGGATGAAGAGT | CTTCTCTTTCTCCTTCTCCGAA | ||
| WGGBH686 | Pm64 | – | – | – | CAGGGTACTGTATCAGTGTGG | AAGTGATAACACAGCTTGTCG | ||
| WGGBH1260 | Pm64 | – | – | – | GACTTGCTCCTGCCTGCTA | TTCTTGGAATGTTCTGCGTGAT | ||
| YTU130–129 | PmCG15–009 | + | + | + | ATCGGGAAGGCATGGTCAAG | CGAGAGGATAAGGCCGAACC | ShiCG15–0 | |
| YTU130–130 | PmCG15–009 | + | + | + | GTGTACGGCAAGGTGACAGA | ATGGCAAGACTGTGGGTACG | 09 | |
| YTU130–97 | PmCG15–009 | + | + | + | CTAGGGCTGGACCAGTTTGG | AGTTGTGGAAATCGGCGGAT | ||
| YTU130–101 | PmCG15–009 | + | + | + | GGGAGAGCCGTCAAAGAACA | CTTCTCATTTTCTCCGCGCG | ||
| YTU130–46 | PmCG15–009 | + | + | + | CTTCCTCCATTGACCACGCT | GCGAGAGATTCATCCAGCGA | ||
| YTU130–105 | PmCG15–009 | + | + | + | TCGAGGCGCTTCTTCACTTT | TTGCAATGGTGTTGCTCTGC | ||
| YTU130–109 | PmCG15–009 | + | + | + | CCGATTACCTGCAGCTCGAT | TCCAGCTTGGACTTGTCGAC | ||
| YTU130–54 | PmCG15–009 | + | + | + | AGGGCAAAAGATGGAGGTCG | TCGTTCAAGGGCATCAGCAT | ||
| YTU130–115 | PmCG15–009 | + | + | + | AGGAGCTTCATGGCCTTCAC | TCACTGTGAGCGACTGACAC | ||
| YTU130–69 | PmCG15–009 | + | + | + | CGAGCGTGATGTAGACCTCC | GTTTTTCCAGGCCAGCAAGG | ||
“+” represents polymorphic or linked, and “–” represents non-polymorphic or unlinked
Genetic diversity comparison with the documented pm genes on the chromosome 2BL
To identify the relationship between PmCG15–009 and the known formally designated Pm genes on chromosome 2BL, 99 closely linked or co-segregated markers, including 57 for Pm6, two for Pm33, six for Pm51, 18 for Pm52, six for Pm63 and ten for Pm64, were tested the polymorphisms between the resistant and susceptible parents and bulks (Table 2). Among them, only ten markers for Pm6 (CINAU130, CIT02g-17, CIT02g-18, CIT02g-20, CISSR02g-6, CISSR02g-3, CINAU141, CINAU143, CIT02g-2, CINAU142) amplified polymorphisms between the resistant and susceptible parents and bulks and were closely linked or co-segregated with PmCG15–009, while other 89 markers showed no polymorphism. Hence, PmCG15–009 is most likely different from the known Pm genes on chromosome arm 2BL.
Prediction and analysis of candidate genes
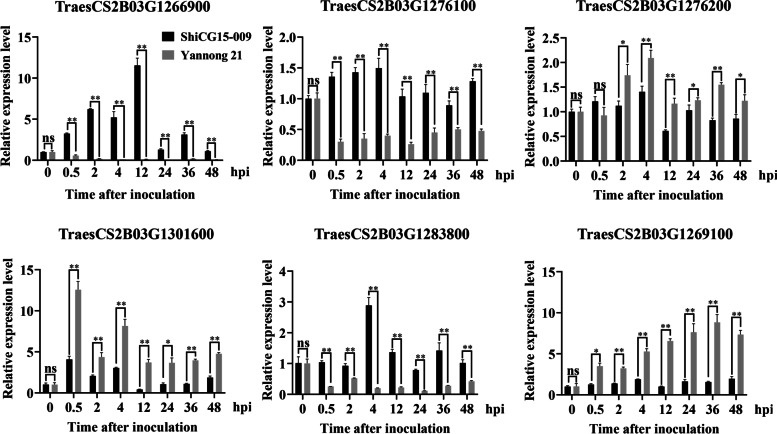
One hundred and ninety-four high confidence genes were annotated in the interval of 705.14–723.48 Mb on chromosome 2BL based on the IWGSC Chinese Spring reference genome v2.1. Among them, only fourteen genes are probably or supposedly associated with disease resistance, including five genes directly related to disease resistance, four genes encoding nucleotide binding site and leucine rich repeat (NBS-LRR) protein, and five genes encoding kinase (Table 3). Then, we used qRT-PCR to investigate the expression patterns of these genes in the resistant parent ShiCG15–009 and susceptible parent Yannong 21 after inoculating with Bgt isolate E09 at different times. As shown in Fig. 4, three genes, including TraesCS2B03G1266900, TraesCS2B03G1276100 and TraesCS2B03G1283800 were induced to express in resistant parent ShiCG15–009, whereas did not change significantly in susceptible parent Yannong 21. In contrast, TraesCS2B03G1276200, TraesCS2B03G1269100 and TraesCS2B03G1301600 were induced in the susceptible parent Yannong 21. The transcript levels of the remaining eight genes were not significantly different between ShiCG15–009 and Yannong 21. Further research is needed to identify the candidate gene for PmCG15–009.
Table 3.
Gene annotation of disease-resistance related in the candidate interval of wheat powdery mildew resistance gene PmCG15–009
| No. | Gene | Physical genomic location | Functional annotation |
|---|---|---|---|
| 1 | TraesCS2B03G1266900 | chr2B:706659806..706669110 | disease resistance |
| 2 | TraesCS2B03G1269100 | chr2B:707563843..707568496 | disease resistance protein |
| 3 | TraesCS2B03G1269800 | chr2B:707673718..707677218 | disease resistance |
| 4 | TraesCS2B03G1276100 | chr2B:712330530..712334826 | disease resistance |
| 5 | TraesCS2B03G1276200 | chr2B:712406234..712410121 | disease resistance |
| 6 | TraesCS2B03G1298600 | chr2B:720465611..720467185 | LRR-repeat protein |
| 7 | TraesCS2B03G1299200 | chr2B:720513971..720515671 | LRR-repeat protein |
| 8 | TraesCS2B03G1300900 | chr2B:721231589..721233256 | LRR-repeat protein |
| 9 | TraesCS2B03G1301600 | chr2B:721291063..721292565 | LRR-repeat protein |
| 10 | TraesCS2B03G1290400 | chr2B:717184726..717191382 | Protein kinase domain |
| 11 | TraesCS2B03G1283800 | chr2B:715213560..715217715 | Serine threonine-protein kinase |
| 12 | TraesCS2B03G1284100 | chr2B:715271978..715273603 | Serine threonine-protein kinase |
| 13 | TraesCS2B03G1272100 | chr2B:709824387..709825406 | cyclin-dependent protein serine/threonine kinase activity |
| 14 | TraesCS2B03G1265500 | chr2B:706166956..706173496 | Phosphatidylinositol-4-phosphate 5-kinase 9 |
Fig. 4.
Expression pattern of TraesCS2B03G1266900, TraesCS2B03G1269100, TraesCS2B03G1276100, TraesCS2B03G1276200, TraesCS2B03G1283800 and TraesCS2B03G1301600 in resistant parent ShiCG15–009 and susceptible parent Yannong 21 after inoculating with Blumeria graminis f. sp. tritici (Bgt) isolate E09 at 0, 0.5, 2, 4, 12, 24, 36 and 48 hours post inoculation (hpi). Normalized values of target genes expression relative to Actin were given as mean ± SD from three replicates. Asterisks indicate significant differences (t-tests) between ShiCG15–009 and Yannong 21 at each time point (*P < 0.05, **P < 0.01, ns: not significant)
Molecular markers for MAS
To better use PmCG15–009 in MAS, 20 markers closely linked or co-segregated with PmCG15–009 were tested for their availability in the 46 susceptible wheat cultivars/lines for MAS. Markers YTU103–130 and CIT02g-18 produced the same genotypes as ShiCG15–009 in 28 and 38 out of the 46 susceptible cultivars/lines, indicating these two markers were not informative despite closely with PmCG15–009. The remaining markers could amplify polymorphic bands between ShiCG15–009 and most of the 46 susceptible cultivars (Fig. 5; Table 4). These results demonstrated that these 18 markers could be used singly or in combination in MAS for tracking PmCG15–009 when transferred into those cultivars.
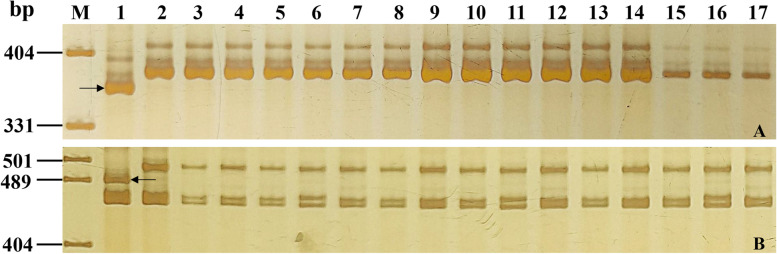
Fig. 5.
Amplification patterns of PmCG15–009-linked markers CISSR02g-6 (A) and CIT02g-17 (B) in ShiCG15–009, Yannong 21 and 15 wheat cultivars/lines susceptible to powdery mildew. M: pUC19/MspI; 1: ShiCG15–009; 2: Yannong 21; 3: Shannong 1538; 4: Hanmai 13; 5: Huaimai 0226; 6: Zhoumai 27; 7: Yannong 1212; 8: Xinong 979; 9: Lumai 185; 10: Zhongyu 1311; 11: Jimai 268; 12: Tainong 1014; 13: Jimai 229; 14: Jimai 21; 15: Jimai 20; 16: Daimai 2173; 17: Zhongmai 1751. The black arrows indicate the polymorphic bands in ShiCG15–009
Table 4.
Validation of PmCG15–009-linked markers on 46 Chinese wheat cultivars/breeding lines and six reference cultivars/lines carrying known genes on the chromosome arm 2BL in marker-assisted selection (MAS) breeding
| Genotypes | Region | Molecular markers | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | ||
| 16P0119 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Daimai 2173 | Shandong | – | – | – | – | – | – | + | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Hanmai 13 | Hebei | – | + | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Huaimai 0226 | Jiangsu | – | – | + | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Huixianhong | Shandong | + | + | + | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Jimai 20 | Shandong | – | – | – | – | + | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Jimai 21 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Jimai 229 | Shandong | – | – | – | – | – | – | + | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Jimai 268 | Shandong | + | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Jinan 17 | Shandong | – | + | + | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Lande 677 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Liangxing 619 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Lumai 185 | Shandong | – | + | + | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Pumai 28 | Henan | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – |
| Qingmai 6 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Shannong 1538 | Shandong | – | + | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Shimai 15 | Hebei | + | + | – | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Taimai 1918 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Tainong 1014 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Womai 8 | Anhui | – | + | + | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – |
| Wunong 6 | Shanxi | – | + | – | – | – | – | – | + | – | – | – | + | – | – | – | – | – | – | – | – |
| Xinluo 4 | Henan | – | – | – | – | + | – | + | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Xinong 979 | Shanxi | – | + | + | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Yannong 1212 | Shandong | – | + | + | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – |
| Yannong 15 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 161 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 17 | Shandong | – | – | – | – | + | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 191 | Shandong | – | – | + | + | – | + | – | + | – | + | + | + | + | + | + | – | – | + | + | + |
| Yannong 199 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 215 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 23 | Shandong | – | + | – | – | – | – | + | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Yannong 24 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 2415 | Shandong | – | + | – | + | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 301 | Shandong | – | + | – | – | – | – | + | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 390 | Shandong | – | + | + | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Yannong 5158 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 572 | Shandong | – | – | + | + | – | + | + | + | + | – | – | + | + | + | + | + | + | + | + | + |
| Yannong 745 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 836 | Shandong | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Yannong 999 | Shandong | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Zhengmai 0856 | Henan | – | + | + | – | – | – | – | – | – | – | – | + | – | + | – | – | – | – | – | – |
| Zhongmai 1751 | Beijing | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Zhongmai 9398 | Beijing | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Zhongxinmai 77 | Hebei | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Zhongyu 1311 | Beijing | – | + | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | – |
| Zhoumai 27 | Henan | – | + | + | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Coker747 (Pm6) | Sweden | – | – | – | – | – | – | – | – | – | + | + | + | – | + | + | + | + | + | + | + |
| Am9/3 (Pm33) | Beijing | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| CH7086 (Pm51) | Shanxi | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Liangxing 99 (Pm52) | Hebei | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| PI 628024 (Pm63) | Iran | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| WE35 (Pm64) | Israel | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
1: YTU103–129; 2: YTU103–130; 3: YTU103–97; 4: YTU103–101; 5: YTU103–46; 6: YTU103–105; 7: YTU103–109; 8: YTU103–54; 9: YTU103–115; 10: CINAU130; 11: CIT02g-17; 12: CIT02g-18; 13: YTU103–69; 14: CIT02g-20; 15: CISSR02g-6; 16: CISSR02g-3; 17: CINAU141; 18: CINAU143; 19: CIT02g-2; 20: CINAU142. ‘-’ represents that the markers can’t amplify the polymorphic products that linked to PmCG15–009 in relevant wheat cultivars/lines, and ‘+’ represents the adverse result
Discussion
The elite wheat breeding line ShiCG15–009 shows a high level of resistance to powdery mildew at the seedling and adult stages. In this study, a dominant gene PmCG15–009 was characterized on the long arm of chromosome 2B in ShiCG15–009, further molecular markers analysis showed that PmCG15–009 was flanked by markers XCINAU130 and XCINAU143 with the genetic distances 0.2 and 0.4 cM, respectively, corresponding to a physic interval of 705.14–723.48 Mb on the Chinese Spring reference genome sequence v2.1 [24]. Previous studies reported that a series of formally designated Pm genes on chromosome 2BL were identified, including dominant genes Pm6 [15], Pm33 [18], Pm51 [19], Pm52 [11], Pm63 [22] and Pm64 [23] which indicated the chromosome 2BL is most likely to be an enrichment region for resistance genes.
Pm6 was derived from T. timopheevii 2B/2G introgression and was moderate to highly susceptible to powdery mildew at the one-leaf stage to the two-leaf stage, but gradually increased resistance from the third leaf stage and reached complete resistance at the fourth leaf stage and later [16]. Wan et al. reported that Pm6 was flanked by markers CIT02g-18 and CIT02g-20, corresponding to the physical interval of 706.75–707.63 Mb and the candidate interval of Pm6 had serious recombination suppression due to the introgression of the 2G chromosome segment. In contrast to those genes, PmCG15–009 (705.14–723.48 Mb), derived from common breeding line ShiCG15–009, was highly resistant to powdery mildew from the first leaf stage to the whole stages shows no significant recombination suppression in our mapping population. Additionally, when tested with 57 co-segregated or closely linked markers of Pm6, only ten markers showed polymorphisms in ShiCG15–009, Yannong 21 and their derivative F2:3 families, which revealed a distinct genetic diversity between the candidate intervals of PmCG15–009 and Pm6. In conclusion, PmCG15–009 was significantly different from Pm6.
Pm33 [18], a dominant powdery mildew resistance gene, was introduced from Triticum carthlicum accession PS5 and was mapped on the interval of 782.3–794.37 Mb. Pm52 [20] was derived from the wheat cultivar Liangxing 99 and flanked by SSR markers Xicssl326 and Xicssl795, referring to the physical interval of 589.14–594.63 Mb. In our study, the dominant gene PmCG15–009 was delimited to an interval of 705.14–723.48 Mb on the Chinese Spring reference genome sequence v2.1, which was significantly different from Pm33 and Pm52 based on the physical interval and/or origins.
Pm51 [19], Pm63 [22] and Pm64 [23] were derived from T. ponticum, Iranian wheat landrace PI 628024, and wild emmer, respectively. Although the physical interval of PmCG15–009 overlapped that of Pm51 (718.30–747.73 Mb), Pm63 (718.80–731.82 Mb,) and Pm64 (703.85–718.80 Mb), their source was different from each other. More importantly, all the closely linked markers or co-segregated markers of these three genes, including six for Pm51, six for Pm63 and ten for Pm64, were not polymorphic between resistant parent ShiCG15–009 and susceptible parent Yannong 21 and two bulks, which indicated a various genetic diversity between the candidate intervals of PmCG15–009 and these of the tested genes. Taken together, PmCG15–009 was different from those documented genes on chromosome 2BL, which might be a novel gene or allele. To further provide more reliable evidence for their relationship, allelism tests and cloning of these genes are necessary in the future to further provide more reliable evidence for their relationship.
So far, 11 race-specific Pm genes have been cloned successively. Among them, Pm3 [25], Pm8 [26], Pm2 [27], Pm17 [6], Pm60 [28], Pm21 [29, 30], Pm5e [31], Pm41 [32] and Pm1a [33] encoded coiled-coil nucleotide-binding site leucine-rich repeat protein (CC-NBS-LRR). Pm4 [34] and Pm24 [35] encoded a putative serine/threonine kinase and tandem kinase protein (TKP) with putative kinase-pseudokinase domains, respectively. In plants, NLR proteins and protein kinases are the major classes of disease resistance genes. NLR functions as intracellular immune receptor that recognizes pathogen effectors and activates effector-triggered immunity (ETI) and protein kinases are important for transmembrane signaling that regulates plant development and adaptation to diverse environmental conditions [36, 37]. In the candidate interval of PmCG15–009, 194 high confidence genes were annotated based on the IWGSC Chinese Spring reference genome v2.1, and only 14 genes are associated with disease resistance. Furtherly, qRT-PCR analysis showed that the transcript levels of six genes were induced at different degree by the Bgt isolate E09 between the resistant parent ShiCG15–009 and susceptible parent Yannong 21. Notably, the gene TraesCS2B03G1283800, encoding a serine threonine-protein kinase, showed high expression in ShiCG15–009 at 4 hpi following Bgt inoculation. TraesCS2B03G1276100 and TraesCS2B03G1266900 were significantly upregulated in resistant parent ShiCG15–009 but not changed in susceptible Yannong 21. Considering the expression patterns, these genes could be the candidate gene of PmCG15–009 or regulatory genes involved in the resistance process. These data provide a significant direction at dissecting the resistance pathways. Of course, further studies are needed to investigate whether these genes are candidate genes of PmCG15–009.
When a novel gene was discovered, the rational utilization was the next challenge in wheat breeding programs. The elite wheat breeding line ShiCG15–009 showed not only highly resistance to powdery mildew at all the stages but excellent agronomic traits, thus should be a valuable resource for genetic research and wheat resistance improvement. To accelerate the transfer of PmCG15–009 in MAS, we evaluated the availability of 20 markers linked or co-segregated with PmCG15–009 in 46 susceptible commercial cultivars/lines. The results showed that 18 of 20 markers could be used singly or in combination in MAS for tracking PmCG15–009 in the background of those susceptible cultivars. Also, we have made many hybrid combinations between ShiCG15–009 and several susceptible commercial wheat cultivars and obtained the BC1F2 and F3 segregation populations. In future, PmCG15–009 will play an important role in wheat breeding programs.
Conclusion
In the present study, a dominant powdery mildew resistance gene PmCG15–009 was identified in wheat breeding line ShiCG15–00 and located within 705.14–723.48 Mb on chromosome 2BL. Based on the physical position, origin and genetic diversity, PmCG15–009 is most likely a novel Pm gene. 18 molecular markers available for marker-assisted selection were selected for tracking PmCG15–009 in breeding. Our study can be valuable for theoretical research and wheat breeding application.
Materials and methods
Plant materials and pathogen isolates
Wheat breeding line ShiCG15–009, released from Hebei Province, was highly resistant to powdery mildew at the adult and seedling stages, whereas wheat cultivar Yannong 21 was highly susceptible. The F1, F2, and F2:3 populations, derived from the cross of ShiCG15–009 and Yannong 21, were used to map the powdery mildew resistance gene(s) in ShiCG15–009. Wheat cultivar Mingxian 169 which didn’t carry any known Pm gene was used as the susceptible control for phenotypic identification and served as the Bgt inoculum spreader. The Bgt isolate E09, collected from Beijing city in 1993 and currently prevalent in the main wheat producing regions of China, which was virulent to powdery mildew resistance gene Pm6 and avirulent to Pm33, Pm51, Pm52, Pm63 and Pm64 on the chromosome arm 2BL [38], was used to evaluate the mapping populations. Prevalent powdery mildew Bgt isolates E20 and E31 with broad virulent spectrum were also used to test the wheat breeding line ShiCG15–009 (Table S1).
Reactions to powdery mildew at the seedling stage
Resistance evaluation to powdery mildew was carried out in a greenhouse. Seedlings were grown in rectangular trays (54 × 28 × 4.2 cm), each tray had 128 cells (3.2 × 3.2 × 4.2 cm) and the susceptible check Mingxian 169 was planted with three cells randomly in the trays. For the F2:3 families derived from the cross ShiCG15–009 and Yannong 21, each of the families was tested with at least 20 seeds to confirm the genotype of the F2 plants. At the one-leaf stage, all seedlings were inoculated with fresh conidiospores increased on Mingxian 169 seedlings and incubated at a greenhouse with a daily cycle of 14 h of light at 22 °C and 10 h of darkness at 18 °C. 10–14 days later, when the spores were fully developed on the first leave of susceptible control Mingxian 169, infection types (ITs) on each plant were assessed on a 0–4 scale, of which 0 = no visible symptoms and signs, 0; = necrotic flecks without sporulation, 1 = sparse aerial hypha and little sporulation, the diameter of colonies less than 1 mm, 2 = moderate aerial hypha and sporulation, diameter of colonies less than 1 mm, 3 = thick aerial hypha and abundant sporulation, diameter of colonies more than 1 mm, and 4 = abundant sporulation with more than 80% of the leaf area covered with aerial hypha, with IT 0, 0;, 1 and 2 being regarded as resistant, and IT 3 and 4 as susceptible [39]. All tests were repeated three times to assure the reliability of the data.
Marker analysis
Total genomic DNA was extracted from young leaf tissues following a procedure described by Sharp et al. (1988) [40]. The resistant and susceptible DNA bulks which consisted of 20 homozygous resistant and 20 homozygous F2:3 families of ShiCG15–009 and Yannong 21 were used in DNA-based Bulked Segregant Analysis (BSA) to validate polymorphic markers [41].
Three hundred and twenty one molecular markers evenly distributed across all the chromosomes [42–47] were selected for an initial survey of polymorphism between resistant and susceptible parents and bulks. Then, the polymorphic markers between the parents and the bulks were used to genotype the F2:3 families of ShiCG15–009 and Yannong 21 for mapping of the Pm gene(s) in ShiCG15–009. In addition, 200 markers based on the simple sequence repeat (SSR) in the target region on chromosome 2BL were designed and were also used to genotype the F2:3 families. The corresponding genomic sequences of PmCG15–009 target region were used as templates to search SSR with the software SSR Hunter, and the parameters as follows: the number of nucleotide repeat units is one to six bp and the number of repeats is more than five. The SSR markers were designed with Primer 5 software. Polymorphism of SSR markers were examined using the parents and the contrasting DNA bulks.
PCR amplification was performed with a 10 μl volume which contained 5 μl 2 × Taq Master Mix (Vazyme, China), 1 μl 50 ng/μl template DNA and 0.5 μl 10 μM/μl primers. The PCR amplification conditions were as follows: pre-denaturation at 94 °C for 5 min followed by 36 cycles of 94 °C for 30 s, 50 to 65 °C (depending on the specific primers) for 40 s, 72 °C for 40 s to 120 s (depending on the target bands), finally extension at 72 °C for 10 min and preservation at 25 °C. PCR products were separated in 8% non-denaturing polyacrylamide gels with a 29:1 ratio of acrylamide and bis-acrylamide, then silver stained and visualized as previously described [48].
Statistical analysis
After obtaining phenotypic data and the genotypic data of the F2:3 families derived from the cross ShiCG15–009 and Yannong 21, Chi-squared (χ2) tests for goodness-of-fit were used to evaluate deviations of observed data from expected segregation ratios. The software MAPMAKER/Exp (version 3.0b) was used to determine linkage with a LOD score of 3.0 as the threshold for declaration of linkage [49]. Genetic distances were estimated from recombination values using the Kosambi mapping function [50].
Genetic diversity comparison with the documented pm genes on the chromosome 2BL
To investigate the genetic diversity of the candidate interval of Pm gene(s) in ShiCG15–009 and the known Pm genes on chromosome 2BL. 99 markers closely linked to those Pm genes were tested for polymorphisms between resistant parent ShiCG15–009 and susceptible parent Yannong 21 and their derived resistant and susceptible bulks.
Prediction and analysis of candidate genes
The flanked markers were aligned to Chinese Spring reference genome sequence v2.1 to obtain the corresponding physical interval of the candidate gene(s) in ShiCG15–009. Then, the annotated disease resistance genes within the mapped interval were used to analyze the expression patterns between resistant parent ShiCG15–009 and susceptible parent Yannong 21 after inoculating with the Bgt isolate E09 at different times.
Total RNA of ShiCG15–009 and Yannong 21 were extracted from leaves after inoculating Bgt isolate E09 at 0, 0.5, 2, 4, 12, 24, 36 and 48 hpi using TRIzol reagent (Invitrogen, USA). About 2 μg of RNA was used for reverse transcription with a FastQuant RT Kit (Tiangen, China). The qRT-PCR assays were performed using SYBR Premix Ex Taq (Takara, China) on the Bio-Rad CFX Connect real-time PCR system (BIO-RAD, USA). The expression pattern of each gene was calculated as a fold change using the comparative CT method [51]. For each sample, three technical replications were analyzed. The TaActin was used as the internal control for normalization. Primers used in this study were listed in Table S1.
Evaluation of the markers for MAS
The 46 powdery mildew-susceptible wheat cultivars/lines from different major wheat producing regions and six reference cultivars/lines carrying known genes on the chromosome arm 2BL, including Coker747 (Pm6), Am9/3 (Pm33), CH7086 (Pm51), Liangxing 99 (Pm52), PI 628024 (Pm63) and WE35 (Pm64) were tested by using the flanked or co-segregated markers. If the polymorphic band(s) amplified by a marker were all same for ShiCG15–009 and the tested cultivars, this marker could not be used for MAS. However, the bands amplified in ShiCG15–009 were different from the tested cultivars, indicating that the marker was considered to be available for MAS in those genetic backgrounds.
Supplementary Information
Additional file 1: Table S1. The virulence frequency of Blumeria graminis f. sp. tritici (Bgt) isolates E09, E31 and E20.
Additional file 2: Fig. S1. The original and unprocessed amplification patterns of PmCG15-009-linked markers YTU103–101 in genotyping resistant parent ShiCG15–009, susceptible parent Yannong 21, and randomly selected F2:3 families of ShiCG15–009 × Yannong 21. Fig. S2. The original and unprocessed amplification patterns of PmCG15–009-linked markers CIT02g–17 in genotyping resistant parent ShiCG15–009, susceptible parent Yannong 21, and randomly selected F2:3 families of ShiCG15–009 × Yannong 21. Fig. S3. The original and unprocessed amplification patterns of PmCG15–009-linked markers CISSR02g-6 in ShiCG15–009, Yannong 21 and 15 wheat cultivars/lines susceptible to powdery mildew. Fig. S4. The original and unprocessed amplification patterns of PmCG15–009-linked markers CIT02g-17 in ShiCG15–009, Yannong 21 and 15 wheat cultivars/lines susceptible to powdery mildew.
Acknowledgements
We are grateful to Prof. Hongxing Xu, School of Life Sciences, Henan University for providing Blumeria graminis f. sp. tritici isolates.
Guideline statement
The authors confirm that all methods were carried out in accordance with relevant guidelines and regulations.
Authors’ contributions
PM, YJ and TY conceived the research. WZ, ZY, DW, LX and FS performed the experiments. YM, JZ, LL and YY developed the experimental materials. WZ, ZY, DW and LL performed the phenotypic assessment. YJ wrote the manuscript. All authors read and approved the final manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (32072053) and (31871450), the Key Research and Development Project of Shandong Province (2020CXGC010805) and Key Research and Development Project of Yantai City (2022XCZX092).
Availability of data and materials
All the data generated or analyzed during the current study were included in the manuscript. The raw data is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All methods complied with relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The author(s) declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wenjing Zhang, Ziyang Yu and Dongmei Wang contributed equally to this work.
Contributor Information
Tianying Yu, Email: tyyubj@sina.com.
Yuli Jin, Email: yulijin@ytu.edu.cn.
Pengtao Ma, Email: ptma@ytu.edu.cn.
References
- 1.Isham K, Wang R, Zhao WD, Wheeler J, Klassen N, Akhunov E, et al. QTL mapping for grain yield and three yield components in a population derived from two high-yielding spring wheat cultivars. Theor Appl Genet. 2021;134:2079–2095. doi: 10.1007/s00122-021-03806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang WR, He HG, Gao HM, Xu HX, Song WY, Zhang X, et al. Characterization of the powdery mildew resistance gene in wheat breeding line KN0816 and its evaluation in marker-assisted selection. Plant Dis. 2021;105:4042–4050. doi: 10.1094/PDIS-05-21-0896-RE. [DOI] [PubMed] [Google Scholar]
- 3.Singh RP, Singh PK, Rutkoski J, Hodson DP, He X, Jørgensen LN, et al. Disease impact on wheat yield potential and prospects of genetic control. Annu Rev Phytopathol. 2016;54:303–322. doi: 10.1146/annurev-phyto-080615-095835. [DOI] [PubMed] [Google Scholar]
- 4.He HG, Liu RK, Ma PT, Du HN, Zhang HH, Wu QH, et al. Characterization of Pm68, a new powdery mildew resistance gene on chromosome 2BS of greek durum wheat TRI 1796. Theor Appl Genet. 2021;134:53–62. doi: 10.1007/s00122-020-03681-2. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh RA, Dubcovsky J, Rogers WJ, Xia XC, Raupp WJ. Catalogue of Gene Symbols For Wheat 2020 Supplement. 2020. [Google Scholar]
- 6.Singh SP, Hurni S, Ruinelli M, Brunner S, Sanchez-Martin J, Krukowski P, et al. Evolutionary divergence of the rye Pm17 and Pm8 resistance genes reveals ancient diversity. Plant Mol Biol. 2018;98:249–260. doi: 10.1007/s11103-018-0780-3. [DOI] [PubMed] [Google Scholar]
- 7.Parks R, Carbone I, Murphy JP, Marshall D, Cowger C. Virulence structure of the eastern U.S. wheat powdery mildew population. Plant Dis. 2008;92:1074–1082. doi: 10.1094/PDIS-92-7-1074. [DOI] [PubMed] [Google Scholar]
- 8.Cowger C, Mehra L, Arellano C, Meyers E, Murphy JP. Virulence differences in Blumeria graminis f. sp. tritici from the central and eastern United States. Phytopathology. 2018;108:402–411. doi: 10.1094/PHYTO-06-17-0211-R. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen JH, Jensen CJ. Gene Pm6 for resistance to powdery mildew in wheat. Euphytica. 1973;22:423. doi: 10.1007/BF00022656. [DOI] [Google Scholar]
- 10.McIntosh RA, Zhang P, Cowger C, Parks R, Lagudah ES, Hoxha S. Rye-derived powdery mildew resistance gene Pm8 in wheat is suppressed by the Pm3 locus. Theor Appl Genet. 2011;123:359–367. doi: 10.1007/s00122-011-1589-5. [DOI] [PubMed] [Google Scholar]
- 11.Zou JW, Qiu D, Sun YL, Zheng CX, Li JT, Wu PP, et al. Pm52: effectiveness of the gene conferring resistance to powdery mildew in wheat cultivar Liangxing 99. Acta Agron Sin. 2017;43:332–342. doi: 10.3724/SP.J.1006.2017.00332. [DOI] [Google Scholar]
- 12.Tan CC, Li GQ, Cowger C, Carver BF, Xu XY. Characterization of Pm59, a novel powdery mildew resistance gene in Afghanistan wheat landrace PI 181356. Theor Appl Genet. 2018;131:1145–1152. doi: 10.1007/s00122-018-3067-9. [DOI] [PubMed] [Google Scholar]
- 13.Li GQ, Cowger C, Wang XW, Carver BF, Xu XY. Characterization of Pm65, a new powdery mildew resistance gene on chromosome 2AL of a facultative wheat cultivar. Theor Appl Genet. 2019;132:2625–2632. doi: 10.1007/s00122-019-03377-2. [DOI] [PubMed] [Google Scholar]
- 14.Huang XQ, Wang LX, Xu MX, Röder MS. Microsatellite mapping of the powdery mildew resistance gene Pm5e in common wheat (Triticum aestivum L.) Theor Appl Genet. 2003;106:858–865. doi: 10.1007/s00122-002-1146-3. [DOI] [PubMed] [Google Scholar]
- 15.Wan WT, Xiao JX, Li ML, Tang X, Wen MX, Cheruiyot AK, et al. Fine mapping of wheat powdery mildew resistance gene Pm6 using 2B/2G homoeologous recombinants induced by the ph1b mutant. Theor Appl Genet. 2020;133:1265–1275. doi: 10.1007/s00122-020-03546-8. [DOI] [PubMed] [Google Scholar]
- 16.Qin B, Cao AZ, Wang HY, Chen TT, You FM, Liu YY, et al. Collinearity-based marker mining for the fine mapping of Pm6, a powdery mildew resistance gene in wheat. Theor Appl Genet. 2011;123:207–218. doi: 10.1007/s00122-011-1577-9. [DOI] [PubMed] [Google Scholar]
- 17.Ji JH, Qin B, Wang HY, Cao AZ, Wang SL, Chen PD, et al. STS markers for powdery mildew resistance gene Pm6 in wheat. Euphytica. 2008;163:159–165. doi: 10.1007/s10681-007-9578-0. [DOI] [Google Scholar]
- 18.Zhu ZD, Zhou RH, Kong XY, Dong YC, Jia JZ. Microsatellite markers linked to 2 powdery mildew resistance genes introgressed from Triticum carthlicum accession PS5 into common wheat. Genome. 2005;48:585–590. doi: 10.1139/g05-016. [DOI] [PubMed] [Google Scholar]
- 19.Zhan HX, Li GR, Zhang XJ, Li X, Guo HJ, Gong WP, et al. Chromosomal location and comparative genomics analysis of powdery mildew resistance gene Pm51 in a putative wheat-Thinopyrum ponticum introgression line. PLoS One. 2014;9:e113455. doi: 10.1371/journal.pone.0113455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu PP, Hu JH, Zou JW, Qiu D, Qu YF, Li YH, et al. Fine mapping of the wheat powdery mildew resistance gene Pm52 using comparative genomics analysis and the Chinese spring reference genomic sequence. Theor Appl Genet. 2019;132:1451–1461. doi: 10.1007/s00122-019-03291-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhao ZH, Sun HG, Song W, Lu M, Huang J, Wu LF, et al. Genetic analysis and detection of the gene MlLX99 on chromosome 2BL conferring resistance to powdery mildew in the wheat cultivar Liangxing 99. Theor Appl Genet. 2013;126:3081–3089. doi: 10.1007/s00122-013-2194-6. [DOI] [PubMed] [Google Scholar]
- 22.Tan CC, Li GQ, Cowger C, Carver BF, Xu XY. Characterization of Pm63, a powdery mildew resistance gene in Iranian landrace PI 628024. Theor Appl Genet. 2019;132:1137–1144. doi: 10.1007/s00122-018-3265-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang DY, Zhu KY, Dong LL, Liang Y, Li GQ, Fang TL, et al. Wheat powdery mildew resistance gene Pm64 derived from wild emmer (Triticumturgidum var. dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5. Crop J. 2019;7:761–770. doi: 10.1016/j.cj.2019.03.003. [DOI] [Google Scholar]
- 24.Zhu TT, Wang L, Rimbert H, Rodriguez JC, Deal KR, Oliveira RD, et al. Optical maps refine the bread wheat Triticum aestivum cv. Chinese Spring genome assembly. Plant J. 2021;107:303–314. doi: 10.1111/tpj.15289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yahiaoui N, Srichumpa P, Dudler R, Keller B. Genome analysis at different ploidy levels allows cloning of the powdery mildew resistance gene Pm3b from hexaploid wheat. Plant J. 2004;37:528–538. doi: 10.1046/j.1365-313X.2003.01977.x. [DOI] [PubMed] [Google Scholar]
- 26.Hurni S, Brunner S, Buchmann G, Herren G, Jordan T, Krukowski P, et al. Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 2013;76:957–969. doi: 10.1111/tpj.12345. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Martín J, Steuernagel B, Ghosh S, Herren G, Hurni S, Adamski N, et al. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016;17:221. doi: 10.1186/s13059-016-1082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou SH, Wang H, Li YW, Kong ZS, Tang DZ. The NB-LRR gene Pm60 confers powdery mildew resistance in wheat. New Phytol. 2018;218:298–309. doi: 10.1111/nph.14964. [DOI] [PubMed] [Google Scholar]
- 29.He HG, Zhu SY, Zhao RH, Jiang ZN, Ji YY, Ji J, et al. Pm21, encoding a typical CC-NBS-LRR protein, confers broad-spectrum resistance to wheat powdery mildew disease. Mol Plant. 2018;11:879–882. doi: 10.1016/j.molp.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Xing LP, Hu P, Liu JQ, Witek K, Zhou S, Xu JF, et al. Pm21 from Haynaldia villosa encodes a CC-NBS-LRR protein conferring powdery mildew resistance in wheat. Mol Plant. 2018;11:874–878. doi: 10.1016/j.molp.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Xie JZ, Guo GH, Wang Y, Hu TZ, Wang LL, Li JT, et al. A rare single nucleotide variant in Pm5e confers powdery mildew resistance in common wheat. New Phytol. 2020;228:1011–1026. doi: 10.1111/nph.16762. [DOI] [PubMed] [Google Scholar]
- 32.Li MM, Dong LL, Li BB, Wang ZZ, Xie JZ, Qiu D, et al. A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol. 2020;228:1027–1037. doi: 10.1111/nph.16761. [DOI] [PubMed] [Google Scholar]
- 33.Hewitt T, Müller MC, Molnár I, Mascher M, Holušová K, Šimková H, et al. A highly differentiated region of wheat chromosome 7AL encodes a Pm1a immune receptor that recognizes its corresponding AvrPm1a effector from Blumeria graminis. New Phytol. 2021;229:2812–2826. doi: 10.1111/nph.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sánchez-Martín J, Widrig V, Herren G, Wicker T, Zbinden H, Gronnier J, et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat Plants. 2021;7:327–341. doi: 10.1038/s41477-021-00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu P, Guo L, Wang ZZ, Li BB, Li J, Li YH, et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat Commun. 2020;11:680. doi: 10.1038/s41467-020-14294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 37.Liang XX, Zhou JM. Receptor-like cytoplasmic kinases: central players in plant receptor kinase-mediated signaling. Annu Rev Plant Biol. 2018;69:267–299. doi: 10.1146/annurev-arplant-042817-040540. [DOI] [PubMed] [Google Scholar]
- 38.Zhou RH, Zhu ZD, Kong XY, Huo NX, Tian QZ, Li P, et al. Development of wheat near-isogenic lines for powdery mildew resistance. Theor Appl Genet. 2005;110:640–648. doi: 10.1007/s00122-004-1889-0. [DOI] [PubMed] [Google Scholar]
- 39.Si QM, Zhang XX, Duan XY, Sheng BQ, Zhou YL. On gene analysis and classification of powdery mildew (Erysiphe graminis f. sp. tritici) resistant wheat varieties. Acta Phytopathol Sin. 1992;22:349–355. [Google Scholar]
- 40.Sharp PJ, Kreis M, Shewry PR, Gale MD. Location of β-amylase sequences in wheat and its relatives. Theor Appl Genet. 1988;75:286–290. doi: 10.1007/BF00303966. [DOI] [Google Scholar]
- 41.Michelmore RW, Paran I, Kesseli RV. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci U S A. 1991;88:9828–9832. doi: 10.1073/pnas.88.21.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, Leroy P, et al. A microsatellite map of wheat. Genetics. 1998;149:2007–2023. doi: 10.1093/genetics/149.4.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paillard S, Schnurbusch T, Winzeler M, Messmer M, Sourdille P, Abderhalden O, et al. An integrative genetic linkage map of winter wheat (Triticum aestivum L.) Theor Appl Genet. 2003;107:1235–1242. doi: 10.1007/s00122-003-1361-6. [DOI] [PubMed] [Google Scholar]
- 44.Somers DJ, Isaac P, Edwards K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.) Theor Appl Genet. 2004;109:1105–1114. doi: 10.1007/s00122-004-1740-7. [DOI] [PubMed] [Google Scholar]
- 45.Sourdille P, Singh S, Cadalen T, Brown-Guedira GL, Gay G, Qi L, et al. Microsatellite-based deletion bin system for the establishment of genetic-physical map relationships in wheat (Triticum aestivum L.) Funct Integr Genomics. 2004;4:12–25. doi: 10.1007/s10142-004-0106-1. [DOI] [PubMed] [Google Scholar]
- 46.Song QJ, Shi JR, Singh S, Fickus EW, Costa JM, Lewis J, et al. Development and mapping of microsatellite (SSR) markers in wheat. Theor Appl Genet. 2005;110:550–560. doi: 10.1007/s00122-004-1871-x. [DOI] [PubMed] [Google Scholar]
- 47.Xue SL, Zhang ZZ, Lin F, Kong ZX, Cao Y, Li CJ, et al. A high-density intervarietal map of the wheat genome enriched with markers derived from expressed sequence tags. Theor Appl Genet. 2008;117:181–189. doi: 10.1007/s00122-008-0764-9. [DOI] [PubMed] [Google Scholar]
- 48.Santos FR, Pena SD, Epplen JT. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum Genet. 1993;90:655–656. doi: 10.1007/BF00202486. [DOI] [PubMed] [Google Scholar]
- 49.Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, et al. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- 50.Kosambi DD. The estimation of map distances from recombination values. Ann Eugenics. 1943;12:172–175. doi: 10.1111/j.1469-1809.1943.tb02321.x. [DOI] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. The virulence frequency of Blumeria graminis f. sp. tritici (Bgt) isolates E09, E31 and E20.
Additional file 2: Fig. S1. The original and unprocessed amplification patterns of PmCG15-009-linked markers YTU103–101 in genotyping resistant parent ShiCG15–009, susceptible parent Yannong 21, and randomly selected F2:3 families of ShiCG15–009 × Yannong 21. Fig. S2. The original and unprocessed amplification patterns of PmCG15–009-linked markers CIT02g–17 in genotyping resistant parent ShiCG15–009, susceptible parent Yannong 21, and randomly selected F2:3 families of ShiCG15–009 × Yannong 21. Fig. S3. The original and unprocessed amplification patterns of PmCG15–009-linked markers CISSR02g-6 in ShiCG15–009, Yannong 21 and 15 wheat cultivars/lines susceptible to powdery mildew. Fig. S4. The original and unprocessed amplification patterns of PmCG15–009-linked markers CIT02g-17 in ShiCG15–009, Yannong 21 and 15 wheat cultivars/lines susceptible to powdery mildew.
Data Availability Statement
All the data generated or analyzed during the current study were included in the manuscript. The raw data is available from the corresponding author on reasonable request.